Review on the transcriptional and epigenetic programs driving hyporesponsive states in T cells.
Keywords: epigenetic, exhaustion, transcription factors
Abstract
Naive CD8+ T cells differentiate into effector and memory cytolytic T cells (CTLs) during an acute infection. In contrast, in scenarios of persistent antigen stimulation, such as chronic infections and cancer, antigen-specific CTLs show a gradual decrease in effector function, a phenomenon that has been termed CD8+ T cell “exhaustion” or “dysfunction.” Another hyporesponsive state, termed “anergy”, is observed when T cells are activated in the absence of positive costimulatory signals. Among the many negative regulators induced in hyporesponsive T cells are inhibitory cell–surface receptors, such as PD-1, LAG-3, CTLA-4, and TIM-3; “checkpoint blockade” therapies that involve treatment of patients with cancer with blocking antibodies to those receptors show considerable promise in the clinic because the blocking antibodies can mitigate hyporesponsiveness and promote tumor rejection. In this review, we describe recent advances in our molecular understanding of these hyporesponsive states. We review evidence for the involvement of diverse transcription factors, metabolic programs, and chromatin accessibility changes in hyporesponsive T cells, and we discuss how checkpoint blockade therapies affect the molecular program of CD8+ T cell exhaustion.
Introduction
Upon Ag encounter, naive CD8+ T cells differentiate into effector and memory CTLs. Memory CTLs confer protective immunity against secondary infection and exhibit a much stronger and more-rapid recall response than naive T cells do upon reexposure to Ags [1–3]. In the face of chronic infections (i.e., persistent Ag stimulation), Ag-specific CTLs show a gradual decrease in effector function, a phenomenon that has been termed CD8+ T cell “exhaustion” [4, 5]. Exhausted CD8+ T cells display a transcriptional program distinct from that of functional effector or memory CD8+ T cells [6], characterized, for example, by the expression of inhibitory cell-surface receptors, including PD-1, LAG-3, TIM-3, TIGIT, and CTLA-4, and by impaired IL-2, TNF, and IFN-γ cytokine production [5, 7]. Exhaustion was first described in mice chronically infected with LCMV clone 13 [4] and has since been observed in patients infected with Mycobacterium tuberculosis, HIV, and hepatitis B and C viruses [8, 9], as well as in several forms of cancer, including cutaneous and metastatic melanoma [10, 11], leukemia [12], breast cancer [13], and gastrointestinal tumors [14], among others. Induction of the exhaustion program may be considered a feedback response to persistent Ag stimulation, which imposes a balance that ensures antiviral or antitumoral responses, albeit at a reduced level; in turn, those reduced responses restrict pathologic outcomes stemming from excessive immune involvement, which could be detrimental to the host.
In this review, we focus on the role of chromatin modifications, TFs, and metabolic pathways that have recently been shown or suggested to participate in regulating exhaustion at a transcriptional level (Fig. 1A and B). This subject has also been covered by several excellent recent reviews [7, 15–18]. Chromatin accessibility patterns are very similar among effector, memory, and exhausted cells [19], suggesting that the chromatin “opening” is driven by the initial encounter with Ags, whereas the actual expression of genes associated with those T cell subsets is largely determined by the transcription factors present in those cells. Indeed, transcriptional networks that operate in exhausted CD4+ and CD8+ T cells and incorporate many different transcription factors have been delineated [20, 21]. Understanding the genome-wide cooperation of those different transcription factors will allow us to comprehend mechanistically how the exhaustion program is driven and/or maintained at the levels of transcription and chromatin accessibility.
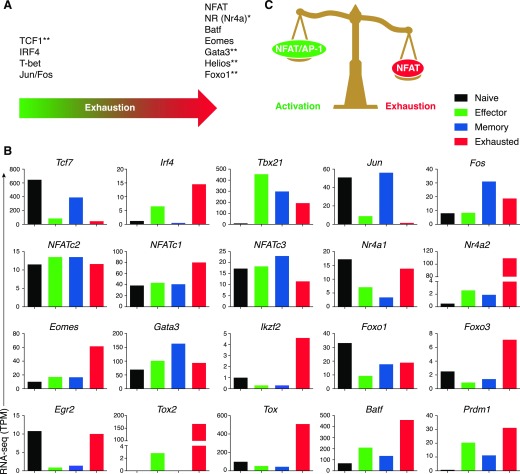
Figure 1. Transcriptional regulators of T cell exhaustion.
(A) Relative importance of the indicated transcription factors in exhaustion. Factors shown toward the right are reported to have a more-prominent role in T cell exhaustion. *Transcription factors whose motifs have been identified by ATAC-seq as enriched in accessible-chromatin regions in exhausted cells. **Transcription factors identified by transcriptomic analyses or genetic approaches, but whose motifs have not been identified by ATAC-seq as enriched in accessible-chromatin regions in one population or the other. (B) Expression of indicated transcription factors measured by RNA-seq and displayed as transcripts per million (TPM) in naive (black bars), effector (green bars), memory (blue bars) or exhausted (red bars) cells. Data from Scott-Browne et al. [19]. (C) Schematic diagram showing how an initiating transcription factor NFAT governs effector and exhaustion programs in T cells in the presence or absence of AP-1 cooperation, respectively.
HYPORESPONSIVE STATES IN CD4+ AND CD8+ T CELLS: EXHAUSTION, ANERGY, AND DYSFUNCTION
A variety of poorly defined terms, including anergy, exhaustion, and dysfunction, have been used to describe various states of decreased response to Ag observed in T cells under various stimulation conditions in vivo and in vitro. In the most common usage, CD4+ T cells that respond poorly because of a lack of sufficient costimulation are said to be anergic (reviewed in Schwartz [22]), whereas CD8+ T cells that respond poorly because of prolonged Ag exposure during chronic viral infections or cancer are referred to as exhausted (reviewed in Wherry [5]). Recently, the term dysfunctional has been used in preference to exhausted to describe tumor-infiltrating T cells that are hyporesponsive and, therefore, are not destroying the tumor effectively (reviewed in [7, 23]). There has been considerable confusion over whether these states are similar or distinct, primarily because various studies have used different model systems and culture conditions and have examined different cell populations at different times during the course of antiviral or antitumoral response.
Much of the confusion arises from the assumption that different cell populations are distinct when they show differences in gene expression patterns. However, lack of expression of a particular gene in a specific T cell population at a specific sampling time does not mean that the gene cannot ever be expressed by that population. The obvious precedent is provided by cytokine genes, which are expressed by T cells only after stimulation. Thus, it is plausible that the transcriptional programs of anergy, exhaustion, and dysfunction are actually more similar than is currently believed.
In this review, we take the position that most hyporesponsive states—whether termed anergy, exhaustion, or dysfunction—represent normal negative feedback processes occurring after early activation, which act to attenuate T cell activity so as to limit immunopathologies caused by inappropriately high and prolonged immune responses. From this viewpoint, the term dysfunctional should be avoided because, even in the tumor environment, the hyporesponsive cells are responding to external signals in a physiologic way. Of course, the transcriptional events occurring during the phase of early activation may well differ qualitatively or quantitatively, depending on whether the cells later become hyporesponsive or develop full effector function.
Based on these considerations, we suggest that the pathways leading to the initial generation of various hyporesponsive states—whether anergy, dysfunction, or exhaustion—reflect overlapping contributions from various negative signaling pathways, whose exact nature depends on signals provided by the local environment, with the resulting patterns of gene expression depending on the cell type and model system used. The results also highlight the importance of unifying the terminology used among publications, or at least defining hyporesponsive states more accurately at a molecular level, so that different model systems can be more readily compared. Until widely accepted definitions are available, we conform to the authors’ terminology when describing specific publications but use the term hyporesponsive when making general observations or conclusions.
INITIATION, MAINTENANCE, AND REVERSIBILITY OF THE HYPORESPONSIVE STATES
Our thesis in this review is that most hyporesponsive states represent, or at least are initiated through, a normal negative feedback response pathway that acts to limit runaway immune responses in conditions of persistent Ag stimulation or TCR stimulation without costimulation. In support of this hypothesis, “dysfunctional” tumor-infiltrating T cells that developed from transferred naive Ag-reactive T cells in a tamoxifen-induced mouse liver cancer model became hyporesponsive within a few days after transfer, presumably because of early up-regulation of diverse negative regulators, including transcription factors previously associated with hyporesponsiveness (Batf, Egr1, Prdm1), phosphatases, such as Ptpn11, Ptpn12, Dusp1, and Dusp6, and the inhibitory cell surface receptors Pdcd1, Lag3, Cd160, and Ctla4, which further promote negative signaling [24].
Specifically, we suggest that facets of the transcriptional network leading to hyporesponsiveness are induced through an initiating transcription factor NFAT, which governs both effector and exhaustion programs in T cells (Fig. 1C). We have shown that NFAT transcription factors, in the absence of AP-1 cooperation, induce a state of reduced responsiveness to subsequent stimulation that is similar between CD4+ and CD8+ T cells and that may be a common initiating stimulus in exhaustion, dysfunction, and anergy [19, 25–27]. In further support of this hypothesis, we have demonstrated that NFAT, in the absence of cooperation with the unrelated transcription factor AP-1 (Fos-Jun), can drive expression of genes associated with anergy and exhaustion in both CD4+ and CD8+ T cells; in contrast, when AP-1 factors are present, NFAT drives expression of molecules such as cytokines that are involved in effector responses [25]. These points are discussed in more detail under the section “The potential roles of NFAT and NFAT-induced transcription factors in the initiation and maintenance of hyporesponsive states”.
Many groups have performed transcriptional profiling of T cells in various hyporesponsive states. These studies have identified numerous transcription factors that are differentially expressed between fully responsive and hyporesponsive states (Fig. 1A and B), and follow-up studies using gene-disrupted mice have shown that, in many cases, cells lacking one or more of these transcription factors show altered responsiveness to stimulation. Studies on selected transcription factors are summarized in the “Other transcription factors implicated in T cell hyporesponsive states” section.
An important question concerns the reversibility of the hyporesponsive state. Whereas the initial NFAT-induced state of diminished signaling is likely reversible with time, prolonged stimulation may lead to the emergence of cells in which the expression of important activation-associated genes is more-permanently repressed [24]. Indeed, when exhausted T cells from chronic infections or cancer were isolated and injected into new mice or tested for in vitro cytotoxicity or cytokine production, they remained hyporesponsive and did not persist, suggesting that they entered a state of potentially “irreversible” exhaustion [24, 28, 29]. Consistent with that hypothesis, only a fraction of patients with cancer (melanoma) respond to “checkpoint inhibitor” therapies (anti-PD-1/anti-PD-L1) [30–32], although another possibility is that combination therapies that block multiple signaling pathways are needed to fully counter exhaustion cells [33–35]. In other studies, detailed analysis of T-bet and Eomes expression identified different populations of terminally exhausted cells, as well as a progenitor subset that proliferated when exhaustion was countered by an anti-PD-1/PD-L1 blockade [36, 37] (see “T-bet and Eomes” section). These conflicting ideas about the reversibility of CD8+ T cell exhaustion are discussed in the “Transcriptional changes and reversibility of exhaustion in response to checkpoint blockade” section.
