Abstract
Background
Arterial dysfunction contributes to cardiovascular disease (CVD) progression and clinical events. Interrelations of aortic stiffness and vasodilator function with incident CVD remain incompletely studied.
Methods and Results
We used proportional hazards models to relate individual measures of vascular function to incident CVD in 4547 participants (mean age 51±11 years, 54% women) in two generations of Framingham Heart Study participants. During follow-up (0.02–13.83 years), 232 participants (5%) experienced new-onset CVD events. In multivariable models adjusted for cardiovascular risk factors, both higher carotid-femoral pulse wave velocity (HR=1.32; 95% CI: 1.07, 1.63; P=0.01) and lower hyperemic mean flow velocity (HR=0.84; 95% CI: 0.71, 0.99; P=0.04) were associated significantly with incident CVD whereas primary pressure wave amplitude (HR=1.12; 95% CI: 0.99, 1.27; P=0.06), baseline brachial diameter (HR=1.09; 95% CI: 0.90, 1.31; P=0.39), and flow-mediated vasodilation (HR=0.85; 95% CI: 0.69, 1.04; P=0.12) were not. In mediation analyses, 8-13% of the relation between aortic stiffness and CVD events was mediated by hyperemic mean flow velocity.
Conclusions
Our results suggest that associations between aortic stiffness and CVD events are mediated by pathways that include microvascular damage and remodeling.
Keywords: aortic stiffness, pulse wave velocity, hyperemic flow, flow-mediated dilation, microvascular damage
Indicators of vascular dysfunction have emerged as surrogate markers for cardiovascular disease (CVD) progression and disease event prediction. Brachial artery flow-mediated dilation (FMD) evaluates conduit artery endothelial function1 and has been shown to predict incident CVD, particularly in individuals who are at high risk.2 Recently, the hyperemic response to ischemia has emerged as a key noninvasive measure of microvascular function. Previously, we showed stronger associations between several CVD risk factors and the hyperemic response compared to brachial artery FMD.3 Additionally, we have shown that the hyperemic response is lower in the setting of higher aortic stiffness assessed by carotid-femoral pulse wave velocity (CFPWV) or pulse pressure.4 Microvascular dysfunction may contribute to progression of target organ damage and hence may represent a potential mediator of relations between aortic stiffness and CVD events.5-7
Recently, hyperemic flow velocity, but not FMD, was shown to be a significant risk predictor for incident CVD events in men.8 We have reported that aortic stiffness predicts incident CVD.9-11 However, the interrelations of aortic stiffness, brachial hyperemic response, and FMD with incident CVD have not been studied comprehensively in community-based cohorts. Thus, we aimed to assess whether microvascular damage or dysfunction, as assessed by hyperemic response, may partially mediate relations between aortic stiffness and incident CVD. We hypothesized that vascular function measures are predictive of first-onset CVD and that microvascular dysfunction (measured by hyperemic flow) is an important mechanistic link between greater aortic stiffness and incident CVD.
Methods
Participants
The study sample was drawn from the Framingham Offspring and Third Generation Cohorts, which have been described.12, 13 Vascular function was assessed in participants in the Offspring Cohort examination seven (1998-2001, N=3333) and Third Generation examination one (2002-2005, N=4095). Participants were excluded for the following reasons: age<35 years (n=1071) because of low CVD risk; missing tonometry (n=1207); prior CVD (n=206); missing vascular measures (n=354); missing laboratory and/or covariate data (n=18); and no follow-up after examination (n=25). All protocols were approved by Boston University Medical Center's Institutional Review Board, and all participants provided written informed consent.
Clinical Evaluation and Definitions
Medical history, physical examination, and electrocardiography were performed routinely at each examination.12 Physician-acquired blood pressures represent the mean of two auscultatory measurements obtained on seated participants at the time of the clinic examination. The physician blood pressures were acquired using a mercury column sphygmomanometer and a standardized protocol with excellent measurement reproducibility. Criteria for diabetes mellitus were a fasting glucose ≥126 mg/dL (7.0 mmol/L) or treatment with insulin or an oral hypoglycemic agent. Smoking was defined as self-reported regular use of cigarettes in the year preceding the examination.
Measures of Brachial Vascular Function
The methodology and reproducibility for assessing the brachial artery diameters and flow have been reported previously.3, 14 With the exception of water or decaffeinated coffee or tea, participants were asked to not eat or drink beverages after 8PM the evening prior to vascular examination. The brachial artery diameter was imaged with a high-resolution ultrasound at rest and again 1 minute after onset of reactive hyperemia, which was induced by a 5-minute forearm cuff occlusion.15 Sonographers blinded to participants' status measured arterial diameter offline using commercially available software.15 FMD was calculated as the percent change in brachial diameter from the resting state. Alternative methods to assess FMD are presented in the Data Supplement. Brachial artery flow velocity was assessed using pulsed Doppler flow at rest and for 15 seconds after cuff release. Technicians blinded to participants' status analyzed Doppler recordings using a semi-automated signal-averaging method with correction for insonation angle.3 Resulting flow waveforms were integrated to assess mean resting and mean hyperemic flow velocities.
