Abstract
It is difficult to pack chromatographic particles having polymeric-bonded phases because solvents used for making a stable slurry cause the polymer layer to swell. Growth of the polymer inside the column (in situ) after packing was investigated and compared with conventional, ex situ polymer growth. The method of activators generated by electron transfer, along with atom-transfer radical polymerization, enabled polymerization under ambient conditions. Nonporous, 0.62 µm silica particles with silane initiators were used. Polyacrylamide films with a hydrated thickness of 23 nm in 75:25 water/isopropanol grew in 55 min for both in situ and ex situ preparations, and the same carbon coverage was observed. Higher chromatographic resolution and better column-to-column reproducibility were observed for in situ polymer growth, as evaluated by hydrophilic interaction liquid chromatography for the model glycoprotein, ribonuclease B. In situ polymer growth was also found to give lower eddy diffusion, as shown by a narrower peak width for injected acetonitrile in 50:50 acetonitrile/water. When columns were packed more loosely, bed collapse occurred quickly for ex situ, but not for in situ, polymer growth. The higher resolution and stability for in situ polymer growth is explained by packing with hard, rather than soft, contacts between particles.
Keywords: glycoproteins, hydrophilic interaction liquid chromatography, packing, polymer, stationary phases
1 INTRODUCTION
Polymeric materials have long been a mainstay in protein chromatography, including gel filtration [1], ion-exchange [2], antibody affinity [3], and hydrophobic interaction [4] resins. More recently monolithic columns formed in situ by cross-linked polymers enable high performance chromatography [5], and molecularly imprinted polymers are used in SPE columns [6]. Grafted bonded phases of polymer chains onto polymer or silica beads are increasingly being used. These have been enabled by the development of solutionphase atom-transfer radical polymerization (ATRP) [7], which allows for controlled molecular weight of linear polymer chains. ATRP was first used to make bonded phases for LC by attaching a silane initiator to silica gel, giving surface-confined growth of polymer chains, and this was demonstrated for size-exclusion chromatography of proteins [8]. Since then, a host of bonded phases have been made for protein chromatography using ATRP, including metal affinity chromatography [9], ion chromatography [10], hydrophobic interaction chromatography [11], hydrophobic charge induction chromatography [12], thermally responsive bonded phases [13], HILIC [14], and lectin affinity chromatography [15]. Furthermore, ATRP-generated polymer surfaces are utilized in other analytical separations and applications [16]. The versatility of ATRP has made polymeric-bonded phases of broad interest in protein chromatography.
Uniform packing of chromatography columns is a longstanding problem, as it has been well documented that columns have radial heterogeneity [17]. The introduction of sonication during slurry-packing for submicrometer particles provides high radial uniformity in capillaries by promoting rapid transverse transport of particles during packing, shown by an eddy diffusion term of less than 10 nm [18]. Extremely long LC columns, 1 m in length, have shown improved eddy diffusion using sonication during slurry packing of 2 µm particles, giving reduced plate heights on the order of unity and plate number in excess of 50 0000 [19]. The challenge of uniform packing remains unmet for particles with polymer bonded phases. For example, 0.7 µm silica particles with bonded phases of linear polyacrylamide chains, when used for HILIC, gave a very high eddy diffusion term of 20 µm for a 2.1 mm I.D. column [14].
One reason for the difficulty in packing polymer-coated particles is a compromise in the choice of solvent for the slurry. A solvent that stably suspends the polymer-coated particles also causes the polymer layer to swell, which impedes tight packing because this layer must be compressed. This issue is illustrated in Fig. 1A. Decompression of the column after packing would cause the polymer layer at the contact points to re-swell, giving a lower packing density. Further, the cycling of swelling and contraction, due to pressurization/depressurization and cycling from the gradient, is possibly why columns with polymer-bonded phases have notoriously short lifetimes. The problems with polymer swelling would conceivably be avoided by first packing the particles when they have only the small-molecule initiator on the surface, then growing the polymer in situ by flowing the ATRP reagents through the packed column, as illustrated in Fig. 1B. For submicrometer particles, this would give the additional advantage of minimizing non-Newtonian rheology, which also impedes uniform packing. This can be understood from a study of slurries of 0.6 µm silica particles, which shows that these exhibit shear-thinning at high slurry concentration [20], which would augment transverse mass transport of the particles during packing for radial homogeneity. By contrast, when the same particles are coated with the hydrophilic polymer, PEG, these exhibit shear-thickening at high slurry concentration, which increases viscosity [20]. Both polymer swelling and increased slurry viscosity work against uniform packing of columns.