CHROMATIN ACCESSIBILITY AND OTHER EPIGENETIC CHANGES IN EXHAUSTED/DYSFUNCTIONAL T CELLS
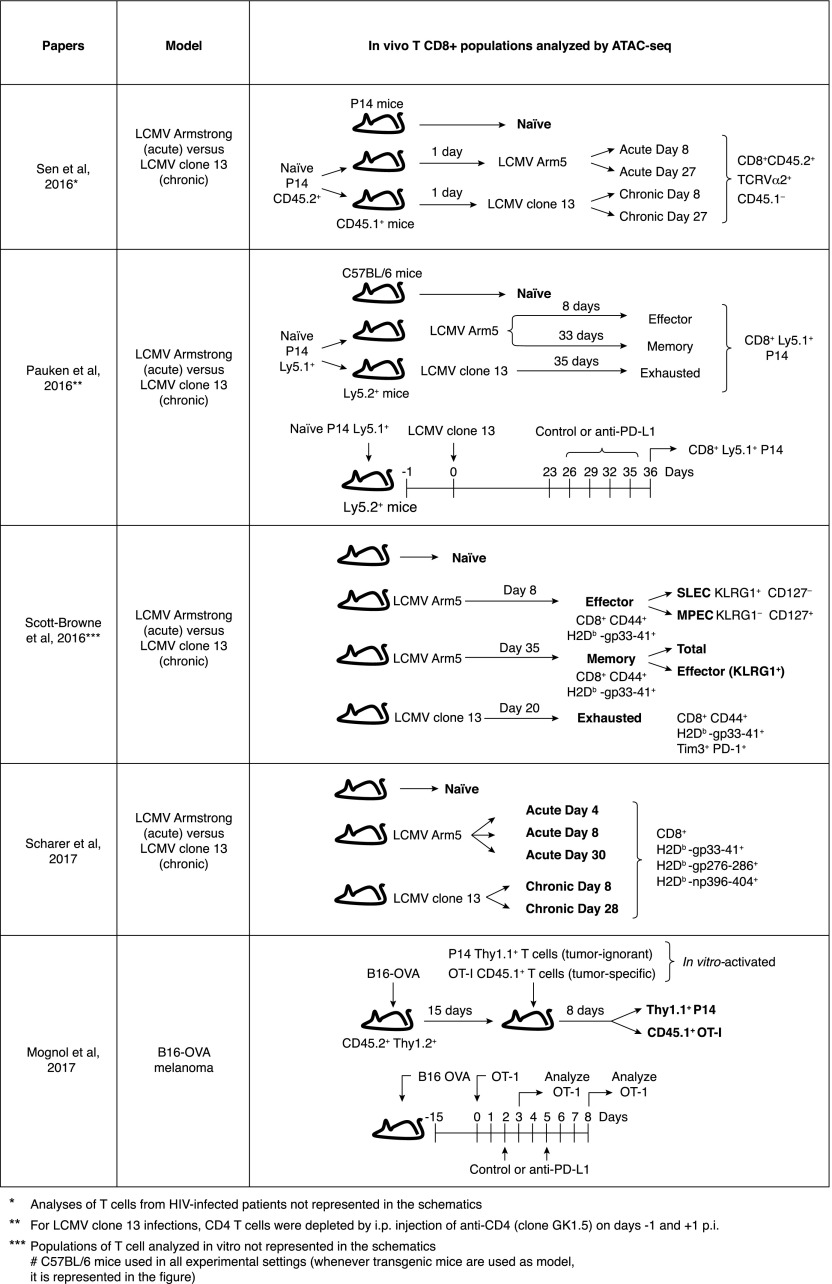
At least 5 recent publications have described the use of ATAC-seq [38] to investigate accessible chromatin regions in exhausted/dysfunctional T cells (Fig. 2). Four of the studies compared exhausted cells from mice infected with LCMV clone 13 to effector and memory CTLs from mice acutely infected with LCMV Armstrong [19, 39–41]. The fifth paper used the B16-OVA melanoma model to compare tumor-infiltrating OT-I TCR transgenic CD8+ T cells that recognize the tumor Ag (OVA) and acquire an exhausted phenotype with bystander P14 TCR transgenic CD8+ T cells that also infiltrate the tumor but do not recognize the tumor Ag and do not become exhausted (Fig. 2) [27]. In the LCMV experiments, 3000–6000 regions were differentially accessible between exhausted cells and either effector or memory cells [19, 39–41]; in the B16-OVA melanoma model, the Ag-reactive OT-I tumor-infiltrating cells contained 1800–1900 accessible regions that were not observed in bystander tumor-infiltrating P14 cells [27]. In all cases, the accessible chromatin regions were mostly found in intergenic or intragenic regions of the genome, corresponding to known or presumed enhancers. On average, differentially accessible regions tended to be associated with activation, rather than repression, of adjacent genes [19, 27, 39–41].
Figure 2. Experimental designs used in studies of chromatin accessibility in hyporesponsive CD8 T cells.
Schematic representations of the experimental approaches of 5 research articles that have recently used ATAC-seq to identify accessible-chromatin regions in CD8+ T cell populations in vivo.
Notably, most accessible chromatin regions present in exhausted cells were shared with effector CD8+ T cells responding to acute LCMV infection and/or with memory cells isolated many weeks later [19]. Similarly, most (1414 of 1864) of the accessible chromatin regions in tumor-infiltrating, Ag-reactive OT-I T cells in the B16-OVA model were shared with in vitro–activated CD8+ T cells [27]. There was a highly significant overlap between exhaustion-specific regions in tumor-infiltrating Ag-specific OT-I cells and cells that became exhausted during chronic viral infection [19, 27]. There was also a significant overlap of accessible regions identified in exhausted mouse CD8+ T cells with those in exhausted Ag-specific T cells from chronically HIV- or HCV-infected individuals [40].
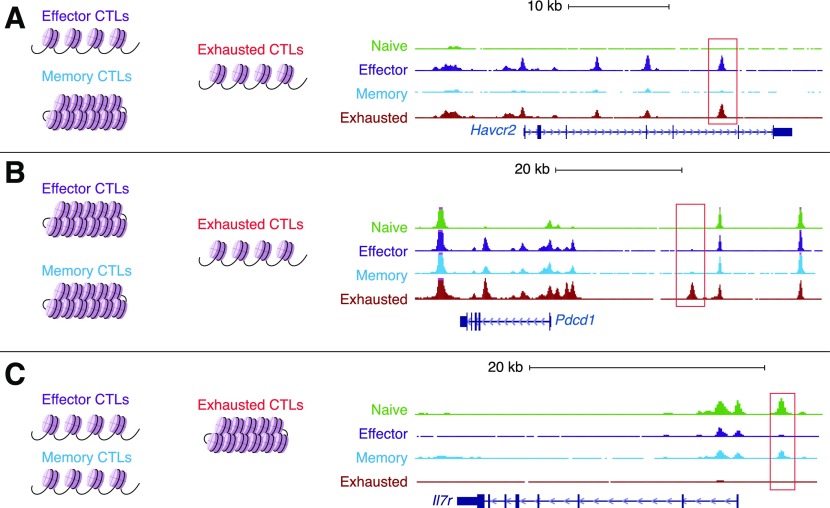
Examples of regions that are differentially accessible in exhausted vs. effector or memory cells are shown in Fig. 3. For instance, chromatin accessibility near key effector-related genes including Ifng, Gzma, Gzmk, Fasl, and Prf1 and genes encoding inhibitory receptors including Havcr2, Lag3, and Ctla4, was similar between effector and exhausted cells (Fig. 3A) [19, 27]. However, a novel enhancer, located ∼22 kb 5′ of the transcriptional start site of the Pdcd1 gene (coding for PD-1), is only accessible in exhausted cells (Fig. 3B) [19, 27, 39, 40]. This enhancer, which is not identifiably conserved in human cells, controls PD-1 expression because its deletion (mediated by CRISPR/Cas9) in EL-4 cells, which constitutively express PD-1, resulted in decreased PD-1 levels [40]. Notably, this exhaustion-specific enhancer was also accessible in cells expressing CA-RIT-NFAT1, a constitutively active NFAT1 that cannot cooperate with AP-1 [19, 27] (see “The potential roles of NFAT and NFAT-induced transcription factors in the initiation and maintenance of hyporesponsive states” section below). A few regions were more accessible in naive and/or memory CTLs compared with effector and exhausted CTLs; an example of one such region in the Il7ra locus is shown in Fig. 3C.
Figure 3. Genome browser views of representative loci showing differential chromatin accessibility when comparing naive, effector, memory, and exhausted Ag-specific cells in acute and chronic LCMV infection.
(A) The Havcr2 locus, which codes for TIM-3, contains several regions in which the chromatin is similarly accessible in effector and exhausted cells but is less accessible in naive or memory T cells. A representative region is highlighted by the red rectangle. (B) The Pdcd1 locus, which codes for PD-1, contains genomic regions at the promoter and at the distal 5′ enhancer, in which the chromatin is more accessible in exhausted cells compared with naive, effector, or memory cells. (C) The Il7r locus contains a genomic region in which the chromatin is more accessible in memory and naive cells compared with exhausted cells. Representative differentially accessible regions are indicated by red rectangles.
There are at least two possible explanations, not mutually exclusive, for the overlap in accessible chromatin between exhausted and effector cell populations. The most striking changes in chromatin accessibility are observed in naive CD8+ T cells upon initial stimulation, and most of those changes are stably maintained in effector, memory, and exhausted cells [19]; in this scenario, exhausted T cells would simply maintain the accessible regions found in effector T cells, and differential gene expression at these accessible regions would be determined primarily by which transcription factors are expressed and active in the different subsets. For instance T-bet, a transcription factor associated with effector function, could be replaced by Eomes, a transcription factor of the same T-box family that is more associated with memory and exhaustion [1, 37]. On the other hand, at the single-cell level, the exhausted cell population could contain individual cells that were recently activated and, therefore, display gene expression and chromatin-accessibility patterns characteristic of activated cells and other cells that, by virtue of longer Ag exposure, display gene expression and chromatin-accessibility patterns that are exhaustion-biased or truly exhaustion specific. In support of the latter hypothesis, single-cell RNA sequencing of tumor-infiltrating lymphocytes has identified a gene expression module of “dysfunction” that correlated negatively with an “activation/dysfunction” module, suggesting that at least some tumor-infiltrating cells can engage in nonoverlapping programs of activation or dysfunction [42]. Single-cell ATAC-seq experiments should establish whether cells whose accessible regions are shared with activated cells are distinct from those in which the accessible regions are primarily exhaustion specific, or whether the same cell can display both activation-related and exhaustion-specific accessible regions.
Analysis of ATAC-seq data revealed enrichment for consensus-binding motifs for several classes of transcription factors in exhaustion-specific accessible regions. Several studies showed high enrichment for binding motifs for NFAT and Nr4a nuclear receptors [19, 27, 39, 40]; Nr4a family transcription factors were also up-regulated at the mRNA level in exhausted cells, as were transcription factors of the bHLH (e.g., bHLHe40), bZIP (e.g., Batf), and high-mobility group (e.g., Tox, Tox2) families [19] (Fig. 1). Also enriched in exhausted-specific accessible regions were consensus-binding motifs for retinoic acid receptors, T-box factors (T-bet and Eomes), and bZIP transcription factors (BATF, Jun, and Fos) [39, 40]. Whereas enrichment for consensus transcription factor binding motifs can be readily assessed by ATAC-seq, whether these motifs are actually occupied by specific transcription factors requires further analysis. With sufficiently high sequence coverage, ATAC-seq data can be mined for footprints characteristic of specific families of transcription factors, providing evidence that one or more members of a particular transcription factor family might occupy a particular site [38]. However, because various members of a transcription factor family tend to have similar DNA binding specificities and therefore ATAC-seq footprints, demonstrating the involvement of a particular family member requires ChIP assays using specific Abs. This, at the moment, represents a technical challenge because ChIP assays for transcription factors typically require large numbers of cells, and only limited numbers of exhausted cells are typically recovered in experimental models.
