Abstract
Numerous studies have revealed an association between particulate matter (PM) and emergency room (ER) visits, although few studies have investigated the association between health and PM components. The present study evaluated the associations of ER visits for cardiovascular and respiratory diseases with PM2.5 components, including organic carbon (OC), elemental carbon (EC), and ion species (SO42-, NO3-, and NH4+). Statistical analyses were performed using the time-series approach, and generalized linear models with natural spline functions were used to adjust for the non-linear relationship between the confounders and ER visits. Our single-pollutant models revealed that the greatest increase in cardiovascular ER visits was associated with NH4+ (relative risk: 1.05; 95% confidence interval: 1.01–1.09), which was followed by OC, SO42-, NO3-, and EC. The associations of cardiovascular ER visits with EC and OC varied according to age and sex, with elderly and female patients exhibiting stronger associations. Lagged SO42- was associated with respiratory ER visits. To the best of our knowledge, this is the first study to evaluate the associations between ER visits and PM components in South Korea. As PM components are related to traffic and industrial sources, and exhibited positive associations with ER visits, our results may help improve air pollution regulation and public health.
1. Introduction
Concerns regarding the health effects of air pollutants have been increasing, and several studies have revealed that adverse health effects are related to increasing concentrations of particulate matter (PM) [1–8]. Previous studies have also examined the association between mortality and PM concentrations, and typically showed that mortality increases with greater population-level exposure to air pollutants [9–16]. Furthermore, researchers have demonstrated that emergency room (ER) visits for cardiovascular and respiratory diseases may increase with elevated PM concentrations [17–25]. Moreover, South Korean studies have revealed an association between PM concentrations and ER visits for cardiovascular and respiratory diseases [26, 27].
Although there is increasing evidence regarding the effects of PM, there is uncertainty regarding the association of health with exposure to specific PM components. In addition, only a few studies have evaluated whether PM components influence the number of ER visits for specific diseases. Chen et al. [28] found that PM2.5 components were more closely related to ER visits for hemorrhagic stroke, compared to the mass of the PM2.5. Furthermore, Qiao et al. [29] have reported a positive association between ER visits and PM2.5 components, such as organic carbon (OC) and elemental carbon (EC) from fossil fuel combustion. However, despite the influence of PM components on ER visits and health, studies regarding this topic are limited by the difficulty of collecting and analyzing PM component data. Therefore, this study evaluated the associations of PM components with ER visits for cardiovascular and respiratory diseases in Seoul, South Korea (2010–2013). To the best of our knowledge, this is the first study to evaluate this association in South Korea using time-series analysis.
2. Materials and methods
2.1 Data collection
The present study was approved by the Seoul National University Institutional Review Board (IRB No: 1501/001-013). This study evaluated daily data (April 17, 2010 to May 10, 2013) regarding PM and ER visits for cardiovascular diseases (ICD-10: I00-I99, G45, G46, M30, M31, R58) and respiratory diseases (ICD-10: J00-J99, I46, I47, I48, I49) in Seoul, South Korea. The ER visit data were obtained from the National Emergency Department Information System (NEDIS), and the hourly PM10 and PM2.5 (PM with diameters of ≤10 μm and ≤2.5 μm, respectively) concentration data were obtained from the National Institute of Environmental Research. The Korea Meteorology Administration provided daily meteorological data, including temperature (°C) and relative humidity (%).
NEDIS is a computerized system that is designed to transmit and analyze emergency care information from 332 emergency medical institutions in 16 metropolitan cities in Korea (based on 2013 values). Fifty-four of the emergency medical institutions were in Seoul. To extract emergency medical center data, we analyzed data from 30 hospitals in Seoul. In addition, NEDIS provides data at the individual level, such as age, sex, primary diagnostic code, ER visit date(s), reasons for the ER visit, and hospital district. Anonymized ER visit data were classified according to the discharge diagnosis.
For our analyses, we considered the average daily concentrations of PM10 and PM2.5. The PM components were defined as carbon species (EC and OC) and ion species (NO3-, NH4+, and SO42-), and related data were obtained during 24-h periods using ambient air samples that were collected on the rooftop of the former School of Public Health building (37.5°N and 127.00°E), which is located in the center of Seoul, between April 2010 and March 2013. The distance between the farthest hospital and the measurement site was 14.09 km. The concentrations of carbon species and ion species were analyzed using thermal and optical transmittance (Sunset Laboratories, Tigard, OR) and ionic chromatography (Dionex DX-120; Thermo Fisher Scientific, Inc., Cambridge, UK), respectively. More detailed information regarding the measurement procedures have been reported by Heo et al. [30] and Kim et al. [31].
