Abstract
Gamma activity has been proposed to promote the feed forward or “bottom-up” flow of information from lower to higher regions of the brain during perception. The pedunculopontine nucleus (PPN) modulates waking and REM sleep, and is part of the reticular activating system (RAS). The properties of PPN cells are unique in that all PPN neurons fire maximally at gamma band frequency regardless of electrophysiological or transmitter type, thus proposed as one origin of “bottom-up” gamma. This property is based on the presence of intrinsic membrane oscillations subserved by high threshold, voltage-dependent calcium channels. Moreover, some PPN cells are electrically coupled. Assuming that the population of PPN neurons has the capacity to fire at ~40 Hz coherently, then the population as a whole can be expected to generate a stable gamma band signal. But what if not all the neurons are firing at the peaks of the oscillations? That means that some cells may fire only at the peaks of every second oscillation. Therefore, the population as a whole can be expected to be firing at a net ~20 Hz. If some cells are firing at the peaks of every fourth oscillation, then the PPN as a whole would be firing at ~10 Hz. Firing at rates below 10 Hz would imply that the system is seldom firing at the peaks of any oscillation, basically asleep, in slow wave sleep, thus the activation of the RAS is insufficient to promote waking. This hypothesis carries certain implications, one of which is that we awaken in stages as more and more cells are recruited to fire at the peaks of more and more oscillations. For this system, it would imply that, as we awaken, we step from ~10 Hz to ~20 Hz to ~30 Hz to ~40 Hz, that is, in stages and presumably at different levels of awareness. A similar process can be expected to take place as we fall asleep. Awakening can then be considered to be stepwise, not linear. That is, the implication is that the process of waking is a stepwise event, not a gradual increase, suggesting that the brain can spend time at each of these different stages of arousal.
Keywords: Coherence, coma, consciousness, N-type calcium channels, P/Q-type calcium channels, preconscious awareness, stepwise waking
INTRODUCTION
The role of gamma band activity
Sensory perception, problem solving, and memory are thought to require gamma band oscillations [1–6]. The coherence of gamma activity may occur at cortical or subcortical levels [7,8], and is thought to contribute to the merger, or “binding”, of information originating from separate regions to promote perception [9]. Gamma oscillations were proposed to emerge from the interaction between intrinsic neuronal and synaptic properties of thalamocortical networks [10]. For example, cortical synaptic connections alone may not be able to maintain firing at gamma frequencies, so that intrinsic membrane properties also appear essential to the maintenance of gamma band activity. One example of the limits of cortical synaptic connectivity is flicker fusion of visual inputs such that cortical circuits cannot “follow” individual visual stimuli presented at rates above 35 Hz. Therefore, it is the ability of cells with intrinsic membrane properties, coupled with synaptic interactions, which allows the circuit as a whole to fire at a preferred frequency, and is essential to maintaining frequencies in the gamma range. The neuronal mechanisms behind such activity include inhibitory cortical interneurons with intrinsic oscillatory activity in the gamma range [7,10,11], many of which are electrically coupled [12], as well as of fast rhythmic bursting pyramidal neurons [13], and layer V pyramidal cells that summate dendritic action potentials at gamma frequency [14]. Moreover, thalamocortical excitatory neurons have intrinsic properties needed to generate subthreshold gamma band oscillations [15]. However, other regions are known to manifest gamma band activity in addition to the cortex and thalamus, including the hippocampus, basal ganglia, cerebellum, and the RAS.