Other epigenetic modifications in exhausted T cells have also been investigated, but in more-limited ways. Studies that focused specifically on the Pdcd1 locus demonstrated that two conserved regions, one of which corresponded to a CpG island in humans, were methylated in naive T cells but demethylated in effector cells and in exhausted Ag-specific cells after chronic exposure to Ags. Both sites partially regained methylation in effector cells isolated at later times and in memory T cells [43]. When Ag-specific cells from chronically infected mice were adoptively transferred into congenic, acutely infected mice, the cells maintained the demethylated state of the Pdcd1 locus even after 45 d of adoptive transfer [44]. Similarly, the Pdcd1 locus remained unmethylated in HIV-infected individuals treated with antiretroviral therapy for >3 yr with undetectable viral load in blood [45].
In a related study, exhausted CTLs from mice chronically infected with LCMV clone 13 showed decreased levels of histone 3 acetylated at lysines 9 and 14, compared with Ag-specific cells from mice infected acutely with the LCMV Armstrong strain [46]. Treatment of the cells with histone deacetylase inhibitors resulted in increased cytokine production in vitro, and adoptive transfer of these cells led to the generation of memory responses and increased cytokine production with reduced viral burden in spleen and liver [46]. This study suggests that the exhaustion phenotype may be ameliorated by targeting epigenetic modifiers; however, these treatments may affect just a fraction of the exhausted cell population or have only a transient effect.
THE POTENTIAL ROLES OF NFAT AND NFAT-INDUCED TRANSCRIPTION FACTORS IN THE INITIATION AND MAINTENANCE OF HYPORESPONSIVE STATES
Transcription factors of the NFAT family are key regulators of T cell activation [47–50]. There are 5 NFAT family members; of which NFAT1-4 (NFATc1-c4) are regulated by Ca2+-calcineurin signaling. NFAT1, NFAT2, and NFAT4 are expressed and have important roles in T cell development, activation, and function. Mice deficient in individual NFAT family members show nonoverlapping phenotypes, suggesting a distinct expression pattern or function by each member [47, 48, 50]. NFAT proteins interact with structurally unrelated Fos-Jun (AP-1) transcription factors to form cooperative NFAT:AP-1 complexes [51] that are critical for the induction of cytokine genes and other activation-associated genes [26].
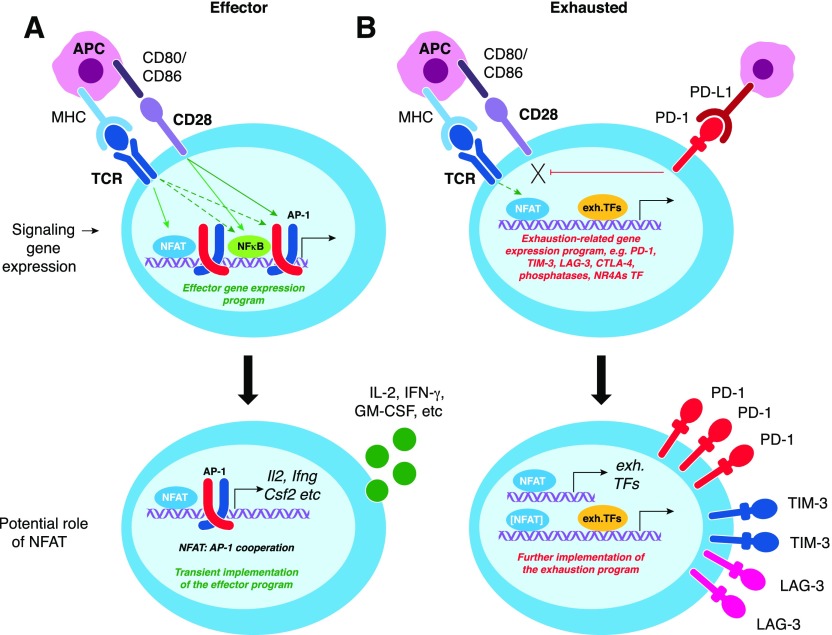
NFAT transcription factors not only initiate transcriptional programs characteristic of productive T cell activation [49] but also are involved in hyporesponsive states, such as anergy and exhaustion [25, 52]. Exhausted cells show increased NFAT expression, whereas AP-1 factors (Fos, Fosb, and Junb) are decreased [6], suggesting that exhaustion can be driven by nuclear NFATs in the absence of AP-1 cooperation, analogous to cells receiving TCR signaling in the absence of costimulation as occurs in anergy. In support of this idea, Tregs can induce exhaustion in tumor-infiltrating lymphocytes through down-regulation of costimulatory molecules on APCs [53]. In the absence of AP-1 cooperation, activated NFAT1 directly induces an exhaustion-associated transcriptional program, including expression of inhibitory receptors, in both CD4+ and CD8+ T cells [25] (Fig. 4), leading to impaired ability of CD8+ T cells to reject colorectal carcinoma CT26 cells expressing HA Ag (CT26-HA) in vivo [25]. Most of the genes induced by active NFAT1 in the absence of AP-1 cooperation have an NFAT1 binding site close to their transcription start site [25], suggesting direct transcriptional induction by NFAT.
Figure 4. In the absence of AP-1 cooperation, NFAT transcription factors drive expression of genes associated with a hyporesponsive state.
(A) Effector CTLs show activated NFAT1, AP-1, and NF-κB transcription factors among others, which together drive the expression of effector-related genes. Many of those genes (e.g., genes encoding cytokines, such as IL-2, IFN-γ, GM-CSF, etc.; see bottom panel) are dependent on NFAT:AP-1 cooperation. (B) Hyporesponsive cells lack or have turned off signals that lead to the induction or activation of AP-1 (Fos-Jun family) factors but sustain calcium signaling at a low level sufficient for NFAT activation. NFAT binds alone, or more likely in cooperation with yet-to-be-determined transcription factors, to NFAT-responsive elements, leading to the induction of genes associated with a hyporesponsive state (e.g., inhibitory receptors and transcription factors as Nr4a members, see bottom panel). In turn, those newly induced transcription factors bind—perhaps with other factors including NFAT itself—to regions that are accessible in hyporesponsive cells, potentially contributing to the maintenance of the hyporesponsive state. Exh. TF, exhaustion-related transcription factors.
The mechanisms by which NFAT family members regulate exhaustion require further elucidation. NFAT2 has been shown to regulate the expression of PD-1 [54]. In our own studies, we have demonstrated by genome-wide ChIP that NFAT1 can bind to the Pdcd1 locus and that both NFAT1 and NFAT2 regulate expression of PD-1 and several other inhibitory receptors [25]. We observed that PD-1 is decreased in cells lacking NFAT2; however, cells deficient in both NFAT1 and NFAT2 have even further reduced expression of PD-1, suggesting an overlapping function of these two NFAT proteins [25, 55].
We have used CA-RIT-NFAT1, an engineered constitutively active NFAT1 that is unable to cooperate with AP-1, to induce a program of hyporesponsiveness resembling anergy/exhaustion in both CD4+ and CD8+ T cells [25, 26]. Emphasizing the role of NFAT in these hyporesponsive states, we found a significant overlap in transcriptional profiles and chromatin-accessibility patterns in CA-RIT-NFAT1–expressing cells, compared with exhausted T cells in both chronic LCMV infection and tumor models [19, 27]. Moreover, accessible regions induced by CA-RIT-NFAT1 resemble accessible regions specific to exhausted cells in vivo and both show enrichment for consensus binding motifs for NFAT- and Nr4a-family transcription factors.
Indeed, our data provide evidence for a potential transcriptional hierarchy in exhausted T cells that connects NFAT with Nr4a-family nuclear receptors [19, 27]. mRNAs encoding both Nr4a2 (Nurr1) and Nr4a3 (Nor1) are highly induced in cells ectopically expressing CA-RIT-NFAT1, and both genes appear to be direct transcriptional targets of NFAT, based on NFAT1 binding to the Nr4a2 and Nr4a3 loci by ChIP-seq analyses [25]. Similarly, Nr4a2 is more highly expressed in exhausted cells arising during chronic LCMV infection than in naive T cells or effector and memory cells generated during acute LCMV infection [19] (Fig. 1), and both Nr4a2 and Nr4a3 are expressed more highly in tumor-infiltrating, tumor Ag-reactive OT-I (exhausted) cells than in bystander (nonexhausted) P14 cells in the tumor model [27]. Nr4a family members have been shown to control the development and function of Tregs [56]. Thus, induction of Nr4a transcription factors by NFAT during hyporesponsive states could create a feedback loop in which Nr4a members could sustain the hyporesponsive phenotype, similar to their function in Tregs.
Several other transcription factors—including Egr2, Tox, Tox2, Ikzf2, Irf4, Nfatc1, and Prdm1—are highly expressed in exhausted cells and are also induced by constitutively active NFAT1 in the absence of AP-1 cooperation [19, 25] (Fig. 1). Moreover, the changes in chromatin accessibility induced by CA-RIT-NFAT1 overlap significantly with those of exhausted cells in vivo [19, 27]. Together, these findings form the basis for our hypothesis that NFAT transcription factors initiate, and potentially also maintain, the transcriptional program of CD8+ T cell exhaustion (Fig. 4). Other mechanisms may operate downstream of inhibitory receptors to maintain the exhausted state: for instance, PD-1 ligation has been reported to induce BATF and to block CD28 signaling [57, 58]. Indeed, some of these downstream mechanisms may again involve NFAT. Further studies will be required to understand how NFAT induces and/or cooperates with other transcription factors to drive the transcriptional network of exhausted cells.
OTHER TRANSCRIPTION FACTORS IMPLICATED IN T CELL HYPORESPONSIVE STATES
Several reports have highlighted the importance of individual transcription factors in the generation or maintenance of hyporesponsive states. Because of space limitations, we focus here on recent reports that highlight the importance of the balance between T-bet and Eomes and the role of TCF-1 in the generation of “progenitor” and terminally exhausted cells (Fig. 5). Other factors that have been shown to have important roles in CD8+ T cells during Ag persistence include Blimp-1 [59–61], BATF [57, 62], FoxO1 [63], FoxO3 [64, 65], and HIF family members [66], among others (Fig. 1).
