Abstract
The strong reinforcing effects of nicotine and the negative symptoms such as anxiety experienced during a quit attempt often lead to relapse and low success rates for smoking cessation. Treatments that not only block the reinforcing effects of nicotine but also attenuate the motivation to relapse are needed to improve cessation rates. Recent genetic and preclinical studies have highlighted the involvement of the α3, β4, and α5 nicotinic acetylcholine receptor (nAChR) subunits and the α3β4 nAChR subtype in nicotine dependence and withdrawal. However, the involvement of these nAChR in relapse is not fully understood. We previously reported that the α3β4 nAChR partial agonist AT-1001 selectively decreases nicotine self-administration in rats without affecting food responding. In the present experiments, we examined the efficacy of AT-1001 in attenuating reinstatement of nicotine-seeking behavior in a model of stress-induced relapse. Rats extinguished from nicotine self-administration were treated with the pharmacological stressor yohimbine prior to AT-1001 treatment and reinstatement testing. We also examined whether AT-1001 produced any withdrawal-related effects when administered to nicotine-dependent rats.
We found that AT-1001 dose-dependently reduced yohimbine stress-induced reinstatement of nicotine seeking. When administered to nicotine-dependent rats at the dose that significantly blocked nicotine reinstatement, AT-1001 elicited minimal somatic withdrawal signs in comparison to the nicotinic antagonist mecamylamine, which is known to produce robust withdrawal. Our data suggest that α3β4 nAChR-targeted compounds may be a promising approach for nicotine addiction treatment because they can not only block nicotine’s reinforcing effects, but also decrease motivation to relapse without producing significant withdrawal effects.
Keywords: Nicotinic acetylcholine receptor, α3β4 nAChR, AT-1001, nicotine reinstatement, relapse, stress-induced reinstatement
1. Introduction
Tobacco use is the leading cause of preventable death and disease, and a major health problem both in the United States and worldwide [1, 2]. Despite the health risks, an estimated 45 million Americans smoke, and nearly 500,000 smoking-related deaths occur each year [1]. Nearly 70% of U.S. adult smokers attempt to quit [3]; however, success rates are modest (~45%) even with the first-line pharmacotherapy varenicline [4]. Overall, only 7% remain abstinent after 1 year due to relapse [5]. There are currently only three FDA-approved pharmacotherapies to assist smokers in quitting: the α4β2 nicotinic acetylcholine receptor (nAChR) partial agonist varenicline; the atypical antidepressant and monoamine uptake inhibitor bupropion; and nicotine replacement therapy such as patch, gum, lozenge, inhaler and nasal spray. These available smoking cessation aids show modest efficacy in promoting long-term abstinence, yielding 17% (nicotine replacement therapy), 14% (bupropion), and 23% (varenicline) success rates after one year [4, 6].
Prevention of relapse is a key factor for successful smoking cessation and improving long-term abstinence rates. Although the neuropharmacology that underlies relapse to smoking is not fully understood, animal models of relapse or reinstatement of nicotine seeking have been developed for investigating potential pharmacotherapies that may attenuate reinstatement following relapse triggers [7–9]. Interestingly, the first-line smoking cessation drug varenicline was shown not to block cue-induced relapse to nicotine seeking in rats [10], although it attenuated reinstatement when cues were combined with a nicotine priming injection [11] or a longer pretreatment time was used [12]. Similarly, bupropion was also shown to ‘increase’, rather than block cue-induced reinstatement of nicotine seeking in animal models [13]. These lines of evidence clearly highlight the need for new approaches to address smoking relapse to enable higher success rates for smoking cessation efforts and abstinence.
Nicotine, the primary addictive component of cigarettes, exerts its positive reinforcing effects by binding to the nicotinic acetylcholine receptors (nAChRs) in the brain which are pentameric ligand-gated ion channels, containing homomeric or heteromeric combinations of α and β subunits [14, 15]. From the various subtype combinations of nAChRs known, the highly abundant heteromeric α4β2* (* indicates possible other subunits present) subtype is linked to the positive reinforcing effects of nicotine [16–18] and is the target for varenicline, which acts as a partial agonist at this nAChR subtype to reduce the rewarding effect of nicotine in humans and in animal models of nicotine self-administration [11, 19]. Recent findings from human genetic studies and preclinical mouse models show that other nAChR subtypes present in the brain also play an important role in the dependence and reinforcing effects of nicotine [20–23].
Among these the α3, β4 and α5 subunit-containing nAChRs have received considerable attention because human genetic association studies show that polymorphisms in the genes encoding these nAChR subunits are associated with heavy smoking and inability to quit [24–26]. Although the α3β4 nAChR subtype has widespread expression in the sensory and autonomic ganglia [27, 28], the neuronal α3β4 nAChR is expressed primarily in the medial habenula (MHb), interpeduncular nucleus (IPN) and the fasciculus retroflexus (fr) [29, 30]. Recent findings suggest that α3β4 nAChRs in the MHb-IPN pathway may serve important functional roles in mediating nicotine reward and withdrawal (reviewed in [31]).
