Abstract
Alcohol dependence is linked to dysregulation of the hypothalamic-pituitary-adrenal axis. Here, we investigated effects of repeated ethanol intoxication-withdrawal cycles (using chronic intermittent ethanol vapor inhalation; CIE) and abstinence from CIE on peak and nadir plasma corticosterone (CORT) levels. Irritability- and anxiety-like behaviors as well as glucocorticoid receptors (GR) in the medial prefrontal cortex (mPFC) were assessed at various intervals (2h-28d) after cessation of CIE. Results show that peak CORT increased during CIE, transiently decreased during early abstinence (1-11d), and returned to pre-abstinence levels during protracted abstinence (17-27d). Acute withdrawal from CIE enhanced aggression- and anxiety-like behaviors. Early abstinence from CIE reduced anxiety-like behavior. mPFC-GR signaling (indexed by relative phosphorylation of GR at Ser211) was transiently decreased when measured at time points during early and protracted abstinence. Further, voluntary ethanol drinking in CIE (CIE-ED) and CIE-naïve (ED) rats, and effects of CIE-ED and ED on peak CORT levels and mPFC-GR were investigated during acute withdrawal (8h) and protracted abstinence (28d). CIE-ED and ED increased peak CORT during drinking. CIE-ED and ED decreased expression and signaling of mPFC-GR during acute withdrawal, an effect that was reversed by systemic mifepristone treatment. CIE-ED and ED demonstrate robust reinstatement of ethanol seeking during protracted abstinence and show increases in mPFC-GR expression. Collectively, the data demonstrate that acute withdrawal from CIE produces robust alterations in GR signaling, CORT and negative affect symptoms which could facilitate excessive drinking. The findings also show that CIE-ED and ED demonstrate enhanced relapse vulnerability triggered by ethanol cues and these changes are partially mediated by altered GR expression in the mPFC. Taken together, transition to alcohol dependence could be accompanied by alterations in mPFC stress-related pathways that may increase negative emotional symptoms and increase vulnerability to relapse.
Keywords: Corticosterone, glucocorticoid receptor, aggression, anxiety, abstinence, medial prefrontal cortex
1. Introduction
Dysfunctional hypothalamic-pituitary-adrenal (HPA) axis activity is a consequence of alcoholism (Adinoff et al., 1998) that is thought to enhance negative emotional state and favor relapse (Breese et al., 2011; Junghanns et al., 2003; Junghanns et al., 2005). As HPA axis dysregulation can underlie anxiety (for review, see (Finsterwald and Alberini, 2014; Timmermans et al., 2013)), it is not surprising that comorbid anxiety disorder in recovering alcoholics is a risk factor for alcohol relapse (Driessen et al., 2001; Zimmermann et al., 2004). Understanding the HPA axis dysfunction in alcohol use disorders may lead to new diagnostic, therapeutic or preventative approaches. Cortisol (the predominant glucocorticoid in humans) links anxiety and alcohol use; for example, elevated plasma cortisol and blunted stress-induced cortisol response were associated with higher anxiety in individuals with alcohol use disorder (Dai et al., 2007; Tennison et al., 2010). Conversely, in some diseases such as post-traumatic stress disorder and Addison's disease (characterized by adrenal insufficiency), increased anxiety is linked to hypocortisolism (Daskalakis et al., 2013; Warmuz-Stangierska et al., 2010). Taken together, these results suggest that both very high and very low levels of basal plasma cortisol, and associated blunted cortisol response are associated with elevated anxiety.
