Abstract
Objective
Experimental studies link oscillatory flow accompanied by flow reversal to impaired endothelial cell function. The relation of flow reversal with vascular function and arterial stiffness remains incompletely defined.
Approach and Results
We measured brachial diastolic flow patterns along with vasodilator function in addition to tonometry-based central and peripheral arterial stiffness in 5708 participants (age 47±13 years, 53% women) in the Framingham Heart Study Offspring and Third Generation cohorts. Brachial artery diastolic flow reversal was present in 35% of participants. In multivariable regression models, the presence of flow reversal was associated with lower flow-mediated dilation (3.9±0.2 vs. 5.0±0.2%, P<0.0001) and reactive hyperemic flow velocity (50±0.99 vs 57±0.93 cm/s, P<0.0001). The presence of flow reversal (compared to absence) was associated with higher central aortic stiffness (carotid-femoral pulse wave velocity 9.3±0.1 vs. 8.9±0.1 m/s), lower muscular artery stiffness (carotid-radial pulse wave velocity 9.6±0.1 vs. 9.8±0.1 m/s) and higher forearm vascular resistance (5.32±0.03 vs. 4.66±0.02 log dyne · s−1 · cm−5), (P<0.0001). The relations of diastolic flow velocity with flow-mediated dilation, aortic stiffness and forearm vascular resistance were nonlinear with a steeper decline in vascular function associated with increasing magnitude of flow reversal.
Conclusions
In our large, community-based sample, brachial artery flow reversal was common and associated with impaired vasodilator function and higher aortic stiffness. Our findings are consistent with the concept that flow reversal may contribute to vascular dysfunction.
Keywords: vascular function, epidemiology, endothelium, arterial stiffness
INTRODUCTION
Arterial blood flow is pulsatile in nature. Experimental studies demonstrate that endothelial cell phenotype and vascular function are strongly influenced by local shear stress, the frictional force exerted by flowing blood.1, 2 Blood flow can be either laminar or turbulent. Atherosclerosis preferentially develops at regions of disturbed flow, and this observation has been attributed to the effects of flow reversal on the vascular endothelium.3–6 Unidirectional laminar flow with pulsatility maintains endothelial health and suppresses inflammatory and thrombotic processes.7–9 Conversely, episodic flow reversal impairs nitric oxide production and generates a pro-atherogenic gene expression profile.10–12 In peripheral arteries, diastolic flow reversal may be present and is more prevalent with aging and sedentary behaviors.13–16
Arterial stiffness may influence flow patterns, and increased stiffness of the central aorta has been proposed to be a determinant of flow reversal in peripheral arteries.17–20 Prior human studies suggest that induction of flow reversal acutely reduces endothelial vasomotor function.21–24 Therefore, we sought to investigate whether flow reversal in the brachial artery relates to measures of endothelial function, including flow-mediated dilation and hyperemic flow. Further, we examined the association of brachial flow reversal and aortic and peripheral arterial stiffness and forearm vascular resistance.
MATERIALS AND METHODS
Materials and methods are available in the online-only Data Supplement.
RESULTS
Participant Characteristics
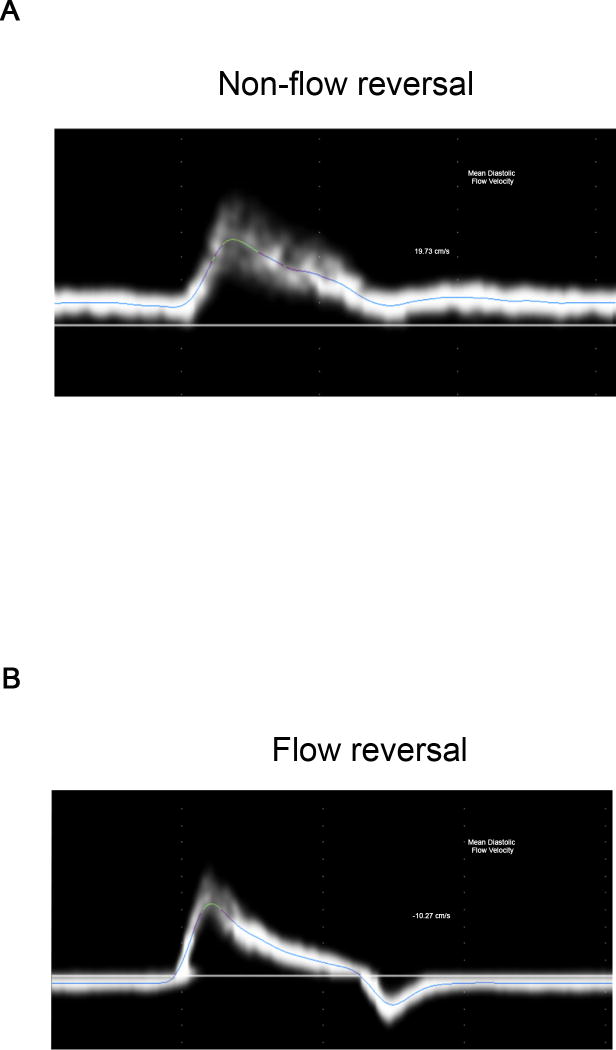
The study sample included 5708 participants, mean age 47±13 years, 53% women. Clinical characteristics and vascular measures are shown in Table 1. Diastolic flow reversal was present in 35% of the participants. Representative examples of flow velocity waveforms are illustrated in Figure 1 showing an individual with unidirectional flow (Panel A) and flow reversal (Panel B).
Table 1.
