PMN-expressed Lea represents a novel glycan target for modulation of PMN trafficking to mucosal tissues during inflammation.
Keywords: mucosal inflammation, transepithelial migration, neutrophil activation
Abstract
PMN-expressed fucosylated glycans from the Lewis glycan family, including Lewis-x (Lex) and sialyl Lewis-x (sLex), have previously been implicated in the regulation of important PMN functions, including selectin-mediated trafficking across vascular endothelium. Although glycans, such as Lex and sLex, which are based on the type 2 sequence (Galβ1-4GlcNAc-R), are abundant on PMNs, the presence of type 1 Galβ1-3GlcNAc-R glycans required for PMN expression of the closely related stereoisomer of Lex, termed Lewis-A (Lea), has not, to our knowledge, been reported. Here, we show that Lea is abundantly expressed by human PMNs and functionally regulates PMN migration. Using mAbs whose precise epitopes were determined using glycan array technology, Lea function was probed using Lea-selective mAbs and lectins, revealing increased PMN transmigration across model intestinal epithelia, which was independent of epithelial-expressed Lea. Analyses of glycan synthetic machinery in PMNs revealed expression of β1-3 galactosyltransferase and α1–4 fucosyltransferase, which are required for Lea synthesis. Specificity of functional effects observed after ligation of Lea was confirmed by failure of anti-Lea mAbs to enhance migration using PMNs from individuals deficient in α1–4 fucosylation. These results demonstrate that Lea is expressed on human PMNs, and its specific engagement enhances PMN migration responses. We propose that PMN Lea represents a new target for modulating inflammation and regulating intestinal, innate immunity.
Introduction
PMNs are critical, innate immune cells, which are essential for successful elimination of invading pathogens. PMNs also have an important role in wound healing and restitution of mucosal homeostasis at later stages of the inflammatory response. However, dysregulated or excessive PMN influx can result in bystander tissue damage, which is pathognomonic of numerous mucosal inflammatory disorders. During inflammation/infection, circulating PMNs exit the microcirculation through a sequential extravasation cascade that encompasses PMN capture, rolling, activation, adhesion, and intraluminal crawling [1].
It is well documented that some ligand-receptor recognition interactions during PMN extravasation are controlled by posttranslational glycosylation modifications. For example, glycans on P-selectin glycoprotein ligand 1 have a key role in regulating PMN rolling on the vascular endothelium during inflammatory responses in vivo. The glycans of P-selectin glycoprotein ligand 1 have been extensively studied, and several key glycan modifications (including α1,3 fucosylation, α2,3 sialylation, and β1,4 galactosylation) have been identified as being important for mediating PMN capture and endothelial rolling [2]. In addition, endothelial P-selectin and E-selectin contain binding sites for PMN fucose, containing Lewis family glycans, including sLex [Neu5Acα2-3Galβ1-4(Fucα1-3)GlcNAcβ-R] and Lex [Galβ1-4(Fucα1-3)GlcNAcβ-R] [3].
Following extravasation, PMNs must migrate through the interstitium and basement membranes before crossing epithelial barriers that line organs such as the lungs and intestine. Although the role of glycan-binding interactions in mediating key steps in PMN transendothelial migration is well accepted, much less is known about the role of glycans once PMNs have exited the microcirculation to undergo the process of migration into epithelial-lined organs. We recently showed that targeting glycans terminating in sLea [Neu5Acα2-3Galβ1-3(Fucα1-4)GlcNAcβ-R] on the apical, epithelial glycoprotein CD44v6 prevents shedding of the CD44v6 extracellular domain and results in PMN accumulation on the apical (luminal) epithelial surface [4, 5]. Similarly, targeting of the related Lewis family member Lex, which is selectively expressed on PMN but not epithelial cells, also inhibits PMN TEM [6].
Despite the importance of Lewis glycans in facilitating both PMN transendothelial migration and PMN trafficking across mucosal epithelium, several Lewis glycans, including Lea have yet to be examined in the context of PMN trafficking. Lea is a blood group Ag glycan, which is usually attached at the nonreducing ends of glycoconjugates and has a role both in cell–cell communication and cell adhesion [7–11]. Further, although Lewis glycans based on the type 2 Lewis sequence (Galβ1-4GlcNAc-R), including Lex, are abundantly expressed on PMNs, the presence of type 1 Galβ1-3GlcNAc-R glycans, which are required for the expression of Lea by PMNs, has not yet, to our knowledge, been investigated.
Here, we show that human PMNs express the glycosyltransferases necessary for Lea synthesis as well as multiple glycoproteins containing terminal Lea glycan structures. Importantly, specific mAb and lectin targeting of Lea increases PMN transepithelial migration, thus demonstrating an important (novel) role for this glycan during PMN intestinal trafficking.
MATERIALS AND METHODS
Antibodies and reagents
Anti-Lea mAb clone 7LE, anti-CD18 mAb, anti-Fut3 mAb, anti-β3GalT1 mAb, and anti-β3GalT4 mAb were purchased from Abcam Inc. (Cambridge, MA, USA). A second anti-Lea mAb (clone SPM 522) was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Antitubulin mAb, Lea trisaccharide, and fMLF were purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-Lex mAb clones H198 and W6D3 were purchased from BD Biosciences (Franklin Lakes, NJ, USA). The anti-CD11b Ab CBRM1/29 and the anti-JAMA mAb J10.4 have been characterized elsewhere [12, 13]. IL-8 and recombinant human MBL were purchased from R&D Systems (Minneapolis, MN, USA). PNGaseF was purchased from New England BioLabs (Ipswich, MA, USA). Primers for Fuc-T3, β3GalT1, β3GalT4, and MBP were purchased from OriGene Technologies (Rockville, MD, USA). Primers for GAPDH were purchased from Integrated DNA Technologies (Coralville, IA, USA). Polymorphprep was purchased from Axis-Shield (Alere Technologies, Oslo, Norway).
Cell culture and PMN isolation
Cultures of T84 and Caco2 IECs (passages 63–68) were grown, as previously described [5, 14]. HL60 cells were obtained from the ATCC (Rockville, MD, USA) and were passaged in RPMI-1640 medium containing 20% heat-inactivated FBS (Atlanta Biologicals, Flowery Branch, GA, USA) with supplements and differentiated with DMSO, as described previously [15]. PMNs were isolated from whole blood obtained from healthy human volunteers, with approval from the Emory University and University of Michigan institutional review boards on human subjects, using a previously described density-gradient centrifugation technique [4]. Briefly, whole blood was layered onto Polymorphprep (Alere Technologies) at a ratio of 1:1 and centrifuged at 500 g for 50 min. The PMN-containing layer was carefully collected, and any contaminating erythrocytes were removed by lysis with ice-cold, sterile water. PMNs were then resuspended in HBSS with 10 mM HEPES, pH 7.4, and without Ca2+ or Mg2+, at a concentration of 5 × 107 cells/ml. PMNs isolated in this way were 97% pure and >95% viable and were used for all assays within 2 h of blood draw. For analysis of PMN gene transcription, eosinophils were removed from isolated PMNs using the Human Eosinophil Isolation kit (Miltenyi Biotec, San Diego, CA, USA) according to the manufacturer’s protocol.
Characterization of Lewis blood group status among RBC donors
Whole blood was isolated from healthy donors via venipuncture into a standard adenine, citrate, and dextrose solution used to immunophenotype an individual’s blood group status. A 3% hematocrit solution was then prepared and incubated with monoclonal anti-Lea or anti-Leb (Immucor, Norcross, GA, USA) using standard blood bank practices. Briefly, RBCs were incubated with anti-Lea, anti-Leb, or no Ab for 20 min at room temperature, followed by centrifugation at 300 g. Cells were then evaluated under a microscope for agglutination. Positive agglutination reactions are used to determine Ag positivity. All experiments were conducting using Emory institutional review board–approved procedures.
