Abstract
Objective
To examine feasibility of a simultaneous high-resolution pharyngeal manometry (HRM) and electromyography (EMG) experimental paradigm to detect swallowing-related patterns of palatal, laryngeal, and pharyngeal muscle activity during expiratory training.
Study Design
Technical report.
Methods
Simultaneous HRM, surface submental, and intramuscular EMG were acquired in two healthy participants during five tasks: 10-cc water swallow, maximum expiratory pressure (MEP) testing, and expiratory muscle strength training (EMST) at three pressure levels (sham, 50%, and 75% MEP).
Results
Experimental conditions were feasible. Velopharyngeal closing pressure, palate EMG activity, and pharyngeal EMG activity increased as expiratory load increased. In contrast, thyroarytenoid EMG activity was low during the expiratory task, consistent with glottic opening during exhalation. Submental EMG patterns were more variable during expiratory tasks. Intraluminal air pressures recorded with HRM were correlated with measured expiratory pressures and target valve-opening pressures of the EMST device.
Conclusion
Results suggest that a simultaneous HRM/EMG/EMST paradigm may be used to detect previously unquantified swallowing-related muscle activity during EMST, particularly in the palate and pharynx. Our approach and initial findings will be helpful to guide future hypothesis-driven studies and may enable investigators to evaluate other muscle groups active during these tasks. Defining mechanisms of action is a critical next step toward refining therapeutic algorithms using EMST and other targeted treatments for populations with dysphagia and airway disorders.
Keywords: High-resolution manometry, electromyography, swallowing, expiratory muscle strength training
INTRODUCTION
Expiratory muscle strength training (EMST) is a simple, inexpensive, device-driven therapy. During EMST, a patient expires forcefully into a one-way, spring loaded valve trainer.1–3 Promising results exist in trials examining a progressive-resistive exercise paradigm of EMST in patients with dysphagia related to neurogenic pathologies, suggesting improvement in airway protection after a 5-week strengthening exercise program.4–6 Expiratory muscle strength training is accordingly gaining popularity as a treatment for populations with swallowing disorders and impaired airway protection. Swallowing-related airway protection is exceedingly complex, coupling sensorimotor processes to prevent airway entry during the pharyngeal swallow and those that promote ejection of foreign materials from the airway via cough. A framework for this continuum of airway protection behaviors and their common neural substrates has been proposed.7 Expiratory muscle strength training is thought to improve swallowing-related airway protection by one of two mechanisms: 1) stronger expiratory pressure generating forces, translating to better cough to clear aspirate from the lower airway; or 2) improved airway closure for swallowing by submental suprahyoid muscle activation.8
Mechanisms by which EMST may help prevent airway entry of a bolus during swallowing are poorly characterized. Primary kinematics observed under videofluoroscopy during EMST include velopharyngeal closure, hyoid elevation (greater than anterior excursion),9 laryngeal framework lift, arytenoid–epiglottic approximation, and pharyngeal shortening (Fig. 1). Collectively, these phenomena represent the proximal seal required for pharyngeal bolus propagation and drivers of supraglottic laryngeal closure.10 In a series of electromyographic studies, EMST was associated with load-dependent activation of submental muscles critical to anterior and superior hyolaryngeal excursion and laryngeal airway closure during swallowing. Increased and prolonged submental EMG activity, relative to swallowing, is suggested to reflect the potential for overload of suprahyoid musculature during EMST.9 Suprahyoid and genioglossus muscle activity during EMST are further supported by morphometric magnetic resonance imaging analyses suggesting load-dependent decreases in midsagittal tongue (extrinsic and intrinsic) area during expiratory training.11 The nature of EMST-dependent muscle activity outside of submental musculature (i.e., in the palate, pharynx, and intrinsic larynx) has not been quantified. As such, the therapeutic potential of this device for dysphagia may be under-recognized.
Fig. 1.

Exemplar of rest-to-peak kinematics during EMST task under video-fluoroscopy. Observations during EMST include velopharyngeal seal (top arrow), hyoid excursion, arytenoid-to-epiglottic approximation (scaled to distance between C2 and C4 vertebrae), and pharyngeal shortening (bottom arrow). EMST = expiratory muscle strength training.