FIGURE 1.
Depiction of the structural distinction between columns packed with particles that (A) had polymer grown before packing (ex situ) versus (B) particles containing initiator but no polymer, with the polymer subsequently grown on the particle surface inside the column (in situ)
The purpose of this work is to test whether growth of the polymer-bonded phase inside the column, i.e., in situ, improves packing. In situ growth of an anionic copolymer on silica particles has very recently been used for capillaries, resulting in minimal swelling-shrinking behavior, a greater mechanical stability, and higher column efficiency than polymer-based monoliths [21]. The in situ and ex situ columns are compared for the case of HILIC using a bonded phase of linear polyacrylamide chains. The previous method of ATRP for preparing HILIC columns [14], where a small amount of Cu(I) was used as catalyst, would be cumbersome for in situ polymer growth because oxygen-free conditions are required. In situ growth is facilitated here by employing AGET ATRP (AGET is activators generated by electron transfer) [22]. AGET maintains a low concentration of Cu(I) through electrochemical reduction of Cu(II) by concentrated ascorbate, thereby buffering against loss of Cu(I) from atmospheric oxygen. Both in situ and ex situ polymer growth are carried out in this work using AGET ATRP with the same reagent composition and reaction time. The performance for each type of column packing is tested by HILIC of the model glycoprotein, ribonuclease B, which has a ladder of 5–9 mannose groups. Polymer growth, column variability and column stability are characterized, and the width of an unretained peak is monitored to assess packing homogeneity.
2 MATERIALS AND METHODS
2.1 Materials
Nonporous silica particles (750 nm) were purchased from Superior Silica (Mesa, AZ). Empty stainless-steel columns (2.1 mm I.D., 30 mm length), reservoirs (4.6 mm I.D., 150 mm), and frits (0.5 µm pore diameter) were purchased from Isolation Technologies (Middleboro, MA). Stainless-steel tubing, ferrules, and internal nuts were all purchased from Valco Instruments (Houston, TX). Methyltri-chlorosilane and [(chloromethyl)phenylethyl]trichlorosilane (Gelest, Morrisville, PA), acrylamide, sodium ascorbate, Tris[2-(dimethylamino)ethyl]amine (Me6TREN), acetonitrile, TFA, and Ribonuclease B (Sigma–Aldrich, St. Louis, MO), and copper(II) chloride (Acros Organics, Morris Plains, NJ) were used. All protein samples were prepared in ultrapure water at a concentration of 1.0 mg/mL.
2.2 Silylation of particles
The silica particles were calcined at 600°C for 12 h, then annealed at 1050°C for 3 h, and rehydroxylated overnight in 0.1 M HNO3. Particles were then rinsed in ultrapure water and dried in a 60°C vacuum oven. SEM showed that the particles decreased in diameter to 0.62 µm from the heating steps. The particles were suspended in dry toluene by sonication and modified with a 2% v/v [(chloromethyl)phenylethyl]trichlorosilane solution. The particles were allowed to react overnight and stirred with a stir bar. After reaction, the particles were rinsed three times with dry toluene, and then dried for 2 h in a 120°C oven to condense the siloxane bonds.
2.3 Ex situ AGET ATRP
In a 25 mL round bottom flask, 500 mg of silylated particles and 4.4 g acrylamide were suspended together by sonication in 20 mL of 75:25 H2O/IPA (v/v). Two other solutions were made: a copper solution containing, 40 mg copper(II) chloride and 80 µLMe6TREN, and a solution containing 20 mg sodium ascorbate. These were also prepared in 2.5 mL of 75:25 H2O/IPA. Afterwards, the copper/Me6TREN solution was added to the suspension, followed by the sodium ascorbate solution; this vessel was left to react for 55 min under sonication. The particles were then rinsed three times with water and dried in a vacuum desiccator at room temperature to allow weighing. Finally, 154 mg of particles were suspended in 75:25 water/IPA and packed into 2.1 mm × 30 mm stainless-steel columns under sonication using a high pressure pump (Laboratory Alliance of Central New York, LLC, Syracuse, NY).
