HSP90 is constantly externalized on the surface of human macrophages; selective inhibition of this HSP90 pool suppresses the macrophages' response to PAMPs.
Keywords: chaperone, geldanamycin, PAMPs, phagocytosis, signalling
Abstract
Heat shock proteins (HSPs) are typical intracellular chaperones which also appear on the cell surface and in extracellular milieu. HSP90, which chaperones many proteins involved in signal transduction, is also a regular component of LPS-signaling complexes on Mϕ. As LPS is a prototypical PAMP, we speculated that HSP90 is engaged in pattern recognition by professional phagocytes. In this report, we provide the first evidence, to our knowledge, of the geldanamycin (Ge)-inhibitable HSP90 on the surface of live monocyte-derived Mϕs (hMDMs). Using cytometry and specific Abs, we showed both HSP90 isoforms (α and β) on the surface of human monocytes and hMDMs. The cell-surface HSP90 pool was also labeled with cell-impermeable Ge derivatives. Confocal analysis of hMDMs revealed that HSP90-inhibitor complexes were rapidly clustered on the cell surface and recycled through the endosomal compartment. This finding suggests that the N-terminal (ATPase) domain of HSP90 is exposed and accessible from the extracellular space. To study the role of cell-surface HSP90 in pattern recognition, we used pathogen (PAMPs)- or apoptotic cell-associated molecular patterns (ACAMPs). We showed that blocking the cell-surface HSP90 pool leads to a dramatic decrease in TNF production by monocytes and hMDMs exposed to soluble (TLRs-specific ligands) and particulate [bacteria Staphylococcus aureus (SA) and Porphyromonas gingivalis (PG)] PAMPs. Surprisingly, in hMDMs the functional cell-surface HSP90 was not necessary for the engulfment of either apoptotic neutrophils or bacteria. The presented data suggest that the cell-surface HSP90 is a “signaling complex chaperone,” with activity that is essential for cytokine response but not for target engulfment by Mϕ.
Introduction
Monocytes and Mϕ, along with dendritic cells create the mononuclear phagocytic system that is necessary to continually sense the extracellular environment and initiate an adequate systemic reaction [1]. Apparently, in the process of evolution, phagocytes were gradually equipped with complex mechanisms of pattern recognition, allowing them not only to identify different pathogens (PAMPs), but also to recognize the molecular patterns of self-derived, apoptotic (ACAMPs) or dying, damaged or stressed cells (DAMPs) [2]. Over the past decade, it has become clear, that studying single cognate receptor–ligand interactions belies the complexity of combinatorial receptor recognition by phagocytic cells [3]. The term “engulfment synapse” has been proposed to depict the synapse-like structures that detect complex molecular patterns on the surface of particulate antigens and integrate them into a nonredundant image [4, 5]. This type of recognition requires large membrane platforms composed of multiple PRRs and other molecules arrayed within lipid rafts. A precedent for cooperative recognition of a ligand by a synapse-like signaling complex has been established for LPS [6]. Numerous molecules that cooperate in LPS signaling including CD11b/CD18, CD14, CD16, and CD36 exist in proximity in the cell membrane, as indicated by FRET between molecules upon ligation with LPS [7]. Using similar techniques, it has also been shown that HSP70, HSP90, CXCR4, GDF5, and TLR4 localize to the site of CD14-LPS ligation within the lipid rafts [8], further emphasizing the potential complexity of the phagocytic interface. LPS-stimulated monocyte HSP90 was unexpectedly also found in proximity to the lipid raft marker—GM1 and TLR4 in the plasma membrane by FRET analysis [9]—but the relation between HSP90 and TLR4 activity has not been elucidated so far.
The surface expression of HSP90, assumed for many years to be typical intracellular proteins, was described for the first time more than 30 yr ago as a tumor-specific antigen [10]. Over time, it became clear that, in cancer cells, surface expression of HSP90α is upregulated and associated with cell migration, invasion, and metastasis [11–13], as well as in tumor immunogenicity [14].
The 90-kDa heat shock protein, certainly the most unexpected member of cell surface receptor platforms, is an essential and ubiquitous molecular chaperone that interacts with >400 client proteins. HSP90 uses the energy generated by ATP binding and hydrolysis to stabilize proteins involved in signal transduction, protein trafficking, receptor maturation and innate and adaptive immunity [15]. Different cochaperones dynamically associate with HSP90 during the maturation of client proteins. They form binary or ternary complexes with HSP90 and regulate its function in various ways, such as recruitment of specific clients and acceleration or inhibition of ATP hydrolysis [16]. The HSP90 family includes two cytoplasm/nucleus localized chaperones, the inducible HSP90α and the constitutive HSP90β [17]. Even under nonstressful conditions, HSP90 comprises ∼1–2% of total cellular protein content and its amount increases ∼2-fold during environmental stress [18–20]. ATP-binding and hydrolysis are essential to the function of HSP90. The presence of an atypical ATP binding pocket in the N-terminal domain of HSP90 explains the remarkable specificity of the natural inhibitors Ge, Rad, and their derivatives. These highly specific inhibitors perfectly mimic the binding of ATP [21], thus blocking the maturation of the client and leading to its proteasomal degradation. Currently, several HSP90 inhibitors have undergone clinical trials, and some of them remain under clinical investigation for cancer therapy and presumably in other treatments [22]. There are many reports demonstrating the effects of HSP90 inhibitors on inflammatory response in the immune system. It has been documented that Ge and its derivative 17-DMAG reduced production of TNF, IL-1, IL-6, and NO by Mϕs stimulated with LPS, Taxol and IFN-γ [23–25]. The secretion of proinflammatory cytokines by monocytes stimulated with mactinin, a fragment of cytoskeletal α-actinin was reduced after treatment with derivatives of Ge, 17-AAG, and 17-DMAG, as well [26]. The anti-inflammatory effect of HSP90 inhibitors were also shown in mouse model of sepsis [27].
Well known for its ability to maintain the conformation of unstable client proteins HSP90 could also exert its chaperone function on the outer side of plasma membrane where signaling requires conformational changes and lateral interactions between receptor proteins. It is particularly important to study the activity of HSP90 on Mϕs because 1) HSP90 is constantly present in signaling complexes, 2) HSP90 is the only stress-induced chaperone that can be inhibited with extremely selective chemical inhibitors, and 3) some of the HSP90 inhibitors do not penetrate to the cytoplasmic compartment, allowing selective inhibition of cell-surface–associated pools. Clearly, any chemical inhibitor altering the recognition of molecular patterns by Mϕs would have profound effects on inflammatory responses. The goal of the present study was to determine whether the presence and mechanochemical cycle of cell-surface HSP90 is necessary for cytokine response and target engulfment after pattern recognition by Mϕs. The results reported below suggest a novel activity of HSP90, which may influence innate recognition of molecular patterns.
MATERIALS AND METHODS
Human peripheral blood monocytes and hMDMs
PBMCs were isolated from citrate-treated blood of healthy donors by standard-density gradient centrifugation (Ficoll-Paque PLUS; Amersham Biosciences, Uppsala, Sweden). To obtain elutriation-purified monocytes, PBMCs were subjected to counterflow centrifugation [28]. Finally monocytes were resuspended in RPMI 1640 (Thermo Fisher Scientific, Paisley, UK) supplemented with 2 mM l-glutamine, 50 μg/ml gentamicin (Sigma-Aldrich St. Louis, MO, USA), and 10% autologous heat-inactivated serum (autologous HS) or 10% FCS (Biochrom, Berlin, Germany) and plated at 0.5 × 106/well in 24-well Cell+ culture plates (Sarstedt, Newton, NC, USA). To obtain monocyte-derived Mϕs, PBMCs were plated at 4 × 106/well in 24-well Primaria Corning culture plates or at 40 × 106/well in 6 cm diameter Primaria Corning culture dishes (Corning Inc., Corning, NY, USA) in RPMI 1640 supplemented with 2 mM l-glutamine, 50 μg/ml gentamicin, and 10% FBS. After 2 h of incubation at 37°C in humidified atmosphere containing 5% CO2, nonadherent cells were removed by washing with complete medium. Adherent cells were cultured in complete medium supplemented with 12% pooled heat-inactivated human serum (HS) for at least 7 d. The medium was changed every 2 d. The hMDM phenotype was routinely controlled, after nonenzymatic detachment of cells, by immunofluorescence staining of CD14 (clone: TŰK4), CD16 (clone: DJ130c) both from DakoCytomation Denmark A/S (Glostrup, Denmark), CD11b (clone: ICRF44) and CD209 (clone: DCN46) both from BD Biosciences (Franklin Lakes, NJ, USA) and subsequent flow cytometry analysis. The cultures selected for further experiments were positive in at least 90% for the first 3 markers and in <1% for CD209. The adherent cells acquired Mϕ morphology. Resting (nonstimulated) cells did not produce cytokines: IL-1, -6, and -10 and TNF.
Apoptotic PMNs
PMNs were obtained as described [29]. In brief, cells were isolated from erythrosediments by sedimentation in 1% polyvinyl alcohol solution (Merck, Hohenbrunn, Germany) for 20 min at RT. PMNs were collected from the upper layer, and contaminated erythrocytes were lysed with distilled water for 20 s. Pappenheim staining indicated that cells isolated in this way were at least 90% homogenous. PMNs were used after incubation for 24 h in RPMI 1640 supplemented with l-glutamine (2 mM), gentamicin (50 μg/ml), and 2% heat-inactivated FCS in a humidified atmosphere containing 5% CO2 at 37°C. The percentage of annexin V+ and PI− cells in populations of aged, apoptotic PMNs ranged from 45 to 83% and the proportion of PI+ cells did not exceed 15%.