The objective of this feasibility report was to examine a multiparametric protocol of swallowing-related upper aerodigestive muscular tract activity during EMST using simultaneous high-resolution pharyngeal manometry (HRM) and intramuscular and surface electromyography (EMG). Through preliminary descriptive analyses, this technical report seeks to demonstrate 1) feasibility and utility of a HRM/EMG paradigm to quantify EMST-related upper aerodigestive activity; 2) optimal data collection procedures; and 3) candidate-dependent and -independent variables for hypothesis-driven studies of EMST mechanisms. With simultaneous HRM and EMG, we hypothesized that it would be feasible to: 1) discriminate between muscles with load-dependent and independent activation during increased expiratory pressure tasks; 2) detect load-dependent differences in EMG activity for actively contracting muscles (submental, palate, and pharynx), and confirm low-activity intrinsic laryngeal muscles (i.e., thyroarytenoid muscle should remain relatively stable given that the glottis would be open during exhalation) during EMST tasks; 3) detect load-dependent differences in velopharyngeal closing pressure on HRM during EMST tasks (75% vs. 50% vs. sham); and 4) observe similar palatal EMG muscle activity patterns with and without the HRM catheter.
MATERIALS AND METHODS
Overview
We present data from two healthy young female participants. Each participant performed five trials each of four expiratory tasks (sham, 50% and 75% of maximum load EMST, and maximum expiratory pressure [MEP] generation) and five 10-cc water swallows during simultaneous EMG/HRM recording.
Participants
Two healthy females (23 and 24 years old) without history of swallowing, respiratory, or neurological deficits participated. Each provided informed consent, and the protocol was approved by the local institutional review board. Participants were instructed to refrain from eating for 4 hours and from drinking for 2 hours before testing to avoid potential effect of satiety.
High-Resolution Manometry and Electromyography
We previously described instrumentation procedures for simultaneous EMG and HRM acquisition.12,13 Pharyngeal pressure was recorded using a solid-state high-resolution manometer (ManoScan360 High-Resolution Manometry System, Given Imaging, Atlanta, GA). The manometric catheter has an outer diameter of 2.75 mm with 36 circumferential pressure sensors, each spanning 2.5 mm, spaced 1-cm apart. Each sensor receives input from 12 circumferential sectors, which are then averaged to yield a single pressure trace. The system and catheter were calibrated before each use in an air pressure chamber to record pressure between −20 to 600 mm Hg, with fidelity of 2 mm Hg. Pressure from each sensor was recorded at a sampling rate of 50 Hz (ManoScan Data Acquisition, Sierra Scientific Instruments, Los Angeles, CA). Topical 2% viscous lidocaine hydrochloride was applied to the nasal passages and to the manometric catheter to ease passage through the nasopharynx. Based on our previous experiments and the small quantity of viscous lidocaine used (< 1 cc), effects on the swallow were negligible.12,13 Velopharyngeal pressures were the HRM parameter of interest for this study.
Electromyography was acquired in both subjects. Bipolar hook-wire intramuscular electrodes (50-micron diameter, MicroProbes, Gaithersburg, MD) were inserted into the palatal levator and pharyngeal muscles (both participants) and into the thyroarytenoid muscle (one participant) using a 27-gauge needle (Table I). The side of electrode insertion was randomly assigned. For tolerability, no more than four intramuscular insertions were attempted in each participant. One participant had two palatal electrodes inserted due to concern that the mouthpiece of the expiratory trainer might dislodge palatal electrodes during experimental tasks. Differences in EMG data acquired between participants are detailed in Table I.
TABLE I.
Experimental Tasks by Participant
| Instrumentation and Recording Parameters | Participant 1 | Participant 2 |
|---|---|---|
| High-resolution manometry: velopharynx | X | X |
| Surface EMG: submental | X | X |
| Intramuscular EMG: palate | X | X |
| Intramuscular EMG: pharynx | X | X |
| Intramuscular EMG: thyroarytenoid | X | |
| Expiratory task repeated with HRM catheter removed | X |
HRM = high-resolution manometry; EMG = electromyography.
Electrode placement was visually targeted and confirmed by examining activation patterns during rest breathing, swallowing, and phonation. Palatal insertion targeted the levator veli palatini. Palatal electrodes were placed into the dimple of the oral surface of the velum in a superior, lateral, and posterior direction just off midline; placement was verified by observing increased activity during spoken production of /k/.14–16 Thyroarytenoid and pharyngeal EMG insertion methods were performed as previously described.15,16 Pharyngeal insertion targeted the superior pharyngeal constrictor. Pharyngeal electrodes were placed lateral to the median raphe of the posterior pharyngeal wall; placement was verified by observing increased activity during the swallow in absence of activity during head rotations. Prior to thyroarytenoid electrode insertion, 1 cc of 1% lidocaine hydrochloride with epinephrine (1:100,000) was subcutaneously injected into the neck through a 30-gauge needle. The characteristic thyroarytenoid pattern of activation during a swallow and vocal fold adduction was consistent with accurate placement. Bilateral surface electromyography electrodes were placed in the submental region between the mandible and the hyoid bone, each at 1 cm from midline, and a surface ground electrode (A10058-SRT, Vermed, Bellows Falls, VT) was placed on the forehead. During each trial, each subject was able to establish a stable quiet EMG baseline before task onset. For the task, we defined the level of change to be 200% above baseline.16 Electromyographic signals were amplified, band-pass filtered from 100 Hz to 6 KHz (Model 15LT, Grass Technologies, Warwick, RI), and digitized at 20 kHz (LabChart version 6.1.3, ADInstruments, Colorado Springs, CO).
