Abstract
Background
Adiposity and diseases associated with it, including cardiovascular disease, are emerging long-term complications of pediatric cancer survivors. Direct evaluations of adiposity and comparisons to contemporary controls that can differentiate recent trends in obesity from cancer-related treatments and sequelae are limited.
Methods
We evaluated demographic, treatment, lifestyle, and endocrine factors at the time of dual energy x-ray absorptiometry testing in 170 non-Hispanic white survivors and 71 sibling controls and compared three measures of adiposity (body mass index [BMI], total body fat, trunk fat). For the survivors alone, we determined factors independently associated with BMI and body fat.
Results
Survivors were 12 years since diagnosis; 58% had leukemia or lymphoma. BMI did not differ between groups. Among males, body fat was greater in survivors than in controls, (25.8% vs. 20.7%; P=0.007), as was trunk fat (26.7% vs 21.3%; P=0.008). Total or trunk fat did not differ among females. Cholesterol, triglycerides, LDL-cholesterol, and television-viewing hours were higher among male survivors than in controls. Independent factors associated with higher BMI, total and trunk fat included any cranial radiation and television-viewing hours, while prior treatment with cyclophosphamide was associated with lower BMI and body fat measures.
Conclusions
Compared to siblings, male survivors have greater body fat and metabolic risks. Cranial irradiation and television hours are important risk factors for adiposity in pediatric cancer survivors.
Impact
Pediatric cancer survivors should be carefully monitored for cardiovascular risk factors and sedentary lifestyles.
Keywords: Child, Cancer, Survivorship, Adiposity, Body Fat, Risk Factors
INTRODUCTION
Treatments for pediatric cancer have advanced substantially in recent years. With over 75% of childhood cancer survivors cured of their original disease, there are now more than 325,000 survivors of childhood cancer in the United States as of January 1, 2005 (1) With increased survivorship, attention has turned to improving the long-term quality of life that can be adversely affected by the late effects of cancer therapy. Common late effects of pediatric cancer therapies include secondary malignancy, cardiotoxicity, reduced pulmonary function, and disturbed growth and pubertal development. (2,3)
Obesity and the diseases associated with it, including cardiovascular disease, are also emerging long-term complications found in pediatric cancer survivors. Problems with overweight can begin at diagnosis, (4) during treatment, (5) and can continue after therapy. (6) The causes of adiposity in cancer survivors are not entirely understood, but a number of contributors have been identified, including reduced physical activity, (7) cranial irradiation with identified or presumed hormonal dysregulation, (8,9) family risk factors,(10) and early adiposity rebound. (11)
Despite the many theories about the mechanism of adiposity and altered metabolism in long-term survivors of childhood cancer, disease- and treatment-related determinants are not often isolated from lifestyle and genetic factors. With the increasing rates of obesity in United States children (12,13) and its emerging importance on cardiovascular health, (14) attributing adiposity to cancer-specific factors without comparison to children with comparable genetic and environmental conditions may produce misleading results. Because treatment recommendations for each cancer diagnosis can change over time, we believe that understanding general treatment and lifestyle factors associated with adiposity, regardless of initial cancer diagnosis, will be more generalizable to pediatric cancer survivors as they age decades later. Knowledge about risk factors for adiposity in cancer survivors may lead to new understandings about its causes and prevention.
The objectives of this study were to 1. compare measures of body fat and metabolic factors associated with body fat between a general cohort of pediatric cancer survivors and their siblings and 2. determine adiposity risks in survivors of childhood cancer receiving a diverse array of treatments. This study builds on previous self-reported outcomes based studies (8,9) by performing direct evaluations on the survivors and their siblings. We hypothesized, compared to a control group with similar genetic and environmental conditions, that pediatric cancer survivors would demonstrate higher levels of adiposity and that lifestyle- and cancer-specific treatments or sequelae would contribute to greater levels of adiposity.
SUBJECTS AND METHODS
Subjects
Subjects were recruited from the Pediatric Oncology Long-Term Survivor Clinic at the University of Rochester, Rochester, NY between 1998 and 2003 as part of enrollment in a prospective, longitudinal study on cardiac risk factors.(15) Patients were eligible for this study if they had been followed in this program for at least 3 years since their diagnosis. Amputees were excluded because of the potential for unreliable body composition studies. Subjects were no longer receiving chemotherapy or radiation and were cancer-free at the time of the current study.
Sibling controls were invited to participate at the time of enrollment for each patient. Ninety-four siblings were invited to participate and 76 siblings enrolled in the study. If there was more than one sibling, the one closest in age was preferred, although not required. Sibling controls could not have a diagnosis or history of cancer and have other medical co-morbidities. All sibling controls that participated in the study were included in the analyses.
The Institutional Review Board at the University of Rochester approved the research protocol, and informed consent from the patient or parent/guardian and assent from the child (when appropriate) were obtained.
Main Outcome Variables
Body Mass Index
Body mass index (BMI), one of our primary outcome variables, is defined as weight/height2 (kg/m2). BMI is a commonly used and validated measure of body fatness among children and adults.(16) Weight and standing height were measured by the recommended techniques(17) at the study visit. BMI was classified as under-weight, healthy-weight, overweight and obese, with the definitions applied that were appropriate for age and sex.(18)
Total Body Fat and Total Trunk Fat
Percent total body and trunk fat were determined by dual energy x-ray absorptiometry (DXA) [GE Lunar Prodigy DF 10370 (Madison, WI)] as previously described.(19) A relatively non-invasive method that quantifies whole-body composition into fat-free and fat masses, DXA also assesses the regional distribution of body fat. Lunar software (Version 6.10) was used to calculate body composition.