2.4 In situ AGET ATRP
A solution containing the same reaction solution as for ex situ polymerization was prepared, but without the particles. The solution was poured into a plugged, 2.1 mm × 150 mm reservoir column. A 2.1 mm × 30 mm column was packed with silylated particles suspended in acetonitrile. The reservoir and column were connected in series. The reaction solution from the reservoir was pumped into the column starting at a high flow rate (200 µL/min) until the reaction mixture dripped from the end of the column. The flow rate was then lowered to 100 µL/min, and the polymerization reaction was allowed to proceed for the desired time period. After reaction, the column was rinsed with water for 10 min at 100 µL/min.
2.5 Chromatography
HILIC separation of ribonuclease B was performed using a Waters Acquity UPLC I-Class system (Milford, MA) with UV absorbance detection. Solvent A was water with 0.1% TFA and solvent B was acetonitrile with 0.1% TFA. The gradient was 75–60% B over 20 min. Absorbance wavelength was set to 215 nm. The flow rates were 100 µL/min and 150 µL/min. The column temperature was 30°C and the injection volume was 2 µL.
3 RESULTS AND DISCUSSION
To monitor in situ polymer growth, the column backpressure was recorded as a function of time while the reagent solution of acrylamide monomer and catalyst were continuously flowed through the column. A plot of column backpressure versus time during polymer growth is provided in Fig. 2A. There is an initial dip because the initial flow rate was set to 200 µL/min to fill the entire length of the column quickly, and after 1 min, the flow rate was switched to 100 µL/min in anticipation of the increase in backpressure. Since the column dead volume is 50 µL, the reagent fills the column in only 15 s to give uniform reaction conditions along the length of the column during the 55 min growth time. The increase in backpressure over the course of the 55 min reaction time is shown to be considerable, changing from 10 000 to 17 000 psi. The 55 min reaction time is the same as that used for the particles coated ex situ. Columns were made for varying reaction time, and the HILIC separation of the ribonuclease B glycoforms is shown for the cases of 20, 40, and 55 min reaction times in Fig. 2B, C and D, respectively. These establish that significantly shorter reaction times are not feasible. Consistent with previous results, a sufficiently thick polymer is needed [14].
FIGURE 2.
Effect of polymer growth time on the HILIC chromatogram of ribonuclease B. (A) Increase in backpressure during in-column polymer growth over 55 min. Pressure drop over the first 2 min is caused by a step-decrease in flow rate from 200 µL/min, for filling the column with reagent, to 100 µL/min during the reaction. The solvent is 25:75 IPA/water. (B, C, D) Chromatograms for growth times of 20, 40 and 55 min, respectively. The gradient 75–60% acetonitrile in water, with 0.1% TFA, over 20 min at 100 µL/min, 30°C, injection volume 2 µL, and detection at 215 nm
The backpressure increase in Fig. 2A is a consequence of the decrease in porosity caused by the growth of the polymer. One can calculate this decrease in porosity over time using the Kozeny–Carman equation.
| (1) |
The porosity is ε, the volume flow rate is Q, the column radius is r, and all other variables have their usual meanings. The viscosity of the 75:25 water/IPA (v/v), which converts to a mole fraction of 0.925 water, is 1.1 mPa·s [23]. Equation (1) is strictly true if the particles are spheres of diameter, dp, which is not exactly the same as spheres of dp with a thin coating, so it is assumed that the polymer is thin compared to the particle diameter. Figure 3A gives a plot of the calculated porosity as a function of time, showing that it decreases from an initial value of 0.36 to 0.31 at the end of the reaction.
FIGURE 3.