Tasks
Once the catheter and electrodes were placed, participants rested for approximately 5 minutes prior to performing the experimental tasks. During the experiment, each participant sat comfortably in a chair and looked straight ahead with the chin in a neutral position.
Seated MEPs were measured using a digital manometer (Micro Respiratory Pressure Meter, CareFusion, Yorba Linda, CA). For each trial, the participant was instructed to inhale to total lung capacity (“fill your lungs as much as possible”), seal the lips fully around the tubed mouthpiece, and exhale forcefully (“blow out as fast and as hard as you can”). Maximum expiratory pressure was calculated as the average of three trials within 10% variance. An EMST training device (EMST 150, Aspire Products, Gainesville, FL) was used to set expiratory targets at 50% and 75% of MEP. The sham trainer has a matching design to the EMST trainer but with negligible expiratory valve-opening pressure of 1- to 3-cm H2O. Expiratory tasks were completed seated with a nose clip in place. For the EMST tasks, participants were asked to take a deep breath, hold the cheeks lightly with the thumb and forefingers, and blow forcefully through the device until the valve opens (hearing air rush out).
Experimental tasks included: a) swallow 10 cc of water; b) exhale into a digital manometer with maximal force while seated (seated MEP); c) exhale into an EMST device set at 50% MEP; d) exhale into an EMST device set at 75% MEP; and e) exhale into a sham device. Twenty-five trials (five for each five tasks) were collected and analyzed for each participant with simultaneous EMG and HRM recording.
After completing the 25 trials, the HRM catheter was removed, and expiratory tasks were repeated only with EMG recording to allow comparison of EMG activity in the palate under catheter and no catheter conditions.
Data Analysis
Time-linked pressure and EMG data for each muscle were analyzed using a customized MATLAB program (MathWorks, Inc., Natick, MA). For this study, we first visually examined the rectified EMG data and the HRM spatiotemporal plots (Fig. 2). Electromyography signals were then normalized to allow for preliminary magnitude comparisons between conditions. The highest value from the EMG signal for a given muscle and participant was set to equal 100%. Therefore, EMG data were computed to be between 0% and 100% (Fig. 3). The velopharyngeal HRM data were also normalized. The highest peak from the velopharyngeal pressure for a given sensor and participant was set to equal 100%. Therefore, relative velopharyngeal data were computed to be between 0% to 100% (Fig. 4). To examine the potential influence of the HRM catheter on velopharyngeal EMG data, we visually examined the palate EMG signals for each task in participant 2, with the HRM catheter in place and again after the catheter was removed (Fig. 5). Finally, peak pressure readings were extracted from the first HRM sensor caudal to the velopharyngeal region and compared to MEP measured on the digital manometer (Fig. 6).
Fig. 2.