Participant Characteristics and Vascular Measures
| Characteristic | Offspring | Third Generation |
Overall |
|---|---|---|---|
| N= 2038 | N = 3670 | N = 5708 | |
| Clinical measures | |||
| Age, years | 61 ± 9 | 40 ± 9 | 47 ± 13 |
| Female, % | 54 | 52 | 53 |
| Systolic blood pressure, mmHg | 127 ± 19 | 116 ± 14 | 120 ± 17 |
| Diastolic blood pressure, mmHg | 74 ± 10 | 75 ± 10 | 75 ± 10 |
| Heart rate, beats per minute | 64 ± 11 | 62 ± 9 | 63 ± 10 |
| Body mass index, kg/m2 | 27.4 ± 4.5 | 26.5 ± 5.0 | 26.8 ± 4.9 |
| Total/HDL cholesterol | 4.0 ± 1.3 | 3.8 ± 1.4 | 3.8 ± 1.4 |
| Triglycerides, mg/dl | 134 ± 90 | 114 ± 89 | 121 ± 90 |
| Glucose, mg/dl | 103 ± 26 | 95 ± 18 | 98 ± 22 |
| Diabetes, % | 12 | 3 | 6 |
| Smoking, % | 13 | 17 | 16 |
| Hypertension, % | 44 | 15 | 26 |
| Hormone replacement therapy, % women | 37 | 5 | 16 |
| Menopause, % women | 85 | 14 | 40 |
| Treatment of hypertensive, % | 32 | 8 | 16 |
| Treatment of Lowering-cholesterol, % | 20 | 7 | 12 |
| Walk test Before, % | 39 | 0 | 14 |
| Prevalent CVD, % | 12 | 1 | 5 |
| Vascular measures | |||
| Baseline brachial diameter, mm | 4.2 ± 0.9 | 4.1 ± 0.8 | 4.2 ± 0.8 |
| Flow-mediated dilation, % | 2.9 ± 2.8 | 5.9 ± 3.7 | 4.8 ± 3.7 |
| Baseline Flow velocity, cm/s | 8.05 ± 4.8 | 7.3 ± 4.2 | 7.6 ± 4.4 |
| Hyperemic Flow velocity, cm/s | 51.1 ± 21.5 | 62.1 ± 18.3 | 58.2 ± 20.2 |
| Carotid-Femoral PWV, m/s | 10.0 ± 3.5 | 7.0 ± 1.4 | 8.1 ± 2.8 |
| Carotid-Radial PWV, m/s | 10.1 ± 1.5 | 9.5 ± 1.5 | 9.7 ± 1.5 |
| Flow reversal, % | 54 | 24 | 35 |
| Baseline FVR (log dyne · s−1 · cm−5) | 4.87 ± 0.73 | 4.99 ± 0.68 | 4.95 ± 0.7 |
Continuous variables expressed as mean ± sd
PWV: pulse wave velocity
FVR: forearm vascular resistance
Figure 1.
A. Representative flow velocity tracing from a participant with no flow reversal B. Representative flow velocity tracing from a participant with flow reversal.
Clinical Correlates of Flow Reversal
Clinical correlates of diastolic flow reversal in age- sex- and cohort- adjusted and multivariable-adjusted models are presented in Table 2. Increasing age and female sex were associated with higher prevalence of flow reversal. Among women there was a modest association of menopause with a lower prevalence of flow reversal in age-adjusted models, which becomes non-significant in multivariable adjusted models. Several cardiovascular disease risk factors, including higher heart rate, higher body mass index, higher total/HDL cholesterol ratio, and smoking were associated with a lower prevalence of flow reversal. Participating in the walk test prior to vascular testing was associated with a lower prevalence of flow reversal.
Table 2.
Relations of Flow Reversal with Clinical Characteristics
|
|
||||
|---|---|---|---|---|
| Flow Reversal | ||||
| Characteristic | Age-Sex-Cohort | Multivariable | ||
| OR (95% CI)* | P | OR (95% CI) | P | |
| Age | 2.60 (2.36, 2.87) | <0.0001 | 2.79 (2.51, 3.09) | <0.0001 |
| Sex, female vs. male | 3.44 (3.03, 3.92) | <0.0001 | 2.94 (2.53, 3.40) | <0.0001 |
| Mean arterial blood pressure | 0.85 (0.79, 0.90) | <.0001 | -- | -- |
| Heart rate | 0.83 (0.77, 0.88) | <0.0001 | 0.90 (0.84, 0.96) | 0.003 |
| BMI | 0.83 (0.77, 0.88) | <0.0001 | 0.63 (0.59, 0.68) | <0.0001 |
| Total/HDL cholesterol | 0.75 (0.69, 0.81) | <0.0001 | 0.90 (0.83, 0.98) | 0.01 |
| Triglycerides, mg/dL | 0.80 (0.74, 0.86) | <0.0001 | -- | -- |
| Fasting glucose, mg/dL | 0.88 (0.82, 0.94) | 0.0003 | -- | -- |
| Diabetes | 0.94 (0.73, 1.20) | 0.61 | -- | -- |
| Smoking | 0.41 (0.34, 0.49) | <0.0001 | 0.39 (0.32, 0.48) | <0.0001 |
| Hormone Replacement Therapy | 0.86 (0.69, 1.07) | 0.17 | -- | -- |
| Menopause, yes vs no | 0.74 (0.58, 0.96) | 0.021 | 0.81 (0.62, 1.06) | 0.12 |
| Hypertension treatment | 0.79 (0.67, 0.94) | 0.009 | -- | -- |
| Walk Test | 0.71 (0.59, 0.87) | 0.0007 | 0.69 (0.56, 0.84) | 0.0003 |
| Lipid lowering treatment | 0.95 (0.78, 1.16) | 0.61 | -- | -- |
| Prevalent CVD | 1.35 (1.03, 1.78) | 0.03 | -- | -- |
OR = odds ratio; CI = Confidence interval.