(A) Calculated porosity and (B) calculated polymer thickness during in situ growth. These graphs are derived from the pressure versus reaction time data in Fig. 2A through the Kozeny–Carman and hydrodynamic radius equations in the text
The decrease in porosity for the growing polymer can be used to estimate the polymer thickness through the relation between the hydrodynamic radius of the fluid channel and the porosity.
| (2) |
The initial hydrodynamic radius, i.e., before polymer growth, is 115 nm. After the polymer has grown for 55 min, the final hydrodynamic radius is calculated to be 92 nm. The difference between the two numbers is the polymer thickness. Figure 3B shows the increase in calculated polymer thickness with reaction time, reaching a thickness of 25 nm after 55 min. This is less than 10% of the particle radius, so the application of the model is reasonable. It is noted that the polymer thickness would change with solvent. Since the AGET ATRP reaction is conducted in more aqueous conditions, the polymer is expected to be more swollen, giving a higher backpressure compared to the less aqueous conditions of HILIC.
Microanalysis of the packing media showed that the carbon loading by mass is 2.0% for both ex situ and in situ polymerization. This means that materials with the same surface coverage are being compared. One can estimate the density of silica on the surface for the in situ polymer growth. Since SEM showed the silica particles to have a final diameter of 0.62 µm after processing, and the coverage of nitrogen, which neglects the initiator layer, is 0.53 ± 0.01%, then the molar coverage of acrylamide monomer is 86 µmol/m2, based on a density of 2.2 g/cm3 for silica. This is 23% of the density of liquid acrylamide. Since non-cross-linked polyacrylamide can swell in water to as much as 10 times its volume in methanol [24], one can presume that water takes account for the large hydrated volume of the polymer layer. The nonlinearity of the polymer growth in Fig. 3B is thus not attributed to steric hindrance; instead, it is owed to radical termination, which is likely from the high catalyst concentration. Whether a more linear polymer growth, or a longer growth time, improvements in HILIC will be investigated in subsequent work, but the quality of the separation in Fig. 2D is comparable to previous results.
Among the multiple columns produced by ex situ growth conditions, more variability in resistance to flow was exhibited compared to in situ grown columns. Columns with lower resistance exhibited less long-term stability for the ex situ case, but not for the in situ case. This is illustrated in Fig. 4. This figure has a high information content, and the first feature to be considered is that most columns were tightly packed, limiting the flow rate to 100 µL/min. More tightly packed columns could not reach as high of a flow rate and had a shorter t0. Tight versus loose packing seems to be happenstance. For one such column, the HILIC chromatogram for ribonuclease B is shown in panel A for ex situ polymerization, showing a good quality separation. The five glycoforms are clearly visible, and the resolution is competitive or better than the commercial columns studied previously [14]. This same panel shows a sampling of replicates: the 1st, 20th, and 40th runs, and these line up well, revealing that the stability exceeds 40 runs. A graph of the column backpressure during the gradient is provided below in panel B, establishing that column resistance is constant from run to run. Backpressure rises during the HILIC separation as the increasing proportion of water increases the viscosity of the mobile phase. The chromatogram for a more loosely packed column made by ex situ polymerization is shown in panel C, where the flow rate was 150 µL/min. The data show that the quality of the separation was initially high, but the quality degraded significantly over the course of 40 runs. Panel D shows the backpressure during the gradient elution. The entire curve also dropped over the course of the 40 runs. The column failure is thus associated with decreasing resistance to flow. This indicates bed collapse, i.e., the particles moved to open lower resistance channels or some polymer was lost to increase porosity. The two columns initially gave similar resolution, they differed only in how tightly the particles were packed. It is noted that the more loosely packed columns did not fail over these time frames at the lower flow rate and pressure, hence it is a combination of loose packing and high compression that causes column failure. Tighter packing helps stability, presumably by locking the particles in place.
FIGURE 4.
HILIC data for ribonuclease B and the corresponding plots of backpressure during the gradient elutions. 40 runs were done for each panel, which show the runs for the 1st, 20th, and 40th runs. The flow rates listed regard the chromatographic separations. (A) HILIC for ex situ growth with a flow rate of 100 µL/min and (B) the corresponding backpressure. (C) HILIC for ex situ growth with a flow rate of 150 µL/min and (D) the corresponding backpressure. (E) HILIC for in situ growth with a flow rate of 100 µL/min and (F) the corresponding backpressure. (G) HILIC for in situ growth with a flow rate of 150 µL/min and (H) the corresponding backpressure. The gradient was the same as in Fig. 2
Figure 4 also presents HILIC data for in situ polymer growth. Panels E and F show, respectively, the chromatograms for the more tightly packed column and the backpressure versus time during the gradient separation. The chromatograms and backpressure curves again line up for the 40 runs. For the more loosely packed column made by in situ polymerization, the stability is now shown to be higher. Panels G and H show that there is minimal degradation of the quality of separation after 40 runs, and the backpressure did not change over 40 runs. This indicates that the bed and the polymer remain intact despite the loose packing for the case of in situ polymerization. This supports the notion that solid contacts between particles, depicted in Fig. 1, enhance column stability.