Data Collection and Covariates
Current clinical and laboratory data were collected during the same visit. Additional information (chemotherapy, radiation) was abstracted retrospectively from medical records. Baseline information was collected on: age, sex, race, type of cancer, age at diagnosis, duration of treatment, time since completion of therapy, type of chemotherapy and type and amount of radiation therapy. Cancer diagnosis was categorized as brain, embryonal, germ, leukemia, lymphoma, sarcoma, or other. All radiation that included the cranium was recorded. Chemotherapeutic categories included anthracyclines, prednisone, methotrexate, cyclophosphamide, and cisplatinum and were not mutually exclusive. The number of hours per day spent watching television, as a surrogate marker for activity level was recorded by study questionnaire.(20)
Laboratory serum biomarkers included markers of endocrine function—fasting insulin, estrogen, follicular stimulating hormone (FSH), luteinizing hormone (LH), insulin-like growth factor-1 (IGF-1), testosterone (males only), thyroid stimulating hormone (TSH), free thyroxine (T4) —and a lipid fasting profile—total cholesterol, HDL-cholesterol, LDL-cholesterol, and triglycerides. Values for these biomarkers were determined in a Clinical Laboratory Improvement Amendments-approved laboratory (Strong Memorial Hospital Clinical Laboratory, Rochester, NY). High-sensitivity C-reactive protein (hsCRP) was assayed by the N High Sensitivity CRP assay (Dade Behring, Newark, DE).
Statistical Methods
The first protocol visit for each participant was used for all statistical analyses. All analyses were performed separately for males and females. First, cancer survivors were compared to controls on anthropometric and metabolic factors. Because anthropometric (such as weight) and metabolic (such as cholesterol) outcome variables change with age, all comparisons of these outcomes were age-adjusted. In these bivariate analyses, the age-adjusted means were calculated from separate linear regression models for cancer survivors and controls, with age as a covariate in each model. When calculating P-values for comparisons of age-adjusted means from cancer survivors and controls, general linear mixed models (21) were used to adjust the P-value for a possible family effect from a sibling control and cancer survivors who are from the same family. In particular, to calculate these P-values, a mixed model was fit with a random sibling effect and covariates: age, cancer/control status, sex, and all possible interactions between sex and age and sex and cancer/control status. Fifty percent of cancer survivors did not have a matched control. However, analyses showed no systematic difference between groups. Thus, all survivors were used in the analyses to increase power.
Second, multivariate analyses were performed in survivors, alone, on the outcomes BMI, percent body fat, and percent trunk fat, with the covariates of type of cancer, type of chemotherapy, radiation, duration of treatment, time since completion of therapy, and hormonal factors (LH, FSH, testosterone (males only), IGF-1, T4). All regression models were age-adjusted. Our a priori hypotheses included analyses of our stated outcomes and predictors. Covariates were kept in a regression model for known biological effects (such as age), and were considered significant if the P-value <0.05.
We performed analyses on these restricted sets of variables. Because this analysis was exploratory, we did not adjust the type-1 error rate to account for multiple comparisons; thus, P-values should be interpreted cautiously. The number of significant associations (P<0.05) in these analyses is much greater than expected by chance if there were no associations in the data. All tests were two-tailed. The SAS statistical software package (SAS Institute, Cary, NC) was used in the analyses.
RESULTS
Patient Characteristics
A total of 201 pediatric cancer survivors and 76 siblings enrolled in the study; however, because the majority (90%) of the cohort was non-Hispanic white, we excluded 26 survivors and siblings of other ethnicities from the data analyses. The remaining survivors were excluded because of previously defined exclusions (amputee). Thus, 170 pediatric cancer survivors and 71 siblings (controls) were included in this analysis.
Enrollment demographic and clinical characteristics of the study participants are shown in Table 1 and approximated the profile of the long-term survivor population in the general clinic that has previously been reported. (15)
Table 1.
Diagnosis and Treatment History of 170 Long-Term Survivors of Pediatric Cancer and 71 Cancer Free Siblings
| Cancer Survivors | All (170) | Females (83) | Males (87) |
|---|---|---|---|
| Mean age at study, years | 19.38 | 20.34 | 18.48 |
| Range, years | (5.9–39.7) | (5.9–39) | (6–39.7) |
| Mean age at diagnosis, years | 7.5 | 8.44 | 6.51 |
| Range, years | (0 – 24.1) | (0.42–17.7) | (0–24) |
| Females, n (%) | 83 (49) | ||
| Mean years since diagnosis | 11.9 | 11.90 | 11.96 |
| Range, years | (4.3 – 31.6) | (4.5–29.9) | (4.3–31.6) |
| Cancer diagnosis, n (%) | |||
| Leukemia | 63 (37) | 32(39) | 31(36) |
| Lymphoma | 35 (21) | 18(22) | 17(20) |
| Embryonal | 33 (20) | 16(19) | 17(20) |
| Brain | 16 (9) | 6(7) | 10(11) |
| Sarcoma | 16 (9) | 9(11) | 7(8) |
| Germ cell | 4 (2) | 2(2) | 2(2) |
| Other | 3 (2) | 3(3) | |
| Chemotherapy, n (%)1 | |||
| Anthracycline | 112 (66) | 58(70) | 54(62) |
| Prednisone | 88 (52) | 44(53) | 44(50) |
| Methotrexate | 80 (47) | 39(47) | 41(47) |
| Cyclophosphamide | 72 (42) | 36(43) | 36(41) |
| Cisplatinum | 32 (19) | 16(19) | 16(18) |
| Any cranial radiation, n (%) | 81 (48) | 34(41) | 47(54) |
|
| |||
| Sibling Controls | All (71) | Female (33) | Male (38) |
|
| |||
| Mean age at study, years | 16 | 15.53 | 16.58 |
| Range, years | (5.3–45.9) | (5.9–31.3) | (5.3–45.9) |
Not mutually exclusive
Anthropometric Characteristics
Among females, survivors were older than their siblings (20.3 vs. 15.5 y; P=0.004; Table 2). The survivors, after adjusting for age and sibling pair, were shorter and weighed less, although the BMIs were similar (24.3 kg/m2 for siblings vs. 23.6 kg/m2 for survivors). Other body composition measures did not differ markedly between survivors and siblings. Thirty-six percent of female cancer survivors (n=30) were classified as overweight or obese, compared to 48% of control females (P=0.22).
Table 2.