Gamma band activity in the RAS
Recently, two important discoveries were made in the properties of neurons that control waking and rapid eye movement (REM) sleep. During waking and REM sleep, the EEG shows low amplitude, high frequency activity at beta/gamma frequencies (~20–30/30–90 Hz). The pedunculopontine nucleus (PPN) is active during waking and REM sleep [16]. The PPN is the arm of the RAS that modulates ascending projections through the intralaminar thalamus (modulating arousal) and descending projections through the pons and medulla (modulating posture and locomotion) [16]. The PPN is composed of different populations of cholinergic, glutamatergic, and GABAergic neurons [17]. Recordings of PPN neurons in vivo identified PPN cells with firing properties related to ponto-geniculo-occipital wave generation [18]. Some neurons had low rates of spontaneous firing (~10 Hz), but most had high rates of tonic firing in the beta/gamma range (~20–80 Hz). PPN neurons also exhibit beta/gamma frequencies in vivo during waking and REM sleep, but not during slow wave sleep [18–23]. Moreover, the presence of gamma band activity has been confirmed in the cortical EEG of the cat in vivo when the animal is active [17,24]; and in the region of the PPN in humans during stepping, but not at rest [25]. In the monkey, PPN neurons fired at low frequencies ~10 Hz at rest, but the same neurons increased firing to gamma band frequencies when the animal woke up, or when the animal began walking on a treadmill [26]. That is, the same cells were involved in both arousal and motor control. Thus, there is ample evidence for gamma band activity during waking and movement in the PPN in vitro, in vivo, and across species, including man.
Mechanisms behind PPN gamma activity
A number of articles described the mechanisms behind gamma band activity in the PPN [27–32]. Ramps instead of steps were required to activate high threshold calcium channels in order to keep from activating potassium channels that would prevent sufficient membrane depolarization [30]. In short, gamma oscillations are mediated by voltage-dependent, high threshold N- and P/Q-type calcium channels that are present in every PPN neuron, regardless of cell or transmitter type. These channels are distributed along the dendrites of PPN cells [33]. It has been proposed that afferent input traveling through “specific” sensory pathways diverges to activate “non-specific” reticular pathways to activate PPN dendrites. This generates activity in the beta/gamma range and basically represents one origin or first way station of “bottom-up” gamma activity [34]. However, it appears that gamma band activity during waking has different mechanisms than gamma band activity during REM sleep. Injections of glutamate into the PPN were found to increase both waking and REM sleep [35], but injections of the glutamatergic receptor agonist N-methyl-D-aspartic acid (NMDA) increased only waking [36], while injections of the glutamatergic receptor agonist kainic acid (KA) increased only REM sleep [37]. Intracellularly, CaMKII, which modulates NMDA receptors, was shown to modulate P/Q-type channel function [38], but protein kinase C (PKC), which modulates KA receptors, enhances N-type channel activity and has no effect on P/Q-type channel function [39].
Thus, PPN calcium channel subtypes are modulated by different intracellular pathways, N-type by the cAMP/PK pathway, and P/Q-type via the CaMKII pathway. Moreover, we found three cell types in the PPN, those bearing only N-type calcium channels, those with both N- and P/Q-type, and those with only P/Q-type calcium channels [40,41]. The implications from all of these results is that there is a “waking” pathway mediated by CaMKII and P/Q-type channels and a “REM sleep” pathway mediated by cAMP/PK and N-type channels, and that different PPN cells fire during waking (those with N+P/Q and only P/Q-type) vs REM sleep (those with N+P/Q and only N-type).
The role of “bottom-up” gamma
Early studies on the RAS suggested that it participates in “tonic” or “continuous” arousal [42], and lesions of the RAS were found to eliminate tonic arousal [43]. Recent findings on the presence of intrinsic gamma oscillations and electrical coupling in PPN cells provided the mechanisms required for the maintenance of gamma band activity [27–32]. The PPN, in which every cell manifests gamma band activity, then becomes a gamma-making machine. We speculate that it is the continued activation of the RAS during waking that allows the maintenance of the background of gamma activity necessary to support the state capable of reliably assessing the world around us on a continuous basis- preconscious awareness. That is, RAS bottom-up gamma provides the maintained arousal necessary for higher functions such as attention, learning, and memory. This activity is relayed through the intralaminar thalamus, specifically the parafascicular nucleus (Pf), whose cells were also found to bear high threshold, voltage-dependent calcium channels all along their dendrites [44,45], to the cortex.
