Abstract
Several studies have suggested functional association between μ-opioid and δ-opioid receptors and showed that μ-activity could be modulated by δ-ligands. The general conclusion is that agonists for the δ-receptor can enhance the analgesic potency and efficacy of μ-agonists. Our preliminary investigations demonstrate that new bivalent ligands constructed from the μ-agonist fentanyl and the δ-agonist enkephalin-like peptides are promising entities for creation of new analgesics with reduced side effects for treatment of neuropathic pain. A new superposition of the mentioned pharmacophores led to novel μ-bivalent/δ-bivalent compounds that demonstrate both μ-opioid and δ-opioid receptor agonist activity and high efficacy in anti-inflammatory and neuropathic pain models with the potential of reduced unwanted side effects.
Introduction
μ-Opioid analgesics are the mainstay for treatment of moderate to severe pain, but they have significant side effects including constipation, respiratory depression, tolerance, addiction, and even death [1]. Convincing data (biochemical, pharmacological, and studies using genetically modified animals) regarding the modulatory interactions between opioid receptors exist in the literature. Several studies indicate that δ-receptor agonists as well as δ-receptor antagonists provide significant modulation of the pharmacological effects of μ-agonists. It has been shown that agonists at the δ-receptor can enhance the analgesic potency and efficacy of μ-agonists while δ-antagonists can prevent or diminish the development of tolerance and physical dependence by μ-agonists; multiple and controversial interpretations of the observed phenomena have been made [2–16].
Functional association between μ-opioid and δ-opioid receptors was first suggested by studies showing that μ-activity could be modulated by δ-ligands [17], but the true role of δ-ligands remains obscure. Some authors insist that δ-agonists increase antinociceptive responses to μ-receptor agonists [18–20] while others state that μ-agonist signaling can be enhanced by co-treatment with δ-selective antagonists [21, 22]. Others indicate differential localizations and pain relieving profiles of the μ-receptors and δ-receptors in dorsal root ganglion cells [23].
One of the promising new approaches to answer the questions raised could be the creation and investigation of bivalent ligands. Bivalent ligands contain two pharmacophores that are fused or variably separated by a chemical spacer, and there are several interesting examples of them in the literature [24–41]. Finding the “correct” pair of two ligands and their “correct” superposition is an attractive yet challenging problem.
Our preliminary investigations demonstrate that new bivalent ligands with mixed μ-profile/δ-profile, which represents one attempt at combining fentanyl 1 and enkephalin-like peptides 2 (Fig. 1), are promising compounds with high binding affinities to both μ-receptors and δ-receptors [42–46].
Figure 1.
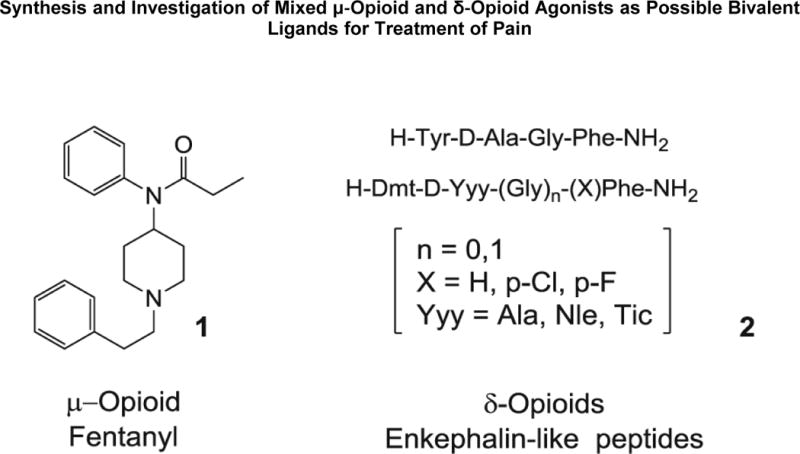
Structures of fentanyl and enkephalin‐like peptides.
Recently, we have designed and synthesized compounds of types 3 and 4 (Fig. 2), which differ mainly in superposition of the μ/δ constituents. Compounds in series 3 display binding affinities in the range of 30–60 nM for both receptors [44–46]. Compounds in series 4 display higher affinity (~0.4 nM) for both receptors with increased hydrophobicity (aLogP 3.01–4.74). Binding affinities of series 4 exceed desired affinities and cross the threshold of nanomolar into picomolar range at both μ-opioid and δ-opioid receptors [42, 43]. The bivalent ligands with synergistic action on different subtypes of the same (opioid) receptors could be called concordant ligands.
Figure 2.

Structures of μ‐bivalent/δ‐bivalent ligands already proposed by our group.
Another novel attempt to create μ-bivalent/δ-bivalent compounds using both a different superposition of ligands and a different linker (carboxy group) and spacer (hydrazino group) is presented in Figure 3 and is the subject of the present publication.
Figure 3.

Structures of novel μ‐bivalent/δ‐bivalent ligands.
Results and Discussion
Chemistry
The gram scale synthesis of functionalized fentanyls—carboxyfentanyl (7a), carboxymethyl-fentanyl (7b), and fentanyl derivative (7c) starting from 1-phenethyl-N-phenylpiperidin-4-amine (6)—was previously described by our group for the synthesis of μ-agonist/NK1-antagonist bivalent ligands [47, 48]. The synthetic Scheme 1 ensures good yields of the desired compounds.
Scheme 1.

Synthesis of novel μ‐bivalent/δ‐bivalent ligands. Reagents and conditions: (a) succinic, glutaric, or diglycolic anhydride in CH2Cl2; (b) Ac2O, HClO4; (c) N‐Boc‐PheNHNH2, EDAC, HOBt, CHCl3; (d) N‐Boc‐PheNHNH2, Et3N, CHCl3; (e) CF3COOH/CH2Cl2; (f) 1. Boc‐Tyr(Boc)‐D‐Ala‐Gly‐OH, BOP, HOBt, DMF; 2. CF3COOH/CH2Cl2.