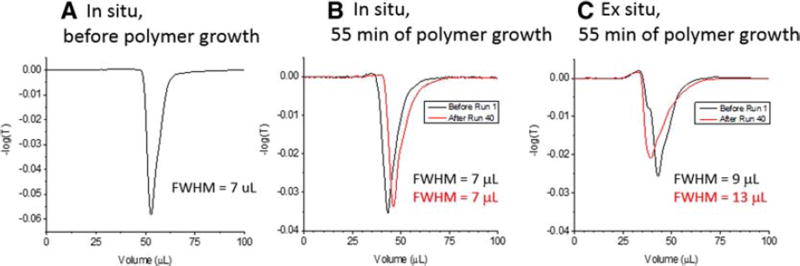
One way of interrogating bed collapse for loosely packed columns is to measure the profile of an unretained peak. This was done by injecting acetonitrile into 50:50 water/acetonitrile and eluting isocratically. The refractive index change gives a negative going peak. Figure 5 provides the results. In Fig. 5A, the unretained peak profile is shown for the in situ polymerization, but before the polymer is grown. This allows a subsequent determination of how much the polymer affects uniformity of the flow paths through the column. Figure 5B shows that the peak width, measured by the full-width at half-maximum (FWHM) is the same before and after in situ polymerization, 7 µL, indicating that the polymer growth does not significantly affect uniformity of flow through the column. Comparing panels 5A and B also shows that the mobile phase volume decreased with polymerization, as expected. Panel B shows that the mobile phase volume increased slightly, by 3 µL, after 40 runs, perhaps due to loss of polymer, but the peak width remained the same, indicating that the flow paths remained homogeneous. For the case of ex situ polymerization, panel C shows, for the first run, the mobile phase volume was virtually identical to that of in situ polymerization, but the peak width was somewhat wider, 9 µL. This is a manifestation of the difficulty in packing particles after they have been polymerized. Panel C also shows that after the 40 runs, the peak width became significantly wider, 13 µL, indicating that the flow paths became more heterogeneous. The mobile phase volume becomes smaller, indicating that wider channels were opened. This interpretation is supported by the column backpressure dropping, as was shown in Fig. 4D. The smaller mobile phase volume and greater heterogeneity of flow paths signal the onset of bed collapse, consistent with the degradation of the chromatographic behavior for this column. Minimizing bed collapse may be achieved by lowering the flow rate for loosely packed ex situ columns.
FIGURE 5.
Profiles of unretained peaks for three different columns (A) column packed with particles bearing only initiator, (B) same column as A but after 55 min of polymer growth, also same column as in Fig. 4G, and (C) column packed with particles polymerized ex situ for 55 min, also same column as in Fig. 4C. In each case, 2 µL of pure acetonitrile was injected into a mobile phase of 50:50 acetonitrile/water and eluted isocratically, with a flow rate 150 µL/min
To assess column-to-column reproducibility of the in situ versus ex situ polymerization, eight columns were made by each method. A different batch of particles was used for each ex situ column presented here. Studies (not shown) of columns made with the same batch of particles showed as much variability. The chromatographic behavior of the 16 columns is shown in Fig. 6. In Fig. 6A and B, the best three columns are compared for ex situ and in situ polymer growth. This shows that the in situ growth results in better resolution. The widths of the unretained peaks, discussed earlier, are much narrower than the protein peaks, hence the packing homogeneity is not responsible for the improved resolution. It is possibly due to the polymer density being uniform for in situ growth, as opposed to the compressed polymer layer near particle contacts for ex situ growth. Figure 6C and D compare the other five columns. For ex situ growth, three of the five columns in Fig. 6C have unacceptably low resolution. These show that the quality is more variable for the ex situ growth. For the in situ growth, the worst column is comparable to the commercial columns previously studied [14]. Improvements can be made for the in situ growth, which gives a 50% yield, with four of the eight columns giving high resolution. Most notably, optimization of the packing process, as well as the AGET ATRP conditions, might improve resolution.