2.2 Statistical analysis
To evaluate the relationships between ER visits and concentrations of PM components, we used a generalized linear model based on the assumption of a quasi-Poisson distribution. The model included various controlling factors, such as influenza status, day of the week (DOW), holiday, and meteorological variables (temperature and relative humidity):
| (1) |
In this model, μt is the expected number of ER visits on day t, Componentt is the concentrations of the PMs and their components on day t (μg/m3), and α is the intercept of the model. DOWt is the day of the week, which was evaluated as a categorical variable on day t (Monday, Tuesday, Wednesday, Thursday, Friday, Saturday, and Sunday). Influenzat was evaluated as a categorical variable on day t (presence: 1, absence: 0), Holidayt was evaluated as a categorical variable on day t (holiday: 1, non-holiday: 0), and ns(Temp1−t,df = 6) was the natural cubic spline function of time with 6 degrees of freedom (df) per year. To control the delayed association between temperature and ER visits, we applied a 5-day moving average for daily temperature. ns(RHt,df = 3) was the natural cubic spline function of humidity with 3 df per year. We used a df of 7 per year for the natural cubic spline function to control for long-term trend and seasonality. Akaike’s information criterion for over-dispersion was applied to examine the statistical model [32]. The choice of the model, the spine functions, and the adjustment for time and seasonality were introduced by Peng et al. [21], Heo et al. [33] and Qiu et al. [34]. PM10, PM2.5, and their components were evaluated by using single-pollutant models. In addition, we created multi-pollutant models that contained each pollutant while simultaneously controlling for the other components. A sensitivity analysis was performed to determine the stability of the fitting model. All statistical analyses were performed using R software.
3. Results
Table 1 describes the basic characteristics of the ER visits for cardiovascular and respiratory diseases between April 17, 2010 and May 10, 2013. We identified 149,452 ER visits for cardiovascular diseases (133.44 visits/day) and 453,868 ER visits for respiratory diseases (405.24 visits/day). Compared to female patients, male patients had a higher average value for cardiovascular and respiratory diseases. Elderly patients (≥65 years old) had lower mean values for cardiovascular and respiratory diseases, compared to younger patients (<65 years old). However, the ER visits for both cardiovascular and respiratory diseases distinctly increased over time among elderly patients (≥65 years old). Compared to 2010, the cardiovascular ER visits among elderly patients increased by 15.51% in 2013. The increases were 9.73% for female patients, 8.72% for male patients, and 3.23% for younger patients. The respiratory ER visits among elderly patients increased by 66.85% in 2013, compared to 2010. The increases were –7.62% for female patients, –12.75% for male patients, and –16.04% for younger patients. Figs A and B in S1 File show the time series distributions of cardiovascular and respiratory ER visits according to sex and age.
Table 1. Characteristics of emergency room visits for cardiovascular and respiratory diseases between April 17, 2010 and May 10, 2013.
| Variables | Cardiovascular disease (n = 149,453) |
Respiratory disease (n = 453,868) |
||
|---|---|---|---|---|
| Daily mean ± SD | Range (daily max–min) |
Daily mean ± SD | Range (daily max–min) |
|
| All cases | 133.44 ± 14.60 | 105 | 405.24 ± 155.86 | 1,725 |
| Age of <65 years | 68.04 ± 9.17 | 62 | 368.53 ± 149.29 | 1,608 |
| Age of ≥ 65 years | 65.40 ± 9.66 | 66 | 36.71 ± 13.52 | 121 |
| Male | 74.50 ± 10.11 | 64 | 220.18 ± 79.44 | 816 |
| Female | 58.94 ± 8.66 | 60 | 185.06 ± 78.38 | 917 |
|
Warm season (March–August) |
68.38 ± 15.25 | 105 | 205.58 ± 122.71 | 650 |
|
Cool season (September–February) |
65.05 ± 13.86 | 89 | 199.65 ± 184.53 | 1,725 |
SD: standard deviation.
Table 2 summarizes the air pollution and meteorological data from the study period. The daily average concentrations of PM10 and PM2.5 were 43.61 μg/m3 and 22.01 μg/m3, respectively. Among the PM components, NO3- had the highest daily concentration value (8.12 μg/m3), which was followed by OC (7.24 μg/m3) and SO42- (6.20 μg/m3). The interquartile ranges (IQR) for PM10 and PM2.5 were 27.05 μg/m3 and 13.90 μg/m3, respectively. Unlike the average daily concentrations, the highest IQR value was observed for NO3- (5.58 μg/m3), which was followed by NH4+ (5.10 μg/m3) and OC (3.90 μg/m3). Histograms of the PM components’ concentrations are shown in Fig C in S1 File.
