Summary
Introduction
Since premature males are more likely to be diagnosed with bronchopulmonary dysplasia we hypothesized that differences in respiratory outcomes after initial hospital discharge and during the first 3 years of life would exist between females and males diagnosed with BPD.
Methods
Subjects with the diagnosis of BPD were recruited from the Johns Hopkins Bronchopulmonary Dysplasia Clinic between 2008 and 2014. Clinical features were assessed through chart review (n = 482). Respiratory morbidities were assessed by caregiver questionnaires at clinic visits (n = 429), including emergency department visits, hospital admissions, systemic steroid use, and antibiotic use for respiratory reasons since the last BPD clinic visit or after initial hospital discharge if assessed at the first visit.
Results
Male infants weighed significantly more at birth, had higher birth weight percentiles and were more likely to be non-white compared to female infants. The frequency of ever acute care use was 36.9% for emergency department visits, 27.4% for hospital admissions, 36.9% for systemic steroid use, and 40.5% for antibiotic use for a respiratory illness. No differences in respiratory morbidities were found between males and females. Females however, tended to be weaned from supplemental oxygen over 3 months later than males.
Conclusions
Compared to females with BPD, males were more likely to weigh more, have higher birth weight percentiles and be non-white. After initial hospital discharge, there were no difference in respiratory morbidities between males and females with BPD. Female infants however were more likely to be weaned from supplemental oxygen at a later age than male infants.
Keywords: bronchopulmonary dysplasia, prematurity, preterm, gender, sex
INTRODUCTION
Advances in neonatal care continue to improve survival of preterm infants.1,2 However, despite the many technological improvements the risk of developing bronchopulmonary dysplasia (BPD) remains high, especially among the lowest birth weight infants (<1,000 g).3,4 Due to the chronicity of respiratory symptoms, many children with BPD require prolong outpatient management for their lung disease following initial hospital discharge.
Studies have demonstrated, a predilection for BPD development in male versus female preterm infants5,6 with some studies reporting that very low birth weight (<1,500g) males have a 1.36–2.00 risk of developing BPD compared to female infants.7–12 Males also have been shown to have a higher risk of developing more severe BPD.3,6 Differences in hormones, surfactant homeostasis, and/or corticosteroid responses may under-lie these gender differences.13 Other studies reported, a higher risk of readmission and respiratory morbidities in male preterm infants with or without BPD, with male infants 1.33 times more likely to be readmitted by 18–22 months of age14 and 1.5 times more likely to have respiratory morbidities at 2 years of age.15
Nevertheless, few studies have examined gender differences in children with BPD after initial hospital discharge, with regard to patient characteristics and respiratory outcomes. For instance, little is known whether gender influences the need for enteral tube use, supplemental oxygen, medication use or need for hospital readmission16–18 in children with BPD after initial hospital discharge. The aims of this study were to determine if differences in patient characteristics and respiratory outcomes exist between female and male children with BPD after hospital discharge. In this study, 482 infants and children diagnosed with BPD were recruited from an outpatient BPD clinic. The potential association of gender on acute care usage, respiratory symptoms and need for medical interventions, including supplemental oxygen and gastric tube placement for nutrition was examined and secondary analysis by race/ethnicity was performed.
METHODS
Study Sample
All subjects (n = 482) were recruited from the Johns Hopkins Bronchopulmonary Dysplasia Clinic between January 2008 and January 2014. Patients are referred to the clinic by area neonatal intensive care units on the basis of prematurity and having respiratory disease. Subjects were recruited by a data coordinator if they met the inclusion criteria, which included (i) a diagnosis of BPD based on available records made by the staffing pediatric pulmonologist (using NICHD criteria)19 and (ii) born at ≤36 weeks of gestation. This study was approved by the Johns Hopkins University Institutional Review Board with informed consent from parents/guardians.
Demographics and Clinical Data
Birth weight percentile was derived from published U.S. norms.20 The presence/absence of gastrostomy tubes and respiratory support were ascertained at the first BPD clinic encounter through chart review. Insurance coverage (private vs. public) was obtained from billing records. Race/ethnicity and primary caregiver education level were self-reported. For the purposes of analysis only, any subject with any reported non-white ancestry was categorized as non-white. Median household income was derived from 2010 U.S. Census data (www.quickfacts.census.gov). Clinical data were obtained through chart review, including dates of initial discharge and first BPD clinic encounter. Supplemental oxygen, ventilators, gastrostomy tubes, and/or Nissen fundoplications were defined by their presence/absence at the first clinic visit. Inhaled corticosteroid use was defined by any documented use in the first 2 years of life. Pulmonary hypertension was defined by the presence of any pulmonary hypertension present on echocardiography on or after 2 months of age.