At the level of the cortex, the difference between gamma band activity during waking compared to REM sleep seems to be decreased coherence [46]. Basically, brainstem driving of gamma band activity during waking carries with it coherence across distant cortical regions, while induction of gamma band activity during REM sleep does not include coherence across distant regions [46,47]. These findings suggest that brainstem centers drive gamma band activity manifested in the cortical EEG. In other words, brainstem PPN activity is ultimately influencing the characteristics of the cortical EEG. Otherwise, there would be no difference between the gamma band manifested during waking compared to during REM sleep. Mainly because, during waking, brainstem-thalamic projections include coherence across regions, but during REM sleep, it drives cortical EEG rhythms without coherence.
Waking up
Roger Sperry, Nobel Laureate in 1971, proposed that the critical organizational features of the neural circuitry for generating conscious awareness are activated through the RAS, and, once activated, become responsive to changing sensory as well as centrally generated input [48]. Therefore, what happens when we first activate the RAS, when we first wake up? Upon waking, blood flow increases first in the upper brainstem and thalamus, and only later increases in the frontal lobes [49]. That is, the process of awakening entails a rapid re-establishment of consciousness (within a few minutes) followed by a relatively slow (20–30 minutes) re-establishment of full awareness. Cerebral blood flow measured using positron emission tomography was found to occur soonest upon waking in the brainstem and thalamus, suggesting that the reactivation of these regions underlies the re-establishment of basic conscious awareness. Over the following 15–20 minutes, increases in cerebral blood flow were evident primarily in anterior cortical regions. These results question ideas that insist that the cortex is solely responsible for achieving conscious awareness.
Changes in arousal state do not occur suddenly unless suprathreshold stimuli ensue. There are relatively slow transitions between waking, slow wave sleep, and REM sleep. The transition from waking to sleep takes time, in the order of minutes, that is, it is not instantaneous. The beginning of REM sleep is also not immediate but appears to be recruited by increasing bursts of ponto-geniculo-occipital waves over a few minutes [11]. In the absence of strong stimuli that induce waking, therefore, waking and sleep appear to be “recruited”, and are part of a stepwise process, in which frequencies decrease from gamma to beta to alpha and lower leading to sleep, or increase from delta to theta to alpha and higher, leading to waking. This stepwise process also suggests that the modulation of waking and sleep is progressive but piecemeal, and that it is the coherence at particular frequencies that determine the particular transition state. That is, the new state may be “recruited” to another level rather than gradually formed.
While gamma band oscillations appear to be a brain-wide property of the awake brain, gamma oscillations are not present simultaneously across the entire cortex. During waking, regions manifesting gamma oscillations do so for some period locally and the oscillations shift to other regions of the cortex. This may also reflect a series of transitions between discrete intermediate states that ultimately gain full consciousness. Therefore, during interactions with a complex environment, there must be a rapidly shifting display of gamma oscillations throughout the cortical mantle, but no one region is always manifesting gamma band activity. Moreover, the resonance of these oscillations is amplified by projections from the cortex to the thalamus, cerebellum, basal ganglia, RAS, etc. That is, a cortical region that received thalamic input sends a reciprocal four- to ten-fold volley to a thalamic target (ratios of corticothalamic to thalamocortical projections are in the range of 4:1 to 10:1 [50]), which in turn projects back to that same or different area of cortex. Therefore, the delayed (by conduction time) return volley represents another stepwise change in the process of sensory perception. We propose that different regions of the cortex are repeatedly shifting from one level to another, not in a linear manner, but in stepwise fashion.