Table 2. Air pollution and meteorological data from April 17, 2010 to May 10, 2013.
| Daily mean ± SD | Interquartile range | ||
|---|---|---|---|
| Air pollution data | PM10 (μg/m3) | 43.61 ± 18.94 | 27.05 |
| PM2.5 (μg/m3) | 22.01 ± 9.84 | 13.90 | |
| Organic carbon (μg/m3) | 7.24 ± 2.98 | 3.90 | |
| Elemental carbon (μg/m3) | 1.46 ± 0.62 | 0.90 | |
| SO42- (μg/m3) | 6.20 ± 3.41 | 3.85 | |
| NO3- (μg/m3) | 8.12 ± 5.11 | 5.58 | |
| NH4+ (μg/m3) | 5.84 ± 3.86 | 5.10 | |
| Meteorological data | Temperature (°C) | 12.17 ± 11.10 | 19.5 |
| Relative humidity (%) | 59.14 ± 15.38 | 22.48 |
SD: standard deviation.
Table 3 shows the Pearson correlation coefficients for the concentrations of PM components. The strongest correlation with PM2.5 was observed for SO42- (r = 0.73). Among the PM components, OC and EC were the strongest correlations (r = 0.79). The weakest correlation was between EC and NH4+ (r = 0.1).
Table 3. Pearson correlation coefficients among concentrations of PM components.
| PM10 | PM2.5 | Organic carbon | Elemental carbon | SO42- | NO3- | NH4+ | |
|---|---|---|---|---|---|---|---|
| PM10 | 1.00 | 0.88 | 0.49 | 0.42 | 0.60 | 0.64 | 0.37 |
| PM2.5 | 1.00 | 0.50 | 0.41 | 0.73 | 0.69 | 0.38 | |
| Organic carbon | 1.00 | 0.79 | 0.26 | 0.40 | 0.12 | ||
| Elemental carbon | 1.00 | 0.28 | 0.31 | 0.10 | |||
| SO42- | 1.00 | 0.63 | 0.58 | ||||
| NO3- | 1.00 | 0.56 | |||||
| NH4+ | 1.00 |
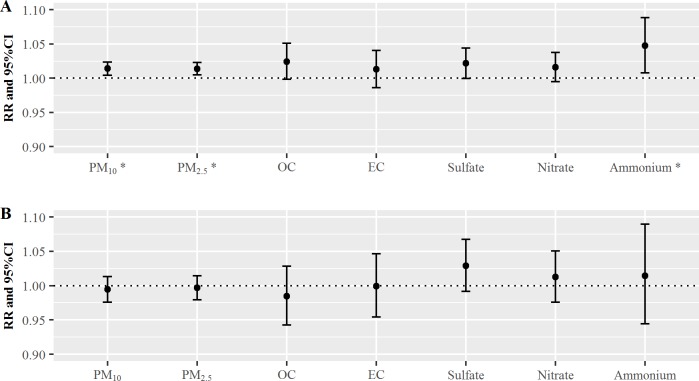
Fig 1 shows the relative risks (RR) of cardiovascular (Fig 1A) and respiratory (Fig 1B) diseases on lag 0 day when exposed to the PMs and their components. The PM10, PM2.5, and component variables exhibited positive associations with ER visits for cardiovascular disease. The estimated RRs on lag 0 day for PM10 and PM2.5 were both 1.01 (95% confidence interval [CI]: 1.00–1.02). The estimated RRs on lag 0 day for OC and EC were 1.02 (95% CI: 1.00–1.05) and 1.01 (95% CI: 0.99–1.04), respectively. The estimated RR on lag 0 day for NH4+ was 1.05 (95% CI: 1.01–1.09). As shown in Fig 1B, the estimated RRs of respiratory ER visits due to SO42- and NO3- in PM2.5 were 1.03 (95% CI: 0.99–1.07) and 1.01 (95% CI: 0.98–1.05), respectively. The largest RR estimate was observed for SO42- (Fig 1B), which was followed by NH4+, NO3-, and EC. Interestingly, the association of respiratory ER visits with SO42- was stronger than that for cardiovascular disease on lag 0 day. The details regarding the RR values in Fig 1 are summarized in Table A in S1 File. In addition, we analyzed multi-pollutant models for each PM2.5 components. For cardiovascular disease, the highest RR on lag 0 day was observed for SO42- (RR: 1.03, 95% CI: 0.98–1.07). For respiratory disease, the highest RR on lag 0 day was observed for EC (RR: 1.09, 95% CI: 0.97–1.23). The estimated RRs of cardiovascular disease in the multi-pollutant models for OC, NO3-, and NH4+ were lower than the values from the single-pollutant models. However, only the estimated RR of respiratory disease in the multi-pollutant model for NO3- was lower than the value from the single-pollutant model. NH4+ was not significantly associated with cardiovascular ER visits in the multi-pollutant models, compared to the single-pollutant models. The details regarding the RR values in the multi-pollutant models are summarized in Table B in S1 File.
Fig 1.
