Abstract
Background
Heart failure (HF) is associated with poor cardiac outcomes and mortality. It is not known if HF leads to poor renal outcomes in patients with normal kidney function. We hypothesized that HF is associated with worse long-term renal outcomes.
Methods and Results
Among 3,570,865 US veterans with estimated glomerular filtration rate (eGFR) ≥60ml/min/1.73m2 during October 1, 2004 to September 30, 2006, we identified 156,743 with an ICD-9 diagnosis of HF. We examined the association of HF with incident chronic kidney disease (CKD), the composite of incident CKD or mortality, and rapid rate of eGFR decline (slopes steeper than −5 ml/min/1.73m2/year) using Cox proportional hazard analyses and logistic regression. Adjustments were made for various confounders. The mean ± standard deviation baseline age and eGFR of HF patients were 68±11 years and 78±14 ml/min/1.73m2 and in patients without HF were 59±14 years and 84±16 ml/min/1.73m2, respectively. HF patients had higher prevalence of hypertension, diabetes, cardiac, peripheral vascular and chronic lung diseases, stroke, and dementia. Incidence of CKD was 69.0/1000 patient years (PY) in HF patients vs. 14.5/1000PY in patients without HF, and 22% of patients with HF had rapid decline in eGFR compared to 8.5% in patients without HF. HF patients had a 2.12-fold, 2.06-fold and 2.13-fold higher multivariable adjusted risk of incident CKD, composite of CKD or mortality and rapid eGFR decline respectively.
Conclusions
HF is associated with significantly higher risk of incident CKD, incident CKD or mortality and rapid eGFR decline. Early diagnosis and management of HF could help reduce the risk of long-term renal complications.
Keywords: Heart failure, chronic kidney disease, outcomes, renal function, mortality, glomerular filtration rate
Journal Subject Terms: Heart Failure, Chronic Kidney Disease
Concurrent renal dysfunction and heart disease is an inter-dependent phenomenon secondary to (among others) changes in hemodynamics,1, 2 and is associated with increased mortality and morbidity.3, 4 Heart failure (HF) and acute myocardial infarction increases the risk of 30-day readmission,5, 6 progression to end stage renal disease (ESRD) 7 and mortality in patients with chronic kidney disease (CKD).8 Of all HF patients admitted to a hospital, those with worsening kidney function on follow-up have higher mortality and recurrent hospital admissions.9, 10 Several studies have evaluated the effects of HF in patients with CKD,11–13 but to the best of our knowledge the risk of incident CKD and progressive loss of kidney function in patients with HF and normal kidney function has not been examined, and it is unclear if interventions aimed at preventing or treating HF in patients with normal kidney function could be useful to prevent the development of de novo CKD. We hypothesized that HF in patients with normal baseline kidney function is associated with higher risk of long-term adverse renal outcomes.
Methods
Cohort Definition
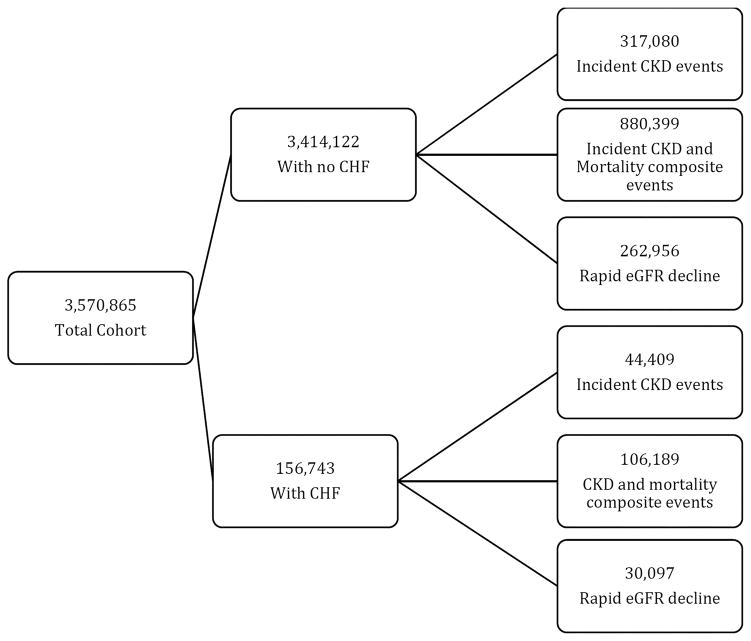
Our study utilized data from a cohort study examining risk factors and outcomes in patients with incident chronic kidney disease (CKD; Racial and Cardiovascular Risk Anomalies in CKD (RCAV) study), as previously described.14–16 Briefly, we identified 3,570,865 US veterans with estimated glomerular filtration rate (eGFR) of ≥60 ml/min/1.73m2 during October 1, 2004–September 30, 2006, calculated according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.17 Out of this cohort, 156,743 patients with a diagnosis of CHF were identified during the same time period using International Classification of Diseases, Ninth Revision (ICD-9) diagnostic codes (Figure 1). Cohort entry was defined as the first date of eGFR ≥60 ml/min/1.73m2 during October 1, 2004–September 30, 2006. Baseline characteristics including income level, body mass index (BMI), blood pressure (BP), ICD 9 code for personal history of noncompliance with medical treatment, service-connectedness (a measure indicating whether one or more of a patient’s comorbidities were caused by their military service, resulting in certain privileges such as preferential access to care and lower copayments), comorbid conditions and laboratory characteristics were obtained as previously described.18, 19 Medication compliance was estimated as the percentage of days a subject had medication available (proportion of days covered, PDC), based on medication dispensation records from any Veteran Administration pharmacy. PDC was calculated as the ratio of the total number of pills and the number of days between the first fill of the medication and the end of the evaluation period.19 Information about race was supplemented from Medicare through the Veterans Administration (VA) -Medicare data merge project. Data on medication exposure throughout the entire follow-up period was collected from VA pharmacy dispensation records. Data on mortality was obtained from the VA Vital Status Files, which contain dates of death or last medical/administrative encounter from all sources in the VA system with sensitivity and specificity of 98.3% and 99.8%, compared to the National Death Index as gold standard.20
Figure 1.
