Abstract
We have developed two linear-dendritic telodendrimers (TDs) with rational design using amphiphilic riboflavin (Rf) as building blocks for efficient doxorubicin (DOX) delivery. Micellar TD nanoparticles (NPs) are composed of a hydrophilic polyethylene glycol (PEG) shell and a Rf-containing affinitive core for DOX encapsulation. Strong DOX-Rf interactions and amphiphilic Rf structure render these nanocarriers with an ultra-high DOX loading capacity (>1/1, DOX/TD, w/w), ~100% loading efficiency, the sustained drug release and the optimal particle sizes (20~40 nm) for efficient tumor-targeted drug delivery. These nanoformulations significantly prolonged DOX circulation time in the blood without the accelerated clearance observed after multiple injections. DOX-TDs target several types of tumors efficiently in vivo, e.g. Raji lymphoma, MDA-MB-231 breast cancer, and SKOV3 ovarian cancer. In vivo maximum tolerated dose (MTD) of DOX was increased by 2~2.5 folds for the nanoformulations in mice relative to those of free DOX and Doxil®. These nanoformulations significantly inhibited tumor growth and prolonged survival of mice bearing SKOV3 ovarian cancer xenografts. In summary, Rf-containing nanoformulations with high DOX loading capacity, improved stability and efficient tumor targeting lead to superior antitumor efficacy, which merit the further development for clinical application.
Keywords: Rational nanocarrier design, Telodendrimer, High loading capacity, Controlled drug release, Targeted therapy, Cancer treatment
1. Introduction
Doxorubicin (DOX) is one of classic anthracycline chemodrugs used as the first line chemotherapy for the treatments of many cancers,[1–4] and is also applied to the patients who relapsed from the previous taxane or platinum-based treatments.[5] Many cancers exhibit either intrinsic or acquired resistance to DOX treatment by the increased expression of drug-efflux proteins.[6–9] The combinational drug regiments or the escalated dose may overcome drug resistance, which are, however, hindered by the toxic side effects, e.g. dose-limiting cardiotoxicity and myelosuppression for DOX.[10, 11] To address this issue, drug delivery systems have been developed to enhance drug accumulation at the tumor site and reduce toxicities.[12]
The emergence of Doxil®, a PEGylated liposomal DOX nanoformulation, dramatically improves the pharmacokinetics (PK), reduces the cardiotoxicity[13] and increases DOX accumulation at the tumor sites by the enhanced permeability and retention (EPR) effect.[13–15] However, Doxil only marginally improves the efficacy in solid tumor treatments in the clinic. [16–18] It was reported that Doxil accumulated at the periphery of blood vessels without further diffusion into interstitial space, which may due to the large particle sizes and slow drug release.[19–23] Various approaches have been reported to improve the intratumoral diffusion[24] and drug release[22, 25] of Doxil in solid tumors in order to improve its efficacy. In addition, up to 48% of patients receiving Doxil® treatment develop a formulation-associated hand-foot syndrome (Palmar-Plantar Erythrodysesthesia), which is caused by the peripheral accumulation of the long-circulating large-sized Doxil® particles.[26, 27] It is well known that different pore sizes and different vasculature degree exist in various types or stages of tumors, which causes the heterogeneity of EPR effects. [28] Therefore, it is key to develop nanocarriers with small particles sizes (10–20 nm), that have better chance to extravasate and penetrate deeper in solid tumors than Doxil with ~100 nm in size.
Various nanocarriers based on amphiphilic block copolymers,[29–31] liposome,[32] gold and carbon NPs,[33, 34] have been developed for DOX delivery.[35] Numbers of DOX nanoformulations have been tested in clinical trials for cancer treatments.[36] A few liposomal formulations, e.g. Myocet[37, 38] and Lipo-DOX[39], were approved for cancer treatments, which reduce the cardiotoxicity of the free DOX and the hand-foot syndrome associated with Doxil without compromising drug efficacy.[40, 41] However, no other type of DOX nanoformulation has yet been approved by any regulatory agencies due to the lack of the merit of efficacy and toxicity profiles in comparison with existing formulations. Therefore, further development of novel nanocarriers with optimal physiochemical properties for DOX delivery is still highly demanded to improve DOX-based cancer therapy.
We have developed a well-defined linear-dendritic telodendrimer (TD) nanoplatform[42], which can be decorated precisely with drug binding molecules (DBMs) identified by virtual screening to optimize in vivo drug delivery.[43] Riboflavin (Rf, vitamin B2) was identified as one of the top DBMs for DOX binding via both hydrophobic π-π stacking and hydrogen bonding.[43] Rf is the functional fragment in flavin mononucleotide (FMN) and Flavin adenine dinucleotide (FAD), which are cofactors in numerous enzymes for metabolic energy transformation.[44] It was reported that the interactions between DOX and Rf are much stronger than the self-association of FAD or FMN and are comparable to DOX-DOX binding.[45] In addition, it has been suggested that Rf can reduce the cardiotoxicity[46] and potentially interfere the efficacy of DOX[47]; and DOX treatment in turn may deplete Rf level in the patients.[48] All these preclinical and clinical evidence further confirm the strong DOX-Rf interactions as revealed in our computational predictions.[43] Rf has both aromatic structure and hydrophilic ribityl hydroxyls, which share the common features with many hydrotropic compounds used in drug formulations to improve drug solubility.[49] Polymer micelles, composed of such hydrotropic compounds[50] or featured with both polar (H-bonding and dipole-dipole interactions) and hydrophobic interactions [51–53] in the core microenvironment, have been developed to improve loading capacity and stability for efficient drug delivery.
Such amphiphilic structure of Rf is the desired and essential property as efficient building blocks to stabilize nanoformulations as demonstrated in our TD systems[42, 54–56] and others, e.g. poly(2-oxazoline) micelles [52]. For example, a facial amphiphilic cholic acid (CA) has been demonstrated to be critical in stabilizing nanocarriers as co-building blocks in TDs, which were decorated with hydrophobic DBMs for the delivery of specific drugs.[43, 54–56] Therefore, the application of Rf affords the TD system with a triple attributes for DOX delivery: (a) enhancing DOX binding affinity; (b) stabilizing nanoformulations; and (c) simplifying TD synthesis as the only building block needed. Unlike the free Rf, the conjugated Rf in TD won’t interfere the activity of DOX after drug release. As expected, Rf-TDs self-assemble into spherical micelles after drug loading that are colloidally stable with small particle sizes (20–40 nm) for storage over months. Surprisingly, these Rf-TDs have a superior DOX loading capacity with 1:1 mass ratio (drug/TD) with ~100% efficiency and sustained drug release profiles. Nanoformulation with high drug loading capacity has been reported to improve both safety and efficacy of chemotherapy.[53] Doxil is commonly used in ovarian cancer treatment in the clinic. [57] Therefore, we chose SKOV-3 ovarian xenograft mouse models to challenge our Rf-containing DOX-TD nanoformulations with the side-by-side comparison with Doxil in cancer treatment. We observed the significantly prolonged circulation, increased MTDs (2~2.5-fold increase), and the improved tumor-growth inhibition in ovarian cancer treatment for our nanoformulations in comparison with both free DOX and Doxil®.
2. Material and methods
2.1 Materials and Nomenclature
Doxorubicin hydrochloride (DOX·HCl) was purchased from AvaChem Scientific LLC (San Antonio, TX), and Doxil® (Ben Venue Laboratories Inc., Bedford, OH) was obtained from the Regional Oncology Center Pharmacy, State University of New York (SUNY) Upstate Medical University. Monomethyl-terminated poly(ethylene glycol) monoamine hydrochloride (MeO-PEG-NH2·HCl, Mw 5 kDa) was purchased from JenKem Technology USA Inc. (Fmoc)Lys(Boc)-OH, and (Fmoc)Lys(Fmoc)-OH were purchased from AnaSpec Inc. (San Jose, CA). CellTiter 96® AQueous MTS reagent powder was purchased from Promega (Madison, WI, USA). Rf, CA, glutaric anhydride and all other chemicals were purchased from Sigma-Aldrich (St. Louis). The preparation of CA derivatives (CA NHS ester) was described in the previous publication.[42]
The nomenclatures of the TDs follow the system used in our previous publications.[42, 43, 58] For example, TD named PEG5kRf8 means that the molecular weight of PEG is 5 kDa with eight Rf molecules conjugated on the periphery amino groups of polylysine; PEG5kCA4Rf4 indicates that four CA and four Rf molecules are conjugated on the α-amino and ε-amino groups, respectively.
2.2 7,8-dimethyl-10-((2R,3R,4S)-2,3,4-trihydroxypentyl-5-carboxybutyryl) benzo[g]pteridine-2,4 (3H,10H)-dione (Rf-COOH) synthesis
0.5 g of Rf was added into a 50-mL round bottom flask, then 10 mL of dimethyl sulfoxide (DMSO), and 6 mL pyridine were added. Solution was heated up to nearly 90 °C until Rf dissolved. 0.5 g glutaric anhydride and 0.1 g 4-dimethylaminopyridine (DMAP) were dissolved in 1 mL DMSO and then added into the flask. The reaction was kept at 70 °C for overnight. After the reaction completion confirmed by thin-layer chromatography (TLC), the system was cooled down to room temperature. Reaction solution was transferred to a 500-mL flask, and 15 mL dichloromethane (DCM) and 200 mL ethyl acetate were added to the reaction solution to precipitate the product. The precipitation was washed twice with pure ethyl acetate: orange solid, yield 70%, 1H NMR (DMSO-d6, 600 MHz) δ:1.73 (m, 2H, CH2), 2.24 (t, J=7.3 Hz, 2H, CH2), 2.34 (t, J=7.4 Hz, 2H, CH2), 2.37 (S, 3H, CH3), 2.46 (S, 3H, CH3), 3.62 (m, 1H, CH2-O-), 3.86 (m, 1H, CH), 4.03 (dd, J1=11.2 Hz, J2=6.4 Hz, 1H, CH2-O-), 4.22 (dd, J1=11.2 Hz, J2= 2.7 Hz, 1H, CH), 4.65 (m, 1H, CH), 4.86 (m, 2H, N-CH2), 7.85 (s, 1H, Ar-H), 7.86(s, 1H, Ar-H), 11.30 (s, 1H, CO-NH-CO), 11.99 (s, 1H, COOH). MALDI-TOF MS calculated (calcd.) for C22H26N4O9 [M+2H]+. : 493.173, found: 493.289 (Fig. S1f & S1k).
