Abstract
Successful application clinical-grade human growth hormone (hGH) immunoassays to the discovery of illegal doping cases has been rare. Indeed, the preferred biological matrix in doping control is urine, where the estimated baseline concentration of hGH falls well below the linear range and sensitivity threshold of all commercially available immunoassays, including hGH isoform differential immunoassays which can discriminate pituitary endogenous hGH from recombinant hGH. We employed hydrogel nanoparticles as a pre-processing step that concentrate urinary hGH into the linear range of isoform differential immunoassays.
We explored the characteristics of immunoassays in urine spiked with both phGH or rhGH, after pre-treatment with the nanoparticles. Subsequently, pre-treatment was applied to urine obtained from 3 healthy volunteers administered during three days with daily subcutaneous injections of 0.026 mg/kg/day rhGH, Genotonorm®.
Linearity between both rhGH and phGH concentrations in urine measured by a chemoluminescent assay (Immulite) and in the particle eluate was evident for differential immunoassays (R square higher than 0.999). In case of treated individuals the recombinant/pituitary concentration ratios remained above the established World Anti-Doping Agency (WADA) criterion for hGH misuse up to 24 h after the last administration dose, using both assays for volunteer 1 and 2 while in case of volunteer 3 results were inconclusive.
The use of nanoparticles appears to open the possibility of assessing rhGH misuse in urine.
Keywords: Differential immunoassays, Hydrogel nanoparticles, Pituitary growth hormone, Recombinant growth hormone
1. Introduction
Clinical-grade human growth hormone (hGH) immunoassays are of no use for application in doping control for a variety of reasons. Firstly, absolute levels of hGH have no value for doping control because of the multiplicity of factors affecting them [1,2]. Secondly, the physicochemical structural differences between pituitary hGH (phGH) and recombinant pharmaceutical preparation of 22 kDa (rhGH) require a detection strategy that specifically addresses this rhGH variant. Thirdly, the half-life of hGH in circulation following subcutaneous administration of rhGH is very short, between 15 (22 kDa hGH) and 19 min (20 kDa hGH) [3], which directly affects the time-frame to detect elevated hGH levels. Lastly, the preferred biological matrix in doping control is urine, where the estimated baseline concentration of hGH is roughly between 100 and 1000 times lower than in blood falling below the linear range and sensitivity threshold (50 pg/mL) of all commercially available immunoassays for hGH. Recently two sensitive luminescent immunoassays with preferential recognition of pituitary (phGH) or recombinant monomeric (rhGH) growth hormone have been developed and validated for serum samples [4]. The method is based on the use of two sandwich type immunoassays that preferentially recognize either mono- and oligomeric phGH variants or the monomeric isoform of rhGH: rec1/pit1 (kit1) and rec2/pit2 (kit2). Assays were successfully used to detect elevated ratios in serum of dosed volunteers from 18 to 36 h, depending of gender and administered dose [4], after a single injection of rhGH. Although these isoform differential immunoassays have a low threshold values (LOQ less than 41 pg/mL), they are above the requirements for direct measurements in urine.
Consequently, the World Anti-Doping Agency (WADA) endorsed the differential immunoassay methodology for serum and released specific guidelines for the application of these hGH isoform assays for anti-doping analyses [5] leading to the unmasking of several cheating athletes.
An adverse analytical finding (AAF) is dependent on the two ratios calculated by dividing the concentration value (in ng/mL) obtained for rhGH by the concentration value for phGH in both assays and these figures must exceed decision limits established for the used kits: ratio1 for rec1/pit1 > 1.81 for males and 1.46 for females and ratio 2 for rec2/pit2 > 1.68 for males and 1.55 for females [5].
To apply these hGH isoform differential immunoassays to urine and overcome the issue of extremely low concentration of hGH, we employed hydrogel nanoparticles [6–10] as a pre-processing step that concentrate urinary hGH into the linear range of the rec1/pit1 and rec2/pit2 isoform differential immunoassays. We here present the preliminary results obtained by applying nanoparticles pre-treatment to urine samples spiked with pituitary and recombinant hGH and to urine samples from healthy volunteers administered with recombinant hGH.