Non-invasive Hemodynamics
Hemodynamic data were acquired as previously described.9 Participants were studied in the supine position after a 5-minute rest. Arterial tonometry with simultaneous electrocardiography was obtained from brachial, radial, femoral, and carotid arteries using a custom tonometer. Tonometric, electrocardiographic, and brachial Doppler data were digitized during the primary acquisition and transferred to the core laboratory for analyses that were performed blinded to clinical data.
Tonometry waveforms were signal-averaged using the electrocardiographic R-wave as a fiducial point.9 Cuff systolic and diastolic blood pressures obtained at the time of tonometry were used to calibrate the peak and trough of the signal-averaged brachial pressure waveform. Diastolic blood pressure and integrated brachial mean arterial pressure were used to calibrate carotid pressure tracings.16 Calibrated carotid pressure was used as a surrogate for central pressure.16 The primary pressure wave amplitude was defined as the pressure difference between the foot of the upstroke and the pressure at the first peak or inflection point of the carotid pressure waveform.17 CFPWV was calculated from tonometry waveforms and body surface measurements, which were adjusted for parallel transmission in the brachiocephalic artery and aortic arch with the use of the suprasternal notch as a fiducial point.17
Outcomes
Criteria for CVD events have been described previously.18, 19 Major CVD events were defined as fatal or nonfatal myocardial infarction, unstable angina, heart failure, and ischemic stroke. Medical records were obtained for hospitalizations and physician visits related to CVD during follow-up and were reviewed by a committee of three physician investigators; events were adjudicated following a written protocol. A separate panel of neurologists adjudicated cerebrovascular events. Follow-up evaluations were performed on data acquired through December 31, 2012.
Statistical Analyses
Baseline characteristics for the study sample were tabulated. CFPWV was inverted in order to limit heteroskedasticity; the inverted value was multiplied by -1000 in order to convert units to ms/m and rectify directionality of the association with aortic stiffness. We estimated partial correlations to assess associations among the various vascular function measures accounting for age, sex, and cohort. To further characterize the interrelations of reactive hyperemia and aortic stiffness, we classified participants into four groups using the median values of hyperemic flow velocity and CFPWV and tabulated the clinical characteristics of each group.
We used Cox models to relate onset of major CVD to individual measures of vascular function. We tested the proportional hazards assumption by assessing the significance of time dependent covariates by creating interactions of each vascular measure and survival time. Covariates were selected a priori and included components of the Framingham risk score.20 Measures of vascular function were added individually to the base model initially adjusting for age, sex, and cohort. For individual hemodynamic variables that showed statistically significant association with incident CVD events, we examined effect modification by age and sex by incorporating corresponding interaction terms in the statistical models. Individual measures of vascular function that were related to the incidence of CVD events in multivariable Cox models were evaluated further in an expanded model adjusted for age, sex, cohort, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, smoking, diabetes mellitus, hypertension treatment, BMI, and correlation among full siblings (relatedness). The correlation due to relatedness among full siblings was accounted for using the robust (sandwich) variance estimator.
To further assess relations between vascular measures and CVD events, continuous predictor variables were categorized into quartiles, and curves of cumulative probability of a first-major CVD event were constructed. We used Cox models to relate onset of major CVD to categorized vascular function measures. Non-CVD death was a censoring event; the Kaplan-Meier plots were not modified for competing events.
Mediating variables are intermediate factors that may provide a link between a predictor variable and an outcome variable. Statistically quantifying their effects on the relationships of interest provides insight into mechanisms that potentially contribute to an observed relation between a predictor variable and an outcome variable and examines the role of the mediator.21-23 To investigate a possible biological mechanism underlying the association between vascular measures and CVD events, mediation analysis was performed. We used the aforementioned partial correlations and Cox proportional hazards regression models to produce conceptual mediation models based on our initial hypothesis using variables that satisfied all of these assumptions. Formal mediation analysis was performed for selected vascular measures (CFPWV and primary pressure wave) with hyperemic flow velocity as potential mediator while adjusting for age, sex, and cohort using a SAS macro for survival outcome.23 Estimates (on the hazard ratio scale) of the total, direct, and indirect effects along with 95% confidence intervals and proportion mediated were tabulated.24
All analyses were performed with SAS version 9.3 for Windows (SAS Institute, Cary, NC). Two-tailed P<0.05 were considered statistically significant.
Results
Study exclusion criteria resulted in a sample of 4547 participants (2446 [54%] women). Baseline characteristics of the study sample are presented in Table 1. A comparison of these characteristics between included and excluded participants is shown in Table S1. Table 2 demonstrates the interrelations of the vascular function measures. As we have previously shown, hyperemic mean flow was associated modestly with higher FMD as expected since hyperemic flow is the stimulus for brachial dilation during hyperemia. Additionally, higher hyperemic flow velocity was modestly associated with lower aortic stiffness measures. Characteristics of individuals across phenotypic groups according to hyperemic flow and CFPWV are shown in Table S2. During follow-up (0.02-13.8 years; median 8.6 years), 232 of 4547 participants (5%) had a first-major CVD event. The most common events were myocardial infarction (n=86), heart failure (n=61), and stroke (n=62); 17 episodes (7% of all events) were fatal.