The relative risks (RRs) of cardiovascular (A) and respiratory (B) diseases on lag 0 day per one-interquartile range increase in PM2.5 and its components. * PM10, PM2.5, and ammonium were associated with increased risks of cardiovascular disease. CI: confidence interval, OC: organic carbon, EC: elemental carbon. Lag 0 day indicates that the RRs were measured on the day of exposure.
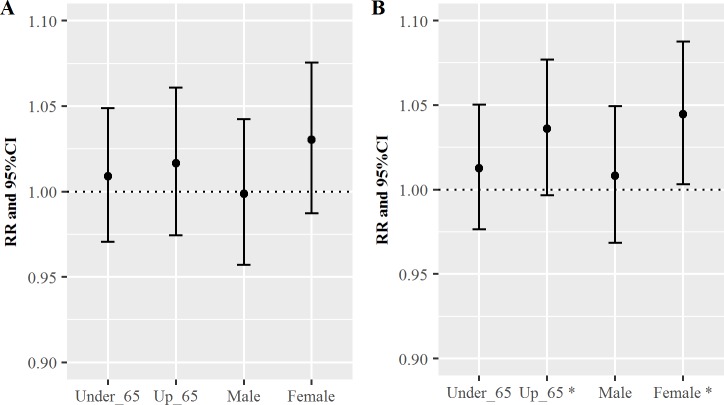
Fig 2 shows the RR values for cardiovascular disease per one-IQR increase in OC (Fig 2A) and EC (Fig 2B) for each age group and sex on lag 0 day. Both OC and EC exhibited positive associations with cardiovascular ER visits. The estimated RRs on lag 0 day for OC in the young and elderly groups were 1.01 (95% CI: 0.98–1.05) and 1.04 (95% CI: 1.00–1.08), respectively. The estimated RRs on lag 0 day for OC in the male and female groups were 1.01 (95% CI: 0.97–1.05) and 1.04 (95% CI: 1.00–1.09), respectively (Fig 2A). Similarly, the estimated RRs on lag 0 day for EC in the young and elderly groups were 1.00 (95% CI: 0.97–1.04) and 1.02 (95% CI: 0.98–1.06), respectively. The estimated RRs on lag 0 day for EC in the male and female groups were 1.00 (95% CI: 0.96–1.04) and 1.03 (95% CI: 0.99–1.08), respectively (Fig 2B). The elderly and female groups exhibited greater RR estimates for EC, compared to OC. The RRs for EC were not significant, although the RRs for OC were significant. In addition, we examined the statistical tests for the comparison, and the choice of the test was introduced by Clogg et al. [35] and Paternoster et al. [36]. The differences in the coefficients for elderly and younger patients, and for male and female patients, were not significant for both OC and EC. The information regarding Fig 2 is summarized in Table C in S1 File, and the information regarding the difference testing is summarized in Table D in S1 File.
Fig 2.
The relative risks (RRs) of cardiovascular disease per one-interquartile range increase in elemental carbon (EC) (A) or organic carbon (OC) (B) for each age group and sex on lag 0 day. CI: confidence interval.
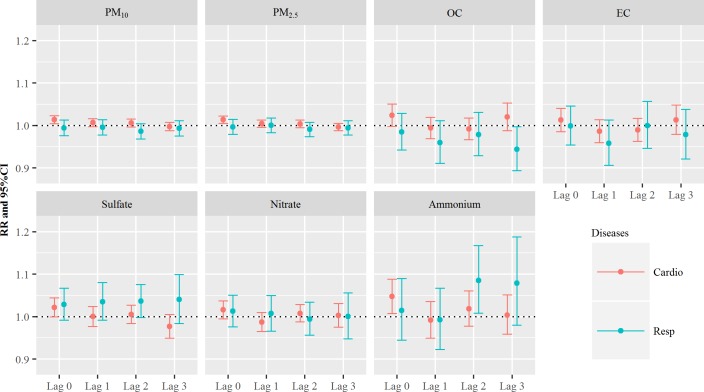
Fig 3 shows the RR values for cardiovascular and respiratory diseases due to PM and their components, while considering the lag. Based on the results in Fig 1B, exposure to SO42- was positively associated with respiratory ER visits. The estimated RRs per one-IQR increase in SO42- were 1.03 on lag 0 day (95% CI: 0.99–1.07) and 1.04 on lag 1 day (95% CI: 0.99–1.08). The RRs in SO42- slightly increased up to lag 3. We also stratified the analyses according to age and sex, and the results revealed that SO42- had the greatest effect on health outcomes in the elderly group on lag 1 day (RR: 1.05, 95% CI: 1.00–1.10). The RRs per one-IQR increase in SO42- for respiratory ER visits for age groups, sex, and lag days are summarized in Table E in S1 File. All information regarding the stratified analyses are described in Fig D in S1 File.
Fig 3. The relative risks (RRs) of cardiovascular and respiratory diseases per one-interquartile range increase in particulate matter and the components on lag 0–3 days.