Respiratory Morbidities
Respiratory morbidities were assessed using Yes/No questions on questionnaires obtained as a sample of convenience from caregivers at clinic visits (see Supplement for questions). Questionnaires were only included if obtained prior to 3 years of age. Primary outcomes included emergency department visits, hospital admissions, systemic steroid use, and antibiotic use for respiratory reasons since the last BPD clinic visit (or since initial hospital discharge if assessed at the first BPD clinic visit). Secondary outcomes included the presence/absence of trouble breathing, rescue beta-agonist use, activity limitations, and nighttime symptoms. A total of 1,099 questionnaires were completed for 429 subjects prior to the age of 3 years with 3.1% of all questions left blank by caregivers (Mean number of questionnaires per subject: 2.6 ± 1.8; Range: 1–13).
Supplemental Oxygen Weaning
Subjects who underwent weaning under the supervision of the BPD clinic were assessed on extended pulse-oximetry in clinic while awake with subsequent weaning during sleep assessed through home pulse-oximetry or overnight polysomnography at the discretion of the staffing pulmonologist. Unsupervised weaning was noted when a subject on oxygen returned to clinic without oxygen and the caregiver reported stopping oxygen use.
Statistical Methods
Demographic frequencies and clinical outcomes stratified by sex were compared using ANOVA with Bonferroni correction for the number of comparisons (six) made between demographic groups (corrected intragroup P values are reported), chi square, and t tests (Tables 1 and 2). Kaplan–Meier methodology was used to analyze the time at which subjects on home supplemental oxygen via nasal cannula were weaned off oxygen stratified by SHS exposure and unadjusted for other factors (Fig. 1A and B). Subjects who were lost to follow-up or who were still on oxygen at the time of analysis had their data censored. The relationship between sex and respiratory morbidities at each clinical visit was assessed using logistic regression adjusted for the age of the subject at the time of questionnaire completion, and factors that differed between males and females, specifically socioeconomic status (log of estimated household income), race/ethnicity, and birth weight percentile (Table 3).21 As caregivers may have completed questionnaires at several clinic visits, the logistic regressions accounted for the possibility of more than one questionnaire per subject using Generalized Estimating Equations (GEE) methodology (clustered by subject). STATAIC 11 (StataCorp LP, College Station, TX) was used for all statistical analyses. P values <0.05 were considered statistically significant.
TABLE 1.
Study Population Demographics by Sex and Race/Ethnicity
| Mean ± S.D. [range] | Entire population (n = 482) | Females (n = 188) | Males (n = 294) | P-value1 | Non-white females (n= 108) | White females (n = 79) | Non-white males (n = 208) | White males (n = 86) | P-value2 |
|---|---|---|---|---|---|---|---|---|---|
| Demographics | |||||||||
| Gestation (weeks) | 26.9 ± 2.9 [22.7–36] |
26.8 ± 2.7 [23–36] |
27.0 ± 3.0 [22.7–36] |
0.44 | 26.2 ± 2.4 [23–32.6] |
27.5±3.0 [23–36] |
26.7 ± 2.8 [22.7–36] |
27.8 ± 3.3 [22.7–36] |
<0.0013 |
| Birth weight (g) | 967 ± 469 [380–3181] (n = 468) |
903 ± 433 [380–3130] (n = 184) |
1008 ± 487 [415–3181] (n = 284) |
0.018 | 792 ± 293 [380–1875] (n= 105) |
1045 ± 537 [450–3130] (n = 78) |
941 ± 415 [469–2760] (n = 203) |
1177 ± 604 [415–3181] (n = 81) |
<0.0014 |