2.3 TD synthesis
General procedure for TD synthesis has been reported in our previous publications[42, 43, 58] and is described briefly as following: the TDs were synthesized by solution-phase peptide chemistry starting from MeO-PEG-NH2·HCl. N-terminal-protected lysine was used to synthesize the branched scaffold of TD. Triethylamine (TEA, 1 equiv) was added to neutralize the hydrochloride on PEG. Diisopropyl carbodiimide (DIC, 3 equiv) and N-hydroxybenzotriazole (HOBt, 3 equiv) were used as coupling reagents in dimethylformamide (DMF) to form amide bonds. The reaction completion was confirmed by the negative Kaiser test result. The ice-chilled ether was added to the reaction solution to precipitate PEGylated intermediates, and then washed by chilled ether twice. Fmoc-protection groups were removed by the treatment with 20% 4-methyl-piperidine in DMF for 30 min. TD was precipitated and washed with chilled ether for three times. Homo TD PEG5kRf8 (TD-1) synthesis: A dendritic polylysine was synthesized by three repeated (Fmoc)Lys(Fmoc)-OH coupling as mentioned above. At the end, the polylysine was capped with NHS ester of Rf carboxylic acid derivative using HOBt/DIC as coupling reagents. The hybrid TD PEG5kCA4Rf4 (TD-2) synthesis: A dendritic polylysine with orthogonally protected α-(N-Fmoc) and ε-(N-Boc) amino groups was synthesized on MEO-PEG-NH2 after two repeated (Fmoc)Lys(Fmoc)-OH couplings followed by a (Fomc)Lys(Boc)-OH coupling using HOBt/DIC chemistry. Then, the Fmoc group was removed by the treatment of 20% 4-methylpiperidine in DMF, followed by the coupling of CA NHS on the α-position of lysine. Then, Boc protecting groups were removed by treatment of 50% trifluoracetic acid (TFA) in DCM for 0.5 h. Rf carboxylic acid derivative reacted with the ε-amine of lysine to generate the hybrid TD. TDs were precipitated twice and washed three times with cold ether, and then dialyzed for purification.
2.4 Drug loading and characterization
DOX was encapsulated into the TD micelles by a thin-film hydration method.[42] DOX·HCl was dissolved in chloroform (CHCl3)/methanol (MeOH) (10:1 v/v) and neutralized by the addition of triethylamine (TEA, 3 equiv) or NaHCO3 (1 equiv, in 1H NMR experiment). The TD was also dissolved in CHCl3/MeOH (10:1 v/v) and transferred into the drug solution at certain TD/drug ratios. Solvents were evaporated to dryness and a thin film of homogeneous drug-TD mixture was coated on the flask wall, which was further dried under a high vacuum for 1 h. Then the film was hydrated in the phosphate buffered saline (PBS). All the DOX formulations were covered by the foil all the time during experiments and storage. The particle size distributions of the drug-loaded NPs were measured by dynamic light scattering (DLS) (Microtrac) and transmission electron microscopy (TEM) (a JEOL JEM-1400 instrument at 80 kV) with negative staining by 1% uranyl acetate. Zeta potential were measured on a Malvern Nano-ZS zetasizer at room temperature in PBS. The particle stability of DOX-loaded NPs upon storage was monitored by DLS. Any unloaded drug precipitation will be removed by filtration through 0.22 μm filter. Drug loading content (DL%) and loading efficiency (LE%) were analyzed by fluorescence and calculated as the following equations: DL% = (mass of encapsulated DOX / mass of TD used for DOX encapsulation) × 100%; LE% = (mass of encapsulated DOX / mass of DOX added) × 100%.
2.5 Nuclear magnetic resonance (NMR) studies
The dried thin film of drug and TD mixture at 1:1 or 1:10 DOX/TD mass ratios will be dispersed in deuterium oxide (D2O) for NMR (Bruker AVANCE III 600 MHz) characterization. As comparisons, the vacuum-dried free DOX and empty TDs were dissolved in D2O, respectively, for 1H NMR studies. DOX concentrations were kept the same at 1 mg/mL for all the samples except blank TD solution (1 mg TD/mL). The loading efficiency (LE%) of DOX were calculated based on the ratio of the peak integrations of the aromatic DOX-proton signals in DOX-nanoformulations over the signals of the free DOX solutions, which were normalized by the signals of the internal reference, i.e. water peak in D2O solvent.
2.6 Size exclusive chromatography (SEC)
The encapsulated DOX and free DOX were separated by SEC using Sephadex G-25 (GE Healthcare Life Sciences). The column (8 × 40 mm) was equilibrated with PBS. A 40 μL sample solution of free DOX or DOX-loaded Rf-containing nanoformulations at a DOX concentration of 1 mg/mL was applied on the column. The flow rate through the column was kept at 0.7 mL/min by gravity during the separation. 96-well plates were used to collect each droplet from the beginning. Then 10 μL solution was taken out from each well and transferred to another 96-well plate. 90 μL DMSO was added into each well and mixed gently to dissolve the micelle structure and release drug into the solution. The fluorescence signals of DOX were measured at ex/em 520/600 nm by a microplate reader (Synergy H1).
2.7 Ultracentrifuge filtration
The Spin-X centrifuge tube filters (MWCO 5,000 Da, Corning) were used to separate free DOX from DOX loaded PEG5kRf8 (DOX-TD-1) and DOX loaded PEG5kCA4Rf4 (DOX-TD-2). 100 μL of DOX-loaded Rf-containing nanoformulations were added onto the filter. After centrifugation at 13,000 g for 10 min, the free DOX was collected through the filter. 2 μL sample from stock solution or collected solution were added into 18 μL DMSO. The fluorescence of DOX were measured by Synergy H1 microplate reader (BioTek, Winooski, VT) at an excitation wavelength of 520 nm and an emission wavelength of 600 nm to determine the drug loading efficiency.
2.8 Agarose gel electrophoresis
Samples with loading buffer (30% glycerol aqueous solutions) were loaded into an agarose gel (1.5% wt) in Tris-acetate-EDTA (TAE) buffer. The gel was running for 2 h at a constant current of 20 mA. The fluorescent signal of DOX on the gel was imaged by a Bio-Rad Universal Hood II Imager (Bio-Rad Laboratories, Inc.) using SYBR green channel (ex/em 497/520 nm). For the stability studies of micelles incubated with fetal bovine serum (FBS), the gel was stained with 1% coomassie blue for 0.25 h followed by detaining overnight. Then the gel was imaged by a Bio-Rad Universal Hood II Imager (Bio-Rad Laboratories, Inc.)
2.9 In vitro drug release
The drug release profiles of DOX formulations were measured by a dialysis method. 300 μL of free DOX, Doxil® and DOX-loaded nanoformulations were loaded into dialysis cartridges with 3.5 kDa MWCO dialysis membranes (Thermo Scientific, Rochford, IL). The cartridge was dialyzed against 50 mL PBS and gently shaken at 37 °C. The PBS solution was changed every 4 h. 2 μL of drug solution within dialysis cartridge will be withdrawn at different time points and diluted with 18 μL of DMSO. Then the concentration of DOX was measured using Synergy H1 microplate reader (BioTek, Winooski, VT) at an excitation wavelength of 520 nm and an emission wavelength of 600 nm. Data were reported as the average percentage of DOX accumulative release for each triplicate samples.
2.10 Cell culture and animals
Ovarian cancer cell line SKOV3, breast cancer cell line MDA-MB-231, T-cell lymphoma cell line Jurkat, B-cell lymphoma cell line Raji, myeloma cell line H929, and myelogenous leukemia cell line K562 were purchased from American Type Culture Collection (Manassas, VA, USA). JurKat, Raji, H929 and K562 cells were cultured in RPMI-1640, and SKOV3, MDA-MB-231 cells were cultured in DMEM medium supplemented with 10% FBS, 100 U/mL penicillin G and 100 μg/mL streptomycin at 37 °C using a humidified 5% CO2 incubator. Specific-pathogen free BALB/c mice aged 5–6 weeks were purchased from Charles river (Hollister, CA); female athymic nude mice (Nu/Nu strain), aged from 5–6 weeks were purchased from the Jackson Laboratory (Bar Harbor, Maine, USA). All the animals were kept under pathogen-free conditions according to the AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care) guidelines and were allowed to acclimatize for at least 4 days before any experiments. All the animal experiments were performed in the compliance with the institutional guidelines and according to the protocol approved by the Committee for the Humane Use of Animals of State University of New York (SUNY) Upstate Medical University.
2.11 Cell viability study
Raji, Jurkat, K562 and H929 cells were seeded in a 96-well plate with the cell density of 8 × 103 cells per well. MDA-MB-231 and SKOV3 cells were seeded in a 96-well plate with the cell density of 4 × 103 cells per well. After an overnight incubation, the cells were treated with different concentrations of DOX formulations, as well as the blank polymers. After 72 h incubation, Cell Titer 96 Aqueous Cell Proliferation Reagent, which is composed of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) and an electron-coupling reagent phenazine methosulphate (PMS), was added to each well according to the manufacturer’s instructions and further incubated for 1–2 h at 37 °C. The cell viability was determined by measuring the absorbance at 490 nm using a microplate reader (BioTek Synergy H1). Untreated cells served as a control. Results were shown as the average cell viability of triplicate wells via a formula: Cell viability % = [(ODtreat-ODblank)/(ODcontrol-ODblank) × 100%].
2.12 Cellular uptake study
2.12.1 Confocal fluorescence microscopy
MDA-MB-231 cells were seeded in a chamber slide with a density of 5 ×104 cells/well in 300 μL of DMEM medium and cultured overnight. Then the cells were incubated with free DOX, Doxil®, and DOX-loaded Rf-containing nanofromulations at the final DOX concentrations of 10 μM for free DOX and 30 μM for Doxil® and DOX-loaded Rf-containing nanoformulations at 37 °C, respectively. After 2 h incubation, the cells were washed three times by cold PBS and fixed by 4% paraformaldehyde for 10 min. The cell nuclei were counterstained by 4’,6-diamidino-2-phenylindole (DAPI). The slides were mounted with cover slips and cells were imaged with a Nikon FV1000 laser scanning confocal scanning microscope.
2.12.2 Cell lysis and drug extraction
1× 105 MDA-MB-231 cells were incubated with free DOX and DOX-loaded nanoformulations with different DOX concentrations at 1, 3 and 9 μM for 2 h at 37 °C and 4 °C, respectively. The cells were washed with PBS for three times. 100 μL extraction buffer containing 10% Triton X-100, deionized water and isopropanol acidified with 0.75 N HCl at a 1:2:15 volume ratio, were added to the cells, and DOX were extracted for overnight at 4 °C. The fluorescence of each cell lysate supernatant was measured at an excitation wavelength of 520 nm and an emission wavelength of 600 nm using microplate reader (BioTek Synergy H1).
2.13 Hemolytic assays
1 mL of fresh blood from a healthy human volunteer was collected into 5 mL PBS solutions with 20 mM EDTA. Red blood cells (RBCs) were separated by centrifugation at 1000 rpm for 10 min. Then the RBCs were washed by PBS for three times and were suspended in 20 mL PBS. DOX formulations or blank polymers were added into 200 μL RBC solutions with the concentration range at 10, 100, 500 and 1000 μg/mL followed by gentle vortex and incubation at 37 °C for 0.5 h, 4 h and overnight, respectively. The samples were centrifuged at 1000 rpm for 5 min. The hemoglobin in supernatant was measured by the UV absorbance at 540 nm using a NanoDrop spectrophotometer (NanoDrop 2000c, Thermo Scientific). PBS and Triton-100 (2%) were also incubated with RBCs for a negative and positive control, respectively. The hemolytic toxicity was calculated by the following formula: Hemolysis % = [(ODsample-ODPBS) / (ODtriton-ODPBS)] × 100%.