We recently reported that systemic (subcutaneous, s.c.) administration of a novel α3β4 nAChR-selective small-molecule AT-1001 blocked self-administration of intravenous nicotine in rats without nonspecific effects on food responding [32]. AT-1001 has single-digit nanomolar binding affinity for the α3β4 nAChR and over 100-fold selectivity over the α4β2 nAChR and α7 nAChR in competition binding experiments with [3H]epibatidine [32, 33]. In electrophysiological experiments, AT-1001 had partial agonist activity at the α3β4 nAChR, evoking 35% of maximum ACh response, and at the same doses, produced desensitization of the ACh response, effectively acting as a functional antagonist at the α3β4 nAChR [34]. Rat brain slice autoradiography with a radio-iodinated analog of AT-1001, viz. [125I]-AT-1012, shows highly localized and selective labeling of areas containing high concentrations of α3β4 nAChR viz. the MHb, IPN and fr, [33]. Interestingly, AT-1001 also selectively decreased self-administration of cigarette smoke extract (CSE), an aqueous extract of cigarette smoke components, without altering natural food intake, when administered systemically to rats trained to self-administer CSE [32, 35].
Although the precise involvement of the α3β4 nAChR in relapse is not fully understood, we found that AT-1001 attenuated reinstatement of nicotine seeking induced by nicotine and interestingly, induced also by varenicline [36]. It is well known that stress and smoking-related cues are often strong triggers for relapse in smokers [37–40]. Therefore, the present series of experiments were designed to investigate the efficacy of AT-1001 in stress-induced reinstatement of nicotine seeking behavior. We have previously shown that AT-1001 had anxiolytic activity in the elevated plus maze assay (EPM) in rats in the presence of a pharmacological stressor yohimbine [41]. Since AT-1001 decreased yohimbine’s anxiogenic effects in the elevated plus maze assay [41], we hypothesized that AT-1001 administration would block yohimbine stress-induced reinstatement of nicotine seeking. Using an extinction-reinstatement paradigm as the model of relapse, here we examined the efficacy of AT-1001 to reduce reinstatement of nicotine seeking in rats exposed to yohimbine stress. Further, since the β4 nAChR subunit has been shown to be involved in nicotine withdrawal,[42] we conducted a preliminary assessment of the effect of the α3β4 nAChR ligand AT-1001 on somatic withdrawal signs when administered to nicotine-dependent rats.
2. Materials and Methods
2.1 Animals
Adult male Sprague-Dawley rats (~ 300–350g; Charles River Labs, Hollister, CA) were group housed in an AAALAC-accredited vivarium and kept on a 12 h light/dark cycle (1900 to 0700 h). All animals were handled for two days prior to testing, and behavioral testing was performed 7 days a week. For withdrawal studies, animals had unlimited access to food and water. For extinction and reinstatement studies, animals were initially food restricted to maintain 85% of their free-feeding body weight during food training and remained at 90–95% of their free-feeding body weight during self-administration tests. Animal care and use were in accordance to NIH standards and protocols were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine.
2.2 Drugs
Nicotine hydrogen tartrate (Sigma, St. Louis, MO) was dissolved in sterile saline and adjusted to pH 7.2–7.4; doses were calculated as free base. AT-1001 was dissolved in a vehicle containing 97% of 0.5% aqueous hydroxypropylcellulose, 2% DMSO, and 1% 0.1M HCl. Propofol (Abbott Laboratories, Chicago, IL), mecamylamine HCl (Tocris Bioscience, Bristol, UK), and yohimbine hydrochloride (Sigma, St. Louis, MO) were dissolved in sterile saline.
2.3 Extinction and Reinstatement of Nicotine-Seeking Behavior
2.3.1 Food training
Animals were trained in 30 minute sessions to lever press for food pellets (45mg rodent purified diet; Bio-Serv, Frenchtown, NJ) (Costello et al., 2014) in an operant chamber containing two levers, cue lights above each lever, and a house light (Med Associates, St Albans, VT). Responses on the reinforced (R) lever delivered a reward, and responses on the nonreinforced (NR) lever were recorded but had no consequence. Upon earning a reward, a cue light above the R lever was illuminated for 5.6s, and the house light was extinguished for the entire duration of the timeout period. Rats began food training under a fixed ratio 1 schedule with a 1 second timeout (FR1TO1) and progressed through to FR1TO10, FR2TO20, and FR5TO20 upon earning 50 pellets (R ≥ 5). Throughout food training, animals were food restricted to maintain 85% of their free-feeding body weight.
2.3.2 Catheter implantation
After acquisition of food responding, rats were anesthetized with equithesin (0.0035ml/g) and surgically implanted with an indwelling catheter in their jugular vein [43]. During the 3-day recovery period, cannulas were flushed daily with heparinized saline solution to maintain catheter patency, and catheter patency was verified by testing for rapid anesthesia with propofol (5 mg/kg, i.v.).
2.3.3 Nicotine self-administration
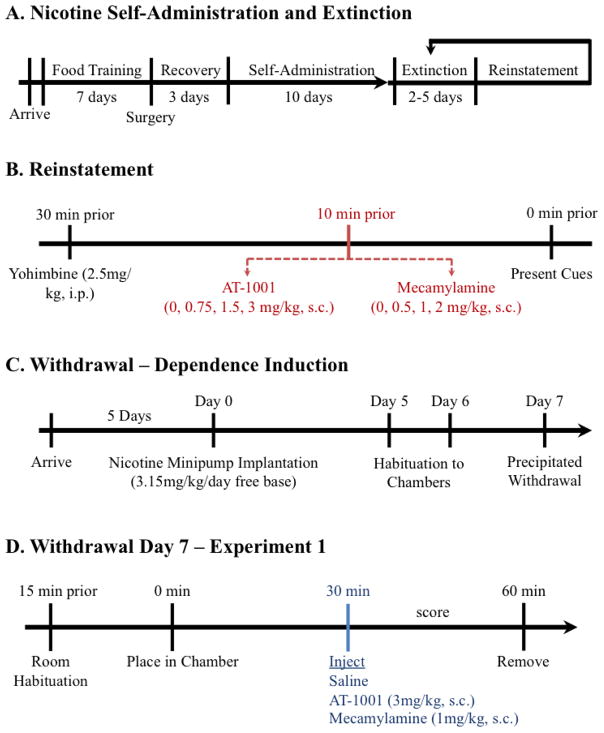
After recovery, animals began 1-hour nicotine self-administration sessions at FR5TO20 (Figure 1A) (Toll et al., 2012). Rats self-administered nicotine (30μg/kg/infusion, 100μl i.v.,5.6s) for a minimum of 10 days until they achieved stable responding (R ± 20% over 3 days; R ≥ 6; R ≥ 2 × NR). Catheter patency was verified by administering propofol and tested for rapid anesthesia after each rat reached stable responding.