A “peptide chemistry” method with carbodiimide activation of the carboxyl function of (7a, 7b, 7c) with EDAC/HOBt [1-ethyl-3-(3-dimethylaminopropyl)carbodiimide/hydroxybenzotriazole] was successfully implemented for the creation of N-Boc-phenylalanine hydrazides (9a, 9b, 9c) acylated with fentanyl carboxylic acids (7a, 7b, 7c).
Another convenient method for creation of substituted fentanyls has been developed using “succinisoimidium perchlorates chemistry”—preparation of succinisoimidium perchlorates from succinamic acids with acetic anhydride and perchloric acid [49–52]. Previously, this method was extended and simplified by us, implementing it for the creation of new type of isoimidium compounds—glutarisoimidium and 3-oxaglutarisoimidium perchlorates (8b, 8c) [47]. All three isoimidium perchlorates smoothly produce appropriate hydrazides (9a, 9b, 9c) when treated with N-Boc-phenylalanine hydrazide, which on further Boc deprotection with trifluoroacetic acid gave (10a, 10b, 10c). Coupling of the obtained products with Boc-protected Tyr-D-Ala-Gly-OH followed by deprotection gave the desired bivalent ligands with mixed μ-profile/δ-profile (11a, 11b, 11c).
Another potentially convenient method for the creation of compounds of the formula (11) by direct coupling of hydrazides (12) (Fig. 4) with enkephalin-like peptides was undertaken, but our various attempts to develop a protocol for their preparative synthesis failed. Direct hydrazination of both (7) and (8) gave very poor yields of an unstable product (12). However, use of Boc or benzylhydrazines gave excellent yields of protected products (13) and (14). But upon deprotection, Boc hydrazides (13) decomposed in different ways depending on conditions, and the nature of the linker between the two carbonyl groups. Bn-hydrazides (14) decomposed on H2/Pd-C hydrogenation to give 1-alkyl-1,2,3,6-tetrahydropyridines (16) (Fig. 4).
Figure 4.

Failed attempts of the synthesis of hydrazides (12).
The bivalent ligands with mixed μ-/δ-profile (11a, 11b, 11c) demonstrated good binding affinity to both μ-opioid and δ-opioid receptors (Table 1).
Table 1.
| Compound | MVD | GPI/LMMP |
|---|---|---|
| 11a | 69 +/− 12 | 44 +/− 9.7 |
| 11b | 39 +/− 8.2 | 63 +/− 13 |
| 11c | 48 +/− 16 | 42 +/− 8.3 Agonist Activity nM IC50[d]+/− sem[e] |
mouse vas deferens.
guinea pig isolated ileum.
longitudinal muscle with myenteric plexus.
concentration of compounds at 50% specific binding.
standard error of mean
Compound (11b) was selected for testing in several in vivo pain models, including inflammatory pain, using the formalin flinch assay and antiallodynia, in a nerve ligation model with no significant sedation, or motor impairment and demonstrates potent and efficacious activity. This is the first report of a fentanyl-based structure with δ-opioid and μ-opioid receptor activity that exhibits outstanding antinociceptive efficacy in neuropathic pain, reducing the propensity of unwanted side effects that occur with current μ-opioid agonist therapies.
Compound (11b) demonstrated significant antinociception using a warm water tail-flick test after the spinal administration. The three doses (1, 3, and 10 μg) produced significant dose-dependent antinociception in non-injured mice compared with vehicle-treated mice 15 min after intrathecal injection (21 ± 6.4%, 36 ± 15.8%, and 77 ± 10.7%, respectively). The A50 dose was calculated to be 3.92 μg.
These effects were blocked by naloxone, indicating that they were opioid receptor-mediated effects. Furthermore, the significant attenuation by the μ-selective antagonist, (D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2) CTAP, and by the δ-selective antagonist, naltrindole, suggests that the antinociception produced by (11b) is indeed mediated by both the μ-opioid and δ-opioid receptors. In order to determine whether (11b) has anti-inflammatory effects, it was tested in a murine formalin flinch model. Compound (11b) resulted in significant inhibition of formalin-induced flinching in both the first and second phases, suggesting antinociception by inhibiting both δ-fiber and C-fiber activity.
Implementing the Kim and Chung model for peripheral neuropathy [53, 54], it was shown that (11b) resulted in significant mechanical antiallodynia and thermal antihypersensitivity in the nerve injured animal. Such studies suggest that a fentanyl-based, mixed mu-delta opioid agonist (11b) can act to inhibit neuropathic pain. Rotorod experiments were performed and demonstrated that the lack of (11b) induced motor paralysis and/or signs of sedation because such behavior would mask paw withdrawal results in our pain behavior tests. Owing to the added analgesic effects of δ-opioid receptor occupation, less μ-receptor occupation is required, essentially decreasing the dose and unwanted side effects driven by μ-opioid receptor agonists. Ultimately, by using a combination of acute and chronic pain models, we demonstrate the efficacy of the novel μ-bivalent/δ-bivalent compound (11b) in treating neuropathic pain without sedation and motor impairment.
Overall, these studies demonstrate a novel fentanyl-based structure that contains both μ-opioid and δ-opioid receptor agonist activity resulting in high efficacy in anti-inflammatory and neuropathic pain models; they also have the potential to reduce unwanted side effects. There may also be the added potential of being anxiolytic, which will need to be further investigated. Detailed pharmacological investigations on (11b) are already published [1].