Treatment with HSP90 inhibitors and stimulation with PAMPs
Cultured monocytes or hMDMs were left untreated or pretreated for 30 min with cell-permeable inhibitors of HSP90: Rad (20 µM), Ge (20 µM) (both from Sigma-Aldrich; 10 mM stock solution in DMSO), and 17-DMAG (1 µM; Invivogen, San Diego, USA; 10 mM stock solution in water) or a cell-impermeable GeB (20 µM; Invivogen; 10 mM stock solution in DMSO). The cells were stimulated with PAMPs: Escherichia coli 0127:B8 LPS (stLPS; Sigma-Aldrich), ultrapure E. coli 011:B4 LPS (upLPS; Invivogen), synthetic lipopeptides: diacylated Pam2CysSerLys4 (Pam2; Invivogen) and triacylated Pam3CysSerLys4 (Pam3; Invivogen) at a final concentration of 10 ng/ml, ultrapure Porphyromonas gingivalis LPS (pgLPS; Invivogen) at a final concentration of 1 μg/ml or heat-killed (72°C for 1 h) SA strain ATCC 25923 (Manassas, VA, USA) and PG strain W83, both used at 1:10 cell:bacteria ratio. SA and PG were kindly provided by Aneta Sroka (Department of Microbiology, Faculty of Biotechnology, Biochemistry and Biophysics, Jagiellonian University, Poland).
DMSO, introduced as a solvent at 0.2%, had no evident effect on the HSP90 staining, cytokine response to PAMPs, or phagocytosis (data not shown).
Measurement of TNF production
For TNF secretion measurement, supernatants were collected 6 h after stimulation and centrifuged at 500 g for 5 min to remove particulate debris and stored at −20°C. The concentrations of TNF were determined by ELISA with the OptEIA Sets (BD Biosciences) according to the instructions provided with each set of Abs. The assay was sensitive down to concentration of 7 pg/ml.
Surface expression of HSP90 on monocytes and hMDMs
To determine HSP90 expression on the cell surface elutriation-purified monocytes or hMDMs (after nonenzymatic detachment) were resuspended in PBS supplemented with 5% FCS and 0.5 µg/ml human IgG Fc fragment (EMS-Millipore, Billerica, MA, USA) and incubated for 30 min on ice. Then, the following anti-human HSP90 mAbs (clone: AC88, K3701, K3705, I6F1, 9D2, 2D12; Enzo Life Sciences, Farmingdale, NY, USA) or (NBP1-77682, NB120-2928; Novus Biologicals, Littleton, CO, USA) polyclonal Abs were added at concentration 10 µg/ml, and cells were incubated for 40 min on ice. After washing in ice-cold PBS staining with appropriate secondary antibody conjugated with fluorochromes: PE-labeled goat anti-mouse Ig (BD Biosciences), FITC-labeled rabbit anti-rat Ig (Abcam, Cambridge, MA, USA) and APC-labeled goat anti-rabbit Ig (Thermo Fisher Scientific) was performed for 30 min on ice. Finally, cells were analyzed by flow cytometry with an LSRII cytometer (BD Biosciences). Appropriate isotype-matched control Abs (R&D Systems, BD Biosciences, and eBioscience) were also used to determine nonspecific binding. The analysis was performed using the FACSDiva program, and the histogram plots were created with the CellQuest program (both from BD Biosciences).
The localization of GeB-HSP90 and FITC-Ge-HSP90 complexes documented by confocal microscopy
To enable confocal microscopy analysis hMDMs were differentiated from monocytes as described above, but on glass coverslips submerged in culture medium in 3.5 cm diameter culture dishes (Sarstedt Inc.). For 3D image collection, live hMDMs were incubated for 15 and 30 min with GeB (20 µM) at 37°C, gently rinsed with culture medium, and incubated with CF640R-conjugated anti-biotin mouse mAbs (Sigma-Aldrich) for 15 min at RT. Alternatively, live hMDMs were incubated for 15 and 30 min with FITC-Ge (Invivogen) at 20 µM concentration at 37°C and then gently rinsed with culture medium. In both runs, before imaging cells were fixed with 4% methanol-free PFA (Thermo Fisher Scientific). In some cases, to visualize cytosolic compartment 24 h before experiment hMDMs were stained with PKH67 green vital stain (Sigma-Aldrich) according to the manufacturer’s recommendations. To analyze the Rac1-dependency of Ge-HSP90 complex formation, live hMDMs were untreated or pretreated with Rac1 inhibitor (NSC23766; Calbiochem, San Diego, CA, USA) at 50 μM concentration for 30 min and then incubated with FITC-Ge at 20 µM concentration for 20 min at 37°C. After gentle washing, the cells were fixed with 4% PFA and labeled with Alexa Fluor 647–conjugated anti-human CD11b Abs (clone ICRF44; BioLegend, San Diego, CA, USA). To quantify the effect of Rac1 inhibition on Ge-HSP90 complex formation, 3D images were analyzed with ImageJ software [30]. Z-stack projections were created for each channel. Areas and the number of cells on each image were identified, with binary mask, based on CD11b staining. On this basis, the number of local fluorescence maxima (foci) per cell were calculated. Results are presented as the mean amount of foci per cell calculated for each field of view. The mean number of cells per field was 17 ± 5, and the overall amount of analyzed cells was 250.
GeB-StrAv-PE complexes were prepared by preincubation of GeB with PE-conjugated streptavidin (StrAvPE; BD Biosciences) at molar ratio 4:1 for 30 min at RT in HBSS. Live hMDMs were placed in a microscope-stage microincubator, incubated with Alexa Fluor 488-conjugated anti-human CD11b Abs (clone Vim12; BD Biosciences) for 5 min, and the solution of GeB-StrAvPE complexes at a concentration equal to 20 µM GeB was added. Images were collected every 30 s for 30 min with a TCS SP5 II confocal microscope (Leica Microsystems GmbH, Wetzlar, Germany). For verification of cell membrane permeability for GeB, confocal microscopy specimens were prepared as described in Table 1, and 3D images were recorded.
TABLE 1.
Schedule of experiment verifying permeability of cell membrane for GeB
| Step no. | Control staining with StrAv-PE | GeB incubated with live hMDMs | GeB incubated with permeabilized hMDMs |
|---|---|---|---|
| 1 | Incubation of live hMDMs in culture medium for 15 min at 37°C | Incubation of live hMDMs with GeB (20 μM) for 15 min at 37°C | Incubation of live hMDMs in culture medium for 15 min at 37°C |
| 2 | Gentle washing with warm PBS | ||
| 3 | Fixation with 4% PFA for 5 min at RT | ||
| 4 | Washing with PBS | ||
| 5 | Permeabilization with ice-cold 90% methanol (MetOH) for 20 min at −20°C | ||
| 6 | Washing with PBS | ||
| 7 | Incubation in culture medium for 15 min at 37°C | Incubation in culture medium for 15 min at 37°C | Incubation with GeB (20 μM) for 15 min at 37°C |
| 8 | Washing with PBS | ||
| 9 | Blocking for 60 min at RT | ||
| 10 | Incubation with PE-conjugated streptavidin (2.5 μg/ml) for 20 min at RT | ||
| 11 | Repeated washing with PBS+0.05% Tween20 | ||
All specimens of fixed cells were mounted on microscope slides (Thermo Fisher Scientific) with Prolong Diamond Antifade Mountant (Thermo Fisher Scientific) and analyzed within 1 wk. All images were acquired with the TCS SP5 II confocal microscope, with LAS AF software (Leica Microsystems GmbH).
Determination of cell viability
The effect of HSP90 inhibitors on cell viability was estimated with methods discriminating the basic types of cell death: apoptosis and necrosis. An early feature of apoptosis, phosphatidylserine exposure to the outer cell membrane, was quantified by the binding of FITC-labeled annexin V and exclusion of PI, according to the manufacturer’s recommendations (BD Biosciences) followed by analysis with an LSR II flow cytometer (BD Biosciences). The proportions of annexin V+, annexin V+PI+, and annexin V−PI+ cells in untreated monocytes and those incubated for 5 h with inhibitors of HSP90 was estimated. For DNA fragmentation assessment, monocytes seeded in complete culture medium were incubated with inhibitors of HSP90 for 5 h. After incubation cells were washed, fixed with 4% PFA and permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate solution using standard protocols. Subsequently, DNA fragmentation was detected by TUNEL staining using the In situ Cell Death Detection Kit (Roche, Mannheim, Germany) according to the instructions provided by manufacturer. TUNEL+ cells were quantified by flow cytometry analysis. Cells treated with SA α-toxin (List Biologic Laboratories Inc., Campbell, CA, USA) served as the positive control [31]. For cytotoxicity assay, hMDMs cultured in RPMI 1640 supplemented with 2 mM l-glutamine, 50 μg/ml gentamicin and 1% FCS were incubated with inhibitors of HSP90. Supernatants were collected after 5 or 20 h, centrifuged at 500 g for 5 min and stored at −20°C. Cytotoxicity of inhibitors was assessed by measurement of lactate dehydrogenase (LDH) release using a Cytotoxicity Detection Kit (Roche) according to the manufacturer’s protocol. Supernatants from Mϕs subjected to sonication with Sonic Ruptor 250 sonicator (Omni International, Kennesaw, GA, USA) was used as a positive control.
Treatment with HSP90 inhibitors and immunofluorescence staining
To analyze the effect of HSP90 inhibitors on the expression of surface receptors on monocytes and hMDMs, the cells were placed in complete medium supplemented with 10% HS and treated with inhibitors for 4 h. After nonenzymatic detachment, the cells were resuspended in PBS supplemented with 5% FCS and 0.5 µg/ml human IgG Fc fragment (EMS-Millipore) and incubated for 30 min on ice. Then, the following anti-human Abs: PE-conjugated mAbs to TLR2 (clone TL2.1; eBioscience, San Diego, CA, USA), TLR4 (clone HTA125; eBioscience), CD14 (clone MP9; BD Biosciences), and CD47 (clone B6412; BD Biosciences); FITC-conjugated mAbs to TLR1 (clone GD2.F4; Abcam), TLR6 (clone TLR6.127; Abcam), CD11b (clone Vim-12; Abcam), and CD16 (clone DJ130c; BD Biosciences), APC-conjugated mAbs to CD36 (clone CB38; BD Biosciences), and CD81 (clone JS-81; BD Biosciences), Alexa Fluor 700-conjugated mAb to CD64 (clone10.1; BD Biosciences), PE-Cy7-conjugated mAb to CD11b (clone ICRF44; BD Biosciences) were added and cells were incubated for 30 min on ice. Isotype matched control Abs were also used to determine nonspecific binding (R&D Systems, BD Biosciences, eBioscience). After washing with ice-cold PBS supplemented with 1% FCS, cells were resuspended and analyzed by flow cytometry using an LSRII cytometer (BD Biosciences). The analysis was performed using the FACSDiva program to determine the percentage and mean fluorescence intensity (MFI) of positive cells. The histograms plots were created with CellQuest software (BD Biosciences).