2.14 Pharmacokinetic studies
Healthy specific pathogen-free BALB/c mice aged 5–6 weeks were administrated intravenously with DOX·HCl, Doxil®, DOX-TD-1, and DOX-TD-2 at a DOX single dose of 10 mg/kg body weight, respectively (n=3). Blood was collected from mice tail vein at different time points in a heparinized tube. Plasma was isolated and collected by centrifugation and diluted by 10-fold with DMSO for fluorescent measurements. The fluorescence of DOX was measured by a microplate reader (Synergy H1, BioTek) at an excitation wavelength of 520 nm and an emission wavelength of 600 nm. The pharmacokinetic parameters were calculated by an add-in program PKsolver in Microsoft Excel. AUC (area under the curve), Cmax (maximum drug concentration), t1/2(terminal half-life), and Cl (total body clearance) were determined.
2.15 Maximum tolerated dose (MTD) studies
6-week old healthy specific pathogen-free BALB/c mice were administrated intravenously with DOX·HCl, Doxil®, DOX-TD-1, DOX-TD-2 at the DOX doses of 10, 15, 20 and 25 mg/kg for either single dose or triple doses with 4-day intervals treatment, respectively (n=3). Mice survival or physical conditions and body weight change were monitored daily for 2 weeks. On day 7 after the last dose, blood was collected from each mouse for blood count analysis by an Hemavet instrument (Hemavet 950FS, Mascot). The MTD was defined as the allowance of 15% loss of median body weight and causing neither death due to toxic effects nor significant changes in the general signs within two weeks after the last dose.
2.16 Fluorescence small animal imaging
Raji lymphoma, MDA-MB-231 breast cancer, and SKOV3 ovary cancer xenograft tumor bearing mice model were established by subcutaneous injection of 1 × 107 cells in 100 μL of Matrigel and PBS (1:1, v/v) at the right or left flank of female nude mice aged 5–6 weeks. The fluorescent nanoformulations for in vivo near infrared fluorescence (NIRF) optical imaging were prepared by co-loading of a hydrophobic near-infrared dye DiD and DOX into TD-1 and TD-2 NPs, respectively, at a ratio of 0.2:1:5 (w/w/w, DiD/DOX/TD). Then the DiD-DOX-co-loaded Rf-containing nanoformulations were filtered with a 0.22 μm sterile filter. Free DiD was used as the control group. All the formulations were intravenously injected through tail vein. The mice were anaesthetized and imaged by IVIS® (In vivo Imaging System) 200 (perkinElmer) at different time points of 0.5, 1, 2, 4, 8, 24, 48 and 72 h with the ex/em 625/700 nm. Finally, the mice were euthanized, tumors and all the major organs were excised for ex vivo imaging. The associated fluorescence intensities were analyzed by Living Image software (Caliper Life Sciences) using operator-defined regions of interest (ROI) measurements.
2.17 Tissue microscopic imaging
Healthy Balb/c mice were treated with free DOX, Doxil, DOX-TD-1D, and DOX-TD-2D at MTD doses. Heart, liver, and kidney were harvested and embedded in O.C.T (Optimal Cutting Temperature Compound, Sakura Finetek USA, Inc) and stored at -80 °C. Tissue were cut into serial 10 μm sections using a Minotome Cryostat and fixed by 95% paraformaldehyde ethanol (EtOH) for 15–20 min, and subjected to pathological analysis by haemotoxylin and eosin (H&E) staining. In addition, the SKOV3 and MDA-MB-231 tumors were obtained from the nude mice used for the in vivo fluorescence imaging study. The tumor tissue slides were prepared by the same processes as described above. The nuclei were stained by DAPI. Cover slips were mounted with the slides and imaged by a Zeiss Axioskop upright fluorescence microscope equipped with a digital camera (Carl Zeiss Microimaging, Inc., Thornwood, NY).
2.18 In vivo efficacy for tumor treatment
SKOV3 xenograft tumor bearing mice model was established by subcutaneous injection of 1 × 107 cells in 100 μL of Matrigel and PBS (1:1, v/v) at the right or left flank in female nude mice. After tumor volume reached 150–200 mm3, the mice were treated with PBS, DOX·HCl (q4d × 3, 8 mg/kg), Doxil® (q4d × 3, 10 mg/kg), DOX-TD-1D (q4d × 3, 20 mg/kg), and dialyzed DOX-TD-2D (q4d × 3, 20 mg/kg), respectively (n=5). Bodyweight changes were monitored and tumor sizes were measured by a digital caliper once a day in the first 3 weeks, then once every other day. The tumor volume was calculated by the equation: Tumor volume = (L×W2) /2, in which L and W are the longest and shortest in tumor diameters (mm), respectively. The mice were euthanized when the tumor volume reached to 2,000 mm3, which was considered as the end of survival data. To compare each group, the relative tumor volume was calculated at each time point (Relative tumor volume = absolute tumor volume/ initial tumor volume). On day 7 of the last dose, blood samples were collected from each mouse for blood cell counting.
2.19 Statistical analysis
Data are presented as means ± standard deviation (SD). Statistical analysis will be performed by Students t-test for the comparison of two groups, and one-way analysis of variance (ANOVA) for multiple groups. The significance level with P < 0.05 is considered statistically significant. Linear regression model was fitted by ordinary least square in the correlation studies. The values of IC50, which were calculated from the curves of cell viability, were fitted by a dose-response model of sigmoidal function with variable Hillslope. A one-compartment model was used to fit the pharmacokinetic data. The sample sizes of in vivo efficacy study were based on our previous studies.[43, 58] In the treatment group, we aimed an effect size of 2 came from tumor volume with estimated 30% reduction and tumor inhibition with anticipated SD of 15% at the end of the experiment. In tumor growth inhibition and survival analysis, an 80% power will be obtained from a sample size of 5 in each group to detect the aimed effect size of 2 with a significance level of 0.05. Kaplan Meier method was used to analyze animal survival data, and the differences of mean survival times between two groups were assessed by Student’s t-test.[59]
3. Results and discussion
3.1 Rf-TD synthesis and DOX loading
A less reactive glutaric anhydride was used to selectively react with the primary alcohol to introduce a carboxylic acid functional group in Rf. The 1H NMR spectrum of the Rf-COOH is shown in Fig. S1f. The carboxylic acid group is verified by a characteristic broad signal at δ= 11.99 ppm. As a comparison, the MS spectrum of Rf and Rf-COOH are shown in Fig. S1j and S1k, respectively. The peak at m/z value of 378 is corresponding to the protonated radical form of the reduced semiquinone species of Rf [M+2H]+·, which has been reported in the previous publication.[60] Accordingly, the carboxylic acid derivative of Rf were also detected as [M+2H]+· at 493.289 (calculated 493.173). As shown in the molecular docking study,[43] Rf-COOH derivative forms the π-π stacking with DOX by adapting a sandwich binding conformation (Fig. 1A). TD-1 and TD-2 were synthesized starting from the MeO-PEG5k-NH2 via peptide chemistry approach. Rf-COOH was conjugated on the peripheral of dendritic oligo-lysine in the TD to yield PEG5kRf8 (TD-1) (Fig. 1A) after three steps of lysine coupling. In parallel, a hybrid TD, PEG5kCA4Rf4 (TD-2), was synthesized as a comparison using the orthogonally protected lysine residues via Fmoc-Boc peptide chemistry as reported in our previous study (Fig. 1A).[58] The chemical structures of PEGylated intermediates of TDs were characterized by 1H NMR, and the mass increase on PEG chain after each coupling matched precisely with the designed structure as detected by MALDI-TOF MS (Fig S1i). Rf moieties in TD-1 and TD-2 were verified by the proton signals of two methyl groups and a signature imide proton at 2.35, 2.43 and 11.29 ppm, respectively (Fig. S1g & S1h). In addition, the CA moieties in TD-2 were confirmed by the integration the proton signals of three methyl groups at 0.54, 0.77 and 0.89 ppm in Fig. S1h. The chemical formulas of TDs were calculated based on these integrations relative to the distal methoxy group on PEG to be: PEG5kRf7.8 and PEG5kCA4Rf3.3, respectively. The molecular entanglement/aggregation of amphiphilic TD can occur even in DMSO, which may cause the underestimation of the polymer composition in NMR measurements. Therefore, the molecular weights of TD-1 (Calculated: 9689.959/ Found: 9614.559, Fig. S1l) and TD-2 (Calculated: 9323.418/ Found: 9262.742, Fig. S1m) were further confirmed by MALDI-TOF MS, which indicated the precise chemical synthesis of TD-1 and TD-2 as designed.
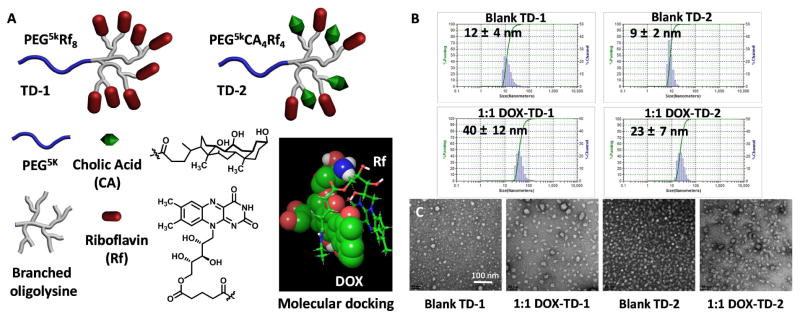
Fig. 1. Chemical structures, particle sizes and morphology of TDs.
(A) The architectures and subunits of PEG5kRf8 (TD-1) and PEG5kCA4Rf4 (TD-2), and the sandwich binding model of Rf-DOX in molecular docking studies. (B) Particle sizes of blank TD-1, blank TD-2, DOX-TD-1 (1/1 w/w), and DOX-TD-2 (1/1 w/w) NPs obtained by DLS. (C) TEM images of blank TD-1, DOX-TD-1, blank TD-2, and DOX-TD-2 NPs.
As expected, TD-1 and TD-2 form well-dispersed spherical micelles with particle sizes of 12 and 9 nm (Fig. 1B) with zeta potential of -5.46 ± 0.34 mV and -4.55 ± 0.10 mV, respectively. Remarkably, both DOX-TD-1 and DOX-TD-2 nanoformulations exhibited very high DOX loading capacities at a 1/1 DOX/TD mass ratio without any drug precipitation observed. The DOX-TD-1 and DOX-TD-2 are well-dispersed in PBS with particle sizes of 40 and 23 nm (Fig. 1B), respectively, detected by DLS. TEM studies revealed the spherical micelles with the mean diameters consistent with DLS results, i.e. 36 ± 5 and 20 ± 7 nm for DOX-TD-1 and DOX-TD-2, respectively (Fig. 1C & S2a). We further challenged the DOX loading capacity at a 2/1 mass feed ratio in TD-1 and TD-2. Complete drug loading was also obtained without any drug precipitation observed. However, after storage over a week at room temperature, part of DOX leaked out from nanocarriers and precipitated in PBS and the loading efficiency decreased to 89% and 93%, corresponding to 1.78/1 and 1.86/1 of DOX/TD loading content for TD-1 and TD-2 respectively (Fig. S2c). Eventually, we chose a stable DOX encapsulation at 1/1 feed mass ratio in TD-1 and TD-2 in the following studies.
