Abstract
Objective
Helicobacter pylori (H. pylori) eradication rarely develops into antibiotic-associated hemorrhagic colitis (AAHC), in which the etiology of colitis remains unclear. We herein report a rare case of AAHC caused by second-line therapy for H. pylori eradication.
Results
A 65-year-old female was administered second-line therapy for H. pylori composed of 1500 mg of amoxicillin, 500 mg of metronidazole and 40 mg of vonoprazan for 7 days because of first-line therapy failure. A day after completing second-line therapy, she complained of abdominal pain and hematochezia. Colonoscopy revealed a hemorrhage and edematous mucosa with no transparent vascular pattern in the transverse colon. A bacterial culture detected Klebsiella oxytoca (K. oxytoca), but no other pathogenic bacteria. A drug-induced lymphocyte stimulation test (DLST) showed positive reactions for both amoxicillin and metronidazole. According to these findings, the patient was diagnosed with AAHC. Bowel rest for 6 days relieved her abdominal pain and hematochezia.
Conclusions
The present case developed AAHC caused by second-line therapy for H. pylori eradication. The pathogenesis is considered to be associated with microbial substitution as well as a delayed-type allergy to antibiotics, suggesting that AAHC is a potential adverse event of second-line therapy for H. pylori eradication.
Background
Helicobacter pylori (H. pylori) infection has been known to cause many gastrointestinal disorders including gastroduodenal ulcers, gastric cancer and lymphoma, and idiopathic thrombocytopenic purpura [1–5]. H. pylori eradication is thus recommended to prevent these gastrointestinal disorders [6, 7]. In Japan, treatment for H. pylori-associated gastritis is now covered by health insurance [8]. The success rate of first-line therapy for H. pylori eradication is considered to be around 70–80% [9]. Second-line therapy for H. pylori eradication is needed for the remaining cases. Although H. pylori eradication occasionally causes adverse events, such as diarrhea and eruptions, it rarely develops into antibiotic-associated hemorrhagic colitis (AAHC), in which the etiology of colitis remains unclear. We herein report a rare case of AAHC caused by second-line therapy for H. pylori eradication.
Case report
A 65-year-old female, who had systemic lupus erythematosus and was taking 5 mg of prednisolone, underwent gastric mucosal resection to remove gastric adenoma. The endoscopic findings of chronic gastritis were confirmed. Histological findings and culture of the gastric specimens obtained from the gastric antrum and body by an endoscopic biopsy procedure detected an H. pylori infection. She was therefore administered first-line therapy composed of 1500 mg of amoxicillin, 800 mg of clarithromycin and 60 mg of lansoprazole for 7 days to eradicate H. pylori in December 2014. A urea breath test was performed to assess the effect of the eradication and revealed a positive result for H. pylori infection. Seven months after the first-line therapy, the patient was prescribed second-line therapy composed of 1500 mg of amoxicillin, 500 mg of metronidazole and 40 mg of vonoprazan for 7 days to eradicate H. pylori in July 2015. A day after completing second-line therapy, she complained of abdominal pain and hematochezia. Blood tests showed increases in neutrophil (6090/µL) and C-reactive protein (1.38 µg/mL) levels, whereas the hemoglobin level was within the normal limits. Colonoscopy revealed a hemorrhage and edematous mucosa with no transparent vascular pattern in the transverse colon (Fig. 1). Histological findings of the biopsy specimens obtained from the transverse colon showed severe infiltrations of neutrophil and lymphocytes and a hemorrhage in the lamina propria (Fig. 2). A bacterial culture detected Klebsiella oxytoca (K. oxytoca), but not Clostridium difficile or other pathogenic bacteria. A drug-induced lymphocyte stimulation test (DLST) showed positive reactions for both amoxicillin and metronidazole. According to these findings, the patient was diagnosed with AAHC.
Fig. 1.

Colonoscopy findings. Diffusely edematous mucosa with mucus, a hemorrhage and no transparent vascular pattern was detected in the transverse colon
Fig. 2.