2. Experimental
2.1. Materials
Among several bait functionalized hydrogel nanoparticles, Remazol Brillant Blue (RBB) nanoparticles (Ceres Nanosciences, Manassas, VA, USA), containing a small organic dye as bait, were employed to capture, preserve and concentrate hGH in urine, since as they were successfully employed in previous experiments [8]. Recombinant 22 kDa hGH Genotonorm® for the administration studies was purchased from Pfizer laboratories (Barcelona, Spain). Spike Recombinant 22 kDa hGH for spiking experiments was Humatrope® from Lilly pharmaceutical company (Indianapolis, USA) and pituitary hGH was a preparation from National Institute for Biological Standards and Controls [11]. GH immunoassay kits for Immulite were from Siemens-DPC (LA, USA). Two sandwich type immunoassays that recognize mono- and oligomeric phGH (rec1/pit1 – kit1) variants or the monomeric isoform of rhGH (rec2/pit2 – kit2) were provided by the CMZ Assay GMBH (Berlin, Germany). Rec is the immuno assay targeting preferentially the recombinant monomeric 22 kDa GH variant and Pit isimmuno assay targeting preferentially the pituitary derived GH variants. All reagents were of analytical-reagent grade purchased from Aldrich (Milan, Italy) and were used without further purification.
2.2. Biological samples
Spiked samples were prepared using urine from healthy volunteers added with four different concentrations of phGH: 379, 38, 9, and 5 pg/mL or four different concentrations of rhGH: 274, 27, 7, and 3 pg/mL.
Real samples were obtained from 3 healthy males administered during three days with daily subcutaneous injections of 0.026 mg/kg/day rhGH, Genotonorm® at time 0, 24 and 48 h.
The study was conducted in compliance with the “ethical principles for medical research involving human subjects” of the Helsinki Declaration, and the protocol was approved by the local ethical review board and authorized by the Spanish Agency for Drugs and Health Products (AEMPS, Madrid, Spain). Serum samples were collected at 0, 6, 12, 24, 30, 48, and 54 h while urine samples at 0 time and at the following intervals:, 6–10, 10–24, 30–34, 34–48, 48–54, 54–58, 58–72, 72–84, and 84–96 h. Spot urine samples from five additional healthy volunteers, not involved in the administration study, were also collected and processed. Details concerning these five subjects can be found elsewhere. All samples were stored at −20°C until analysis.
2.3. Conditions and instrumentation
First we explored the characteristics of rec1/pit1 and rec2/pit2 immunoassays in urine samples spiked with both phGH or rhGH, after pre-treatment with nanoparticles. Twenty mL spiked urine were incubated with 2 mL functionalized RBB particles for 30 min as indicated by the manufacturer. Particles were isolated by centrifugation (19,000 rpm, 50 min, 18 °C) and washed twice with water. Proteins captured by the particles were eluted with organic solvents (1 mL acetonitrile–ammonium hydroxide, 70–30), split in two aliquots and lyophilized in presence of trehalose (1 mL for every 10 mL of urine sample processed) as pellet builder. One aliquot was reconstituted in 500 μL Immulite sample diluent and measured with Immulite-1000 (Siemens Corp., USA). The other aliquot was reconstituted in 500 μL sheep serum (Sigma–Aldrich Corp., St. Louis, MO, USA) and analyzed with the isoform differential immunoassays by an AutoLumat LB 953 Multi-Tube Luminometer (Berthold Technologies, Germany).
3. Results and discussion
Linearity between both rhGH and phGH concentrations in urine measured by Immulite and in the particle eluate was evident for rec2/pit2 immunoassay and the relationship could be mathematically described by linear equations with values of R squared higher than 0.999 (Table 1). Similar results were obtained when comparing Immulite results with those attained with rec1/pit1 immunoassay.