There must be a sufficient number of regions manifesting gamma band activity to maintain full awareness. However, upon waking, as regions are recruited, activity rises from low frequencies (delta, theta) to higher and higher frequencies (alpha ~10 Hz, beta ~20 Hz, and gamma ~40 Hz). This may mean that different levels of awareness are achieved during these stepwise transitions. A recent study on recovery from anesthesia found that recovery passes through several discreet activity states [51]. The study reported that there was an orderly progression through intermediate states rather than a continuous and gradual recovery of consciousness from anesthesia. What if the person cannot wake up? The EEG of the comatose patient appears to be more similar to that during general anesthesia, which has been referred to as a “drug-induced coma”, and both states are characterized by burst suppression [52]. In coma, for example, it may be that either the ability to generate significant stepwise levels of gamma activity, and/or the mechanism for maintaining gamma oscillations, are disrupted. Moreover, it is possible that some pathological insults may affect one or both processes, which further explains the variability in the symptomatic manifestations during coma. Given the potential causes of coma, from trauma to metabolic (e.g. glucose) to organ failure (e.g. hepatic) to toxicological to drug induced to infection, it is difficult to determine how each of these insults could affect stepwise gamma band generation or maintenance mechanisms specifically. Nevertheless, it is the attainment of a sufficiently stable process that appears to be blocked in coma. The insult or damage to the brain makes it unable to step through all the levels needed to reach full consciousness. We assume that, based on the amount or degree of damage, partial “levels” of consciousness may be achieved in different patients. For example, in the chronic vegetative state, the EEG changes may reflect stepwise shifts that do not reach higher levels of consciousness, whereas in the minimally conscious state, some degree of consciousness is attained, but the next higher level cannot be fully manifested.
What mechanism accounts for the stepwise progression through different states of awareness?
HYPOTHESIS
The hypothesis proposed takes into consideration the background reviewed and suggests that, given the intrinsic membrane oscillations manifested and other properties of PPN neurons, there may be different levels of arousal during the waking process. Assuming that the population of PPN neurons has the capacity to fire at ~40 Hz and higher when all of its neurons have been recruited to fire coherently (due to the fact that some cells, particularly GABAergic cells, are electrically coupled), then the population as a whole can be expected to generate a stable gamma band signal. But what if not all the neurons are firing at the peaks of the oscillations? That means that some cells may fire only at the peaks of every second oscillation. Therefore, the population as a whole can be expected to be firing at ~20 Hz. If the population as a whole is firing at the peaks of every fourth oscillation, then the PPN would be firing at ~10 Hz. Figure 1 illustrates the idea of differential activity at some but not all the peaks of the intrinsic membrane oscillations. Firing at rates below 10 Hz would imply that the system is seldom firing at the peaks of any oscillation, basically in slow wave sleep, thus the activation of the RAS is insufficient to promote waking. This hypothesis carries certain implications, one of which is that we awaken in stages as more and more cells are recruited to fire at the peaks of more and more oscillations. The frequency is unlikely to deviate from this progression since the cells are more likely to fire at the peaks of the oscillations and not in between. For this system, it would imply that, as we awaken, we step from ~10 Hz to ~20 Hz to ~30 Hz to ~40 Hz, that is, in stages and at different levels of awareness. A similar process can be expected to take place as we fall asleep. Awakening can be considered to be stepwise, not linear. That is, the implication is that the process of waking is a stepwise event, not a linearly increasing ramp, suggesting that the brain can spend time at each of these stages of arousal.
Figure 1.
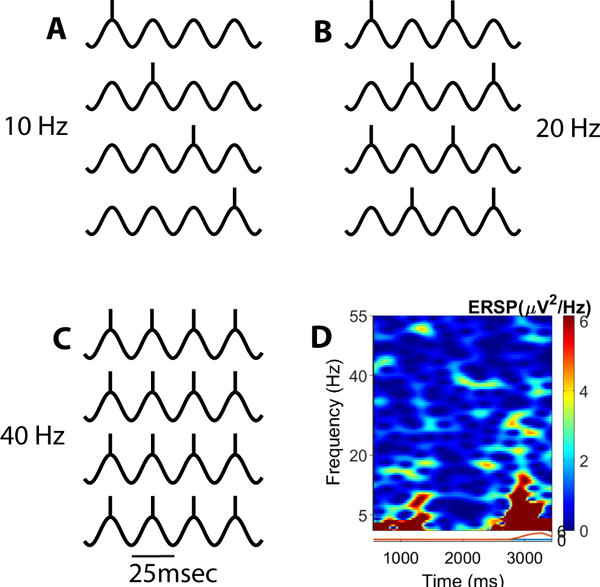
A–C. Gamma frequency waveforms depicting intrinsic membrane oscillations during stages when a cell fires an action potential (vertical line atop the waveform) at the peak of every fourth wave (A, 10 Hz), every second wave (B, 20 Hz), or every wave (C, 40 Hz). D. Event Related Spectral perturbation (ERSP) plot of frequency (left axis) over time (bottom axis) in relation to ongoing activity (right axis). The ERSP is essentially a running power spectrum revealing changes in frequency of activity over time.