Conclusion
The result of these studies indicate that our design and synthesis of novel non-peptide μ-agonists/δ-agonists resulted in new series of compounds such as (11), which have potent analgesic effects. Novel fentanyl-based structures that contain both μ-opioid and δ-opioid receptor agonist constituents reveal high efficacy in anti-inflammatory and neuropathic pain models with the potential of reduced unwanted side effects. In addition, μ-agonists/δ-agonists have been shown to significantly decrease anxiety in mice and rats. This latter aspect needs to be further investigated. Detailed pharmacological investigation on (11b) is in progress.
Experimental
Chemistry
The compounds were characterized by 1H and 13C nuclear magnetic resonance (NMR). NMR spectra were recorded on a Bruker (Billerica, MA) DRX-600 spectrometer using tetramethylsilane as an internal standard. The compounds were analyzed by electrospray mass spectrometry (MS) on a Thermo Electron (Waltham, MA) (Finnigan) LCQ classic ion trap mass spectrometer. Direct infusion (10 μL/min) was applied to about 50 μM solutions of the samples in MeOH/H2O 1:1 2% AcOH. Standard electrospray ionization conditions were applied, and the masses of protonated molecules [MH]+ were measured. Optical rotations were measured on a Rudolph Research Analytical (Hackettstown, NJ) AutoPol III polarimeter using the Na-D line. The purity of the compounds was determined by thin-layer chromatography on silica gel plates (Analtech, Newark, DE; 02521), solvent system MeOH/CHCl3 1:4. Melting points are uncorrected. The purity of the compounds was also checked by analytical reversed-phase high-performance liquid chromatography using an Amersham Pharmacia Biotech (Piscataway, NJ) Äkta Basic 10F with a ODS-A C18 (120 Å, 5 μm, 250 mm × 4.6 mm) column (Omnicrom; YMC Europe GmbH, Dinslaken, Germany) monitored at 220 and 254 nm and by high-resolution mass spectral analysis (Supporting Information).
General method for the synthesis of the 4-oxo-4-((1-phenethylpiperidin-4-yl)(phenyl)amino)alcanoic acids (7a, 7b, 7c)
A mixture of [1-(2-phenyl)-ethyl-piperidin-4-yl]-phenyl-amine (2.8 g, 10 mmol), appropriate acid anhydride (succinic, glutaric, or diglycolic, 11 mmol) and two to three drops of acetic acid in dichloromethane (30 mL) was heated in a closed pressure-proof flask in a boiling water bath for 5 h and left overnight at room temperature. The solvent was evaporated; ethyl acetate (50 mL) was added, and the mixture was heated to dissolve the content of the flask. For the cases (7b) and (7c), it is necessary to add some ethanol (5–10 mL). Crystals of the appropriate acid separated on cooling with 80–90% yield.
4-Oxo-4-((1-phenethylpiperidin-4-yl)(phenyl)amino)butanoic acid (7a)
Crystalline solid; yield: 3.4 g (89%); mp 119–120 °C; 1H-NMR (600 MHz, methanol-d4): δ = 7.50 (t, J = 7.3 Hz, 2H), 7.48–7.43 (m, 1H), 7.32–7.25 (m, 4H), 7.25–7.18 (m, 3H), 4.70 (tt, J = 12.0, 3.5 Hz, 1H), 3.42 (d, J = 12.2 Hz, 2H), 3.00 (m, 2H), 2.90 (dd, J = 10.5, 6.1 Hz, 2H), 2.80 (t, J = 12.0 Hz, 2H), 2.42 (t, J = 6.8 Hz, 2H), 2.18 (t, J = 6.8 Hz, 2H), 2.00 (d, J = 12.2 Hz, 2H), 1.62 ppm (dq, J = 3.3, 13.1 Hz, 2H); 13C-NMR (150 MHz, methanol-d4): δ = 172.77, 138.08, 137.48, 130.16, 129.40, 128.65, 128.31, 126.44, 58.09, 51.92, 50.76, 30.85, 30.47, 30.25, 28.14 ppm; MS [electron impact (EI), 70 eV] m/z (%): 381; high-resolution MS fast atom bombardment (HRMS-FAB) m/z [M+H]+ calcd for C23H29N2O3:381.2178; found: 381.2183.
5-Oxo-5-((1-phenethylpiperidin-4-yl)(phenyl)amino)pentanoic acid (7b)
Crystalline solid; 3.42 g (87%); mp 104–105 °C; 1H-NMR (600 MHz, methanol-d4): δ = 7.49 (t, J = 7.3 Hz, 2H), 7.45 (d, J = 7.2 Hz, 1H), 7.29 (t, J = 7.2 Hz, 2H), 7.24–7.19 (m, 5H), 4.72 (tt, J = 12.0, 3.6 Hz, 1H), 3.42 (d, J = 12.3 Hz, 2H), 2.99 (m, 2H), 2.89 (m, 2H), 2.79 (t, J = 11.9 Hz, 2H), 2.10 (t, J = 7.3 Hz, 2H), 2.05–1.95 (m, 4H), 1.79 (dd, J = 14.7, 7.3 Hz, 2H), 1.61 ppm (dd, J = 12.8, 3.2 Hz, 2H); 13C-NMR (150 MHz, methanol-d4): δ = 173.27, 138.07, 137.56, 130.05, 129.44, 128.66, 128.32, 126.43, 58.11, 51.87, 50.78, 34.52, 33.99, 30.89, 28.19, 21.05 ppm; MS (EI, 70 eV) m/z (%): 395; HRMS-FAB m/z [M+H]+ calcd for C24H31N2O3: 395.2325; found: 395.2323.