Figure 1. Schematic of Experimental Design.
A) Experimental timeline for nicotine self-administration, extinction, and reinstatement. B) Dose-response comparison of AT-1001 versus mecamylamine in blocking reinstatement of nicotine-seeking behavior. C) Induction of nicotine dependence over 7 days. D) For Experiment 1, comparison of AT-1001 versus mecamylamine in precipitating nicotine withdrawal.
2.3.4 Extinction
Upon reaching stable responding, nicotine-seeking behavior was extinguished by removal of drug and drug-associated cues (Figure 1A). During extinction, the house light was on continuously, but the animal was not connected to the infusion tubing and responses on the levers had no consequence. Extinction lasted until rats met criteria (≥ 5 days; R ≤ 20% last day of nicotine self-administration).
2.3.5 Reinstatement
Once responding was extinguished, animals began reinstatement testing the following day (Figure 1B). Thirty minutes before testing, reinstatement was primed by a pharmacological stressor (yohimbine 2.5 mg/kg, i.p). Ten minutes before testing, separate groups of animals received injections of AT-1001 (0, 0.75, 1.5, 3 mg/kg, s.c.) or drug control mecamylamine (0, 0.5, 1, 2 mg/kg s.c.). The doses of AT-1001 were selected based on our previous studies [32, 35, 36]. Doses of mecamylamine were selected based on previous reports [44]. Animals were placed in the self-administration chamber and exposed to the drug-associated cue light. This combination of using yohimbine stress with nicotine-paired cues has been shown to induce robust reinstatement of nicotine-seeking behavior, compared to using yohimbine alone [45]. Between reinstatement tests animals were returned to extinction conditions for a minimum of two days or until extinction criteria were met (Figure 1A). They were then retested with another dose of antagonist in a within subjects, Latin-square design.
2.4 Effect of AT-1001 and mecamylamine in nicotine-dependent rats
2.4.1 Dependence induction
A separate group of rats were anesthetized with equithesin (0.0035ml/g) and implanted with a subcutaneous (s.c.) Alzet 2ML1 minipump (DURECT, Cupertino, CA) to infuse 3.15 mg/kg/day of nicotine free-base for 7 days using a protocol previously described by Malin et al. [46]. Following surgery, rats were weighed daily to monitor health. On day 5 and 6 following minipump implantation, rats were placed in clear plexiglass, open field chambers (43.2 × 43.2 × 30.5 cm3) for 30 minutes to acclimate to the testing environment (Figure 1C).
2.4.2 Treatment of nicotine-dependent rats with AT-1001 or mecamylamine
On day 7 of chronic nicotine exposure, the rats were placed in the chambers and scored for baseline somatic withdrawal signs. After 30 minutes, the rats were given vehicle, AT-1001 (3 mg/kg, s.c.), or mecamylamine (1 mg/kg, s.c.), and scored for an additional 30 minutes (Figure 1D). The dose of AT-1001 used was the highest dose that blocked stable responding of nicotine self-administration [32], whereas the dose of mecamylamine used is known to produce robust precipitated withdrawal [47]. After drug treatment, behavior was digitally recorded and scored live by a blind observer for 10 seconds of every minute. Somatic nicotine withdrawal signs included teeth chatter/chewing, gasps/writhing, ptosis, “wet dog” shakes/tremors, and a miscellaneous category of less frequent symptoms such as hop, yawn, scratch, ejaculation, and dyspnea [based on Malin et al., 46, 48]. Simultaneously, locomotor activity and center time were recorded in the open field chambers through 16 photobeams along the sides of each wall (MED Associates, Inc., St. Albans, VT).
2.5 Statistical Analysis
Both AT-1001 and mecamylamine reinstatement experiments were performed as a within-subjects counterbalanced design. Reinstatement lever responses were analyzed by 1-way, repeated measures ANOVA with drug dose (AT-1001 or mecamylamine) as the repeated measure. Significant main effects were further analyzed with appropriate post-hoc tests.
Withdrawal data were analyzed by 2-way ANOVA comparing drug × total somatic withdrawal signs. Individual categories of withdrawal measures were analyzed by 2-way ANOVA comparing drug × somatic withdrawal signs, with repeated measures on somatic withdrawal signs. Likewise, locomotor activity and center time were analyzed by 2-way ANOVA comparing drug × ambulatory counts and drug × seconds, respectively. All significant main effects were further analyzed by one-way ANOVA as well as Dunnett’s and Bonferroni post hoc tests.