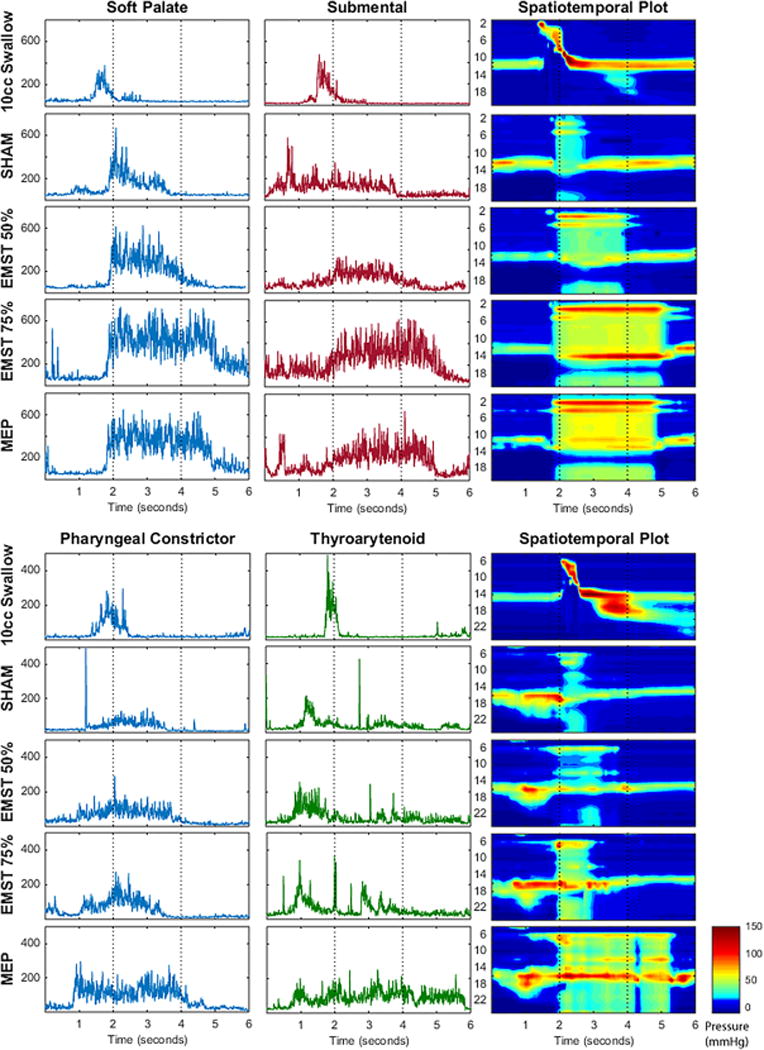
Representative EMG waveforms and HRM spatiotemporal plots for both participants. Each row presents the time-aligned rectified EMG waveform (top: palate and submental for participant 1; bottom: pharynx and thyroarytenoid for participant 2) and corresponding spatiotemporal plot for each task.
The vertical axes for the EMG waveforms are in microvolts. The vertical axes for the spatiotemporal plots are labeled by HRM sensor number. Two seconds on the horizontal axes marks approximate onset of the expiratory training task.
EMG = electromyography; EMST = expiratory muscle strength training; HRM = high-resolution manometry; MEP = maximum expiratory pressure.
Fig. 3.
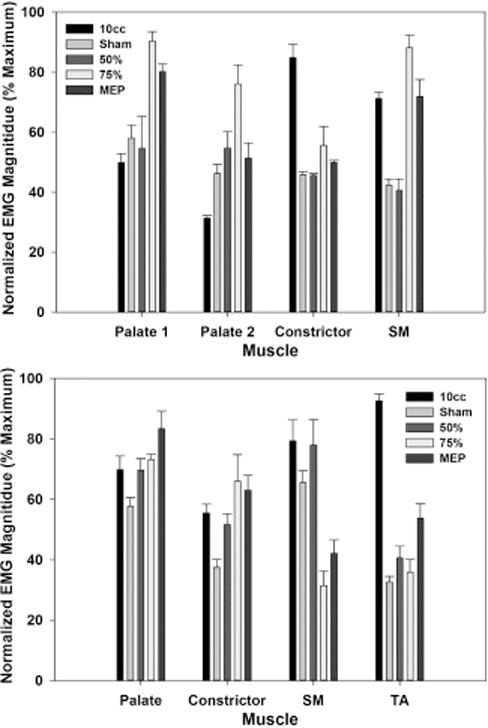
Peak EMG magnitude. Each bar represents the mean (and standard error) of the maximum EMG magnitude for five trials of each task for palate, pharynx, thyroartyenoid, and submental electrodes (top: participant 1; bottom: participant 2).
EMG data were normalized based on the maximum EMG magnitude in the data set for each individual EMG channel.
EMG = electromyography; MEP = maximum expiratory pressure; SM = submental; TA = thyroarytenoid.
Fig. 4.

Peak HRM VP pressure. Each bar represents the mean (and standard error) of the maximum VP pressure for five trials of each task (top: participant 1; bottom: participant 2).
Pressure data were normalized based on the maximum pressure in the data set for each HRM sensor. For each participant, there were two sensors within the VP region.
EMG = electromyography; HRM = high-resolution manometry; MEP = maximum expiratory pressure; VP = velopharyngeal.
Fig. 5.