Table 1.
Concentration in urine samples spiked with rhGH or phGH after nanoparticles extraction as measured by Immulite and rec2/pit2 (Kit2) differential immunoassay.
| Spiked urine samples rhGH (pg/ml) | Immulite hGH (pg/ml) | Kit 2 differential immunoassay rhGH (pg/ml) | Kit 2 differential immunoassay phGH (pg/ml) |
|---|---|---|---|
| 274 | 272.50 | 235.95 | 119.30 |
| 27 | 13.28 | 26.50 | 16.75 |
| 7 | 2.35 | 4.05 | 4.70 |
| 3 | 2.15 | 2.75 | 3.35 |
| phGH (pg/ml) | hGH (pg/ml) | rhGH (pg/ml) | phGH (pg/ml) |
|
| |||
| 379 | 207.25 | 162.60 | 176.15 |
| 38 | 2.42 | 2.14 | 20.50 |
| 9 | 4.73 | 3.20 | 5.10 |
| 5 | 1.78 | 2.05 | 4.05 |
In the samples spiked with rhGH the response in the pit assay was approximately half that of the rec assay whereas spiking with phGH yielded more equivalent results. This is in good agreement with the design of the kits where the antibody of the rec assay was selected for a preference for monomeric 22 kDa hGH and with the stated recoveries for the kits [4].
Subsequently, nanoparticle pre-treatment was applied to urine samples obtained from 3 healthy males administered with three daily subcutaneous injections of rhGH. Serum samples of all three volunteers were analyzed following WADA specific guidelines for hGH analysis using isoform differential immunoassays [4,5,12] and urine samples as above described for the samples spiked with rhGh and phGH.
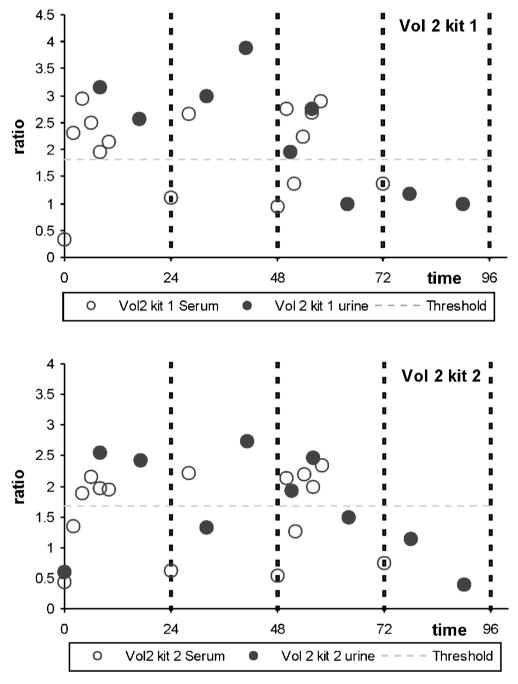
The profile of the ratios for volunteer 2 as a representative example, throughout the three-days treatment follows the same trend both in amplitude as well as tendency (Fig. 1), with a potential shift in secretion maximum the time frame of application of the test could be slightly longer.
Fig. 1.
Pharmacokinetics of the rec/pit ratios for volunteer 2 in urine (black circles) and serum (white circles). Urine samples correspond to complete collection periods (each circle is depicted at the mean time of the period). Horizontal dotted line indicates the present threshold for positivity in serum, according to WADA guidelines (5). (kit1: rec1/pit 1 kit; kit2: rec2/pit 2 kit.)
The pharmacokinetics following rhGH administration was in line with what is known for this hormone [12,13] under similar administration regimes, with a maximum concentration at approximately 6–10 h, post-administration, and near baseline levels after 24 h. In urine, a very similar trend was observed, albeit that the maximum concentration appeared to be shifted slightly with respect to that encountered in serum. Serum measures correspond to a defined extraction time whereas the urine sample represents the total collected with respect to the previous collection point. This way of processing may artificially prolong the timepoint of maximum secretion in urine, but also include a dilution factor due to the timeframe of collection.