3. Results
3.1 AT-1001 blocks reinstatement of nicotine seeking induced by yohimbine stress
Since AT-1001 attenuated yohimbine-induced anxiogenic responses in rat, as we previously showed [41], we tested whether AT-1001 could block yohimbine stress-induced reinstatement of nicotine-seeking behavior in rats. Given that varenicline or bupropion do not significantly block reinstatement of nicotine-seeking,[10, 13] we used mecamylamine as a positive control, since it has been shown to decrease cue-induced nicotine reinstatement [44]. Rats that extinguished stable responding to nicotine self-administration were primed to reinstate with yohimbine stress. Vehicle-treated animals significantly reinstated responding (Figure 2, gray bar). There was a significant effect of AT-1001 dose (F4, 20 = 16.375, p < 0.001), where at both the 1.5 and 3 mg/kg doses, AT-1001 significantly reducing responding on the nicotine-associated (reinforced, R) lever compared to vehicle-treated controls (Figure 2A). AT-1001 had no significant effects on the nonreinforced (NR) lever. Similarly, there was a significant effect of mecamylamine dose (F4, 20 = 9.903, p < 0.001), with mecamylamine significantly attenuating reinforced lever responses at both 1 and 2 mg/kg doses (Figure 2B).
Figure 2. Effect of AT-1001 and mecamylamine on yohimbine-induced reinstatement of nicotine-seeking behavior.
A) AT-1001 dose-dependently attenuated reinstatement of nicotine-seeking behavior. Rats significantly reinstated R lever responding at lower doses of AT-1001 (** p < 0.01). At higher doses, AT-1001 blocked yohimbine-induced reinstatement (+ p < 0.05, +++ p < 0.001). B) Nicotine-seeking behavior was significantly reinstated following vehicle injection (* p < 0.05), and mecamylamine dose-dependently blocked reinstatement of R responding (+ p < 0.05, ++ p < 0.01). n = 6/group.
3.2 AT-1001 administration to nicotine-dependent rats produces minimal withdrawal signs
Since the β4 nAChR subunit is thought to be involved in nicotine withdrawal, we examined whether AT-1001 administration to nicotine-dependent rats elicited any somatic withdrawal signs, using a protocol described by Malin et al [47]. Mecamylamine, which produces robust somatic signs of withdrawal, was used as a positive control and a comparator for the somatic withdrawal signs.
AT-1001 (or mecamylamine, as a positive control that precipitates withdrawal) was administered on the 7th day after chronic nicotine exposure, and somatic withdrawal signs were evaluated in the 30 minutes after compound administration. For somatic signs scored following drug treatment, there was a significant main effect of drug (F2, 22 = 27.898, p < 0.001). As expected, the positive control mecamylamine precipitated a significant increase in somatic withdrawal signs compared to vehicle controls (Figure 3A, black bar, p < 0.001). Rats treated with AT-1001, on the other hand, also exhibited higher somatic signs compared to vehicle (p < 0.05, respectively; Figure 3A). However, Bonferroni post hoc analysis showed that AT-1001 produced significantly less total somatic withdrawal signs compared to mecamylamine (p < 0.001; Figure 3A). Analysis of the individual categories of each withdrawal measure indicated significant within effects of somatic withdrawal signs (F1, 22 = 13.643, p = 0.001) and a drug × somatic withdrawal signs interaction (F2, 22 = 9.830, p = 0.001). Mecamylamine significantly enhanced teeth chatter/chewing and ptosis compared to AT-1001 (p < 0.001) and vehicle (p < 0.001; Figure 3B), indicating nonspecific ganglionic effects [49, 50]. In contrast, AT-1001 and vehicle-treated rats do not exhibit significant ptosis signs (Figure 3B). Moreover, AT-1001 had no significant effect on any individual somatic withdrawal sign (Figure 3B). AT-1001 and mecamylamine had no significant effects on locomotor activity or center time compared to vehicle (Figure 3C and 3D).
Figure 3. Effect of AT-1001 on somatic withdrawal signs in nicotine dependent rats.
A) Total somatic signs during the 30 minutes after vehicle, AT-1001, or mecamylamine injection. Rats administered AT-1001 (3 mg/kg, s.c.) or mecamylamine (1 mg/kg, s.c.) showed enhanced somatic nicotine withdrawal signs compared to vehicle controls (* p < 0.05, *** p < 0.001). AT-1001-injected rats displayed significantly fewer somatic signs compared to mecamylamine-injected rats (+++ p < 0.001). B) Individual somatic signs after drug injection. Rats that received mecamylamine exhibited more occurrences of teeth chatter/chewing and ptosis compared to rats that received vehicle or AT-1001 (*** p < 0.001, +++ p < 0.001, respectively). AT-1001 was not significantly different from saline in any individual category. C) Effect of AT-1001 on locomotor activity. No significant differences in locomotor activity between drug treatments and vehicle, as measured by ambulatory counts. D) Effect of AT-1001 on center time. No significant differences in center time (s) after drug treatment compared to vehicle treatment. n = 8–9/group.
4. Discussion
Our studies show that AT-1001 dose-dependently attenuates reinstatement of nicotine seeking induced by pharmacological stress in a rat model of relapse. Our results add to our previous findings and demonstrate, that at a dose that blocks nicotine self-administration [32] the α3β4 nAChR-selective AT-1001 also blocks relapse of nicotine seeking, induced by nicotine, varenicline [36] as well as stress. We have previously shown that AT-1001 does not exhibit reinforcing properties per se and does not induce reinstatement of nicotine seeking in extinguished rats [32, 36]. We have also shown that AT-1001 does not induce a conditioned place preference or aversion in mice [51].