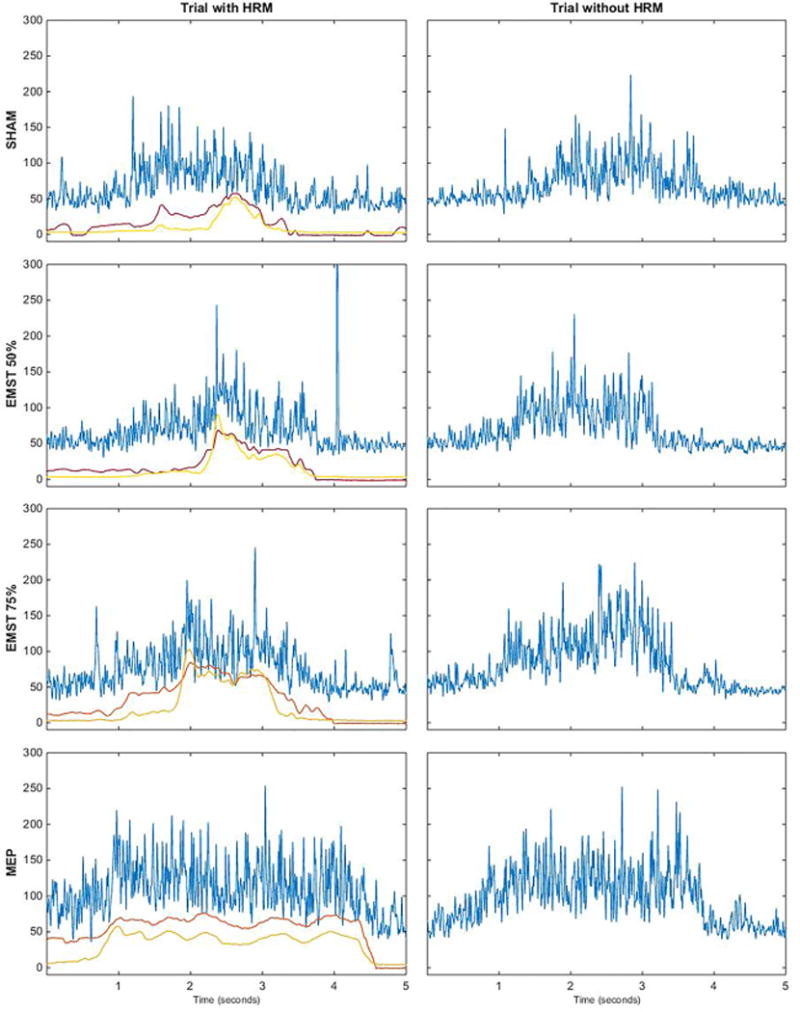
Representative palate EMG waveforms (blue) and HRM velopharyngeal pressure waveforms (red and yellow, left column only) for each task for participant 2. The palate EMG waveforms are similar between the left column (with the HRM catheter in place) and the right column (after the HRM catheter was removed).
Vertical axes for the EMG waveforms are in microvolts. Vertical axes for the pressure waveforms are in mm Hg. Horizontal axes are time in seconds.
EMG = electromyography; EMST = expiratory muscle strength training; HRM = high-resolution manometry; MEP = maximum expiratory pressure.
Fig. 6.
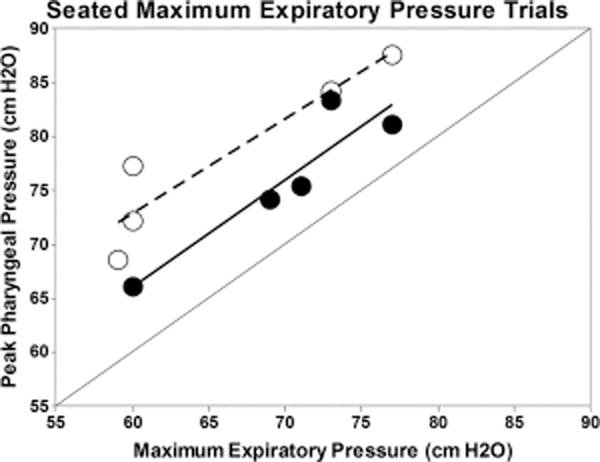
Peak pharyngeal pressure for seated MEP trials compared with high-resolution manometry (HRM) detected pharyngeal air pressures (converted from mmHg to cm H2O). Black circles are for participant 1; open circles are for participant 2. Although the data suggest a positive linear relationship, HRM-detected pressures measured in the pharynx were consistently higher than MEP pressures.
HRM = high-resolution manometry; MEP = maximum expiratory pressure.