Adiposity Characteristics of 170 Pediatric Cancer Survivors and 71 Sibling Controls*
| Variable (mean, IQR) | Females | Males | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Survivors n = 83 |
Controls n = 33 |
P | Survivors n = 87 |
Controls n = 38 |
P | |
| Age, y | 20.3 (14.2–26.2) | 15.5 (12.2–17.9) | 0.004 | 18.5 (12.4–23.4) | 16.6 (10.8–19.9) | 0.13 |
| Height, cm | 155.0 (147.7–163.1) | 161.3 (158.1–169–3) | 0.03 | 163.2 (152.7–173–9) | 167.0 (160.5–178.2) | 0.11 |
| Weight, kg | 57.9 (44.8–67.6) | 63.4 (53.0–73.4) | 0.14 | 64.9 (51.1–76.3) | 66.2 (60.1–80.1) | 0.68 |
| BMI, kg/m2 | 23.6 (19.0–26.4) | 24.3 (22.0–27.6) | 0.49 | 23.5 (20.6–24.9) | 22.6 (19.9–26.3) | 0.38 |
| Body fat, % | 36.3 (28.3–43.0) | 34.5 (28.8–41.7) | 0.43 | 25.8 (17.9–33.2) | 20.7 (13.8–28.2) | 0.007 |
| Trunk fat, % | 35.0 (25.9–43.4) | 33.5 (26.9–41.6) | 0.55 | 26.7 (19.3–34.8) | 21.3 (13.3–29.2) | 0.008 |
Mixed models (SAS Proc Mixed) were used to estimate means by sex while adjusting for age and sibling pairs.
BMI = Body mass index
IQR = Inter-quartile range
Among males, survivors were older than their siblings (18.5 vs. 16.6 y; P=0.13; Table 2), although this did not reach statistical significance. Heights, weights, and BMIs, after adjusting for age and sibling pair, were similar between survivors and siblings. However, percent body fat (25.8% vs. 20.7%; P=0.007) and percent trunk fat (26.7% vs. 21.3%; P=0.008) were greater in survivors than in siblings. Thirty-seven percent of male cancer survivors (n=32) were classified as overweight or obese, compared to 29% of control males (P=0.40).
Metabolic Factors and Television Viewing
For females, the age-adjusted IGF-1 levels of survivors were significantly lower than controls (213.2 vs. 283.8 ng/mL; P=0.004). All other factors were similar in both groups (Table 3). For males, after adjusting for age and sibling pair, survivors had significantly lower T4 (1.06 vs. 1.32 ng/dL; P<0.001), higher FSH (7.28 vs. 4.92 μU/mL; P=0.01) and lower IGF-1 (197.9 vs. 258.2 ng/mL; P=0.04) values. Male survivors watched a greater number of hours of television than their siblings (2.3 vs. 1.8 hours/day; P=0.04).
Table 3.
Non-Oncologic Predictors of Obesity in 170 Cancer Survivors and 71 Sibling Controls*
| Variable (mean, IQR) | Females | Males | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Survivors n = 83 |
Controls n = 33 |
P | Survivors n = 87 |
Controls n = 38 |
P | |
| TSH, μU/mL | 2.27 (1.28–2.55) | 2.04 (1.17–2.68) | 0.52 | 2.19 (1.23–2.52) | 1.96 (1.16–2.27) | 0.43 |
| T4, ng/dL | 1.09 (1.00–1.24) | 1.05 (1.00–1.10) | 0.64 | 1.06 (1.00–1.10) | 1.32 (1.00–1.30) | <0.001 |
| FSH, μU/mL | 4.89 (1.74–6.52) | 4.42 (2.77–5.93) | 0.68 | 7.28 (3.00–9.30) | 4.92 (1.84–5.00) | 0.01 |
| LH, μU/mL | 6.57 (0.95–6.12) | 6.47 (1.57–8.37) | 0.94 | 3.80 (1.84–5.14) | 5.55 (1.58–3.97) | 0.13 |
| Estrogen, pg/mL | 58.4 (20.00–70.23) | 53.2 (20.00–97.9) | 0.59 | 24.5 (20.00–25.89) | 23.3 (20.00–23.40) | 0.88 |
| Testosterone, ng/dL | … | … | … | 358.2 (187.2–486.8) | 357.8 (186.3–560.2) | 0.99 |
| IGF-1, ng/mL | 213.2 (149.9–270.2) | 283.8 (204.2–349.7) | 0.004 | 197.9 (122.0–237.0) | 258.2 (152.8–308.2) | 0.004 |
| TV, hours/day | 2.2 (1.4–2.7) | 2.1 (0.8–2.7) | 0.63 | 2.3 (1.4–2.9) | 1.8 (0.8–2.7) | 0.04 |
Mixed models (SAS Proc Mixed) were used to estimate means by sex while adjusting for age and sibling pairs.
IQR = inter-quartile range; TSH = thyroid stimulating hormone; T4 = free thyroxine; FSH = follicular stimulating hormone; LH = luteinizing hormone; IGF-1 = insulin-like growth factor-1; TV = television-viewing
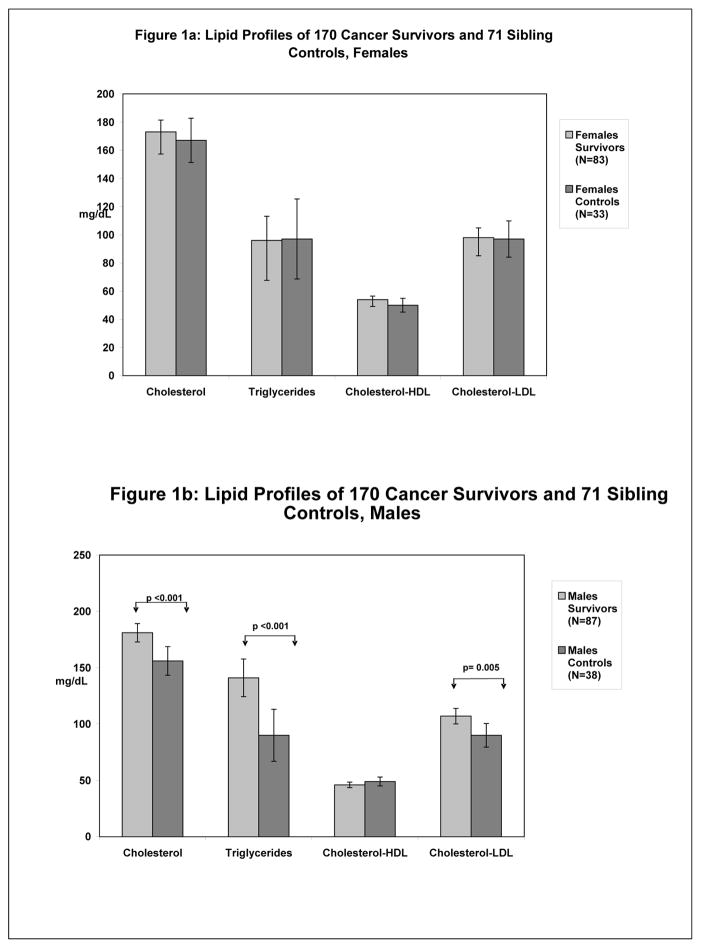
Serum lipid levels that may be associated with, yet not causal for, higher BMI and body fat are compared in Figure 1. For males, total cholesterol, LDL-cholesterol, and triglycerides were significantly higher in survivors. No differences were found for females. High-sensitivity CRP was significantly higher in female survivors compared to siblings (0.35 vs. 0.07 mg/dL; P=0.02), but there were no differences among males (0.18 vs. 0.09 mg/dL; P=0.38). No differences were found in fasting insulin levels for either sex.