CONSEQUENCES
Two proposals led to the hypothesis offered here. We previously proposed that there is a 10 Hz frequency fulcrum at the transition between waking and sleep that is replaced on the one hand by lower frequencies during sleep, or on the other hand by higher frequencies during volition and cognition [53]. The 10 Hz frequency fulcrum was proposed as the natural frequency of the brain during quiet waking, one that is replaced by higher frequencies capable of permitting more complex functions, or by lower frequencies during sleep and inactivity. At the center of the transition shifts to and from the resting rhythm is the RAS, a phylogenetically preserved area of the brain essential for preconscious awareness. The PPN, as the only part of the RAS that is active during states of high frequency cortical activity, namely waking and REM sleep, is the main purveyor of this basic signal. As sensory afferents provide additional load to PPN dendrites, cells are activated and more likely to fire during more of the peaks of the intrinsic membrane oscillations at higher frequencies [31,34].
We also proposed that the calcium channel-mediated gamma band activity in the PPN is one origin of “bottom-up” gamma that is relayed through the intralaminar thalamus to the cortex [32,33]. The hypothesis proposed herein builds upon these proposals to suggest that, because, a) the underlying intrinsic membrane oscillations are manifested at ~40 Hz frequency, and b) electrical coupling helps the population as a whole become coherent, then c) the population of PPN neurons at large will fire at the peaks of some or all of these oscillations. Firing at each peak, the net frequency of the population will be ~40 Hz, but if they fire at every second or fourth peak, the frequency as a whole will be 20 Hz or 10 Hz, respectively. This provides a potential mechanism for the stepwise process described above.
The implications of this hypothesis are testable. For example, it should be demonstrable that, as the cortex transitions from waking to sleep, there will be steps, not slow ramps, in the spectral analysis of the EEG. Similarly, in transitions from sleep to waking, a stepwise increase in spectral power should be visible, such as is evident in recovery from anesthesia [51]. Moreover, in cases of chronic vegetative state, net frequencies will be lower than those in the minimally conscious state. In addition, the transitions across these states should follow a stepwise progression in frequency rather than a smooth, gradual increase in frequency. These studies could be performed on both humans and animals.
It may be possible to demonstrate the stepwise progression of recruited frequencies of activity by spectral analysis of population responses in slices in vitro. On the one hand, manipulation of NMDA receptors, P/Q-type channels, and/or CaMKII may result in activity more related to waking that to REM sleep, which may manifest upon manipulation of KA, N-type channels, and/or cAMP/PKC mechanisms. Spectral maps may be similar in frequency during the two conditions, but coherence should be lacking in the REM sleep condition (e.g. see Figure 1, part D).
In general, the likelihood that waking takes place in measurable steps accounts for the findings outlined above and introduce a novel manner of thinking about states of awareness. Not only is this of interest to those seeking answers to the problem of consciousness, but also will have significant implications for the treatment of coma, vegetative states, and minimally conscious states. Knowledge of how these separate states are achieved will be clinically invaluable. If the mechanism proposed is at least partly responsible for these effects, then therapeutic interventions can be developed to modulate the frequency and efficiency of PPN gamma band intrinsic membrane oscillations.
Acknowledgments
We are grateful for the assistance of Susan Mahaffey in generating the figure shown, as well as comments on the manuscript by Dr. F.J. Urbano. The research described was supported by NIH awards from the IDeA program at NIGMS P20 GM103425 and P30 GM1102702.
Support: Supported by NIH awards from the IDeA program at NIGMS P20 GM103425 and P30 GM110702.
Footnotes
CONFLICT OF INTEREST
The author certifies that he has NO affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.