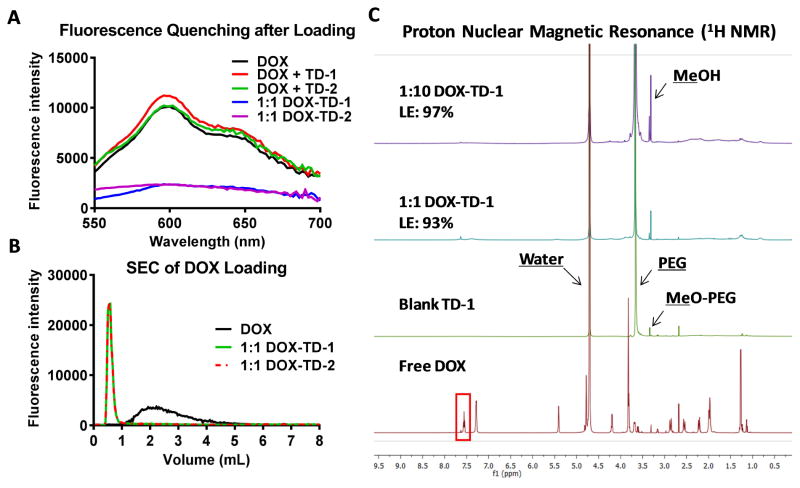
To confirm that such high DOX loading content in both TD-1 and TD-2, we applied several different methods to characterize and quantify DOX encapsulation efficiency, including fluorescence quenching, size exclusive chromatography (SEC), 1H NMR, and ultracentrifuge filtration. DOX has a strong intrinsic fluorescence signal when dissolved molecularly in aqueous solutions or organic solvents. The fluorescent signal of DOX is usually quenched when molecular aggregation occurs, especially for π-π stacking after being encapsulated into nanocarriers containing aromatic structures.[43, 61] As shown in Fig. 2A, the fluorescent signal of DOX was quenched significantly after being loaded in Rf-containing TD nanocarriers at a 1:1 mass ratio, in comparison with that of the free DOX. In contrast, fluorescence signal of the free DOX maintained after being mixed with these TD micelles in aqueous solution without loading process. Additionally, it was found that the fluorescent signal of Rf in TD nanocarrier was also significantly quenched by the encapsulation of DOX (Fig. S4a), which also indicates the π- π which also indicates the stacking between Rf and DOX in the nanocarrier. In the SEC studies, no free DOX was detected in the DOX nanoformulations after passing through the column, indicating almost 100% DOX loading efficiency (Fig. 2B). From the 1H NMR spectra, the proton signal of free DOX was clearly detected in D2O, while the signals of the encapsulated DOX in the nanocarriers were significantly decreased due to the reduced molecular motion by molecular stacking in nanocarriers. Based on the integration of proton signals on the aromatic rings of DOX, the loading efficiencies (LEs) of both Rf-containing nanoformulations were calculated to be over 93% (Fig. 2C & S3). The relatively low LE detected by NMR in comparison with SEC method is likely due to the detection of DOX loosely bounded in the shell of nanocarriers, which may not be the free DOX in solution. The LE% for DOX-TD-1 and DOX-TD-2 were detected to be 97% and 100%, respectively, by an ultracentrifuge filtration (molecular weight cutoff: 3.5 kDa) method to separate free DOX from NPs. These results indicate both Rf-containing TD NPs indeed have an almost complete loading even at an ultra-high DOX loading content of 1:1 DOX/TD in mass ratio.
Fig. 2. DOX loading efficiency.
(A) Fluorescence spectra of free DOX, mixtures of free DOX and TD solutions, and the drug loaded solutions DOX-TD-1, and DOX-TD-2 at a concentration of 1 mg DOX/mL in PBS. (B) The retention time of free DOX, DOX-TD-1, and DOX-TD-2 on SEC chromatograph. (C) 1H NMR spectra of free DOX, blank TD-1, and DOX-TD-1 (1/10 and 1/1, w/w) at a DOX concentration of 1 mg DOX/mL. The drug loading efficiency (LE) was calculated based on the DOX integrations at 7.75 ppm relative to the free DOX solution, calibrated by the water peak in D2O.
3.2 Stability of DOX-loaded Rf-containing NPs
DOX-loaded Rf-containing NPs were stable upon storage at room temperature. The particle sizes and fluorescence signals of these samples were monitored over a month. The particle sizes of DOX-TD-1 and DOX-TD-2 remain around 37 and 24 nm, respectively, without significant changes from the freshly loaded samples. Compared to free DOX, the nanoformulations of DOX exhibited significant fluorescence quenching, due to the molecular stacking between DOX and Rf (Fig. 2A). Interestingly, the fluorescent signal in 1:1 nanoformulations were further gradually quenched during the storage within the first 5 days in PBS solution (Fig. 3A), but not in pure water (Fig. S4b). In contrast, the fluorescence of DOX-TD nanoformulations with a lower drug loading ratio of 1/10 (w/w) were quenched to a greater extent due to the higher ratios of Rf/DOX in nanocarriers, and the signal remained unchanged during the storage in both PBS (Fig 3A) and pure water (Fig. S4b). It indicates that DOX-TDs at 1:1 mass ratio can still undergo a process of molecular rearrangement after the initial encapsulation. This curing process can be accelerated by the increased temperatures, e.g. 37 °C or 60 °C (Fig. S4c). As a control, fluorescence of free DOX was monitored and kept constant during the storage in water or PBS (Fig. S4b). All these phenomena indicated that some DOX molecules can be loosely encapsulated (higher fluorescence) in the core-shell interface of the nanocarriers at 1/1 DOX/TDs loading ratio, which are not free drug in solution as shown in NMR studies in Fig. 2C. Indicated by the reduced fluorescence, these loosely bounded DOX can be gradually annealed by forming molecular stacking driven by the reduced DOX solubility in the presence of both ionic strength and neutral pH in PBS (Fig. 3B). These phenomena indicate that Rf serves as an efficient anchor to seed DOX in nanocarriers. In comparison with the hydrophobic DBMs in TDs [43], the amphiphilic structure of Rf also contribute significantly to the stability and dispersion of DOX-loaded micelles. We further dialyzed the cured samples for 10 h to achieve nanoformulations with superior stability, which have 70% of DOX stably encapsulated with the fluorescence efficiently quenched.
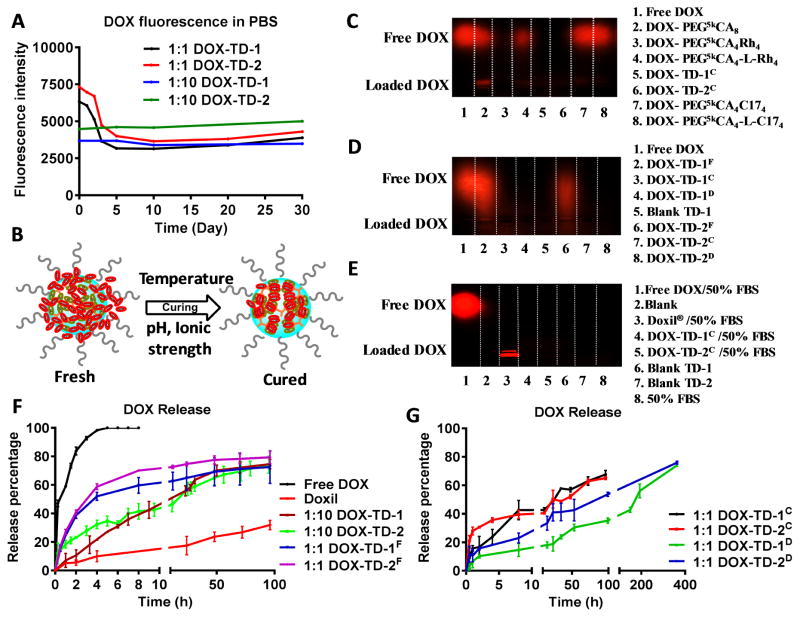
Fig. 3. DOX loading stability and in vitro DOX release profiles.
(A) Fluorescence quenching of DOX-TD-1 and DOX-TD-2 at a DOX concentration of 1 mg/mL in PBS monitored for 1-month. (B) Cartoon illustrates the dynamic molecular stacking during the curing processes by time or the increased temperature and ionic strength. (C–E) Agarose gel electrophoresis of (C) free DOX, DOX-TD-1, DOX-TD-2, and other previously reported TD-based DOX nanoformulations,[43, 58] (D) fresh, cured, and dialyzed DOX-TD-1 and DOX-TD-2, and (E) free DOX, Doxil®, cured DOX-TD-1 and DOX-TD-2 incubated with 50% FBS. (F) In vitro cumulative DOX release profiles of free DOX, Doxil®, fresh 1:1 and 1:10 DOX-TD-1 and DOX-TD-2 nanoformulations in PBS at 37 °C. ( G) In vitro cumulative DOX release profiles of cured and dialyzed DOX-TD-1 and DOX-TD-2 nanoformulations in PBS at 37 °C. The fluorescence of DOX remained in t he dialysis bag at different time points were measured. Data are represented as a mean ± SD of triplicate samples.
Electrophoresis is generally used to characterize the loading of gene or protein molecules in NPs.[62, 63] DOX has a positively charged amine group, which should be able to migrate under electric field. Being stably loaded in a nanocarrier, DOX should be trapped in the loading well due to the size effects. As expected, free DOX migrate efficiently to the cathode (Fig. 3C). The cured DOX-TD-1 and DOX-TD-2 nanoformulations at a 1:1 mass loading ratio (denoted as DOX-TD-1C & DOX-TD-2C) were characterized in comparison with our previously reported sub-optimal and optimized DOX-TD nanoformulations via structure-based design[43, 58] at a 1:10 (DOX:TD) mass loading ratio, including DOX-PEG5kCA8, DOX-PEG5kCA4Rh4, DOX-PEG5kCA4-L-Rh4, DOX-PEG5kCA4C174, and DOX-PEG5kCA4-L-C174 (Scheme S1). As shown in Fig. 3C, DOX-TD-1C, DOX-TD-2C and DOX-PEG5kCA4Rh4 were completely trapped in the loading well with the fluorescence efficiently quenched; while DOX leaked out from the nanocarriers formed by TDs with weaker DBMs, i.e. PEG5kCA8, PEG5kCA4C174, and PEG5kCA4-L-C174. As expected, TD-1, TD-2 and a previously demonstrated optimized hybrid PEG5kCA4Rh4 with a strong DBM of aromatic rhein (Rh)[43], can encapsulate DOX stably without free DOX detected in electrophoresis. Some DOX leaked out from a three-layered PEG5kCA4-L-Rh4, which may be because some DOX molecules are encapsulated loosely in the CA-containing intermediate layer and may leak out driven by electrical force as shown in DOX-PEG5kCA8. All these results indicated that our newly developed DOX-TD-1 and DOX-TD-2 nanoformulations have superior DOX loading capacity and stability. To the best of our knowledge, this is the first attempt to apply agarose gel electrophoresis to characterize drug loading efficiency and stability in nanocarriers.