Table 2 reports the comparative ratios (ratio1 = rec1/pit1 and ratio2 = rec2/pit2) obtained for urine and serum samples from the three volunteers at similar times after short rhGH treatment. The ratios obtained for urine samples from five non-treated healthy volunteers are also included (controls 1–5).
Table 2.
rec/pit ratios obtained in urine samples and time related serum samples. Values above the threshold for positivity in serum, according to WADA guidelines (5), are in bold type.
| Urine samples | Serum samples | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Collection time | Ratio 1 rec1/pit1 | Ratio 2 rec2/pit2 | Collection time | Ratio 1 rec1/pit1 | Ratio 2 rec2/pit2 | |
| Volunteer 1 | 0 h (pre-treatment) | ND* | 0.25 | 0 h (pre-treatment) | 0.29 | 0.34 |
| 6–10 h (<10 h 1st dose) | 3.62 | 2.41 | 10 h (10 h 1 st dose) | 3.32 | 2.58 | |
| 10–24 h (<24 h 1st dose) | 1.71 | 1.19 | 24 h (24 h 1st dose) | 0.49 | 0.51 | |
| 30–34 h (<10 h 2n dose) | 4.14 | 1.57 | 28 h (10 h 2n dose) | 2.95 | 2.45 | |
| 34–48 h (<24 h 2n dose) | 1.85 | 1.08 | 48 h (24 h 2n dose) | 0.81 | 0.75 | |
| 48–54 h (<6 h 3r dose) | 4.20 | 1.50 | 54 h (6 h 3r dose) | 3.04 | 2.86 | |
| 54–58 h (<10 h 3r dose) | 3.44 | 2.25 | 58 h (10 h 3r dose) | 3.28 | 2.97 | |
| 58–72 h (<24 h 3r dose) | 2.15 | 1.78 | 72 h (24 h 3r dose) | 1.16 | 0.71 | |
| 72–84 h (<36 h 3r dose) | 1.00 | 0.43 | ||||
| Volunteer 2 | Basal | ND* | 0.60 | 0 h (pre-treatment) | 0.34 | 0.43 |
| 6–10 h (<10 h 1st dose) | 3.16 | 2.55 | 10 h (10 h 1st dose) | 2.15 | 1.94 | |
| 10–24 h (<24 h 1st dose) | 2.56 | 2.42 | 24 h (24 h 1st dose) | 1.11 | 0.63 | |
| 30–34 h (<10 h 2n dose) | 3.00 | 1.33 | 28 h (10 h 2n dose) | 2.66 | 2.21 | |
| 34–48 h (<24 h 2n dose) | 3.88 | 2.74 | 48 h (24 h 2n dose) | 0.94 | 0.54 | |
| 48–54 h (<6 h 3r dose) | 1.96 | 1.92 | 54 h (6 h 3r dose) | 2.5 | 2.19 | |
| 54–58 h (<10 h 3r dose) | 2.76 | 2.47 | 58 h (10 h 3r dose) | 2.89 | 2.34 | |
| 58–72 h (<24 h 3r dose) | 1.00 | 1.50 | 72 h (24 h 3r dose) | 1.36 | 0.75 | |
| 72–84 h (<36 h 3r dose) | 1.17 | 1.14 | ||||
| Volunteer 3 | Basal | ND* | 0.50 | 0 h (pre-treatment) | 0.32 | 0.31 |
| 6–10 h (<10 h 1st dose) | 2.09 | 2.29 | 10 h (10 h 1st dose) | 3.54 | 2.55 | |
| 10–24 h (<24 h 1st dose) | ND* | ND* | 24 h (24 h 1st dose) | 0.78 | 1.06 | |
| 30–34 h (<10 h 2n dose) | 0.53 | 0.69 | 28 h (10 h 2n dose) | 3.62 | 3.20 | |
| 34–48 h (<24 h 2n dose) | ND* | 1.00 | 48 h (24 h 2n dose) | 1.92 | 1.25 | |
| 48–54 h (<6 h 3r dose) | 2.50 | 2.23 | 54 h (6 h 3r dose) | 3.34 | 2.03 | |
| 54–58 h (<10 h 3r dose) | 1.92 | 2.45 | 58 h (10 h 3r dose) | 3.93 | 1.60 | |
| 58–72 h (<24 h 3r dose) | 2.00 | 3.43 | 72 h (24 h 3r dose) | 0.86 | 0.79 | |
| 72–84 h (<36 h 3r dose) | 0.33 | 1.33 | ||||
| Control 1 | ND* | ND* | ||||
| Control 2 | 1.07 | 0.76 | ||||
| Control 3 | 1.16 | 0.98 | ||||
| Control 4 | 1.02 | 1.01 | ||||
| Control 5 | 1.22 | 0.95 | ||||
ND* = ratio could not be calculated due to pit measurement under the LOD of the assay.