Figure 1.
Comparisons of lipid profiles between female cancer survivors and sibling controls (Figure 1a) and male cancer survivors and sibling controls (Figure 1b). No significant differences were found among females. Male cancer survivors had significantly elevated levels of total and LDL-cholesterol and triglycerides.
Predictors of Adiposity in Survivors
We performed bivariate (adjusting for age in all analyses) and multivariate analyses to identify specific factors associated with BMI and percent body and trunk fat in survivors. We analyzed data for males and females separately (Tables 4a through 4c).
Table 4a.
Bivariate* and Multivariate** Determinants of Body Mass Index in 170 Long-Term Pediatric Cancer Survivors, by Sex
| Variable | Bivariate | Multivariate | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Estimate | SE | P | Estimate | SE | P | |
| Females | ||||||
| Age, years | 0.25 | 0.08 | <0.003 | 0.20 | 0.08 | 0.02 |
| Cyclophosphamide | −3.34 | 1.33 | 0.01 | −3.00 | 1.34 | 0.03 |
| Methotrexate | 2.13 | 1.36 | 0.12 | … | … | … |
| Cisplatinum | −3.49 | 1.71 | 0.04 | … | … | … |
| LH, μU/mL | −0.09 | 0.05 | 0.09 | … | … | … |
| Cranial irradiation | 3.44 | 1.35 | 0.01 | … | … | … |
| TV hours/day | 1.17 | 0.54 | 0.03 | 1.02 | 0.52 | 0.06 |
|
| ||||||
| Males | ||||||
| Age, years | 0.38 | 0.06 | <0.001 | 0.43 | 0.06 | <0.001 |
| Cyclophosphamide | −2.77 | 1.02 | 0.008 | −2.84 | 1.06 | 0.009 |
| Cranial irradiation | 1.95 | 1.02 | 0.06 | … | … | … |
| IGF-1, ng/mL | 0.01 | 0.005 | 0.04 | 0.012 | 0.005 | 0.01 |
Age-adjusted bivariate analyses
All variables listed in the bivariate analysis were considered for inclusion in the multivariate analysis
LH = Luteinizing hormone
IGF-1 = Insulin-like growth factor-1
TV = Television-viewing
Table 4c.
Bivariate* and Multivariate** Determinants of Percent Trunk Fat in 170 Long-Term Pediatric Cancer Survivors, by Sex
| Variable | Bivariate | Multivariate | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Estimate | SE | P | Estimate | SE | P | |
| Females | ||||||
| Age, years | 0.44 | 0.15 | 0.003 | 0.36 | 0.15 | 0.01 |
| Cyclophosphamide | −6.03 | 2.41 | 0.01 | −4.95 | 2.43 | 0.05 |
| Methotrexate | 5.84 | 2.42 | 0.02 | … | … | … |
| Cranial irradiation | 8.21 | 2.30 | <0.006 | 4.85 | 2.50 | 0.05 |
| TV, hours/day | 2.97 | 0.94 | 0.002 | 2.09 | 0.94 | 0.03 |
|
| ||||||
| Males | ||||||
| Age, years | 0.55 | 0.13 | <0.001 | 0.64 | 0.12 | <0.0001 |
| Cranial irradiation | 6.73 | 2.15 | 0.002 | 6.44 | 2 | 0.002 |
| TV hours/day | 3.38 | 0.82 | <0.001 | 2.98 | 0.78 | <0.001 |
Age-adjusted analyses
All variables listed in the bivariate analysis were considered for inclusion in the multivariate analysis
TV = Television-viewing
For BMI in females, cranial irradiation and more television-viewing hours were associated with greater BMI (P=0.01 and 0.03, respectively), whereas exposure to cyclophosphamide and cisplatinum were associated with a lower BMI (P=0.01 and 0.04, respectively) in the bivariate analysis. In similar bivariate analyses for percent body fat and percent trunk fat in females, methotrexate, cranial irradiation, and more television hours had increased these outcomes, whereas past exposure to cyclophosphamide decreased them. For males, higher BMI was positively associated with IGF-1 (P=0.04), and lower BMI was associated with cyclophosphamide exposure (P=0.008). Furthermore, bivariate results in a similar model for males showed cranial irradiation and more television viewing hours were associated with higher percent body fat and higher percent trunk fat.
Although not considered in the multivariate model, the diagnosis of leukemia, after adjusting for age and sex, was associated with increased BMI (estimate 1.93; P=0.03), body fat (estimate 4.36; P=0.005) and trunk fat (estimate 0.02; P=0.04). Furthermore, a history of brain tumor, after adjusting for age and sex, was also associated with increased BMI (estimate 3.54; P=0.02), and total body fat (estimate 9.17; P<0.001).
Multivariate models for females and males were then developed (Tables 4a through 4c). For females, BMI was positively associated with age and negatively associated with cyclophosphamide exposure. Percent body and trunk fat were positively associated with age, cranial irradiation, television-viewing hours, and negatively associated with cyclophosphamide use.
For males, BMI was independently and positively associated with age and IGF-1 levels and negatively associated with cyclophosphamide use. Percent body and trunk fat in males were independently and positively associated with age, cranial irradiation, and television viewing hours. As an example, the male model in Table 4b shows that an average male cancer survivor who received cranial irradiation had 5.82% more body fat than an average male cancer survivor who did not receive cranial irradiation, after controlling for other characteristics.
Table 4b.