Analysis of mAb specificity by glycan microarray assay
The anti-Lea Ab 7LE was submitted to the CFG (http://www.functionalglycomics.org; Scripps Research Institute, San Diego, CA, USA) at 2 μg/ml and screened for glycoepitope binding using the version 5.1 glycan array (CFG). This glycan microarray contains 610 individual glycans printed on activated glass slides, representing a library of known, natural and synthetic, mammalian glycans, in replicates of 6. After washing, binding of Abs to specific glycan epitopes was detected with Alexa Fluor 488–labeled goat anti-mouse IgG (Thermo Fisher Scientific) as described previously [5, 16]. The RFUs of the bound Ab–glycan complexes were detected on a PerkinElmer (Waltham, MA, USA) ProScanArray 4 laser scanner and quantified using ImaGene software (BioDiscovery, El Segundo, CA, USA).
Immunoblotting and protein purification
PMN cell lysates for immunoblotting were prepared with the following lysis buffer (20 mM Tris, pH 7.5; 150 mM NaCl; 1 mM EDTA; 1% TX-100; 1 mM Na3VO4; and 1 mM PMSF) supplemented with 10% mammalian tissue protease inhibitor cocktail (Sigma-Aldrich). PMN cell lysates were boiled in SDS-PAGE sample buffer under reducing conditions, and then subjected to SDS-PAGE followed by transfer to PVDF under standard conditions. Membranes were blocked with 4% milk powder or 3% BSA and incubated with 1 μg/ml of indicated mAb. Primary Ab binding was detected using appropriate HRP-linked secondary Abs (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). N-glycans were removed from PMN protein lysate PNGaseF, according to manufacturer’s instructions. Immunoblots are representative of PMNs isolated from 5 independent donors. Functionally active, human CD11b/CD18 was purified from PMNs by LM2/1 immunoaffinity chromatography as described previously [3, 17] and immunoblotted using anti-Lex, anti-Lea, anti-CD18, and anti-CD11b mAbs.
PMN and intestinal epithelial cell immunostaining
Isolated PMNs were fixed with 3.7% paraformaldehyde and, where indicated, permeabilized with 0.5% Triton-X. After blocking with 3% BSA, PMNs were incubated with 10 μg/ml of the indicated Abs. After washing, PMNs were incubated with an FITC-labeled secondary Ab and mounted in ProLong antifading embedding solution (Thermo Fisher Scientific). Nuclei were visualized by staining with TO-PRO-1 (Thermo Fisher Scientific). Images shown are representative of PMN from ≥3 independent PMN donors with multiple images captured per donor.
Immunofluorescent labeling of IECs was achieved as follows. T84 and Caco2 monolayers were fixed using methanol and, subsequently, blocked with 3% BSA. Monolayers were then incubated with primary Abs (10 μg/ml) and fluorescently labeled secondary Abs. After Ab incubations, IECs were mounted in ProLong antifading embedding solution. Images shown are representative of ≥3 experiments with multiple images taken per monolayer using a Leica Inverted SP5 Confocal Microscope System (Leica Biosystems, Bannockburn, IL, USA).
PMN transmigration assays
For transepithelial migration experiments, IECs were grown on collagen-coated, permeable, 0.33-cm2, polycarbonate filters (5 μm pore size; CoStar Group, Washington DC, USA), as described previously [4, 14, 18]. All epithelial migration experiments were performed in the physiologically relevant basolateral-to-apical direction (i.e., inverted monolayers), in the presence of a chemotactic gradient of 100 nM fMLF. For migration experiments, 1 × 106 PMNs pretreated with 10 μg/ml of the indicated Abs, lectins, or soluble Lewis A trisaccharide were added to the upper chambers of transwell inserts and migration was measured at 37°C for 1 h. Transmigrated PMNs were quantified by assaying for the PMN azurophilic marker myeloperoxidase, as published previously [18]. Briefly, Triton X-100 was added to the lower reservoir of the transwell (final concentration 0.5%), and the pH was adjusted to 4.2 with citrate buffer. For each sample, color development was assayed at 405 nm on a microtiter plate reader after mixing equal parts of sample and a solution containing 1 mM 2,2′-azino-di-(3-ethyl)dithiazoline sulfonic acid and 10 mM H2O2 in 100 nM citrate buffer pH 4.2. The assay was standardized with known dilutions of the same PMNs used in each experiment and was linear in the range used (0.05 × 106–1 × 106 PMNs). The percentage of migration represents the percentage of the 1 × 106 PMNs added (for each sample) to the upper portion of the transwell system. For PMN chemotaxis assays, PMNs were incubated with 10 μg/ml of the indicated Abs, lectins, or soluble Lewis A trisaccharides before migration across collagen-coated, permeable, 0.33-cm2, polycarbonate filters to 100 nm fMLF, or 100 nM IL-8 was assessed by measurement myeloperoxidase, as described above. Data are means ± se (n = 5).
Flow cytometry and phagocytosis analyses
For flow cytometry analyses, nonstimulated PMNs or PMNs stimulated with 10 nM fMLF were blocked in 3% BSA with Human TruStain FcX (FC Receptor Blocking Solution; BioLegend, San Diego, CA, USA) before incubation with 10 μg/ml anti-Lea mAb, 10 μg/ml anti-CD11b mAb, 10 μg/ml anti-Lex mAb, or an IgG isotype-matched control mAb. Following primary Ab incubation, PMNs were washed and incubated with relevant, fluorescently labeled, secondary mAbs and fixed in 3.7% paraformaldehyde, before analysis by flow cytometry. Flow cytometric analysis was carried out using a FACScan (Becton Dickinson, Franklin Lakes, NJ, USA), equipped with an argon ion laser tuned at a 488-nm wavelength. Data are representative of PMNs isolated from 3–5 healthy donors. For analysis of changes in surface expression of Lex and CD18/CD11b, PMNs were incubated with 10 μg/ml anti-Lea mAb (7LE), 10 μg/ml isotype matched control mAb, or 10 nm fMLF, before blocking in 3% BSA with Human TruStain FcX and subsequent incubation with 10 μg/ml FITC-labeled anti-Lex and FITC-labeled anti-CD11b mAbs. For phagocytosis assays, PMNs were incubated with 10 μg/ml relevant Abs and FITC-conjugated FluoSpheres (Thermo Fisher Scientific) at a ratio of 1:100 (PMN to FluoSpheres) in the presence of 10 nM fMLF, 10 μg/ml anti-Lea mAb (7LE), 10 μg/ml IgG isotype-matched control mAb, or 10 μg/ml MBL for 30 min at 37°C. Uptake of FluoSpheres by PMNs was assessed by flow cytometry using an argon laser tuned at a 488-nm wavelength.
Transcriptional analysis
HL60 cells or human PMNs were lysed in TRIzol (Thermo Fisher Scientific) then subjected to phenol-chloroform extraction, according to the manufacturer’s protocol [19]. RNA was digested with DNaseI (Ambion, Austin, TX, USA) to remove contamination with genomic DNA; then, DNA was synthesized by reverse transcription using oligo(dt12–18) primers and Superscript II reverse transcriptase (Thermo Fisher Scientific). Real-time PCR was performed with a MyIQ real-time PCR machine and SYBR Green supermix (Bio-Rad Laboratories, Hercules, CA, USA). Data were analyzed by the ΔΔCt threshold cycle method and normalized to the housekeeping gene GAPDH. Data are means ± se from 5 healthy blood donors (n = 5).