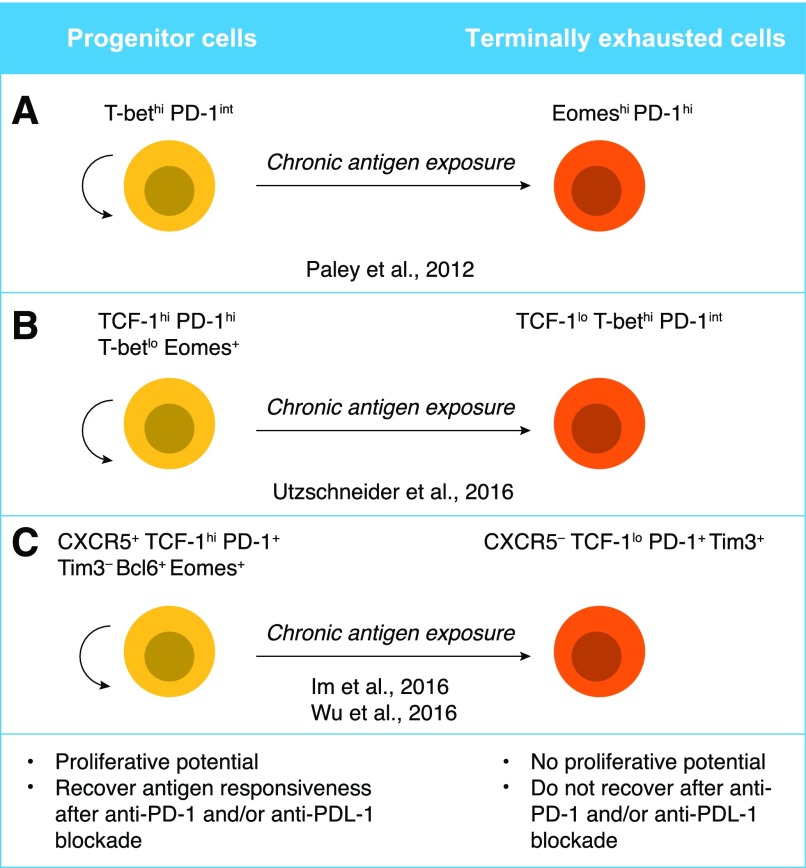
Figure 5. Proposed models for progenitor and terminally differentiated exhausted T cells.
(A, Left) T-bethi PD-1int cells retain some proliferation potential as well as the ability to produce cytokines. (A, Right) Eomeshi PD-1hi cells have lower proliferation potential as well as lower cytokine production. T-bethi PD-1int cells can give rise to the Eomeshi PD-1hi population upon persistent Ag exposure, thus, are suggested to be “progenitor” and “terminally differentiated” cells, respectively [36, 37]. (B) Progenitor cells have a distinct phenotype characterized by TCF-1hi PD-1hi Eomes+ T-betlo, and that population can give rise to TCF-1lo PD-1int T-bethi [75]. (C) Cells expressing the surface markers CXCR5 and PD-1, as well as transcription factors TCF-1, Bcl6, and Eomes, have a greater proliferation potential and act as progenitors, giving rise to more terminally differentiated, exhausted T cells, which show a reduction in CXCR5 and TCF-1 and an increase in Blimp-1 and TIM-3 expression [78, 79].
T-bet and Eomes
The differentiation and function of CTLs are largely regulated by the T-box transcription factors T-bet and Eomes. Although T-bet regulates effector function, Eomes mainly controls the expression of proteins that maintain a memory CD8+ T cell pool (reviewed by Kaech and Cui [1]). The ratio between T-bet and Eomes is crucial for determining the fate of CD8+ T cells during acute [1] and chronic [37] viral infection (Fig. 1). T-bethi cells displayed low intrinsic turnover but proliferated in response to persisting Ags, giving rise to Eomeshi terminal progeny [37]. The T-bethi subset expresses intermediate levels of PD-1 (PD-1int), whereas Eomeshi cells express high levels of PD-1 (PD-1hi) [37]; PD-1int exhausted cells can be reinvigorated by PD-1 blockade, whereas the PD-1hi subset cannot [36] (Fig. 5).
Consistent with the fact that T-bethi cells express lower levels of PD-1 compared with Eomeshi cells (Fig. 5), T-bet can directly repress PD-1 expression during chronic viral infection [67]. Mice with specific deletion of Tbx21 (the gene coding for T-bet) in T cells showed increased viral titers during chronic viral infection, with Ag-specific T cells, in particular, showing an inverse correlation between PD-1 and T-bet expression. Conversely, forced expression of T-bet led to a decrease not only of PD-1 but also of several other inhibitory receptors, including LAG-3, CD160, and BTLA [67]. Moreover, inhibition of the kinase GSK3 was associated with increased T-bet expression and decreased PD-1 expression both in vitro and in vivo; and in vivo administration of the GSK3 inhibitor resulted in decreased viral loads in LCMV clone 13 and MHV-68 viral models, with a concomitant increase in CTL effector function, including cytokine production and target killing [68].
T-bet has also been suggested to affect TIM-3 expression, albeit the regulation is more complex. Tbx21−/− cells showed less TIM-3 expression at early times after viral infection with either acute or chronic LCMV infection models (Armstrong 5 and clone 13, respectively) [67]. Similarly, T-bet, but not STAT4, directly regulated TIM-3 expression in CD4+ Th1 cells [69]. However, forced expression of T-bet also resulted in a slight decrease in TIM-3 expression after infection with LCMV clone 13 [67]. Thus, the exact contribution of T-bet to TIM-3 expression throughout the course of viral infection still needs further investigation. Because NFAT1 directly induces TIM-3 [25], T-bet may depend on, or cooperate with, NFAT and/or other transcription factors to regulate TIM-3 expression.
TCR signal strength directly regulates the relative expression of T-bet and Eomes in Ag-specific CD8+ T cells by modulating IRF4 levels, as shown by using P14 TCR transgenic cells and variants of the LCMV gp33–41 peptide with different affinities for the P14 TCR [70]. Reduced IRF4 expression skewed this ratio in favor of Eomes during chronic LCMV infection, resulting in a more pronounced exhaustion phenotype and impaired viral control [70]. These data suggest that IRF4 has a role in regulating T cell exhaustion by controlling the ratio between T-bet and Eomes during chronic infection.
HIV persistence in humans is also associated with an altered balance of Eomes and T-bet expression [71]. HIV-specific CD8+ T cells from long-term nonprogressors retained high T-bet expression [72]. At the single-cell level, HIV-specific CD8+ T cells in chronic infection were marked by high levels of Eomes, but lower T-bet expression (T-betdim), and this T-betdim Eomeshi profile was associated with up-regulation of the inhibitory receptors PD-1, CD160, and 2B4, impaired effector function, and a transitional memory differentiation phenotype. Eomes levels were persistently high in virus-specific cells, even after antiretroviral therapy, potentially explaining the inability of HIV-specific CD8+ T cells to control viral replication after cessation of therapy [71].
TCF-1
TCF-1 (encoded by the Tcf7 gene) is critical for the generation of memory precursor T cells and for protective immunity after resolution of acute LCMV infection [73, 74]. During chronic infections, CD8+ T cells expressing TCF-1 display hallmarks of the exhausted phenotype (e.g., expression of PD-1 and LAG-3). Transcriptional profiling showed that these cells lose the effector signature but acquire some characteristics of central memory cells, as judged by expression of CD62L and Eomes. Indeed, memory CTLs show greater TCF-1 expression compared with either exhausted or effector CTLs (Fig. 1). Consistent with these findings, TCF-1 expression is dispensable for initial T cell expansion, but essential for reexpansion of chronically stimulated CD8+ T cells and for long-term pathogen control [75]. A TCF-1+ subpopulation was also detected among virus-specific CD8+ T cells in patients with chronic HCV infection, and as in chronic LCMV infection, TCF-1+ HCV-specific cells expressed greater levels of CD127 (the IL-7 receptor, a marker of memory T cells) compared with TCF-1− cells [75].
Blocking PD-1/PD-L1 interactions can increase effector functions and proliferation of Ag-specific CD8+ T cells, which correlates with a decrease in pathogen load and an increase in tumor control [35, 76, 77] (see “Transcriptional changes and reversibility of exhaustion in response to checkpoint blockade” section). Whereas the PD-1/PD-L1 blockade in LCMV clone 13-infected mice resulted in increased expansion of Ag-specific P14 TCR transgenic T cells compared with their expansion in mice treated with isotype control Abs, Tcf7−/− P14 T cells failed to expand [75]. Additionally, when Tcf7-GFP reporter mice were subjected to chronic LCMV infection, only GFP+, but not GFP−, P14 T cells showed significant expansion after transfer into secondary infected recipients that were treated with anti–PD-1. These data demonstrate that, at least in a chronic infection mouse model, the effectiveness of inhibitory receptor blockade depended on the TCF-1+ CD8+ T cell population (Fig. 5).
TCF-1–expressing cells identified during chronic infection exhibit a unique gene signature that is similar to that of CD4+ Tfhs, memory precursor CD8+ T cells, and hematopoietic stem cell progenitors [78]. These cells, characterized as CXCR5+PD-1+CD8+, depend on TCF-1 for their generation and reside predominantly in the T cell zone of lymphoid tissue in chronically LCMV-infected mice [78]. Similarly, TCF-1–expressing CD8 T cells in chronic infection and cancer resembled Tfhs, with increased expression of CXCR5 and Bcl6 and reduced expression of Blimp-1; they were intrinsically able to persist and show better recall responses than TCF-1low cells, suggesting a less-exhausted state. In fact, those cells acted as progenitors, giving rise to more terminally differentiated exhausted T cells, which showed a reduction in TCF-1 and an increase in Blimp-1 [79]. Overexpression of TCF-1 led to increased expression of CXCR5 and Bcl-6, and reduced expression of Blimp-1 and TIM-3; because TCF-1 bound the promoters of these genes, it likely regulated their expression directly [79].
Finally, neutralization of IFN-α using cells lacking IFNARI (IFN receptor 1) or a neutralizing Ab against this receptor, led to an increase in TCF-1hi cells, suggesting that type-I IFNs act upstream of TCF-1, directly inducing a more-exhausted TCF-1low cell pool [79]. Indeed, it has been previously suggested that type I IFNs induce exhaustion and viral persistence [80, 81]. Consistent with that hypothesis, blocking type I IFN signaling was associated with reduced expression of exhaustion-associated markers in T cells in a humanized mouse model of chronic HIV infection. Moreover, the combination of antiretroviral therapy and blockade of type I IFN signaling led to a faster decrease in viral loads [82, 83].
Transcriptional regulation in exhausted CD4 T cells
Upon chronic LCMV infection, dysfunctional CD4+ Th cells were also identified. These cells clearly showed a bias against Th1 cells, but not against or in favor of other Th subsets, including Th2, Th17, Tregs, or Tfh cells [21]. Interestingly, those dysfunctional CD4+ T cells seemed to comprise a heterogeneous population, with Blimp-1hi Bcl-6lo and Bcl-6hi Blimp-1lo cells and a peculiar pattern of expression of T-box transcription factors: T-bet expression was greater in Blimp-1–expressing cells, whereas Eomes was greatest in Bcl-6–expressing cells. However, Blimp-1+ cells also expressed greater levels of Eomes compared with naive cells [21].
Dysfunctional CD4+ T cells shared some of the transcriptional signature of exhausted Ag-specific CD8+ T cells but also displayed distinct features. Among the transcription factors expressed both in CD4+ and CD8+ T cells in chronic viral infection are the Nr4a family of transcription factors, Eomes, Zeb2, Bach2, Nfil3, Batf, Nfkbiz, Prdm1, and others [21]. Among the transcription factors whose expression was reported to be biased toward CD4+ T cells in chronic viral infection were Egr2 and Helios [21], but recent reports indicate that those two transcription factors are highly expressed in exhausted CD8+ T cells as well. Egr2 was shown to be up-regulated in TIM-3+ PD-1+ CD8 T cells in chronic LCMV infection [19] or in 4-1BB+ LAG-3+ tumor-infiltrating lymphocytes [84] and Helios in dysfunctional CD8+ T cells in a tumor model [42]. Thus, although certain features might indeed be specific for CD4+ or CD8+ T cells, multiple reports now highlight the possibility that the program of hyporesponsiveness induced by persistent Ag exposure in CD4+ and CD8+ T cells shows greater similarity than initially assumed.