2-(2-Oxo-2-((1-phenethylpiperidin-4-yl)(phenyl)amino)ethoxy)acetic acid (7c)
Crystalline solid; 3.12 g (79%); mp 143–144 °C; 1H-NMR (600 MHz, methanol-d4): δ = 7.55–7.45 (m, 3H), 7.35–7.26 (m, 4H), 7.25–7.22 (m, 3H), 4.74 (tt, J = 12.1, 3.6 Hz, 1H), 3.86 (d, J = 6.0 Hz, 4H), 3.56 (d, J = 12.3 Hz, 2H), 3.15 (dd, J = 10.3, 6.6 Hz, 2H), 3.01 (t, J = 12.2 Hz, 2H), 2.95 (dd, J = 10.3, 6.6 Hz, 2H), 2.09 (d, J = 12.7 Hz, 2H), 1.72 ppm (dd, J = 12.8, 2.8 Hz, 2H); 13C-NMR (150 MHz, methanol-d4): δ = 174.52, 170.05, 136.78, 136.17, 129.90, 129.70, 129.28, 128.46, 128.33, 126.67, 69.40, 68.52, 57.60, 51.69, 50.81, 30.36, 27.41, 19.81 ppm; MS (EI, 70 eV) m/z (%): 397; HRMS-FAB m/z [M+H]+ calcd for C23H29N2O4: 397.2127; found: 397.2123.
General method for the synthesis of the isoimidium perchlorates (8a, 8b, 8c)
The stirred mixture of acid (7a, 7b, 7c, 0.5 mmol) in acetic anhydride (1.5 mL) was slightly heated until the beginning of dissolution of the starting material. Then, the heater was turned off, but stirring was continued for 1 h. The mixture was cooled (ice bath), and 70% HClO4 (0.15 mL) was carefully added dropwise. The obtained solution was allowed to come to room temperature overnight, and 5 mL of dry ether was added with stirring. After 15–20 min, the liquid was separated from the precipitated perchlorate, and the solid residue was washed with three portions of ether (5 mL). For analytical purposes, the residue was dried overnight in a vacuum desiccator over phosphorous pentoxide. For synthetic purposes, it was dissolved in CHCl3/CH3CN (5 mL/1 mL) mixture and added to a solution of N-Boc-phenylalanine hydrazide.
General method for the synthesis of the N-Boc-phenylalanine hydrazides acylated with fentanyl carboxylic acids(9a, 9b, 9c)
Peptide chemistry method
To the stirred mixture of acid (7a, 7b, 7c, 0.25 mmol) in CHCl3 (25 mL) cooling in ice bath, EDAC (0.048 mL, 0.275 mmol) was added. After 15 min, HOBt (0.04 g, 0.275 mmol) was added, and after stirring for additional 15 min, N-Boc-PheNHNH2(0.07 g, 0.275 mmol) was added. The reaction mixture was allowed to come to room temperature and left overnight. The solvent was removed in vacuo, and acetone (15 mL) was added to the remaining mixture, which was then refluxed for 5–10 min and allowed to cool. Precipitate of the obtained acetone hydrazone formed from excess of N-Boc-PheNHNH2 was filtered, acetone was removed in vacuo, CHCl3 (25 mL) was added to remaining solid, and the solution was washed twice with 5% NaHCO3 (10 mL) and with water and dried on MgSO4. Solvent evaporation gave pure (7a, 7b, 7c) with 70–80% yield. If necessary, product can be purified on silica gel column with the 4:1 chloroform/methanol solvent mixture.
Isoimidium perchlorates method
N-Boc-PheNHNH2 (0.07 g, 0.275 mmol) was added to the stirred suspension of perchlorate (8) (0.25 mmol) in CHCl3 (25 mL), and the solution was cooled in an ice bath before a dropwise addition of triethylamine (0.13 mL, 1 mmol) in CHCl3 (5 mL). The reaction mixture was allowed to come to room temperature and left overnight. The solvent was removed in vacuo, and acetone (15 mL) was added to the remaining mixture, which was then refluxed for 5–10 min and allowed to cool. Precipitate of the obtained acetone hydrazone was filtered, acetone was removed in vacuo, CHCl3 (25 mL) was added to the remaining solid, and the solution was washed twice with 5% NaHCO3 (10 mL) and with water before it was dried on MgSO4. Solvent evaporation gave pure (7a, 7b, 7c) with 65–70% yield. If necessary, the product can be purified on silica gel column with the 4:1 chloroform/methanol mixture.
Tert-butyl (1-oxo-1-(2-(4-oxo-4-((1-phenethylpiperidin-4-yl) (phenyl)amino)butanoyl)hydrazinyl)-3-phenylpropan-2-yl)carbamate (9a)
Viscous oil; 0.12 g (75%); 1H-NMR (600 MHz, CDCl3): δ = 7.42–7.39 (m, 2H), 7.27–7.09 (m, 13H), 5.69 (d, J = 8.8 Hz, 1H), 5.39 (d, J = 8.8 Hz, 1H), 5.38 (d, J = 9.6 Hz, 1H), 4.65 (m, 2H), 4.10 (s, 2H), 3.83 (s, 2H), 3.16 (m, 2H), 3.01 (d, J = 10.3 Hz, 2H), 2.72 (m, 2H), 2.54 (m, 2H), 2.16 (m, 2H), 1.82 (m, 2H), 1.46 (qd, J = 12.3, 3.8 Hz, 2H), 1.36 ppm (s, 9H); 13C-NMR (150 MHz, CDCl3) δ = 171.64, 169.01, 167.63, 155.49, 139.80, 136.95, 136.61, 130.28, 129.62, 129.49, 129.48, 128.70, 128.53, 128.49, 126.84, 126.22, 77.37, 60.12, 54.06, 52.88, 52.83, 38.66, 33.38, 30.55, 30.07, 29.18, 28.36; MS (EI, 70 eV) m/z (%): 641; HRMS-FAB m/z [M+H]+ calcd for C37H47N5O5: 641.3577; found: 641.3583.