Table 1. Baseline characteristics of the sample (N=4547).
| Variable | Value |
|---|---|
| Clinical measures | |
| Age, y | 51±11 |
| Women, N (%) | 2446 (54) |
| Height, cm | 169±10 |
| Weight, kg | 77.9±17.1 |
| Body mass index, kg/m2 | 27.0±4.9 |
| Systolic blood pressure, mm Hg | 121±17 |
| Diastolic blood pressure, mm Hg | 75±10 |
| Heart rate, bpm | 63±10 |
| Total cholesterol, mg/dL | 197±36 |
| High-density lipoprotein cholesterol, mg/dL | 55±17 |
| Triglycerides, mg/dL | 123±90 |
| Hypertension treatment, N (%) | 826 (18) |
| Diabetes mellitus, N (%) | 291 (6) |
| Smoker, N (%) | 703 (15) |
| Generation 3 Exam 1, N (%) | 2626 (58) |
| Vascular measures | |
| Baseline brachial diameter, mm | 4.2±0.9 |
| Flow-mediated vasodilation, % | 4.5±3.6 |
| Hyperemic mean flow velocity, cm/sec | 58±20 |
| Carotid-femoral pulse wave velocity, m/s | 8.4±2.7 |
| Tonometry primary pressure wave, mm Hg | 42±11 |
All values are mean±standard deviation except as noted.
Table 2. Matrix of Pearson partial correlation coefficients for measures of vascular function (N=4547).
| Baseline brachial diameter | Flow-mediated dilation | Tonometry primary pressure wave | Hyperemic mean flow velocity | |
|---|---|---|---|---|
| Carotid-femoral pulse wave velocity | 0.15 <0.001 | -0.14 <0.001 | 0.28 <0.001 | -0.13 <0.001 |
| Baseline brachial diameter | -0.31 <0.001 | 0.02 0.11 | -0.12 <0.001 | |
| Flow-mediated dilation | -0.10 <0.001 | 0.35 <0.001 | ||
| Tonometry primary pressure wave | -0.12 <0.001 |
Top value is partial r adjusted for age, sex, and cohort; bottom value is P in each cell.
Cox proportional hazards models for individual measures of vascular function as predictors of a major CVD event are presented in Table 3. After adjusting for age, sex, and cohort, each measure of vascular function that was evaluated individually was predictive of first-major CVD event. After further adjustment for total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, smoking, diabetes mellitus, hypertension treatment, cohort, BMI, and relatedness, the relation between CFPWV (HR=1.32; 95% CI: 1.07, 1.63; P=0.01) and incident CVD events and the relation between hyperemic mean flow velocity (HR=0.84; 95% CI: 0.71, 0.99; P=0.04) and incident CVD events persisted. In the expanded model, the association between incident CVD and primary pressure wave amplitude (HR=1.12; 95% CI: 0.99, 1.27; P=0.06) had a tendency toward significance; whereas, the associations between CVD events and both baseline brachial diameter (HR=1.09; 95% CI: 0.90, 1.31; P=0.39) and FMD (HR=0.85; 95% CI: 0.69, 1.04; P=0.12) were no longer significant. Table S3 presents similar models further adjusted for pulse pressure.
Table 3. Individual measures of vascular function as predictors of a major CVD event (N=4547).
| Vascular measure | Hazard Ratio (LCL, UCL) Minimal Model* | P | Hazard Ratio (LCL, UCL) Expanded Model† | P |
|---|---|---|---|---|
| Baseline brachial diameter | 1.24 (1.04, 1.48) | 0.02 | 1.09 (0.90, 1.31) | 0.39 |
| Flow-mediated vasodilation | 0.78 (0.64, 0.95) | 0.01 | 0.85 (0.69, 1.04) | 0.12 |
| Hyperemic mean flow velocity | 0.76 (0.65, 0.89) | <0.001 | 0.84 (0.71, 0.99) | 0.04 |
| Carotid-femoral pulse wave velocity | 1.69 (1.41, 2.03) | <0.001 | 1.32 (1.07, 1.63) | 0.01 |
| Tonometry primary pressure wave | 1.25 (1.12, 1.39) | <0.001 | 1.12 (0.99, 1.27) | 0.06 |
Models add covariates to the vascular variables one at a time.
Minimal models adjusted for age, sex, and cohort.
Expanded models adjusted for age, sex, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, smoking, diabetes mellitus, hypertension treatment, cohort, BMI, and relatedness. LCL, UCL, lower and upper limits of the 95% confidence intervals. HRs expressed per 1 SD higher value, 232 events (5%) with median of 8.6 years of follow-up.
Figure 1 depicts the cumulative probability of a first-major CVD event when participants were grouped according to quartiles of each measure of vascular function. In a model adjusted for age, sex, and cohort, participants in the lowest (<6.7 m/s) CFPWV group vs. participants in the highest (≥9.2 m/s) group had an adjusted HR of 4.4 (95% CI, 1.8-10.7; P=0.001). In a model adjusted for age, sex, and cohort, participants in the highest (≥47.9 mm Hg) primary pressure wave amplitude group vs. participants in the lowest (<34.1 mm Hg) group had an adjusted HR of 1.8 (95% CI, 1.2-2.7; P=0.003). In a model adjusted for age, sex, and cohort, participants in the highest (≥71.0 cm/sec) hyperemic flow velocity group vs. participants in the lowest (<42.8 cm/sec) group had an adjusted HR of 0.5 (95% CI, 0.3-0.9; P=0.01). We observed no significant differences between the quartile groups for FMD and baseline brachial diameter.