TRANSCRIPTIONAL CHANGES AND REVERSIBILITY OF EXHAUSTION IN RESPONSE TO CHECKPOINT BLOCKADE
Reversal of the exhaustion phenotype is a very important clinical goal and, as such, has been a major focus of research in the past several years. Blockade of the PD-1/PD-L1 pathway during chronic LCMV infection reinvigorates virus-specific CD8+ T cell responses, resulting in lower viral loads [76]. Moreover, blockade of other inhibitory checkpoint receptors, including CTLA-4, TIM-3, and LAG-3 (individually or combined with PD-1 blockade), has been shown to reverse exhaustion efficiently in CD8+ T cells [33–35].
Blocking Abs to PD-1 and CTLA-4 have emerged as effective agents for cancer immunotherapy [7]. Compared with patients who received conventional therapies, patients with advanced melanoma, non–small cell lung cancer and renal cell carcinoma exhibited objective responses accompanied by extended overall survival when treated with mAbs that inhibited the PD-1/PD-L1 pathway, resulting in recent regulatory approvals by the US Food and Drug Administration (FDA) for the use of two different anti–PD-1 Abs (nivolumab and pembrolizumab) or anti–PD-L1 Ab (atezolizumab) in those diseases. Increasing evidence also supports the use of these drugs in other cancer types, such as bladder cancer, Hodgkin lymphoma, and head and neck cancers. CTLA-4 blockade using the mAb ipilimumab, as well as combined therapy with ipilimumab and nivolumab, has also been approved by the FDA for advanced melanoma [85]. Besides PD-1 and CTLA-4, additional checkpoint pathways are being targeted clinically, including LAG-3 and TIM-3, and combinations of those blocking therapies are showing promise in the clinic [85].
CD8+ T cell responses improve with anti–PD-1 checkpoint blockade [76], suggesting that the effector function of CD8+ T cells is not completely abolished during chronic infections. In fact, a subpopulation of memory-like cells, which express the transcription factor TCF-1 and have characteristics of central memory and exhausted cells, maintain long-term responses during chronic LCMV infection [75, 78]. As mentioned in the “TCF-1” section, TCF-1 is needed for reexpansion of chronically stimulated CD8+ T cells in response to PD-1 blockade [75, 78]. The expanded cells also express PD-1 and several costimulatory molecules (ICOS, OX-40, CD28) and exhibit a transcriptional signature related to that of CD4+ Tfh cells [78]. The TCF-1+ T cells able to proliferate after checkpoint inhibitors, however, are predominantly PD-1hi T-betlo and Eomes+. Those results contradict previous published data showing that PD-1mid Tbethi Eomeslo cells could be reinvigorated by PD1 blockade, whereas CD8+ T cells expressing high levels of PD-1 could not [36] (Figs. 5 and 6). Additional studies will be needed to resolve these discrepancies.
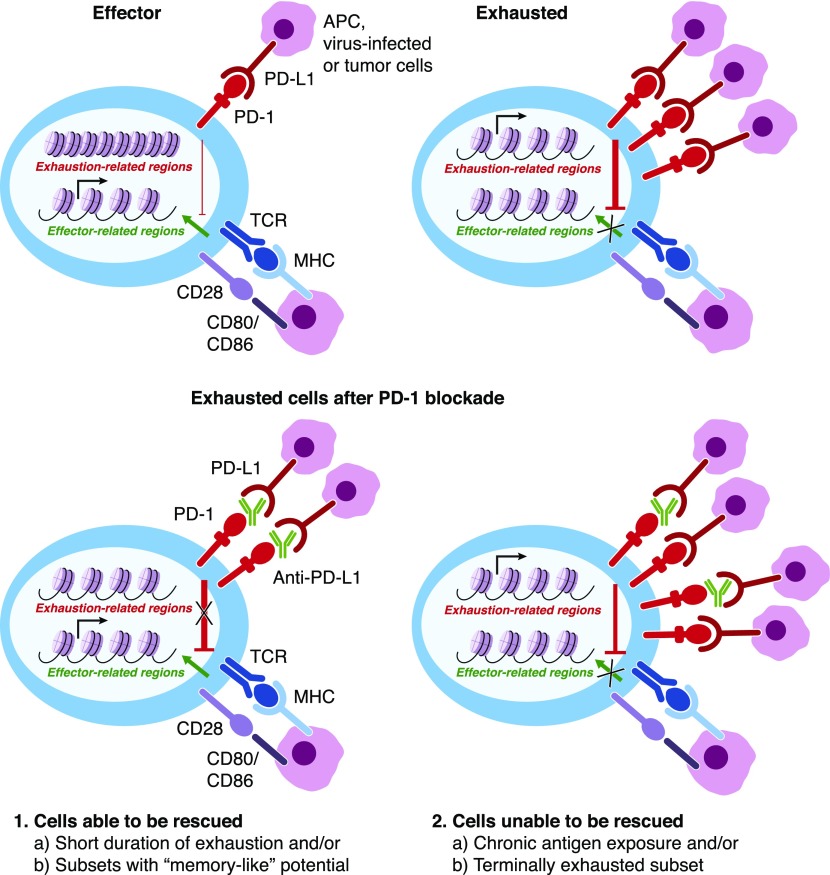
Figure 6. Anti–PD-1/PD-L1 blockade affects a distinct population of exhausted cells.
Anti–PD-1 or anti–PD-L1 therapies have been recently shown to affect “progenitor” cells (see Fig. 5) and/or cells that have been exposed to Ags for shorter periods [24] and result in only a few chromatin-accessibility changes [27, 39]. Cells able to be reverted temporarily from the exhaustion phenotype are depicted in the left panel, whereas “terminally differentiated” cells that are unable to be rescued from checkpoint blockade are depicted in the right panel.
Transcriptional analyses demonstrated that exhausted T cells from anti-PD-L1–treated mice exhibited a gene-expression program that partially overlapped with the effector T cell program, in that it was largely driven by cell-cycle pathways. However, there was minimal overlap with the memory T cell program, suggesting limited acquisition of memory potential [86]. These analyses were performed on the total pool of exhausted cells, and single-cell analyses or additional experiments with purified subpopulations of exhausted T cells are needed to elucidate whether checkpoint inhibitor therapies give rise to small subsets of cells with memory potential. Several metabolic genes were also altered after PD-L1 blockade [86], consistent with the previous demonstration that key metabolic events driven by PD-1 were implicated in the establishment of exhaustion [86] (see “Connection between metabolic reprograming and transcriptional regulation of exhausted T cells” section).
Although checkpoint blockade therapies are promising, many patients either develop side effects or fail to develop sustainable responses [30, 87]. PD-1/PD-L1 blockade during chronic LCMV infection induced an effector-like transcriptional program in exhausted T cells that was not sustained after cessation of anti–PD-L1 treatment [39] (Fig. 2). T cells from anti-PD-L1–treated mice showed increased expansion and concomitant changes in the expression of genes related to cell division and genes associated with effector function, compared with T cells from control-treated mice. However, there were far fewer changes in chromatin-accessibility patterns between these two groups of exhausted T cells. Motif enrichment analysis of the few differentially accessible regions suggested that cells from anti-PD-L1–treated mice augmented activity of NF-κB, AP-1, and IRF family members but decreased activity of NFAT, Egr2, and Nur77 (Nr4a1) [39]. Combined treatment with anti–PD-L1 and IL-7 led to a stronger effector response, with enhanced production of the cytokines IFN-γ and TNF [39]. Whether this combined treatment would be more beneficial than anti–PD-L1 treatment alone in the longer term was not addressed.
Chromatin accessibility in exhausted CTLs has also been studied using a tumor model [27] (Fig. 2), in which previously activated OT-I and P14 TCR transgenic cells were adoptively transferred into congenic mice previously inoculated with B16-OVA tumor cells, and OT-I, but not P14, cells acquired characteristics of exhaustion. Again, treatment with anti–PD-L1 Abs—which was effective because it caused tumor regression—resulted in only minor changes in chromatin accessibility and gene expression in the tumor-infiltrating OT-I cells, but the up-regulated genes (encoding granzymes and serpins) correlated with increased effector function. This study and the one discussed in the preceding paragraph [39] are in agreement that anti–PD-L1 treatment leads to relatively minor changes in chromatin accessibility and gene expression. The interpretations are also similar: both studies agreed that anti–PD-L1 treatment (presumably by blocking the negative signals known to be transmitted to T cells through PD-1, an ITIM-containing protein), leads to a “rewiring” of the transcriptional profile of exhausted T cells due to increased binding of transcription factors, such as NF-κB and AP-1, to accessible regions already present in exhausted cells, allowing them to acquire effector gene expression.
The B16–OVA model described above [27] suffers from the drawbacks that the tumor is ectopically introduced and the T cells are preactivated before transfer. To eliminate these drawbacks, Schietinger et al. [24] used a tamoxifen-inducible liver-cancer mouse model with a defined oncogenic driver Ag (SV40 large T-Ag) to track the activation of tumor-specific CD8+ T cells. Naive TCR transgenic T cells (TCRSV40-I) were adoptively transferred to congenic mice 2 d before the induction of the driver oncogene and were analyzed on d 8 and 35 after tumor induction. When d-8 TCRSV40-I cells were isolated from malignant lesions and cultured for 3–4 d in vitro in the presence of IL-2 and anti–PD-1 blocking Abs, they appeared to be in a plastic cell state and were reprogrammed to become functional T cells (as measured by IFN-γ and TNF production). However d-35 TCRSV40-I cells failed to restore effector functions, suggesting that a fixed state of dysfunction persists even in the absence of Ags (Fig. 6). Similar results were obtained in vivo, where PD-1 and PD-L1 blockade partially rescued the effector function of d-8 cells (measured as granzyme B expression) but failed to restore the effector functions of d-35+ TCRSV40-I cells. Together, these recent reports suggest that the therapeutic potential of anti–PD-1 or anti–PD-L1 treatments is limited and further reinforce the necessity of delineating the molecular mechanisms driving and/or maintaining hyporesponsive states, so that better therapeutics can be developed.
CONNECTION BETWEEN METABOLIC REPROGRAMING AND TRANSCRIPTIONAL REGULATION OF EXHAUSTED T CELLS
The role of metabolic pathways and metabolites in T cell biology and how they can affect T cell differentiation has been a growing area of research in the past years. It is now widely accepted that effector T cells use primarily anabolic pathways of metabolism, such as aerobic glycolysis, whereas memory T cells employ catabolic pathways, such as fatty acid oxidation (reviewed in [88, 89]). However, the role of different metabolic pathways in driving or contributing to exhaustion has remained unclear.
In models of both chronic infection and antitumor responses, exhausted CD8+ T cells have been shown to exhibit metabolic changes compared with effector T cells [86, 90]. Transcriptional profiling of exhausted T cells in early chronic LCMV infection, compared with later stages, revealed enrichment of genes whose products are involved in oxidative phosphorylation, citrate cycle, fatty acid and amino acid metabolism, paradoxically coupled to defects in glycolysis and oxidative phosphorylation [86]. Although mitochondrial respiration was reduced, mitochondrial mass was higher in early exhausted T cells compared with effector cells, consistent with the altered transcriptional profile observed. However, early exhausted T cells showed depolarization of the mitochondrial membrane, explaining the reduced oxidative phosphorylation. Greater mitochondrial mass was also observed at the stage of established chronic infection, although it was reduced compared with early infection [86]. In contrast, in the tumor model, tumor-infiltrating T lymphocytes showed reduced mitochondrial mass and reduced oxidative phosphorylation, which were induced upon entry into the tumor microenvironment [90]. Interestingly, PD-1 blockade did not alter the metabolic dysfunction observed in the tumor model but did have an effect in the chronic-infection model, particularly in PD-1int exhausted T cells [86, 90].