RESULTS
Participants
For participant 1, seated MEP was 74-cm H2O, with EMST settings at 37-cm H2O (50%) and 56-cm H2O (75%). For participant 2, seated MEP was 60-cm H2O, with EMST settings at 30-cm H2O (50%) and 45-cm H2O (75%). For all tasks, there was an increase in EMG activity for each muscle compared with baseline. Expiratory pressure measures recorded from the digital expiratory manometer during MEPs testing showed a linear relationship with peak HRM pharyngeal pressure (Fig. 6). For each participant, peak HRM pressures within the pharynx exceeded the expiratory pressures by about 30% to 40%.
Palatal Activity
For both participants, EMG activity of the palatal levator muscle generally increased in a load-dependent fashion with increased expiratory pressure (i.e., sham < 50% EMST < 75% EMST; Figs. 2 and 3). The EMG-detected pattern of palatal activity was paralleled by increased closure pressure in the velopharynx detected on HRM (Fig. 4) alongside increased pressure in the upper esophageal sphincter (Fig. 2, spatiotemporal plot). Electromyography recordings during high-intensity expiratory tasks (75% EMST or MEP) generated peak amplitudes in the palatal musculature equivalent to or above those generated during swallowing tasks (Fig. 3). In contrast, palatal activity during similar expiratory tasks, as measured by velopharyngeal pressures on HRM, was at levels below those observed during 10-cc swallows (Fig. 4). Finally, patterns of palatal EMG activity in participant 2 appeared similar with and without the HRM catheter in place (Fig. 5), suggesting that the nasopharyngeal manometry catheter did not interfere with patterns of muscle activity detected by palatal EMG recording.
Submental Activity
Consistent with previous reports,9 load-dependent submental EMG activity was detectable with surface electrode readings, but the pattern was not consistent across the participants (Figs. 2 and 3). We observed that some submental EMG activity readings reflected movements while posturing and preparing for the expiratory task, as well as effort during the expiratory task effort itself, particularly at the low load (sham, 50% of MEP) EMST tasks.
Pharyngeal Activity
Pharyngeal muscle EMG recordings only were taken from participant 1, in whom pharyngeal muscle EMG activity increased with the load of expiratory effort (Figs. 2 and 3). The peak amplitude of EMG activity during expiratory tasks appeared less than that observed during the swallow; however, the duration of activation extended throughout the EMST task.
Intrinsic Laryngeal Activity
Thyroarytenoid EMG recordings only were taken from participant 2. Thyroarytenoid EMG activity showed the expected pattern during the 10-cc swallow task associated with vocal fold adduction. As predicted, we did not detect graded load-dependent differences in EMG activity in the thyroarytenoid during the EMST tasks. Thyroarytenoid EMG activity was low during the actual the expiratory task, consistent with opening of the glottis during exhalation. Instead, we observed that thyroarytenoid EMG activity for this participant appeared to precede exhalation (Fig. 2), corresponding with impounding air pressure in preparation for the expiratory task.
DISCUSSION
In this report, our primary goal was to record activity from the muscles of the palate, pharynx, larynx, and submental region with simultaneous HRM and EMG to examine the feasibility of detecting load-dependent changes in swallowing-specific muscle regions of interest during EMST use. As hypothesized, using the EMG/HRM paradigm we were able to identify activity in the swallowing-associated muscles of interest with the use of EMST. Our preliminary findings suggest graded increases in previously unmeasured pharyngeal and velar muscle EMG activity because expiratory pressure generation increased during use of the EMST device. The utility of HRM adjunctive to EMG was supported by our observation that the EMG-measured activity of the palatal levator muscle increased in parallel to HRM-detected velopharyngeal closure pressure, as well as the observation that air pressure measures on the catheter may also serve as an external calibration of the expiratory load of the task. Although group-level analysis in a larger series is necessary to statistically examine swallowing-associated muscle interactions with EMST use, to our knowledge we are the first to document load-dependent activity in the palate and pharynx during EMST tasks. This likely reflects the velar musculature working to actively seal the velopharyngeal port as expiratory pressure rises. Pharyngeal muscle activity could reflect either vertical pharyngeal shortening as the laryngeal framework lifts during the EMST task and/or active reduction of pharyngeal cross-sectional area as the airway compresses during the expiratory tasks.