Data analysis
Statistical differences were determined by 2-factor ANOVA using PRISM 5 for Mac OSX (version 5.0a 1992–1998; GraphPad Software, La Jolla, CA, USA). Values are expressed as the means ± se from ≥3 separate experiments.
RESULTS
PMNs express the type 1 glycan Lea
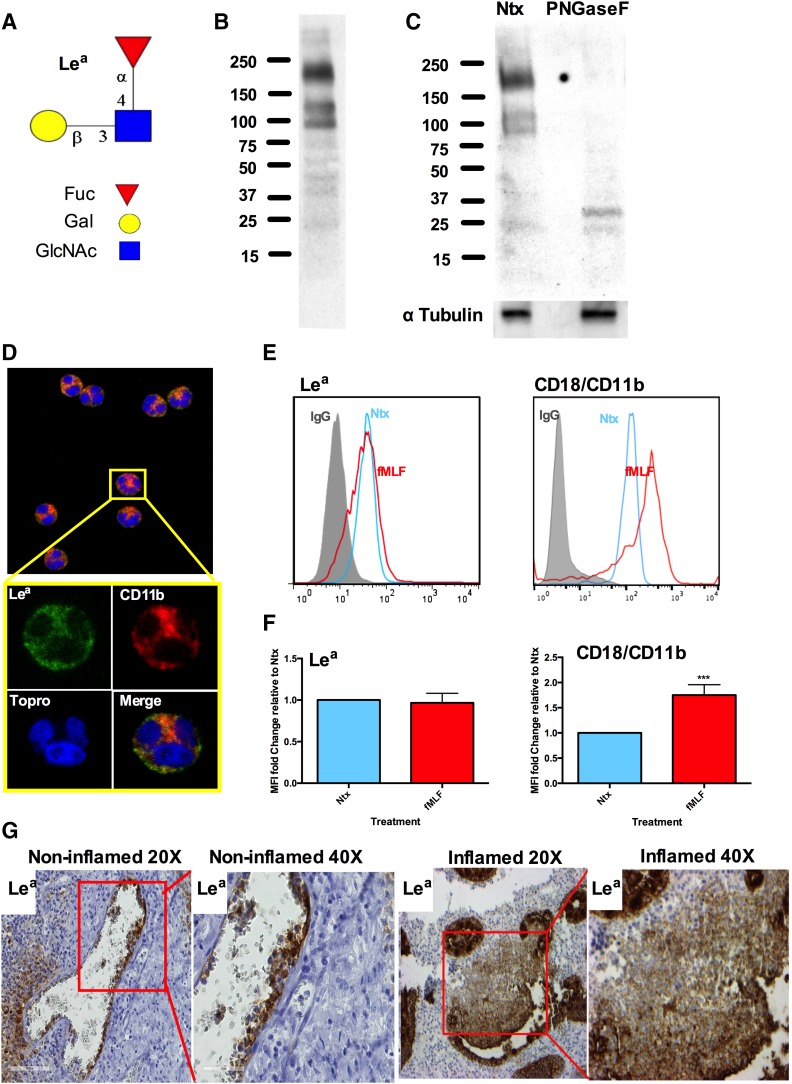
Although it has been widely reported that PMNs express type 2 Lewis glycans, including Lex and sLex, abundantly [6, 20], and that these glycans regulate important functions, including migration, phagocytosis, and degranulation, it is not known whether PMNs express the type 1 Lewis glycan Lea (Fig. 1A) and what its function might be. Immunoblotting of lysates from human PMNs revealed expression of several large Lea glycans containing glycoproteins between 100 and 250 kDa (Fig. 1B). To determine whether PMN-expressed Lea was displayed as part of larger N- or O-linked glycan structures, PMN lysates were treated with PNGaseF, an N-glycosidase that cleaves all types of asparagine-bound N-glycans. As shown in Fig. 1C, PNGaseF treatment prevented detection of PMN Lea by immunoblotting, suggesting that this trisaccharide is displayed as part of larger N-linked PMN glycan structures.
Figure 1. Human PMN express N-glycan-linked Lea.
(A) Schematic of Lea trisaccharide glycan. (B) Immunoblot of lysates from human PMNs showing expression of multiple Lea-containing glycoproteins. Data shown are representative of blotting from PMNs isolated from 5 independent donors. (C) Treatment with PNGaseF removes N-glycan–linked Lea from human PMN glycoproteins. Data shown are representative of blotting from PMNs isolated from 5 independent donors. (D) Immunofluorescence showing expression of Lea in permeabilized human PMNs with Lea in green and CD18/CD11b in red. Data shown are representative of imaging from PMNs isolated from 5 independent donors. (E) Representative flow plots showing surface expression of Lea and CD118/CD11b on nonstimulated (Ntx) PMNs (blue) and 10-nm fMLF-treated PMNs (red) relative to an IgG control (gray). (F) Quantification of changes in surface expression of Lea and CD18/CD11b after stimulation with fMLF. Data expressed as mean fluorescence intensity are means ± se from n = 5 independent blood donors. ***P < 0.001. (G) Immunohistochemistry demonstrating expression of Lea on circulating PMNs and on PMNs in the inflamed intestinal mucosa of individuals with ulcerative colitis (original magnifications, ×20 and ×40). Images are representative of tissue extracted from n = 3 human tissue samples.
Surface expression of Lea on PMNs was confirmed by immunofluorescence staining and flow cytometry (Fig. 1D and E). No increase in expression of Lea was observed after stimulation with 10 nm fMLF (Fig. 1E and F). As a positive control for fMLF-mediated PMN activation, surface expression of the β2 integrin CD18/CD11b was also measured. In contrast to the unchanging expression of Lea, there was a significant increase in surface expression of CD18/CD11b after PMN activation with fMLF (Fig. 1E and F; ***P > 0.001) suggesting that PMN Lea glycans are displayed on proteins other than CD18/CD11b. Additional confirmation of PMN Lea expression was obtained by immunohistochemical analysis of human tissue sections containing inflamed and noninflamed intestinal mucosa. In addition to the expected expression of Lea on intestinal epithelial cells [21, 22], expression of Lea was also observed on circulating PMNs in noninflamed tissue as well as on infiltrating PMNs in the intestinal mucosa of individuals with ulcerative colitis (Fig. 1G).
Binding specificity of anti-Lea mAb 7LE
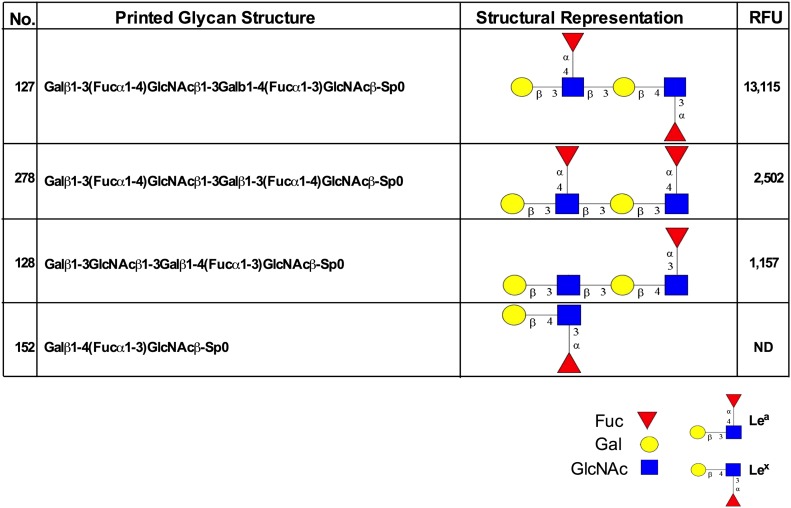
To exclude the possibility of nonspecific binding of anti-Lea mAbs to the closely related PMN-expressed Lewis glycan stereoisomer Lex, glycan array analyses were performed. Such analyses afford identification of highly specific presentations of Lea glycans recognized by anti-Lea mAbs at a molecular/structural level. The anti-Lea mAb 7LE was assayed for binding to a panel of 610 mammalian glycan structures using the version 5.1 glycan microarray from the CFG. Analyses revealed that 7LE bound with highest affinity to glycans expressing Galβ1-3(Fucα1-4)GlcNAc (Lea), in which the Lea structure was expressed in a terminal, nonreducing position (13,115 RFU, glycan 127; Fig. 2). The 7LE also recognized glycans containing terminal Lea determinants in repeating poly-Lea structures (2502 RFU, glycan 278). Importantly, there was no recognition by the anti-Lea mAb of the stereoisomer glycan structure Lex (glycan 152; Fig. 2). Further, 7LE showed no binding to glycans containing only an internal Lea sequence (glycans 395, 473, Supplemental Fig. 1). Interestingly, 7LE also failed to recognize glycans terminating with the sialylated version of Lea (sLea) (glycans 240, 331, 239). Taken together, these results highlight the highly restricted and differential recognition of discrete, terminal Lea glycan structures by the anti-Lea mAb 7LE.