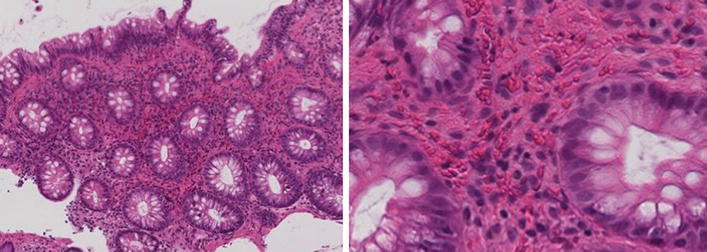
Histological findings. Severe infiltrations of neutrophil and lymphocytes and a hemorrhage in the lamina propria were detected in the biopsy specimens obtained from the transverse colon (magnifications: 100/400)
Bowel rest for 6 days under the oral administration of 5 mg of prednisolone relieved her abdominal pain and hematochezia. Typical symptoms due to SLE have been relieved for 20 months with conservative treatment alone, and no vasculitis was histologically detected in biopsy specimens of the colon. Therefore, lupus enteritis was not thought to be associated with the pathogenesis of the enteric disorder in the patient.
Discussion
We herein report a rare case of AAHC that developed after the completion of second-line therapy using amoxicillin, metronidazole and vonoprazan for H. pylori eradication. Clinicians must pay careful attention to the possibility of this disorder, even when no side effects develop after the first-line therapy for H. pylori eradication.
While the incidence rate of AAHC after the first-line therapy for H. pylori eradication has been reported to range from 0.35 to 0.6% [10, 11], the frequency of AAHC after second-line therapy for H. pylori eradication is still unclear. A total of five previous cases that exhibited AAHC after completing second-line therapy for H. pylori eradication were identified in a literature search on the PubMed and Ichushi databases (Table 1) [12, 13]. Including the present case, there were 2 males and 4 females, and the mean age of the cases was 59.3 years old. Five cases exhibited AAHC after completing second-line H. pylori eradication therapy, suggesting that an immediate-type allergy was not a cause of AAHC. In two cases, K. oxytoca was isolated from the stool culture. It is known that stool testing reveals K. oxytoca in significant amounts in the majority of patients with AAHC [14]. K. oxytoca strains isolated from patients with AAHC are known to produce a cytotoxin, which were shown to cause cell death in cultured Hep2, Vero, CHOK1, and HeLa cell lines as well as in an isolated intestinal loop rabbit model [15, 16]. These studies indicated that microbial substitution by amoxicillin- and/or metronidazole-induced overgrowth of K. oxytoca could be associated with the development of AAHC in patients with the administration of second-line therapy for H. pylori eradication. The DLST was performed only in our case, which detected positive reactions for amoxicillin and metronidazole. Collectively, two mechanisms are thought to be involved in the pathogenesis of AAHC. First, microbial alterations accumulate by repeated antibiotic administration, thereby leading to AAHC at the second-line treatment. Second, amoxicillin sensitization may have been acquired at the time of first-line therapy, and a delayed allergic reaction may have occurred at the time of second-line therapy. Although the delayed-type allergy was a potential cause of AAHC in our case, it is unclear whether this type of allergy is a major cause of AAHC in patients receiving second-line therapy for H. pylori eradication. A greater accumulation of cases is warranted to understand the etiology of AAHC due to second-line therapy for H. pylori eradication.
Table 1.