We evaluated the statistical models by changing the df for time and temperature, and observed a maximum change in the RR of 0.62% (Table F in S1 File).
4. Discussion
The present study is the first to investigate the relationship between cardiovascular/respiratory diseases and PM components in Seoul, South Korea. Our findings indicate that PMs and their components were more positively associated with ER visits for cardiovascular disease, compared to those for respiratory disease, and these findings were consistent with findings from previous studies [21, 28, 37]. Peng et al. [21] reported that nitrate, EC, OC, and ammonium could increase the risk of ER visits for cardiovascular disease, with ammonium exhibiting the strongest association. Peng et al. [21] estimated that one-IQR increases in EC, OC, and ammonium were associated with 0.72% (95% CI: 0.43–1.01%), 0.66% (95% CI: 0.29–1.02%), and 0.68% (95% CI: 0.31–1.06%) increases in cardiovascular hospital admissions. Another study investigated the associations of ER visits for specific cardiovascular diseases with PM components, and revealed that exposure to EC and OC may induce ER visits [28]. For example, EC exposure increased the risk of ER visits for ischemic and hemorrhagic stroke (RR: 0.99 per 1.0 μg/m3, 95% CI: 0.95–1.03 and RR: 1.07 per 1.0 μg/m3, 95% CI: 1.00–1.14; respectively). Similar results were observed for OC exposure (RR: 1.03 per 3.0 μg/m3, 95% CI: 0.98–1.09 and RR: 1.10 per 3.0 μg/m3, 95% CI: 0.99–1.22, respectively). Kim et al. [35] also reported that sulfate, EC, and OC were associated with increasing risks of cardiovascular ER visits. Those authors adjusted the lag models for 14 days and estimated the cumulative RRs for cardiovascular hospital admissions on lag 0–1 day, and reported that one-IQR increases in sulfate, EC, and OC provided RRs of 1.004 (95% CI: 0.995–1.013), 1.018 (95% CI: 1.006–1.030), and 1.015 (95% CI: 1.002–1.029), respectively. However, a recent study by Wang and Lin [38] revealed that the lag effects of sulfate varied according to the cause-specific ER visits. For respiratory disease, the estimated RRs decreased until lag 2 day and then increased. Although we observed a different trend (slightly increasing RRs until lag 3 day), both their study and our study revealed positive associations after considering lag effects.
The present study also revealed that EC and OC were associated with more frequent ER visits for both cardiovascular and respiratory diseases among elderly and female patients. Previous studies regarding the biological mechanisms for these relationships support our finding [39–43], and inflammation that is induced by EC/OC may be responsible for the cardiovascular ER visits. In this context, EC exposure may decrease the myocardial oxygen supply and increase the risk of cardiac ischemia [42, 43], which may induce cardiac disease through potentially interrelated mechanisms, including systemic inflammation and oxidative stress [42, 43]. Moreover, EC exposure is associated with ST-segment depression, which possibly represents myocardial ischemia or inflammation [41]. Delfino et al. [39] have reported that OC may be associated with higher concentrations of nitric oxide in exhaled breath, which might disrupt cardiovascular function. Seaton et al. [44, 45] have also proposed that fine particles (e.g., OC) could provoke pulmonary inflammation by inducing oxidative stress, which could lead to increased blood coagulability and systemic inflammation [44, 45].
The present study has several limitations. First, the sampling of PM2.5 and the PM components was performed over a period of several days, because of the sampling schedule at the Atmospheric Environment & Climate Change laboratory in Seoul National University. This prevented us from using a distributed lag model, because the data were not measured every day. However, we applied the moving averages concept to consider the short-term lag effects, which allows us to investigate the short-term associations between PM components and ER visits. Another limitation is that we used stationary air pollution data, rather than the individual’s exposures to PM and PM components, as it is difficult to examine the influence of air pollution exposure at the individual level. This approach may have introduced random errors in the PM component data and led to a reduction in the related RR values [46]. In addition, PM components influence people when they are near ground level, while we collected PM component data on a rooftop of a large building, which may have created discordance between the air pollutant data and the exposure location. Furthermore, we could not consider repeated admissions for the same person because our ER visit data were anonymized, and our results may have been overestimated because we could not address the correlated data [47].
Our findings regarding the influences of EC, OC, and sulfate suggest that traffic- and industrial-related sources play important roles in driving ER visits for cardiovascular and respiratory diseases. In this context, sulfate is emitted from sulfur in diesel fuel [48]. Similarly, the major sources of EC and OC are related to urban development, such as motorized vehicles, coal burning, shipping emissions, and industrial sources [49]. As Seoul is a representative traffic-congested region in South Korea, studies regarding the health effects of traffic-related air pollution have recently increased in importance. Thus, our data may help promote further research regarding the effects of PM components, and may facilitate the control and regulation of air pollution.