Continuous variables expressed per 1 SD change; see table 1 for SD values
Association of Flow Reversal with Vasodilator Function
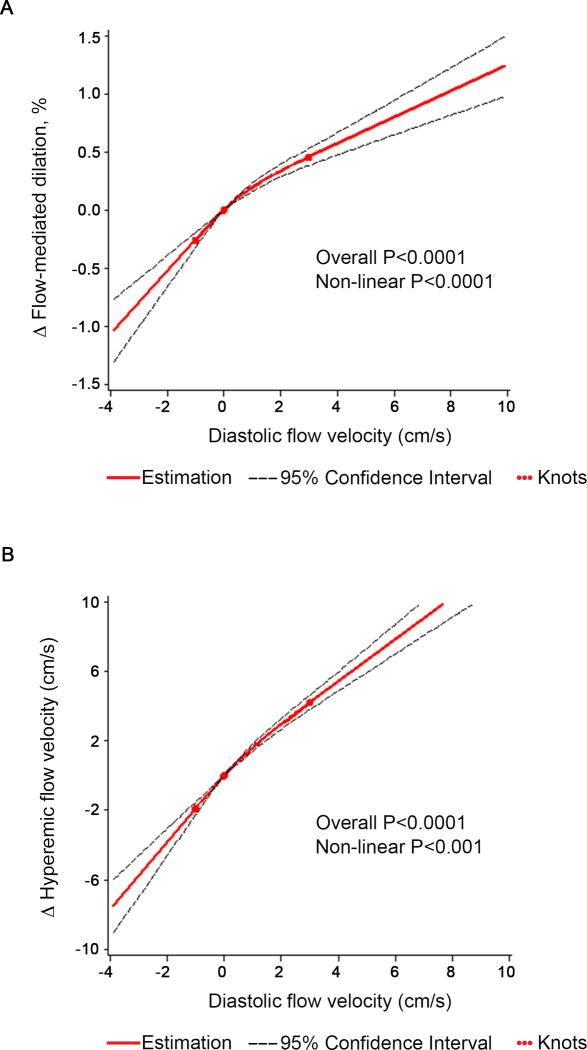
To gain insight into the association of flow reversal with endothelial function, we compared mean values of vascular measures between participants with and without diastolic flow reversal. We used linear regression models adjusted for age, sex and cohort initially, and additionally adjusted for clinical covariates, including mean arterial pressure, heart rate, body-mass index, total/HDL cholesterol ratio, triglycerides, glucose, diabetes, current smoking, hypertension treatment, lowering lipid treatment, walk test, and prevalent CVD (Table 3). Both flow-mediated dilation and reactive hyperemia were lower in individuals with flow reversal compared to individuals without flow reversal. Further, we evaluated the continuous relation of diastolic flow and vasodilator measures using multivariable-adjusted spline analyses with knots at the 20th, 50th (0cm/s) and 80th percentiles of diastolic flow velocity (Figure 2). Lower diastolic flow velocity was associated with lower flow-mediated dilation; however, the relation was non-linear (p<0.0001). Greater degrees of flow reversal were associated with a steep decline in flow-mediated dilation. In contrast, the difference in flow-mediated dilation was more gradual with increasingly positive diastolic flow velocity (Figure 2A). A similar pattern was observed for reactive hyperemia (Figure 2B). Further, the association of diastolic flow velocity with flow-mediated dilation adjusted for hyperemic flow showed the same pattern (Figure I in the online-only Data Supplement).
Table 3.
Relations of Vascular Measures with Brachial Artery Flow Reversal
| Age-Sex-Cohort | Multivariable* | |||||
|---|---|---|---|---|---|---|
| Flow Reversal | Flow Reversal | |||||
| Yes N=1994 |
No N=3714 |
Yes N=1994 |
No N=3714 |
|||
| Measurement | LSM (SE)** | LSM(SE) | P | LSM (SE) | LSM(SE) | P |
| Baseline brachial diameter, mm | 4.11 (0.01) | 4.22 (0.01) | <0.0001 | 4.16 (0.03) | 4.20 (0.03) | <0.01 |
| Flow-mediated dilation, % | 3.9 (0.08) | 4.9 (0.06) | <0.0001 | 3.9 (0.17) | 5.0 (0.16) | <0.0001 |
| Hyperemic Flow velocity, cm/s | 53 (0.46) | 60 (0.33) | <0.0001 | 50 (0.99) | 57 (0.93) | <0.0001 |
| Carotid-Femoral PWV***, m/s | 8.3 (0.05) | 8.1 (0.04) | 0.0016 | 9.3 (0.10) | 8.9 (0.09) | <0.0001 |
| Carotid-Radial PWV, m/s | 9.5 (0.03) | 9.8 (0.02) | <0.0001 | 9.6 (0.07) | 9.8 (0.06) | <0.0001 |
| Baseline FVR (resting), (log dyne · s−1 · cm−5) | 5.38 (0.01) | 4.64 (0.01) | <0.0001 | 5.32 (0.03) | 4.66 (0.02) | <0.0001 |
Multivariable model adjusted for age, sex, cohort, mean arterial pressure, heart rate, body-mass index, total/high density lipoprotein cholesterol ratio, triglycerides, glucose, diabetes, current smoking, hypertension treatment, lowering lipid treatment, walk test (before), prevalent CVD.
LSM = least squares mean, SE = standard error.
PWV = pulse wave velocity.
Figure 2.
Multivariable-adjusted association of diastolic flow velocity and vasodilator measures (flow-mediated dilation and hyperemic flow velocity) using restricted cubic splines with 3 knots at diastolic flow velocity at the 20th, 50th, and 80th percentile. Analyses were based on the entire cohort; for visual display the range of mean diastolic flow shown on the x-axis was limited to the 5th to 95th percentile. The y-axis represents the difference in vasodilator response. The solid line shows the association of mean diastolic flow velocity and vasodilator measures. The dashed lines represent the 95% confidence interval.
As shown in Table 4, the associations of diastolic flow velocity with flow-mediated dilation and with hyperemic flow velocity were stronger in the presence of flow reversal compared to no flow reversal.