Reported cases of antibiotic-associated hemorrhagic colitis due to the second-line therapy for Helicobacter pylori eradication
| Age | Gender | Symptoms | Onset | First-line therapy | Second-line therapy | Fecal bacterial culture | DLST | References |
|---|---|---|---|---|---|---|---|---|
| 58 | F | Abdominal pain, bloody diarrhea | 8 days after starting eradication | CAM + AMPC + RPZ | MNZ 500 mg + AMPC 1500 mg + RPZ 20 mg | Pathogenic bacteria was not detected | Not described | #12 |
| 54 | M | Abdominal pain, bloody diarrhea | 7 days after starting eradication | CAM + AMPC + RPZ | MNZ 500 mg + AMPC 1500 mg + RPZ 20 mg | Pathogenic bacteria was not detected | Not described | #12 |
| 59 | F | Abdominal pain, bloody diarrhea | 9 days after starting eradication | CAM + AMPC + RPZ | MNZ 500 mg + AMPC 1500 mg + RPZ 20 mg | Pathogenic bacteria was not detected | Not described | #12 |
| 60 | F | Abdominal pain, bloody diarrhea | 9 days after starting eradication | CAM + AMPC + PPI | MNZ + AMPC + PPI | Klebsiella oxytoca | Not described | #13 |
| 50 | M | Abdominal pain, hematochezia | 6 days after starting eradication | CAM + AMPC + PPI | MNZ + AMPC + PPI | Not described | Not described | #13 |
| 65 | F | Abdominal pain, hematochezia | 8 days after starting eradication | CAM 800 mg + AMPC 1500 mg + LPZ 60 mg | MNZ 500 mg + AMPC 1500 mg + Vonoprazan 40 mg | Klebsiella oxytoca | Positive for both AMPC and MNZ | Present case |
CAM clarithromycin, AMPC amoxicillin, MNZ metronidazole, RPZ rabeprazole, LPZ lansoprazole, PPI proton pump inhibitor
Conclusions
In summary, we herein reported a rare case of AAHC caused by second-line therapy for H. pylori eradication. Due to the detection of K. oxytoca and positive reaction on the DLST for amoxicillin and metronidazole, the pathogenesis of the present case was considered to be associated with microbial substitution as well as a delayed-type allergy to amoxicillin and/or metronidazole. Clinicians must pay careful attention to the possibility of AAHC, even when administering second-line therapy for H. pylori eradication.
Authors’ contributions
KT and MF contributed equally to this study. KT and MF conceived the report, collected data, and wrote the first draft of the report. KT, AS and YN followed up the patient. SF, NU and SK performed endoscopy and evaluated the disease severity. TG and JS evaluated radiological images including computed tomography. KM and TO supervised the study. All authors contributed to the critical revision of the report for important intellectual content. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank Dr. Masami Ijiri, Dr. Tatsuya Utsumi, Dr. Takuya Iwama, and Dr. Hiroki Sato for significant assistances.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Ethics approval and consent to participate
Not applicable.
Funding
The authors declare that they have no funding.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- H. pylori
Helicobacter pylori
- AAHC
antibiotic-associated hemorrhagic colitis
- K. oxytoca
Klebsiella oxytoca
- DLST
drug-induced lymphocyte stimulation test
- CAM
clarithromycin
- AMPC
amoxicillin
- MNZ
metronidazole
- RPZ
rabeprazole
- LPZ
lansoprazole
- PPI
proton pump inhibitor
Contributor Information
Kazuyuki Tanaka, Email: kazuyuki@asahikawa-med.ac.jp.
Mikihiro Fujiya, Phone: +81-166-68-2462, Email: fjym@asahikawa-med.ac.jp.
Aki Sakatani, Email: sakatani@asahikawa-med.ac.jp.
Shugo Fujibayashi, Email: fujishu@asahikawa-med.ac.jp.
Yoshiki Nomura, Email: nomuzo@asahikawa-med.ac.jp.
Nobuhiro Ueno, Email: u-eno@asahikawa-med.ac.jp.
Shin Kashima, Email: shin1014@asahikawa-med.ac.jp.
Takuma Goto, Email: t-gotti@asahikawa-med.ac.jp.
Junpei Sasajima, Email: junsasaji@gmail.com.
Kentaro Moriichi, Email: morimori@asahikawa-med.ac.jp.
Toshikatsu Okumura, Email: okumurat@asahikawa-med.ac.jp.