Figure 1. Kaplan-Meier estimators of the cumulative probability of a first-major CVD event when participants were grouped according to quartiles of vascular function measures (N=4547).
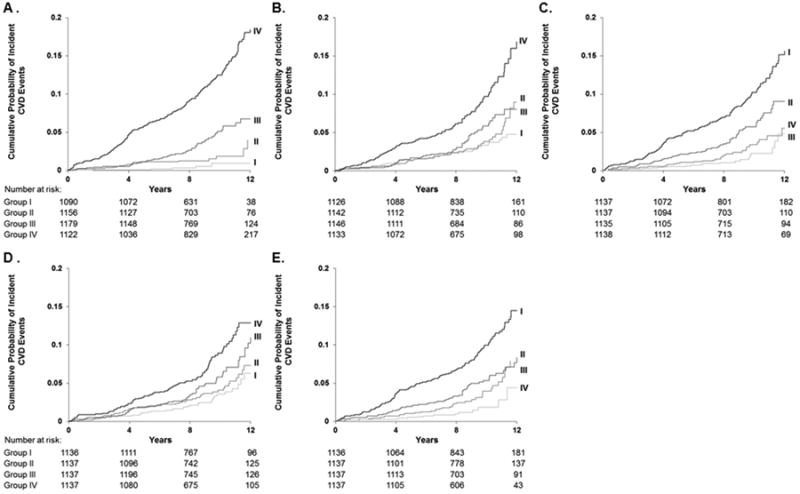
CVD events per person for quartile groups of vascular function measures (A-E). A. Carotid-femoral pulse wave velocity: Group I (<6.7 m/s, 6/1090 [0.55%]); Group II (6.7 to <7.7 m/s, 18/1156 [1.56%]), Group III (7.7 to <9.2 m/s, 50/1179 [4.24%]); and Group IV (≥9.2 m/s, 158/1122 [14.08%]). B. Primary pressure wave amplitude: Group I (<34.1 mm Hg, 41/1126 [3.64%]); Group II (34.1 to <40.4 mm Hg, 43/1142 [3.77%]); Group III (40.4 to <47.9 mm Hg, 50/1146 [4.36%]); and Group IV (≥47.9 mm Hg, 98/1133 [8.65%]). C. Hyperemic flow velocity: Group I (<42.80 cm/sec, 122/1137 [10.73%]); Group II (42.80 to <57.53 cm/sec, 59/1137 [5.19%]); Group III (57.53 to <71.00 cm/sec, 31/1135 [2.73%]); and Group IV (≥71.00 cm/sec, 20/1138 [1.76%]). D. Baseline brachial diameter: Group I (<3.51 mm, 34/1136 [2.99%]); Group II (3.51 to <4.08 mm, 48/1137 [4.22%]); Group III (4.08 to <4.82 mm, 65/1137 [5.72%]); and Group IV (≥4.82 mm, 85/1137 [7.48%]). E. Flow-mediated vasodilation: Group I (<1.81%, 119/1136 [10.48%]); Group II (1.81 to <4.05%, 61/1137 [5.36%]), Group III (4.05 to <6.77%, 37/1137 [3.25%]); and Group IV (≥6.77%,15/1137 [1.32%]).
Results of the mediation analyses are summarized in Figure 2, which depicts models demonstrating that hyperemic mean flow velocity partially mediated the relation of aortic stiffness (measured by CFPWV or forward wave) with incident CVD events. Effect estimates are reported as hazard ratios. The analyses for both CFPWV and primary pressure wave confirmed a significant direct effect and demonstrated a significant indirect effect of hyperemic mean flow velocity consistent with the presence of mediation. Calculation of the proportion mediated showed that hyperemic mean flow velocity was estimated to mediate 7.5% of the effect of CFPWV on time to CVD event. When primary pressure wave was assessed as the predictor variable (lower panel), hyperemic mean flow velocity was estimated to mediate 12.7% of the effect of primary pressure wave on time to CVD event.
Figure 2. Analysis for the effect of aortic stiffness on incident CVD events mediated by hyperemic flow (N=4547).