Succinctly, when considering the WADA decision limits for males of 1.81 (rec1/pit1 assay) and 1.68 (rec2/pit2) [5] for the rhGH/phGH ratio analyzed with isoform differential immunoassays we notice that:
In case of volunteer 1, the recombinant/pituitary concentration ratio in urine remained above the established WADA criterion for HGH misuse up to 24 h after the last administration dose, using both assays; for of volunteer 2, the positive ratio lasted for about 10 h after the last rhGH dose, while in case of volunteer 3 results were inconclusive, particularly on first and second day. In this latter case, several measures fell under the assay limit of detection (LOD) and substantiate the choice for 30, rather than 20 mL of starting volume of urine.
The evaluation of 5 control urine samples yielded ratios in the range between 1.02 and 1.22 in case of rec1/pit1 kit and between 0.76 and 1.01 in case of rec2/pit2 kit. Even though this is a very limited number of individuals, and larger studies with both treated and untreated subjects are required, the basal ratio in urine appears to be clearly in the non-AAF of the test but different than in serum where extensive studies have yielded an average value of approximately 0.5. This could be explained by the renal filtration processes discriminating between the different variants present in serum. Furthermore, the potential size-exclusion of the nanoparticles could contribute to the selective analysis of monomers. Despite the potential difference in basal ratios between serum and urine, and the fact that the established values should not be translated directly, the preliminary data indicate that the seem to be valid for both as non treated values remained below the threshold while following administration the ratios rapidly exceeded these values for several hours, as happens in serum.
4. Conclusions
In summary, the use of nanoparticles appears to open the possibility of assessing rhGH misuse in urine and this sample preparation protocol could be of use to other proteins that are targeted in urine for doping control such as erythropoietin. Spiking urinary samples with either pituitary or recombinant hGH yields differential immunoassay results that are in line with the assay characteristics. With a limited number of blank subjects and samples from an administration study we have shown that rhGH misuse is reflected in urine and that the same approach as for serum can be pursued. However, these preliminary proofs of principle require further studies with rhGH administration to larger number of subjects and with non-treated healthy volunteers to accurately establish baseline values from which new potential decision limits could be established.
Acknowledgments
The authors thank Silvia Graziano for technical assistance. This investigation was supported by the Italian Anti-Doping Commission from Ministry of Health (2009 funds) and Italia – USA Collaboration Programme, and financial aid from the Catalan Government (DIUE2009SGR492).