FIGURE 6.
Chromatograms of ribonuclease B for the best three of eight columns made by (A) ex situ and (B) in situ polymer growth. Chromatograms for the remaining five of eight columns made by (C) ex situ and (D) in situ polymer growth. The gradient was the same as in Figs. 2 and 4
4 CONCLUDING REMARKS
The problem of preparing stable, reproducible columns with a polyacrylamide bonded phase for HILIC of intact proteins is addressed by growing the polymer-bonded phase in the column after the solid particles are packed. This in situ growth is shown to give a better HILIC stationary phase, in addition to improvements in stability and reproducibility. The use of the AGET ATRP reaction scheme facilitates in situ growth by removing the necessity of maintaining an oxygen-free environment for the Cu(I) catalyst. The inherent slowness of the reaction is overcome by use of a higher Cu(I) concentration. This gives some radical termination, but is able to generate a polymer thickness estimated to be 23 nm in 75:25 v/v water/IPA after a 55 min reaction time. The methodology can presumably be adapted to other types of polymeric-bonded phases made by radical polymerization.
Acknowledgments
This work was supported by NIH under grant R01GM101464.
Abbreviations
- AGET
activators generated by electron transfer
- ATRP
atom-transfer radical polymerization
- Me6TREN
Tris[2-(dimethylamino)ethyl]amine
Footnotes
Conflict of interest: Dr. Mary J. Wirth has a financial interest in a company, bioVidria, Inc., which has licensed the polymer growth technology described in this manuscript.
References
- 1.Williams T. Gel permeation chromatography: a review. J. Mater. Sci. 1970;5:811–820. [Google Scholar]
- 2.Stein A, Kiesewetter A. Cation exchange chromatography in antibody purification: pH screening for optimised binding and HCP removal. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2007;848:151–158. doi: 10.1016/j.jchromb.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Fahrner RL, Blank GS, Zapata GA. Expanded bed protein a affinity chromatography of a recombinant humanized monoclonal antibody: process development, operation, and comparison with a packed bed method. J. Biotechnol. 1999;75:273–280. doi: 10.1016/s0168-1656(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 4.Mata-Gomez MA, Yaman S, Valencia-Gallegos JA, Tari C, Rito-Palomares M, Gonzalez-Valdez J. Synthesis of adsorbents with dendronic structures for protein hydrophobic interaction chromatography. J. Chromatogr. A. 2016;1443:191–200. doi: 10.1016/j.chroma.2016.03.057. [DOI] [PubMed] [Google Scholar]
- 5.Svec F. Quest for organic polymer-based monolithic columns affording enhanced efficiency in high performance liquid chromatography separations of small molecules in isocratic mode. J. Chromatogr. A. 2012;1228:250–262. doi: 10.1016/j.chroma.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarafraz-Yazdi A, Razavi N. Application of molecularly-imprinted polymers in solid-phase microextraction techniques. TrAC Trends Anal. Chem. 2015;73:81–90. [Google Scholar]
- 7.Patten TE, Xia JH, Abernathy T, Matyjaszewski K. Polymers with very low polydispersities from atom transfer radical polymerization. Science. 1996;272:866–868. doi: 10.1126/science.272.5263.866. [DOI] [PubMed] [Google Scholar]
- 8.Huang XY, Wirth MJ. Surface-initiated radical polymerization on porous silica. Anal. Chem. 1997;69:4577–4580. [Google Scholar]