Table 4.
Multivariable-Adjusted Association of Diastolic Flow Velocity with Vascular Function Measures by Flow Reversal Status
| Flow Reversal | No Flow Reversal | |||||
|---|---|---|---|---|---|---|
| Est. β* (SE) | P | Est. β (SE) | P | Δβ (SE)† | P‡ | |
| Flow-mediated dilation, % | 0.23 (0.04) | <0.0001 | 0.13 (0.01) | <0.0001 | −0.10 (0.04) | 0.04 |
| Hyperemic flow velocity, cm/s | 1.87 (0.23) | <0.0001 | 1.27 (0.08) | <0.0001 | −0.60 (0.26) | 0.02 |
| Carotid femoral PWV §, m/s | −0.26 (0.02) | <0.0001 | −0.02 (0.02) | 0.004 | 0.24 (0.02) | <0.0001 |
| Carotid radial PWV, m/s | 0.14 (0.02) | <0.0001 | −0.01 (0.01) | 0.62 | −0.13 (0.02) | <0.0001 |
| Log baseline FVR (resting), (dyne · s−1 · cm−5) | −0.15 (0.01) | <0.0001 | −0.12 (0.01) | <0.0001 | 0.03 (0.01) | <0.0001 |
Multivariable model adjusted for age, sex, cohort, mean arterial pressure, heart rate, body mass index, total/hdl cholesterol ratio, triglycerides, glucose, diabetes, current smoking, hypertension treatment, lowering lipid treatment, hormone replacement therapy, walk test (before), prevalent CVD.
β-represents the slopes of outcome variable versus diastolic flow velocity.
Difference in β estimates comparing flow reversal to no flow reversal (Δβ = β (no flow) − β (flow).
For comparison of β for the association of diastolic flow velocity with vascular measures for flow reversal compared to no flow reversal, from test of Ho: Δβ = 0.
PWV denotes pulse wave velocity
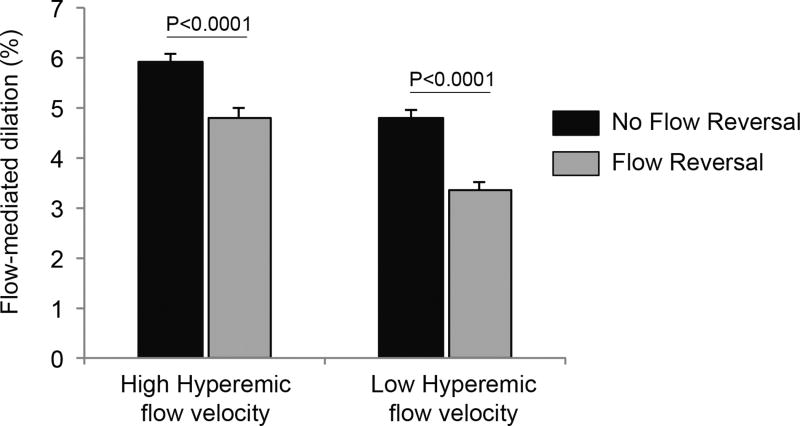
Further, there was evidence of effect modification by diastolic flow reversal of the association between hyperemic flow velocity and flow-mediated dilation (P-value for interaction=0.03). As shown in Figure 3, the presence of diastolic flow reversal is associated with lower flow-mediated dilation function for both individuals with high and low hyperemic flow. These findings suggest that the association of flow reversal and flow-mediated dilation is not simply reflective of lower stimulus for dilation.
Figure 3.
Multivariable adjusted least square means with standard error of flow-mediated dilation in high and low hyperemic flow velocity categories according to presence or absence of flow reversal. The hyperemic flow velocity categories are defined as high (hyperemic flow velocity above the overall median ≥ 58 cm/s), and low (hyperemic flow velocity below the overall median < 58 cm/s).
Association of Flow Reversal with Arterial Stiffness
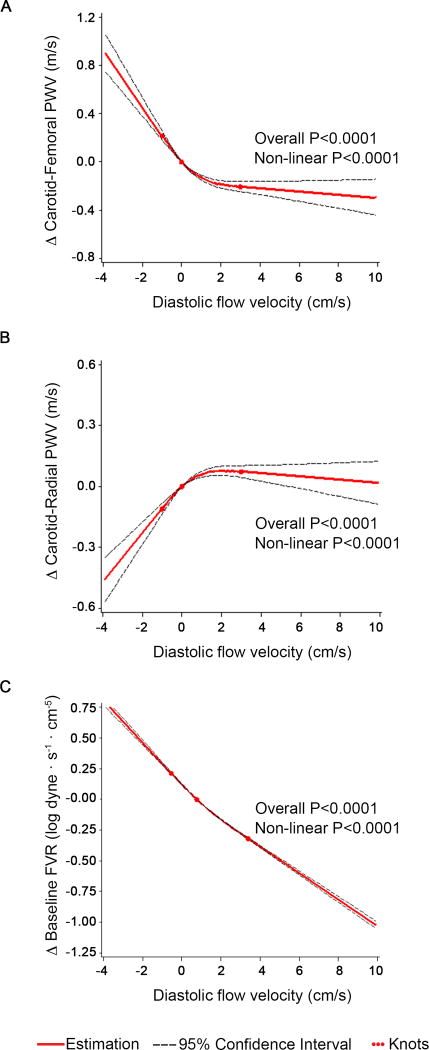
In multivariable models adjusting for clinical covariates, central aortic stiffness measured by carotid femoral pulse wave velocity was higher in individuals with flow reversal compared to individuals without flow reversal (Table 3). In spline analysis, the relation of diastolic flow velocity with carotid femoral pulse wave velocity was also non-linear (Figure 4). Increasingly negative diastolic flow velocity was associated with marked increase in carotid femoral pulse wave velocity consistent with greater aortic stiffness. The difference in carotid femoral pulse wave velocity was minimal with increasingly positive diastolic flow velocity above the median. As shown in Table 4, the association of diastolic flow velocity with carotid-femoral pulse wave velocity was stronger in the presence of flow reversal compared to no flow reversal.