Luminex-based analysis of cytokines and chemokines
For the secretome analysis, supernatants were collected 24 h after stimulation and centrifuged at 500 g for 5 min to remove particulate debris and stored at −20°C. The analyses of the cytokines and chemokines secreted by Mϕs were performed with FlexMAP 3D (Luminex, Austin, TX, USA) platform using the Human Cytokine Magnetic 30-Plex Panel kit (Thermo Fisher Scientific). The detection thresholds (in pg/ml) for particular protein were as follows: epidermal growth factor (EGF, 40), eotaxin (5), fibroblast growth factor (FGF basic; 22), G-CSF (30), GM-CSF (15), hepatocyte growth factor (HGF; 50), IFN-α (25), IFN-γ (5), IL-1 receptor antagonist (IL-1RA; 60), IL-1β (25), IL-2 (10), IL-2R (60), IL-4 (5), IL-5 (3), IL-6 (3), IL-7 (30), IL-8 (3), IL-10 (5), IL-12 (15), IL-13 (10), IL-15 (125), IL-17 (50), IFN-γ-inducible protein 10 (IP-10, 5), MCP-1 (10), monokine induced by γ-IFN (MIG, 45), MIP-1α (16), MIP-1beta (100), RANTES (20), TNF (10) and VEGF (10). The procedure was performed according to the manufacturer’s instructions. To confirm the results obtained by multiplexed bead-based immunoassay we applied sandwich ELISA for IL-6, IL-10, and TNF and produced consistent results.
Phagocytosis of bacteria
To enable analysis of phagocytosis by flow cytometry, the heat-killed SA (25923; ATCC) bacteria were incubated for 30 min at 65°C in PBS containing 10 μM TOTO-3 (Thermo Fisher Scientific) and then washed 3 times with a large volume of PBS. The density of bacterial cells was measured spectrophotometrically (540 nm), and the number of cells was calculated by using previously determined standard curves (based on CFU counts). hMDMs differentiated in 24-well culture plates were incubated at 37°C with suspensions of TOTO-3 labeled bacteria in a total volume of 1 ml and the Mϕ:bacterium ratio 1:50. Cells without bacteria were also incubated in parallel. After 90 min of incubation, the cells were washed twice with 1 ml of ice-cold PBS. Nonenzymatically detached cells were analyzed by flow cytometry. The intensity of phagocytosis was expressed as phagocytic index: percentage of positive (with ingested bacteria) cells × MFI of positive population.
Phagocytosis of PMNs: elastase activity assay
A suspension of apoptotic PMNs in complete medium was incubated with hMDMs differentiated in 24-well culture plates (2.5 × 106 PMNs/well) for 2 h in a humidified atmosphere containing 5% CO2 at 37°C. The monolayer was then washed vigorously with ice-cold PBS to remove nonphagocytosed PMNs, including those that were only Mϕ-surface associated. The Mϕ monolayer was lysed with 0.1% hexadecyltrimethyl ammonium bromide (CTAB) at 37°C for 15 min. Lysate (100 µl) was transferred to a 96-well plate with 4 replicates, followed by the addition of 100 µl of 1 mM solution of N-methoxysuccinyl-Ala-Ala-Pro-Val-p-nitroanilide in 0.2 M Tris-HCl (pH 7.5). Hydrolysis of the substrate by PMN elastase was measured as the increase in absorbance at 405 nm after incubation at 37°C for 30 min using a microplate reader (model 200; Tecan, Männedorf, Switzerland). The activity was expressed as an increase in absorbance at OD 405 nm in milliunits per minute (mOD405 nm/min). In addition, the lysates of the macrophage monolayer, which had not been exposed to PMNs, were used in each experiment as a negative control. Routinely, Mϕs alone were found negative for elastase activity.
Statistical analyses
All experiments were performed at least in triplicate. The data are presented as means ± sem. All statistics were calculated using Origin 8.1 (OriginLab Corp., Northampton, MA, USA). Statistical significance was assessed at 0.05 and calculated with the 1-way ANOVA.
RESULTS
Inhibition of HSP90 reduces PAMP-induced TNF secretion by human monocytes and hMDMs
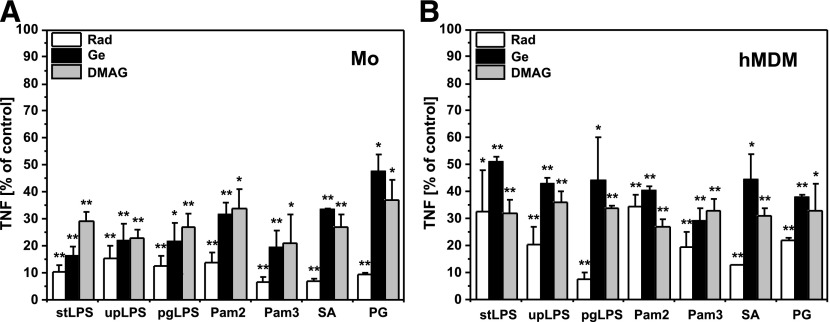
The first series of experiments was designed to investigate the effect of HSP90 inhibitors on PAMP-induced TNF production by human monocytes and hMDMs. Elutriation-purified monocytes or hMDMs were cultured alone or pretreated for 30 min with cell-permeable inhibitors of HSP90: Rad, Ge, or DMAG, and then stimulated with defined PAMPs as indicated in Fig. 1. Supernatants were collected 6 h after stimulation, and TNF was subsequently measured by ELISA. Unstimulated cells did not secrete TNF, and HSP90 inhibitors alone did not activate cytokine production (data not shown). The data presented in Fig. 1 show that inhibition of HSP90 significantly suppressed TNF production by monocytes and Mϕs stimulated with defined PAMPs specific for TLR4, TLR2, or its heterodimers with TLR1 or -6, as well as TNF production induced by heat-killed bacteria SA and PG.
Figure 1. Inhibition of HSP90 reduces TNF production by monocytes and hMDMs.
(A) Monocytes were isolated from PBMCs by elutriation and cultured in medium supplemented with 10% autologous serum. (B) hMDMs were differentiated from adherent monocytes for at least 7 d in medium supplemented with 10% HS. Cultured cells were left untreated or pretreated for 30 min with Rad and Ge (20 µM) or DMAG (1 µM), the inhibitors of HSP90, and then stimulated with the PAMPs: stLPS, upLPS, pgLPS, Pam2, Pam3, or the bacteria SA or PG. TNF production by monocytes (Mo) or Mϕs (hMDMs) stimulated with PAMPs or bacteria in the absence of HSP90 inhibitors was arbitrarily set at 100% and served as the positive control. Results are expressed as a percentage (mean ± sem: from 3 independent experiments) of corresponding positive controls. *P < 0.05; **P < 0.01.
From the data in Fig. 1 (and see also Fig. 7), it is apparent that 17-DMAG demonstrated much higher inhibitory activity than the other Ges. 17-DMAG is a semisynthetic Ge that has been optimized for maximum cellular bioavailability necessary in human cancer therapy [32]. First, 17-DMAG is water-soluble: 1.4 mg/ml vs. 0.1 mg/ml for Ge [33]. Second, wild-type human HSP90 binds 17-DMAG with higher affinity than Ge, Kd ∼0.35 μM vs. Kd ∼1.2 μM, respectively [21, 34].
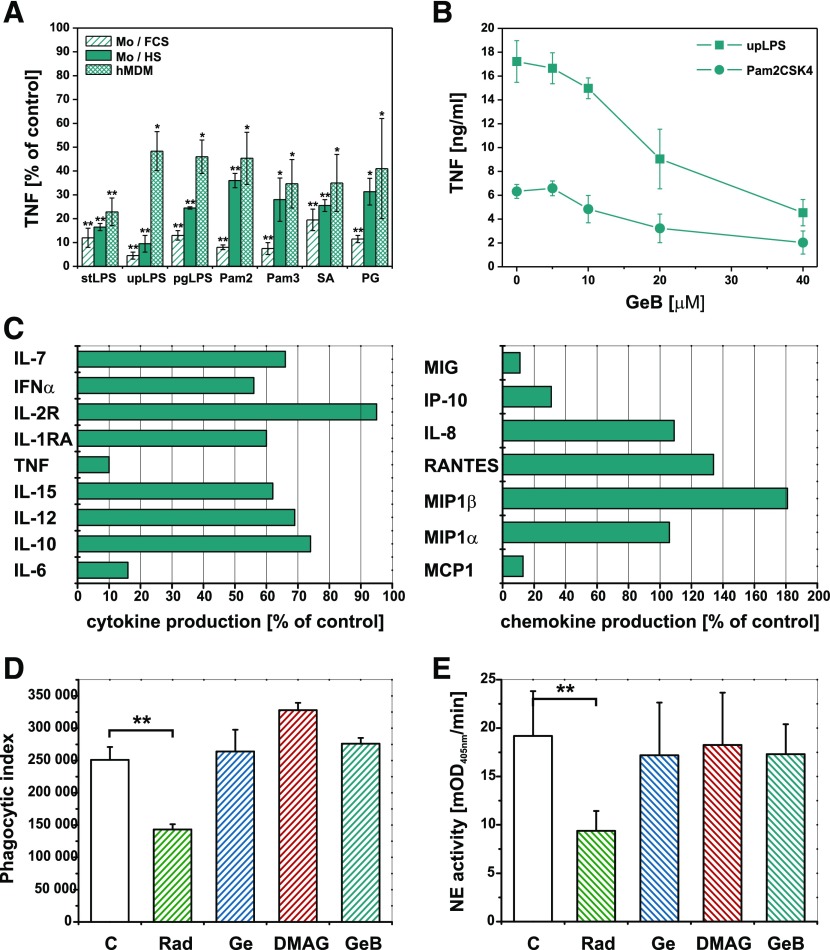
Figure 7. Activity of cell surface HSP90 is necessary for cytokine response, but not for target engulfment after pattern recognition by Mϕs.