Bivariate* and Multivariate** Determinants of Percent Body Fat in 170 Long-Term Pediatric Cancer Survivors, by Sex
| Variable | Bivariate | Multivariate | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Estimate | SE | P | Estimate | SE | P | |
| Females | ||||||
| Age, years | 0.37 | 0.13 | 0.005 | 0.31 | 0.13 | 0.02 |
| Cyclophosphamide | −5.56 | 2.10 | 0.01 | −4.66 | 2.09 | 0.03 |
| Methotrexate | 5.10 | 2.13 | 0.02 | … | … | … |
| Cranial irradiation | 7.10 | 2.02 | <0.001 | 3.93 | 2.15 | 0.07 |
| TV, hours/day | 2.92 | 0.81 | <0.006 | 2.18 | 0.81 | 0.009 |
|
| ||||||
| Males | ||||||
| Age, years | 0.27 | 0.13 | 0.04 | 0.34 | 0.11 | 0.003 |
| Cyclophosphamide | −3.65 | 2.07 | 0.08 | … | … | … |
| Cranial irradiation | 5.92 | 2.02 | 0.004 | 5.82 | 1.91 | 0.003 |
| TV, hours/day | 2.96 | 0.77 | <0.003 | 2.6 | 0.74 | <0.001 |
Age-adjusted bivariate analyses
All variables listed in the bivariate analysis were considered for inclusion in the multivariate analysis
TV = television-viewing
Dose Effect of Cranial Radiation on Adiposity
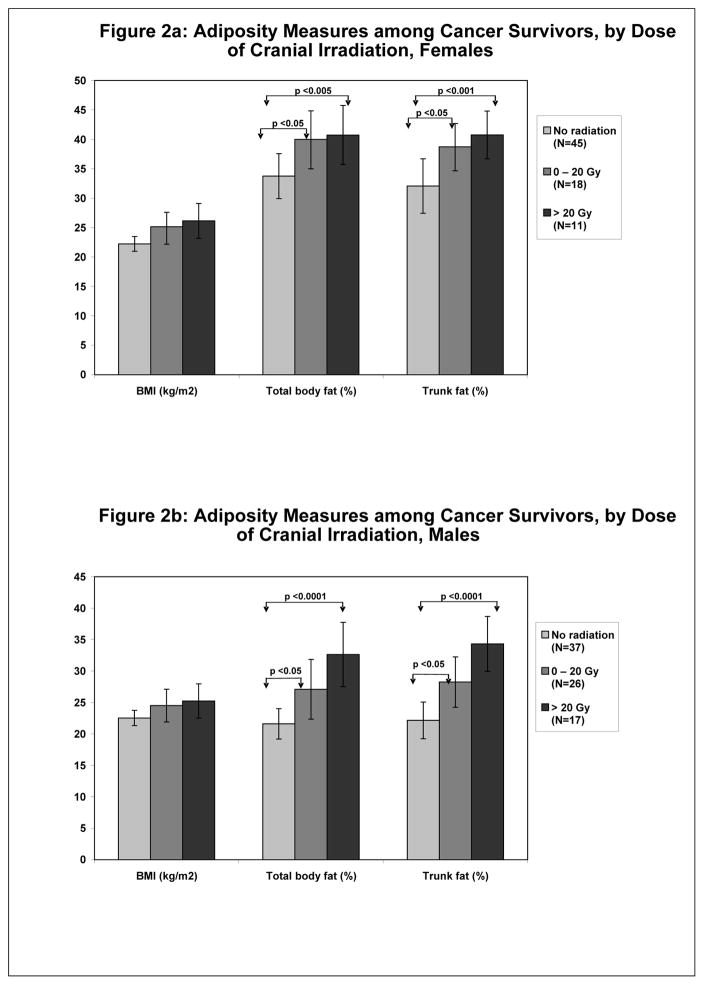
We were especially interested in the effect of cranial radiation on adiposity because of the known effects of cranial irradiation on the hypothalamic/pituitary axes. (22) Cranial radiation, as a categorical variable, in addition to television-viewing hours, were the most consistent predictors of our outcomes in the multivariate analyses. We explored the effect of cranial radiation according to increasing dose (none, 0–20 Gy, and >20 Gy [Figure 2]) and after adjustment for age on our outcomes. Females who received any cranial radiation (compared to those who received none) had significantly higher percent body and trunk fat. There were no significant differences in body composition measures between those children who received 0–20 Gy and those who received >20 Gy of cranial irradiation. Although there were no statistically significant differences in BMI between these three radiation dose categories, BMI increased from 22.2 kg/m2 (with no cranial irradiation) to 26.1 kg/m2 when >20 Gy had been administered.
Figure 2.
Comparisons of adiposity measures (BMI, total body fat and trunk fat) by increasing dosages of cranial irradiation in female cancer survivors (Figure 2a) and male cancer survivors (Figure 2b). Increasing dosages of cranial irradiation did not significantly affect BMI, whereas there were increased levels of total body and trunk fat with cranial irradiation in both females and males.
The results were similar for males, with no significant differences in BMI by cranial irradiation dose. However, percent body and trunk fat both increased with any cranial irradiation. Percent body and trunk fat did not differ between the radiation dose groups.
DISCUSSION
In this study, we evaluated BMI, total body and trunk fat in a convenience sample of pediatric cancer survivors with several tumor types from one geographical region of the United States. As opposed to focusing on original diagnoses, our analyses identified demographic, lifestyle (television viewing), and treatment-related factors associated with adiposity that can be applied to non-Hispanic white pediatric cancer survivors as a general group.
We compared anthropometric and metabolic indices associated with body fat between pediatric cancer survivors and their siblings. With ballooning rates of childhood obesity and its sequelae, (12,13) a comparison group is necessary to discriminate late cancer effects from contemporary trends. BMI was similar among male and female survivors, each compared to their siblings. Over a third of the cancer survivors met criteria for overweight or obese, yet these percentiles were not statistically different than the controls. However, male survivors had more trunk and total body fat than their siblings. Lipid profiles and television viewing were also different in male survivors. Cranial irradiation and television viewing were associated with higher total fat and trunk fat (not BMI) for both males and females.