Figure 4.
Multivariable-adjusted association of diastolic flow velocity with arterial stiffness and resistance measures (carotid-femoral pulse wave velocity, carotid-radial pulse wave velocity and forearm vascular resistance) using restricted cubic splines with 3 knots at diastolic flow velocity at 20th, 50th, and 80th percentiles. Analyses were based on the entire cohort; for visual display the range of mean diastolic flow velocity shown on the x-axis was limited to the 5th to 95th percentile. The y-axis represents the difference in arterial stiffness measures or forearm vascular resistance respectively. The solid line shows the association of mean diastolic flow velocity and stiffness and resistance measures. The dashed lines represent the 95% confidence interval.
In contrast, muscular artery stiffness measured by carotid radial pulse wave velocity was lower in the presence of flow reversal (Table 3). In spline analysis, the relation of diastolic flow velocity with carotid radial pulse wave velocity was also non-linear (Figure 4B). More negative diastolic flow velocity was associated with lower carotid radial pulse wave velocity consistent with lower muscular artery stiffness. The difference in carotid radial pulse wave velocity was minimal with increasingly positive diastolic flow velocity above the median. As shown in Table 4, the association of diastolic flow velocity with carotid-radial pulse wave velocity was stronger in the presence of flow reversal compared to no flow reversal.
Association of Flow Reversal with Vascular Resistance
Peripheral artery resistance may contribute to flow reversal. In both minimally and multivariable adjusted models, baseline forearm vascular resistance (FVR) was higher in individuals with flow reversal when comparing with individuals with non-reversal flow (Table 3). In spline analysis, the relation of diastolic flow velocity with forearm vascular resistance was non-linear (Figure 4). Greater degrees of flow reversal were associated with markedly higher FVR. As shown in Table 4, the association of diastolic flow velocity with FVR was stronger in the presence of flow reversal compared to no flow reversal. In age-, sex- and cohort-adjusted analyses, there was not a significant correlation of carotid-radial pulse wave velocity (partial r=−0.013, P=0.31).
DISCUSSION
In the present study, we investigated the relations of flow patterns in the brachial artery with vasodilator function and arterial stiffness. In a community-based study, we observed that flow reversal during diastole was present in more than a third of participants. In models adjusting for conventional risk factors, we noted that the presence of diastolic flow reversal was related to lower vasodilation in both the conduit brachial artery and forearm microcirculation. Diastolic flow reversal was associated with impaired flow-mediated dilation in individuals with low or high reactive hyperemic responses suggesting an independent association with conduit vasodilator function. Further, we demonstrated that an increasing magnitude of flow reversal was associated with a greater decrease in vasodilator function. Flow reversal related to higher carotid femoral pulse wave velocity and higher forearm vascular resistance, consistent with the possibility that reduced central arterial compliance alters flow patterns in the peripheral arteries. Conversely, muscular arterial stiffness was lower in the presence of flow reversal. Thus, our findings indicate that flow reversal in peripheral arteries is accompanied by vascular dysfunction and aortic stiffening.
Extensive experimental evidence links local flow disturbance to altered endothelial cell properties.2 Multiple studies in cell culture and animal models have shown that oscillatory flow induces a pathologic endothelial state and flow reversal suppresses endothelial nitric oxide synthase expression and activation as well increases oxidative stress.4, 25–28 Prior human studies suggest an impact of flow patterns on endothelial vasodilator function. In coronary arteries, endothelial dysfunction is more pronounced at branch points, regions characterized by flow turbulence.29 External counterpulsation therapy in patients with coronary artery disease improved flow-mediated dilation potentially through reduction of brachial flow reversal in diastole.30 Importantly, intervention studies by Thijssen et al provide compelling evidence that flow reversal impacts endothelial function acutely and over a two-week period.21, 23 In healthy volunteers, manipulation of brachial artery blood flow and shear to generate acute flow reversal lead reduced flow-mediated dilation.23 Further, augmentation of retrograde flow with a compression sleeve worn for two weeks reduced flow-mediated dilation in young but not old men.21 Reduction of retrograde flow is related to the endothelial benefit of exercise interventions.31, 32 The association of flow reversal with vascular function and arterial stiffness in a community-based sample remains uncertain.
The current study evaluates flow reversal and vascular function in a large, unselected sample with comprehensive risk factor assessment. Flow-mediated dilation is a key physiologic regulator of blood flow that occurs in response to acute changes in shear stress through endothelial release of nitric oxide and other vasodilators.33 Reactive hyperemia reflects microvessel dilation produced by ischemia-mediated vasodilator generation including nitric oxide.34–36 By evaluating average diastolic flow, we identified that more than a third of the participants had evidence of flow reversal, with mean diastolic flow ≤0. Consistent with prior reports, advancing age was associated with higher prevalence of flow reversal.13–15 Interestingly several cardiovascular risk factors were associated with a lower prevalence of flow reversal higher total/HDL cholesterol ratio, smoking and higher body mass index. We have previously demonstrated that a similar set of cardiovascular risk factors was associated with higher baseline forearm flow and reduced FVR potentially reflecting resting vasodilation or microvascular remodeling.36–39 Similarly, female sex was previously associated with lower resting flow and we now report a higher prevalence of flow reversal.37 Higher resting flow with lower FVR may limit flow reversal.