Our preliminary observations also suggest that the thyroarytenoid and submental muscles appear to be as much or more involved in preparatory activity before the expiratory resistance task than during the task itself. Simultaneous HRM and EMG used in this study allowed visualization of pharyngeal pressure on the manometry catheter with EMG muscle activity before and during the expiratory task. In this paradigm, much of the observed submental muscles activity appeared to coincide with opening the mouth for inhalation and holding the device in position during the task. That is, our observations suggested that submental muscle activity may correspond with posturing of the mouth and preparation for the expiratory task, in addition to or at lower resistance loads of expiratory effort. The set of muscle groups that contribute to the submental surface EMG waveform can be influenced by a variety of actions, including jaw/mouth opening, posturing, and tongue protrusion. Therefore, this complex submental region of interest will be the focus of further detailed, group-level investigation.
Thyroarytenoid muscle activity often appeared to correspond with impounding respiratory air in preparation for the expiratory task rather than playing an active role during the expiratory task. A relatively brief burst of thyroarytenoid activity was present before exhalation, when the vocal folds would be expected to close as subglottal pressure is compounded prior to the release of pulmonary air. Thyroarytenoid activity then decreased when the vocal folds are expected to open during exhalation. Therefore, previous reports of decreased aspiration after EMST appear to involve mechanisms beyond the intrinsic larynx.5,6 Any treatment benefit to the thyroarytenoid may stem from the preparation for the EMST task rather than the expiratory task itself. Our experimental approach will enable us to examine these mechanisms in further detail.4–6
The data presented are intended to demonstrate feasibility of detecting load-dependent change in palate, pharyngeal, and submental muscles using simultaneous EMG and HRM during EMST tasks. Acknowledging fully the limitations of an exploratory technical report such as this, there are hypothesis-generating trends detectable using this novel simultaneous EMG and HRM paradigm during swallowing and EMST tasks. On the basis of these preliminary observations, a hypothesis-driven study with group-level analysis is planned to statistically test the hypothesis that EMST is associated with load-dependent activity in swallowing-associated muscles. The mechanisms by which EMST may improve swallowing-related airway protection may likely involve muscles beyond the suprahyoid region and laryngeal introitus. This preliminary study represents the first step toward defining these mechanisms in healthy and clinical populations. Although it is likely that the experimental paradigm described herein can characterize these complex motor mechanisms, it will be important to also consider paradigms that seek to understand how EMST modulates sensory receptors17,18 and cough7 as components of airway protection in patients with dysphagia.
These initial findings and the work that will follow may have important implications for understanding the mechanisms of EMST as we seek to define its potential as a treatment option for swallowing disorders and other aspects of airway sensorimotor control. Electromyography was initially used to improve respiration in healthy elderly, athlete, and musician participants before it was explored as a treatment of respiratory, cough, airway protection, and voice in clinical populations. Earlier reports support the premise that expiratory mechanisms by which EMST improves cough and subglottic pressure generation may include thoracic and abdominal muscles of respiration.9 Our initial objective of this pilot work was to explore a paradigm for hypothesis-driven studies to characterize the swallow-related muscle activity during EMST training. Although swallowing is the focus of this line of work, more fully characterizing muscle activity during EMST beyond the thorax, including muscles of the larynx, pharynx, and palate, will hopefully have broader utility. Directly measuring the mechanisms of EMST may enable clinicians and scientists to determine how EMST may target specific muscle groups, understand the potential limitations of EMST, match therapy to the pathophysiology of dysphagia within disordered populations, and identify potential treatment benefits for other clinical populations.
CONCLUSION
In this work, we have demonstrated the feasibility of a simultaneous EMG and HRM experimental paradigm to detect load dependent differences in the palate, pharynx, and submental region during use of an EMST device.
Acknowledgments
This study was supported by National Institutes of Health (NIH) grant number R21 DC011130 from the National institute on Deafness and other Communicative Disorders (NIDCD). Dr. Hammer’s research is also funded by NIH grants DC014519 and DC010900. Ms. jones receives support from the NIH grants DC011130 and T32 GM007507. Dr. Rosen receives support from NIH, NIDCD grant number T32 DC009401. Dr. Hutcheson’s research is also funded by NIH grants R01 DE025248 and R03 CA188162 and the MD Anderson Institutional Research Grant Program.
Footnotes
Financial Disclosure: The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Institution where work was performed: University of Wisconsin-Madison, Madison, Wisconsin, U.S.A.