| Birth weight (Percentile) | 41.0 ± 23.5 [1–95] (n = 468) |
35.6 ±20.8 [1–88] (n = 184) |
44.6 ± 24.5 [1–95] (n = 284) |
<0.001 | 33.5 ± 21.2 [1–88] (n = 105) |
38.0 ± 19.9 [1–82] (n = 78) |
43.2 ± 24.5 [1–95] (n = 203) |
48.0 ± 24.3 [1–95] (n = 81) |
<0.0015 |
| Household factors | |||||||||
| Median household income ($ 000′s) | 63.1 ± 21.4 [15.6–156.6] |
66.1 ± 23.0 [25.2–156.6] |
61.2 ± 20.2 [15.6–156.6] |
0.015 | 60.3 ± 23.1 [25.2–156.6] |
73.0 ± 19.2 [36.2–126.7] |
58.2 ± 20.6 [15.6–156.6] |
68.6 ± 17.2 [40.8–103.6] |
<0.0016 |
| Public insurance (% yes) | 60.4 | 58.5 | 61.6 | 0.50 | 72.2 | 40.5 | 69.7 | 41.9 | <0.001 |
| Education (% high school or less) | 30.1 (n = 346) | 28.9 (n= 135) | 30.8 (n = 211) | 0.70 | 32.9 (n = 76) | 23.7 (n = 59) | 32.7 (n = 150) | 26.2 (n = 61) | 0.51 |
| Environmental tobacco smoke exposure in the home (% yes) | 27.7 (n = 480) | 30.9 | 25.7 (n = 292) | 0.22 | 34.3 | 26.6 | 34.2 (n = 207) | 29.4 (n = 85) | 0.29 |
| Clinical data | |||||||||
| Age at discharge from NICU (months) | 4.1 ± 2.8 [0.1–24.5] (n = 480) |
4.1 ± 2.6 [0.1–24.4] (n = 187) |
4.2 ± 2.9 [0.1–24.5] (n = 293) |
0.77 | 4.5 ± 2.9 [0.5–24.4] (n = 107) |
3.6 ± 2.0 [0.1–13.6] |
4.5 ± 3.1 [0.2–24.5] |
3.4 ± 2.2 [0.1–16.6] (n = 85) |
0.0437 |
| Age at first pulmonary clinic visit (months) | 7.7 ± 5.8 [0.9–51.3] |
7.8 ± 5.9 [0.9–48.9] |
7.6 ± 5.8 [2.5–51.3] |
0.82 | 7.7 ± 4.3 [3.3–30.9] |
7.3 ± 6.0 [0.9–34.0] |
7.1 ± 3.5 [2.5–25.7] |
8.8 ± 9.2 [2.5–51.3] |
0.11 |
| Home supplemental oxygen (% yes) | 36.3 | 35.1 | 37.1 | 0.66 | 37.0 | 32.9 | 35.1 | 41.9 | 0.64 |
| Home ventilator (% yes) | 2.7 | 2.7 | 2.7 | 0.97 | 3.7 | 1.3 | 2.9 | 2.3 | 0.78 |
| Gastrostomy tube (% yes) | 23.7 | 27.1 | 21.4 | 0.15 | 28.8 | 26.6 | 22.6 | 18.6 | 0.44 |
| Nissen (% yes) | 16.2 | 19.2 | 14.3 | 0.16 | 22.2 | 15.2 | 15.4 | 11.6 | 0.22 |
| Inhaled corticosteroid use prior to age 2yo (% yes) | 78.6 | 80.0 | 77.9 | 0.62 | 84.3 | 74.7 | 84.6 | 61.6 | <0.001 |
P values reported in this column are comparisons on males versus females based on t tests for continuous variables and chi-square tests for categorical variables.
P values reported in this column are comparisons on the four sex and race/ethnicity groups based on ANOVA testing for continuous variables and chi-square tests for categorical variables. P values reported in the footnotes are Bonferroni corrected P values from ANOVA testing unless otherwise specified.
Non-white females are born at earlier gestational ages than white females (P = 0.009) and white males (P = 0.001). White males are born at later gestational ages than non-white females (P = 0.001) and non-white males (P = 0.011).
Non-white females have lower birth weights than white females (P = 0.001), non-white males (P = 0.039), and white males (P < 0.001). White males have higher birth weights than non-white females (P < 0.001) and non-white males (P < 0.001).
Non-white females have a lower birth weight percentile than white females (P = 0.003) and white males (P < 0.001). White males have a higher birth percentile than non-white females (P < 0.001) and white females (P = 0.041).
There was no difference between non-white females and non-white males (P = 1.00) or white females and white males (P = 0.97), but all comparisons between any non-white gender group and any white gender group were significant (P range: <0.001–0.030).
White males were discharged from the NICU at an earlier age than non-white males (P = 0.019), and all whites were discharged earlier than non-whites (t test P-value <0.001).
TABLE 2.