(A) Monocytes were cultured in medium supplemented with 10% FCS or 10% autologous serum. hMDMs were cultured in medium supplemented with 10% HS. Cultured cells were untreated or pretreated for 30 min with GeB (20 µM) and then stimulated with PAMPs: E. coli LPS (stLPS and upLPS), PG LPS (pgLPS), Pam2CSK4 (Pam2), Pam3CSK4 (Pam3) or bacteria SA or PG. TNF production stimulated with PAMPs or bacteria in the absence of GeB was arbitrarily set at 100% and served as the positive control. Results are expressed as percentage of corresponding positive controls (means ± sem of results from at least 3 independent experiments). *P < 0.05; **P < 0.01. (B) Cultured hMDMs were untreated or pretreated for 30 min with GeB at the concentrations indicated and then stimulated with upLPS or Pam2CSK4. Data presented are means ± sem of TNF concentration in supernatants from 3 independent experiments. (C) Cultured hMDMs were untreated or pretreated for 30 min with GeB (20 µM) and then stimulated with E. coli LPS. The levels of cytokines were measured by a bead-based multiplexing immunoassay. Cytokine production stimulated in the absence of GeB was arbitrarily set at 100% and served as the positive control. Results are expressed as percentage of corresponding positive controls. Phagocytosis of SA (D) or apoptotic PMNs (E) by hMDMs untreated or pretreated for 30 min with Rad, Ge, or GeB (20 µM) or with DMAG (1 µM). (D) After 90 min incubation with fluorescently labeled SA hMDMs were nonenzymatically detached and analyzed by flow cytometry. The intensity of phagocytosis is expressed as the phagocytic index: percentage of positive cells × MFI of positive population. **P < 0.01. The representative, raw flow cytometry data are depicted in Supplemental Fig. 2. (E) After 2 h incubation with apoptotic PMNs, noningested neutrophils were removed by intensive washing of the hMDM monolayer. The cells were solubilized with detergent, and the neutrophil elastase activity was measured in lysates. The intensity of phagocytosis is expressed as mOD405nm / min of the substrate turnover catalyzed by neutrophil-derived elastase. **P < 0.01.
HSP90 is expressed at the cell surface
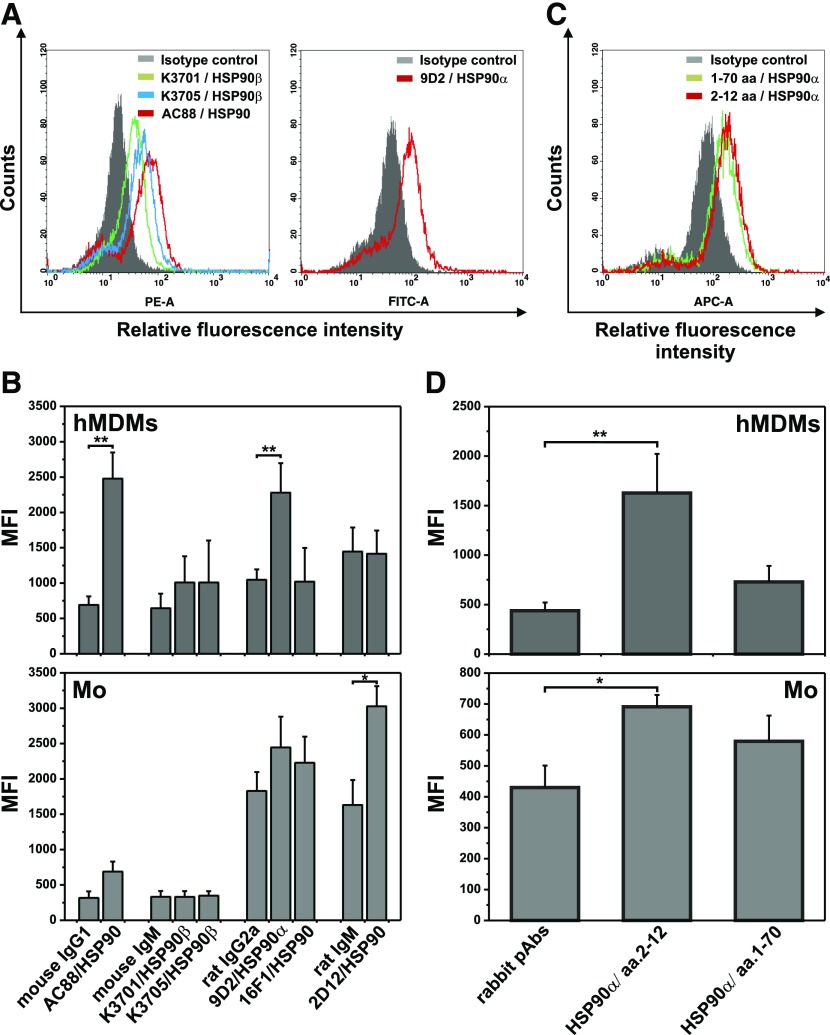
As the presence of HSP90 on the surface of normal cells is still uncertain we extensively examined its expression by immunofluorescence staining followed by flow cytometry analysis. Using different polyclonal or mAbs we have found the expression of both HSP90 isoforms on the surface of hMDMs. Data from 1 experiment representative of 3 performed depicted in Fig. 2A clearly revealed cells positive for HSP90 (clone AC88) as well as its both isoforms: HSP90α (clone 9D2) and HSP90β (clones K3705 and K3701). Surface expression of HSP90 on monocytes was also observed with AC88 and 2D12 anti-HSP90 clones. Figure 2B summarize results from 3 independent experiments presented as MFI for specific staining in comparison to MFI of corresponding isotype controls. We also noticed positive staining of monocytes and hMDMs with Abs against an N-terminal portion of HSP90α (Fig. 2C and D) pointing at the accessibility of ATPase domain on the cell surface. In contrast, we did not observe binding of anti-HSP90 Abs clone AC88 by human neutrophils or lymphocytes (data not shown).
Figure 2. Surface expression of HSP90 on human monocytes and hMDMs.
Monocytes were isolated from PBMC by elutriation. hMDMs were differentiated from adherent monocytes for at least 7 d in medium supplemented with 10% HS and then were nonenzymatically detached. Cells were indirectly labeled with anti-HSP90 mAbs (A and B) (clones denoted in figure captions) or rabbit pAbs (C and D) specific for the N-domain of HSP90 (epitopes denoted in figure captions) and corresponding fluorescent secondary Abs and analyzed by flow cytometry. (A and C) Flow cytometry data from 1 experiment representative of 3 performed showing the expression of HSP90 on hMDMs. The gray-shaded histograms represent background staining obtained with isotype-matched controls. (B and D) HSP90 expression on hMDMs and monocytes, presented as MFI of specific staining in comparison to MFI of corresponding isotype controls (mean ± sem from 3 independent experiments). *P < 0,05; **P < 0.01.
GeB-inhibited HSP90 are detected on the hMDM surface
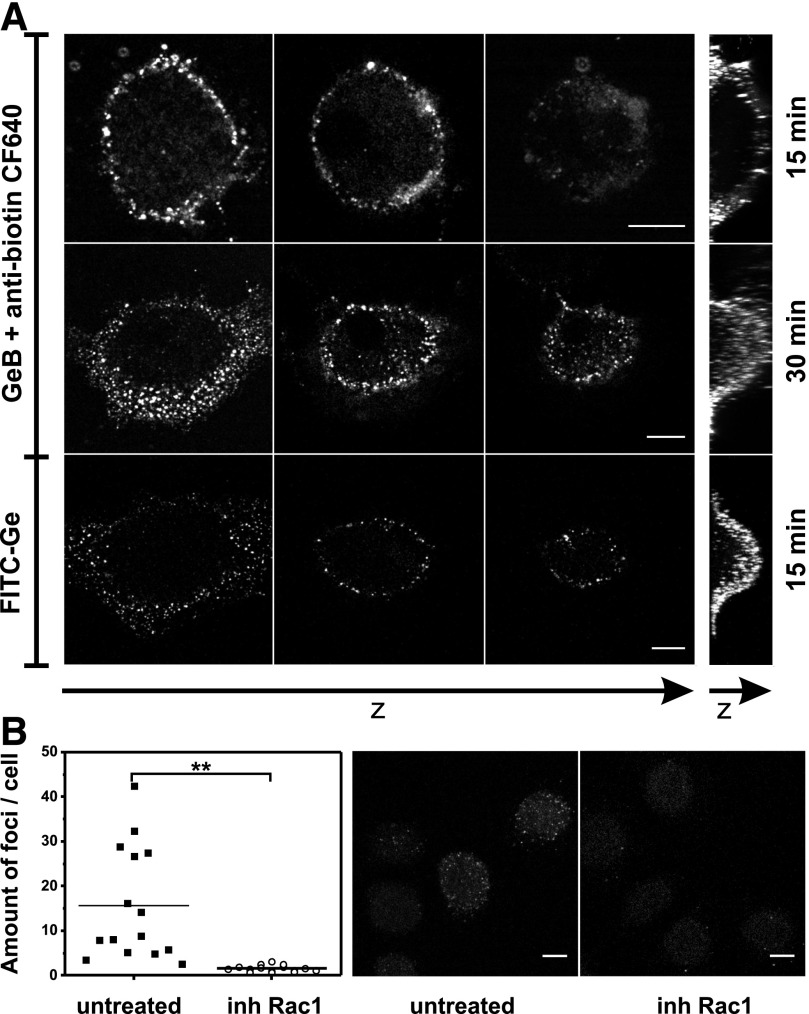
In the next part of this study, we used an HSP90 inhibitor, GeB—Ge coupled to the biotin molecule, through a long, hydrophobic spacer. Using the inhibitor, we were able to label cell surface HSP90 confirming our previous immunofluorescence results. Confocal analysis of live hMDMs labeled with GeB and CF640R-conjugated anti-biotin Abs (Fig. 3A) showed that GeB-inhibited HSP90 molecules were rapidly (about 15 min) clustered on the Mϕ surface (top row). The finding proved that N-terminal (ATPase) domain of HSP90 is exposed to and accessible from the outside of the cell. The specificity of the observed staining was verified by analogical analysis of cells untreated with GeB and labeled with CF640-conjugated anti-biotin antibody. Confocal images presented in Supplemental Fig. 1 demonstrate some heterogeneous, low-intensity staining of control cells but significantly lower compared with that of GeB-pretreated hMDMs. After prolonged incubation (about 30 min) with GeB, HSP90 was recycled from the cell surface through the endosomal compartment and was clustered at the border of perinuclear cytoplasm (Fig. 3A, middle row). Using PKH67—green vital stain to visualize cytosolic compartment—no superposition of red and green was observed, suggesting that PKH67 and GeB stayed in different compartments (data not shown). In addition, HSP90 was detected with FITC-Ge, a construct similar to GeB, where the FITC molecule was linked through a long spacer to Ge at the C17 position. The bottom panel in Fig. 3A presents characteristic, membrane-associated, and submembrane puncta of FITC-Ge-inhibited HSP90 molecules almost identically distributed, as visualized with GeB. Clustering and recycling of Ge-inhibited HSP90 was Rac1-dependent. As shown in Fig. 3B, confocal imaging followed by quantitative analysis of the mean amount of foci per cell revealed that, pretreatment of hMDMs with NSC23766 (Rac1 inhibitor) slowed down the endocytosis and significantly diminished the FITC-Ge staining.