Figure 2. Glycan array analysis demonstrating exquisite specificity of an anti-Lea mAb 7LE.
The mAb 7LE (2 μg/ml) was incubated with an array of 610 glycan structures (version 5.1 glycan microarray; CFG) and detected with Alexa Fluor-488–labeled anti-mouse IgG. As shown, 7LE specifically recognized glycans terminating in Lea. ND, not detectable (<500 RFU); SP, spacer.
PMNs express glycosyltransferases required for Lea biosynthesis
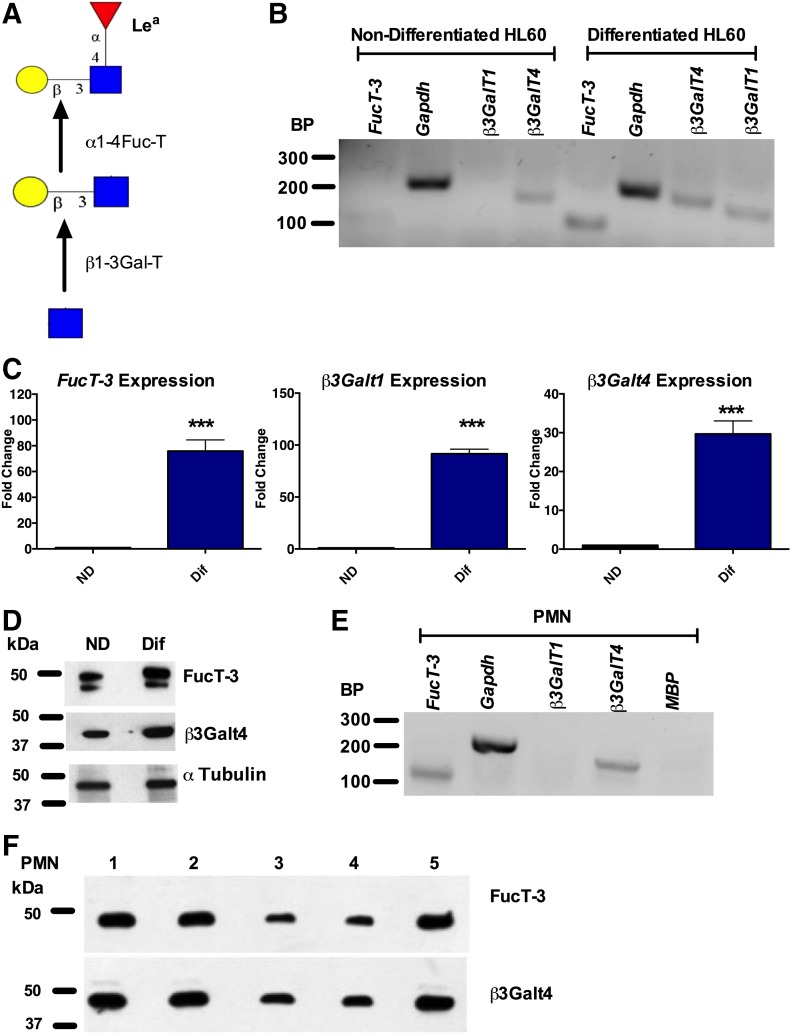
Having demonstrated specific recognition of Lea by the anti-Lea mAb 7LE, as well as Lea expression by human PMNs, we next examined changes in expression of glycosyltransferases required for generation of Lea, including an α1-4 fucosyltransferase and a β1-3 galactosyltransferase [23] (Fig. 3A) in ND vs. Dif HL60 cells. Interestingly, increased expression of the α1,4 fucosyltransferase FUT3 as well as the galactosyltransferase β3Galt1 was observed when HL60 cells were Dif toward a PMN-like state (Fig. 3B). These differences in expression were further quantified by real-time PCR. Analyses revealed an 80-fold increase in the expression of FUT3 (***P < 0.001), a 90-fold increase in the expression of β3Galt1 (*** P < 0.001), and a 30-fold increase in the expression of β3Galt4 (*** P < 0.001) in Dif HL60 cells relative to ND HL60 cells (Fig. 3C). Analysis of protein expression revealed a similar increase in the expression of FUT3 and β3Galt4 upon HL60 differentiation (Fig. 3D). Analysis of gene expression in human PMNs confirmed expression of the α1,4 fucosyltransferase FUT3 as well as the galactosyltransferase β3Galt4 (Fig. 3E). However, in contrast to Dif HL60 cells, no expression of β3Galt1 was observed in human PMNs (Fig. 3E). The absence of contaminating eosinophils in PMN cDNA samples was confirmed by the lack of expression of the eosinophil marker MBP. Robust protein expression of both FUT3 and β3Galt4 was observed in PMNs from 5 healthy donors (Fig. 3F).
Figure 3. Glycosyltransferases that synthesize Lea are expressed by differentiated HL60 cells and by human PMNs.
(A) Schematic outlining the glycosyltransferases required for Lea synthesis. PCR (B) and real-time PCR (C) analyses show increased expression of β3Galt1, β3Galt4, and FUT3 in differentiated (Dif) vs. nondifferentiated (ND) HL60 cells. Real-time PCR results are depicted as the fold change relative to ND HL60s and are normalized for expression of the housekeeping gene GAPDH. Data are means ± se from n = 4 HL60 differentiation assays. ***P < 0.001. (D) Immunoblots showing increased protein expression of β3Galt4 and FUT3 in Dif (D) vs. ND HL60 cells. Blots are representative from n = 4 HL60 differentiation assays. PCR (E) and immunoblotting (F) analyses demonstrate expression of FUT3 and β3Galt4 in human PMN. As a control for removal of eosinophils expression of MBP, an eosinophil marker was also examined. Data shown are representative of results obtained from n = 5 independent blood donors.