Subjects on Home Respiratory Support Only1
| Mean ± S.D. [range] | Entire population (n = 181) | Females (n = 68) | Males (n = 113) | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Race (% non-white) | 64.6 | 60.3 | 67.3 | 0.34 |
| Gestation (weeks) | 26.3 ± 2.6 [23–35.5] | 25.9 ± 2.5 [23–34.4] | 26.5 ± 2.7 [23–35.5] | 0.16 |
| Birth weight (g) | 875 ± 450 [380–3181] (n=175) |
796 ± 414 [380–3130] (n=67) |
923 ± 466 [415–3181] (n = 108) |
0.07 |
| Birth weight (Percentile) | 38.7 ± 24.2 [1–95] (n=175) |
34.0 ± 19.7 [2–78] (n=67) |
41.6 ± 26.2 [1–95] (n = 108) |
0.044 |
| Household factors | ||||
| Median household income ($ 000′s) | 62.6 ± 19.8 [15.6–156.6] | 68.0 ± 20.1 [25.2–156.6] | 59.4 ± 18.9 [15.6–156.6] | 0.004 |
| Public insurance (% yes) | 60.2 | 55.9 | 62.8 | 0.36 |
| Education (% high school or less) | 33.3 (n = 141) | 37.0 (n=54) | 31.0 (n = 87) | 0.46 |
| Environmental tobacco smoke exposure in the home (% yes) | 24.4 (n = 180) | 29.4 | 21.4 (n = 112) | 0.23 |
| Clinical Data | ||||
| Age at discharge from NICU (months) | 5.1 ± 3.6 [0.1–24.5] (n=180) |
5.2 ± 3.4 [1.5–24.4] | 5.0 ± 3.7 [0.1–24.5] (n = 112) |
0.76 |
| Age at first pulmonary clinic visit (months) | 7.6 ± 5.5 [2.5–51.3] | 8.0 ± 5.4 [2.5–30.9] | 7.3 ± 5.5 [2.5–51.3] | 0.42 |
| Gastrostomy tube (% yes) | 37.6 | 47.1 | 31.9 | 0.041 |
| Nissen (% yes) | 28.2 | 35.3 | 23.9 | 0.10 |
| Inhaled corticosteroid use prior to age 2yo (% yes) | 85.6 | 89.7 | 83.2 | 0.23 |
A total of 181 of the 482 subjects in this study on home respiratory support. Specifically, 168 were on supplemental oxygen via nasal cannula, 7 were on home ventilators with supplemental oxygen entrainment, and 6 were on home ventilators without supplemental oxygen.
Fig. 1.
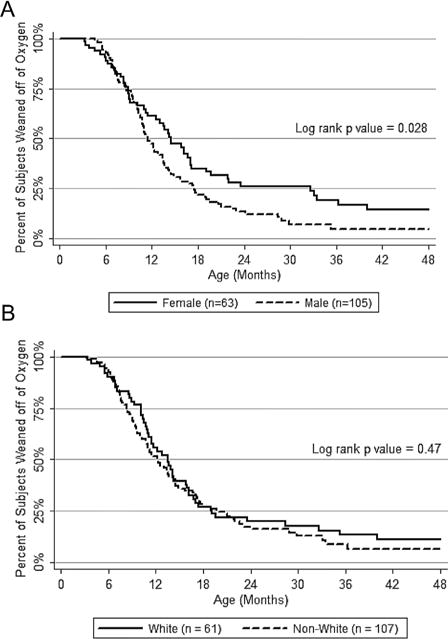
Age of weaning from home supplemental oxygen (not corrected for gestational age) for all infants on oxygen using censored data when appropriate (Graph A: Females vs. males; Graph B: Non-whites vs. Whites).
TABLE 3.