Flow chart of patient selection
Outcomes
We defined three different outcomes: 1) incident CKD, 2) composite of incident CKD or mortality, and 3) rate of kidney function decline. Incident CKD was defined as two eGFR values of <60ml/min/1.73m2 occurring ≥3 months apart and a decrease from baseline eGFR of at least 25%. 21, 22 The 25% drop was measured from the baseline eGFR to the second of the two eGFR values used to define CKD. A rapid rate of eGFR decline was defined as slopes of longitudinal eGFR (calculated by ordinary least squares regressions for individual patients from minimum of three eGFR values steeper than −5 ml/min/1.73m2/year.21
Statistical Analysis
Descriptive analyses were performed and skewed variables were log-transformed. Data were summarized using proportions, means (±standard deviation, SD) or medians ( 25th – 75th percentile) as appropriate. Due to the large sample size traditional comparisons were all statistically significant, and we hence determined significance based on biologically relevant differences. The association of HF with incident CKD and with the composite of incident CKD or mortality was assessed using unadjusted and multivariable adjusted Cox proportional hazard models and the association of HF with rapid decline in eGFR was assessed using unadjusted and multivariable adjusted logistic regression models (for slope as a categorical variable) and linear regression models (for slope as a continuous variable). All models were adjusted sequentially for the following confounders based on a priori considerations: model 1: unadjusted; model 2: age, gender, race/ethnicity; model 3: model 2 variables and marital status, income level, service connection and non-compliance; model 4: model 3 variables and diabetes mellitus, hypertension, cardiovascular disease, peripheral arterial disease, lung disease and malignancy; model 5: model 4 variables and body mass index (BMI), systolic and diastolic BP; model 6: model 5 variables and eGFR. Medications which could be initiated in response to HF (angiotensin converting enzyme inhibitors, statins, beta blockers, calcium channel blockers, vasodilators, thiazide diuretic, loop diuretic, potassium sparing diuretics, anticoagulants, anti-platelet agents, digoxin and inotropes), and which could act as effect mediators, were not included in the main multivariable analyses, but adjustment for these medications was performed in sensitivity analyses (Model 7).
78.5% of patients in the final multivariable model had complete data for analysis. Missing data was not imputed.
Analyses were repeated in subgroups of patients divided by age, race, presence or absence of diabetes and cardiovascular disease, systolic blood pressure level, use of inotropes, anticoagulants and loop diuretics. Statistical analyses were performed using STATA MP Versions 13 and 14 (STATA Corporation, College Station, Texas). The study protocol with waiver of informed consent was approved by the Institutional Review Boards at the Memphis and Long Beach VA Medical Centers and complied with Declaration of Helsinki.
Results
Baseline characteristics
Baseline characteristics of patients categorized by HF status are shown in Table 1. HF patients were older, had lower baseline eGFR, and higher prevalence of various comorbid conditions. Medication use was higher, and medication compliance was lower in HF patients.
Table 1.
Baseline characteristics of HF patients and patients without HF.
| HF (N=156,743) | No HF (N=3,414,122) | |
|---|---|---|
| Demographics | ||
| Age (years) | 68 ± 11 | 59 ± 14 |
| Gender (% male) | 98 | 93 |
| Race (% black) | 16 | 17 |
| Marital Status | ||
| Married (%) | 54 | 56 |
| Single | 7 | 11 |
| Divorced | 26 | 26 |
| Widowed | 13 | 7 |
| Income ($) | 19,859 (11,225–31,460) | 23,129 (11,752–36,490) |
| Service connection (%) | 36 | 40 |
| Medication use (%) | ||
| Statin | 68 | 53 |
| ACEI/ARB | 87 | 50 |
| Beta blocker | 84 | 42 |
| CCB | 44 | 31 |
| Vasodilator | 14 | 4 |
| Thiazide | 33 | 32 |
| Loop diuretic | 79 | 16 |
| Potassium sparing diuretic | 32 | 8 |
| Anticoagulation | 37 | 17 |
| Antiplatelet agents | 16 | 9 |
| Digoxin | 35 | 4 |
| Inotropic agent | 3 | 0 |
| Comorbidities (%) | ||
| Medication compliance | 89 | 94 |
| Hypertension | 82 | 58 |
| Diabetes Mellitus | 45 | 23 |
| CVD | 42 | 10 |
| CVA | 15 | 6 |
| PAD | 16 | 5 |
| Chronic lung disease | 43 | 17 |
| Dementia | 2 | 1 |
| Rheumatologic disease | 2 | 1 |
| Liver disease | 2 | 1 |
| Malignancy | 16 | 10 |
| HIV | 0 | 1 |
| Depression | 9 | 9 |
| Vital signs | ||
| SBP (mmHg) | 129±15 | 133±14 |
| DBP (mmHg) | 71±9 | 76±9 |
| BMI (kg/m2) | 30.4±7.2 | 29.1±5.6 |
| Laboratory Findings | ||
| eGFR (ml/min/1.73m2) | 78±14 | 84±16 |
| Serum BNP (ng/l) | 262 (84–728) | 15 (1–74) |
| Serum Cholesterol (mg/dl) | 163±38 | 183±38 |
| Serum Sodium (mmol/l) | 139±3 | 139±3 |
| Serum Potassium (mmol/l) | 4.3±0.3 | 4.3±0.4 |
| Serum Bicarbonate (mmol/l) | 28±3 | 28±3 |
| Serum BUN (mg/dl) | 19±8 | 15±5 |
Data presented as mean ± standard deviation or median and interquartile range. Abbreviations: HF- heart failure; ACEI – angiotensin converting enzyme inhibitors; ARB – angiotensin receptor blocker; CCB – calcium channel blocker; CVD – cardiovascular disease; CVA – cerebrovascular accident; PAD – peripheral arterial disease; SBP – systolic blood pressure; DBP – diastolic blood pressure; BMI – body mass index; BNP – brain natriuretic peptide; BUN – blood urea nitrogen; SD – standard deviation.