5. Conclusions
Our analyses revealed that PM2.5 mass and PM2.5 components were associated with cardiovascular ER visits, with the strongest association observed for NH4+. When we performed stratified analyses according to age and sex, OC was significantly associated with cardiovascular ER visits among elderly and female patients. Similarly, EC was positively associated with cardiovascular ER visits among elderly and female patients. Lagged SO42- was associated with respiratory ER visits. Based on our results, it appears that elderly and female patients are more vulnerable to both OC and EC. Furthermore, as SO42-, EC, and OC are generally categorized as traffic-related air pollutants, our findings may facilitate health policy development and promote the management of traffic-related air pollutants.
Supporting information
(ZIP)
Acknowledgments
This research was supported by a grant from the Natural Hazard Mitigation Research Group [MPSS-NH-2015-81], which is funded by the Korean Ministry of Public Safety and Security. This work was supported by Basic Science Research Program (2014R1A2A2A04007801) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT (Information and Communication Technologies) & Future Planning. The authors declare that they have no actual or potential competing financial interests.
Data Availability
All relevant data are included within the paper and its Supporting Information files.
Funding Statement
This research was supported by a grant from the Natural Hazard Mitigation Research Group (MPSS-NH-2015-81), which is funded by the Korean Ministry of Public Safety and Security (https://www.mpss.go.kr/), and the Basic Science Research Program (2014R1A2A2A04007801) through the National Research Foundation of Korea (NRF; www.nrf.re.kr/), funded by the Ministry of Science, ICT (Information and Communication Technologies) & Future Planning.
References
- 1.Bascom R, Bromberg PA, Costa DL, Devlin R, Dockery DW, Frampton MW, et al. Health effects of outdoor air pollution. Am J Respir Crit Care Med 1996;153:477–498. doi: 10.1164/ajrccm.153.2.8564086 [DOI] [PubMed] [Google Scholar]
- 2.Bernstein JA, Alexis N, Barnes C, Bernstein IL, Nel A, Peden D, et al. Health effects of air pollution. J Allergy Clin Immunol 2004;114:1116–1123. doi: 10.1016/j.jaci.2004.08.030 [DOI] [PubMed] [Google Scholar]
- 3.Brunekreef B, Holgate ST. Air pollution and health. Lancet 2002;360:1233–1242. doi: 10.1016/S0140-6736(02)11274-8 [DOI] [PubMed] [Google Scholar]
- 4.Dab W, Medina S, Quenel P, Le Moullec Y, Le Tertre A, Thelot B, et al. Short term respiratory health effects of ambient air pollution: Results of the Aphea Project in Paris. J Epidemiol Community Health 1996;50:s42–s46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kampa M, Castanas E. Human health effects of air pollution. Environ Pollut 2008;151:362–367. doi: 10.1016/j.envpol.2007.06.012 [DOI] [PubMed] [Google Scholar]
- 6.Katsouyanni K, Touloumi G, Spix C, Schwartz J, Balducci F, Medina S, et al. Short-term effects of ambient sulphur dioxide and particulate matter on mortality in 12 European cities: results from time series data from the APHEA project. Air Pollution and Health: a European Approach. BMJ 1997;314:1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neidell MJ. Air pollution, health, and socio-economic status: The effect of outdoor air quality on childhood asthma. J Health Econ 2004;23:1209–1236. doi: 10.1016/j.jhealeco.2004.05.002 [DOI] [PubMed] [Google Scholar]
- 8.Romieu I, Meneses F, Ruiz S, Sienra JJ, Huerta J, White MC, et al. Effects of air pollution on the respiratory health of asthmatic children living in Mexico City. Am J Respir Crit Care Med 1996;154:300–307. doi: 10.1164/ajrccm.154.2.8756798 [DOI] [PubMed] [Google Scholar]
- 9.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8 [DOI] [PubMed] [Google Scholar]
- 10.Hatzakis A, Katsouyanni K, Kalandidi A, Day N, Trichopoulos D. Short-term effects of air pollution on mortality in Athens. Int J Epidemiol 1986;15:73–81. [DOI] [PubMed] [Google Scholar]
- 11.Hoek G, Brunekreef B, Verhoeff A, Wijnen JV, Fischer P. Daily mortality and air pollution in the Netherlands. Journal of the Air & Waste Management Association 2000;50:1380–1389. [DOI] [PubMed] [Google Scholar]