- 9.McCarthy P, Chattopadhyay M, Millhauser GL, Tsarevsky NV, Bombalski L, Matyjaszewski K, Shimmin D, Avdalovic N, Pohl C. Nano-engineered analytical immobilized metal affinity chromatography stationary phase by atom transfer radical polymerization: separation of synthetic prion peptides. Anal. Biochem. 2007;366:1–8. doi: 10.1016/j.ab.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwellenbach J, Kosiol P, Solter B, Taft F, Villain L, Strube J. Controlling the polymer-nanolayer architecture on anion-exchange membrane adsorbers via surface-initiated atom transfer radical polymerization. React. Funct. Polym. 2016;106:32–42. [Google Scholar]
- 11.Shen Y, Qi L, Wei XY, Zhang RY, Mao LQ. Preparation of well-defined environmentally responsive polymer brushes on monolithic surface by two-step atom transfer radical polymerization method for HPLC. Polymer. 2011;52:3725–3731. [Google Scholar]
- 12.Liu T, Lin DQ, Wu QC, Zhang QL, Wang CX, Yao SJ. A novel polymer-grafted hydrophobic charge-induction chromatographic resin for enhancing protein adsorption capacity. Chem. Eng. J. 2016;304:251–258. [Google Scholar]
- 13.Kanazawa H, Okano T. Temperature-responsive chromatography for the separation of biomolecules. J. Chromatogr. A. 2011;1218:8738–8747. doi: 10.1016/j.chroma.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z, Wu Z, Wirth MJ. Polyacrylamide brush layer for hydrophilic interaction liquid chromatography of intact glycoproteins. J Chromatogr. A. 2013;1301:156–161. doi: 10.1016/j.chroma.2013.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lazar J, Park H, Rosencrantz RR, Boker A, Elling L, Schnakenberg U. Evaluating the thickness of multivalent glycopolymer brushes for lectin binding. Macromol. Rapid Commun. 2015;36:1472–1478. doi: 10.1002/marc.201500118. [DOI] [PubMed] [Google Scholar]
- 16.Jain P, Baker GL, Bruening ML. Applications of polymer brushes in protein analysis and purification. Annu. Rev. Anal. Chem. 2009;2:387–408. doi: 10.1146/annurev-anchem-060908-155153. [DOI] [PubMed] [Google Scholar]
- 17.Guiochon G, Farkas T, Guan-Sajonz H, Koh JH, Sarker M, Stanley BJ, Yun T. Consolidation of particle beds and packing of chromatographic columns. J. Chromatogr. A. 1997;762:83–88. doi: 10.1016/s0021-9673(96)00642-5. [DOI] [PubMed] [Google Scholar]
- 18.Wei BC, Rogers BJ, Wirth MJ. Slip flow in colloidal crystals for ultraefficient chromatography. J. Am. Chem. Soc. 2012;134:10780–10782. doi: 10.1021/ja304177m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godinho JM, Reising AE, Tallarek U, Jorgenson JW. Implementation of high slurry concentration and sonication to pack high-efficiency, meter-long capillary ultrahigh pressure liquid chromatography columns. J. Chromatogr. A. 2016;1462:165–169. doi: 10.1016/j.chroma.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaman AA, Bjelopavlic M, Moudgil BM. Effect of adsorbed polyethylene oxide on the rheology of colloidal silica suspensions. J. Colloid Interf. Sci. 2000;226:290–298. [Google Scholar]
- 21.Ren H, Zhang X, Li Z, Liu Z, Li J. Silica-supported polymeric monolithic column with a mixed mode of hydrophilic and strong cation-exchange interactions for microcolumn liquid chromatography. J Sep Sci. 2017;40:826–833. doi: 10.1002/jssc.201601035. [DOI] [PubMed] [Google Scholar]
- 22.Jakubowski W, Matyjaszewski K. Activator generated by electron transfer for atom transfer radical polymerization. Macromolecules. 2005;38:4139–4146. [Google Scholar]
- 23.Pang FM, Seng CE, Teng TT, Ibrahim MH. Densities and viscosities of aqueous solutions of 1-propanol and 2-propanol at temperatures from 293.15 K to 333.15 K. J. Mol. Liq. 2007;136:71–78. [Google Scholar]
- 24.Li A, Ramakrishna SN, Kooij ES, Espinosa-Marzal RM, Spencer ND. Poly(acrylamide) films at the solvent-induced glass transition: adhesion, tribology, and the influence of crosslinking. Soft Matter. 2012;8:9092–9100. [Google Scholar]