Due to the large sample size traditional comparisons were all statistically significant, and we hence determined significance based on biologically relevant differences.
Incident CKD
A total of 361,488 incident CKD events occurred at an event rate of 16.04/1000 patient-years (PY) (95% confidence interval (CI): 15.98 to 16.09) over a median follow-up of 7.6 years. The CKD incidence in HF patients was higher at 68.90/1000PY (95%CI: 68.22 to 69.51) compared to 14.48/1000PY (95%CI: 14.43 to 14.53) in patients without HF.
The crude and multivariable adjusted hazard ratios (HR) of incident CKD associated with HF were 4.30 (95% CI 4.26 to 4.35) and 2.12 (95% CI 2.10 to 2.14) respectively (Table 2). The association of HF with higher risk of incident CKD was present in all examined subgroups (Figure 2). The association between HF and incident CKD was attenuated, but remained significant after adjustment for medication use (Table 2).
Table 2.
Multivariable adjusted models, showing the risk of various outcomes associated with CHF status.
| Model number (N) | Incident CKD (HR, 95% CI) | Incident CKD or Mortality (HR, 95% CI) | Rapid Decline in eGFR (OR, 95% CI) | Slopes of eGFR (Coefficient, 95% CI) |
|---|---|---|---|---|
| Model 1 (3,212,315) | 4.30 (4.26–4.35) | 3.96 (3.94–3.99) | 2.96 (2.92–3.00) | −1.53 (−1.51 to −1.56) |
| Model 2 (2,941,052) | 3.21 (3.18–3.24) | 2.79 (2.78–2.81) | 2.55 (2.52–2.59) | −1.28 (−1.26 to −1.31) |
| Model 3 (2,693,381) | 3.00 (2.96–3.03) | 2.65 (2.63–2.67) | 2.45 (2.42–2.49) | −1.22 (−1.20 to −1.25) |
| Model 4 (2,693,381) | 2.13 (2.10–2.15) | 2.07 (2.05–2.08) | 2.02 (1.99–2.06) | −1.01 (−0.99 to −1.03) |
| Model 5 (2,599,864) | 2.14 (2.12–2.17) | 2.06 (2.05–2.08) | 2.06 (2.03–2.09) | −1.03 (−1.01 to −1.06) |
| Model 6 (2,599,864) | 2.12 (2.10–2.14) | 2.06 (2.05–2.08) | 2.13 (2.10–2.17) | −1.09 (−1.07 to −1.11) |
| Model 7 (2,599,864) | 1.34 (1.32–1.36) | 1.41 (1.39–1.42) | 1.41 (1.39–1.44) | −0.62 (−0.59 to −0.64) |
Model 1: unadjusted; Model 2: baseline age, gender, race/ethnicity; Model 3: model 2 variables and marital status, income level, service connection and non-compliance; Model 4: model 3 variables and diabetes mellitus, hypertension, cardiovascular disease, peripheral arterial disease, lung disease and malignancy; Model 5: model 4 variables and body mass index, systolic blood pressure and diastolic blood pressure; Model 6 (main multivariable model): model 5 variables and eGFR; Model 7 (adjustment for medication use): model 6 variables and use of ACE inhibitors, statins, beta blockers, calcium channel blockers, vasodilators, thiazide diuretic, loop diuretic, potassium sparing diuretics, anticoagulants, anti-platelet agents, digoxin and inotropes.. N denotes total number of observation in each model.
Figure 2.