Figure 3. GeB or FITC-Ge-inhibited HSP90 molecules are detected on the hMDMs surface.
(A) Confocal microscopy 3D image of hMDMs incubated with biotinylated GeB (20 µM) for 15 min or 30 min and then labeled with CF640R-conjugated anti-biotin mAbs, or hMDMs incubated with FITC-Ge (20 µM) for 15 min. Before imaging, all samples were fixed with 4% PFA. Rows represent 3 horizontal single cross sections of the cell, followed by vertical maximum pixel value Z-stack projections. Scale bar, 10 µm. Control staining for CF640R-conjugated anti-biotin mAbs is presented in Supplemental Fig. 1. (B) Cultured hMDMs were left untreated or pretreated with Rac1 inhibitor (NSC23766) and then incubated with FITC-Ge (20 µM) for 20 min. Before imaging, samples were fixed with 4% PFA. Confocal microscopy 3D images were analyzed, and the mean amount of foci per cell from separate fields of view was determined. The images are Z-stack projections of the representative cells. Scale bar, 10 µm.
GeB does not penetrate the cell membrane
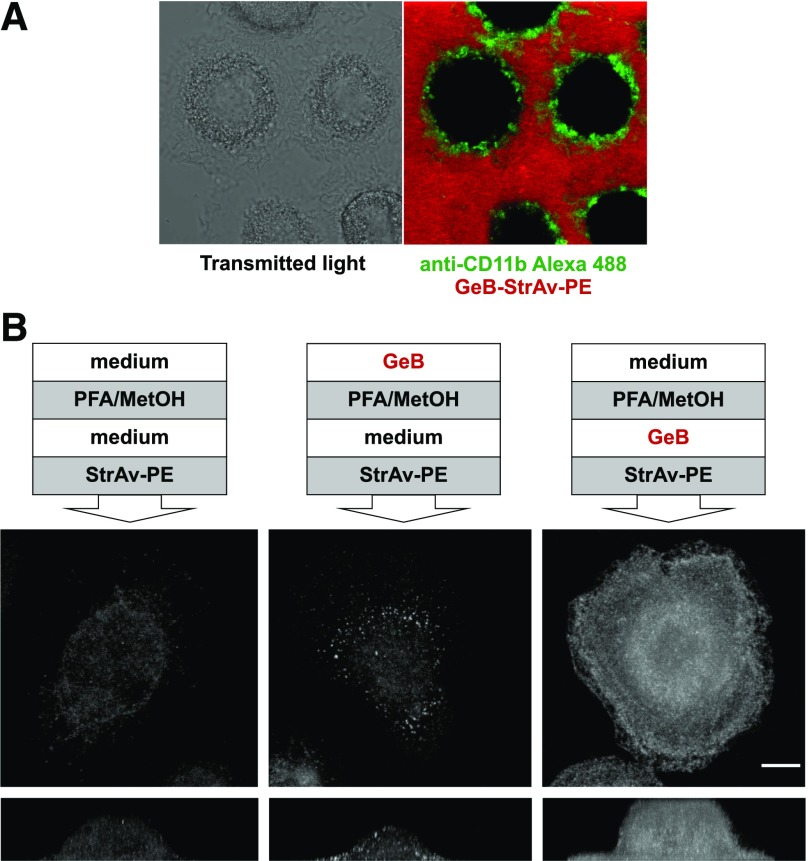
Structural and confocal microscopy data indicate that GeB is a cell-impermeable inhibitor and could be used to inhibit only the surface pool of HSP90 molecules. Further confocal microscopy analysis of live hMDMs incubated with GeB-streptavidin-PE was conducted (Fig. 4A). The cell membrane was visualized with Alexa Fluor 488-conjugated anti-CD11b Abs (green rings), and it was apparent that GeB stayed outside the cells (diffuse red staining) and did not penetrate cell membranes. Because cells are nonpermeable to streptavidin, which and can bind only extracellular GeB, additional control experiments were advisable. The experimental schedule, presented in Materials and Methods, is shown in a simplified version in Fig. 4B. In brief, 2 modes of GeB binding to HSP90 were performed in parallel: live cells were incubated with GeB, then fixed and permeabilized, or cells were first fixed and permeabilized and then incubated with GeB. Subsequently, both samples were labeled with PE-conjugated streptavidin. Appropriate staining controls (i.e., cells fixed, permeabilized and labeled with PE-conjugated streptavidin, without GeB treatment) were also prepared. In fixed and permeabilized cells, where GeB freely diffused to the cell interior, the horizontal and vertical projections revealed massive staining, which can be attributed mostly to the intracellular pool of HSP90 (Fig. 4B). A strikingly different staining pattern was observed when GeB was incubated with live hMDMs before their fixation and permeabilization (middle column). Besides clustered staining on the surface of hMDMs some signal was also observed inside the cells, but it could be attributed to endogenous biotin interfering with biotin-streptavidin-detection schemes, as staining with PE-conjugated streptavidin without GeB treatment resulted in nearly identical intracellular staining pattern.
Figure 4. Neither GeB-StrAv-PE nor GeB alone penetrates cell membrane.
(A) Confocal images of live hMDMs labeled with Alexa Fluor 488-conjugated anti-CD11b mAbs and incubated with GeB-StrAv-PE for 15 min at 37°C. GeB-StrAv-PE was prepared by preincubation of GeB with PE-conjugated streptavidin at a molar ratio of 4:1 for 30 min. (B) Confocal microscopy 3D images of hMDMs incubated with GeB (20 μM, 15 min, 37°C) before (middle column) or after (right column) fixation (4%PFA, 5 min, RT) and permeabilization (90% MetOH, 20 min, −20°C) and finally labeled with PE-conjugated streptavidin (20 min, RT). Left column shows cell untreated with GeB and labeled with PE-conjugated streptavidin after fixation and permeabilization. The images show maximum pixel value Z-stack projections of the representative cells from 1 experiment of 3 conducted. Scale bar, 10 µm.
Inhibitors of HSP90 had no effect on monocyte and hMDM viability and expression of major PRRs
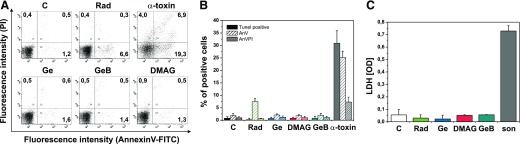
Because of the pronounced proapoptotic effect of the HSP90 inhibitors, we carefully studied the viability of monocytes and Mϕs incubated with Rad and Ge or its derivatives. Methods discriminating 2 basic types of cell death were applied: apoptosis and necrosis. Monocytes were cultured alone (C) or treated with inhibitors of HSP90. After 5 h, monocytes were stained with annexin V-FITC and PI or for DNA nicks labeled with the TUNEL kit followed by flow cytometry analysis. As clearly indicated in Fig. 5A and B, treatment with HSP90 inhibitors did not influence early feature of apoptosis—externalization of phosphatidylserine—detected by FITC-conjugated annexin V binding. We observed annexin V+ monocytes only when treated with Rad, but still the proportion did not exceed 10%. TUNEL assay, that estimate DNA fragmentation also failed to detect committed apoptosis following treatment with HSP90 inhibitors (Fig. 5B). PI exclusion test applied to determine cytotoxic effect of HSP90 inhibitors revealed that, in control as well as in treated monocytes, the percentage of PI+ cells did not exceed 2% (Fig. 5A and B). The advanced apoptosis was also excluded as no ladder-like DNA fragmentation was observed in monocytes (data not shown). Cells treated with SA α-toxin, a well-known inductor of apoptosis in monocytes [31], served as a positive control, and, in similar conditions, we observed significant proportion of annexin V or TUNEL+ cells and visible ladder-like DNA fragmentation. LDH release from Mϕs incubated with HSP90 inhibitors was comparable to control cells and considerably lower than in supernatants from necrotic cells (positive control; Fig. 5C).
Figure 5. Inhibitors of HSP90 do not affect the viability of monocytes and hMDMs.
(A and B) Monocytes were isolated from PBMCs by elutriation and cultured in media supplemented with 10% autologous serum. (C) hMDMs were differentiated from adherent monocytes for at least 7 d in medium supplemented with 10% HS. Before the experiment, cells were placed in medium supplemented with 1%FCS. (A) Flow cytometry analysis of monocytes labeled with FITC-conjugated annexin V and PI after 5 h culture, with or without the inhibitors of HSP90 Rad, Ge, and GeB (20 µM)or DMAG (1 µM). Dot-plots are from one representative experiment out of 3. The numbers correspond to percentage of cells in quadrants set on the base of autofluorescence. (B) Percentage of TUNEL positive, AnV+PI− and AnV+PI+ monocytes from 3 independent experiments were shown (mean ± sem from 3 independent experiments). Cells treated with S. aureus α-toxin (1 µg/ml) served as positive control. (C) Membrane integrity in Mϕs treated for 5 h with the inhibitors of HSP90 Rad, Ge, and GeB (20 µM) or DMAG (1 µM) was controlled by LDH released into the culture medium (mean ± sem of results from 3 independent experiments). Enzyme released from necrotic (sonicated) hMDMs acted as a positive control.