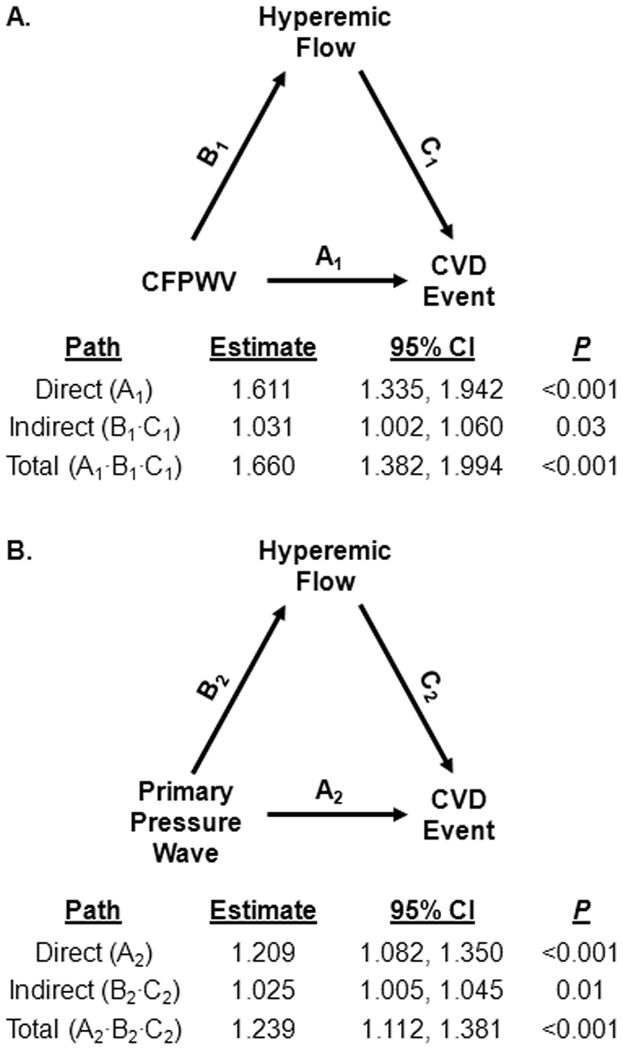
The effect estimate (hazard ratio) and 95% confidence intervals are reported for all paths: direct effect, A; indirect effect, B·C; and total effect, A·B·C. Models adjusted for age, sex, and cohort. A. Mediation analysis of the effect of CFPWV on CVD events. Proportion mediation=7.5%. B. Mediation analysis of the effect of primary pressure wave amplitude on CVD events. Proportion mediation=12.7%.
Discussion
Principal Findings
We investigated the interrelations of aortic stiffness and markers of vascular dysfunction with incidence of first-onset CVD events in a wide age range of participants in the community-based Framingham Heart Study. In multivariable models adjusted for standard CVD risk factors, cohort, BMI, and relatedness, both elevated CFPWV and reduced hyperemic mean flow velocity individually associated with incident CVD. In the expanded risk factor model, elevated primary pressure wave amplitude was marginally associated with incident CVD, but baseline brachial diameter and FMD were not. We used mediation analysis to examine the putative role of microvascular function (indicated by hyperemic flow velocity) as a mediator of the relation between aortic stiffness (indicated by CFPWV and primary pressure wave) and incident CVD. Mediation analysis revealed that hyperemic flow velocity partially mediated the relation between aortic stiffness and incident CVD. Our results are consistent with the hypothesis that elevated aortic stiffness may promote damage or remodeling in the microcirculation, which contributed to the observed relation of aortic stiffness with time to onset of a first-major CVD event in our community-based sample.
Vascular Measures and Incident CVD
Several studies have evaluated the relations of hemodynamic load, including novel measures of aortic stiffness, such as peripheral and central pulse pressure and aortic pulse wave velocity, with incident CVD and disease progression.9, 25-32 More specifically, CFPWV is a well-established marker of aortic stiffness, which has been extensively studied as a predictor of CVD risk. In an older Framingham cohort, we showed that elevated aortic stiffness, as assessed by CFPWV, is related to incident CVD events.9 The present study is consistent with these forgoing analyses.
On the other hand, the relative predictive value of hyperemic flow and FMD continues to be a point of debate. FMD is traditionally measured after ischemia induced by occlusion of the forearm vessels. The resulting hyperemic flow, which is attributable to dilation of forearm microvessels, reflects microvascular function and is in part mediated by nitric oxide.33 In previous cross-sectional studies, we reported that hyperemic flow velocity correlated more strongly with CVD risk factors than FMD.3 Recently, Yeboah and colleagues showed significant relations between FMD and CVD events in two different large cohorts.2, 34 Conversely, the recent Firefighters and Their Endothelium (FATE) study showed that hyperemic flow velocity, but not FMD, was predictive of incident CVD events in healthy men.8 Among patients with prevalent peripheral arterial disease, Huang et al. reported that both FMD and reactive hyperemia related to CVD risk, but FMD was the stronger predictor within this population with severe atherosclerotic disease.35 Demographic differences among the study populations, such as age and prevalent risk factor and CVD burden, likely underlie the inconsistent results regarding the associations among FMD, hyperemic flow, and CVD events.8
In the present study, we observed associations of reactive hyperemia but not FMD with incident events in expanded multivariable-adjusted models. Since hyperemic flow is a marker of microvascular function, whereas FMD is an index of endothelial function of large vessels, these data suggest that microvascular dysfunction may be a stronger marker of cardiovascular risk. In light of previous studies among participants of various ages, our data further suggest differential relevance for FMD and reactive hyperemia. For example, in older individuals and persons with prevalent CVD pathology, endothelial dysfunction of large conduit arteries may be a more relevant predictor of CVD risk and progression; whereas, small artery and microvascular dysfunction may be a more important pre-clinical indicator of CVD risk among healthier and younger individuals.8 However, we did not find significant effect modification by age of the relation of FMD (P=0.89) or reactive hyperemia (P=0.25) with events in our cohort of relatively younger participants.