Female Sex as a Predictor of Selected Respiratory Outcomes (Adjusted Odds Ratios)
| Odds ratio1 [95% C.I.] | All subjects | P-value | Subjects on home respiratory support2 | P-value |
|---|---|---|---|---|
| Emergency department visit | 0.97 [0.67, 1.41] (1071 forms; n = 417) | 0.87 | 1.02 [0.59, 1.78] (459 forms; n = 157) | 0.94 |
| Inpatient hospitalization | 1.16 [0.78, 1.72] (1072 forms; n = 418) | 0.47 | 1.33 [0.75, 2.36] (459 forms; n = 158) | 0.33 |
| Systemic steroid use | 1.20 [0.81, 1.76] (1065 forms; n = 416) | 0.36 | 1.41 [0.84, 2.36] (459 forms; n = 158) | 0.20 |
| Antibiotic use | 0.78 [0.55, 1.09] (1071 forms; n = 418) | 0.15 | 0.83 [0.48, 1.43] (460 forms; n = 158) | 0.49 |
| Cough or wheeze | 0.77 [0.53, 1.12] (1040 forms; n = 412) | 0.17 | 0.75 [0.44, 1.28] (447 forms; n = 155) | 0.30 |
| Rescue β-agonist use | 1.00 [0.67, 1.50] (1026 forms; n = 410) | 1.00 | 0.94 [0.55, 1.62] (438 forms; n = 153) | 0.83 |
| Activity limitations | 1.19 [0.72, 1.97] (1014 forms; n = 405) | 0.49 | 1.31 [0.73, 2.35] (437 forms; n = 153) | 0.37 |
| Nighttime symptoms | 1.16 [0.74, 1.82] (1033 forms; n = 409) | 0.52 | 0.57 [0.26, 1.26] (443 forms; n = 154) | 0.16 |
Odds ratios were generated through logistic regression with a dependent variable for specified respiratory morbidity, and an independent variable for sex (female = 1; male = 1), and were clustered by individual. Odds ratios were adjusted for race/ethnicity, birth weight percentile, log of median household income, and age at the time of form completion. Only forms completed prior to 3 years of age were included.
A total of 181 of the 482 subjects in this study on home respiratory support. Specifically, 168 were on supplemental oxygen via nasal cannula, seven were on home ventilators with supplemental oxygen entrainment, and six were on home ventilators without supplemental oxygen.
RESULTS
Demographics
A total of 515 subjects were approached to participate in this study. Six declined to participate and an additional 27 did not meet study inclusion criteria. Among the 482 subjects recruited for this study (including 31 pairs of twins or two triplets from a trio), there was a predominance of male subjects (61%) which was similar to the non-participating subjects (61%; n = 33; P = 0.97). In our study, the majority of subjects were non-white (65.7% with 92.4% of non-whites self-reporting being African-American or black).
Interestingly, the predominance of non-white subjects in our study was greater than that found in the surrounding general population and may reflect the greater risk of preterm birth in minority populations.1 Of the 28.6% (n = 138) subjects who resided within Baltimore City, 89.1% were non-white compared to 71.7% of the general population within Baltimore City as reported on the 2010 U.S. census. Of the 30.5% (n = 147) who resided in the surrounding Baltimore County, 64.0% were non-white, compared to 39.5% of the general population of Baltimore County. Of the remaining subjects, 38.0% (n = 183) resided elsewhere in Maryland and 2.9% (n = 14) resided in an adjacent state. In this study population, male children with BPD were more likely to be non-white (70.8%) compared to female children with BPD (57.8%; P = 0.003). There were no sex differences by residential location defined as categories of Baltimore City, Baltimore County, other Maryland locations, and out-of-state (P = 0.08). The mean gestational age at birth for the population was 26.9 ± 2.9 weeks and this did not differ by sex; however, females had significantly lower birth weight (P = 0.018) and lower birth weight percentile (P < 0.001) than males of similar gestational age (Table 1).
Female subjects resided in households with a higher estimated median household income ($66,101) than males ($61,218; P = 0.015). As reference, the median household income for the state of Maryland, where 97.3% of subjects reside, is $70,004. However, this difference between males and females became non-significant in a regression adjusted for race/ethnicity (sex co-efficient P = 0.12) as a higher proportion of males (70.8%) were self-reported as non-white compared to females (57.8%). There were no differences between sexes in rates of public versus private insurance coverage, highest level of caregiver education, or environmental tobacco smoke exposure in the home.
Clinical Data
There were no differences in the mean age of discharge from the neonatal intensive care unit (4.1 ± 2.8 months) or mean age at the time of the first visit to BPD clinic (7.7 ± 5.8 months) between sexes. Among the study population, 36.3% were on supplemental oxygen, 2.7% were on home ventilators, 23.7% had gastrostomy tubes, and 16.2% had Nissen fundoplications (68.4% of those with gastrostomy tubes) at the first clinic visit, and again there were no differences between sexes. A total of 14.1% of subjects had pulmonary hypertension present after 2 months of age, but this was not different between males and females. Additionally, a majority of subjects (78.6%) received inhaled corticosteroids during the first 2 years of life with no difference between the sexes in terms of frequency in being prescribed. To capture a population of children with more severe BPD (Table 2), we examined demographic differences between males and females for subjects who required home respiratory support (supplemental oxygen and/or home ventilator), at their first clinic visit. The 181 subjects receiving respiratory support at home included 168 children on supplemental oxygen via nasal cannula and 13 children on home mechanical ventilation. Among these subjects (n = 181), females had a lower birth weight percentile (P = 0.044) and higher median household income (P = 0.004) compared to males. Females on respiratory support at home were also more likely to have a gastrostomy tube (47.1%) compared to males (31.9%; P = 0.041).