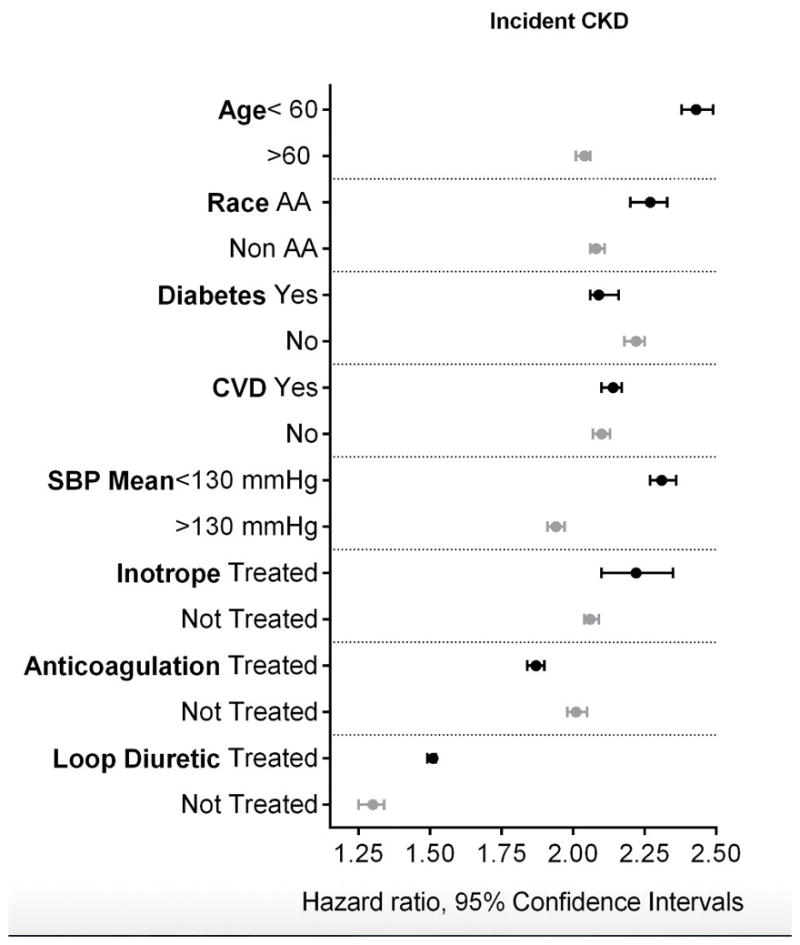
Association between HF and the risk of incident CKD in various subgroups. Adjusted for baseline age, gender, race/ethnicity, marital status, income level, service connection and non-compliance, diabetes mellitus, hypertension, cardiovascular disease, peripheral arterial disease, lung disease and malignancy, body mass index, systolic blood pressure and diastolic blood pressure and eGFR. The light and dark markers denote the hazard ratio and 95% confidence intervals of two categories of the same variable.
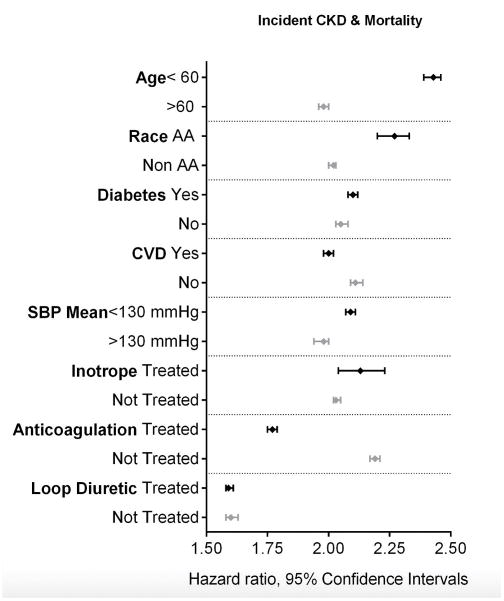
Composite of incident CKD or mortality
A total of 986,588 composite events of CKD or mortality were identified. The incidence of the composite end point in HF patients was higher at 164.66/1000 patient years (95%CI: 163.67 to 165.65) compared to 40.20/1000 patient years (95%CI: 40.12 to 40.29) in patients without HF.
The crude and multivariable adjusted hazard ratios of the composite outcome associated with HF vs. no HF was 3.96 (95%CI: 3.94 to 3.99) and 2.06 (95%CI: 2.05 to 2.08), respectively (Table 2). The association of HF with the composite outcome remained significant in all the studied subgroups (Figure 3). The association between HF and the composite of incident CKD or mortality was attenuated, but remained significant after adjustment for medication use (Table 2).
Figure 3.
Association between HF and the risk of the composite endpoint of incident CKD or mortality in various subgroups. Adjusted for baseline age, gender, race/ethnicity, marital status, income level, service connection and non-compliance, diabetes mellitus, hypertension, cardiovascular disease, peripheral arterial disease, lung disease and malignancy, body mass index, systolic blood pressure and diastolic blood pressure and eGFR. The light and dark markers denote the hazard ratio and 95% confidence intervals of two categories of the same variable.
Rapid decline in eGFR
The prevalence of rapid eGFR decline was 9.12%. Among patients with and without HF 21.69% and 8.56% had a rapid decline in eGFR, respectively. The crude and adjusted odds ratios of rapid decline in eGFR were 2.96 (95%CI 2.92 to 3.00) and 2.13 (95%CI 2.10 to 2.17), respectively (Table 2). The association of HF with rapid decline in eGFR remained significant in all examined subgroups (Figure 4). The association between HF and the risk of rapid loss of kidney function was attenuated, but remained significant after adjustment for medication use (Table 2).
Figure 4.