Putative Microvascular Mechanism Linking Aortic Stiffness with Incident CVD
Although crosstalk between small and large vessels has been described,36 the underlying mechanisms linking hemodynamics of large vessels to the development and progression of CVD remain to be completely elucidated. In the present study, elevated CFPWV and reduced hyperemic flow were the two strongest vascular measures predictive of incident CVD, suggesting that aortic stiffness and microvascular dysfunction may be implicated independently or synergistically in incident CVD. Growing evidence has shown that elevated aortic stiffness may lead to targeted organ damage and pathological events, including incident CVD. Around midlife, the aorta begins to stiffen; aortic impedance increases disproportionately to the muscular arteries leading to impedance matching and a reduction in wave reflection. This reduction in proximal wave reflection, relative to the muscular and resistance arteries, removes a protective mechanism that normally shields the peripheral microcirculation from excessive pulsatility and thereby increases transmission of pulsatile energy into the microcirculation.17, 37 Higher pulsatility leads to hypertrophic remodeling and progressive encroachment of arterial lumen, possibly as a means to protect the microcirculation from pulsatile damage.38, 39
In this investigation, we performed formal mediation analysis to elucidate a possible biological mechanism underlying the association between aortic stiffness and CVD events. Hyperemic flow velocity modestly attenuated the relation between CFPWV and incident CVD events. Although aortic stiffness is independently associated with risk for CVD events, our results suggest that elevated aortic stiffness may be associated with microvascular remodeling and damage, which may be an important causal contributor to incident CVD. Since early microvascular dysfunction is mostly asymptomatic, we posit that assessment among healthier and younger individuals with a family history or perceived higher risk for CVD may be beneficial and may provide an early marker of future risk. Although risk reclassification analysis is beyond the scope of this investigation, studies with stratification of individuals by both elevated aortic stiffness and reduced hyperemic flow reserve may be warranted and may potentially lead to further insight toward prevention of incident CVD.
Limitations
Our prospective study is observational with many years of follow-up. Although we were able to infer temporal associations, our data only suggest causality regarding the partial mediation that may underlie the observed associations among aortic stiffness, microvascular function, and CVD events. We cannot dismiss the possibility that hyperemic flow may serve as a proxy, and there may be residual confounding by duration or severity of unknown or associated risk factors. Cardiovascular risk factors are key contributors to the development of vascular dysfunction. Additionally, although we present paths by which aortic stiffness leads to microvascular damage, Stefanadis et al. showed that removing the vasa vasorum network led to deterioration of aortic elasticity in animal models, suggesting that small vessel dysfunction may promote functional and structural remodeling of the aorta.40, 41 Thus, it is possible that microvascular dysfunction promotes arterial stiffness or that stiffness and microvascular dysfunction are mediators of traditional risk factors. Furthermore, the measures of vascular function examined in the mediation analysis (i.e., CFPWV, primary pressure wave, and hyperemic flow velocity) have inherent variability, and the estimates of the associations are modest. However, our findings have pathophysiologic implications regarding the relevance of microvascular dysfunction to vascular health. In addition, we did not obtain true forward wave amplitude derived from pressure and flow for both cohorts. Therefore, we used the primary pressure wave amplitude as a pressure-only surrogate, which could result in variable underestimation of forward wave amplitude. We recorded Doppler information immediately after cuff release; thus, we are unable to assess the impact of the entire hyperemic response that may have a different association with events.8 Participants were permitted to consume water, decaffeinated coffee, or tea prior to the examination. The effects of these beverages on vascular function were presumed to be minimal; however, it remains possible that their consumption induced residual confounding or increased heterogeneity of vascular testing. We did not account for multiple testing; thus, the reader should consider that our investigation is more susceptible to type-1 error. Finally, because our cohort was comprised primarily of white participants of European descent, our findings may not be generalizable to other racial/ethnic groups.
Conclusion
We have shown that measures of aortic stiffness and hyperemic flow velocity were associated with incident CVD in an ambulatory community-based sample. In addition, we showed that hyperemic flow velocity partially mediated the relation between aortic stiffness and incident CVD. This novel hemodynamic mechanism was shown in a broad age range of men and women participants. With age, the aorta becomes increasingly stiff and sends excessive pulsatile energy into the microcirculation, leading to microvascular injury, target organ damage, and ultimately incident CVD. Since aortic stiffening may be preventable, further clinical research on earlier preventive measures that focus on aortic stiffness and microvascular remodeling within younger individuals is warranted.
Supplementary Material
Clinical Perspective.