We applied electrophoresis to further monitor the curing process of DOX nanoformulations. As shown in Fig. 3D, DOX leaked out partially from the freshly prepared DOX-TD-1 and DOX-TD-2 (denoted as DOX-TD-1F & DOX-TD-2F) nanoformulations and migrated as a smeared band, indicating the leaked DOX molecules are loosely bounded other than free DOX. While after curing, DOX-TD-1C & DOX-TD-2C stably retain DOX in the loading vials, which were also observed for the dialyzed nanoformulations DOX-TD-1D & DOX-TD-2D. These observations are consistent with the previous fluorescence quenching studies.
The stability of NPs in the blood circulation, e.g. interactions with serum proteins, is important for the in vivo fate and efficacy of nanoformulations. Therefore, we investigated the stability of DOX encapsulation in DOX-TD-1 and DOX-TD-2 NPs in the presence of fetal bovine serum (FBS) by both agarose gel electrophoresis and fluorescence measurements. Doxil® is known as a “stealth” nanoformulation and therefore was tested as a comparison. Once the FBS was added into DOX-TD-1C and DOX-TD-2C, the fluorescence of these solutions only slightly increased after 24 h incubation with 50% FBS, which was minimum relative to the fluorescence intensity of the free DOX mixed with FBS (Fig. S4d–S4f). It indicates that only trace amount of DOX may be extracted by FBS and became fluorescent. As shown in Fig. 3E, no significant DOX leakages were observed in electrophoresis for both Doxil and our formulations after FBS incubation.
3.3 In vitro drug release profiles
The in vitro drug release behaviors of the fresh, cured and dialyzed DOX-TD-1 and DOX-TD-2 were evaluated in PBS at 37 °C under a sink condition, i.e. frequently refreshed PBS. Free DOX diffused very fast through the dialysis membrane with a complete drug release in 8 h (Fig. 3F). Doxil® exhibited the slowest DOX release profile, which, however, may reduce the drug bioavailability and hinder cancer treatment.[25] The freshly loaded DOX-TD-1F and DOX-TD-2F with a drug loading content of 1:1 in mass ratio exhibited a two-phase drug-release profile with an initial burst release of 50% of drug within 4 h, followed by a slow linear release profile (Fig. 3F). At a relatively lower drug loading ratio, e.g. 1/10 (w/w), the drug release profiles were significantly slower, due to the efficient drug encapsulation. As expected, the cured nanoformulations significantly reduced the initial burst release to ~20% followed by the slow drug release profiles with 50% of drug released at about 24 h (Fig. 3G). Further, the dialyzed nanoformulations DOX-TD-1D & DOX-TD-2D exhibited even slower DOX release profiles in comparison with those cured nanoformulations (Fig. 3G). DOX-TD-1 exhibited a slightly slower release profile than DOX-TD-2, due to the higher density of Rf as DBMs.
3.4 In vitro cytotoxicity assay
DOX has been applied to treat various cancers in clinic.[1, 2] Therefore, we chose an array of cancer cell lines to evaluate the efficacies of our DOX nanoformulations. After a 72 h incubation with the DOX formulations, there was no significant difference in IC50 between free DOX and our DOX nanoformulations in both suspension cell lines, such as Raji, K562, H929 and Jurkat cells (Fig. S5) and adherent cancer cell lines, such as MDA-MB-231 and SKOV3 (Fig. 4A & 4B). However, Doxil exhibited 5–10 times higher IC50 than free DOX (Table S1), due to the inefficient drug release. The blank TD-1 and TD-2 have no significant cytotoxicity against all these cells tested up to 625 μg/mL (Fig. S5).
Fig. 4. Cytotoxicity, hemolytic toxicity evaluation and cellular uptake imaging of DOX formulations.
(A, B) Cell viability of MDA-MB-231 (A), SKOV3 (B) cells after 72 h incubation with free DOX, Doxil, DOX-TD-1 and DOX-TD-2 nanoformulations. (C) Cell viability of SKOV3 cells treated with free DOX, Doxil®, DOX-loaded Rf-containing nanoformulations for 2 h followed by an incubation for 72 h. (D) Hemolytic properties of free DOX, Doxil®, DOX-TD-1, and DOX-TD-2 nanoformulations after incubation with red blood cells at 37 °C for 24 h. ( E) Confocal fluorescence microscopy images of MDA-MB-231 cells incubated with free DOX (10 μM), Doxil®, DOX-loaded Rf-containing nanoformulations at a DOX concentration of 30 μM for 2 h. The scale bars of images in the lanes for DOX, Lysotracker, and Overlay are the same. Data are expressed as means ± SD, n=3 for all groups. ** P < 0.01, *** P < 0.001.
SKOV3 cells were exposed to DOX formulations for 2 h, and cell culture medium were refreshed for continuous culture for 72 h before MTS assays. As shown in Fig. 4C, cells treated with nanoformulations, e.g. Doxil®, DOX-TD-1, and DOX-TD-2, displayed significantly higher cell viabilities at both 5 and 20 μM, when compared to the cells treated with the free DOX, suggesting potentially reduced systemic toxicity of nanoformulations during blood circulation. The dialyzed DOX-TDD formulations showed significant lower cytotoxicity in line with Doxil, indicating the improved controlled drug release than the cured samples at both high and low DOX loading ratios. However, the maintained DOX potency in the dialyzed nanoformulations after 72 h incubation (Fig. 4A & 4B) indicates the potential in vivo efficacy and justifies for further in vivo evaluation.
The hemolytic toxicity of DOX formulations were evaluated via red blood cell (RBC) incubation for 0.5 h, 4 h and overnight. Free DOX showed strong hemolytic activity, while all the DOX nanoformulations exhibited ≤ 4% hemolysis rate at a concentration up to 1000 μg/mL (Fig. 4D & S6), suggesting safe systemic administration through intravenous (i.v.) injection.
3.5 Cellular uptake
The cellular uptake profiles of DOX formulations were studied in MDA-MB-231 cells using a confocal microscopy. As shown in Fig. 4E, free DOX rapidly translocated into the cell nucleus after 2 h incubation at 37 °C. In contrast, DOX nanoformulations mainly located in the cytosol and were co-localized with lysotracker. Among the nanoformulations, Doxil® exhibited the lowest fluorescent signal, indicating inefficient cellular uptake due to its “hyper”-stealth properties and slow drug release, which may reduce drug bioavailability in cancer treatment.[25] Dialyzed samples showed lower intracellular fluorescent intensity than the corresponding cured nanoformulations, which is correlated with the less burst release and the significant fluorescence quench. After 5 h, the encapsulated DOX released out from NPs and translocated in the cell nucleus for efficient cancer treatment (Fig. S7a). Considering the fluorescence quenching in the nanoformulations, we extracted DOX from cell lysis after drug incubation for 2 h at 37 °C and 4 °C. The fluorescent analysis showed the same trend as the cell imaging studies (Fig. S7b & S7c). It suggested that the dialyzed nanoformulations may further reduce the off-target toxicity caused by the premature drug release during the blood circulation.
3.6 Pharmacokinetic (PK) studies
The PK profiles of DOX-loaded Rf-containing nanoformulations were investigated in BALB/c mice after intravenous administration in comparison with free DOX and Doxil®. As shown in Fig. 5A, free DOX administration has a rapid clearance from blood circulation with a half-life (t1/2) of 0.004 h analyzed by fluorescent measurements of plasma at the different time points. Doxil® has a very long blood circulation time (t1/2=20 h), which contributes to a 32-fold increase in area under the curve (AUC) relative to free DOX. Our DOX nanoformulations present the significantly improved PK profiles with a nearly 500 times prolonged t1/2 than the free DOX. The dialyzed nanoformulations exhibited a longer circulation time than that of the cured nanoformulations, and the DOX-TD-1 nanoformulations had a longer t1/2 than that of DOX-TD-2 due to the stronger drug binding and minimized initial burst drug release as demonstrated in Fig. 3F & 3G. A more than 12-fold increase in plasma AUC for the dialyzed DOX-TD-1D nanoformulation was observed in comparison with free DOX. Such PK profiles via the optimized drug encapsulation have been revealed to be efficient for tumor-targeted drug delivery by EPR effects.[43]
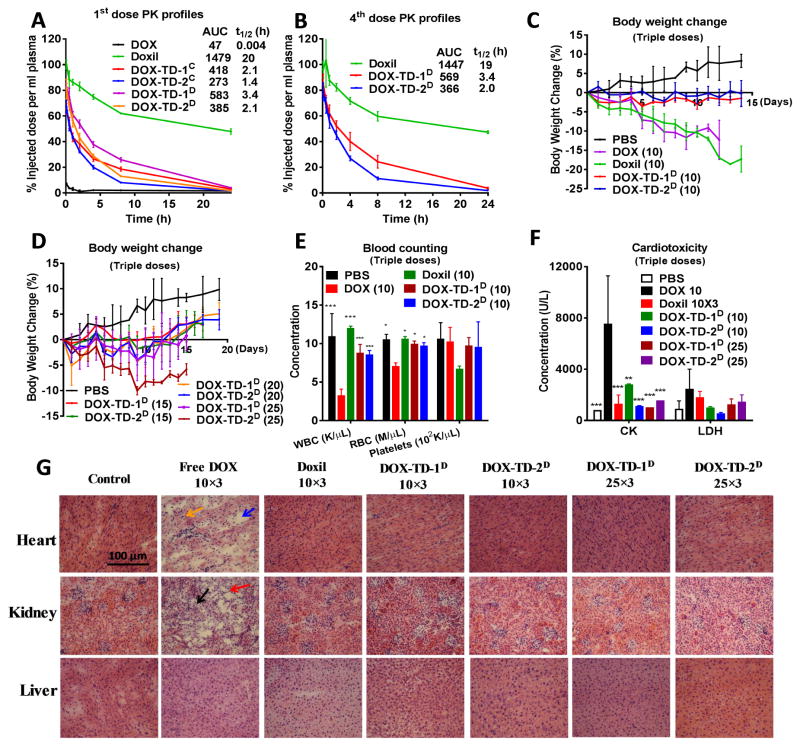
Fig. 5. Pharmacokinetics, MTD and toxicology profiles of DOX formulations in BALB/c mice.
(A) Pharmacokinetics of free DOX, Doxil®, and DOX-loaded nanoformulations at a dose of 10 mg/kg by single dose intravenous injection; (B) The 4th dose pharmacokinetics of DOX formulations in the BALB/c mice treated with four consecutive doses of DOX nanoformulations on days 0, 4, 8 and 12. (C, D) Body weight changes of healthy BALB/c mice administrated intravenously with q4d × 3 10 mg/kg of free DOX, Doxil®, and dialyzed DOX-TD-1D, DOX-TD-2D (C), and with the escalated q4d × 3 doses ranging from 15 mg/kg to 25 mg/kg of DOX-TD-1D and DOX-TD-2D (D). (E) Blood cell counts on day 7 after the last dose in MTD studies. (F) CK, LDH levels on day 7 after the last dose. (G) Histological examinations of heart, kidney and liver tissues by H&E staining from animals treated with free DOX, Doxil (10 mg/kg × 3), DOX-TD-1, and DOX-TD-2 (10 mg/kg × 3, 25 mg/kg × 3) or PBS. Tissues were obtained on day 7 after the last dose of q4d × 3 regimen. Yellow arrow indicates the myofibrillar atrophy and loss; blue arrow indicates the extracellular edema; black arrow indicates the tubular necrosis; and red arrow indicates the inflammatory cells infiltration. For A–F, data are expressed as means ± SD, n=3 for all groups. For E and F, * P < 0.05, ** P < 0.01, *** P < 0.001 for each group versus the free DOX.