Although not considered in the final model because of our objectives to study the effects of past treatment regimens, we did find leukemia survivors and those with brain tumors had higher estimates of body fat than other diagnoses. The associations between these diagnoses and body fat are likely confounded by these patients’ past receipt of cranial irradiation. Because the location of the brain tumor may have produced specific abnormalities that would affect body fat, we did consider this tumor type in the final model, however this variable was not retained (data not shown).
Adiposity in survivors of childhood leukemia (8,9,23–27) and brain tumors (28, 29) has been studied. The prevalence of overweight and obesity, as measured by BMI, in childhood acute lymphoblastic leukemia survivors ranges between 21% and 54% (overweight) and 14% and 30% (obese) in some studies. (8) Some investigators have found that pediatric cancer survivors do not have higher-than-normal BMIs (28, 30–32) and some survivors are at significant risk for being underweight. (33) Variability in patient characteristics and study design, including length of follow-up, treatment, and appropriateness of controls, might explain these conflicting results.
Body composition is not routinely measured in childhood cancer survivor studies because these instruments are not easily accessed in the clinical setting. However, a European study, (24) among others, (6) confirmed that BMI was not as sensitive in detecting differences in body composition as fat estimates obtained by DXA. (24) Male childhood acute lymphoblastic leukemia survivors who received cranial radiation had higher body fat, trunk fat, and serum lipids than did males who did not receive cranial radiation. (24) The findings from our study are similar and show that these results can be generalized to a population-based cohort of pediatric cancer survivors.
High body fat can be caused by endocrine dysfunction that can lead to growth hormone dysregulation. Abnormal endocrine function has been associated with cranial irradiation (8) in cancer survivors. We found IGF-1 levels to be lower in survivors compared to sibling controls. Furthermore, cranial irradiation emerged as one of the strongest predictors of higher body fat (even after considering IGF-1 levels), suggesting a more global influence of cranial irradiation on other factors that affect body fat, such as thyroid and sex hormones. This association was stronger for total body or trunk fat outcomes than BMI. Higher cranial irradiation doses were associated with greater body fat or BMI, although we could not statistically discriminate between 0–20 Gy and >20 Gy.
The findings of adverse lipid profiles, along with higher body fat in male cancer survivors suggest that they might be a high-risk group for adverse metabolic cardiovascular outcomes. Fat, a metabolically active tissue, produces pro-inflammatory factors that contribute to vascular inflammation that can ultimately predispose the individual to atherosclerotic vascular lesions. (34,35) Total and LDL-cholesterol are increased and HDL-cholesterol is decreased in childhood cancer survivors. (36–39) In our study, higher lipids and body fat, along with lower overall physical activity (as determined indirectly by the number of hours per day watching television) may contribute to these children being at potentially a greater risk for premature atherosclerotic cardiovascular disease.
Although our study did not evaluate the potential additive effects of video games and computer screen time, the possible effects of a sedentary lifestyle are evident because the television viewing hours was one of the strongest and most consistent predictors of body and trunk fat. Several studies, (39–41) including a meta-analysis of physical activity interventions in cancer survivors by Schmitz, (7) found overall physical deconditioning in survivors. Schmitz’s meta-analysis of 32 studies, (7) and others, (42) show exercise interventions in survivors had a moderate to large positive effect on cardiorespiratory outcomes. (7) However, the child’s risk from exercise must be carefully evaluated before recommending an exercise program because the cause of deconditioning can be multifactorial, including not only cardiac dysfunction, but also pulmonary abnormalities, hormonal disturbances, obesity, and psychosocial issues. (42)
Finally, therapy with the alkylating cytostatic agent, cyclophosphamide, is a significant independent predictor of lower BMI and body fat, an association that was found more often in females than males. This has not been well studied but is biologically plausible because of this drug’s other late effects in long-term survivors. Cyclophosphamide is associated with gonadal failure (43) and other late effects including cardiac disease, (44) decreased growth velocity (45,46) and in experimental animal models, (47) apoptosis in bone (48,49) and altered lipid metabolism. (50,51) Because our study design included a diverse study population, we cannot exclude the possibility that this finding may have been confounded by changes associated with other simultaneous treatments. However, extensive analyses that included propensity scores relating to cyclophosphamide therapy found no other associations (data not shown).
This study has some limitations. Non-Hispanic whites were our focus and inferences to pediatric cancer survivors of other ethnicities must be cautiously considered. Our control group was smaller than our survivor group. We attempted to control for genetic and environmental influences by recruiting cancer survivors and their siblings and control for sibling pairs in the analyses.
In conclusion, body and trunk fat are greater in male pediatric cancer survivors than in their siblings, while BMI is a less sensitive indicator of adiposity. Male cancer survivors have worse lipid profiles, watch a greater amount of television, and have other metabolic abnormalities associated with adiposity. For both female and male survivors, cranial irradiation and television viewing are associated with adiposity. Future studies should focus on early detection and promotion of safe lifestyle and physical activities in these patients at risk for both metabolic and drug- or radiation-related premature cardiovascular disease.
Acknowledgments
Financial Support: This work was supported by grants from National Institutes of Health (NCI: CA-79060 and CA-127642; NIDDK: P01 DK-45734 and the General Clinical Research Center, which was funded by the Division of Research Resources of the NIH under grant numbers: M01-RR00054 and M01-RR00044).
Footnotes
Disclosures: The authors have no conflicts of interest and all work is original in content. There are no financial disclosures.