To our knowledge, this study is the first to demonstrate that selectively targeting the α3β4 nAChR (with selective ligands such as AT-1001) attenuates stress-induced nicotine seeking behavior. The only other selective α3β4* nAChR ligand shown to attenuate the rewarding effects of nicotine is the peptide α-conotoxin AuIB, which has been shown to attenuate nicotine conditioned place preference (CPP) (nicotine reward) in C57BL/6 mice after local intracerebral injection [52] and to decrease intravenous (IV) nicotine self-administration when injected directly into the MHb [53]. AuIB has not been investigated in nicotine reinstatement, likely due to confounding difficulties associated with the requirement for direct intracerebral administration of this peptide. The systemically active but modestly-selective α3β4* nAChR antagonist, 18-methoxycoronaridine (18-MC) has also not been investigated for efficacy in blocking reinstatement to nicotine seeking, although it was found to decrease IV and oral nicotine self-administration in rats when administered systemically [54, 55]. 18-MC also decreases IV nicotine self-administration when injected directly into the MHb, but increased nicotine self-administration when injected into the IPN [53]. Interestingly, a recent study showed that 18-MC enhanced reinstatement of cocaine CPP following extinction even though it blocked acquisition of cocaine CPP [56]. The lack of selectivity of 18-MC for the α3β4 nAChR, particularly after systemic administration, preclude a conclusion about the role of α3β4 nAChR in the effects of 18-MC in drug seeking [57].
While the role of the α3β4* nAChR in nicotine reinstatement is not extensively studied, recent reports showing the involvement of the medial habenula, IPN and α3β4* nAChR in nicotine-mediated behaviors offer intriguing clues about the possible role of α3β4* nAChR activation in relapse. Elegant studies reported by Gorlich et al. found that heightened sensitivity to nicotine in mice undergoing withdrawal occurs from an increase in the firing and pacemaking activity of the cholinergic MHb neurons and involve the activation of only the α3β4* nAChR but not the α4β2*, α4β4*, α3β2*, α7, β3* nAChR subunits [58]. These studies suggest that α3β4* nAChR activation in the MHb may play a role in triggering relapse by driving the increased sensitivity to nicotine during an abstinent state. These findings suggest the intriguing possibility that suppression of α3β4* nAChR activation during the abstinent phase may decrease the heightened sensitivity to nicotine during abstinence and prevent relapse. The studies of Gorlich et al. appear to support our observations that AT-1001 blocks reinstatement of nicotine-primed drug seeking [36] as well as stress-induced nicotine seeking. Together, these studies suggest that modulation of the α3β4* nAChR may be a promising approach to decrease sensitivity to relapse triggers during abstinence, reduce smoking relapse and improve cessation rates.
The results of the withdrawal experiments (Figure 3) further confirm the α3β4*-selective action of AT-1001 in vivo. We found that AT-1001 administration to nicotine-dependent rats induces minimal withdrawal signs, when compared to those produced by mecamylamine (Figure 3). The lack of significant signs of somatic withdrawal when AT-1001 was injected (on day 7) is consistent with its selective action at the α3β4* nAChR. The selective α3β4* nAChR antagonist AuIB was also shown to produce lesser somatic withdrawal signs in mice [42, 52]. The nonspecific nicotinic antagonist mecamylamine, on the other hand, elicited robust withdrawal in nicotine-dependent rats, as expected (Figure 3B). Mecamylamine is routinely used in experimental settings to precipitate withdrawal in nicotine-dependent animals [47, 59, 60]. Given that mecamylamine also produces withdrawal, albeit reduced, in β4 null mice further confirms that the withdrawal produced by mecamylamine is likely due to its actions at receptors other than just the α3β4 nAChRs and even other non-nicotinic targets [61]. These withdrawal-promoting adverse effects of mecamylamine probably precluded its development as a smoking cessation medication [62, 63]. Overall, our findings of minimal withdrawal produced by AT-1001 further support the notion that selective modulation of the α3β4 nAChR subtype may be a better approach for smoking cessation treatment and not produce withdrawal-induced effects observed with non-selective antagonists such as mecamylamine and [64, 65].
Both current approved medications for smoking cessation, bupropion as well as varenicline do not demonstrate a robust inhibition of nicotine-seeking behavior in animal models of relapse and have low long-term abstinence rates (<25%) in the clinic.[10, 11, 13] The robust efficacy of α3β4-selective compounds such as AT-1001, to block reinstatement of nicotine-seeking, demonstrated in this and previous studies, clearly differentiate it from available medications and from nonspecific antagonists like mecamylamine, and offers a new approach for smoking cessation treatment and improved abstinence rates. The present findings also lend support the human genetic association and functional studies implicating the α3 and β4 nAChRs in nicotine dependence and addictive behaviors. Together, our results support a role of the α3β4 nAChR as a potential target for developing medications that may provide improved abstinence rates.
Highlights.
AT-1001, a high affinity, selective α3β4 nAChR partial agonist attenuates reinstatement of nicotine seeking induced by pharmacological stress in a rat model of relapse.
When administered to nicotine-dependent rats, AT-1001 induces minimum withdrawal signs.
The robust efficacy of α3β4 nAChR-selective compounds such as AT-1001 to block reinstatement of nicotine-seeking differentiate it from current smoking cessation medications like bupropion and varenicline which do not attenuate relapse to nicotine seeking.
Targeting the α3β4 nAChR offers a new approach for smoking cessation treatment and improved long term abstinence rates.
Acknowledgments
This work was supported by the National Institutes of Health’s National Institute on Drug Abuse Grants R43DA033744 and R44DA033744 to Astraea Therapeutics.