Abbreviations
- Rec
recombinant
- Pit
pituitary
- hGH
human growth hormone
- AAF
adverse analytical finding
References
- 1.Segura J, Gutiérrez-Gallego R, Ventura R, Pascual JA, Bosch J, San-martín Such-G, Nikolovski Z, Pinyot A, Pichini S. Growth hormone in sport: beyond Beijing 2008. Therapeutic Drug Monitoring. 2009;31:3–13. doi: 10.1097/FTD.0b013e318194cc94. [DOI] [PubMed] [Google Scholar]
- 2.Baumann GP. Growth hormone doping in sports: a critical review of use and detection strategies. Endocrine Reviews. 2012;33:155–186. doi: 10.1210/er.2011-1035. [DOI] [PubMed] [Google Scholar]
- 3.Leung KC, Howe C, Gui LY, Trout G, Veldhuis JD, Ho KK. Physiological and pharmacological regulation of 20-kDa growth hormone. American Journal of Physiology – Endocrinology and Metabolism. 2002;283:E836–E843. doi: 10.1152/ajpendo.00122.2002. [DOI] [PubMed] [Google Scholar]
- 4.Bidlingmaier M, Suhr J, Ernst A, Wu Z, Keller A, Strasburger CJ, Bergmann A. High-sensitivity chemiluminescence immunoassays for detection of growth hormone doping in sports. Clinical Chemistry. 2009;55:445–453. doi: 10.1373/clinchem.2008.112458. [DOI] [PubMed] [Google Scholar]
- 5.World Anti-Doping Agency. hGH isoform differential immunoassays for anti-doping analyses. Version 1.0. World Anti-Doping Program WADA. 2010 Guidelines: http://www.wada-ama.org/Documents/Resources/Guidelines/WADAGuidelineshGH%20Differential%20ImmunoassaysENJune10.pdf.
- 6.Fredolini C, Meani F, Reeder KA, Rucker S, Patanarut A, Botterell PJ, et al. Concentration and preservation of very low abundance biomarkers in urine, such as human growth hormone (hGH), by Cibacron Blue F3G-A loaded hydrogel particles. Nano Research. 2008;1:502–518. doi: 10.1007/s12274-008-8054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luchini A, Geho DH, Bishop B, Tran D, Xia C, Dufour RL, et al. Smart hydrogel particles: biomarker harvesting: one-step affinity purification, size exclusion, and protection against degradation. Nano Letters. 2008;8:350–361. doi: 10.1021/nl072174l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fredolini C, Tamburro D, Gambara G, Lepene BS, Espina V, Petricoin EF, III, et al. Nanoparticle technology: amplifying the effective sensitivity of biomarker detection to create a urine test for hGH. Drug Testing and Analysis. 2009;1:447–454. doi: 10.1002/dta.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luchini A, Longo C, Espina V, Petricoin EF, III, Liotta LA. Nanoparticle technology: addressing the fundamental roadblocks to protein biomarker discovery. Journal of Materials Chemistry. 2009;19:5071–5077. doi: 10.1039/b822264a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luchini A, Fredolini C, Espina BH, Meani F, Reeder A, Rucker S, et al. Nanoparticle technology: addressing the fundamental roadblocks to protein biomarker discovery. Current Molecular Medicine. 2010;10:133–141. doi: 10.2174/156652410790963268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.NIBSC. WHO International Standard Growth Hormone Human Pituitary. NIBSC report. 2007 code 80/505:1–3, http://www.nibsc.ac.uk/documents/ifu/80-505.pdf.
- 12.Luchini A, Tamburro D, Magni R, Fredolini C, Espina V, Bosch J, Garaci E, Petricoin EF, III, Liotta LA. Application of analyte harvesting nanoparticle technology to the measurement of urinary HGH in healthy individuals. Journal of Sports Medicine & Doping Studies. 2012;2:2–4. doi: 10.4172/2161-0673.1000e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosch J, Ueki M, Such-Sanmartin G, Segura J, Gutiérrez-Gallego R. Tracking growth hormone abuse in sport: a comparison of distinct isoform-based assays. Analytica Chimica Acta. 2012;733:56–63. doi: 10.1016/j.aca.2012.04.028. [DOI] [PubMed] [Google Scholar]