Tert-butyl (1-oxo-1-(2-(5-oxo-5-((1-phenethylpiperidin-4-yl)(phenyl)amino)pentanoyl)hydrazinyl)-3-phenylpropan-2-yl)carbamate (9b)
Viscous oil; 0.12 g (73%); 1H-NMR (600 MHz, CDCl3): δ = 7.41–7.34 (m, 2H), 7.29–7.05 (m, 13H), 5.57 (d, J = 8.8 Hz, 1H), 5.41 (d, J = 8.8 Hz, 1H), 5.33 (d, J = 9.6 Hz, 1H), 4.64 (m, 2H), 3.13 (m, 2H), 3.01 (d, J = 10.3 Hz, 2H), 2.72 (m, 2H), 2.52 (m, 2H), 2.26 (t, J = 7.3 Hz), 2.13 (m, 2H), 2.03 (m, 2H), 1.84 (quintet, J = 7.3 Hz), 1.79 (m, 2H), 1.42 (m, 2H), 1.35 ppm (s, 9H); 13C-NMR (150 MHz, CDCl3) δ = 172.25, 168.65, 167.09, 155.61, 140.29, 136.96, 136.53, 130.37, 129.56, 129.49, 129.48, 128.72, 128.62, 128.49, 126.96, 126.14, 77.37, 60.55, 54.15, 53.18, 53.14, 38.61, 33.91, 33.72, 33.15, 30.61, 28.40, 21.37; MS (EI, 70 eV) m/z (%): 655; HRMS-FAB m/z [M+H]+ calcd for C38H49N5O5: 655.3734 found: 655.3743
Tert-butyl (1-oxo-1-(2-(2-(2-oxo-2-((1-phenethylpiperidin-4-yl)(phenyl)amino)ethoxy)acetyl)hydrazinyl)-3-phenylpropan-2-yl)carbamate (9c)
Viscous oil; 0.11 g (67%); 1H-NMR (600 MHz, CDCl3): δ = 7.39–7.33 (m, 2H), 7.27–7.07 (m, 13H), 5.58 (d, J = 8.8 Hz, 1H), 5.36 (d, J = 8.8 Hz, 1H), 5.33 (d, J = 9.6 Hz, 1H), 4.63 (m, 2H), 3.14 (m, 2H), 3.04 (d, J = 10.3 Hz, 2H), 2.74 (m, 2H), 2.59 (m, 2H), 2.50 (t, J = 7.3 Hz), 2.26 (t, J = 7.3 Hz), 2.19 (m, 2H), 1.79 (m, 2H), 1.47 (qd, J = 12.3, 3.8 Hz, 2H), 1.34 ppm (s, 9H); 13C-NMR (150 MHz, CDCl3) δ = 169.34, 168.93, 167.50, 155.62, 140.11, 136.59, 136.29, 130.14, 129.79, 129.51, 129.46, 128.64, 128.50, 128.42, 126.80, 126.10, 77.37, 71.23, 70.68, 60.38, 54.04, 52.93, 52.87, 38.66, 33.76, 30.16, 28.33; MS (EI, 70 eV) m/z (%): 657; HRMS-FAB m/z [M+H]+ calcd for C37H47N5O6: 657.3526; found: 657.3543
General method for the Boc deprotection of the N-Boc-phenylalanine hydrazides acylated with fentanyl carboxylic acids (10a, 10b, 10c)
To the stirred mixture of hydrazide (10, 0.25 mmol) in CH2Cl2 (10 mL), on cooling, a solution of CF3COOH (7 mL) in CH2Cl2 (7 mL) was added dropwise. The reaction mixture was allowed to come to room temperature overnight. The solvent and excess of CF3COOH were removed in vacuo, and CH2Cl2 (25 mL) was added to the remaining mixture. The mixture was cooled in an ice bath and basified with 28–30% NH4OH solution. The organic layer was separated and dried on MgSO4, and obtained (10a, 10b, 10c) were acylated with Boc-Tyr(Boc)-D-Ala-Gly-OH.
General method for the synthesis of ligands with mixed μ-profile/δ-profile (11a, 11b, 11c)
Fmoc-Gly-OH (0.35 g, 1.2 mmol) and diisopropylethylamine (DIEA) (0.84 mL, 4.8 mmol) was added to a stirred suspension of 2-chlorotrityl chloride resin (0.834 g, 1.0 mmol) in CH2Cl2 (10 mL). After 2 h, the resin was filtered and washed with dimethylformamide (DMF) and CH2Cl2. The resin was treated twice with 20% piperidine in DMF with mixing for 5 and 13 min and washed with DMF and CH2Cl2. >Fmoc-D-Ala-OH (1.558 g, 5.0 mmol), HCTU [2-(6-chloro-1H-benzotriazole-1-yl)-1,1,3,3-tetramethylaminium hexafluorophosphate] (2.06 g, 5.0 mmol), and DIEA (1.74 mL, 10.0 mmol) in DMF (12 mL) were added, and the mixture was shaken for 30 min, followed by washing with DMF and CH2Cl2. After subsequent Fmoc deprotection 5 and 13 min and washing, Boc-Tyr(tBu)-OH(1.69 g, 5.0 mmol), HCTU (2.06 g, 5.0 mmol), and DIEA (1.74 mL, 10.0 mmol) in DMF (12 mL) were added, followed by mixing for 30 min and washing with DMF and CH2Cl2. The protected tripeptide was cleaved from the resin with 12 mL of 20% hexafluoroisopropanol in CH2Cl2 for 1.5 h, which was evaporated to provide (0.406 g, 87.1%) of the product. Boc-Tyr(tBu)-D-Ala-Gly-OH (0.122 g, 0.24 mmol), 10a, 10b, 10c (0.22 mmol), BOP (0.115 g, 0.26 mmol), HOBt (0.035 g, 0.26 mmol), and DIEA (114 μL, 0.65 mmol) in DMF (1.5 mL) were stirred overnight. The reaction mixture was diluted with ethyl acetate and washed with 10% (aq.) potassium carbonate and then dried over solid potassium carbonate. After filtering, the solvent was evaporated. The resulting residue was dissolved in 1:1 CH2Cl2/CF3COOH (4 mL) and stirred for 30 min. After evaporating the reaction solvent and coevaporation of excess CF3COOH with toluene and diethyl ether, the residue was purified by reversed-phase high-performance liquid chromatography (C18) using a 30–70% gradient of acetonitrile in 0.1% aq. CF3COOH and lyophilized. Owing to the limited sensitivity of natural-abundance 13C and the relatively large size of compounds 11a, 11b, 11c, the 13C spectra were not conclusive. Instead, the 1H spectra were resolved using 2D total correlation spectroscopy and 2D rotational Overhauser effect spectroscopy to confirm the connectivity.