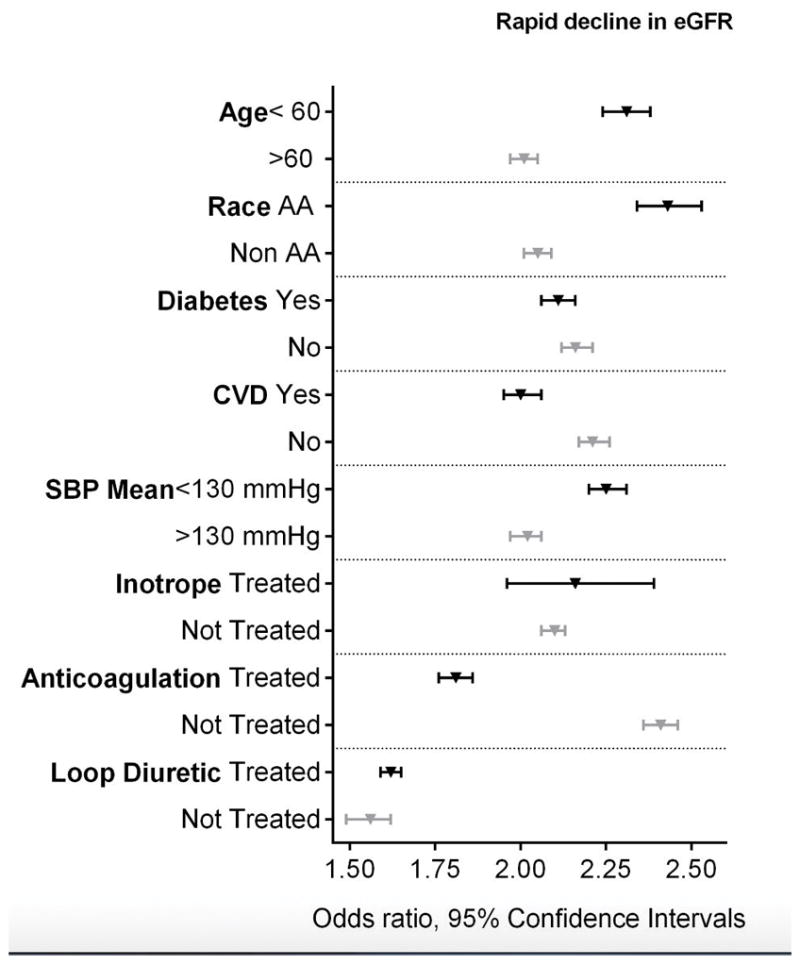
Association between HF and the risk of rapid decline in eGFR (≥5 ml/min/1.73m2/year) in various subgroups. Adjusted for baseline age, gender, race/ethnicity, marital status, income level, service connection and non-compliance, diabetes mellitus, hypertension, cardiovascular disease, peripheral arterial disease, lung disease and malignancy, body mass index, systolic blood pressure and diastolic blood pressure and eGFR. The light and dark markers denote the odds ratio and 95% confidence intervals of two categories of the same variable.
Discussion
We describe an association between HF and a significantly higher risk of new onset chronic kidney disease (incident CKD) and rapid decline in kidney function among a large cohort of patients with normal baseline eGFR. The risk of the composite end point of incident CKD or mortality was also significantly higher in patients with HF.
There are several potential biological explanations for the association between HF and kidney disease. Among patients with preexisting CKD, the presence of HF has been linked with poorer outcomes, including significant worsening of kidney function.23–28 HF is characterized by numerous pathophysiologic abnormalities, which could lead to long-term kidney damage. In addition to hemodynamic mechanisms such as low cardiac output and increased renal venous pressure, complex neurohomonal changes such as activation of the sympathetic nervous system and the renin angiotensin system, increased oxidative stress, and inflammatory activation can affect renal blood flow and glomerular perfusion.29, 30 Further contributing to potential renal damage are recurrent episodes of acute kidney injury 31 and potential direct nephrotoxicity of medications received during the course of HF. 32 Much less is known about the effects of HF on long-term kidney function in patients with normal eGFR, but the above mentioned pathophysiologic changes postulated to cause poorer renal outcomes in those with preexisting CKD could also be instrumental in engendering new onset kidney damage and incident CKD. It is thought that the elevated renal G-Protein Coupled Receptor – G protein βγ (GPCR-Gβγ) signaling and endothelin system expression seen in heart failure may cause renal tissue damage, fibrosis and inflammation, which can manifest as acute renal failure and cardiorenal syndrome 2. 33
The concomitant presence of HF and CKD has also been associated with a higher risk of mortality.13, 27, 34 The putative pathophysiological mechanism for the increased mortality are similar to the ones responsible for kidney damage, including exaggerated neurohormonal activation and oxidative stress;30 these, along with diffuse atherosclerosis especially in cases of ischemic cardiomyopathy and vascular calcification, can result in increased risk of cardiovascular events and mortality.35, 36
Treatment strategies aimed at preventing and treating HF may prevent worsening kidney function and mortality in these patients. Angiotensin converting enzyme inhibitors, angiotensin receptor antagonists and aldosterone inhibitors have been shown to reduce the risk of mortality in patients with HF in numerous studies,24, 37–41 but the renoprotective effect of these agents in this population is not well validated. Slight worsening of kidney function is often seen at initiation of therapy with renin-angiotensin-aldosterone system inhibitors, but the progressive decline in kidney function and the severity of proteinuria is mitigated upon continuation of these medications.42 It is therefore possible that early interventions aimed at treating HF could also help prevent the development of CKD and progressive loss of kidney function. Though we cannot make practical recommendations on the beneficial effect of early treatment based on our study, this should stimulate further research. This hypothesis will have to be proven in dedicated randomized controlled clinical trials.