We observed that brachial flow reversal was associated with impaired flow-mediated dilation and reactive hyperemia even after adjusting for risk factors suggesting that local flow dynamics alter vascular function. In addition, flow reversal was associated with impaired flow-mediated dilation both in individuals with low and high hyperemic responses consistent with an association flow reversal with endothelial dysfunction. Our findings support the possibility that flow reversal influences vascular function. Alternatively, abnormal endothelial function may contribute to the generation of diastolic flow reversal.14
Arterial stiffness may influence flow patterns in peripheral arteries. In the present study we observed elevated aortic stiffness in association with brachial flow reversal supporting an intersection of central arterial structure and peripheral arterial dynamics. Under physiological conditions, elastic recoil of the aorta helps maintain forward flow in peripheral arteries during diastole. Stiffening of the central aorta tends to increase systolic flow amplitude and induce diastolic flow reversal in the periphery.16, 40 Furthermore, several studies have suggested that elevated aortic stiffness may lead to increased peripheral resistance and have detrimental effects on microvessel structure and function that may augment flow reversal in the periphery.36, 37 Alternatively endothelial dysfunction may promote arterial stiffness that in turn influences peripheral flow patterns.41, 42 Central flow patterns in the aorta were not measured in the current study thus the contribution of aortic flow reversal to aortic stiffness cannot be assessed. However, prior studies indicate a contribution of endothelial nitric oxide to functional aortic stiffness that may account for the association of carotid femoral pulse wave velocity with peripheral flow reversal.41 The cross-sectional nature of the present study precludes a precise determination of the temporal sequence of central arterial stiffening, endothelial dysfunction, and peripheral flow reversal. However, the observed association of carotid femoral pulse wave velocity and flow reversal is consistent with an intersection of central aortic properties and peripheral flow patterns.
Stiffness of the brachial artery may alter the effects of flow on the endothelium. We observed divergent patterns in the association of central and muscular artery stiffness with arterial flow patterns. In the presence of flow reversal, lower peripheral stiffness was associated with a greater degree of flow reversal; however, in the absence of flow reversal, there was no association between diastolic flow velocity and carotid-radial pulse wave velocity. Our finding of lower peripheral artery stiffness in the presence of flow reversal suggests complex interrelations of regional stiffness, flow patterns, and vascular function. In experimental models, local compliance modulates the effects of oscillatory flow on endothelial phenotype.43 Circumferential stretch of the arterial wall, which occurs during systole, and relates to higher arterial distensibility, is associated with increased oxidative stress.14 Further, a stiffened central aorta accompanied by compliant muscular arteries permits increased forward wave penetration that may damage the microcirculation thereby augmenting resting flow reversal.37, 40, 44 Importantly, peripheral conductance measure as forearm vascular resistance does not associate with peripheral stiffness, confirming that forearm vascular resistance is largely determined at the arterial level. Thus, it appears that flow reversal is associated with higher small vessel resistance potentially related to microvascular vasodilator dysfunction (indicated by lower reactive hyperemia) but not with higher muscular artery stiffness. Lower muscular artery stiffness may promote small vessel damage by transmission of pulsatile forces into the microcirculation.37
Several limitations of the current study must be considered. The study design is cross-sectional. Thus, the directionality of the associations between flow patterns, endothelial function, and arterial stiffness cannot be determined. It is likely that flow patterns both alter and are altered by vasomotor function. However, the present study cannot evaluate the temporal sequence of these changes. We based our evaluation of diastolic flow patterns on mean diastolic flow velocity and did not measure minimum diastolic flow. Therefore, participants with transient, lesser degrees of diastolic flow reversal may have been assigned to the no flow reversal group. We would expect that misclassification of flow reversal would tend to make associations with vascular function measures more difficult to detect. Further, the spline analysis using diastolic flow velocity as a continuous measure confirmed the findings of the categorical analysis. The cohort consists of predominantly white participants potentially limiting generalizability to other racial and ethnic groups. Prior studies have suggested variability in flow-mediated dilation across the menstrual cycle and the scheduling of participant visits was not timed to menstrual cycle that may have introduced variability into the vascular measurements. Based on scheduling, some of the participants had a walk test performed prior to vascular testing that may influence flow patterns. We included an adjustment for this parameter in multivariable modeling. Given the community-based study design, we did not withhold medications or administer nitroglycerin to assess endothelium-independent vasodilation.
In summary, we provide evidence supporting a link between peripheral artery flow reversal and vascular function. Our observations are consistent with the hypothesis that flow reversal may influence endothelial function and impair vasodilator capacity. Taken together, our findings highlight flow reversal as an emerging vascular measure that relates to both central arterial stiffness and vasodilator function. Future longitudinal studies are required to evaluate the potential contribution of flow patterns to the risk for hypertension and cardiovascular events.
Supplementary Material
Highlights.
-
-
Retrograde flow impairs endothelial cell function and phenotype. Arterial stiffness may contribute to peripheral flow reversal.
-
-
We measured brachial diastolic flow patterns in a large community-based cohort. Flow reversal was present in more than a third of the participants and associated with advancing age.
-
-
Greater degrees of flow reversal were associated with reduced flow-mediated dilation and reactive hyperemia flow velocity consistent with conduit and small vessel endothelial dysfunction.
-
-
Brachial artery flow reversal was associated with higher central aortic stiffness, lower muscular artery stiffness and higher forearm vascular resistance.
-
-
Our findings support a link between flow reversal in the peripheral arteries with impaired vascular function and higher central arterial stiffness, and are consistent with the hypothesis that flow reversal may contribute to endothelial dysfunction and impair vasodilator capacity.
Acknowledgments
Sources of Funding
The Framingham Heart Study is funded by NIH/NHLBI contract N01-HC25195 and HHSN268201500001l. The project was supported NIH grants R01HL107385, 1R01HL126136-01, 1R01HL60040, HL70100, HL076784, AG028321, HL080124, 2K24-HL4334 and the Donald W. Reynolds Foundation. Dr. Hamburg is supported by NIH grants HL083781 and HL102299. Dr. Breton-Romero is supported by an American Heart Association Postdoctoral Fellowship 16POST27260178. Dr. Vasan is partly supported by the Evans Scholar award from the Department of Medicine at Boston University School of Medicine.