- 12.Lin H, Tao J, Du Y, Liu T, Qian Z, Tian L, et al. Differentiating the effects of characteristics of PM pollution on mortality from ischemic and hemorrhagic strokes. Int J Hyg Environ Health 2016;219:204–211. doi: 10.1016/j.ijheh.2015.11.002 [DOI] [PubMed] [Google Scholar]
- 13.Schwartz J. Air pollution and daily mortality: A review and meta analysis. Environ Res 1994;64:36–52. doi: 10.1006/enrs.1994.1005 [DOI] [PubMed] [Google Scholar]
- 14.Spix C, Heinrich J, Dockery D, Schwartz J, Völksch G, Schwinkowski K, et al. Air pollution and daily mortality in Erfurt, East Germany, 1980–1989. Environ Health Perspect 1993;101:518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Touloumi G, Pocock S, Katsouyanni K, Trichopoulos D. Short-term effects of air pollution on daily mortality in Athens: A time-series analysis. Int J Epidemiol 1994;23:957–967. [DOI] [PubMed] [Google Scholar]
- 16.Wong CM, Vichit-Vadakan N, Kan H, Qian Z. Public Health and Air Pollution in Asia (PAPA): a multicity study of short-term effects of air pollution on mortality. Environ Health Perspect 2008;116:1195 doi: 10.1289/ehp.11257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Y, Jia Y, Pan X, Liu L, Wichmann HE. The association between fine particulate air pollution and hospital emergency room visits for cardiovascular diseases in Beijing, China. Sci Total Environ 2009;407:4826–4830. doi: 10.1016/j.scitotenv.2009.05.022 [DOI] [PubMed] [Google Scholar]
- 18.Hwang JS, Hu TH, Chan CC. Air pollution mix and emergency room visits for respiratory and cardiac diseases in Taipei. Journal of Data Science 2004;2:311–327. [Google Scholar]
- 19.Pönkä A, Virtanen M. Low-level air pollution and hospital admissions for cardiac and cerebrovascular diseases in helsinki. Am J Public Health 1996;86:1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peel JL, Metzger KB, Klein M, Flanders WD, Mulholland JA, Tolbert PE. Ambient air pollution and cardiovascular emergency department visits in potentially sensitive groups. Am J Epidemiol 2007;165:625–633. doi: 10.1093/aje/kwk051 [DOI] [PubMed] [Google Scholar]
- 21.Peng RD, Bell ML, Geyh AS, McDermott A, Zeger SL, Samet JM, et al. Emergency admissions for cardiovascular and respiratory diseases and the chemical composition of fine particle air pollution. Environ Health Perspect 2009;117:957 doi: 10.1289/ehp.0800185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spix C, Anderson HR, Schwartz J, Vigotti MA, Letertre A, Vonk JM, et al. Short-term effects of air pollution on hospital admissions of respiratory diseases in Europe: a quantitative summary of APHEA study results. Air Pollution and Health: a European Approach. Arch Environ Health 1998;53:54–64. doi: 10.1080/00039899809605689 [DOI] [PubMed] [Google Scholar]
- 23.Stieb DM, Beveridge RC, Brook JR, Smith-Doiron M, Burnett RT, Dales RE, et al. Air pollution, aeroallergens and cardiorespiratory emergency department visits in Saint John, Canada. J Expo Sci Environ Epidemiol 2000;10:461–477. [DOI] [PubMed] [Google Scholar]
- 24.Stieb DM, Szyszkowicz M, Rowe BH, Leech JA. Air pollution and emergency department visits for cardiac and respiratory conditions: A multi-city time-series analysis. Environ Health 2009;8:1 doi: 10.1186/1476-069X-8-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tolbert PE, Klein M, Peel JL, Sarnat SE, Sarnat JA. Multipollutant modeling issues in a study of ambient air quality and emergency department visits in Atlanta. J Expo Sci Environ Epidemiol 2007;17:S29–S35. doi: 10.1038/sj.jes.7500625 [DOI] [PubMed] [Google Scholar]
- 26.Son JY, Lee JT, Park YH, Bell ML. Short-term effects of air pollution on hospital admissions in Korea. Epidemiology 2013;24:545–554. doi: 10.1097/EDE.0b013e3182953244 [DOI] [PubMed] [Google Scholar]
- 27.Yi O, Hong YC, Kim H. Seasonal effect of pm 10 concentrations on mortality and morbidity in seoul, korea: A temperature-matched case-crossover analysis. Environ Res 2010;110:89–95. doi: 10.1016/j.envres.2009.09.009 [DOI] [PubMed] [Google Scholar]
- 28.Chen SY, Lin YL, Chang WT, Lee CT, Chan CC. Increasing emergency room visits for stroke by elevated levels of fine particulate constituents. Science of The Total Environment 2014;473:446–450. doi: 10.1016/j.scitotenv.2013.12.035 [DOI] [PubMed] [Google Scholar]
- 29.Qiao L, Cai J, Wang H, Wang W, Zhou M, Lou S, et al. PM2.5 constituents and hospital emergency-room visits in Shanghai, China. Environ Sci Technol 2014;48:10406–10414. doi: 10.1021/es501305k [DOI] [PubMed] [Google Scholar]