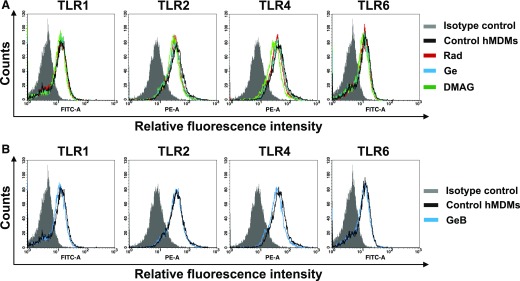
To determine whether the observed decrease in TLR-induced cytokine production was caused by disturbance in TLR expression, we incubated hMDMs with HSP90 inhibitors for 4 h, stained with fluorescently-labeled antihuman mAbs to TLR1, -2, -4, and -6 and analyze using flow cytometry. The general HSP90 inhibitors (Fig. 6A) as well as cell-impermeable biotinylated Ge (Fig. 6B) did not substantially change the TLR expression. The mean values calculated from all experiments performed (Table 2) indicate only minor increase in TLR2 and -4 expression in case of Ge or DMAG treatment. We also verified the expression of other surface molecules present on monocytes and hMDMs that may influence or participate in TLR signaling. The results obtained for hMDMs treated with HSP90 inhibitors were expressed as a ratio to MFI measured for control, untreated cells and presented in Table 2. We observed mild reduction of CD11b and CD81 after 17-DMAG or Ge treatment. Although Ge or 17-DMAG diminished CD11b expression on the cells, its biotinylated derivative had no effect on the expression of the analyzed molecules (Table 2). Consequently, we assumed that diminished TNF production after treatment with HSP90 inhibitors cannot be attributed, either to reduced viability or lower expression of TLRs and their coreceptors.
Figure 6. HSP90 inhibitors do not affect surface expression of TLR1, -2, -4, and -6 on hMDMs.
hMDMs left untreated (control) or treated for 4 h with HSP90 inhibitors were nonenzymatically detached, stained with fluorescence-labeled mAbs and analyzed by flow cytometry. (A) Open histograms depict results for control cells or cells treated with Rad or Ge (20 µM) or DMAG (1 µM). The gray-shaded histogram represents background staining obtained with the isotype-matched controls. (B) Open histograms show results for control and cells treated with GeB (20 µM). The gray-shaded histogram represents background staining obtained with isotype-matched controls. Data are from 1 experiment representative of 3 performed.
TABLE 2.
Flow cytometry analysis of hMDM surface markers expression upon treatment with HSP90 inhibitors
| Rad |
Ge |
DMAG |
GeB |
|||||
|---|---|---|---|---|---|---|---|---|
| Markers | Ratio | SEM | Ratio | SEM | Ratio | SEM | Ratio | SEM |
| TLR1 | 1.03 | 0.07 | 1.01 | 0.01 | 0.94 | 0.03 | 0.96 | 0.04 |
| TLR2 | 0.98 | 0.14 | 1.01 | 0.14 | 1.10 | 0.08 | 1.10 | 0.08 |
| TLR4 | 1.00 | 0.18 | 1.08 | 0.18 | 1.11 | 0.11 | 1.05 | 0.11 |
| TLR6 | 1.08 | 0.16 | 1.07 | 0.12 | 1.01 | 0.13 | 1.04 | 0.09 |
| CD14 | 1.05 | 0.12 | 1.01 | 0.13 | 1.09 | 0.02 | 1.12 | 0.09 |
| CD11b | 0.96 | 0.01 | 0.83 | 0.02 | 0.86 | 0.05 | 0.97 | 0.12 |
| CD64 | 0.90 | 0.16 | 0.81 | 0.06 | 0.91 | 0.10 | 0.89 | 0.15 |
| CD16 | 0.98 | 0.16 | 0.93 | 0.14 | 1.01 | 0.14 | 1.00 | 0.12 |
| CD36 | 1.09 | 0.09 | 1.03 | 0.01 | 0.85 | 0.11 | 0.92 | 0.05 |
| CD47 | 0.89 | 0.04 | 0.90 | 0.08 | 0.95 | 0.12 | 0.90 | 0.11 |
| CD81 | 0.84 | 0.09 | 0.88 | 0.18 | 0.83 | 0.07 | 0.89 | 0.09 |
Cultured hMDMs left untreated or treated for 4 h with HSP90 inhibitors were nonenzymatically detached, stained with fluorescence-labeled mAbs, and analyzed by flow cytometry. Data are expressed as the ratio of MFI measured for hMDMs treated with inhibitors to MFI of control, untreated cells. Values are means ± sem of results from 3 independent experiments.
Activity of cell surface HSP90 is necessary for cytokine response but not for target engulfment following pattern recognition by Mϕs
Seeing that cell membrane is impermeable for GeB and GeB-inhibited HSP90s are present on the surface of Mϕs, we studied whether this inhibitor could affect the response of monocytes and MDMs stimulated with PAMPs. Figure 7A presents a series of experiments in which monocytes or hMDMs were cultured untreated or pretreated for 30 min with a cell-impermeable GeB, and then stimulated with PAMPs. Supernatants were collected 6 h after stimulation and TNF was subsequently measured by ELISA. The cell-impermeable HSP90 inhibitor (GeB) unexpectedly suppressed the PAMPs-induced TNF production by monocytes and hMDMs as effectively as the cell-permeable inhibitors. Likewise, FITC-labeled Ge showed an effect nearly identical to biotin-coupled inhibitor (data not shown). From the presented data, it is also apparent that serum factors play significant role in modulating the response to PAMPs after inhibition of surface HSP90 as autologous HS supplementing culture media to some extent revealed protective effect. It has also been clearly visible that the inhibitory activity of GeB was PAMP-specific. This observation is coherent with previously expressed views, which considered signaling complexes as modular and variable structures, differentially dependent on HSP90. A selected example was shown in Figure 7B where ultra pure E. coli LPS was compared to synthetic diacylated lipoprotein Pam2CSK4. Mϕs are able to detect and integrate complex signals derived from molecular patterns and respond with the wide range of immunoregulatory mediators. Thus, the next purpose of the study was to perform a comparative analysis of the LPS-induced secretome of Mϕs preincubated with or without cell-impermeable HSP90 inhibitor, GeB. Supernatants were collected after 24 h and cytokine content were determined with Luminex. Cytokines below the lower limit of assay sensitivity (IL-1β, IL-2, IL-3, IL-4, IL-13, IFN-γ, and eotaxin) were not included in further analysis. On account of considerable variability among donors, protein secretion profile of Mϕs stimulated with LPS in the presence of GeB was presented relative to control cells, stimulated with LPS in the absence of GeB. We observed that inhibition of surface pool of HSP90 resulted in moderate to significant alteration in both IL and chemokine family secretion. Specifically, we noted significant inhibition of TNF (similar to ELISA results) but also another proinflammatory cytokine: IL-6. Moreover, chemokine production was also considerably regulated. In hMDMs treated with GeB LPS-induced secretion of MCP1, MIG, and IP-10 was almost completely abrogated, whereas production of MIP-1α, IL-8, and RANTES remained unaffected or even enhanced in case of MIP-1β. Phagocytes not only define the consequences of molecular pattern recognition as either proinflammatory or anti-inflammatory, but also are able to engulf recognized targets. Therefore, we used 2 kinds of particulate molecular patterns: bacteria SA as PAMPs and apoptotic PMNs as ACAMPs to outline the role of surface HSP90 in phagocytic activity of hMDMs. Phagocytosis of SA (Fig. 7D; Supplemental Fig. 2) and apoptotic PMNs (Fig. 7E) was unexpectedly not inhibited by Ge, its derivative 17-DMAG, and the cell-impermeable inhibitor GeB. Among all inhibitors used, only Rad, which is also known to inhibit c-Src kinase in a HSP90-independent manner [35] hindered the phagocytic activity of hMDMs.
Collectively, we have shown that blocking the cell-surface HSP90 pool leads to the dramatic decrease of TNF production by primary human monocytes and hMDMs exposed to wide range of soluble and particulate PAMPs. In addition, LPS-induced production of IL-6, MCP1, MIG, and IP-10 was also susceptible to inhibition of surface HSP90. In human Mϕs, the functional cell-surface HSP90 was surprisingly unnecessary for the engulfment of either apoptotic neutrophils or bacteria cells. The presented data suggest that the surface HSP90 is a “signalling complex chaperone,” the activity of which is indispensable for signal transduction resulting from PAMPs recognition but is not essential for the engulfment of particulate antigens.
DISCUSSION
In this report, we describe the first comprehensive immunocytochemistry studies, to our knowledge, of HSP90 on the surface of human monocytes and hMDMs. Using numerous anti-HSP90 Abs and flow cytometry analysis, we found the expression of both (α and β) HSP90 isoforms on the cell surface (Fig. 2). Exposure of HSP90 epitopes on hMDMs and monocytes seemed to be disparate. Although, both cell types bound anti-HSP90 Abs clone AC88, only monocytes showed positive staining with clone 2D12 (Fig. 2B). In addition, surface expression of HSP90 on hMDMs was confirmed by confocal microscopy analysis of cells labeled with anti-HSP90 Abs (data not shown). The Abs directed against the N-terminal domain of HSP90 also efficiently labeled the cell surface, which leads to the conclusion that N-terminal ATPase domain of HSP90 is accessible from the outside of the cell (Fig. 2C and D). Our observation is consistent with reported expression of HSP90 on the surface of human monocytes determined with an antibody specific for N-terminal domain (N17) and with the proposed existence of multiple complexes comprising HSP90 and other receptors, involved in activation of immune cells by microbial patterns [6, 36, 37]. The immunofluorescence results mentioned above were confirmed in the next part of our study, in which we used one of the remarkably specific inhibitors of HSP90 - geldanamycin coupled to biotin or FITC molecule through a long, hydrophobic spacer. Confocal analysis of live hMDMs labeled with FITC-Ge or GeB and CF640R-conjugated anti-biotin Abs proved that the N-terminal (ATPase) domain of HSP90 is exposed to and accessible from outside the cells. Based on the 3D images of hMDMs, surface localization of HSP90 was unequivocally confirmed (Fig. 3A, top and bottom row). We provide the first evidence, to our knowledge, of the presence of Ge-inhibitable HSP90 on the surface of normal primary hMDMs.