References
- 1.Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, et al. Coherent oscillations: a mechanism of feature linking in the visual system? Biol Cybern. 1988;60:121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- 2.Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Nat Acad Sci USA. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones EG. Calcium channels in higher-level brain function. Proc Nat Acad Sci USA. 2007;14:17903–17904. doi: 10.1073/pnas.0709509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Philips S, Takeda Y. Greater frontal-parietal synchrony at low gamma-band frequencies for inefficient then efficient visual search in human EEG. Int J Psychophysiol. 2009;73:350–354. doi: 10.1016/j.ijpsycho.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Palva S, Monto S, Palva JM. Graph properties of synchronized cortical networks during visual working memory maintenance. Neuroimage. 2009;49:3257–3268. doi: 10.1016/j.neuroimage.2009.11.031. [DOI] [PubMed] [Google Scholar]
- 6.Voss U, Holzmann R, Tuin I, Hobson JA. Lucid dreaming: a state of consciousness with features of both waking and non-lucid dreaming. Sleep. 2009;32:1191–1200. doi: 10.1093/sleep/32.9.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llinas RR, Grace AA, Yarom Y. In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range. Proc Nat Acad Sci USA. 1991;88:897–901. doi: 10.1073/pnas.88.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singer W. Synchronization of cortical activity and its putative role in informtion processing and learning. Annu Rev Physiol. 1993;55:349–374. doi: 10.1146/annurev.ph.55.030193.002025. [DOI] [PubMed] [Google Scholar]
- 9.Llinás RR, Paré D. Of dreaming and wakefulness. Neurosci. 1991;44:521–535. doi: 10.1016/0306-4522(91)90075-y. [DOI] [PubMed] [Google Scholar]
- 10.Steriade M, Llinás RR. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988;68:649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- 11.Steriade M. Cellular substrates of oscillations in corticothalamic systems during states of vigilance. In: Lydic R, Baghdoyan HA, editors. Handbook of Behavioral State Control Cellular and molecular mechanisms. New York: CRC Press; 1999. pp. 327–347. [Google Scholar]
- 12.Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham MO, Whittington MA, Bibbig A, Roopun A, LeBeau FE, et al. A role for fast rhythmic bursting neurons in cortical gamma oscillations in vitro. Proc Nat Acad Sci USA. 2004;101:7152–7157. doi: 10.1073/pnas.0402060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larkum ME, Zhu JJ, Sakmann B. Dendritic mechanisms underlying the coupling of the dendritic with the axonal action potential initiation zone of adult rat layer 5 pyramidal neurons. J Physiol. 2001;533:447–466. doi: 10.1111/j.1469-7793.2001.0447a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pedroarena C, Llinás RR. Dendritic calcium conductances generate high-frequency oscillation in thalamocortical neurons. Proc Natl Acad Sci USA. 1997;94:724–728. doi: 10.1073/pnas.94.2.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Rill E. Waking and the Reticular Activating System. New York: Academic Press; 2015. p. 330. [Google Scholar]
- 17.Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci. 2009;29:340–358. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steriade M, Paré D, Datta S, Oakson G, Curro Dossi R. Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J Neurosci. 1990;10:2560–2579. doi: 10.1523/JNEUROSCI.10-08-02560.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakai K, El Mansari M, Jouvet M. Inhibition by carbachol microinjections of presumptive cholinergic PGO-on neurons in freely moving cats. Brain Res. 1990;527:213–223. doi: 10.1016/0006-8993(90)91140-c. [DOI] [PubMed] [Google Scholar]