Strengths and limitations
The strength of this study is in its scale and rigorous follow up in a closed patient care system, and the inclusion of veterans from the entire United States. Our study also has limitations. This being an observational study, we can only report associations, and we cannot claim that HF was indeed the cause of the worse renal outcomes. Additionally, models could only be adjusted for confounders for which we had available data, and we cannot rule out residual confounding from unobserved variables. The study population consisted of mostly male patients; hence, the results may not be generalizable to females. We used ICD-9 codes to define HF, and hence may have misclassified patients with HF who did not have a diagnostic code entered in their record. However, such misclassification would have biased results towards the null. We did not have access to information about the severity of HF (New York Heart Association class), etiology of heart failure, presence of cardiac resynchronization therapy or implantable cardiac defibrillator device, average 24 hour ambulatory heart rate or blood pressure readings, heart transplant status, left ventricular assist device support, and the baseline left ventricular ejection fraction (LVEF). Hence, we could not assess the presence or absence of a graded relationship between its severity and the various outcomes, and more importantly the differential impact of heart failure with preserved or reduced LVEF on the outcomes. Finally, we did not have information about proteinuria, hematuria or evidence of structural or pathological changes in kidneys; consequently the diagnosis of CKD is less accurate and patients with proteinuria and normal eGFR (eGFR ≥60 ml/min/1.73m2) may be misclassified as not having CKD.
Conclusions
Heart failure is associated with significantly higher risk of incident CKD, incident CKD or mortality and more rapid GFR decline. Early diagnosis and management of HF or its risk factors may have the potential to reduce the risk of long-term renal complications. The benefits of such strategies will have to be examined in randomized clinical trials.
Clinical Perspective.
What’s New
This article describes an association between HF and a high risk of new onset CKD and rapid decline in kidney function among a large cohort of patients with normal baseline estimated glomerular filtration rate (eGFR).
The risk of the composite end point of incident CKD or mortality was also significantly higher in patients with HF.
What are the Clinical Implications
Both patients and their treating physicians need to be aware that HF could hasten the development of CKD, and could lead to faster decline in kidney function even in patients with normal eGFR.
It is thus important that kidney function be monitored closely in patients with HF, and potentially nephrotoxic exposures (e.g. non-steroidal anti-inflammatory medications, radio-contrast agents) are minimized or avoided.
Though this observational study shows increased risk for CKD and rapid decline in renal function in patients with HF, prospective studies are required to look into the effects of early reno-protective strategies in these patients
Acknowledgments
We thank Praveen Potukuchi, B Pharm, MSc, MS, for his help with preparing figures.
Sources of Funding: This work was supported by grant R01DK096920 to CPK and KKZ and is the result of work supported with resources and the use of facilities at the Memphis VA Medical Center and the Long Beach VA Medical Center. Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004).
Footnotes
Conflict of Interest Disclosures: No industry support or industry affiliation pertaining to this study. Dr. Kalantar-Zadeh reports personal fees from Abbott, personal fees from Abbvie, non-financial support from DaVita, personal fees from Fresenius, personal fees from Genentech, personal fees from Genzyme/Sanofi, personal fees from Hospira, personal fees from Keryx, personal fees from Amgen, personal fees from Shire, personal fees from Vifor, grants from NIH, outside the submitted work
References
- 1.Leithe ME, Margorien RD, Hermiller JB, Unverferth DV, Leier CV. Relationship between central hemodynamics and regional blood flow in normal subjects and in patients with congestive heart failure. Circulation. 1984;69:57–64. doi: 10.1161/01.cir.69.1.57. [DOI] [PubMed] [Google Scholar]
- 2.Ljungman S, Laragh JH, Cody RJ. Role of the kidney in congestive heart failure. Relationship of cardiac index to kidney function. Drugs. 1990;39(Suppl 4):10–21. doi: 10.2165/00003495-199000394-00004. discussion 22–4. [DOI] [PubMed] [Google Scholar]
- 3.Loffler AI, Cappola TP, Fang J, Hetzel SJ, Kadlec A, Astor B, Sweitzer NK. Effect of renal function on prognosis in chronic heart failure. Am J Cardiol. 2015;115:62–8. doi: 10.1016/j.amjcard.2014.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McClellan WM, Langston RD, Presley R. Medicare patients with cardiovascular disease have a high prevalence of chronic kidney disease and a high rate of progression to end-stage renal disease. J Am Soc Nephrol. 2004;15:1912–9. doi: 10.1097/01.asn.0000129982.10611.4c. [DOI] [PubMed] [Google Scholar]