Vascular dysfunction contributes to cardiovascular disease (CVD) progression and clinical events. We assessed several functional indicators of vascular function (hyperemic mean flow velocity, flow-mediated vasodilation, carotid-femoral pulse wave velocity, primary pressure wave amplitude, and baseline brachial diameter) in Framingham Heart Study participants (N=4547). In age- and sex-adjusted models, both impaired vasodilation and higher arterial stiffness were associated with higher CVD risk. Multivariable models adjusted for cardiovascular risk factors showed that higher carotid-femoral pulse wave velocity and lower hyperemic mean flow velocity remained associated with incident CVD events. Furthermore, mediation analysis indicated that 8-13% of the relation between measures of aortic stiffness and CVD events was mediated by hyperemic mean flow velocity. Our findings reveal a putative but novel mechanism by which the associations between aortic stiffness and CVD events are partially mediated by pathways that include microvascular damage and remodeling. Aortic stiffening and vascular dysfunction are modifiable and may be preventable; therefore, earlier prevention and measures that focus on reducing aortic stiffness and microvascular remodeling may reduce CVD risk.
Acknowledgments
From the Framingham Heart Study of the National Heart Lung and Blood Institute of the National Institutes of Health and Boston University School of Medicine.
Sources of Funding: This work was supported by the National Heart, Lung and Blood Institute's Framingham Heart Study (Contracts No. N01-HC-25195 and HHSN268201500001I) and by HL076784, AG028321, HL070100, HL060040, HL080124, HL071039, HL077447, HL107385, HL126136, HL128914, and 2-K24-HL04334. Dr. Cooper is supported by the UNCF/Merck Science Initiative.
Dr. Mitchell is owner of Cardiovascular Engineering, Inc., a company that develops and manufactures devices to measure vascular stiffness, serves as a consultant to and receives honoraria from Novartis, Merck, Servier and Philips, and was funded by research grants HL094898, DK082447, HL107385, HL104184 and HL126136 from the National Institutes of Health.
Footnotes
Disclosures: The remaining authors report no conflicts.
References
- 1.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Noninvasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 2.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF, Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension. 2004;44:134–139. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell GF, Vita JA, Larson MG, Parise H, Keyes MJ, Warner E, Vasan RS, Levy D, Benjamin EJ. Cross-sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness--the Framingham Heart Study. Circulation. 2005;112:3722–3728. doi: 10.1161/CIRCULATIONAHA.105.551168. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility--Reykjavik Study. Brain. 2011;134:3398–3407. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodard T, Sigurdsson S, Gotal JD, Torjesen AA, Inker LA, Aspelund T, Eiriksdottir G, Gudnason V, Harris TB, Launer LJ, Levey AS, Mitchell GF. Mediation analysis of aortic stiffness and renal microvascular function. J Am Soc Nephrol. 2015;26:1181–1187. doi: 10.1681/ASN.2014050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper LL, Woodard T, Sigurdsson S, van Buchem MA, Torjesen AA, Inker LA, Aspelund T, Eiriksdottir G, Harris TB, Gudnason V, Launer LJ, Mitchell GF. Cerebrovascular damage mediates relations between aortic stiffness and memory. Hypertension. 2016;67:176–182. doi: 10.1161/HYPERTENSIONAHA.115.06398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, Hildebrand K, Fung M, Verma S, Lonn EM. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the Firefighters and Their Endothelium (FATE) Study. Circulation. 2011;123:163–169. doi: 10.1161/CIRCULATIONAHA.110.953653. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsao CW, Lyass A, Larson MG, Levy D, Hamburg NM, Vita JA, Benjamin EJ, Mitchell GF, Vasan RS. Relation of central arterial stiffness to incident heart failure in the community. J Am Heart Assoc. 2015;4:4. doi: 10.1161/JAHA.115.002189. e002189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper LL, Rong J, Benjamin EJ, Larson MG, Levy D, Vita JA, Hamburg NM, Vasan RS, Mitchell GF. Components of hemodynamic load and cardiovascular events: the Framingham Heart Study. Circulation. 2015;131:354–361. doi: 10.1161/CIRCULATIONAHA.114.011357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. the Framingham Offspring Study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 13.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB, Sr, Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D. The third generation cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 14.Benjamin EJ, Larson MG, Keyes MJ, Mitchell GF, Vasan RS, Keaney JF, Jr, Lehman BT, Fan S, Osypiuk E, Vita JA. Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham Heart Study. Circulation. 2004;109:613–619. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- 15.Hamburg NM, Palmisano J, Larson MG, Sullivan LM, Lehman BT, Vasan RS, Levy D, Mitchell GF, Vita JA, Benjamin EJ. Relation of brachial and digital measures of vascular function in the community: the Framingham Heart Study. Hypertension. 2011;57:390–396. doi: 10.1161/HYPERTENSIONAHA.110.160812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly R, Fitchett D. Noninvasive determination of aortic input impedance and external left ventricular power output: a validation and repeatability study of a new technique. J Am Coll Cardiol. 1992;20:952–963. doi: 10.1016/0735-1097(92)90198-v. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 18.Cupple LA, D'Agostino RB. Some risk factors related to the annual incidence of cardiovascular disease and death using pooled repeated biennial measurments. In: Kannel WB, Wolff PA, Garrison RJ, editors. The Framingham Heart Study; an Epidemiologic Investigation of Cardiovascular Disease. Washington DC: National Institutes of Health; 1987. pp. 87–203. [Google Scholar]