References
- 1.Mariotto AB, Rowland JH, Yabroff KR, et al. Long-term survivors of childhood cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18:1033–40. doi: 10.1158/1055-9965.EPI-08-0988. [DOI] [PubMed] [Google Scholar]
- 2.Hewitt M, Weiner SL, Simone JV, editors. National Cancer Policy Board (U.S.) Childhood cancer survivorship: improving care and quality of life. Washington, D.C: National Academies Press; 2003. [PubMed] [Google Scholar]
- 3.Alvarez JA, Scully RE, Miller TL, et al. Long-term effects of treatments for childhood cancers. Curr Opin Pediatr. 2007;19:23–31. doi: 10.1097/MOP.0b013e328013c89e. [DOI] [PubMed] [Google Scholar]
- 4.Larsson SC, Wolk A. Overweight and obesity and incidence of leukemia: a meta-analysis of cohort studies. Int J Cancer. 2008;122:1418–21. doi: 10.1002/ijc.23176. [DOI] [PubMed] [Google Scholar]
- 5.Reilly JJ, Ventham JC, Newell J, et al. Risk factors for excess weight gain in children treated for acute lymphoblastic leukaemia. Int J Obes Relat Metab Disord. 2000;24:1537–41. doi: 10.1038/sj.ijo.0801403. [DOI] [PubMed] [Google Scholar]
- 6.Nysom K, Holm K, Michaelsen KF, et al. Degree of fatness after treatment for acute lymphoblastic leukemia in childhood. J Clin Endocrinol Metab. 1999;84:4591–6. doi: 10.1210/jcem.84.12.6205. [DOI] [PubMed] [Google Scholar]
- 7.Schmitz KH, Holtzman J, Courneya KS, et al. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;14:1588–95. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- 8.Oeffinger KC, Mertens AC, Sklar CA, et al. Obesity in adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2003;21:1359–65. doi: 10.1200/JCO.2003.06.131. [DOI] [PubMed] [Google Scholar]
- 9.Garmey EG, Liu Q, Sklar CA, et al. Longitudinal changes in obesity and body mass index among adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2008;26:4639–45. doi: 10.1200/JCO.2008.16.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross JA, Oeffinger KC, Davies SM, et al. Genetic variation in the leptin receptor gene and obesity in survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2004;22:3558–62. doi: 10.1200/JCO.2004.11.152. [DOI] [PubMed] [Google Scholar]
- 11.Reilly JJ, Kelly A, Ness P, et al. Premature adiposity rebound in children treated for acute lymphoblastic leukemia. J Clin Endocrinol Metab. 2001;86:2775–8. doi: 10.1210/jcem.86.6.7554. [DOI] [PubMed] [Google Scholar]
- 12.Messiah SE, Arheart KL, Luke B, et al. Relationship between body mass index and metabolic syndrome risk factors among US 8- to 14-year-olds, 1999 to 2002. J Pediatr. 2008;153:215–21. doi: 10.1016/j.jpeds.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Messiah SE, Arheart KL, Lipshultz SE, et al. Body mass index, waist circumference, and cardiovascular risk factors in adolescents. J Pediatr. 2008;153:845–50. doi: 10.1016/j.jpeds.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez JA, Miller TL, Starc TJ, McGrath K, Lipshultz SE. Preventive Cardiology. In: McInarney T, Hoekelman RA, Adams HM, Nelson NM, Weitzman ML, Wilson MH, editors. American Academy of Pediatrics: Pediatric Primary Care, 5/e. St Louis: Elsevier Mosby; 2008. [Google Scholar]
- 15.Hinkle AS, Proukou C, French CA, et al. A clinic-based, comprehensive care model for studying late effects in long-term survivors of pediatric illnesses. Pediatrics. 2004;113(4 Suppl):1141–5. [PubMed] [Google Scholar]
- 16.Freedman DS, Ogden CL, Berenson GS, et al. Body mass index and body fatness in childhood. Curr Opin Clin Nutr Metab Care. 2005;8:618–23. doi: 10.1097/01.mco.0000171128.21655.93. [DOI] [PubMed] [Google Scholar]
- 17.Lohman TG, Roche AF, Marttorell R. Anthropometric Standardization Manual. Champaign, Ill: Human Kinetics Publishers; 1988. [Google Scholar]
- 18.Centers for Disease Control and Prevention. [accessed February 7, 2010];Defining Overweight and Obesity. http://www.cdc.gov/nccdphp/dnpa/obesity/defining.htm.
- 19.Margulies L, Horlick M, Thornton JC, et al. Reproducibility of pediatric whole body bone and body composition measures by dual-energy X-ray absorptiometry using the GE Lunar Prodigy. J Clin Densitom. 2005;8:298–304. doi: 10.1385/jcd:8:3:298. [DOI] [PubMed] [Google Scholar]
- 20.Dietz WH, Jr, Gortmaker SL. Do we fatten our children at the television set? Obesity and television viewing in children and adolescents. Pediatrics. 1985;75:807–12. [PubMed] [Google Scholar]
- 21.Littell RC, Milliken GA, Stroup WW, et al. SAS System for Mixed Models. SAS Institute; Cary, NC: 1996. [Google Scholar]
- 22.Constine LS, Woolf PD, Cann D, et al. Hypothalamic-pituitary dysfunction after radiation for brain tumors. N Engl J Med. 1993;328:87–94. doi: 10.1056/NEJM199301143280203. [DOI] [PubMed] [Google Scholar]
- 23.Baillargeon J, Langevin AM, Lewis M, et al. Therapy-related changes in body size in Hispanic children with acute lymphoblastic leukemia. Cancer. 2005;103:1725–9. doi: 10.1002/cncr.20948. [DOI] [PubMed] [Google Scholar]
- 24.Jarfelt M, Lannering B, Bosaeus I, et al. Body composition in young adult survivors of childhood acute lymphoblastic leukaemia. Eur J Endocrinol. 2005;153:81–9. doi: 10.1530/eje.1.01931. [DOI] [PubMed] [Google Scholar]
- 25.Murphy AJ, Wells JC, Williams JE, et al. Body composition in children in remission from acute lymphoblastic leukemia. Am J Clin Nutr. 2006;83:70–4. doi: 10.1093/ajcn/83.1.70. [DOI] [PubMed] [Google Scholar]
- 26.Warner JT, Evans WD, Webb DK, et al. Body composition of long-term survivors of acute lymphoblastic leukaemia. Med Pediatr Oncol. 2002;38:165–72. doi: 10.1002/mpo.1304. [DOI] [PubMed] [Google Scholar]
- 27.Janiszewski PM, Oeffinger KC, Church TS, et al. Abdominal obesity, liver fat, and muscle composition in survivors of childhood acute lymphoblastic leukemia. J Clin Endocrinol Metab. 2007;92:3816–21. doi: 10.1210/jc.2006-2178. [DOI] [PubMed] [Google Scholar]