- 5.Ross JS, Chen J, Lin Z, Bueno H, Curtis JP, Keenan PS, Normand SL, Schreiner G, Spertus JA, Vidan MT, Wang Y, Wang Y, Krumholz HM. Recent national trends in readmission rates after heart failure hospitalization. Circ Heart Fail. 2010;3:97–103. doi: 10.1161/CIRCHEARTFAILURE.109.885210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heidenreich PA, Sahay A, Kapoor JR, Pham MX, Massie B. Divergent Trends in Survival and Readmission Following a Hospitalization for Heart Failure in the Veterans Affairs Health Care System 2002 to 2006. J Am Coll Cardiol. 2010;56:362–368. doi: 10.1016/j.jacc.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 7.Sud MTN, Pintilie M, Levey A, Naimark DM. ESRD and death after heart failure in CKD. J Am Soc Nephrol. 2015;26:715–22. doi: 10.1681/ASN.2014030253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang W, Xie D, Anderson AH, Joffe MM, Greene T, Teal V, Hsu CY, Fink JC, He J, Lash JP, Ojo A, Rahman M, Nessel L, Kusek JW, Feldman HI Investigators CS. Association of kidney disease outcomes with risk factors for CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2014;63:236–43. doi: 10.1053/j.ajkd.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damman K, Jaarsma T, Voors AA, Navis G, Hillege HL, van Veldhuisen DJ. Both in- and out-hospital worsening of renal function predict outcome in patients with heart failure: results from the Coordinating Study Evaluating Outcome of Advising and Counseling in Heart Failure (COACH) Eur J Heart Fail. 2009;11:847–54. doi: 10.1093/eurjhf/hfp108. [DOI] [PubMed] [Google Scholar]
- 10.Bansal N, Hyre Anderson A, Yang W, Christenson RH, deFilippi CR, Deo R, Dries DL, Go AS, He J, Kusek JW, Lash JP, Raj D, Rosas S, Wolf M, Zhang X, Shlipak MG, Feldman HI. High-Sensitivity Troponin T and N-Terminal Pro-B-Type Natriuretic Peptide (NT-proBNP) and Risk of Incident Heart Failure in Patients with CKD: The Chronic Renal Insufficiency Cohort (CRIC) Study. J Am Soc Nephrol. 2015;26(4):946–56. doi: 10.1681/ASN.2014010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beck H, Titze SI, Hübner S, Busch M, Schlieper G, Schultheiss UT, Wanner C, Kronenberg F, Krane V, Eckardt K-U, Köttgen A Investigators G. Heart Failure in a Cohort of Patients with Chronic Kidney Disease: The GCKD Study. PLoS One. 2015;10(4):e0122552. doi: 10.1371/journal.pone.0122552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson SE, Shroff GR, Li S, Herzog CA. Impact of Chronic Kidney Disease on Risk of Incident Atrial Fibrillation and Subsequent Survival in Medicare Patients. J Am Heart Assoc. 2012;1(4):e002097. doi: 10.1161/JAHA.112.002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Payne J, Sharma S, De Leon D, Lu JL, Alemu F, Balogun RA, Malakauskas SM, Kalantar-Zadeh K, Kovesdy CP. Association of echocardiographic abnormalities with mortality in men with non-dialysis-dependent chronic kidney disease. Nephrol Dial Transplant. 2012;27:694–700. doi: 10.1093/ndt/gfr282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.George LK, Molnar MZ, Lu JL, Kalantar-Zadeh K, Koshy SK, Kovesdy CP. Association of Pre-Operative Albuminuria with Post-Operative Outcomes after Coronary Artery Bypass Grafting. Sci Rep. 2015;5:16458. doi: 10.1038/srep16458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovesdy CP, Norris KC, Boulware LE, Lu JL, Ma JZ, Streja E, Molnar MZ, Kalantar-Zadeh K. Association of Race With Mortality and Cardiovascular Events in a Large Cohort of US Veterans. Circulation. 2015;132:1538–48. doi: 10.1161/CIRCULATIONAHA.114.015124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu JL, Molnar MZ, Naseer A, Mikkelsen MK, Kalantar-Zadeh K, Kovesdy CP. Association of age and BMI with kidney function and mortality: a cohort study. Lancet Diabetes Endocrinol. 2015;3:704–14. doi: 10.1016/S2213-8587(15)00128-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovesdy CP, Lott EH, Lu JL, Malakauskas SM, Ma JZ, Molnar MZ, Kalantar-Zadeh K. Outcomes associated with microalbuminuria: effect modification by chronic kidney disease. J Am Coll Cardiol. 2013;61:1626–33. doi: 10.1016/j.jacc.2012.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gosmanova EO, Lu JL, Streja E, Cushman WC, Kalantar-Zadeh K, Kovesdy CP. Association of medical treatment nonadherence with all-cause mortality in newly treated hypertensive US veterans. Hypertension. 2014;64:951–7. doi: 10.1161/HYPERTENSIONAHA.114.03805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 22.Stevens PE, Levin A Members KDIGOCKDGDWG. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–30. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 23.Lofman I, Szummer K, Hagerman I, Dahlstrom U, Lund LH, Jernberg T. Prevalence and prognostic impact of kidney disease on heart failure patients. Open Heart. 2016;3:e000324. doi: 10.1136/openhrt-2015-000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damman K, Valente MA, Voors AA, O’Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014;35:455–69. doi: 10.1093/eurheartj/eht386. [DOI] [PubMed] [Google Scholar]