4-(2-((R)-2-(2-((S)-2-((R)-2-Amino-3-(4-hydroxyphenyl)propanamido)propanamido) acetamido)-3-phenylpropanoyl)hydrazinyl)-4-oxo-N-(1-phenethylpiperidin-4-yl)-N-phenylbutanamide trifluoroacetate (11a)
Viscous oil; 0.13 g (71%); 1H-NMR (600 MHz, DMSO-d6): δ = 10.03 (d, J = 11.1 Hz, 1H), 9.82 (d, J = 13.7 Hz, 1H), 9.36 (s, 1H), 8.53 (d, J = 7.6 Hz, 1H), 8.15 (t, J = 6.4 Hz, 1H), 8.09 (d, J = 8.2 Hz, 1H), 7.51 (t, J = 7.5 Hz, 2H), 7.46 (t, J = 7.5 Hz, 1H), 7.31 (t, J = 7.5 Hz, 2H), 7.29–7.15 (m, 10H), 7.00 (d, J = 8.5 Hz, 2H), 6.69 (d, J = 8.5 Hz, 2H), 4.69 (m, 1H), 4.56 (m, 1H), 4.30 (m, 1H), 3.97 (m, 1H), 3.72 (m, 1H), 3.58 (m, 1H), 3.51 (m, 2H), 3.16 (m, 2H), 3.06 (m, 2H), 2.99 (m, 1H), 2.87 (m, 4H), 2.76 (m, 1H), 2.33 (m, 2H), 2.07 (t, J = 7.1 Hz, 2H), 1.92 (m, 2H), 1.49 (m, 2H), 1.03 ppm (d, J = 7.3 Hz, 3H); (c 0.2; DMSO); MS (EI, 70 eV) m/z (%): 832; HRMS-FAB m/z [M+H]+ calcd for C46H56N8O7: 832.4272; found: 832.4283.
5-(2-((R)-2-(2-((S)-2-((R)-2-Amino-3-(4-hydroxyphenyl)propanamido)propanamido)acetamido)-3-phenylpropanoyl)hydrazinyl)-5-oxo-N-(1-phenethylpiperidin-4-yl)-N-phenylpentanamide trifluoroacetate (11b)
Viscous oil; 0.14 g (75%); 1H-NMR (600 MHz, DMSO-d6): δ = 10.06 (s, 1H), 9.89 (s, 1H), 9.47 (s, 1H), 8.59 (d, J = 7.1 Hz, 1H), 8.20 (t, J = 6.5 Hz, 1H), 8.16 (d, J = 8.2 Hz, 1H), 7.49 (t, J = 6.8 Hz, 2H), 7.44 (t, J = 6.8 Hz, 1H), 7.31 (t, J = 7.2 Hz, 2H), 7.27–7.15 (m, 10H), 7.00 (d, J = 8.2 Hz, 2H), 6.69 (d, J = 8.2 Hz, 2H), 4.72 (m, 1H), 4.55 (m, 1H), 4.29 (m, 1H), 3.97 (m, 1H), 3.70 (m, 1H), 3.57 (m, 1H), 3.50 (m, 2H), 3.16 (m, 2H), 3.08 (m, 2H), 2.98 (m, 1H), 2.87 (m, 4H), 2.75 (m, 1H), 2.01 (t, J = 7.1 Hz, 2H), 1.94 (m, 2H), 1.87 (t, J = 7.1 Hz, 2H), 1.66 (quintet, J = 7.1 Hz, 2H) 1.48 (m, 2H), 1.02 ppm (d, J = 7.3 Hz, 3H); (c 0.2; DMSO); MS (EI, 70 eV) m/z (%): 846; HRMS-FAB m/z [M+H]+calcd for C47H58N8O7: 846.4418; found: 846.4428.