Some studies reported that anti-PEG IgM might be induced after repeated administration of PEGylated NPs, resulting in the fast in vivo clearance of nanoformulations in the repeated administrations.[64, 65] To address this concern, we continuously treated BALB/c mice with DOX nanoformulations every four days for four times at a dose level of 10 mg/kg. Right after the fourth treatment, blood samples were collected for PK analysis. As shown in Fig. 5B, DOX-TD-1D, DOX-TD-2D and Doxil® exhibited similar PK profiles to the first injection (Fig. 5A). No accelerated clearance of nanoformulations was observed after repeated administration, indicating the low immunogenicity of our micelle formulations. In addition, it has been reported that suppression of the contacted immune cells by the payload DOX within nanocarriers prevents anti-PEG IgM production.[66, 67]
3.7 Maximum tolerance dose (MTD) and toxicology studies
We estimated MTD by dose escalation in BALB/c mice using a single dose injection or a schedule of every fourth day for a total three injections. In the triple-dose treatment studies (Fig. 5C & 5D), mice lost nearly over 15% body weight in the groups treated with 10 mg/kg of free DOX and Doxil®. All the mice in free DOX treatment group were found to have abdominal dropsy on day 4 after the last injections. Dry eye syndrome was found in the mice treated with Doxil®. The mice treated with the dialyzed DOX-TD-1 and DOX-TD-2 barely showed any weight loss or noticeable changes in behavior up to 25 mg/kg dose. In clinic, myelosuppression is a common side effect of chemotherapy.[68] Therefore, the blood cells were collected and counted on day 7 after the last dose (Fig. 5E & S8c). Consistently, the WBC and RBC in mice treated with free DOX were significantly decreased, while the dialyzed nanoformulations and Doxil® exhibited normal blood counts similar to PBS group. The accumulative cardiomyopathy is a dose-limiting side effect for DOX in the clinic.[10] Therefore, blood enzyme levels of mice in the triple-dose treatments were analyzed to uncover any possible organ dysfunctions. As shown in Fig. 5F, creatine kinase (CK) was found nearly 6-fold higher in mice treated with free DOX than those in PBS and all other treatment groups, indicating severe cardiotoxicity caused by free DOX. No alteration in BUN, ALT and AST were observed in mice treated with DOX formulations at 10 mg/kg, indicating normal function of kidney and liver. A noticed increase in ALT, but not AST was observed in mice treated with triple-dose of DOX-TD-1 and DOX-TD-2 at 25 mg/kg (Fig. S8d), which may indicate possible liver toxicity and calls for further pathological examination. In addition, single dose i.v. injections at 500 mg/kg of blank TD-1 and TD-2 micelles were well tolerated without noticeable changes in general activity and body weight (Fig. S8a). Only moderate platelet decrease, although still within the normal range, was observed in the mice treated with blank TD-2 at 500 mg/kg when compared to the PBS control group (Fig. S8b). This dose is nearly 6-fold higher than that needed in the treatment studies.
To further characterize the potential organ damage in animals after MTD studies, mice were sacrificed, and heart, liver and kidney were harvested for histology analysis on day 7 after the last dose. We carefully examined the liver structures in the mice treated with our nanoformulations. It didn’t reveal any significant pathologic changes for all treatment groups, including high dose treatments at 25 mg/kg triple-dose, when compared to the PBS control group. The increased ALT levels might only indicate the transient alteration in liver function, since the magnitude of change in ALT has no relationship to the prognosis or severity of liver damage.[69] As shown in Fig. 5G, myocardium in the mice treated with all the nanoformulations showed no significant pathologic changes in comparison with those in PBS control group. In contrast, myofibrillar atrophy and loss, and extracellular edema were observed in the mice treated with free DOX, which was consistent with DOX cardiomyopathy and correlated with the increased CK level. In addition, the kidney tissue from the mice treated with free DOX showed markedly degenerative changes, including glomerular loss, tubular necrosis, inflammatory cells infiltration, which was evidenced by the white-off kidney at harvest. Such kidney dysfunction may explain the abdomen fluid accumulation observed in these mice as well.
Given the alteration of ALT levels in mice treated with 25 mg/kg, we determined the MTD for DOX-TD-1D and DOX-TD-2D nanoformulations to be 20 mg/kg as a q4d×3 regimen. Both groups showed the minimum body weight changes, which will be applied in the following treatment studies. Free DOX at 10 mg/kg has shown significant body weight loss and organ toxicity in mice, therefore, we chose 8 mg/kg as MTD dose for free DOX according to the literature.[70] The mice treated with Doxil (10 mg/kg triple-dose) experienced body weight loss close to 15–20% in BALB/c mice. However, all the blood chemistry and histopathologic studies still revealed the normal health of mice. Therefore, MTD level of Doxil® was determined to be 10 mg/kg, similar to our previous observations.[43, 58] As a result, our nanoformulation doubles the MTD level of Doxil® and even reaches to a 2.5-fold higher than the MTD of free DOX.
3.8 In vivo and ex vivo tumor targeting
DOX nanoformulations with small particle sizes of 23–40 nm are expected to diffuse through the leakier tumor blood vessels and penetrate tumor tissues efficiently. Nude mice bearing tumor xenografts, e.g. Raji lymphoma, SKOV-3 ovarian cancer, and MDA-MB-231 breast cancer, were treated with the DOX nanoformulations co-loaded with a near-infrared (NIR) dye DiD and monitored with a small animal fluorescence imager. After co-loading DOX with trace amount of DiD (2% by weight) in TD nanocarriers, the particle sizes remained 41 and 28 nm for DiD-DOX-TD-1 and DiD-DOX-TD-2, respectively (Fig. S9a & S9b). The release profile of DiD from the co-loading NPs synchronized with the DOX release profile, which validates DiD as a surrogate to probe DOX biodistribution (Fig. S9c). Interestingly, the fluorescence signal of DiD was significantly quenched similar to DOX after loaded in TD micelles (Fig. S9d). Therefore, the in vivo NIR fluorescent imaging can only detect the released DiD and reflect the biodistribution of the bioavailable and bioactive form of the released DOX, which may serve as theranostic imaging modality and predict the efficacy of cancer treatments.
The in vivo tumor-targeting of DiD-DOX nanoformulations in the nude mice bearing different tumor xenografts, e.g. SKOV-3 ovarian cancer (Fig. 6A), Raji lymphoma (Fig. S10a) and MDA-MB-231 (Fig. S11a), respectively, were monitored at different time points after tail vein injections. In SKOV-3 ovarian cancer xenografted mouse models, tumor uptake can barely be seen in vivo in mice treated with free DiD. Instead, the accumulation of the free DiD in spleen was observed in vivo as early as 1 h post-injection due to macrophage clearance of the hydrophobic DiD aggregates, which was still seen in the ex vivo imaging 70 h post-injection (Fig. 6B). Only very weak fluorescence in tumor was visualized for the free DiD in the ex vivo imaging (Fig. 6B). In contrast, TD nanoformulations were gradually detected in mice and accumulated preferably at tumor sites over 24 to 48 hours (Fig. 6A). Interestingly, TD-1 lights up slower and slightly weaker than TD-2 in the in vivo imaging (Fig. 6A & 6C), which may be partially due to the relatively slower drug release and stronger fluorescent quenching by higher density of Rf in TD-1. In the semi-quantitative analysis of the in vivo tumor accumulation, both nanoformulations significant increase tumor uptakes with around 6~7-fold higher in AUC than free DiD (Fig. 6C). The ex vivo images revealed the tumor specific accumulations for both nanoformulations in comparison with the normal organs, which is in a sharp contrast to the preferred accumulation of the free DiD in spleen and lung rather than tumor. DiD-DOX-TD-1 exhibited ~3-fold higher ex vivo tumor uptake than the free DiD (Fig. 6D). Surprisingly, TD-2 nanoformulation was observed to have another ~3-fold higher tumor uptake than TD-1 in SKOV-3 ovarian cancer xenograft models (Fig. 6D), as well as in MDA-MB-231 breast cancer xenograft mouse models (Fig. S11D), which were confirmed by the fluorescent measurements of the tumor lysis (Data not shown). Then, the question comes why TD-1 nanoformulation showed similar strong tumor uptake in the in vivo imaging in Fig. 6A? It may be due to the specific top-view tumor position, which receives vertical and maximized excitation than the side-view tumors. It is worthwhile to notice that TD-1 and TD-2 showed the similar tumor uptake in lymphoma xenograft mouse models, indicating the heterogeneity of tumor blood vessel and microenvironments in different tumor types (Fig. S10D).
Fig. 6. Tumor targeting and tissue biodistribution of DiD-DOX-co-loading nanoformulations in SKOV3 ovarian xenograft tumor bearing nude mice.
(A) In vivo real-time imaging of SKOV3 xenograft tumor bearing nude mice treated by i.v. injections of free DiD and DiD-DOX-co-loaded Rf-containing nanoformulations. (B) Representative ex vivo optical images of tumors and major organs taken out at 70 h after i.v. injection of free DiD and DiD-DOX-co-loaded Rf-containing nanoformulations. For clear display, the rainbow spectrum for each image represents different scale range of fluorescent intensity. (C) Semi-quantitative fluorescent intensity of SKOV3 xenograft tumors overtime during in vivo imaging in the duplicated experiments. (D) Ex vivo semi-quantitative fluorescent intensity of the SKOV3 tumors and major organs at 70 h post-injection in the duplicated experiments. (E) Fluorescence microscopy images of SKOV3 tumors obtained from mice treated with free DiD, DiD-DOX-TD-1, and DiD-DOX-TD-2.