From the further set of experiments using confocal microscopy analysis, we also concluded that GeB did not penetrate the cell (Fig. 4A and B). Instead GeB bound only to a surface pool of HSP90, as we found GeB-inhibited HSP90 molecules clustered on the Mϕ surface (Fig. 3A, top row). After prolonged incubation with GeB, HSP90 still did not enter the cytoplasm, it was recycled from the cell surface and accumulated in endosomes at the border of perinuclear cytoplasm (Fig. 3A, middle row).
In the first part of this study we demonstrated that inhibition of HSP90 significantly suppressed TNF production by monocytes and Mϕs stimulated with soluble and particulate PAMPs (Fig. 1). This observation is consistent with the well-known role of HSP90 as the main chaperone of signaling machinery in the cell. A potent anti-inflammatory activity of HSP90 inhibitors has already been shown by many groups. Similar to our results, Ge and its derivatives decreased production of proinflammatory cytokines by monocytes and Mϕs stimulated with LPS, Taxol, IFN-γ, and mactinin, a fragment of cytoskeletal α-actinin [23–26], as well. The postulated mechanism was the inhibitor-induced dissociation of HSP90 clients—Akt kinase and IKK inhibitor—resulting in its degradation and reduction of inflammatory response [25]. However, results obtained in our study challenge the concept of cytoplasmic HSP90 as a key factor in PAMP-induced TNF production. We have demonstrated that cell-impermeable HSP90 inhibitors suppressed the TNF production as effectively as the cell-permeable inhibitors (Figs. 1 and 7A). Moreover, secretome analysis of LPS-stimulated hMDMs indicated that inhibition of surface pool of HSP90 was cytokine selective. It was clearly visible that secretion of 2 major proinflammatory cytokines TNF and IL-6 was more strongly inhibited than the production of remaining proteins (Fig. 7B). Even more diverse effect of GeB on chemokine production was observed. We noted dramatic inhibition of MCP1, IP-10, and MIG and unchanged secretion of RANTES and IL-8. In the case of particulate PAMPs recognition is often followed by their engulfment by interacting phagocytes. To our surprise, in hMDMs the functional cell-surface HSP90 was not necessary for the engulfment of either apoptotic neutrophils or bacteria (Fig. 7D and E; Supplemental Fig. 2). We did not observe the effect of Ge, 17-DMAG, and cell-impermeable GeB on phagocytic activity of hMDMs. Only Rad treatment resulted in pronounced reduction of both SA and apoptotic PMNs engulfment, probably related to inhibition of c-Src kinase in a HSP90-independent manner [35]. Our data are reminiscent of results demonstrating that HSP90 was not required for recombinant adhesin of Neisseria meningitis (NadAD351–405) binding to monocytes, but was nevertheless engaged in the formation of a postreceptor complex that is necessary for cell activation and the ensuing induction of cytokines [36]. In addition, the same paper provided evidence that contribution of surface HSP90 in cell activation by NadAD351–405 was selective, affecting the secretion of most, although not all, cytokines. Sidera et al. [38] described the interaction of plasma-membrane associated HSP90 with extracellular domain of receptor tyrosine kinase HER2. An anti-HSP90 antibody significantly reduced phosphorylated forms of HER2. This interaction did not affect the expression and stability of HER2 in the cell membrane, but was necessary for HER2 activation resulting in MAPK and PI3K-Akt signaling, leading to actin rearrangement and cell mobility.
Although little is known about the function and origin of phagocyte surface HSP90, it is probably externalized as a closed HSP90 dimer (molecular clamp) holding a client protein. It is tempting to speculate that in such a way HSP90 protects receptor proteins and other membrane proteins during their transport to the cell surface. Novel structural research on HSP90-client complexes seem to provide a molecular basis for the speculation. Recently Verba et al. [39] revealed that HSP90 wedging itself between 2 client kinase lobes clamps around the unfolded client domain and forms a stable ternary complex. In light of our results the chaperoning function of surface HSP90 is indispensable during pattern recognition by monocytes and Mϕs, when signal transduction pathways from many heterologous receptors must be orchestrated to adjust the response.
We demonstrated that the modified response of hMDMs after treatment with HSP90 inhibitors cannot be attributed, either to reduced viability (Fig. 5) or to diminished expression of TLRs (Fig. 6) and their coreceptors (Table 2). Among chaperone proteins endoplasmic HSP90 paralog, Grp94 protein, has been shown to be a master chaperone of TLRs, which determine proper posttranslational maturation and surface transport of these proteins [40, 41]. Moreover, Randow and Seed [42] presented that cell surface exposition of TLR1, -2, and -4 and also some integrins (CD11a, CD18, and CD49d) is critically dependent on functional Grp94. In addition, short treatment with HSP90 inhibitor, Ge has been described to reduce CD14 expression (by enhancement of CD14 internalization and retention within the cell) in murine Mϕs J774 [43]. There is only limited data available with regard to regulation of surface expression of PRR receptors by HSP90 inhibitors in human Mϕs. In our hands, 4 h incubation either with HSP90 cell-permeable inhibitors (Rad, Ge, and 17-DAMG) or cell-impermeable GeB did not cause a significant change in the surface expression level of TLR and their coreceptors.
Reassuming, the present study demonstrates that HSP90, a cytoplasmic heat shock-induced chaperone, is constantly present at the surface of human monocytes and Mϕs. The cell-membrane HSP90 exposes its N-terminal (ATPase) domain, which binds specific mAbs. Such exposed HSP90 also binds Ge and that results in rapid recycling of the protein by endocytosis. For the first time, to our knowledge, we describe the translocation of cell-surface HSP90 on live hMDMs by binding of a fluorochrome-coupled Ge and subsequent confocal microscopy. Selective inhibition of cell-surface HSP90 pool suppresses the inflammatory cytokine response of Mϕ to PAMPs. Our identification of HSP90 as a crucial regulator of cytokine response to PAMPs suggests a potential new target for the treatment of inflammatory diseases associated with pattern recognition. It will also be interesting to investigate in further studies whether the cell-surface HSP90 is externalized with client proteins.
AUTHORSHIP
M.B. conducted the experiments, performed analyses, and wrote the manuscript; M.Z. provided expertise in confocal microscopy and image analysis; A.N., K.B., and M.Z. conducted the experiments; J.D. provided intellectual input and revised the manuscript; K.G. designed the study, analyzed data, and wrote the manuscript.
ACKNOWLEDGMENTS
This work was supported by Grants NN303 8086 40 (to M.B.) and UMO-2012/05/B/NZ6/00677 (to K.G.) from the National Science Centre (Kraków, Poland). Jagiellonian University is a partner of the Leading National Research Center (KNOW) supported by the Polish Ministry of Science and Higher Education.