Footnotes
6. Disclosures
NTZ and DY are employees of Astraea Therapeutics. The other authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.USDHHS. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 2.WHO. WHO Report on the Global Tobacco Epidemic, Raising Taxes on tobacco. World Health Organization; Geneva: 2015. [Google Scholar]
- 3.CDC. Morbidity and Mortality Weekly Report. Centers for Disease Control and Prevention; 2015. Trends in Quit Attempts Among Adult Cigarette Smokers — United States, 2001–2013; pp. 1129–1135. [DOI] [PubMed] [Google Scholar]
- 4.Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 5.CDC. Morbidity and Mortality Weekly Report. Centers for Disease Control and Prevention; 2011. Quitting Smoking Among Adults — United States, 2001–2010; pp. 1513–1519. [PubMed] [Google Scholar]
- 6.Reus VI, Smith BJ. Multimodal techniques for smoking cessation: a review of their efficacy and utilisation and clinical practice guidelines. Int J Clin Pract. 2008;62(11):1753–68. doi: 10.1111/j.1742-1241.2008.01885.x. [DOI] [PubMed] [Google Scholar]
- 7.Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168(1–2):3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 8.Napier TC, Herrold AA, de Wit H. Using conditioned place preference to identify relapse prevention medications. Neuroscience & Biobehavioral Reviews. 2013;37(9 Part A):2081–2086. doi: 10.1016/j.neubiorev.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X. Effects of blockade of alpha4beta2 and alpha7 nicotinic acetylcholine receptors on cue-induced reinstatement of nicotine-seeking behaviour in rats. The International Journal of Neuropsychopharmacology. 2014;17(01):105–116. doi: 10.1017/S1461145713000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wouda JA, Riga D, De Vries W, Stegeman M, van Mourik Y, Schetters D, Schoffelmeer AN, Pattij T, De Vries TJ. Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacology (Berl) 2011;216(2):267–77. doi: 10.1007/s00213-011-2213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Connor EC, Parker D, Rollema H, Mead AN. The alpha4beta2 nicotinic acetylcholine-receptor partial agonist varenicline inhibits both nicotine self-administration following repeated dosing and reinstatement of nicotine seeking in rats. Psychopharmacology (Berl) 2010;208(3):365–76. doi: 10.1007/s00213-009-1739-5. [DOI] [PubMed] [Google Scholar]
- 12.Le Foll B, Chakraborty-Chatterjee M, Lev-Ran S, Barnes C, Pushparaj A, Gamaleddin I, Yan Y, Khaled M, Goldberg SR. Varenicline decreases nicotine self-administration and cue-induced reinstatement of nicotine-seeking behaviour in rats when a long pretreatment time is used. Int J Neuropsychopharmacol. 2012;15(9):1265–74. doi: 10.1017/S1461145711001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Caggiula AR, Palmatier MI, Donny EC, Sved AF. Cue-induced reinstatement of nicotine-seeking behavior in rats: effect of bupropion, persistence over repeated tests, and its dependence on training dose. Psychopharmacology (Berl) 2008;196(3):365–75. doi: 10.1007/s00213-007-0967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Novere N, Corringer PJ, Changeux JP. The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J Neurobiol. 2002;53(4):447–56. doi: 10.1002/neu.10153. [DOI] [PubMed] [Google Scholar]
- 15.Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L, Zoli M. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol. 2009;78(7):703–11. doi: 10.1016/j.bcp.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391(6663):173–7. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- 17.Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306(5698):1029–32. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- 18.Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, Dufour N, Cloez-Tayarani I, Bemelmans AP, Mallet J, Gardier AM, David V, Faure P, Granon S, Changeux JP. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436(7047):103–7. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- 19.Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD, 3rd, Williams KE. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52(3):985–94. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Picciotto MR, Kenny PJ. Molecular mechanisms underlying behaviors related to nicotine addiction. Cold Spring Harbor perspectives in medicine. 2013;3(1):a012112. doi: 10.1101/cshperspect.a012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fowler CD, Arends MA, Kenny PJ. Subtypes of nicotinic acetylcholine receptors in nicotine reward, dependence, and withdrawal: evidence from genetically modified mice. Behav Pharmacol. 2008;19(5–6):461–84. doi: 10.1097/FBP.0b013e32830c360e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenbaum L, Lerer B. Differential contribution of genetic variation in multiple brain nicotinic cholinergic receptors to nicotine dependence: recent progress and emerging open questions. Mol Psychiatry. 2009;14(10):912–45. doi: 10.1038/mp.2009.59. [DOI] [PubMed] [Google Scholar]
- 23.Wen L, Jiang K, Yuan W, Cui W, Li MD. Contribution of Variants in CHRNA5/A3/B4 Gene Cluster on Chromosome 15 to Tobacco Smoking: From Genetic Association to Mechanism. Molecular Neurobiology. 2016;53(1):472–484. doi: 10.1007/s12035-014-8997-x. [DOI] [PubMed] [Google Scholar]