Selective targeting of Lea increases PMN migration
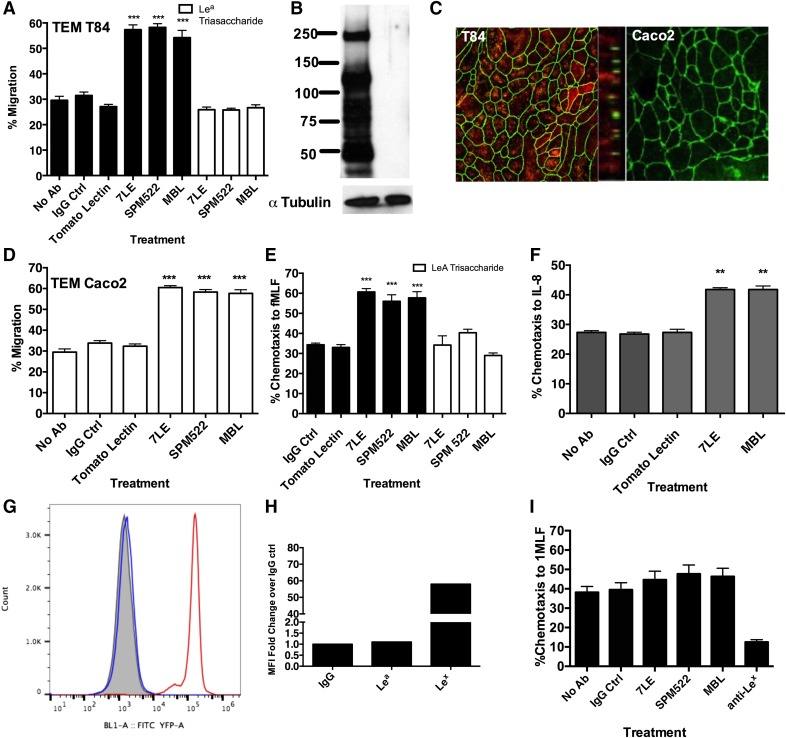
Glycan mediated-binding interactions have previously been shown to have a role in PMN trafficking across both endothelial and epithelial barriers [2, 3, 5, 6]. Therefore, we next examined the role of Lea in regulating PMN TEMs. As shown in Fig. 4A, specific mAb engagement of terminal Lea glycans with the anti-Lea mAb 7LE (10 μg/ml) resulted in a significant increase in PMN TEMs across T84 IECs relative to PMN treated with no mAbs or with 10 μg/ml J10.4, an IgG matched-binding control mAb (***P < 0.001). A similar increase in PMN TEMs was observed after incubation of PMN with 10 μg/ml of a second anti-Lea mAb (SPM522; ***P < 0.001). In addition, targeting of Lea with 1 of its physiologic ligands (MBL, 10 μg/ml) also increased PMN TEMs relative to treatment with a non-Lea binding lectin (Tomato lectin, ***P < 0.001, 10 μg/ml). Specificity of MBL and anti-Lea mAbs for Lea was demonstrated by incubating PMNs with 10 μg/ml anti-Lea mAbs/lectins in the presence of soluble Lea trisaccharide (10 μg/ml). As shown in Fig. 4A, incubation with soluble Lea trisaccharide prevented all mAb/lectin-mediated increases in PMN trafficking.
Figure 4. Engagement of PMN Lea increases TEM and transmigration.
(A) Anti-Lea mAbs 7LE (10 μg/ml) and SPM522 (10 μg/ml) and the Lea-biding lectin MBL (10 μg/ml) increase PMN TEM to the chemoattractant fMLF (100 nM) across T84 IECs relative to IgG or lectin controls. Data shown are means ± se using PMNs from n = 5 independent donors. ***P < 0.001. (B) Immunoblot demonstrating expression of Lea in T84, but not Caco2, IECs. Data shown are representative of n = 3 Western blots. (C) Confluent T84 or Caco2 monolayers were costained with 10 μg/ml anti-ZO1 (green) or 10 μg/ml anti Lea mAb 7LE (red). Protein localization was determined by confocal microscopy analysis. Representative images from n = 3 experiments are shown both en face and in the x–z plane of the section. (D) Anti-Lea mAbs 7LE (10 μg/ml) and SPM522 (10 μg/ml) and the Lea-biding lectin MBL (10 μg/ml) increase PMN TEM to fMLF (100 nM) across Caco2 IECs. Data are means ± se from n = 3 blood donors. ***P < 0.001. Engagement of Lea increases PMN transmigration across collagen-coated transwells to fMLF (100 nM) (E) or IL-8 (100 nM) (F). Data are means ± se from n = 5 blood donors. ***P < 0.001; **P < 0.01. (G) Flow plot of PMN from a Lea-negative blood donor comparing surface expression of Lea (blue) and Lex (red), relative to an IgG control (gray). (H) Quantification of flow-plot data showing lack of surface expression of Lea in the PMN from a Lea-negative donor. (I) No effect on PMN transmigration across collagen-coated transwells to 100 nm fMLF was observed when PMN from a Lea-negative donor were treated with 10 μg/ml anti-Lea mAbs (7LE/SPM522) or 10 μg/ml MBLs. Ctrl, control.
To confirm that anti-Lea mAbs/MBL were specifically targeting PMN-expressed Lea, expression of Lea in T84 and Caco2 IECs were next examined. Western blotting revealed robust expression of numerous Lea-containing glycoproteins by T84 IECs. In contrast, there was no detectable expression of Lea-containing glycans in Caco2 IECs (Fig. 4B). Confocal microscopy analyses (Fig. 4C) confirmed the lack of expression of Lea in Caco2 IECs and revealed non tight junction associated, apical expression of Lea in T84 IECs. Further, the lack of Lea expression on Caco2 IECs did not prevent the increase in PMN TEMs observed downstream of the ligation of Lea by anti-Lea mAbs or MBL (***P < 0.001, Fig. 4D), suggesting that it is Lea expressed on PMN that is crucial for mediating the enhanced transepithelial migration. The effects of directly targeting PMN Lea on PMN trafficking were also studied in transmigration assays, where PMNs migrate across acellular, collagen-coated transwell filters. These data demonstrate that targeting of PMN-expressed Lea with 10 μg/ml anti-Lea mAbs (7LE and SPM522) and 10 μg/ml MBL results in enhanced transmigration across collagen to both fMLF and IL-8 (**P > 0.01, ***P > 0. 001, Fig. 4E and F).
Specificity of Lea glycans in mediating observed increases in PMN migration was confirmed in transmigration experiments with PMN derived from human donors that lack Lea. Such individuals have a point mutation in FUT3, which results in its inability to fucosylate in the α1-4 position. As shown in Fig.4G, PMNs from a donor with a loss of function mutation in Fuc-T3 do not express Lea. In contrast, PMNs from the same donor express normal levels of Lex (Fig. 4G and H). In addition, treatment of PMN from a Lea-negative donor with anti-Lea mAbs or MBLs did not result in significantly increased PMN transmigration relative to PMN treated with J10.4, an IgG binding control mAb or control PMN not treated with any mAb (Fig. 4I). Although targeting of Lea had no effect on PMN transmigration across collagen, treatment of PMNs deficient in α1-4 fucosylation with mAbs to Lex (which requires α1-3 and not α1-4 fucosylation for its synthesis) did result in a large decrease in PMN transmigration (Fig. 4I) as we have previously reported [6]. Taken together, these data highlight specific recognition of PMN-expressed Lea trisaccharide by anti-Lea mAbs and MBLs, as well as the functional effects of targeting Lea during PMN transmigration.
DISCUSSION
Although PMN migration into mucosal tissues is essential for effective host defense against invading pathogens, dysregulated PMN migration is implicated in the pathology of many chronic inflammatory conditions, including rheumatoid arthritis, myocardial reperfusion injury, chronic obstructive pulmonary disease, and inflammatory bowel disease [24–28]. Recent evidence has shown that migration of PMNs into epithelial-lined organs, such as the intestine, is, in part, regulated through glycan-mediated adhesion events. For example, we have demonstrated that mAb targeting of sLea on the epithelial protein CD44v6 blocks PMN TEM into the intestinal lumen [4, 5] and that targeting of the related PMN-expressed Lewis glycan Lex also blocks PMN TEM [6].