- 28.Gurney JG, Ness KK, Stovall M, et al. Final height and body mass index among adult survivors of childhood brain cancer: Childhood Cancer Survivor Study. J Clin Endocrinol Metab. 2003;88:4731–9. doi: 10.1210/jc.2003-030784. [DOI] [PubMed] [Google Scholar]
- 29.Lustig RH, Post SR, Srivannaboon K, et al. Risk factors for the development of obesity in children surviving brain tumors. J Clin Endocrinol Metab. 2003;88:611–6. doi: 10.1210/jc.2002-021180. [DOI] [PubMed] [Google Scholar]
- 30.Meacham LR, Gurney JG, Mertens AC, et al. Body mass index in long-term adult survivors of childhood cancer: a report of the Childhood Cancer Survivor Study. Cancer. 2003;103:1730–9. doi: 10.1002/cncr.20960. [DOI] [PubMed] [Google Scholar]
- 31.Van Dongen-Melman JE, Hokken-Koelega AC, Hahlen K, et al. Obesity after successful treatment of acute lymphoblastic leukemia in childhood. Pediatr Res. 1995;38:86–90. doi: 10.1203/00006450-199507000-00015. [DOI] [PubMed] [Google Scholar]
- 32.Davies HA, Didcock E, Didi M, et al. Growth, puberty and obesity after treatment for leukaemia. Acta Paediatr. 1995;(Suppl 411):45–50. doi: 10.1111/j.1651-2227.1995.tb13862.x. [DOI] [PubMed] [Google Scholar]
- 33.Meacham LR, Gurney JG, Mertens AC, et al. Body mass index in long-term adult survivors of childhood cancer: a report of the Childhood Cancer Survivor Study. Cancer. 2005;103:1730–9. doi: 10.1002/cncr.20960. [DOI] [PubMed] [Google Scholar]
- 34.Murdolo G, Smith U. The dysregulated adipose tissue: a connecting link between insulin resistance, type 2 diabetes mellitus and atherosclerosis. Nutr Metab Cardiovasc Dis. 2006;16(Suppl 1):S35–8. doi: 10.1016/j.numecd.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 35.Fisher SD, Miller TL, Lipshultz SE. Impact of HIV and highly active antiretroviral therapy on leukocyte adhesion molecules, arterial inflammation, dyslipidemia, and atherosclerosis. Atherosclerosis. 2006;185:1–11. doi: 10.1016/j.atherosclerosis.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 36.Lipshultz SE, Lipsitz SR, Hinkle AS, et al. Cardiovascular status, subsequent risk, and associated factors in long-term survivors of childhood cancer in a population-based NCI study. Circulation. 2005;112:II–476. [Google Scholar]
- 37.Oeffinger KC, Buchanan GR, Eshelman DA, et al. Cardiovascular risk factors in young adult survivors of acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2001;23:424–430. doi: 10.1097/00043426-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Link K, Moell C, Garwicz S, et al. Growth hormone deficiency predicts cardiovascular risk in young adults treated for acute lymphoblastic leukemia in childhood. J Clin Endocrinol Metab. 2004;89:5003–5012. doi: 10.1210/jc.2004-0126. [DOI] [PubMed] [Google Scholar]
- 39.Meacham LR, Chow EJ, Ness KK, et al. Cardiovascular risk factors in adult survivors of pediatric cancer--a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2010;19:170–81. doi: 10.1158/1055-9965.EPI-09-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Caro E, Fioredda F, Calevo MG, et al. Exercise capacity in apparently healthy survivors of cancer. Arch Dis Child. 2006;91:47–51. doi: 10.1136/adc.2004.071241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adams MJ, Lipsitz SR, Colan SD, et al. Cardiovascular status in long-term survivors of Hodgkin’s disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–48. doi: 10.1200/JCO.2004.09.109. [DOI] [PubMed] [Google Scholar]
- 42.Miller TL, Horgan S, Lipshultz SE. Exercise rehabilitation of pediatric patients with cardiovascular disease. Prog Pediatr Cardiol. 2005;20:27–37. [Google Scholar]
- 43.Grigg AP, McLachlan R, Zaja J, et al. Reproductive status in long-term bone marrow transplant survivors receiving busulfan-cyclophosphamide (120 mg/kg) Bone Marrow Transplant. 2000;26:1089–95. doi: 10.1038/sj.bmt.1702695. [DOI] [PubMed] [Google Scholar]
- 44.Simbre VC, Duffy SA, Dadlani GH, et al. Cardiotoxicity of cancer chemotherapy: implications for children. Paediatr Drugs. 2005;7:187–202. doi: 10.2165/00148581-200507030-00005. [DOI] [PubMed] [Google Scholar]
- 45.Wojcik D, Barq E, Niedzielska E, et al. Analysis of some risk factors for abnormal growth velocity in children treated with haematopoetic stem cell transplantation. Med Wieku Rozwoi. 2006;10:841–8. [PubMed] [Google Scholar]
- 46.Okunieff P, Barrett AJ, Phang SE, et al. Circulating basic fibroblast growth factor declines during CyTBI bone marrow transplantation. Bone Marrow Transplant. 1999;23:1117–21. doi: 10.1038/sj.bmt.1701778. [DOI] [PubMed] [Google Scholar]
- 47.Kim Y, Brown TP, Pantin-Jackwood MJ. Lesions induced in broiler chickens by cyclophosphamide treatment. Vet Hum Toxicol. 2003;45:121–3. [PubMed] [Google Scholar]
- 48.Xian CJ, Cool JC, van Gangelen J, et al. Effects of etoposide and cyclophosphamide acute chemotherapy on growth plate and metaphyseal bone in rats. Cancer Bill Ther. 2006;6:e1–e8. doi: 10.4161/cbt.6.2.3576. [DOI] [PubMed] [Google Scholar]
- 49.Zilberman O, Nasman M, Forsberg C-M, et al. Effects of cyclophosphamide on the femoral epiphyseal growth plate in young Sprague-Dawley rats. Acta Odontologica Scandinavica. 2002;60:208–12. doi: 10.1080/000163502760147963. [DOI] [PubMed] [Google Scholar]
- 50.Lespine A, Azema C, Gafvels M, et al. Lipoprotein lipase regulation in the cyclophosphamide-treated rabbit: dependence on nutritional status. J Lipid Res. 1993;34:23–36. [PubMed] [Google Scholar]
- 51.Lespine A, Chap H, Perret B. Impaired secretion of heart lipoprotein lipase in cyclophosphamide-treated rabbit. Biochim Biophys Acta. 1997;1345:77–85. doi: 10.1016/s0005-2760(96)00167-1. [DOI] [PubMed] [Google Scholar]