- 24.Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, Waterworth D, Muglia P, Mooser V. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Molecular Psychiatry. 2008;13(4):368–73. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saccone NL, Wang JC, Breslau N, Johnson EO, Hatsukami D, Saccone SF, Grucza RA, Sun L, Duan W, Budde J, Culverhouse RC, Fox L, Hinrichs AL, Steinbach JH, Wu M, Rice JP, Goate AM, Bierut LJ. The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Res. 2009;69(17):6848–56. doi: 10.1158/0008-5472.CAN-09-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss RB, Baker TB, Cannon DS, von Niederhausern A, Dunn DM, Matsunami N, Singh NA, Baird L, Coon H, McMahon WM, Piper ME, Fiore MC, Scholand MB, Connett JE, Kanner RE, Gahring LC, Rogers SW, Hoidal JR, Leppert MF. A candidate gene approach identifies the CHRNA5-A3-B4 region as a risk factor for age-dependent nicotine addiction. PLoS Genetics. 2008;4(7):e1000125. doi: 10.1371/journal.pgen.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandelzys A, Pie B, Deneris ES, Cooper E. The developmental increase in ACh current densities on rat sympathetic neurons correlates with changes in nicotinic ACh receptor alpha-subunit gene expression and occurs independent of innervation. J Neurosci. 1994;14(4):2357–64. doi: 10.1523/JNEUROSCI.14-04-02357.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poth K, Nutter TJ, Cuevas J, Parker MJ, Adams DJ, Luetje CW. Heterogeneity of nicotinic receptor class and subunit mRNA expression among individual parasympathetic neurons from rat intracardiac ganglia. J Neurosci. 1997;17(2):586–96. doi: 10.1523/JNEUROSCI.17-02-00586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quick MW, Ceballos RM, Kasten M, McIntosh JM, Lester RA. Alpha3beta4 subunit-containing nicotinic receptors dominate function in rat medial habenula neurons. Neuropharmacology. 1999;38(6):769–83. doi: 10.1016/s0028-3908(99)00024-6. [DOI] [PubMed] [Google Scholar]
- 30.Perry DC, Xiao Y, Nguyen HN, Musachio JL, Davila-Garcia MI, Kellar KJ. Measuring nicotinic receptors with characteristics of alpha4beta2, alpha3beta2 and alpha3beta4 subtypes in rat tissues by autoradiography. J Neurochem. 2002;82(3):468–81. doi: 10.1046/j.1471-4159.2002.00951.x. [DOI] [PubMed] [Google Scholar]
- 31.Leslie FM, Mojica CY, Reynaga DD. Nicotinic receptors in addiction pathways. Mol Pharmacol. 2013;83(4):753–8. doi: 10.1124/mol.112.083659. [DOI] [PubMed] [Google Scholar]
- 32.Toll L, Zaveri NT, Polgar WE, Jiang F, Khroyan TV, Zhou W, Xie XS, Stauber GB, Costello MR, Leslie FM. AT-1001: a high affinity and selective alpha3beta4 nicotinic acetylcholine receptor antagonist blocks nicotine self-administration in rats. Neuropsychopharmacology. 2012;37(6):1367–76. doi: 10.1038/npp.2011.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu J, Perry DC, Bupp J, Jiang F, Polgar WE, Toll L, Zaveri NT. [125I]AT-1012, a New High Affinity Radioligand for the α3β4 Nicotinic Acetylcholine Receptors. Neuropharmacology. 2014;77:193–199. doi: 10.1016/j.neuropharm.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaveri NT, Bertrand S, Yasuda D, Bertrand D. Functional characterization of AT-1001, an alpha3beta4 nicotinic acetylcholine receptor ligand, at human alpha3beta4 and alpha4beta2 nAChR. Nicotine Tob Res. 2015;17(3):361–7. doi: 10.1093/ntr/ntu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costello MR, Reynaga DD, Mojica CY, Zaveri NT, Belluzzi JD, Leslie FM. Comparison of the reinforcing properties of nicotine and cigarette smoke extract in rats. Neuropsychopharmacology. 2014;39(8):1843–51. doi: 10.1038/npp.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cippitelli A, Wu J, Gaiolini KA, Mercatelli D, Schoch J, Gorman M, Ramirez A, Ciccocioppo R, Khroyan TV, Yasuda D, Zaveri NT, Pascual C, Xie XS, Toll L. AT-1001: a high-affinity alpha3beta4 nAChR ligand with novel nicotine-suppressive pharmacology. Br J Pharmacol. 2015;172(7):1834–45. doi: 10.1111/bph.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brandon TH, Tiffany ST, Obremski KM, Baker TB. Postcessation cigarette use: the process of relapse. Addict Behav. 1990;15(2):105–14. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- 38.USDHHS. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. [PubMed] [Google Scholar]
- 39.Cummings KM, Jaen CR, Giovino G. Circumstances surrounding relapse in a group of recent exsmokers. Prev Med. 1985;14(2):195–202. doi: 10.1016/0091-7435(85)90035-0. [DOI] [PubMed] [Google Scholar]
- 40.Bruijnzeel AW. Tobacco addiction and the dysregulation of brain stress systems. Neurosci Biobehav Rev. 2012;36(5):1418–41. doi: 10.1016/j.neubiorev.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cippitelli A, Brunori G, Gaiolini KA, Zaveri NT, Toll L. Pharmacological stress is required for the anti-alcohol effect of the α3β4* nAChR partial agonist AT-1001. Neuropharmacology. 2015;93(0):229–236. doi: 10.1016/j.neuropharm.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. J Neurosci. 2004;24(45):10035–9. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30(4):705–12. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- 44.Liu X, Caggiula AR, Yee SK, Nobuta H, Sved AF, Pechnick RN, Poland RE. Mecamylamine attenuates cue-induced reinstatement of nicotine-seeking behavior in rats. Neuropsychopharmacology. 2007;32(3):710–8. doi: 10.1038/sj.npp.1301129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feltenstein MW, Ghee SM, See RE. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Depend. 2012;121(3):240–6. doi: 10.1016/j.drugalcdep.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malin DH, Lake JR, Newlin-Maultsby P, Roberts LK, Lanier JG, Carter VA, Cunningham JS, Wilson OB. Rodent model of nicotine abstinence syndrome. Pharmacol Biochem Behav. 1992;43(3):779–84. doi: 10.1016/0091-3057(92)90408-8. [DOI] [PubMed] [Google Scholar]