Blood group Ags, including ABH Ags and Lea/Leb, represent glycan residues attached at the nonreducing ends of glycoconjugates, which have fundamental roles in mammalian cell-to-cell communications at sites of inflammation [7–11]. Although it has been previously demonstrated that human PMNs express type 2 Lewis glycans (Lex and sLex) [3, 20, 29], it has also been previously reported that PMNs do not express the related Lewis glycans sLea [5] and Leb [6]. Additionally, it has been reported that Lea is expressed by inflamed and noninflamed intestinal epithelium [21, 22]. However, to date, there have been no studies, to our knowledge, looking at expression of Lea by PMNs.
Given the important role of Lex, sLex, and sLea in regulating PMN trafficking, we examined expression of Lea in human PMNs and demonstrated expression of several Lea-containing, N-linked glycoproteins. Consistent with that, generation of N-glycan–linked Lea has been reported previously in other cell types [30, 31]. In addition to PMN-expressed Lea, expression of Lea in noninflamed and inflamed mucosal epithelial tissue, as well as in T84 model IECs, was observed in the current study. Consistent with these data, there is a report of Lea expression in the mucosa from individuals with ulcerative colitis that compared the extent of epithelial Lea staining between inflammatory lesions and adjacent noninflammatory mucosal regions [21, 22]. Although the purpose of these studies was to evaluate differences in Lea expression in mucosal epithelial cells, immunohistochemistry images appear to contain Lea-positive immune cells infiltrating the inflamed mucosa of patients with ulcerative colitis [21].
Although the above publications reported Lea expression in IECs, this study is the first, to our knowledge, to report expression of Lea by human PMNs. Furthermore, this study demonstrates that specific engagement of Lea results in enhanced PMN transmigration. Because regulatory functions of glycans are often mediated through interactions with complementary GBPs that have carbohydrate-recognition domains binding specific groupings of 2–7 terminal sugar units in precise configurations, we next examined the specific conformations of Lea recognized by one of the anti-Lea mAbs (7LE) used in this study.
Glycan-binding analysis revealed specific recognition by 7LE of glycans terminating with the basic Lea trisaccharide structure. It has recently become better appreciated that glycans at the termini of longer oligosaccharide chains can mediate discrete functional effects [2]. Specifically, mAb targeting of terminal Lex at the reducing ends of glycan chains blocks PMN TEM [6]. In addition to binding terminal Lea, glycan array analysis also revealed binding of 7LE to terminal Lea displayed as part of a Lea–Lea tandem-repeat oligosaccharide. Interestingly, N-linked glycoproteins terminating with tandem Lea repeats have been reported to bind to C type serum lectin MBL to form a complex that has potent inhibitory activity against growth of human colorectal cancer cells [32, 33]. The Lea–Lea binding protein MBL is also known to have a role in innate immunity through activation of complement in addition to functioning as an opsonin through direct binding of pathogen glycans [34]. Finally, array analysis also revealed that there was no recognition of glycan structures containing only internal Lea structures by 7LE, once again highlighting the importance of the specific affinity of GBPs for terminally expressed glycan residues.
Glycan recognition by GBPs (including mAbs and lectins) is accomplished through interactions between protein amino acid side chains and multiple chemical moieties, including hydroxyl groups, N acetyl groups, and carboxylic acids on glycans. The chemical nature and stereochemical placement of each moiety can drive GBP-mediated recognition, such that even small differences at an atomic level are often important for binding interactions. In keeping with the latter, there was no recognition by the anti-Lea mAb 7LE of glycan structures very similar to Lea, including sLea and Lex present on the version 5.1 glycan array. In addition, 7LE failed to recognize sulfated Lea glycans present on the array. Interestingly, sLea glycans can act as potent ligands for E-selectin [35].
The biosynthesis of terminal glycan structures including Lewis glycans proceeds from precursors by stepwise addition of monosaccharide units through the actions of discrete glycosyltransferases. From the type 1 chain the biosynthesis of Lea proceeds through the activities of a β3GalT and α1,4 FucT [23]. In support of Lea biosynthesis by PMN, we show that when HL60 myelomonocytic cells are differentiated toward a PMN-like state there is a marked increase in the expression of β3Galt1, βGalt4 and FUT3. HL60 cells induced to differentiate with DMSO have many of the functional characteristics of circulating peripheral blood granulocytes such as phagocytosis, complement receptor activity, chemotaxis and the ability to reduce NBT. However, it has also been reported that differentiated HL60 cells retain surface glycan features characteristic of immature undifferentiated cells [36, 37]. Given this, expression of glycosyltransferases was also examined in isolated human PMN where robust RNA and protein expression of both β3Galt4 and FUT3 was observed. Further, the lack of expression of β3Galt1 observed in PMN is consistent with previous studies reporting that differentiated HL60 cells express glycosyltransferases that are not found in mature circulating PMN [36].
Given the importance of glycan-mediated binding interactions during PMN trafficking [5, 6], we examined functional consequences of ligation of Lea in PMN.
We show that specific mAb targeting of Lea increases PMN TEM across 2 well-characterized model IEC lines. Furthermore, incubation of PMNs with the C-type serum lectin MBL, which is a physiologic ligand of N-linked poly Lea–Lea structures [32, 33], also resulted in increased PMN transmigration. Unlike the anti-Lea mAbs used in this study, MBL can recognize other non-Lea ligands. However, specificity for MBL–Lea interactions in mediating increased PMN TEM was highlighted by the loss of effect of MBL (and anti-Lea mAbs) on PMN TEM in the presence of soluble Lea trisaccharide. Although ligation of Lea results in enhanced PMN TEM, we have previously shown that specific targeting of Lex (the stereoisomer of Lea) blocks PMN TEM [6], suggesting that these related Lewis glycans are on different PMN glycoproteins that have opposing roles during TEM. Other studies have shown that PMN Lex is displayed on the β2 integrin CD11b/CD18 [6, 20]. However, our data support Lex and Lea being displayed on different PMN glycoproteins because there is no change in the surface expression of Lea after stimulation of PMN with fMLF in contrast to a marked increase in the surface expression of both Lex and CD11b/CD18 after PMN activation with fMLF [6]. Analysis of CD18/CD11b (purified from human PMN by LM2/1 immunoaffinity) by immunoblotting revealed 2 protein bands recognized by anti-CD18 and anti-CD11b mAbs (Supplemental Fig. 2A). Further, immunoblotting of purified CD18/CD11b with an anti-Lex mAb (H198) confirmed expression of Lex on both the CD11b and CD18 subunits. In contrast, there was no detection of Lea on purified CD18/CD11b consistent with Lea and Lex being displayed on different PMN glycoproteins. Consistent with that, targeting of PMN-expressed Lex increased phagocytosis [6], whereas ligation of Lea had no effect on other PMN functions (Supplemental Fig. 2B and C). In addition, increases in PMN TEM downstream of Lea engagement (by anti-Lea mAbs or by MBL) were not mediated through Lea-induced changes in the surface expression of CD18/CD11b or Lex (Supplemental Fig. 2D–G). Taken together, these data demonstrate that, in contrast to Lex, functional effects downstream of Lea engagement are specific to PMN trafficking and are not mediated through CD18/CD11b.
Our studies also demonstrated that epithelial-expressed Lea is not required for an enhanced migratory response because PMN TEM across Caco2 IECs (which do not express Lea) was also increased downstream of engagement of PMN-expressed Lea. This lack of expression of Lea by Caco2 is consistent with a previous report showing no expression of FUT3 in these IECs [5]. Consistent with the role of PMN-expressed Lea mediating increased PMN trafficking, engagement of PMN Lea also resulted in increased PMN trafficking across collagen-coated support filters, an effect that was lost using PMN from a Lea-deficient individual. Because FUT3 encodes the α1-3/4 fucosyltransferase required to synthesize Lea, homozygotic carriers of 1 of several point mutations that inactivate FUT3 lack Lea Ags and, as such, are denoted as Lewis-negative individuals (constituting about 5% of the white population) [38, 39]. Consistent with specific mAb/MBL targeting of PMN Lea mediating functional effects observed during PMN TEM, treatment of PMN from an individual incapable of α1-4 fucosylation (that do not express Lea on their surface) with anti-Lea mAbs/Lea binding lectin did not result in significantly increased PMN transmigration.