- 20.Kayama Y, Ohta M, Jodo E. Firing of ‘possibly’ cholinergic neurons in the rat laterodorsal tegmental nucleus during sleep and wakefulness. Brain Res. 1992;569:210–220. doi: 10.1016/0006-8993(92)90632-j. [DOI] [PubMed] [Google Scholar]
- 21.Datta S, Siwek DF. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J Neurosci Res. 2002;70:79–82. doi: 10.1002/jnr.10405. [DOI] [PubMed] [Google Scholar]
- 22.Datta S, Siwek DF, Stack EC. Identification of cholinergic and non-cholinergic neurons in the pons expressing phosphorylated cyclic adenosine monophosphate response element-binding protein as a function of rapid eye movement sleep. Neurosci. 2009;163:397–414. doi: 10.1016/j.neuroscience.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boucetta S, Cisse Y, Mainville L, Morales M, Jones BE. Discharge profiles across the sleep-waking cycle of identified cholinergic, gabaergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat. J Neurosci. 2014;34:4708–4727. doi: 10.1523/JNEUROSCI.2617-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steriade M, Curro Dossi R, Paré D, Oakson G. Fast oscillations (20–40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proc Nat Acad Sci USA. 1991;88:4396–4400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fraix V, Bastin J, David O, Goetz L, Ferraye M, et al. Pedunculopontine nucleus area oscillations during stance, stepping and freezing in Parkinson’s disease. PLOS ONE. 2013;8:e83919. doi: 10.1371/journal.pone.0083919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goetz L, Piallat B, Bhattacharjee M, Mathieu H, David O, et al. The primate pedunculopontine nucleus region: towards a dual role in locomotion and waking state. J Neural Transm. 2016;123:667–678. doi: 10.1007/s00702-016-1577-7. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Rill E, Kezunovic N, Hyde J, Beck P, Urbano FJ. Coherence and frequency in the reticular activating system (RAS) Sleep Med Rev. 2013;17:227–238. doi: 10.1016/j.smrv.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Rill E, Luster B, D’Onofrio S, Mahaffey S, Bisagno V, et al. Implications of gamma band activity in the pedunculopontine nucleus. J Neural Transm. 2015;123:655–665. doi: 10.1007/s00702-015-1485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Rill E, D’Onofrio S, Luster B, Mahaffey S, Urbano FJ, et al. The 10 Hz Frequency: a fulcrum for transitional brain states. Translat Brain Rhyth. 2016;1:7–13. [PMC free article] [PubMed] [Google Scholar]
- 30.Kezunovic N, Urbano FJ, Simon C, Hyde J, Smith K, et al. Mechanism behind gamma band activity in the pedunculopontine nucleus (PPN) Eur J Neurosci. 2011;34:404–415. doi: 10.1111/j.1460-9568.2011.07766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simon C, Kezunovic N, Ye M, Hyde J, Hayar A, Williams DK, Garcia-Rill E. Gamma band unit activity and population responses in the pedunculopontine nucleus (PPN) J Neurophysiol. 2010;104:463–474. doi: 10.1152/jn.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urbano FJ, D’Onofrio SM, Luster BR, Hyde JR, Bosagno V, et al. Pedunculopontine nucleus gamma band activity- preconscious awareness, waking, and REM sleep. Frontiers in Sleep and Chronobiol. 2014;5:210. doi: 10.3389/fneur.2014.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyde JR, Kezunovic N, Urbano FJ, Garcia-Rill E. Spatiotemporal properties of high speed calcium oscillations in the pedunculopontine nucleus. J Appl Physiol. 2013;115:1402–1414. doi: 10.1152/japplphysiol.00762.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Rill E, D’Onofrio S, Mahaffey S. Bottom-up Gamma: the Pedunculopontine Nucleus and Reticular Activating System. Transl Brain Rhythm. 2016;1:49–53. doi: 10.15761/TBR.1000109. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Datta S, Spoley EE, Patterson EH. Microinjection of glutamate into the pedunculopontine tegmentum induces REM sleep and wakefulness in the rat. Amer J Physiol Reg Integ Comp Physiol. 2001;280:R752–R759. doi: 10.1152/ajpregu.2001.280.3.R752. [DOI] [PubMed] [Google Scholar]