Glossary
- 3D
3-dimensional
- 17-AAG
17-demethoxy-17-allylamino geldanamycin
- 17-DMAG
17-dimethylaminoethylamino-17-demethoxygeldanamycin
- ACAMP
apoptotic cell-associated molecular pattern
- APC
allophycocyanin
- CTAB
hexadecyltrimethyl ammonium bromide
- CD
cluster of differentiation
- FITC-Ge
FITC-conjugated geldanamycin
- FRET
fluorescence resonance energy transfer
- GDF
growth/differentiation factor
- Ge
geldanamycin
- GeB
biotinylated geldanamycin
- GM1
ganglioside-monosialic acid
- HER
human epidermal growth factor receptor
- hMDM
human monocyte-derived Mϕ
- HS
human serum
- HSP
heat shock protein
- IL-1RA
IL-1 receptor antagonist
- IP-10
IFN-γ-inducible protein 10
- LDH
lactate dehydrogenase
- MFI
mean fluorescence intensity
- MIG
monokine induced by γ-IFN
- Pam2
synthetic lipopeptide Pam2CysSerLys4
- Pam3
synthetic lipopeptide Pam3CysSerLys4
- PAMP
pathogen-associated molecular pattern
- PE-Cy7
phycoerythrin-cyanine 7
- PFA
paraformaldehyde
- PG
Porphyromonas gingivalis
- pgLPS
ultrapure P. gingivalis LPS
- PI
propidium iodide
- PMN
polymorphonuclear cell
- PRR
pattern recognition receptor
- Rad
radicicol
- RT
room temperature
- SA
Staphylococcus aureus
- stLPS
Escherichia coli 0127:B8 LPS
- StrAv-PE
phycoerythrin-conjugated streptavidin
- upLPS
ultrapure E. coli 011:B4 LPS
Footnotes
The online version of this paper, found at www.jleukbio.org, contains supplemental information.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Murray P. J., Wynn T. A. (2011) Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11, 723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green D. R., Ferguson T., Zitvogel L., Kroemer G. (2009) Immunogenic and tolerogenic cell death. Nat. Rev. Immunol. 9, 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Triantafilou M., Lepper P. M., Olden R., Dias I. S., Triantafilou K. (2011) Location, location, location: is membrane partitioning everything when it comes to innate immune activation? Mediators Inflamm. 2011, 186093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lauber K., Blumenthal S. G., Waibel M., Wesselborg S. (2004) Clearance of apoptotic cells: getting rid of the corpses. Mol. Cell 14, 277–287. [DOI] [PubMed] [Google Scholar]
- 5.Stuart L. M., Ezekowitz R. A. (2005) Phagocytosis: elegant complexity. Immunity 22, 539–550. [DOI] [PubMed] [Google Scholar]
- 6.Triantafilou K., Triantafilou M., Dedrick R. L. (2001) A CD14-independent LPS receptor cluster. Nat. Immunol. 2, 338–345. [DOI] [PubMed] [Google Scholar]
- 7.Pfeiffer A., Böttcher A., Orsó E., Kapinsky M., Nagy P., Bodnár A., Spreitzer I., Liebisch G., Drobnik W., Gempel K., Horn M., Holmer S., Hartung T., Multhoff G., Schütz G., Schindler H., Ulmer A. J., Heine H., Stelter F., Schütt C., Rothe G., Szöllôsi J., Damjanovich S., Schmitz G. (2001) Lipopolysaccharide and ceramide docking to CD14 provokes ligand-specific receptor clustering in rafts. Eur. J. Immunol. 31, 3153–3164. [DOI] [PubMed] [Google Scholar]
- 8.Triantafilou M., Triantafilou K. (2004) Heat-shock protein 70 and heat-shock protein 90 associate with Toll-like receptor 4 in response to bacterial lipopolysaccharide. Biochem. Soc. Trans. 32, 636–639. [DOI] [PubMed] [Google Scholar]
- 9.Triantafilou M., Miyake K., Golenbock D. T., Triantafilou K. (2002) Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J. Cell Sci. 115, 2603–2611. [DOI] [PubMed] [Google Scholar]
- 10.Ullrich S. J., Robinson E. A., Law L. W., Willingham M., Appella E. (1986) A mouse tumor-specific transplantation antigen is a heat shock-related protein. Proc. Natl. Acad. Sci. USA 83, 3121–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsutsumi S., Neckers L. (2007) Extracellular heat shock protein 90: a role for a molecular chaperone in cell motility and cancer metastasis. Cancer Sci. 98, 1536–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsutsumi S., Scroggins B., Koga F., Lee M. J., Trepel J., Felts S., Carreras C., Neckers L. (2008) A small molecule cell-impermeant Hsp90 antagonist inhibits tumor cell motility and invasion. Oncogene 27, 2478–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X., Yan Z., Huang L., Guo M., Zhang Z., Guo C. (2011) Cell surface heat shock protein 90 modulates prostate cancer cell adhesion and invasion through the integrin-β1/focal adhesion kinase/c-Src signaling pathway. Oncol. Rep. 25, 1343–1351. [DOI] [PubMed] [Google Scholar]
- 14.Panzarini E., Inguscio V., Fimia G. M., Dini L. (2014) Rose Bengal acetate photodynamic therapy (RBAc-PDT) induces exposure and release of Damage-Associated Molecular Patterns (DAMPs) in human HeLa cells. PLoS One 9, e105778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taipale M., Jarosz D. F., Lindquist S. (2010) HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 11, 515–528. [DOI] [PubMed] [Google Scholar]
- 16.Röhl A., Rohrberg J., Buchner J. (2013) The chaperone Hsp90: changing partners for demanding clients. Trends Biochem. Sci. 38, 253–262. [DOI] [PubMed] [Google Scholar]
- 17.Chen B., Piel W. H., Gui L., Bruford E., Monteiro A. (2005) The HSP90 family of genes in the human genome: insights into their divergence and evolution. Genomics 86, 627–637. [DOI] [PubMed] [Google Scholar]
- 18.Buchner J. (1999) Hsp90 & Co. - a holding for folding. Trends Biochem. Sci. 24, 136–141. [DOI] [PubMed] [Google Scholar]
- 19.Welch W. J., Feramisco J. R. (1982) Purification of the major mammalian heat shock proteins. J. Biol. Chem. 257, 14949–14959. [PubMed] [Google Scholar]
- 20.Whitesell L., Lindquist S. L. (2005) HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 5, 761–772. [DOI] [PubMed] [Google Scholar]
- 21.Roe S. M., Prodromou C., O’Brien R., Ladbury J. E., Piper P. W., Pearl L. H. (1999) Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J. Med. Chem. 42, 260–266. [DOI] [PubMed] [Google Scholar]
- 22.Neckers L., Trepel J. B. (2014) Stressing the development of small molecules targeting HSP90. Clin. Cancer Res. 20, 275–277. [DOI] [PubMed] [Google Scholar]
- 23.Byrd C. A., Bornmann W., Erdjument-Bromage H., Tempst P., Pavletich N., Rosen N., Nathan C. F., Ding A. (1999) Heat shock protein 90 mediates macrophage activation by Taxol and bacterial lipopolysaccharide. Proc. Natl. Acad. Sci. USA 96, 5645–5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu H. Y., Wu H. L., Tan S. K., Li V. P., Wang W. T., Hsu J., Cheng C. H. (2007) Geldanamycin interferes with the 90-kDa heat shock protein, affecting lipopolysaccharide-mediated interleukin-1 expression and apoptosis within macrophages. Mol. Pharmacol. 71, 344–356. [DOI] [PubMed] [Google Scholar]
- 25.Shimp S. K. III, Parson C. D., Regna N. L., Thomas A. N., Chafin C. B., Reilly C. M., Nichole Rylander M. (2012) HSP90 inhibition by 17-DMAG reduces inflammation in J774 macrophages through suppression of Akt and nuclear factor-κB pathways. Inflamm. Res. 61, 521–533. [DOI] [PubMed] [Google Scholar]
- 26.Luikart S. D., Panoskaltsis-Mortari A., Hinkel T., Perri R. T., Gupta K., Oegema T. R., Gupta P. (2009) Mactinin, a fragment of cytoskeletal alpha-actinin, is a novel inducer of heat shock protein (Hsp)-90 mediated monocyte activation. BMC Cell Biol. 10, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatterjee A., Dimitropoulou C., Drakopanayiotakis F., Antonova G., Snead C., Cannon J., Venema R. C., Catravas J. D. (2007) Heat shock protein 90 inhibitors prolong survival, attenuate inflammation, and reduce lung injury in murine sepsis. Am. J. Respir. Crit. Care Med. 176, 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bzowska M., Guzik K., Barczyk K., Ernst M., Flad H. D., Pryjma J. (2002) Increased IL-10 production during spontaneous apoptosis of monocytes. Eur. J. Immunol. 32, 2011–2020. [DOI] [PubMed] [Google Scholar]
- 29.Bzowska M., Hamczyk M., Skalniak A., Guzik K. (2011) Rapid decrease of CD16 (Fcγcpid) expression on heat-shocked neutrophils and their recognition by macrophages. J. Biomed. Biotechnol. 2011, 284759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider C. A., Rasband W. S., Eliceiri K. W. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bantel H., Sinha B., Domschke W., Peters G., Schulze-Osthoff K., Jänicke R. U. (2001) alpha-Toxin is a mediator of Staphylococcus aureus-induced cell death and activates caspases via the intrinsic death pathway independently of death receptor signaling. J. Cell Biol. 155, 637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith V., Sausville E. A., Camalier R. F., Fiebig H. H., Burger A. M. (2005) Comparison of 17-dimethylaminoethylamino-17-demethoxy-geldanamycin (17DMAG) and 17-allylamino-17-demethoxygeldanamycin (17AAG) in vitro: effects on Hsp90 and client proteins in melanoma models. Cancer Chemother. Pharmacol. 56, 126–137. [DOI] [PubMed] [Google Scholar]
- 33.Tian Z. Q., Liu Y., Zhang D., Wang Z., Dong S. D., Carreras C. W., Zhou Y., Rastelli G., Santi D. V., Myles D. C. (2004) Synthesis and biological activities of novel 17-aminogeldanamycin derivatives. Bioorg. Med. Chem. 12, 5317–5329. [DOI] [PubMed] [Google Scholar]
- 34.Onuoha S. C., Mukund S. R., Coulstock E. T., Sengerovà B., Shaw J., McLaughlin S. H., Jackson S. E. (2007) Mechanistic studies on Hsp90 inhibition by ansamycin derivatives. J. Mol. Biol. 372, 287–297. [DOI] [PubMed] [Google Scholar]
- 35.Pillay I., Nakano H., Sharma S. V. (1996) Radicicol inhibits tyrosine phosphorylation of the mitotic Src substrate Sam68 and retards subsequent exit from mitosis of Src-transformed cells. Cell Growth Differ. 7, 1487–1499. [PubMed] [Google Scholar]
- 36.Cecchini P., Tavano R., Polverino de Laureto P., Franzoso S., Mazzon C., Montanari P., Papini E. (2011) The soluble recombinant Neisseria meningitidis adhesin NadA(Δ351-405) stimulates human monocytes by binding to extracellular Hsp90. PLoS One 6, e25089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saito K., Kukita K., Kutomi G., Okuya K., Asanuma H., Tabeya T., Naishiro Y., Yamamoto M., Takahashi H., Torigoe T., Nakai A., Shinomura Y., Hirata K., Sato N., Tamura Y. (2015) Heat shock protein 90 associates with Toll-like receptors 7/9 and mediates self-nucleic acid recognition in SLE. Eur. J. Immunol. 45, 2028–2041. [DOI] [PubMed] [Google Scholar]
- 38.Sidera K., Gaitanou M., Stellas D., Matsas R., Patsavoudi E. (2008) A critical role for HSP90 in cancer cell invasion involves interaction with the extracellular domain of HER-2. J. Biol. Chem. 283, 2031–2041. [DOI] [PubMed] [Google Scholar]
- 39.Verba K. A., Wang R. Y., Arakawa A., Liu Y., Shirouzu M., Yokoyama S., Agard D. A. (2016) Atomic structure of Hsp90-Cdc37-Cdk4 reveals that Hsp90 traps and stabilizes an unfolded kinase. Science 352, 1542–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Y., Liu B., Dai J., Srivastava P. K., Zammit D. J., Lefrançois L., Li Z. (2007) Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity 26, 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu B., Yang Y., Qiu Z., Staron M., Hong F., Li Y., Wu S., Li Y., Hao B., Bona R., Han D., Li Z. (2010) Folding of Toll-like receptors by the HSP90 paralogue gp96 requires a substrate-specific cochaperone. Nat. Commun. 1, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Randow F., Seed B. (2001) Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat. Cell Biol. 3, 891–896. [DOI] [PubMed] [Google Scholar]
- 43.Vega V. L., De Maio A. (2003) Geldanamycin treatment ameliorates the response to LPS in murine macrophages by decreasing CD14 surface expression. Mol. Biol. Cell 14, 764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]