Highlighting the relevance of glycans to mucosal inflammation, it has been previously reported that frequencies of FUT3 polymorphisms are higher in patients with ulcerative colitis than in healthy controls. Furthermore, stratified analyses indicate that mutant alleles of FUT3 are more common in patients with distal colitis than in those with more extensive colitis [21]. In addition, although the precise etiology of inflammatory bowel disease is not yet fully understood, abnormal host–microbial interactions have been implicated in disease pathogenesis. Interestingly, it has been reported that terminal Lewis Ags (including Lea) can act as binding sites for invading microbes, including Helicobacter pylori, Campylobacter jejuni, norovirus, and rotavirus [40–42]. Indeed, reports have already shown that Lea-negative individuals lack attachment factors for most norovirus strains and are thus less prone to norovirus infection [43, 44]. Therefore, the expression of Lea on human PMNs could have important implications for host immune responses beyond PMN trafficking during intestinal inflammation. Deeper understanding of the interactions between specific glycans (including Lea) and GBPs will likely lead to new insights into, and understanding of, human disease and, thus, has great potential to uncover novel mechanism-based anti-inflammatory therapeutics.
AUTHORSHIP
J.C.B. was involved in the planning, design, execution and analysis of all experiments and was responsible for writing and editing the manuscript. R.S., S.R.S., and G.L. were involved in the execution of immunofluorescence, blood screening, and immunohistochemistry experiments. N.A.L., R.D.C., and C.A.P. were involved in experimental design and conception, as well as manuscript editing.
ACKNOWLEDGMENTS
The authors acknowledge support from U.S. National Institutes of Health Grants DK079392 and DK072564 (C.A.P.) and the Crohn’s & Colitis Foundation of America Career Development Award (J.C.B.).
Glossary
- β3Galt
β3galactosyltransferase
- CFG
Consortium for Functional Glycomics
- Dif
differentiated
- Fuc
fucose
- FUT3
fucosyltransferase
- Gal
galactose
- GBF
glycan-binding protein
- GlcNAc
N-acetylglucosamine
- Lea
Lewis a
- Leb
Lewis b
- Lex
Lewis x
- MBL
mannan-binding lectin
- MBP
major basic protein
- ND
nondifferentiated
- PMN
polymorphonuclear leukocyte
- RFU
relative fluorescent units
- sLea
sialyl Lewis a
- sLex
sialyl Lewis x
- TEM
transepithelial migration
Footnotes
The online version of this paper, found at www.jleukbio.org, contains supplemental information.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Ley K., Laudanna C., Cybulsky M. I., Nourshargh S. (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689. [DOI] [PubMed] [Google Scholar]
- 2.Sperandio M., Frommhold D., Babushkina I., Ellies L. G., Olson T. S., Smith M. L., Fritzsching B., Pauly E., Smith D. F., Nobiling R., Linderkamp O., Marth J. D., Ley K. (2006) Alpha 2,3-sialyltransferase-IV is essential for L-selectin ligand function in inflammation. Eur. J. Immunol. 36, 3207–3215. [DOI] [PubMed] [Google Scholar]
- 3.Beauharnois M. E., Lindquist K. C., Marathe D., Vanderslice P., Xia J., Matta K. L., Neelamegham S. (2005) Affinity and kinetics of sialyl Lewis-X and core-2 based oligosaccharides binding to L- and P-selectin. Biochemistry 44, 9507–9519. [DOI] [PubMed] [Google Scholar]
- 4.Brazil J. C., Lee W. Y., Kolegraff K. N., Nusrat A., Parkos C. A., Louis N. A. (2010) Neutrophil migration across intestinal epithelium: evidence for a role of CD44 in regulating detachment of migrating cells from the luminal surface. J. Immunol. 185, 7026–7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brazil J. C., Liu R., Sumagin R., Kolegraff K. N., Nusrat A., Cummings R. D., Parkos C. A., Louis N. A. (2013) α3/4 Fucosyltransferase 3-dependent synthesis of sialyl Lewis A on CD44 variant containing exon 6 mediates polymorphonuclear leukocyte detachment from intestinal epithelium during transepithelial migration. J. Immunol. 191, 4804–4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brazil J. C., Sumagin R., Cummings R. D., Louis N. A., Parkos C. A. (2016) Targeting of neutrophil lewis X blocks transepithelial migration and increases phagocytosis and degranulation. Am. J. Pathol. 186, 297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto F., Clausen H., White T., Marken J., Hakomori S. (1990) Molecular genetic basis of the histo-blood group ABO system. Nature 345, 229–233. [DOI] [PubMed] [Google Scholar]
- 8.Greenwell P. (1997) Blood group antigens: molecules seeking a function? Glycoconj. J. 14, 159–173. [DOI] [PubMed] [Google Scholar]
- 9.Inoue M., Sasagawa T., Saito J., Shimizu H., Ueda G., Tanizawa O., Nakayama M. (1987) Expression of blood group antigens A, B, H, Lewis-a, and Lewis-b in fetal, normal, and malignant tissues of the uterine endometrium. Cancer 60, 2985–2993. [DOI] [PubMed] [Google Scholar]
- 10.Phillips M. L., Nudelman E., Gaeta F. C., Perez M., Singhal A. K., Hakomori S., Paulson J. C. (1990) ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science 250, 1130–1132. [DOI] [PubMed] [Google Scholar]
- 11.Becker D. J., Lowe J. B. (2003) Fucose: biosynthesis and biological function in mammals. Glycobiology 13, 41R–53R. [DOI] [PubMed] [Google Scholar]
- 12.Balsam L. B., Liang T. W., Parkos C. A. (1998) Functional mapping of CD11b/CD18 epitopes important in neutrophil-epithelial interactions: a central role of the I domain. J. Immunol. 160, 5058–5065. [PubMed] [Google Scholar]
- 13.Liu Y., Nusrat A., Schnell F. J., Reaves T. A., Walsh S., Pochet M., Parkos C. A. (2000) Human junction adhesion molecule regulates tight junction resealing in epithelia. J. Cell Sci. 113, 2363–2374. [DOI] [PubMed] [Google Scholar]
- 14.Parkos C. A., Delp C., Arnaout M. A., Madara J. L. (1991) Neutrophil migration across a cultured intestinal epithelium: dependence on a CD11b/CD18-mediated event and enhanced efficiency in physiological direction. J. Clin. Invest. 88, 1605–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. (1979) Normal functional characteristics of cultured human promyelocytic leukemia cells (HL-60) after induction of differentiation by dimethylsulfoxide. J. Exp. Med. 149, 969–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heimburg-Molinaro J., Song X., Smith D. F., Cummings R. D. (2011) Preparation and analysis of glycan microarrays. Curr. Protoc. Protein Sci. Chapter 12, Unit12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diamond M. S., Staunton D. E., de Fougerolles A. R., Stacker S. A., Garcia-Aguilar J., Hibbs M. L., Springer T. A. (1990) ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18). J. Cell Biol. 111, 3129–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colgan S. P., Parkos C. A., Delp C., Arnaout M. A., Madara J. L. (1993) Neutrophil migration across cultured intestinal epithelial monolayers is modulated by epithelial exposure to IFN-gamma in a highly polarized fashion. J. Cell Biol. 120, 785–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karhausen J., Furuta G. T., Tomaszewski J. E., Johnson R. S., Colgan S. P., Haase V. H. (2004) Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J. Clin. Invest. 114, 1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stöckl J., Majdic O., Rosenkranz A., Fiebiger E., Kniep B., Stockinger H., Knapp W. (1993) Monoclonal antibodies to the carbohydrate structure Lewis(x) stimulate the adhesive activity of leukocyte integrin CD11b/CD18 (CR3, Mac-1, alpha m beta 2) on human granulocytes. J. Leukoc. Biol. 53, 541–549. [DOI] [PubMed] [Google Scholar]