Further, Tumor tissue was cryo-sectioned for fluorescence microscopic imaging studies. Compared to the free DiD treatment, strong and homogenous fluorescence signals were observed in the tumor tissues treated by both DOX-DiD-TD-1D and DOX-DiD-TD-2D (Fig. 6E). Accordingly, TD-2 exhibited higher fluorescent signal in the tumor tissues than TD-1 in both SKOV-3 ovarian cancer tumors (Fig. 6E) and MDA-MB-231 breast cancer tumors as shown in Fig. S11e.The difference of the efficiency in tumor uptake between TD-1 and TD-2 may be due to the relative larger particle sizes of TD-1 especially in the solid tumors.[42, 71]
3.9 Antitumor efficacy
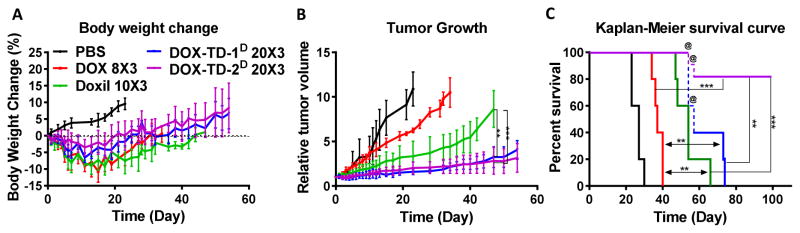
In our previous studies, we have demonstrated that our optimized TD nanoformulations for DOX delivery significantly improved anticancer effects in lymphoma xenograft mouse models.[43] Solid tumor treatment, e.g. ovarian cancer, is more challenging, where Doxil only exhibits marginally improved efficacy in the clinic.[16–18] SKOV-3 ovarian xenograft mouse models were selected to challenge and compare our Rf-containing DOX-TD nanoformulations with Doxil side-by-side in cancer treatment. Animals were treated on days 0, 4 and 8 for three consecutive doses with various DOX formulations, i.e. DOX-TD-1D (20 mg/kg×3), DOX-TD-2D (20 mg/kg×3), DOX (8 mg/kg×3), Doxil® (10 mg/kg×3), in comparison with PBS control. The treatment started at the tumor sizes of 150–200 mm3. No bodyweight loss over 15% was observed in all groups (Fig. 7A). The tumor growth was efficiently inhibited by DOX-TD-1 and DOX-TD-2 nanoformulations during the period of drug administration, while tumors were continuously progressing in DOX and Doxil® treatment groups (Fig. 7B & S12). Compared to PBS control group, free DOX at 8 mg/kg delayed tumor growth. With the increased dose and tumor targeted drug delivery, Doxil® further improved tumor inhibition in the first two weeks, which were followed by the rapid tumor progression lately. In contrast, TD nanoformulations still inhibited tumor progression efficiently when the endpoints of tumor control met in both DOX and Doxil groups on day 47 (Fig. 7B & S12). Unexpectedly, 3 of 5 mice in DOX-TD-1D, and 2 of 5 mice in DOX-TD-2D groups experienced tumor necrosis after day 54 even though the tumor sizes were still relatively small tumor, which may be due to the efficient anticancer effects of nanoformulations. These mice were euthanized for humane reason after day 54 and the tumor growth curves were stopped as shown in Fig. 7B. All the groups treated by DOX formulations showed significant therapeutic effects in comparison with PBS control with the prolong median survival time: i.e. PBS (27 days), DOX (37 days), Doxil (54 days), TD-1 (apparently 57 days) and TD-2 (undefined). No mice in the free DOX and Doxil group survived longer than 40 and 66 days, respectively (Fig. 7C). The last two of the mice (40%) treated by DOX-TD-1D survived longer than 73 days and three mice (60%) in DOX-TD-2D group survived without tumor progression even over 100 days (Fig. S12).
Fig. 7. Antitumor efficacy of DOX formulations in SKOV3 xenograft bearing nude mice.
(A) Body weight changes, (B) relative tumor volume and (C) cumulative mice survival of mice was monitored. @ mice were euthanatized due to tumor necrosis before tumor size grew beyond size limitation (Fig. S12). Three doses were given at days 0, 4, 8. Data are expressed as means ± SD, n=5 for all groups. ** P < 0.01; *** P < 0.001.
4. Conclusion
In this study, biocompatible well-defined Rf-containing telodendrimer nanocarriers have been rationally designed and synthesized efficiently for DOX delivery. The DOX-TD nanoformulations have stable and small particle sizes (23~40 nm) with superior DOX loading capacity (1/1 w/w) and efficiency (~100%), which are desired for tumor-targeted drug delivery. The initial burst release was significantly minimized by a simple cure process in PBS, which can also be efficiently eliminated via a pre-dialysis treatment with over 70% DOX stably encapsulated. Sustained drug release profiles were observed, which is promising to reduce the systemic toxic side effects of DOX. In addition, the efficient interstitial diffusion of small-sized NPs and readily local drug release are promising to further improve anticancer effects over Doxil®. As a result, the drug-loaded TD micelles exhibited prolonged blood circulation time, enhanced tumor accumulation, reduced toxic side effects, and significantly improved antitumor efficacy than free DOX and Doxil in SKOV3 ovarian cancer xenograft mouse models. We believe that the stable and efficient drug encapsulation in the small-sized NPs with the optimized release profile is crucial for the tumor-targeted drug delivery, the intratumoral penetration and the in vivo efficacy. Both DOX-TD-1 and DOX-TD-2 significantly enhanced cancer treatments the clinical formulations, which hold a great potential for clinical translation.
Supplementary Material
Acknowledgments
We thank Professor Golam Mohi and Professor Jennifer F. Moffat for kindly sharing instruments for this study in blood sample analysis and small animal imaging, respectively. We acknowledge the financial supports from NIH/NCI R01CA140449 (Luo), NIH/NIBIB 1R21EB019607 (Luo), Napi Family Research Awards (Luo), New York State Department of Health/PETER T. ROWLEY breast cancer research award (Luo).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wooster R, Weber BL. Breast and ovarian cancer. N Engl J Med. 2003;348(23):2339–2347. doi: 10.1056/NEJMra012284. [DOI] [PubMed] [Google Scholar]
- 2.Bramwell V, Anderson D, Charette M. The Cochrane Library. 2003. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deconinck E, Foussard C, Milpied N, Bertrand P, Michenet P, Cornillet-LeFebvre P, Escoffre-Barbe M, Maisonneuve H, Delwail V, Gressin R. High-dose therapy followed by autologous purged stem-cell transplantation and doxorubicin-based chemotherapy in patients with advanced follicular lymphoma: a randomized multicenter study by GOELAMS. Blood. 2005;105(10):3817–3823. doi: 10.1182/blood-2004-10-3920. [DOI] [PubMed] [Google Scholar]
- 4.Dana BW, Dahlberg S, Nathwani BN, Chase E, Coltman C, Miller TP, Fisher RI. Long-term follow-up of patients with low-grade malignant lymphomas treated with doxorubicin-based chemotherapy or chemoimmunotherapy. J Clin Oncol. 1993;11(4):644–651. doi: 10.1200/JCO.1993.11.4.644. [DOI] [PubMed] [Google Scholar]
- 5.Thigpen T, Vance R, Puneky L, Khansur T. Chemotherapy in advanced ovarian carcinoma: current standards of care based on randomized trials. Gynecol Oncol. 1994;55(3):S97–S107. doi: 10.1006/gyno.1994.1347. [DOI] [PubMed] [Google Scholar]
- 6.Thomas ES, Gomez HL, Li RK, Chung HC, Fein LE, Chan VF, Jassem J, Pivot XB, Klimovsky JV, De Mendoza FH. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol. 2007;25(33):5210–5217. doi: 10.1200/JCO.2007.12.6557. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3(7):502–516. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 8.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP–dependent transporters. Nat Rev Cancer. 2002;2(1):48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 9.Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv. 2003;55(1):3–29. doi: 10.1016/s0169-409x(02)00169-2. [DOI] [PubMed] [Google Scholar]
- 10.Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments: what the cardiologist needs to know. Nat Rev Cardiol. 2010;7(10) doi: 10.1038/nrcardio.2010.121. [DOI] [PubMed] [Google Scholar]
- 11.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339(13):900–905. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 13.Barenholz YC. DoxilR—the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160(2):117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal doxorubicin. Clin Pharmacokinet. 2003;42(5):419–436. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 15.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 16.Batist G, Ramakrishnan G, Rao CS, Chandrasekharan A, Gutheil J, Guthrie T, Shah P, Khojasteh A, Nair MK, Hoelzer K, Tkaczuk K, Park YC, Lee LW. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J Clin Oncol. 2001;19(5):1444–54. doi: 10.1200/JCO.2001.19.5.1444. [DOI] [PubMed] [Google Scholar]
- 17.O'Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, Catane R, Kieback DG, Tomczak P, Ackland SP, Orlandi F, Mellars L, Alland L, Tendler C, Group CBCS. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15(3):440–9. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 18.Chang HI, Yeh MK. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. Int J Nanomedicin. 2012;7:49. doi: 10.2147/IJN.S26766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang S, Martin F, Jay G, Vogel J, Papahadjopoulos D, Friend D. Extravasation and transcytosis of liposomes in Kaposi's sarcoma-like dermal lesions of transgenic mice bearing the HIV tat gene. Am J Pathol. 1993;143(1):10. [PMC free article] [PubMed] [Google Scholar]
- 20.Huang SK, Lee KD, Hong K, Friend DS, Papahadjopoulos D. Microscopic localization of sterically stabilized liposomes in colon carcinoma-bearing mice. Cancer Res. 1992;52(19):5135–43. [PubMed] [Google Scholar]
- 21.Yuan F, Leunig M, Huang SK, Berk DA, Papahadjopoulos D, Jain RK. Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res. 1994;54(13):3352–6. [PubMed] [Google Scholar]
- 22.Manzoor AA, Lindner LH, Landon CD, Park JY, Simnick AJ, Dreher MR, Das S, Hanna G, Park W, Chilkoti A. Overcoming limitations in nanoparticle drug delivery: triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 2012;72(21):5566–5575. doi: 10.1158/0008-5472.CAN-12-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tailor TD, Hanna G, Yarmolenko PS, Dreher MR, Betof AS, Nixon AB, Spasojevic I, Dewhirst MW. Effect of pazopanib on tumor microenvironment and liposome delivery. Mol Cancer Ther. 2010;9(6):1798–808. doi: 10.1158/1535-7163.MCT-09-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci U S A. 2011;108(7):2909–14. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y, Alakhova DY, Kim JO, Bronich TK, Kabanov AV. A simple way to enhance DoxilR therapy: drug release from liposomes at the tumor site by amphiphilic block copolymer. J Control Release. 2013;168(1):61–69. doi: 10.1016/j.jconrel.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martschick A, Sehouli J, Patzelt A, Richter H, Jacobi U, Oskay-Ozcelik G, Sterry W, Lademann J. The pathogenetic mechanism of anthracycline-induced palmar-plantar erythrodysesthesia. Anticancer Res. 2009;29(6):2307–2313. [PubMed] [Google Scholar]
- 27.Lorusso D, Di Stefano A, Carone V, Fagotti A, Pisconti S, Scambia G. Pegylated liposomal doxorubicin-related palmar-plantar erythrodysesthesia (‘hand-foot’syndrome) Ann Oncol. 2007;18(7):1159–1164. doi: 10.1093/annonc/mdl477. [DOI] [PubMed] [Google Scholar]