- 47.Malin DH, Lake JR, Carter VA, Cunningham JS, Hebert KM, Conrad DL, Wilson OB. The nicotinic antagonist mecamylamine precipitates nicotine abstinence syndrome in the rat. Psychopharmacology (Berl) 1994;115(1–2):180–4. doi: 10.1007/BF02244770. [DOI] [PubMed] [Google Scholar]
- 48.Malin DH, Lake JR, Smith TD, Khambati HN, Meyers-Paal RL, Montellano AL, Jennings RE, Erwin DS, Presley SE, Perales BA. Bupropion attenuates nicotine abstinence syndrome in the rat. Psychopharmacology (Berl) 2006;184(3–4):494–503. doi: 10.1007/s00213-005-0135-z. [DOI] [PubMed] [Google Scholar]
- 49.Hildebrand EB, Nomikos GG, Bondjers C, Nisell M, Svensson HT. Behavioral manifestations of the nicotine abstinence syndrome in the rat: peripheral versus central mechanisms. Psychopharmacology. 1997;129(4):348–356. doi: 10.1007/s002130050200. [DOI] [PubMed] [Google Scholar]
- 50.Clarke PB, Chaudieu I, el-Bizri H, Boksa P, Quik M, Esplin BA, Capek R. The pharmacology of the nicotinic antagonist, chlorisondamine, investigated in rat brain and autonomic ganglion. Br J Pharmacol. 1994;111(2):397–405. doi: 10.1111/j.1476-5381.1994.tb14748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khroyan TV, Yasuda D, Toll L, Polgar WE, Zaveri NT. High affinity α3β4 nicotinic acetylcholine receptor ligands AT-1001 and AT-1012 attenuate cocaine-induced conditioned place preference and behavioral sensitization in mice. Biochemical Pharmacology. 2015;97(4):531–541. doi: 10.1016/j.bcp.2015.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jackson KJ, Sanjakdar SS, Muldoon PP, McIntosh JM, Damaj MI. The alpha3beta4* nicotinic acetylcholine receptor subtype mediates nicotine reward and physical nicotine withdrawal signs independently of the alpha5 subunit in the mouse. Neuropharmacology. 2013;70:228–35. doi: 10.1016/j.neuropharm.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glick SD, Sell EM, McCallum SE, Maisonneuve IM. Brain regions mediating alpha3beta4 nicotinic antagonist effects of 18-MC on nicotine self-administration. Eur J Pharmacol. 2011;669(1–3):71–5. doi: 10.1016/j.ejphar.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glick SD, Maisonneuve IM, Dickinson HA. 18-MC reduces methamphetamine and nicotine self-administration in rats. Neuroreport. 2000a;11(9):2013–5. doi: 10.1097/00001756-200006260-00041. [DOI] [PubMed] [Google Scholar]
- 55.Glick SD, Maisonneuve IM, Visker KE, Fritz KA, Bandarage UK, Kuehne ME. 18-Methoxycoronardine attenuates nicotine-induced dopamine release and nicotine preferences in rats. Psychopharmacology (Berl) 1998;139(3):274–80. doi: 10.1007/s002130050716. [DOI] [PubMed] [Google Scholar]
- 56.McCallum SE, Glick SD. 18-Methoxycoronaridine blocks acquisition but enhances reinstatement of a cocaine place preference. Neurosci Lett. 2009;458(2):57–9. doi: 10.1016/j.neulet.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuehne ME, He L, Jokiel PA, Pace CJ, Fleck MW, Maisonneuve IM, Glick SD, Bidlack JM. Synthesis and biological evaluation of 18-methoxycoronaridine congeners. Potential antiaddiction agents. J Med Chem. 2003;46(13):2716–30. doi: 10.1021/jm020562o. [DOI] [PubMed] [Google Scholar]
- 58.Gorlich A, Antolin-Fontes B, Ables JL, Frahm S, Slimak MA, Dougherty JD, Ibanez-Tallon I. Reexposure to nicotine during withdrawal increases the pacemaking activity of cholinergic habenular neurons. Proc Natl Acad Sci U S A. 2013;110(42):17077–82. doi: 10.1073/pnas.1313103110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology (Berl) 1995;122(4):390–94. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- 60.Malin DH, Goyarzu P. Rodent models of nicotine withdrawal syndrome. Handb Exp Pharmacol. 2009;(192):401–34. doi: 10.1007/978-3-540-69248-5_14. [DOI] [PubMed] [Google Scholar]
- 61.Papke RL, Sanberg PR, Shytle RD. Analysis of mecamylamine stereoisomers on human nicotinic receptor subtypes. J Pharmacol Exp Ther. 2001;297(2):646–56. [PubMed] [Google Scholar]
- 62.Elrashidi MY, Ebbert JO. Emerging drugs for the treatment of tobacco dependence: 2014 update. Expert opinion on emerging drugs. 2014;19(2):243–60. doi: 10.1517/14728214.2014.899580. [DOI] [PubMed] [Google Scholar]
- 63.Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. The Cochrane database of systematic reviews. 2013;5:Cd009329. doi: 10.1002/14651858.CD009329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lancaster T, Stead LF. The Cochrane Library. 1998. Mecamylamine (a nicotine antagonist) for smoking cessation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tennant FS, Jr, Tarver AL. Withdrawal from nicotine dependence using mecamylamine: comparison of three-week and six-week dosage schedules. NIDA Res Monogr. 1984;55:291–7. [PubMed] [Google Scholar]