- 21.Hu D., Zhang D., Zheng S., Guo M., Lin X., Jiang Y. (2016) Association of ulcerative colitis with FUT2 and FUT3 polymorphisms in patients from Southeast China. PLoS One 11, e0146557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan M., Itzkowitz S. H., Palekar A., Shamsuddin A. M., Phelps P. C., Trump B. F., Kim Y. S. (1985) Distribution of blood group antigens A, B, H, Lewisa, and Lewisb in human normal, fetal, and malignant colonic tissue. Cancer Res. 45, 4499–4511. [PubMed] [Google Scholar]
- 23.Holgersson J., Löfling J. (2006) Glycosyltransferases involved in type 1 chain and Lewis antigen biosynthesis exhibit glycan and core chain specificity. Glycobiology 16, 584–593. [DOI] [PubMed] [Google Scholar]
- 24.Kucharzik T., Walsh S. V., Chen J., Parkos C. A., Nusrat A. (2001) Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am. J. Pathol. 159, 2001–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoenderdos K., Condliffe A. (2013) The neutrophil in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 48, 531–539. [DOI] [PubMed] [Google Scholar]
- 26.Fattori V., Amaral F. A., Verri W. A. Jr (2016) Neutrophils and arthritis: role in disease and pharmacological perspectives. Pharmacol. Res. 112, 84–98. [DOI] [PubMed] [Google Scholar]
- 27.Saverymuttu S. H., Chadwick V. S., Hodgson H. J. (1985) Granulocyte migration in ulcerative colitis. Eur. J. Clin. Invest. 15, 60–63. [DOI] [PubMed] [Google Scholar]
- 28.Saverymuttu S. H., Peters A. M., Lavender J. P., Chadwick V. S., Hodgson H. J. (1985) In vivo assessment of granulocyte migration to diseased bowel in Crohn’s disease. Gut 26, 378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foxall C., Watson S. R., Dowbenko D., Fennie C., Lasky L. A., Kiso M., Hasegawa A., Asa D., Brandley B. K. (1992) The three members of the selectin receptor family recognize a common carbohydrate epitope, the sialyl Lewis(x) oligosaccharide. J. Cell Biol. 117, 895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fitchette-Lainé A. C., Gomord V., Cabanes M., Michalski J. C., Saint Macary M., Foucher B., Cavelier B., Hawes C., Lerouge P., Faye L. (1997) N-glycans harboring the Lewis a epitope are expressed at the surface of plant cells. Plant J. 12, 1411–1417. [DOI] [PubMed] [Google Scholar]
- 31.Fitchette A. C., Cabanes-Macheteau M., Marvin L., Martin B., Satiat-Jeunemaitre B., Gomord V., Crooks K., Lerouge P., Faye L., Hawes C. (1999) Biosynthesis and immunolocalization of Lewis a-containing N-glycans in the plant cell. Plant Physiol. 121, 333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terada M., Khoo K. H., Inoue R., Chen C. I., Yamada K., Sakaguchi H., Kadowaki N., Ma B. Y., Oka S., Kawasaki T., Kawasaki N. (2005) Characterization of oligosaccharide ligands expressed on SW1116 cells recognized by mannan-binding protein: a highly fucosylated polylactosamine type N-glycan. J. Biol. Chem. 280, 10897–10913. [DOI] [PubMed] [Google Scholar]
- 33.Ma Y., Uemura K., Oka S., Kozutsumi Y., Kawasaki N., Kawasaki T. (1999) Antitumor activity of mannan-binding protein in vivo as revealed by a virus expression system: mannan-binding proteindependent cell-mediated cytotoxicity. Proc. Natl. Acad. Sci. USA 96, 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawasaki N., Kawasaki T., Yamashina I. (1989) A serum lectin (mannan-binding protein) has complement-dependent bactericidal activity. J. Biochem. 106, 483–489. [DOI] [PubMed] [Google Scholar]
- 35.Jakab Z., Fekete A., Csávás M., Borbás A., Lipták A., Antus S. (2012) Synthesis of a sulfonic acid mimetic of the sulfated Lewis A pentasaccharide. Carbohydr. Res. 350, 90–93. [DOI] [PubMed] [Google Scholar]
- 36.Pasqualetto V., Néel D., Feugeas J. P., Aubery M., Derappe C. (1995) HL 60 leukaemia cells chemically induced to differentiate retain some surface glycan features of undifferentiated cells not found in normal leukocytes. Glycobiology 5, 59–66. [DOI] [PubMed] [Google Scholar]
- 37.Pasqualetto V., Lemaire S., Neel D., Aubery M., Berger E. G., Derappe C. (2000) Phorbol ester treatment of HL 60 leukemia cells results in increase of β-(1→4)-galactosyltransferase. Carbohydr. Res. 328, 301–305. [DOI] [PubMed] [Google Scholar]
- 38.Mollicone R., Reguigne I., Kelly R. J., Fletcher A., Watt J., Chatfield S., Aziz A., Cameron H. S., Weston B. W., Lowe J. B. (1994) Molecular basis for Lewis α(1,3/1,4)-fucosyltransferase gene deficiency (FUT3) found in Lewis-negative Indonesian pedigrees. J. Biol. Chem. 269, 20987–20994. [PubMed] [Google Scholar]
- 39.Carmona-Vicente N., Allen D. J., Rodríguez-Díaz J., Iturriza-Gómara M., Buesa J. (2016) Antibodies against Lewis antigens inhibit the binding of human norovirus GII.4 virus-like particles to saliva but not to intestinal Caco-2 cells. Virol. J. 13, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shirato H., Ogawa S., Ito H., Sato T., Kameyama A., Narimatsu H., Xiaofan Z., Miyamura T., Wakita T., Ishii K., Takeda N. (2008) Noroviruses distinguish between type 1 and type 2 histo-blood group antigens for binding. J. Virol. 82, 10756–10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heneghan M. A., McCarthy C. F., Moran A. P. (2000) Relationship of blood group determinants on Helicobacter pylori lipopolysaccharide with host lewis phenotype and inflammatory response. Infect. Immun. 68, 937–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moran A. P., Prendergast M. M. (2001) Molecular mimicry in Campylobacter jejuni and Helicobacter pylori lipopolysaccharides: contribution of gastrointestinal infections to autoimmunity. J. Autoimmun. 16, 241–256. [DOI] [PubMed] [Google Scholar]
- 43.Larsson M. M., Rydell G. E., Grahn A., Rodriguez-Diaz J., Akerlind B., Hutson A. M., Estes M. K., Larson G., Svensson L. (2006) Antibody prevalence and titer to norovirus (genogroup II) correlate with secretor (FUT2) but not with ABO phenotype or Lewis (FUT3) genotype. J. Infect. Dis. 194, 1422–1427. [DOI] [PubMed] [Google Scholar]
- 44.Tan M., Jiang X. (2005) Norovirus and its histo-blood group antigen receptors: an answer to a historical puzzle. Trends Microbiol. 13, 285–293. [DOI] [PubMed] [Google Scholar]