Respiratory Morbidities
Out of the 482 subjects in the study population, 429 had completed at least one questionnaire assessing for respiratory morbidities prior to 3 years of age. On average these subjects completed 2.6 ± 1.8 questionnaires [Range: 1–13]. There was no difference in the frequency of questionnaires completed between male and female subjects (P = 0.25). The frequency of acute care use on any questionnaire ever was 36.9% for emergency department visits, 27.4% for hospital admissions, 36.9% for systemic steroid use, and 40.5% for antibiotic use for a respiratory illness. The frequency of chronic symptoms within the past week was 53.8% for symptoms, such as coughing or wheezing during the day, 40.4% for beta-agonist rescue medication use, 25.2% for activity limitations, and 33.1% for nighttime symptoms. We tested for the association between respiratory morbidities and sex using logistic regression models adjusted for age at the time of form completion, and clustered by subject to account for caregivers who completed more than one questionnaire at different clinic visits prior to 3 years of age. We also adjusted for factors that differed between sexes, specifically race/ethnicity, birth weight percentile, and median household income. We did not find any differences in respiratory morbidities by sex in either the study population or the subset of the population that required home respiratory support (Table 3).
Supplemental Oxygen Weaning
Using Kaplan–Meier methodology, we found that males and females differed by age in which they weaned off supplemental oxygen supplied by nasal cannula (Fig. 1A). Of the 168 subjects who received supplemental oxygen at home, 136 had oxygen discontinued at the time of analysis. All 168 individuals were used for time-to-weaning analysis, including the use of censored data for 21 subjects who were still on oxygen at the time of analysis, and 11 who were lost to follow-up prior to oxygen discontinuation. Females tended to be weaned from supplemental oxygen over 3 months later (median age: 14.6 months) than males (median age: 11.4 months; log rank P = 0.028).
Gender/Race Ethnicity
We also wished to assess whether there were differences in demographics and clinical outcomes by race/ethnicity. Overall, the non-white females presenting to BPD clinic tended to be born at earlier gestational ages with lower birth weights and birth weight percentiles, especially compared to white males (Table 1). Although there were no differences by gender, non-whites tended to have lower estimated median household incomes and a longer initial NICU admission, and were more likely to be covered by public insurance and prescribed inhaled corticosteroids. Using logistic regression clustered by subject and adjusted for factors that differed between the groups (gestational age, birth weight percentile, median household income, insurance status, and prescription of inhaled corticosteroids), we found no differences between the groups (non-white females, non-white males, white females) and the reference group (white males) in terms of respiratory morbidities. Although female children weaned off supplemental oxygen later than males, we found no difference in age of weaning supplemental oxygen by race/ethnicity (Fig. 1B).
DISCUSSION
In this study, the prevalence of male children attending an outpatient BPD clinic was significantly higher than females nevertheless both males and females had similar respiratory morbidities during the first 3 years of life. Females with BPD were more likely to have lower birth weights and lower birth weight percentiles compared to males. In addition, females receiving home respiratory support were more likely to receive enteral tube feedings and to be weaned off supplemental oxygen significantly, later than males on home respiratory support. Findings from this study suggest that although males had a higher prevalence of BPD in the outpatient setting, there were no gender differences with respect to respiratory morbidities after initial hospital discharge during the preschool years. Furthermore, females that required respiratory support after initial hospital discharge were more likely to receive supplemental nutrition and require supplemental oxygen for a greater period of time than males.