BIBLIOGRAPHY
- 1.Baker S, Davenport P, Sapienza C. Examination of strength training and detraining effects in expiratory muscles. J Speech Lang Hear Res. 2005;48:1325–1333. doi: 10.1044/1092-4388(2005/092). [DOI] [PubMed] [Google Scholar]
- 2.Sapienza CM, Wheeler K. Respiratory muscle strength training: functional outcomes versus plasticity. Semin Speech Lang. 2006;27:236–244. doi: 10.1055/s-2006-955114. [DOI] [PubMed] [Google Scholar]
- 3.Sapienza CM. Respiratory muscle strength training applications. Curr Opin Otolaryngol Head Neck Surg. 2008;16:216–220. doi: 10.1097/MOO.0b013e3282fe96bd. [DOI] [PubMed] [Google Scholar]
- 4.Pitts T, Bolser D, Rosenbek J, Troche M, Okun MS, Sapienza C. Impact of expiratory muscle strength training on voluntary cough and swallow function in Parkinson disease. Chest. 2009;135:1301–1308. doi: 10.1378/chest.08-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Troche MS, Okun MS, Rosenbek JC, et al. Aspiration and swallowing in Parkinson disease and rehabilitation with EMST: a randomized trial. Neurology. 2010;75:1912–1919. doi: 10.1212/WNL.0b013e3181fef115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hegland KW, Davenport PW, Brandimore AE, Singletary FF, Troche MS. Rehabilitation of swallowing and cough functions following stroke: an expiratory muscle strength training trial. Arch Phys Med Rehabil. 2016;97:1345–1351. doi: 10.1016/j.apmr.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 7.Troche MS, Brandimore AE, Godoy J, Hegland KW. A framework for understanding shared substrates of airway protection. J Appl Oral Sci. 2014;22:251–260. doi: 10.1590/1678-775720140132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sapienza C, Troche M, Pitts T, Davenport P. Respiratory strength training: concept and intervention outcomes. Semin Speech Lang. 2011;32:21–30. doi: 10.1055/s-0031-1271972. [DOI] [PubMed] [Google Scholar]
- 9.Wheeler-Hegland KM, Rosenbek JC, Sapienza CM. Submental sEMG and hyoid movement during Mendelsohn maneuver, effortful swallow, and expiratory muscle strength training. J Speech Lang Hear Res. 2008;51:1072–1087. doi: 10.1044/1092-4388(2008/07-0016). [DOI] [PubMed] [Google Scholar]
- 10.Pearson WG, Jr, Langmore SE, Yu LB, Zumwalt AC. Structural analysis of muscles elevating the hyolaryngeal complex. Dysphagia. 2012;27:445–451. doi: 10.1007/s00455-011-9392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yanagisawa Y, Matsuo Y, Shuntoh H, Mitamura M, Horiuchi N. Change in tongue morphology in response to expiratory resistance loading investigated by magnetic resonance imaging. J Phys Ther Sci. 2013;25:667–669. doi: 10.1589/jpts.25.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammer MJ, Jones CA, Mielens JD, Kim CH, McCulloch TM. Evaluating the tongue-hold maneuver using high-resolution manometry and electromyography. Dysphagia. 2014;29:564–570. doi: 10.1007/s00455-014-9545-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones CA, Hammer MJ, Hoffman MR, McCulloch TM. Quantifying contributions of the cricopharyngeus to upper esophageal sphincter pressure changes by means of intramuscular electromyography and high-resolution manometry. Ann Otol Rhinol Laryngol. 2014;123:174–182. doi: 10.1177/0003489414522975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon JB, Collins DR, Canady JW. Single motor unit activity in levator veli palatini during speech and nonspeech tasks. Cleft Palate Craniofac J. 2003;40:256–262. doi: 10.1597/1545-1569_2003_040_0256_smuail_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 15.Van Daele DJ, McCulloch TM, Palmer PM, Langmore SE. Timing of glottic closure during swallowing: a combined electromyographic and endoscopic analysis. Ann Otol Rhinol Laryngol. 2005;114:478–487. doi: 10.1177/000348940511400610. [DOI] [PubMed] [Google Scholar]
- 16.Perlman AL, Palmer PM, McCulloch TM, Vandaele DJ. Electromyographic activity from human laryngeal, pharyngeal, and submental muscles during swallowing. J Appl Physiol. 1999;86:1663–1669. doi: 10.1152/jappl.1999.86.5.1663. [DOI] [PubMed] [Google Scholar]
- 17.Hammer MJ. Design of a new somatosensory stimulus delivery device for measuring laryngeal mechanosensory detection thresholds in humans. IEEE Trans Biomed Eng. 2009;56:1154–1159. doi: 10.1109/TBME.2008.2007968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammer MJ, Barlow SM. Laryngeal somatosensory deficits in Parkinson’s disease: implications for speech respiratory and phonatory control. Exp Brain Res. 2010;201:401–409. doi: 10.1007/s00221-009-2048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