- 19.Frankel DS, Vasan RS, D'Agostino RB, Sr, Benjamin EJ, Levy D, Wang TJ, Meigs JB. Resistin, adiponectin, and risk of heart failure the Framingham Offspring Study. J Am Coll Cardiol. 2009;53:754–762. doi: 10.1016/j.jacc.2008.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 21.Mackinnon DP, Fairchild AJ. Current directions in mediation analysis. Curr Dir Psychol Sci. 2009;18:16–20. doi: 10.1111/j.1467-8721.2009.01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes AF. An Introduction to Mediation, Moderation, and Conditional Process Analysis: a Regression-Based Approach. New York, NY: Guilford Press; 2013. [Google Scholar]
- 23.Valeri L, VanderWeele TJ. SAS macro for causal mediation analysis with survival data. Epidemiology. 2015;26:e23–24. doi: 10.1097/EDE.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 24.Valeri L, VanderWeele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18:137–150. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber T, Wassertheurer S, Rammer M, Haiden A, Hametner B, Eber B. Wave reflections, assessed with a novel method for pulse wave separation, are associated with end-organ damage and clinical outcomes. Hypertension. 2012;60:534–541. doi: 10.1161/HYPERTENSIONAHA.112.194571. [DOI] [PubMed] [Google Scholar]
- 26.Russo C, Jin ZZ, Palmieri V, Homma S, Rundek T, Elkind MSV, Sacco RL, Di Tullio MR. Arterial stiffness and wave reflection sex differences and relationship with left ventricular diastolic function. Hypertension. 2012;60:362–368. doi: 10.1161/HYPERTENSIONAHA.112.191148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Regnault V, Thomas F, Safar ME, Osborne-Pellegrin M, Khalil RA, Pannier B, Lacolley P. Sex difference in cardiovascular risk. J Am Coll Cardiol. 2012;59:1771–1777. doi: 10.1016/j.jacc.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glasser SP, Halberg DL, Sands C, Gamboa CM, Muntner P, Safford M. Is pulse pressure an independent risk factor for incident acute coronary heart disease events? the REGARDS Study. Am J Hypertens. 2014;27:555–563. doi: 10.1093/ajh/hpt168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berard E, Bongard V, Ruidavets JB, Amar J, Ferrieres J. Pulse wave velocity, pulse pressure and number of carotid or femoral plaques improve prediction of cardiovascular death in a population at low risk. J Hum Hypertens. 2013;27:529–534. doi: 10.1038/jhh.2013.8. [DOI] [PubMed] [Google Scholar]
- 31.Baba Y, Ishikawa S, Kayaba K, Gotoh T, Kajii E. High pulse pressure is associated with increased risk of stroke in Japanese: the JMS Cohort Study. Blood Press. 2011;20:10–14. doi: 10.3109/08037051.2010.516075. [DOI] [PubMed] [Google Scholar]
- 32.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R, International Brachial Artery Reactivity Task Force Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 34.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the Multi-Ethnic Study of Atherosclerosis. Circulation. 2009;120:502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang AL, Silver AE, Shvenke E, Schopfer DW, Jahangir E, Titas MA, Shpilman A, Menzoian JO, Watkins MT, Raffetto JD, Gibbons G, Woodson J, Shaw PM, Dhadly M, Eberhardt RT, Keaney JF, Jr, Gokce N, Vita JA. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol. 2007;27:2113–2119. doi: 10.1161/ATVBAHA.107.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laurent S, Briet M, Boutouyrie P. Large and small artery cross-talk and recent morbidity-mortality trials in hypertension. Hypertension. 2009;54:388–392. doi: 10.1161/HYPERTENSIONAHA.109.133116. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol. 2008;105:1652–1660. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell GF, Lacourciere Y, Ouellet JP, Izzo JL, Neutel J, Kerwin LJ, Block AJ, Pfeffer MA. Determinants of elevated pulse pressure in middle-aged and older subjects with uncomplicated systolic hypertension--the role of proximal aortic diameter and the aortic pressure-flow relationship. Circulation. 2003;108:1592–1598. doi: 10.1161/01.CIR.0000093435.04334.1F. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell GF. Impedance progress: aortic diameter rears its head again? Hypertension. 2007;49:1207–1209. doi: 10.1161/HYPERTENSIONAHA.107.087239. [DOI] [PubMed] [Google Scholar]
- 40.Stefanadis C, Vlachopoulos C, Karayannacos P, Boudoulas H, Stratos C, Filippides T, Agapitos M, Toutouzas P. Effect of vasa vasorum flow on structure and function of the aorta in experimental animals. Circulation. 1995;91:2669–2678. doi: 10.1161/01.cir.91.10.2669. [DOI] [PubMed] [Google Scholar]
- 41.Stefanadis CI, Karayannacos PE, Boudoulas HK, Stratos CG, Vlachopoulos CV, Dontas IA, Toutouzas PK. Medial necrosis and acute alterations in aortic distensibility following removal of the vasa vasorum of canine ascending aorta. Cardiovasc Res. 1993;27:951–956. doi: 10.1093/cvr/27.6.951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