(R)-2-Amino-N-((9R,15S)-9-benzyl-1,5,8,11,14-pentaoxo-1-((1-phenethylpiperidin-4-yl)(phenyl)amino)-3-oxa-6,7,10,13-tetraazahexadecan-15-yl)-3-(4-hydroxyphenyl)propanamide trifluoroacetate (11c)
Viscous oil; 0.14 g (75%); 1H-NMR (600 MHz, DMSO-d6): δ = 10.09 (s, 1H), 9.89 (s, 1H), 9.47 (s, 1H), 8.59 (d, J = 7.3 Hz, 1H), 8.20 (m, 1H), 8.16 (d, J = 8.4 Hz, 1H), 7.52–7.44 (m, 3H), 7.30 (t, J = 7.3 Hz, 2H), 7.29–7.15 (m, 10H), 7.00 (d, J = 8.1 Hz, 2H), 6.69 (d, J = 8.1 Hz, 2H), 4.71 (m, 1H), 4.56 (td, J = 9.3, 4.3 Hz, 1H), 4.28 (m, 1H), 4.01 (s, 2H), 3.97 (m, 1H), 3.76 (s, 1H), 3.71 (m, 1H), 3.57 (m, 1H), 3.53 (m, 2H), 3.16 (m, 2H), 3.10 (m, 2H), 2.98 (m, 1H) 2.87 (m, 4H), 2.76 (m, 1H), 1.96 (m, 2H), 1.51 (m, 2H), 1.02 ppm (d, J = 6.8 Hz, 3H); (c 0.2; DMSO); MS (EI, 70 eV) m/z (%): 848; HRMS-FAB m/z [M+H]+ calcd for C46H56N8O7: 848.4221; found: 848.4233.
Supplementary Material
Acknowledgments
This work was supported by the U.S. Public Health Service National Institute of Health DA 13449, DA 06284, and DA 06789.
References
- 1.Podolsky AT, Sandweiss A, Hu J, Bilsky EJ, Cain JP, Kumirov VK, Lee Y, Hruby VJ, Vardanyan RS, Vanderah TW. Life Sci. 2013;93:1010–1016. doi: 10.1016/j.lfs.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ananthan S. AAPS J. 2006;8:E118. doi: 10.1208/aapsj080114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hruby VJ, Porreca F, Yamamura HI, Tollin G, Agnes RS, Lee Y-S, Cai M, Alves I, Cowell S, Varga E, Davis P, Salamon Z, Roeske W, Vanderah T, Lai J. AAPS J. 2006;8:E450. doi: 10.1208/aapsj080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dietis N, Guerrini R, Calo G, Salvadori S, Rowbotham DJ, Lambert DG. Brit J Anaesth. 2009;103:38. doi: 10.1093/bja/aep129. [DOI] [PubMed] [Google Scholar]
- 5.Balboni G, Salvadori S, Trapella C, Knapp BI, Bidlack JM, Lazarus LH, Peng X, Neumeyer JL. ACS Chem Neurosci. 2010;1:155. doi: 10.1021/cn900025j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rozenfeld R, Devi LA. Trends Pharm Sci. 2010;31:124. doi: 10.1016/j.tips.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasternak GW, Pan Y-X. Neuron. 2011;69:6. doi: 10.1016/j.neuron.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao PL, Law P-Y, Loh HH. IUBMB Life. 2010;62:103. doi: 10.1002/iub.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rozenfeld R, Abul-Husn NS, Gomes I, Devi LA. ScientificWorld. 2007;7(Suppl. 2):64. doi: 10.1100/tsw.2007.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kabli N, Martin N, Fan T, Nguyen T, Hasbi A, Balboni G, O’Dowd BF, George SR. Brit J Pharmacol. 2010;161:1122–1136. doi: 10.1111/j.1476-5381.2010.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi L. A Proc Natl Acad Sci USA. 2004;101:5135. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Z, Pan ZZJ. Neurosci. 2010;30:4735. doi: 10.1523/JNEUROSCI.5968-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta A, Decaillot FM, Devi LA. AAPS J. 2006;8:E153. doi: 10.1208/aapsj080118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kabli N, Martin N, Fan T, Nguyen T, Hasbi A, Balboni G, O’Dowd BF, George SR. Brit J Pharm. 2010;161:1122. doi: 10.1111/j.1476-5381.2010.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, O’Dowd BFJ. Biol Chem. 2000;275:26128. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- 16.He S-Q, Zhang Z-N, Guan J-S, Liu H-R, Zhao B, Wang H-B, Yang Q, Li H, Luo J, Li Z-Y, Wang Q, Lu Y-J, Bao L, Zhang X. Neuron. 2011;69:120. doi: 10.1016/j.neuron.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Lee NM, Leybin L, Chang JK, Loh HH. Eur J Pharmacol. 1980;68:181. doi: 10.1016/0014-2999(80)90319-2. [DOI] [PubMed] [Google Scholar]
- 18.Porreca F, Takemori AE, Sultana M, Portoghese PS, Bowen WD, Mosberg HIJ. Pharm Exp Therapeutics. 1992;263:147. [PubMed] [Google Scholar]
- 19.Heyman JS, Vaught JL, Mosberg HI, Haaseth RC, Porreca F. Eur J Pharm. 1989;165:1. doi: 10.1016/0014-2999(89)90764-4. [DOI] [PubMed] [Google Scholar]
- 20.Heyman JS, Jang Q, Rothman RB, Mosberg HI, Porreca F. Eur J Pharm. 1989;169:43. doi: 10.1016/0014-2999(89)90815-7. [DOI] [PubMed] [Google Scholar]
- 21.Rozenfeld R, Devi LA. FASEB J. 2007;21:2455. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi L. A Proc Natl Acad Sci USA. 2004;101:5135. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O’Donnell D, Kieffer BL, Basbaum AI. Cell. 2009;137:1148. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dietis N, Guerrini R, Calo G, Salvadori S, Rowbotham DJ, Lambert DG. Brit J Anaesth. 2009;103:38. doi: 10.1093/bja/aep129. [DOI] [PubMed] [Google Scholar]