Dr Mitchell is owner of Cardiovascular Engineering, Inc., a company that designs and manufactures vascular stiffness measurement devices.
Footnotes
Disclosures
The other authors report no conflicts.
References
- 1.Silver AE, Vita JA. Shear-stress-mediated arterial remodeling in atherosclerosis: Too much of a good thing? Circulation. 2006;113:2787–2789. doi: 10.1161/CIRCULATIONAHA.106.634378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conway DE, Williams MR, Eskin SG, McIntire LV. Endothelial cell responses to atheroprone flow are driven by two separate flow components: Low time-average shear stress and fluid flow reversal. Am J Physiol Heart Circ Physiol. 2010;298:H367–374. doi: 10.1152/ajpheart.00565.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, Garcia-Cardena G, Gimbrone MA., Jr Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc. Natl. Acad. Sci. U. S. A. 2004;101:14871–14876. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gambillara V, Chambaz C, Montorzi G, Roy S, Stergiopulos N, Silacci P. Plaque-prone hemodynamics impair endothelial function in pig carotid arteries. Am J Physiol Heart Circ Physiol. 2006;290:H2320–2328. doi: 10.1152/ajpheart.00486.2005. [DOI] [PubMed] [Google Scholar]
- 6.Hastings NE, Simmers MB, McDonald OG, Wamhoff BR, Blackman BR. Atherosclerosis-prone hemodynamics differentially regulates endothelial and smooth muscle cell phenotypes and promotes pro-inflammatory priming. Am J Physiol Cell Physiol. 2007;293:C1824–1833. doi: 10.1152/ajpcell.00385.2007. [DOI] [PubMed] [Google Scholar]
- 7.Sorescu GP, Sykes M, Weiss D, Platt MO, Saha A, Hwang J, Boyd N, Boo YC, Vega JD, Taylor WR, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J. Biol. Chem. 2003;278:31128–31135. doi: 10.1074/jbc.M300703200. [DOI] [PubMed] [Google Scholar]
- 8.Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, Dikalov S, Giddens DP, Griendling KK, Harrison DG, Jo H. Oscillatory shear stress stimulates endothelial production of o2- from p47phox-dependent nad(p)h oxidases, leading to monocyte adhesion. The Journal of biological chemistry. 2003;278:47291–47298. doi: 10.1074/jbc.M305150200. [DOI] [PubMed] [Google Scholar]
- 9.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu X, Kassab GS. Nitric oxide is significantly reduced in ex vivo porcine arteries during reverse flow because of increased superoxide production. J Physiol. 2004;561:575–582. doi: 10.1113/jphysiol.2004.075218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: Molecular, cellular, and vascular behavior. J. Am. Coll. Cardiol. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 12.Zhou J, Li YS, Chien S. Shear stress-initiated signaling and its regulation of endothelial function. Arterioscler Thromb Vasc Biol. 2014;34:2191–2198. doi: 10.1161/ATVBAHA.114.303422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Credeur DP, Dobrosielski DA, Arce-Esquivel AA, Welsch MA. Brachial artery retrograde flow increases with age: Relationship to physical function. Eur J Appl Physiol. 2009;107:219–225. doi: 10.1007/s00421-009-1117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padilla J, Simmons GH, Fadel PJ, Laughlin MH, Joyner MJ, Casey DP. Impact of aging on conduit artery retrograde and oscillatory shear at rest and during exercise: Role of nitric oxide. Hypertension. 2011;57:484–489. doi: 10.1161/HYPERTENSIONAHA.110.165365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young CN, Deo SH, Padilla J, Laughlin MH, Fadel PJ. Pro-atherogenic shear rate patterns in the femoral artery of healthy older adults. Atherosclerosis. 2010;211:390–392. doi: 10.1016/j.atherosclerosis.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baccelli G, Pignoli P, Corbellini E, Pizzolati PL, Bassini M, Longo T, Zanchetti A. Hemodynamic factors changing blood flow velocity waveform and profile in normal human brachial artery. Angiology. 1985;36:1–8. doi: 10.1177/000331978503600101. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell GF, Vita JA, Larson MG, Parise H, Keyes MJ, Warner E, Vasan RS, Levy D, Benjamin EJ. Cross-sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: The framingham heart study. Circulation. 2005;112:3722–3728. doi: 10.1161/CIRCULATIONAHA.105.551168. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto J, Ito S. Aortic stiffness determines diastolic blood flow reversal in the descending thoracic aorta: Potential implication for retrograde embolic stroke in hypertension. Hypertension. 2013;62:542–549. doi: 10.1161/HYPERTENSIONAHA.113.01318. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell GF. Arterial stiffness and wave reflection: Biomarkers of cardiovascular risk. Artery Res. 2009;3:56–64. doi: 10.1016/j.artres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vita JA, Mitchell GF. Effects of shear stress and flow pulsatility on endothelial function: Insights gleaned from external counterpulsation therapy. J Am Coll Cardiol. 2003;42:2096–2098. doi: 10.1016/j.jacc.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Thijssen DH, Schreuder TH, Newcomer SW, Laughlin MH, Hopman MT, Green DJ. Impact of 2-weeks continuous increase in retrograde shear stress on brachial artery vasomotor function in young and older men. J Am Heart Assoc. 2015;4:e001968. doi: 10.1161/JAHA.115.001968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreuder TH, Green DJ, Hopman MT, Thijssen DH. Acute impact of retrograde shear rate on brachial and superficial femoral artery flow-mediated dilation in humans. Physiol Rep. 2014;2:e00193. doi: 10.1002/phy2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension. 2009;53:986–992. doi: 10.1161/HYPERTENSIONAHA.109.131508. [DOI] [PubMed] [Google Scholar]