- 28.Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC, Barry ST, Gabizon A, Grodzinski P, Blakey DC. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73(8):2412–7. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shuai X, Ai H, Nasongkla N, Kim S, Gao J. Micellar carriers based on block copolymers of poly (ε-caprolactone) and poly (ethylene glycol) for doxorubicin delivery. J Control Release. 2004;98(3):415–426. doi: 10.1016/j.jconrel.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Nakanishi T, Fukushima S, Okamoto K, Suzuki M, Matsumura Y, Yokoyama M, Okano T, Sakurai Y, Kataoka K. Development of the polymer micelle carrier system for doxorubicin. J Control Release. 2001;74(1):295–302. doi: 10.1016/s0168-3659(01)00341-8. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y, Zou W, Bian S, Huang Y, Tan Y, Liang J, Fan Y, Zhang X. Bioreducible PAA-g-PEG graft micelles with high doxorubicin loading for targeted antitumor effect against mouse breast carcinoma. Biomaterials. 2013;34(28):6818–6828. doi: 10.1016/j.biomaterials.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 32.Banerjee R, Tyagi P, Li S, Huang L. Anisamide-targeted stealth liposomes: A potent carrier for targeting doxorubicin to human prostate cancer cells. Int J Cancer. 2004;112(4):693–700. doi: 10.1002/ijc.20452. [DOI] [PubMed] [Google Scholar]
- 33.You J, Zhang G, Li C. Exceptionally high payload of doxorubicin in hollow gold nanospheres for near-infrared light-triggered drug release. ACS nano. 2010;4(2):1033–1041. doi: 10.1021/nn901181c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang X, Zhang X, Liu Z, Ma Y, Huang Y, Chen Y. High-efficiency loading and controlled release of doxorubicin hydrochloride on graphene oxide. J Phys Chem C. 2008;112(45):17554–17558. [Google Scholar]
- 35.Wang Y, Wei X, Zhang C, Zhang F, Liang W. Nanoparticle delivery strategies to target doxorubicin to tumor cells and reduce side effects. Ther Deliv. 2010;1(2):273–87. doi: 10.4155/tde.10.24. [DOI] [PubMed] [Google Scholar]
- 36.Cagel M, Grotz E, Bernabeu E, Moretton MA, Chiappetta DA. Doxorubicin: nanotechnological overviews from bench to bedside. Drug Discov Today. 2017;22(2):270–281. doi: 10.1016/j.drudis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Harris L, Batist G, Belt R, Rovira D, Navari R, Azarnia N, Welles L, Winer E, Group TDS. Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer. 2002;94(1):25–36. doi: 10.1002/cncr.10201. [DOI] [PubMed] [Google Scholar]
- 38.Waterhouse DN, Tardi PG, Mayer LD, Bally MB. A comparison of liposomal formulations of doxorubicin with drug administered in free form: changing toxicity profiles. Drug Saf. 2001;24(12):903–20. doi: 10.2165/00002018-200124120-00004. [DOI] [PubMed] [Google Scholar]
- 39.Hsiao SM, Chen CA, Lin HH, Hsieh CY, Wei LH. Phase II trial of carboplatin and distearoylphosphatidylcholine pegylated liposomal doxorubicin (Lipo-Dox) in recurrent platinum-sensitive ovarian cancer following front-line therapy with paclitaxel and platinum. Gynecol Oncol. 2009;112(1):35–9. doi: 10.1016/j.ygyno.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 40.Rafiyath SM, Rasul M, Lee B, Wei G, Lamba G, Liu D. Comparison of safety and toxicity of liposomal doxorubicin vs. conventional anthracyclines: a meta-analysis. Exp Hematol Oncol. 2012;1(1):10. doi: 10.1186/2162-3619-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marty M. Liposomal doxorubicin (Myocet™) and conventional anthracyclines: a comparison. The Breast. 2001;10:28–33. [Google Scholar]
- 42.Luo J, Xiao K, Li Y, Lee JS, Shi L, Tan YH, Xing L, Holland Cheng R, Liu GY, Lam KS. Well-defined, size-tunable, multifunctional micelles for efficient paclitaxel delivery for cancer treatment. Bioconjug Chem. 2010;21(7):1216–24. doi: 10.1021/bc1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi C, Guo D, Xiao K, Wang X, Wang L, Luo J. A drug-specific nanocarrier design for efficient anticancer therapy. Nat Commun. 2015;6 doi: 10.1038/ncomms8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldsmith GA. Riboflavin deficiency, Riboflavin. Springer; 1975. pp. 221–244. [Google Scholar]
- 45.Kharasch ED, Novak RF. The molecular basis for complexation of adriamycin with flavin mononucleotide and flavin adenine dinucleotide. Arch Biochem Biophs. 1981;212(1):20–36. doi: 10.1016/0003-9861(81)90339-8. [DOI] [PubMed] [Google Scholar]
- 46.Evstigneev MP. Physicochemical mechanisms of synergistic biological action of combinations of aromatic heterocyclic compounds. Org Chem Int. 2013;2013 [Google Scholar]
- 47.Evstigneev M, Mykhina YV, Davies D. Complexation of daunomycin with a DNA oligomer in the presence of an aromatic vitamin (B 2) determined by NMR spectroscopy. Biophys Chem. 2005;118(2):118–127. doi: 10.1016/j.bpc.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Pinto J, Raiczyk GB, Huang YP, Rivlin RS. New approaches to the possible prevention of side effects of chemotherapy by nutrition. Cancer. 1986;58(8 Suppl):1911–1914. doi: 10.1002/1097-0142(19861015)58:8+<1911::aid-cncr2820581420>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 49.Kim JY, Kim S, Papp M, Park K, Pinal R. Hydrotropic solubilization of poorly water-soluble drugs. J Pharm Sci. 2010;99(9):3953–3965. doi: 10.1002/jps.22241. [DOI] [PubMed] [Google Scholar]
- 50.Huh KM, Min HS, Lee SC, Lee HJ, Kim S, Park K. A new hydrotropic block copolymer micelle system for aqueous solubilization of paclitaxel. J Control Release. 2008;126(2):122–129. doi: 10.1016/j.jconrel.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han Y, He Z, Schulz A, Bronich TK, Jordan R, Luxenhofer R, Kabanov AV. Synergistic combinations of multiple chemotherapeutic agents in high capacity poly (2-oxazoline) micelles. Mol Pharm. 2012;9(8):2302. doi: 10.1021/mp300159u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luxenhofer R, Schulz A, Roques C, Li S, Bronich TK, Batrakova EV, Jordan R, Kabanov AV. Doubly amphiphilic poly (2-oxazoline) s as high-capacity delivery systems for hydrophobic drugs. Biomaterials. 2010;31(18):4972–4979. doi: 10.1016/j.biomaterials.2010.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He Z, Wan X, Schulz A, Bludau H, Dobrovolskaia MA, Stern ST, Montgomery SA, Yuan H, Li Z, Alakhova D. A High Capacity Polymeric Micelle of Paclitaxel: Implication of High Dose Drug Therapy to Safety and In Vivo Anti-Cancer Activity. Biomaterials. 2016 doi: 10.1016/j.biomaterials.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang W, Wang X, Shi C, Guo D, Xu G, Wang L, Bodman A, Luo J. Fine-tuning vitamin E-containing telodendrimers for efficient delivery of gambogic acid in colon cancer treatment. Mol Pharm. 2015;12(4):1216–29. doi: 10.1021/acs.molpharmaceut.5b00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang W, Luo J, Nangia S. Multiscale approach to investigate self-assembly of telodendrimer based nanocarriers for anticancer drug delivery. Langmuir. 2015;31(14):4270–80. doi: 10.1021/la503949b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu G, Shi C, Guo D, Wang L, Ling Y, Han X, Luo J. Functional-segregated coumarin-containing telodendrimer nanocarriers for efficient delivery of SN-38 for colon cancer treatment. Acta Biomater. 2015;21:85–98. doi: 10.1016/j.actbio.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pisano C, Cecere SC, Di Napoli M, Cavaliere C, Tambaro R, Facchini G, Scaffa C, Losito S, Pizzolorusso A, Pignata S. Clinical trials with pegylated liposomal Doxorubicin in the treatment of ovarian cancer. J Drug Deliv. 2013;2013:898146. doi: 10.1155/2013/898146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Y, Xiao K, Luo J, Xiao W, Lee JS, Gonik AM, Kato J, Dong TA, Lam KS. Well-defined, reversible disulfide cross-linked micelles for on-demand paclitaxel delivery. Biomaterials. 2011;32(27):6633–45. doi: 10.1016/j.biomaterials.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pepe MS, Fleming TR. Weighted Kaplan-Meier statistics: a class of distance tests for censored survival data. Biometrics. 1989;45(2):497–507. [PubMed] [Google Scholar]
- 60.Duurkens RH, Tol MB, Geertsma ER, Permentier HP, Slotboom DJ. Flavin binding to the high affinity riboflavin transporter RibU. J Biol Chem. 2007;282(14):10380–10386. doi: 10.1074/jbc.M608583200. [DOI] [PubMed] [Google Scholar]
- 61.Mohan P, Rapoport N. Doxorubicin as a molecular nanotheranostic agent: effect of doxorubicin encapsulation in micelles or nanoemulsions on the ultrasound-mediated intracellular delivery and nuclear trafficking. Mol Pharm. 2010;7(6):1959–1973. doi: 10.1021/mp100269f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X, Shi C, Zhang L, Bodman A, Guo D, Wang L, Hall WA, Wilkens S, Luo J. Affinity-Controlled Protein Encapsulation into Sub-30 nm Telodendrimer Nanocarriers by Multivalent and Synergistic Interactions. Biomaterials. 2016 doi: 10.1016/j.biomaterials.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X, Bodman A, Shi C, Guo D, Wang L, Luo J, Hall WA. Tunable Lipidoid-Telodendrimer Hybrid Nanoparticles for Intracellular Protein Delivery in Brain Tumor Treatment. Small. 2016;12(31):4185–4192. doi: 10.1002/smll.201601234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang X, Ishida T, Kiwada H. Anti-PEG IgM elicited by injection of liposomes is involved in the enhanced blood clearance of a subsequent dose of PEGylated liposomes. J Control Release. 2007;119(2):236–244. doi: 10.1016/j.jconrel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 65.Ishihara T, Takeda M, Sakamoto H, Kimoto A, Kobayashi C, Takasaki N, Yuki K, Tanaka K-i, Takenaga M, Igarashi R. Accelerated blood clearance phenomenon upon repeated injection of PEG-modified PLA-nanoparticles. Pharm Res. 2009;26(10):2270–2279. doi: 10.1007/s11095-009-9943-x. [DOI] [PubMed] [Google Scholar]
- 66.Laverman P, Carstens MG, Boerman OC, Dams ETM, Oyen WJ, van Rooijen N, Corstens FH, Storm G. Factors affecting the accelerated blood clearance of polyethylene glycol-liposomes upon repeated injection. J Pharm Exp Ther. 2001;298(2):607–612. [PubMed] [Google Scholar]
- 67.Koide H, Asai T, Hatanaka K, Akai S, Ishii T, Kenjo E, Ishida T, Kiwada H, Tsukada H, Oku N. T cell-independent B cell response is responsible for ABC phenomenon induced by repeated injection of PEGylated liposomes. Int J Pharm. 2010;392(1):218–223. doi: 10.1016/j.ijpharm.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 68.Fernandez HF, Sun Z, Yao X, Litzow MR, Luger SM, Paietta EM, Racevskis J, Dewald GW, Ketterling RP, Bennett JM. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361(13):1249–1259. doi: 10.1056/NEJMoa0904544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ennulat D, Magid-Slav M, Rehm S, Tatsuoka KS. Diagnostic performance of traditional hepatobiliary biomarkers of drug-induced liver injury in the rat. Toxicol Sci. 2010:kfq144. doi: 10.1093/toxsci/kfq144. [DOI] [PubMed] [Google Scholar]
- 70.Houba P, Boven E, van der Meulen-Muileman I, Leenders R, Scheeren J, Pinedo H, Haisma H. Pronounced antitumor efficacy of doxorubicin when given as the prodrug DOX-GA3 in combination with a monoclonal antibody β-glucuronidase conjugate. Int J Cancer. 2001;91(4):550–554. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1075>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 71.Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, Terada Y, Kano M, Miyazono K, Uesaka M. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol. 2011;6(12):815–823. doi: 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.