- 25.Hruby VJ, Porreca F, Yamamura HI, Tollin G, Agnes RS, Lee YS, Cai M, Alves I, Cowell S, Varga E, Davis P, Salamon Z, Roeske W, Vanderah T, Lai J. AAPS J. 2006;8:E450. doi: 10.1208/aapsj080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schiller PW. Life Sci. 2010;86:598–603. doi: 10.1016/j.lfs.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ballet S, Pietsch M, Abell AD. Protein & Peptide Lett. 2008;15:668. doi: 10.2174/092986608785133672. [DOI] [PubMed] [Google Scholar]
- 28.Liu Z, Zhang J, Zhang A. Curr Pharm Design. 2009;15:682. doi: 10.2174/138161209787315639. [DOI] [PubMed] [Google Scholar]
- 29.Zhang A, Liu Z, Kan Y. Curr Top Med Chem. 2007;7:343. doi: 10.2174/156802607779941279. [DOI] [PubMed] [Google Scholar]
- 30.Messer JR, William S. Curr Pharm Design. 2004;10:2015. doi: 10.2174/1381612043384213. [DOI] [PubMed] [Google Scholar]
- 31.Pasternak G. W Neuropharmacol. 2004;47(Suppl. 1):312. doi: 10.1016/j.neuropharm.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Vance D, Shah M, Joshi A, Kane RS. Biotechnol Bioengineer. 2008;101:429. doi: 10.1002/bit.22056. [DOI] [PubMed] [Google Scholar]
- 33.Zhang S, Yekkirala A, Tang Y, Portoghese PS. Bioorg Med Chem Lett. 2009;19:6978. doi: 10.1016/j.bmcl.2009.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng Y, Akgun E, Harikumar KG, Hopson J, Powers MD, Lunzer MM, Miller LJ, Portoghese PSJ. Med Chem. 2009;52:247. doi: 10.1021/jm800174p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yekkirala AS, Lunzer MM, McCurdy CR, Powers MD, Kalyuzhny AE, Roerig SC, Portoghese P. S Proc Natl Acad Sci USA. 2011;108:5098. doi: 10.1073/pnas.1016277108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balboni G, Salvadori S, Marczak ED, Knapp BI, Bidlack JM, Lazarus LH, Peng X, Si YG, Neumeyer JL. Eur J Med Chem. 2011;46:799. doi: 10.1016/j.ejmech.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valant C, Lane JR, Sexton PM, Christopoulos A. Ann Rev Pharmacol Toxicol. 2012;52:153. doi: 10.1146/annurev-pharmtox-010611-134514. [DOI] [PubMed] [Google Scholar]
- 38.Halazy S. Exp Opin Ther Pat. 1999;9:431. [Google Scholar]
- 39.Shonberg J, Scammells PJ, Capuano B. ChemMedChem. 2011;6:963. doi: 10.1002/cmdc.201100101. [DOI] [PubMed] [Google Scholar]
- 40.Erlanson DA. ACS Chem Biol. 2007;2:779. doi: 10.1021/cb700240b. [DOI] [PubMed] [Google Scholar]
- 41.Hruby VJ, Agnes RS, Davis P, Ma SW, Lee YS, Vanderah TW, Lai J, Porreca F. Life Sci. 2003;73:699. doi: 10.1016/s0024-3205(03)00390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee YS, Nyberg J, Moye S, Agnes RS, Davis P, Wu S, Ma S, Lai J, Porreca F, Vardanyan R, Hruby VJ. Bioorg Med Chem Lett. 2007;17:2161. doi: 10.1016/j.bmcl.2007.01.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee YS, Kulkarani V, Cowell SM, Ma SW, Davis P, Hanlon KE, Vanderah TW, Lai J, Porreca F, Vardanyan R, Hruby VJ. J Med Chem. 2011;(54):382. doi: 10.1021/jm100982d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee YS, Petrov R, Kulkarni V, Min BJ, Ma S, Davis P, Oyarzo J, Vanderah T, Lai J, Porreca F, Vardanyan R, Hruby VJ. Adv Exp Med Biol. 2009;(611):517. doi: 10.1007/978-0-387-73657-0_225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee YS, Agnes RS, Cain JP, Kulkarni V, Cai M, Salibay C, Ciano K, Petrov R, Mayorov A, Vagner J, Trivedi D, Davis P, Ma S, Lai J, Porreca F, Vardanyan R, Hruby VJ. Pept Sci. 2008;90:433. doi: 10.1002/bip.20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee YS, Petrov R, Park CK, Ma S, Davis P, Lai J, Porreca F, Vardanyan R, Hruby VJJ. Med Chem. 2007;50:5528. doi: 10.1021/jm061465o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vardanyan R, Kumirov VK, Nichol GS, Davis P, Liktor-Busa E, Rankin D, Varga E, Vanderah T, Porreca F, Lai J, Hruby VJ. Bioorg Med Chem. 2011;(19):6135. doi: 10.1016/j.bmc.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nichol GS, Kumirov VK, Vardanyan R, Hruby VJ. CrystEngComm. 2010;12:3651. doi: 10.1039/B923698H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoogewerff S, van Dorp WA. Rec Trav Chim. 1892;11:84. [Google Scholar]
- 50.Baydar AE, Boyd GV. J Chem Soc, Perkin Trans 1. 1978;11:1360. [Google Scholar]
- 51.Boyd GV, Monteil RL. J Chem Soc, Perkin Trans 1. 1978;11:1338. [Google Scholar]
- 52.Balaban AR, Balaban TS, Boyd GV. Synthesis. 1987;6:577. [Google Scholar]
- 53.Kim SH, Chung JM. Pain. 1992;50:355. [Google Scholar]
- 54.Schiller PW, Fundytus ME, Merovitz L, Weltrowska G, Nguyen TM, Lemieux C, Chung NN, Coderre TJJ. Med Chem. 1999;42:3520. doi: 10.1021/jm980724+. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