Both reports identified the transcriptional regulator PGC-1α (PPAR-γ coactivator 1 α) as a potential mediator of metabolic dysfunction in exhausted T cells [86, 90]. PGC-1α was previously shown to control mitochondrial biogenesis [91], and overexpression of PGC-1α rendered cells more functional in both models [86, 90]. The details of transcriptional regulation leading to mitochondrial dysfunction in exhausted cells remains to be fully determined, but it is possible that PPAR-γ or mitochondrial transcription factor A might have roles in this process with additional, yet-to-be-determined transcription factors.
Intratumor T lymphocytes are exposed to lower concentrations of glucose, leading to impaired calcium signaling and NFAT activation with no defect in Akt or Erk phosphorylation [92]. The effect was attributed to blockade of SERCA-mediated calcium reuptake in the ER by phosphoenolpyruvate. T cell overexpression of phosphoenolpyruvate carboxykinase 1 (PCK1), which generates the phosphoenolpyruvate metabolite, led to enhanced activation of the NFAT pathway, resulting in increased antitumor activity [92]. Whether AP-1 or other transcription factors cooperate with NFAT in the induction of that antitumor activity was not addressed. If overexpression of PCK1 resulted in increased activation of AP-1 as well, the resulting NFAT:AP-1 cooperation would be expected to produce enhanced effector function.
Lower glucose concentration in the tumor microenvironment also led to enhanced expression of microRNAs miR-101 and miR-26a, which bind the 3′-untranslated region of Ezh2 mRNA, leading to decreased Ezh2 expression [93]. In human T cells, Ezh2 directly regulated expression of the Notch inhibitors Numb and Fbxw7, and cells expressing Ezh2 and Notch intracellular domain showed effector properties, including simultaneous expression of IL-2, IFN-γ, and TNF [93]. Whether other transcriptional or epigenetic modifiers are regulated by glucose levels in the tumor microenvironment and the extent through which that modulation affects T exhaustion remain to be investigated.
In a mouse model of B cell leukemia, the generation of exhausted T cells involved impaired metabolism, including decreased activity of the Akt/mTORC1 pathway and decreased expression of the glucose transporter Glut1, leading to reduced glucose uptake and reduced levels of the glycolytic enzyme hexokinase. Interestingly, although treatment with anti–PD-1 in vivo had no effect on T cell function, forced Akt/mTORC1 signaling partially reverted T cell exhaustion, leading to a delay in disease progression. A similar dysfunctional metabolic state was also observed in T cells from patient samples with B cell leukemias [94].
Overall, these reports highlight the importance of metabolites and shifts in the use of metabolic pathways as contributors to the induction of T cell exhaustion. How those changes in metabolic pathways are coupled to the transcriptional and epigenetic changes that occur in hyporesponsive T cells is not completely understood. Further research in this area would help elucidate the link between metabolic changes and transcriptional networks in the hyporesponsive states that develop during Ag persistence.
CONCLUDING REMARKS
T cell exhaustion has emerged as an important topic of investigation, relevant not only in T cell biology but also in the clinic because the presence of exhausted cells correlates with poor patient prognosis in several types of cancers and chronic infections. Although checkpoint inhibitor therapies show considerable promise for restoring T cell function and improving the survival of patients with cancer, the unresponsiveness of some types of tumors (including mixed metastatic tumors) to this therapeutic approach and the reacquisition of features of exhaustion by tumor-infiltrating T cells still represent a substantial challenge [85]. A clearer understanding of the biologic and molecular aspects of T cell exhaustion is an essential step, not only to design more efficient strategies of checkpoint blockade but also to develop new therapies (combined or otherwise) to avoid the generation of exhausted cells. There is increasing evidence that exhausted cells are not a homogeneous population and that their heterogeneity can dictate the success of immunotherapy.
It was originally suggested that levels of T-bet, Eomes, and PD-1 define T cell subpopulations that can or cannot be reinvigorated by PD-1 blockade [36]. Important studies from the past year [75] have added to our comprehension of the subpopulations that comprise the pool of exhausted cells. Two additional important markers, TCF-1 and CXCR5, have been implicated in the generation of cells that have memory potential and are able to re-exhibit effector function [75]. However, our understanding of this population is still somewhat limited because TCF-1+ cells that are able to proliferate after PD-1 pathway blockade [75] are predominantly PD-1hi T-betlo and Eomes+, not PD-1int T-bethi Eomes−, as previously suggested [37] (Fig. 5). Not only is the heterogeneity of the exhausted T cell population an obstacle for the development of better immunotherapies, but a challenge is also posed by the fact that current markers, transcriptional signatures, and chromatin accessibility are very conserved between exhausted and activated cells.
Recent single-cell profiling of tumor-infiltrating lymphocytes has begun to circumvent these challenges and to identify distinct gene modules for T cell dysfunction and activation [42]. In this study, Gata-3 was suggested as a potential regulator of dysfunction in a tumor model: depletion of Gata-3 led to increased cytokine production and tumor rejection, without affecting expression of the inhibitory receptors TIM-3 and PD-1 [42]. However, Gata-3 expression was not significantly up-regulated in PD-1+ TIM-3+ Ag-specific CD8+ T cells from LCMV clone 13–infected mice [19] (Fig. 1), suggesting that hyporesponsive cells might have different transcriptional programs depending on the particular microenvironment to which they are exposed. Additional studies addressing protein expression and epigenetic modifications in different Ag-persistence models will be informative for further characterization of cellular subpopulations expressing various transcriptional profiles. For instance, expanding chromatin-accessibility assays to the single-cell scale may help correlate dysfunctional gene expression with the accessibility of gene regulatory regions. Moreover deleting exhaustion-specific enhancers or modulating enhancer function by targeting inhibitory or activating Cas9 fusion proteins may provide more-specific and more-adaptable approaches to modulating the function of T cells than do coding-region deletions.
In an attempt to circumvent immune evasion in the tumor microenvironment [95], CAR T cell therapies directed toward solid tumors have emerged as important tools to rescue T cell tumor-specific activity [96]. T cells genetically engineered to express artificial TCRs can enhance tumor specificity, and their ex vivo expansion enables the production of high doses of these therapeutic cells, with striking and unprecedented results in the clinic [96]. However, solid tumors up-regulate coinhibitory ligands that bind to inhibitory receptors on transferred T cells, which also compromises the efficacy of CAR T cell therapies [97]. Thus, the combination of CAR T cells with other therapeutic strategies has considerable promise in the clinic. For instance, PD-1/PD-L1 blockade may be an effective strategy for improving the potency of CAR T cell therapies, as demonstrated in a clinically relevant model of pleural mesothelioma [97]. In the same sense, editing of genomic regulatory regions associated with T cell dysfunction could potentially result in a major improvement of CAR T cell therapies. Although a newly identified exhaustion related PD-1 enhancer [19, 39, 40] might not be conserved in humans, the fact that its disruption by CRISPR/Cas9 led to decreased PD-1 expression in a cell line [39] is encouraging and highlights the potential of genome modification as a therapeutic approach. Thus, a better understanding of the molecular mechanisms that drive T cell hyporesponsiveness (anergy, exhaustion, dysfunction) is a timely and important goal, and data emerging from such studies will drive the next generation of therapies against cancer and chronic infectious diseases.
AUTHORSHIP
R.M.P. and G.J.M. performed bibliographical search, conceptualized the manuscript and made the figures; R.M.P., P.G.H., A.R. and G.J.M. wrote the manuscript.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health Grants R01 AI109842 and AI 40127 (to A.R. and P.G.H.), Rosalind Franklin University start-up (to G.J.M.), and International Centre for Genetic Engineering and Biotechnology Research Grant CRP/BRA16-05_EC (to R.M.P.).
Glossary
- ATAC-seq
assay for transposase-accessible chromatin with high-throughput sequencing
- bHLH
basic helix-loop-helix
- bZIP
basic region-leucine zipper
- CAR
chimeric Ag receptor
- ChIP
chromatin immunoprecipitation
- CRISPR
clustered regularly interspaced short palindromic repeats
- HCV
hepatitis C virus
- IRF
IFN regulatory factor
- LCMV
lymphocytic choriomeningitis virus
- NFAT
nuclear factor of activated T cell
- TCF-1
transcription factor t cell factor 1
- TF
transcription factor
- Tfh
follicular helper T cell
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Kaech S. M., Cui W. (2012) Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 12, 749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weng N. P., Araki Y., Subedi K. (2012) The molecular basis of the memory T cell response: differential gene expression and its epigenetic regulation. Nat. Rev. Immunol. 12, 306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zediak V. P., Johnnidis J. B., Wherry E. J., Berger S. L. (2011) Cutting edge: persistently open chromatin at effector gene loci in resting memory CD8+ T cells independent of transcriptional status. J. Immunol. 186, 2705–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zajac A. J., Blattman J. N., Murali-Krishna K., Sourdive D. J., Suresh M., Altman J. D., Ahmed R. (1998) Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188, 2205–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wherry E. J. (2011) T cell exhaustion. Nat. Immunol. 12, 492–499. [DOI] [PubMed] [Google Scholar]
- 6.Wherry E. J., Ha S. J., Kaech S. M., Haining W. N., Sarkar S., Kalia V., Subramaniam S., Blattman J. N., Barber D. L., Ahmed R. (2007) Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27, 670–684. [DOI] [PubMed] [Google Scholar]
- 7.Schietinger A., Greenberg P. D. (2014) Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 35, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin H., Wherry E. J. (2007) CD8 T cell dysfunction during chronic viral infection. Curr. Opin. Immunol. 19, 408–415. [DOI] [PubMed] [Google Scholar]
- 9.Jayaraman P., Jacques M. K., Zhu C., Steblenko K. M., Stowell B. L., Madi A., Anderson A. C., Kuchroo V. K., Behar S. M. (2016) TIM3 mediates T cell exhaustion during mycobacterium tuberculosis infection. PLoS Pathog. 12, e1005490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baitsch L., Baumgaertner P., Devêvre E., Raghav S. K., Legat A., Barba L., Wieckowski S., Bouzourene H., Deplancke B., Romero P., Rufer N., Speiser D. E. (2011) Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J. Clin. Invest. 121, 2350–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fourcade J., Sun Z., Benallaoua M., Guillaume P., Luescher I. F., Sander C., Kirkwood J. M., Kuchroo V., Zarour H. M. (2010) Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J. Exp. Med. 207, 2175–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mumprecht S., Schürch C., Schwaller J., Solenthaler M., Ochsenbein A. F. (2009) Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood 114, 1528–1536. [DOI] [PubMed] [Google Scholar]