References
- 1.Marshall BJ. Helicobacter pylori. Am J Gastroenterol. 1994;89:S116–S128. [PubMed] [Google Scholar]
- 2.Malfertheiner P, Leodolter A, Peitz U. Cure of Helicobacter pylori—associated ulcer disease through eradication. Baillieres Best Pract Res Clin Gastroenterol. 2000;14:119–132. doi: 10.1053/bega.1999.0063. [DOI] [PubMed] [Google Scholar]
- 3.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 4.Walt RP. Regression of MALT lymphoma and treatment for Helicobacter pylori. Lancet. 1996;348:1041–1042. doi: 10.1016/S0140-6736(05)64980-X. [DOI] [PubMed] [Google Scholar]
- 5.Choe YH, Kim SK, Son BK, Lee DH, Hong YC, Pai SH. Randomized placebo-controlled trial of Helicobacter pylori eradication for iron- deficiency anemia in preadolescent children and adolescents. Helicobacter. 1999;4:135–139. doi: 10.1046/j.1523-5378.1999.98066.x. [DOI] [PubMed] [Google Scholar]
- 6.Gisbert JP, Khorrami S, Carballo F, Calvet X, Gene E, Dominguez-Muñoz E. Helicobacter pylori eradication therapy vs. antisecretory non-eradication therapy (with or without long-term maintenance antisecretory therapy) for the prevention of recurrent bleeding from peptic ulcer. Cochrane Database Syst Rev. 2004;2:004062. doi: 10.1002/14651858.CD004062.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Fukase K, Kato M, Kikuchi S. Eradication of Helicobacter pylori after endoscopic resection of early gastric cancer reduced the incidence of metachronous gastric cancer. Lancet. 2008;372:392–397. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 8.Asaka M, Takahashi S, et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter. 2010;15:1–20. doi: 10.1111/j.1523-5378.2009.00738.x. [DOI] [PubMed] [Google Scholar]
- 9.Laheij RJ, Rossum LG, Jansen JB, Straatman H, Verbeek AL. Evaluation of treatment regimens to cure Helicobacter pylori infection—a meta-analysis. Aliment Pharmacol Ther. 1999;13:857–864. doi: 10.1046/j.1365-2036.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa S, Hayami Y, Sugawara K, Tanaka T, Yamamoto N, Hashimoto M, et al. A study on 19 cases of antibiotic-associated hemorrhagic colitis. Progr Dig Endosc. 1998;53:132–133. doi: 10.11641/pdensks.53.0_132. [DOI] [Google Scholar]
- 11.Kashihara W, Hashimoto A, Kou H, Kin Y, Nishitani H, Okui M, Fukui S, et al. Case of antibiotic-associated hemorrhagic colitis during eradication therapy for Helicobacter pylori. Helicobacter Res. 1998;2:580–581. [Google Scholar]
- 12.Ikegami R, Kamiya K, Iwashige H. Three cases of antibiotic-associated hemorrhagic colitis after second-line therapy for Helicobacter pylori. J Jpn Soc Coloproctol. 2015;68:419–424. doi: 10.3862/jcoloproctology.68.419. [DOI] [Google Scholar]
- 13.Kakinoki K. Two cases of antibiotic-associated hemorrhagic colitis after second-line therapy for Helicobacter pylori. J Jpn Assoc Infect Dis. 2015;89:247. [Google Scholar]
- 14.Zollner-Schwetz I, Hogenauer C, Joainig M, Weberhofer P, Gorkiewicz G, Valentin T, et al. Role of Klebsiella oxytoca in antibiotic-associated diarrhea. Clin Infect Dis. 2008;47:e74–e78. doi: 10.1086/592074. [DOI] [PubMed] [Google Scholar]
- 15.Higaki M, Chida T, Takano H, Nakaya R. Cytotoxic component(s) of Klebsiella oxytoca on HEp-2 cells. Microbiol Immunol. 1990;34:147–151. doi: 10.1111/j.1348-0421.1990.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 16.Minami J, Katayama S, Matsushita O, Sakamoto H, Okabe A. Enterotoxic activity of Klebsiella oxytoca cytotoxin in rabbit intestinal loops. Infect Immun. 1994;62:172–177. doi: 10.1128/iai.62.1.172-177.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