Preterm males have previously been reported to have a higher incidence of pulmonary morbidity compared to females during initial hospitalization.10,22 In this study, we found that the majority of infants presenting to an outpatient BPD clinic were male, consistent with the higher prevalence of BPD in male infants reported in the literature.23 Nevertheless, we found that females and males with BPD had similar respiratory outcomes during the first 3 years of life with regard to acute care usage including ER visits, hospitalizations and antibiotic use for respiratory conditions. Of interest was our finding that females with BPD who presented to their first visit on supplemental oxygen were weaned off supplemental oxygen significantly later than males with BPD. Compared to males of similar gestational age, female infants who required supplemental oxygen at their initial outpatient clinic visit were more likely to have lower birth weight percentiles. Among females, race did not account for lower birth weight percentiles and no difference in birth weight percentiles were found between white and non-white females with BPD. Taken together, these findings suggest that a lower birth weight percentile in females with the diagnosis of BPD may be a risk factor for requiring supplemental oxygen after initial hospital discharge. Animal studies have shown an association between intrauterine growth retardation and impaired alveolar growth.24 Indeed, an interpretation of our results suggest that a lower birth weight percentile as a contributing factor to BPD severity, may disproportionately affect preterm females who may otherwise be relatively protected due to other factors. This was also supported by our finding that females requiring respiratory support in the outpatient setting were more likely to require supplemental oxygen for a longer duration compared to males. Future prospective studies are needed to examine the relationship between gender, birth weight percentile, growth curve after birth and need for supplemental oxygen and postnatal lung growth in infants with BPD after initial discharge from the hospital.
There are limitations to the study. This study focused on respiratory outcomes in children with BPD in the outpatient settings during the first 3 years of life. The respiratory outcomes of children with BPD who attend a single-center disease specific clinic may not be representative of all outpatient children with BPD. Other limitations include the primarily urban population served by our tertiary care center, which may increase the severity of cases that were referred to the BPD clinic, and we also included infants born at up to 36 weeks gestation, who may not have similar lung disease to that of very preterm infants. Our results may not be also generalizable to children who live in a geographical area that does not have a BPD clinic. The questionnaire used for this study is not validated; however, no validated questionnaires to assess respiratory outcomes in this population exist. Finally, the acute respiratory outcomes reported in this study were based on questionnaires that may be subject to recall bias. Limited published data suggest that hospital admissions and antibiotic use may be underestimated on questionnaires, thus if anything, our results are biased towards the null.25,26 Questions regarding chronic symptoms were based on familial observations over the past week prior to the clinic and less subject to recall bias.
In conclusion, while the literature and our own data would suggest that males are more likely to develop BPD than females, once having developed BPD, we found no difference in respiratory morbidities among female and male children with BPD following initial hospital discharge during the first 3 years of life. We did however, find that female infants with BPD who presented to an outpatient BPD clinic were more likely to have lower birth weight percentiles and to be weaned from supplemental oxygen at a later age than male infants.
Supplementary Material
Acknowledgments
The authors wish to thank the families who participated in this study. This study received funding from the Flight Attendant Medical Research Institute and the American Academy of Pediatrics.
Funding source: Flight Attendant Medical Research Institute; American Academy of Pediatrics; National Institutes of Health, Number: RHL114800A.
Footnotes
Conflicts of Interest: None.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- 1.Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1–65. [PubMed] [Google Scholar]