- 24.Tinken TM, Thijssen DH, Hopkins N, Black MA, Dawson EA, Minson CT, Newcomer SC, Laughlin MH, Cable NT, Green DJ. Impact of shear rate modulation on vascular function in humans. Hypertension. 2009;54:278–285. doi: 10.1161/HYPERTENSIONAHA.109.134361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng C, van HR, de WM, van Damme LC, Tempel D, Hanemaaijer L, van Cappellen GW, Bos J, Slager CJ, Duncker DJ, van der Steen AF, de CR, Krams R. Shear stress affects the intracellular distribution of enos: Direct demonstration by a novel in vivo technique. Blood. 2005;106:3691–3698. doi: 10.1182/blood-2005-06-2326. [DOI] [PubMed] [Google Scholar]
- 26.De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: Role of a superoxide-producing nadh oxidase. Circ Res. 1998;82:1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- 27.Silacci P, Desgeorges A, Mazzolai L, Chambaz C, Hayoz D. Flow pulsatility is a critical determinant of oxidative stress in endothelial cells. Hypertension. 2001;38:1162–1166. doi: 10.1161/hy1101.095993. [DOI] [PubMed] [Google Scholar]
- 28.Choy JS, Lu X, Yang J, Zhang ZD, Kassab GS. Endothelial actin depolymerization mediates nadph oxidase-superoxide production during flow reversal. Am J Physiol Heart Circ Physiol. 2014;306:H69–77. doi: 10.1152/ajpheart.00402.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLenachan JM, Vita J, Fish DR, Treasure CB, Cox DA, Ganz P, Selwyn AP. Early evidence of endothelial vasodilator dysfunction at coronary branch points. Circulation. 1990;82:1169–1173. doi: 10.1161/01.cir.82.4.1169. [DOI] [PubMed] [Google Scholar]
- 30.Vita JA, Mitchell GF. Effects of shear stress and flow pulsatility on endothelial function: Insights gleaned from external counterpulsation therapy. J Am Coll Cardiol. 2003;42:2096–2098. doi: 10.1016/j.jacc.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 31.Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension. 2010;55:312–318. doi: 10.1161/HYPERTENSIONAHA.109.146282. [DOI] [PubMed] [Google Scholar]
- 32.Simmons GH, Padilla J, Young CN, Wong BJ, Lang JA, Davis MJ, Laughlin MH, Fadel PJ. Increased brachial artery retrograde shear rate at exercise onset is abolished during prolonged cycling: Role of thermoregulatory vasodilation. J Appl. Physiol. 2011;110:389–397. doi: 10.1152/japplphysiol.00936.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ganz P, Vita JA. Testing endothelial vasomotor function: Nitric oxide, a multipotent molecule. Circulation. 2003;108:2049–2053. doi: 10.1161/01.CIR.0000089507.19675.F9. [DOI] [PubMed] [Google Scholar]
- 34.Philpott A, Anderson TJ. Reactive hyperemia and cardiovascular risk. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:2065–2067. doi: 10.1161/ATVBAHA.107.149740. [DOI] [PubMed] [Google Scholar]
- 35.Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, Hildebrand K, Fung M, Verma S, Lonn EM. Microvascular function predicts cardiovascular events in primary prevention: Long-term results from the firefighters and their endothelium (fate) study. Circulation. 2011;123:163–169. doi: 10.1161/CIRCULATIONAHA.110.953653. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF, Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: The framingham heart study. Hypertension. 2004;44:134–139. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell GF, Vita JA, Larson MG, Parise H, Keyes MJ, Warner E, Vasan RS, Levy D, Benjamin EJ. Cross-sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness. The framingham heart study. Circulation. 2005 doi: 10.1161/CIRCULATIONAHA.105.551168. [DOI] [PubMed] [Google Scholar]
- 38.Chung WB, Hamburg NM, Holbrook M, Shenouda SM, Dohadwala MM, Terry DF, Gokce N, Vita JA. The brachial artery remodels to maintain local shear stress despite the presence of cardiovascular risk factors. Arterioscler. Thromb. Vasc. Biol. 2009;29:606–612. doi: 10.1161/ATVBAHA.108.181495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamburg NM, Mott MM, Bigornia SJ, Duess MA, Kluge MA, Hess DT, Apovian CM, Vita JA, Gokce N. Maladaptive enlargement of the brachial artery in severe obesity is reversed with weight loss. Vasc Med. 2010;15:215–222. doi: 10.1177/1358863X10362831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS. Hemodynamic correlates of blood pressure across the adult age spectrum: Noninvasive evaluation in the framingham heart study. Circulation. 2010;122:1379–1386. doi: 10.1161/CIRCULATIONAHA.109.914507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilkinson IB, MacCallum H, Cockcroft JR, Webb DJ. Inhibition of basal nitric oxide synthesis increases aortic augmentation index and pulse wave velocity in vivo. Br. J. Clin. Pharmacol. 2002;53:189–192. doi: 10.1046/j.1365-2125.2002.1528adoc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilkinson IB, Franklin SS, Cockcroft JR. Nitric oxide and the regulation of large artery stiffness: From physiology to pharmacology. Hypertension. 2004;44:112–116. doi: 10.1161/01.HYP.0000138068.03893.40. [DOI] [PubMed] [Google Scholar]
- 43.Peng X, Haldar S, Deshpande S, Irani K, Kass DA. Wall stiffness suppresses akt/enos and cytoprotection in pulse-perfused endothelium. Hypertension. 2003;41:378–381. doi: 10.1161/01.hyp.0000049624.99844.3d. [DOI] [PubMed] [Google Scholar]
- 44.Halliwill JR, Minson CT. Retrograde shear: Backwards into the future? Am J Physiol Heart Circ. Physiol. 2010;298:H1126–H1127. doi: 10.1152/ajpheart.00174.2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.