- 25.Kajimoto K, Sato N, Takano T. eGFR and Outcomes in Patients with Acute Decompensated Heart Failure with or without Elevated BUN. Clin J Am Soc Nephrol. 2016;11(3):405–12. doi: 10.2215/CJN.08210815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma K, Hill T, Grams M, Daya NR, Hays AG, Fine D, Thiemann DR, Weiss RG, Tedford RJ, Kass DA, Schulman SP, Russell SD. Outcomes and worsening renal function in patients hospitalized with heart failure with preserved ejection fraction. Am J Cardiol. 2015;116:1534–40. doi: 10.1016/j.amjcard.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 27.Khan NA, Ma I, Thompson CR, Humphries K, Salem DN, Sarnak MJ, Levin A. Kidney function and mortality among patients with left ventricular systolic dysfunction. J Am Soc Nephrol. 2006;17:244–53. doi: 10.1681/ASN.2005030270. [DOI] [PubMed] [Google Scholar]
- 28.Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35:681–9. doi: 10.1016/s0735-1097(99)00608-7. [DOI] [PubMed] [Google Scholar]
- 29.Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, Hampton JR, van Veldhuisen DJ. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102:203–10. doi: 10.1161/01.cir.102.2.203. [DOI] [PubMed] [Google Scholar]
- 30.Metra M, Cotter G, Gheorghiade M, Cas LD, Voors AA. The role of the kidney in heart failure. Eur Heart J. 2012;33(17):2135–42. doi: 10.1093/eurheartj/ehs205. [DOI] [PubMed] [Google Scholar]
- 31.Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–96. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunner-La Rocca HP, Knackstedt C, Eurlings L, Rolny V, Krause F, Pfisterer ME, Tobler D, Rickenbacher P, Maeder MT. Impact of worsening renal function related to medication in heart failure. Eur J Heart Fail. 2015;17:159–68. doi: 10.1002/ejhf.210. [DOI] [PubMed] [Google Scholar]
- 33.Kamal FA, Travers JG, Schafer AE, Ma Q, Devarajan P, Blaxall BC. G Protein-Coupled Receptor-G-Protein betagamma-Subunit Signaling Mediates Renal Dysfunction and Fibrosis in Heart Failure. J Am Soc Nephrol. 2017;28(1):197–208. doi: 10.1681/ASN.2015080852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Silva R, Nikitin NP, Witte KK, Rigby AS, Goode K, Bhandari S, Clark AL, Cleland JG. Incidence of renal dysfunction over 6 months in patients with chronic heart failure due to left ventricular systolic dysfunction: contributing factors and relationship to prognosis. Eur Heart J. 2006;27:569–81. doi: 10.1093/eurheartj/ehi696. [DOI] [PubMed] [Google Scholar]
- 35.Palazzuoli A, Ruocco G, Pellegrini M, Martini S, Del Castillo G, Beltrami M, Franci B, Lucani B, Nuti R. Patients with cardiorenal syndrome revealed increased neurohormonal activity, tubular and myocardial damage compared to heart failure patients with preserved renal function. Cardiorenal Med. 2014;4:257–68. doi: 10.1159/000368375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smilde TD, Hillege HL, Navis G, Boomsma F, de Zeeuw D, van Veldhuisen DJ. Impaired renal function in patients with ischemic and nonischemic chronic heart failure: association with neurohormonal activation and survival. Am Heart J. 2004;148:165–72. doi: 10.1016/j.ahj.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Logeart D, Tabet JY, Hittinger L, Thabut G, Jourdain P, Maison P, Tartiere JM, Solal AC. Transient worsening of renal function during hospitalization for acute heart failure alters outcome. Int J Cardiol. 2008;127:228–32. doi: 10.1016/j.ijcard.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Molnar MZ, Kalantar-Zadeh K, Lott EH, Lu JL, Malakauskas SM, Ma JZ, Quarles DL, Kovesdy CP. ACE Inhibitor and Angiotensin Receptor Blocker Use and Mortality in Patients with Chronic Kidney Disease. J Am Coll Cardiol. 2014;63:650–658. doi: 10.1016/j.jacc.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chin KL, Collier TJ, Pitt B, McMurray JJ, Swedberg K, van Veldhuisen DJ, Pocock SJ, Vincent J, Turgonyi E, Zannad F, Krum H. Aspirin does not reduce the clinical benefits of the mineralocorticoid receptor antagonist eplerenone in patients with systolic heart failure and mild symptoms: an analysis of the EMPHASIS-HF study. Eur J Heart Fail. 2016;18(9):1175–81. doi: 10.1002/ejhf.485. [DOI] [PubMed] [Google Scholar]
- 40.Clark H, Krum H, Hopper I. Worsening renal function during renin-angiotensin-aldosterone system inhibitor initiation and long-term outcomes in patients with left ventricular systolic dysfunction. Eur J Heart Fail. 2014;16:41–8. doi: 10.1002/ejhf.13. [DOI] [PubMed] [Google Scholar]
- 41.Rossignol P, Menard J, Fay R, Gustafsson F, Pitt B, Zannad F. Eplerenone survival benefits in heart failure patients post-myocardial infarction are independent from its diuretic and potassium-sparing effects. Insights from an EPHESUS (Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study) substudy. J Am Coll Cardiol. 2011;58:1958–66. doi: 10.1016/j.jacc.2011.04.049. [DOI] [PubMed] [Google Scholar]
- 42.Lambers Heerspink HJ, Weldegiorgis M, Inker LA, Gansevoort R, Parving HH, Dwyer JP, Mondal H, Coresh J, Greene T, Levey AS, de Zeeuw D. Estimated GFR decline as a surrogate end point for kidney failure: a post hoc analysis from the Reduction of End Points in Non-Insulin-Dependent Diabetes With the Angiotensin II Antagonist Losartan (RENAAL) study and Irbesartan Diabetic Nephropathy Trial (IDNT) Am J Kidney Dis. 2014;63:244–50. doi: 10.1053/j.ajkd.2013.09.016. [DOI] [PubMed] [Google Scholar]