- 2.Hamilton BE, Hoyert DL, Martin JA, Strobino DM, Guyer B. Annual summary of vital statistics: 2010–2011. Pediatrics. 2013;131:548–558. doi: 10.1542/peds.2012-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, Wrage LA, Poole K, National Institutes of Child Health and Human Development Neonatal Research Network Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–1360. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 4.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, Kennedy KA, Stoll BJ, Buchter S, Laptook AR, Ehrenkranz RA, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. 2011;183:1715–1722. doi: 10.1164/rccm.201101-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zysman-Colman Z, Tremblay GM, Bandeali S, Landry JS. Bronchopulmonary dysplasia—trends over three decades. Paediatr Child Health. 2013;18:86–90. doi: 10.1093/pch/18.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gursoy T, Hayran M, Derin H, Ovali F. A clinical scoring system to predict the development of bronchopulmonary dysplasia. Am J Perinatol. 2015;32:659–666. doi: 10.1055/s-0034-1393935. [DOI] [PubMed] [Google Scholar]
- 8.Gortner L, Misselwitz B, Milligan D, Zeitlin J, Kollée L, Boerch K, Agostino R, Van Reempts P, Chabernaud JL, Bréart G, et al. Rates of bronchopulmonary dysplasia in very preterm neonates in Europe: results from the MOSAIC cohort. Neonatology. 2011;99:112–117. doi: 10.1159/000313024. [DOI] [PubMed] [Google Scholar]
- 9.Jones HP, Karuri S, Cronin CM, Ohlsson A, Peliowski A, Synnes A, Lee SK, Canadian Neonatal Network Actuarial survival of a large Canadian cohort of preterm infants. BMC Pediatr. 2005;5:40. doi: 10.1186/1471-2431-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevenson DK, Verter J, Fanaroff AA, Oh W, Ehrenkranz RA, Shankaran S, Donovan EF, Wright LL, Lemons JA, Tyson JE, et al. Sex differences in outcomes of very low birthweight infants: the newborn male disadvantage. Arch Dis Child Fetal Neonatal Ed. 2000;83:F182–F185. doi: 10.1136/fn.83.3.F182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klinger G, Sokolover N, Boyko V, Sirota L, Lerner-Geva L, Reichman B. Perinatal risk factors for bronchopulmonary dysplasia in a national cohort of very-low-birthweight infants. Am J Obstet Gynecol. 2013;208:115–119. doi: 10.1016/j.ajog.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 12.Binet ME, Bujold E, Lefebvre F, Tremblay Y, Piedboeuf B. Role of gender in morbidity and mortality of extremely premature neonates. Am J Perinatol. 2012;29:159–166. doi: 10.1055/s-0031-1284225. [DOI] [PubMed] [Google Scholar]
- 13.Gortner L, Shen J, Tutdibi E. Sexual dimorphism of neonatal lung development. Klin Padiatr. 2013;225:64–69. doi: 10.1055/s-0033-1333758. [DOI] [PubMed] [Google Scholar]
- 14.Ambalavanan N, Carlo WA, McDonald SA, Yao Q, Das A, Higgins RD. Identification of extremely premature infants at high risk of rehospitalization. Pediatrics. 2011;128:e1216–e1225. doi: 10.1542/peds.2011-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teune MJ, van Wassenaer AG, van BS, Mol BW, Opmeer BC. Perinatal risk-indicators for long-term respiratory morbidity among preterm or very low birth weight neonates. Eur J Obstet Gynecol Reprod Biol. 2012;163:134–141. doi: 10.1016/j.ejogrb.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Smith VC, Zupancic JA, McCormick MC, Croen LA, Greene J, Escobar GJ, Richardson DK. Rehospitalization in the first year of life among infants with bronchopulmonary dysplasia. J Pediatr. 2004;144:799–803. doi: 10.1016/j.jpeds.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 17.Furman L, Baley J, Borawski-Clark E, Aucott S, Hack M. Hospitalization as a measure of morbidity among very low birth weight infants with chronic lung disease. J Pediatr. 1996;128:447–452. doi: 10.1016/s0022-3476(96)70353-0. [DOI] [PubMed] [Google Scholar]
- 18.Chye JK, Gray PH. Rehospitalization and growth of infants with bronchopulmonary dysplasia: a matched control study. J Paediatr Child Health. 1995;31:105–111. doi: 10.1111/j.1440-1754.1995.tb00756.x. [DOI] [PubMed] [Google Scholar]
- 19.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 20.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang K-L, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 22.Mehta P, Berger J, Bucholz E, Bhandari V. Factors affecting nasal intermittent positive pressure ventilation failure and impact on bronchopulmonary dysplasia in neonates. J Perinatol. 2014;34:754–760. doi: 10.1038/jp.2014.100. [DOI] [PubMed] [Google Scholar]
- 23.Ali Z, Schmidt P, Dodd J, Jeppesen DL. Bronchopulmonary dysplasia: a review. Arch Gynecol Obstet. 2013;288:325–333. doi: 10.1007/s00404-013-2753-8. [DOI] [PubMed] [Google Scholar]
- 24.Zana-Taieb E, Pham H, Franco-Montoya ML, Jacques S, Letourneur F, Baud O, Jarreau PH, Vaiman D. Impaired alveolarization and intra-uterine growth restriction in rats: a postnatal genome-wide analysis. J Pathol. 2015;235:420–430. doi: 10.1002/path.4470. [DOI] [PubMed] [Google Scholar]
- 25.Seidl H, Meisinger C, Kirchberger I, Burkhardt K, Kuch B, Holle R. Validity of self-reported hospital admissions in clinical trials depends on recall period length and individual characteristics. J Eval Clin Pract. 2015 doi: 10.1111/jep.12506. [DOI] [PubMed] [Google Scholar]
- 26.Dasanayake AP, Macaluso M, Roseman JM, Caufield PW. Validity of the mother’s recall of her child’s antibiotic use. ASDC J Dent Child. 1995;62:118–122. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