- 13.Poschke I., De Boniface J., Mao Y., Kiessling R. (2012) Tumor-induced changes in the phenotype of blood-derived and tumor-associated T cells of early-stage breast cancer patients. Int. J. Cancer 131, 1611–1620. [DOI] [PubMed] [Google Scholar]
- 14.Lu B., Chen L., Liu L., Zhu Y., Wu C., Jiang J., Zhang X. (2011) T-cell-mediated tumor immune surveillance and expression of B7 co-inhibitory molecules in cancers of the upper gastrointestinal tract. Immunol. Res. 50, 269–275. [DOI] [PubMed] [Google Scholar]
- 15.Wherry E. J., Kurachi M. (2015) Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pauken K. E., Wherry E. J. (2015) Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 36, 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Topalian S. L., Drake C. G., Pardoll D. M. (2015) Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27, 450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuniga E. I., Macal M., Lewis G. M., Harker J. A. (2015) Innate and adaptive immune regulation during chronic viral infections. Annu. Rev. Virol. 2, 573–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott-Browne J. P., Lopez-Moyado I. F., Trifari S., Wong V., Chavez L., Rao A., Pereira R. M. (2016) Dynamic changes in chromatin accessibility occur in CD8+ T cells responding to viral infection. Immunity 45, 1327–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doering T. A., Crawford A., Angelosanto J. M., Paley M. A., Ziegler C. G., Wherry E. J. (2012) Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity 37, 1130–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crawford A., Angelosanto J. M., Kao C., Doering T. A., Odorizzi P. M., Barnett B. E., Wherry E. J. (2014) Molecular and transcriptional basis of CD4+ T cell dysfunction during chronic infection. Immunity 40, 289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz R. H. (2003) T cell anergy. Annu. Rev. Immunol. 21, 305–334. [DOI] [PubMed] [Google Scholar]
- 23.Apetoh L., Smyth M. J., Drake C. G., Abastado J. P., Apte R. N., Ayyoub M., Blay J. Y., Bonneville M., Butterfield L. H., Caignard A., Castelli C., Cavallo F., Celis E., Chen L., Colombo M. P., Comin-Anduix B., Coukos G., Dhodapkar M. V., Dranoff G., Frazer I. H., Fridman W. H., Gabrilovich D. I., Gilboa E., Gnjatic S., Jäger D., Kalinski P., Kaufman H. L., Kiessling R., Kirkwood J., Knuth A., Liblau R., Lotze M. T., Lugli E., Marincola F., Melero I., Melief C. J., Mempel T. R., Mittendorf E. A., Odun K., Overwijk W. W., Palucka A. K., Parmiani G., Ribas A., Romero P., Schreiber R. D., Schuler G., Srivastava P. K., Tartour E., Valmori D., van der Burg S. H., van der Bruggen P., van den Eynde B. J., Wang E., Zou W., Whiteside T. L., Speiser D. E., Pardoll D. M., Restifo N. P., Anderson A. C. (2015) Consensus nomenclature for CD8+ T cell phenotypes in cancer. OncoImmunology 4, e998538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schietinger A., Philip M., Krisnawan V. E., Chiu E. Y., Delrow J. J., Basom R. S., Lauer P., Brockstedt D. G., Knoblaugh S. E., Hämmerling G. J., Schell T. D., Garbi N., Greenberg P. D. (2016) Tumor-specific T cell dysfunction is a dynamic antigen-driven differentiation program initiated early during tumorigenesis. Immunity 45, 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez G. J., Pereira R. M., Äijö T., Kim E. Y., Marangoni F., Pipkin M. E., Togher S., Heissmeyer V., Zhang Y. C., Crotty S., Lamperti E. D., Ansel K. M., Mempel T. R., Lähdesmäki H., Hogan P. G., Rao A. (2015) The transcription factor NFAT promotes exhaustion of activated CD8+ T cells. Immunity 42, 265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macián F., García-Cózar F., Im S. H., Horton H. F., Byrne M. C., Rao A. (2002) Transcriptional mechanisms underlying lymphocyte tolerance. Cell 109, 719–731. [DOI] [PubMed] [Google Scholar]
- 27.Mognol G. P., Spreafico R., Wong V., Scott-Browne J. P., Togher S., Hoffmann A., Hogan P. G., Rao A., Trifari S. (2017) Exhaustion-associated regulatory regions in CD8+ tumor-infiltrating T cells. Proc. Natl. Acad. Sci. USA 114, E2776–E2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wherry E. J., Barber D. L., Kaech S. M., Blattman J. N., Ahmed R. (2004) Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl. Acad. Sci. USA 101, 16004–16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wherry E. J., Blattman J. N., Murali-Krishna K., van der Most R., Ahmed R. (2003) Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77, 4911–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma P., Allison J. P. (2015) The future of immune checkpoint therapy. Science 348, 56–61. [DOI] [PubMed] [Google Scholar]
- 31.Hamid O., Robert C., Daud A., Hodi F. S., Hwu W. J., Kefford R., Wolchok J. D., Hersey P., Joseph R. W., Weber J. S., Dronca R., Gangadhar T. C., Patnaik A., Zarour H., Joshua A. M., Gergich K., Elassaiss-Schaap J., Algazi A., Mateus C., Boasberg P., Tumeh P. C., Chmielowski B., Ebbinghaus S. W., Li X. N., Kang S. P., Ribas A. (2013) Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 369, 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J. J., Cowey C. L., Lao C. D., Schadendorf D., Dummer R., Smylie M., Rutkowski P., Ferrucci P. F., Hill A., Wagstaff J., Carlino M. S., Haanen J. B., Maio M., Marquez-Rodas I., McArthur G. A., Ascierto P. A., Long G. V., Callahan M. K., Postow M. A., Grossmann K., Sznol M., Dreno B., Bastholt L., Yang A., Rollin L. M., Horak C., Hodi F. S., Wolchok J. D. (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373, 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blackburn S. D., Shin H., Haining W. N., Zou T., Workman C. J., Polley A., Betts M. R., Freeman G. J., Vignali D. A., Wherry E. J. (2009) Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 10, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin H. T., Anderson A. C., Tan W. G., West E. E., Ha S. J., Araki K., Freeman G. J., Kuchroo V. K., Ahmed R. (2010) Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. USA 107, 14733–14738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakuishi K., Apetoh L., Sullivan J. M., Blazar B. R., Kuchroo V. K., Anderson A. C. (2010) Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 207, 2187–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blackburn S. D., Shin H., Freeman G. J., Wherry E. J. (2008) Selective expansion of a subset of exhausted CD8 T cells by αPD-L1 blockade. Proc. Natl. Acad. Sci. USA 105, 15016–15021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paley M. A., Kroy D. C., Odorizzi P. M., Johnnidis J. B., Dolfi D. V., Barnett B. E., Bikoff E. K., Robertson E. J., Lauer G. M., Reiner S. L., Wherry E. J. (2012) Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 338, 1220–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buenrostro J. D., Giresi P. G., Zaba L. C., Chang H. Y., Greenleaf W. J. (2013) Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pauken K. E., Sammons M. A., Odorizzi P. M., Manne S., Godec J., Khan O., Drake A. M., Chen Z., Sen D. R., Kurachi M., Barnitz R. A., Bartman C., Bengsch B., Huang A. C., Schenkel J. M., Vahedi G., Haining W. N., Berger S. L., Wherry E. J. (2016) Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 354, 1160–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sen D. R., Kaminski J., Barnitz R. A., Kurachi M., Gerdemann U., Yates K. B., Tsao H. W., Godec J., LaFleur M. W., Brown F. D., Tonnerre P., Chung R. T., Tully D. C., Allen T. M., Frahm N., Lauer G. M., Wherry E. J., Yosef N., Haining W. N. (2016) The epigenetic landscape of T cell exhaustion. Science 354, 1165–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scharer C. D., Bally A. P., Gandham B., Boss J. M. (2017) Cutting edge: chromatin accessibility programs CD8 T cell memory. J. Immunol. 198, 2238–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singer M., Wang C., Cong L., Marjanovic N. D., Kowalczyk M. S., Zhang H., Nyman J., Sakuishi K., Kurtulus S., Gennert D., Xia J., Kwon J. Y., Nevin J., Herbst R. H., Yanai I., Rozenblatt-Rosen O., Kuchroo V. K., Regev A., Anderson A. C. (2016) A distinct gene module for dysfunction uncoupled from activation in tumor-infiltrating T cells. Cell 166, 1500–1511.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Youngblood B., Oestreich K. J., Ha S. J., Duraiswamy J., Akondy R. S., West E. E., Wei Z., Lu P., Austin J. W., Riley J. L., Boss J. M., Ahmed R. (2011) Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8+ T cells. Immunity 35, 400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahn E., Youngblood B., Lee J., Lee J., Sarkar S., Ahmed R. (2016) Demethylation of the PD-1 promoter is imprinted during the effector phase of CD8 T cell exhaustion. J. Virol. 90, 8934–8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Youngblood B., Noto A., Porichis F., Akondy R. S., Ndhlovu Z. M., Austin J. W., Bordi R., Procopio F. A., Miura T., Allen T. M., Sidney J., Sette A., Walker B. D., Ahmed R., Boss J. M., Sékaly R. P., Kaufmann D. E. (2013) Cutting edge: prolonged exposure to HIV reinforces a poised epigenetic program for PD-1 expression in virus-specific CD8 T cells. J. Immunol. 191, 540–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang F., Zhou X., DiSpirito J. R., Wang C., Wang Y., Shen H. (2014) Epigenetic manipulation restores functions of defective CD8+ T cells from chronic viral infection. Mol. Ther. 22, 1698–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macian F. (2005) NFAT proteins: key regulators of T-cell development and function. Nat. Rev. Immunol. 5, 472–484. [DOI] [PubMed] [Google Scholar]
- 48.Hogan P. G., Chen L., Nardone J., Rao A. (2003) Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 17, 2205–2232. [DOI] [PubMed] [Google Scholar]
- 49.Rao A., Luo C., Hogan P. G. (1997) Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 15, 707–747. [DOI] [PubMed] [Google Scholar]
- 50.Crabtree G. R., Olson E. N. (2002) NFAT signaling: choreographing the social lives of cells. Cell 109 (Suppl), S67–S79. [DOI] [PubMed] [Google Scholar]
- 51.Chen L., Glover J. N., Hogan P. G., Rao A., Harrison S. C. (1998) Structure of the DNA-binding domains from NFAT, Fos and Jun bound specifically to DNA. Nature 392, 42–48. [DOI] [PubMed] [Google Scholar]
- 52.Macián F., García-Rodríguez C., Rao A. (2000) Gene expression elicited by NFAT in the presence or absence of cooperative recruitment of Fos and Jun. EMBO J. 19, 4783–4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bauer C. A., Kim E. Y., Marangoni F., Carrizosa E., Claudio N. M., Mempel T. R. (2014) Dynamic Treg interactions with intratumoral APCs promote local CTL dysfunction. J. Clin. Invest. 124, 2425–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oestreich K. J., Yoon H., Ahmed R., Boss J. M. (2008) NFATc1 regulates PD-1 expression upon T cell activation. J. Immunol. 181, 4832–4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martinez G. J., Hu J. K., Pereira R. M., Crampton J. S., Togher S., Bild N., Crotty S., Rao A. (2016) Cutting edge: NFAT transcription factors promote the generation of follicular helper T cells in response to acute viral infection. J. Immunol. 196, 2015–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sekiya T., Kashiwagi I., Yoshida R., Fukaya T., Morita R., Kimura A., Ichinose H., Metzger D., Chambon P., Yoshimura A. (2013) Nr4a receptors are essential for thymic regulatory T cell development and immune homeostasis. Nat. Immunol. 14, 230–237. [DOI] [PubMed] [Google Scholar]