- 30.Heo J-B, Hopke P, Yi SM. Source apportionment of PM 2.5 in Seoul, Korea. Atmos Chem Phys 2009;9:4957–4971. [Google Scholar]
- 31.Kim HS, Huh JB, Hopke PK, Holsen TM, Yi SM. Characteristics of the major chemical constituents of PM 2.5 and smog events in Seoul, Korea in 2003 and 2004. Atmost Environ 2007;41:6762–6770. [Google Scholar]
- 32.Ver Hoef JM, Boveng PL. Quasi-poisson vs Negative binomial regression: How should we model overdispersed count data? Ecology 2007;88:2766–2772. [DOI] [PubMed] [Google Scholar]
- 33.Heo J, Schauer JJ, Yi O, Paek D, Kim H, Yi SM. Fine particle air pollution and mortality: Importance of specific sources and chemical species. Epidemiology 2014;25:379–388. doi: 10.1097/EDE.0000000000000044 [DOI] [PubMed] [Google Scholar]
- 34.Qiu H, Yu IT, Wang X, Tian L, Tse LA, Wong TW. Differential effects of fine and coarse particles on daily emergency cardiovascular hospitalizations in hong kong. Atmos Environ 2013;64:296–302. [Google Scholar]
- 35.Clogg CC, Petkova E, Haritou A. Statistical methods for comparing regression coefficients between models. Am J Sociol 1995;100:1261–1293. [Google Scholar]
- 36.Paternoster R, Brame R, Mazerolle P, Piquero A. Using the correct statistical test for equality of regression coefficients. Criminology 1998;36:859–866. [Google Scholar]
- 37.Kim SY, Peel JL, Hannigan MP, Dutton SJ, Sheppard L, Clark ML, et al. The temporal lag structure of short-term associations of fine particulate matter chemical constituents and cardiovascular and respiratory hospitalizations. Environ Health Perspect 2012;120:1094–1099. doi: 10.1289/ehp.1104721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang YC, Lin YK. Mortality and emergency room visits associated with ambient particulate matter constituents in metropolitan Taipei. Sci Total Environ 2016;569:1427–1434. doi: 10.1016/j.scitotenv.2016.06.230 [DOI] [PubMed] [Google Scholar]
- 39.Delfino RJ, Staimer N, Gillen D, Tjoa T, Sioutas C, Fung K, et al. Personal and ambient air pollution is associated with increased exhaled nitric oxide in children with asthma. Environ Health Perspect 2006;114:1736–1743. doi: 10.1289/ehp.9141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J, Zhu T, Kipen H, Wang G, Huang W, Rich D, et al. Cardiorespiratory biomarker responses in healthy young adults to drastic air quality changes surrounding the 2008 Beijing Olympics. Res Rep Health Eff Inst 2013;(174):5–174. [PMC free article] [PubMed] [Google Scholar]
- 41.Gold DR, Litonjua AA, Zanobetti A, Coull BA, Schwartz J, MacCallum G, et al. Air pollution and ST-segment depression in elderly subjects. Environ Health Perspect 2005;113:883–887. doi: 10.1289/ehp.7737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gold DR, Litonjua A, Schwartz J, Lovett E, Larson A, Nearing B, et al. Ambient pollution and heart rate variability. Circulation 2000;101:1267–1273. [DOI] [PubMed] [Google Scholar]
- 43.Liao D, Creason J, Shy C, Williams R, Watts R, Zweidinger R. Daily variation of particulate air pollution and poor cardiac autonomic control in the elderly. Environ Health Perspect 1999;107:521–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seaton A, MacNee W, Donaldson K, Godden D. Particulate air pollution and acute health effects. Lancet 1995;345:176–178. [DOI] [PubMed] [Google Scholar]
- 45.Seaton A, Soutar A, Crawford V, Elton R, McNerlan S, Cherrie J, et al. Particulate air pollution and the blood. Thorax 1999;54:1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JY, Kim H. Projection of future temperature-related mortality due to climate and demographic changes. Environ Int 2016;94:489–494. doi: 10.1016/j.envint.2016.06.007 [DOI] [PubMed] [Google Scholar]
- 47.Sainani K. The importance of accounting for correlated observations. PM R 2010;2:858–861. doi: 10.1016/j.pmrj.2010.07.482 [DOI] [PubMed] [Google Scholar]
- 48.Truex TJ, Pierson WR, McKee DE. Sulfate in diesel exhaust. Environ Sci Technol 1980;14:1118–1121. [Google Scholar]
- 49.Yang F, He K, Ye B, Chen X, Cha L, Cadle S, et al. One-year record of organic and elemental carbon in fine particles in downtown Beijing and Shanghai. Atmospheric Chemistry and Physics 2005;5:1449–1457. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(ZIP)
Data Availability Statement
All relevant data are included within the paper and its Supporting Information files.