- 36.Datta S, Patterson EH, Spoley EE. Excitation of the pedunculopontine tegmental NMDA receptors induces wakefulness and cortical activation in the rat. J Neurosci Res. 2002;66:109–116. doi: 10.1002/jnr.1202. [DOI] [PubMed] [Google Scholar]
- 37.Datta S. Evidence that REM sleep is controlled by the activation of brain stem pedunculopontine tegmental kainite receptor. J Neurophysiol. 2002;87:1790–1798. doi: 10.1152/jn.00763.2001. [DOI] [PubMed] [Google Scholar]
- 38.Jiang X, Lautermilch NJ, Watari H, Westenbroek RE, Scheuer T, et al. Modulation of Cav2.1 channels by Ca+/calmodulin-dependent kinase II bound to the C-terminal domain. Proc Nat Acad Sci USA. 2008;105:341–346. doi: 10.1073/pnas.0710213105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stea A, Soomg TW, Snutch TP. Determinants of PKC-dependent modulation of a family of neuronal Ca2+ channels. Neuron. 1995;15:929–940. doi: 10.1016/0896-6273(95)90183-3. [DOI] [PubMed] [Google Scholar]
- 40.Luster B, D’Onofrio S, Urbano FJ, Garcia-Rill E. High-Threshold Ca2+ channels behind gamma band activity in the pedunculopontine nucleus (PPN) Physiol Rep. 2015;3:e12431. doi: 10.14814/phy2.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luster B, Urbano FJ, Garcia-Rill E. Intracellular mechanisms modulating gamma band activity in the pedunculopontine nucleus (PPN) Physiol Rep. 2016:e12787. doi: 10.14814/phy2.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroenceph Clin Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- 43.Watson RT, Heilman KM, Miller BD. Neglect after mesencephalic reticular formation lesions. Neurol. 1974;24:294–298. doi: 10.1212/wnl.24.3.294. [DOI] [PubMed] [Google Scholar]
- 44.Kezunovic N, Hyde J, Simon C, Urbano FJ, Garcia-Rill E. Gamma band activity in the developing parafascicular nucleus (Pf) J Neurophysiol. 2012;107:772–784. doi: 10.1152/jn.00677.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hyde J, Kezunovic N, Urbano FJ, Garcia-Rill E. Visualization of fast calcium oscillations in the parafascicular nucleus. J Eur Physiol (Pflug Arch) 2013;465:1327–1340. doi: 10.1007/s00424-013-1264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castro S, Falconi A, Chase M, Torterolo P. Coherent neocortical 40-Hz oscillations are not present during REM sleep. Eur J Neurosci. 2013;37:1330–1339. doi: 10.1111/ejn.12143. [DOI] [PubMed] [Google Scholar]
- 47.Cavelli M, Castro S, Schwartzkopf N, Chase M, Falconi A, Torterolo P. Coherent cortical oscillations decrease during REM sleep in the rat. Behav Brain Res. 2015;281:318–325. doi: 10.1016/j.bbr.2014.12.050. [DOI] [PubMed] [Google Scholar]
- 48.Sperry RW. A modified concept of consciousness. Psychol Rev. 1969;76:532–536. doi: 10.1037/h0028156. [DOI] [PubMed] [Google Scholar]
- 49.Balkin TJ, Braun AR, Wesensten NJ, Jeffries K, et al. The process of awakening: a PET study of regional brain activity patterns mediating the re-establishment of alertness and consciousness. Brain. 2002;125:2308–2319. doi: 10.1093/brain/awf228. [DOI] [PubMed] [Google Scholar]
- 50.Deschenes M, Veinante P, Zhang Z-W. The organization of corticothalamic projections: reciprocity versus parity. Brain Res Rev. 1998;28:286–308. doi: 10.1016/s0165-0173(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 51.Hudson AE, Calderon DP, Pfaff DW, Proekt A. Recovery of consciousness is mediated by a network of discrete metastable activity states. Proc Natl Acad Sci USA. 2014;111:9283–9288. doi: 10.1073/pnas.1408296111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. New Engl J Med. 2010;363:2638–2650. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia-Rill E, Virmani T, Hyde JR, D’Onofrio S, Mahaffey S. Arousal and the control of perception and movement. Curr Trends Neurol. 2016;10:53–64. [PMC free article] [PubMed] [Google Scholar]