- 57.Quigley M., Pereyra F., Nilsson B., Porichis F., Fonseca C., Eichbaum Q., Julg B., Jesneck J. L., Brosnahan K., Imam S., Russell K., Toth I., Piechocka-Trocha A., Dolfi D., Angelosanto J., Crawford A., Shin H., Kwon D. S., Zupkosky J., Francisco L., Freeman G. J., Wherry E. J., Kaufmann D. E., Walker B. D., Ebert B., Haining W. N. (2010) Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat. Med. 16, 1147–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Latchman Y., Wood C. R., Chernova T., Chaudhary D., Borde M., Chernova I., Iwai Y., Long A. J., Brown J. A., Nunes R., Greenfield E. A., Bourque K., Boussiotis V. A., Carter L. L., Carreno B. M., Malenkovich N., Nishimura H., Okazaki T., Honjo T., Sharpe A. H., Freeman G. J. (2001) PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2, 261–268. [DOI] [PubMed] [Google Scholar]
- 59.Shin H., Blackburn S. D., Intlekofer A. M., Kao C., Angelosanto J. M., Reiner S. L., Wherry E. J. (2009) A role for the transcriptional repressor Blimp-1 in CD8+ T cell exhaustion during chronic viral infection. Immunity 31, 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hwang S., Cobb D. A., Bhadra R., Youngblood B., Khan I. A. (2016) Blimp-1-mediated CD4 T cell exhaustion causes CD8 T cell dysfunction during chronic toxoplasmosis. J. Exp. Med. 213, 1799–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seddiki N., Phetsouphanh C., Swaminathan S., Xu Y., Rao S., Li J., Sutcliffe E. L., Denyer G., Finlayson R., Gelgor L., Cooper D. A., Zaunders J., Kelleher A. D. (2013) The microRNA-9/B-lymphocyte-induced maturation protein-1/IL-2 axis is differentially regulated in progressive HIV infection. Eur. J. Immunol. 43, 510–520. [DOI] [PubMed] [Google Scholar]
- 62.Xin G., Schauder D. M., Lainez B., Weinstein J. S., Dai Z., Chen Y., Esplugues E., Wen R., Wang D., Parish I. A., Zajac A. J., Craft J., Cui W. (2015) A critical role of IL-21-induced BATF in sustaining CD8-T-cell-mediated chronic viral control. Cell Rep. 13, 1118–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Staron M. M., Gray S. M., Marshall H. D., Parish I. A., Chen J. H., Perry C. J., Cui G., Li M. O., Kaech S. M. (2014) The transcription factor FoxO1 sustains expression of the inhibitory receptor PD-1 and survival of antiviral CD8+ T cells during chronic infection. Immunity 41, 802–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sullivan J. A., Kim E. H., Plisch E. H., Suresh M. (2012) FOXO3 regulates the CD8 T cell response to a chronic viral infection. J. Virol. 86, 9025–9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Grevenynghe J., Procopio F. A., He Z., Chomont N., Riou C., Zhang Y., Gimmig S., Boucher G., Wilkinson P., Shi Y., Yassine-Diab B., Said E. A., Trautmann L., El Far M., Balderas R. S., Boulassel M. R., Routy J. P., Haddad E. K., Sekaly R. P. (2008) Transcription factor FOXO3a controls the persistence of memory CD4+ T cells during HIV infection. Nat. Med. 14, 266–274. [DOI] [PubMed] [Google Scholar]
- 66.Doedens A. L., Phan A. T., Stradner M. H., Fujimoto J. K., Nguyen J. V., Yang E., Johnson R. S., Goldrath A. W. (2013) Hypoxia-inducible factors enhance the effector responses of CD8+ T cells to persistent antigen. Nat. Immunol. 14, 1173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kao C., Oestreich K. J., Paley M. A., Crawford A., Angelosanto J. M., Ali M. A., Intlekofer A. M., Boss J. M., Reiner S. L., Weinmann A. S., Wherry E. J. (2011) Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat. Immunol. 12, 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taylor A., Harker J. A., Chanthong K., Stevenson P. G., Zuniga E. I., Rudd C. E. (2016) Glycogen synthase kinase 3 inactivation drives T-bet-mediated downregulation of co-receptor PD-1 to enhance CD8(+) cytolytic T cell responses. Immunity 44, 274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anderson A. C., Lord G. M., Dardalhon V., Lee D. H., Sabatos-Peyton C. A., Glimcher L. H., Kuchroo V. K. (2010) T-bet, a Th1 transcription factor regulates the expression of Tim-3. Eur. J. Immunol. 40, 859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nayar R., Schutten E., Jangalwe S., Durost P. A., Kenney L. L., Conley J. M., Daniels K., Brehm M. A., Welsh R. M., Berg L. J. (2015) IRF4 regulates the ratio of T-bet to Eomesodermin in CD8+ T cells responding to persistent LCMV infection. PLoS One 10, e0144826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buggert M., Tauriainen J., Yamamoto T., Frederiksen J., Ivarsson M. A., Michaëlsson J., Lund O., Hejdeman B., Jansson M., Sönnerborg A., Koup R. A., Betts M. R., Karlsson A. C. (2014) T-bet and Eomes are differentially linked to the exhausted phenotype of CD8+ T cells in HIV infection. PLoS Pathog. 10, e1004251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hersperger A. R., Martin J. N., Shin L. Y., Sheth P. M., Kovacs C. M., Cosma G. L., Makedonas G., Pereyra F., Walker B. D., Kaul R., Deeks S. G., Betts M. R. (2011) Increased HIV-specific CD8+ T-cell cytotoxic potential in HIV elite controllers is associated with T-bet expression. Blood 117, 3799–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jeannet G., Boudousquié C., Gardiol N., Kang J., Huelsken J., Held W. (2010) Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc. Natl. Acad. Sci. USA 107, 9777–9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou X., Yu S., Zhao D. M., Harty J. T., Badovinac V. P., Xue H. H. (2010) Differentiation and persistence of memory CD8+ T cells depend on T cell factor 1. Immunity 33, 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Utzschneider D. T., Charmoy M., Chennupati V., Pousse L., Ferreira D. P., Calderon-Copete S., Danilo M., Alfei F., Hofmann M., Wieland D., Pradervand S., Thimme R., Zehn D., Held W. (2016) T cell factor 1-expressing memory-like CD8+ T cells sustain the immune response to chronic viral infections. Immunity 45, 415–427. [DOI] [PubMed] [Google Scholar]
- 76.Barber D. L., Wherry E. J., Masopust D., Zhu B., Allison J. P., Sharpe A. H., Freeman G. J., Ahmed R. (2006) Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687. [DOI] [PubMed] [Google Scholar]
- 77.Herbst R. S., Soria J. C., Kowanetz M., Fine G. D., Hamid O., Gordon M. S., Sosman J. A., McDermott D. F., Powderly J. D., Gettinger S. N., Kohrt H. E., Horn L., Lawrence D. P., Rost S., Leabman M., Xiao Y., Mokatrin A., Koeppen H., Hegde P. S., Mellman I., Chen D. S., Hodi F. S. (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Im S. J., Hashimoto M., Gerner M. Y., Lee J., Kissick H. T., Burger M. C., Shan Q., Hale J. S., Lee J., Nasti T. H., Sharpe A. H., Freeman G. J., Germain R. N., Nakaya H. I., Xue H. H., Ahmed R. (2016) Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu T., Ji Y., Moseman E. A., Xu H. C., Manglani M., Kirby M., Anderson S. M., Handon R., Kenyon E., Elkahloun A., Wu W., Lang P. A., Gattinoni L., McGavern D. B., Schwartzberg P. L. (2016) The TCF1-Bcl6 axis counteracts type I interferon to repress exhaustion and maintain T cell stemness. Sci. Immunol. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Teijaro J. R., Ng C., Lee A. M., Sullivan B. M., Sheehan K. C., Welch M., Schreiber R. D., de la Torre J. C., Oldstone M. B. (2013) Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science 340, 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilson E. B., Yamada D. H., Elsaesser H., Herskovitz J., Deng J., Cheng G., Aronow B. J., Karp C. L., Brooks D. G. (2013) Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 340, 202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhen A., Rezek V., Youn C., Lam B., Chang N., Rick J., Carrillo M., Martin H., Kasparian S., Syed P., Rice N., Brooks D. G., Kitchen S. G. (2017) Targeting type I interferon-mediated activation restores immune function in chronic HIV infection. J. Clin. Invest. 127, 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheng L., Ma J., Li J., Li D., Li G., Li F., Zhang Q., Yu H., Yasui F., Ye C., Tsao L. C., Hu Z., Su L., Zhang L. (2017) Blocking type I interferon signaling enhances T cell recovery and reduces HIV-1 reservoirs. J. Clin. Invest. 127, 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Williams J. B., Horton B. L., Zheng Y., Duan Y., Powell J. D., Gajewski T. F. (2017) The EGR2 targets LAG-3 and 4-1BB describe and regulate dysfunctional antigen-specific CD8+ T cells in the tumor microenvironment. J. Exp. Med. 214, 381–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Topalian S. L., Taube J. M., Anders R. A., Pardoll D. M. (2016) Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 16, 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bengsch B., Johnson A. L., Kurachi M., Odorizzi P. M., Pauken K. E., Attanasio J., Stelekati E., McLane L. M., Paley M. A., Delgoffe G. M., Wherry E. J. (2016) Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD-1 are an early driver of CD8+ T cell exhaustion. Immunity 45, 358–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Maleissye M. F., Nicolas G., Saiag P. (2016) Pembrolizumab-induced demyelinating polyradiculoneuropathy. N. Engl. J. Med. 375, 296–297. [DOI] [PubMed] [Google Scholar]
- 88.Chang C. H., Pearce E. L. (2016) Emerging concepts of T cell metabolism as a target of immunotherapy. Nat. Immunol. 17, 364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Buck M. D., O’Sullivan D., Pearce E. L. (2015) T cell metabolism drives immunity. J. Exp. Med. 212, 1345–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scharping N. E., Menk A. V., Moreci R. S., Whetstone R. D., Dadey R. E., Watkins S. C., Ferris R. L., Delgoffe G. M. (2016) The tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity 45, 374–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Austin S., St-Pierre J. (2012) PGC1α and mitochondrial metabolism--emerging concepts and relevance in ageing and neurodegenerative disorders. J. Cell Sci. 125, 4963–4971. [DOI] [PubMed] [Google Scholar]
- 92.Ho P. C., Bihuniak J. D., Macintyre A. N., Staron M., Liu X., Amezquita R., Tsui Y. C., Cui G., Micevic G., Perales J. C., Kleinstein S. H., Abel E. D., Insogna K. L., Feske S., Locasale J. W., Bosenberg M. W., Rathmell J. C., Kaech S. M. (2015) Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell 162, 1217–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao E., Maj T., Kryczek I., Li W., Wu K., Zhao L., Wei S., Crespo J., Wan S., Vatan L., Szeliga W., Shao I., Wang Y., Liu Y., Varambally S., Chinnaiyan A. M., Welling T. H., Marquez V., Kotarski J., Wang H., Wang Z., Zhang Y., Liu R., Wang G., Zou W. (2016) Cancer mediates effector T cell dysfunction by targeting microRNAs and EZH2 via glycolysis restriction. Nat. Immunol. 17, 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Siska P. J., van der Windt G. J., Kishton R. J., Cohen S., Eisner W., MacIver N. J., Kater A. P., Weinberg J. B., Rathmell J. C. (2016) Suppression of Glut1 and glucose metabolism by decreased Akt/mTORC1 signaling drives T cell impairment in B cell Leukemia. J. Immunol. 197, 2532–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hanahan D., Weinberg R. A. (2011) Hallmarks of cancer: the next generation. Cell 144, 646–674. [DOI] [PubMed] [Google Scholar]
- 96.Fesnak A. D., June C. H., Levine B. L. (2016) Engineered T cells: the promise and challenges of cancer immunotherapy. Nat. Rev. Cancer 16, 566–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cherkassky L., Morello A., Villena-Vargas J., Feng Y., Dimitrov D. S., Jones D. R., Sadelain M., Adusumilli P. S. (2016) Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J. Clin. Invest. 126, 3130–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]