Aggressive behavior is also linked with HPA-axis dysregulation, often to low glucocorticoid levels. For example, Addison's disease and adrenal insufficiency are associated with increased irritability, which is mitigated typically by hydrocortisone replacement therapy (Bender et al., 2013; Hahner et al., 2007; Tiemensma et al., 2014). Preclinical research also supports a relation of low corticosterone (CORT, the predominant glucocorticoid in rats) to high aggression (Haller et al., 2001; Miczek et al., 2015b). Particularly, high aggression-like behavior was observed in adrenalectomized rats and was mitigated by CORT replacement prior to testing behavior in a resident-intruder paradigm (Haller et al., 2001). One interpretation of this is that aggression may be compensatory manifestation for hypoarousal, comparable to that seen in sensation seeking (Wilson and Scarpa, 2011), reflected and possibly resulting from low CORT release. Therefore, it was not surprising that irritability (a form of aggression-like behavior) was found to be greater in adolescents at higher risk for alcohol and substance use (Tarter et al., 1995). Cortisol-aggression relationship in alcohol use disorders is currently unclear; some suggest that low plasma corticosterone is associated with aggression in alcoholics with a history of violent behavior (Bergman and Brismar, 1994; von der et al., 2002), while others report higher plasma corticosterone in violent alcoholics (Buydens-Branchey and Branchey, 1992). Importantly, while violent behavior under alcohol intoxication has been studied extensively, the CORT versus-aggression relation during alcohol abstinence in alcohol dependent subjects is less understood (Miczek et al., 2015a). Thus, alcohol abstinence in rat models of alcohol dependence may help to identify the temporal relations of anxiety/aggression with changes in plasma CORT.
HPA axis dysfunction as well as alterations in stress-related neural pathways are observed in animals subjected to chronic intermittent ethanol vapor inhalation (CIE), a rodent model of ethanol dependence and addiction (for review, see (Heilig and Koob, 2007; Rivier, 2014; Vendruscolo and Roberts, 2014; Zorrilla et al., 2014)). It has been hypothesized that CIE increases the amplitude of diurnal CORT fluctuations, and such alterations in basal CORT may lead to neuroendocrine tolerance. This tolerance has been posited to produce a dampening of CORT response to ethanol challenge during acute withdrawal that persists into protracted abstinence (Lu and Richardson, 2014). Importantly, inhibition of CORT-mediated activation of glucocorticoid receptor (GR) via systemic administration of mifepristone prevented the enhanced voluntary drinking observed in rats with CIE, but did not affect moderate/low drinking observed in CIE naïve rats (Vendruscolo et al., 2012; Vendruscolo et al., 2015). These effects were observed during acute withdrawal (or 6-10 h after cessation of CIE) and following protracted abstinence (22-31 d after cessation of CIE). Taken together, the development and maintenance of enhanced voluntary drinking due to alcohol dependence is affected by brain GR-mediated signaling due to potentially enhanced CORT and/or amplitude of diurnal CORT fluctuation. However, to the best of our knowledge, the effect of CIE and protracted abstinence from CIE on the diurnal fluctuations of CORT have not been empirically evaluated previously.
The interplay between ethanol and stress responses in the medial prefrontal cortex (mPFC) is hypothesized to be critical for the transition from nondependent drinking to dependence (for review see (Lu and Richardson, 2014)). For example, expression of the stress-related peptide corticotropin-releasing factor (CRF) in the mPFC was increased during the transition from low alcohol drinking to escalated alcohol drinking, and thus, was suggested to affect alcohol seeking and relapse (George et al., 2012; Lu and Richardson, 2014). Potentially, aberrant fluctuations in plasma CORT levels and changes in GR function in the mPFC could regulate CRF responses in the mPFC and other stress-related regions (Myers et al., 2014; Watts, 2005). Alternatively, negative affect symptoms associated with escalated drinking in CIE rats may be modulated by mPFC GR via mechanisms unrelated to CRF. For example, activation of excitatory neurons in the mPFC was shown to inhibit aggression and inhibition of these neurons increased aggression (Takahashi et al., 2014). GR function in the mPFC can regulate response to stressors via modulation of glutamatergic projections to the VTA (Butts and Phillips, 2013; Gourley et al., 2012). These results suggest that mPFC, in part via GR signaling, can modulate the mesocorticolimbic pathway and thereby regulate anxiety-like and aggression-like negative affect symptoms (Butts and Phillips, 2013; Miczek et al., 2015b). Previous research shows that acute withdrawal (2-10 h) from ethanol in CIE rats altered structural neuroplasticity in the mPFC pyramidal neurons (Kim et al., 2014) and decreased expression of GR mRNA in the mPFC without altering GR protein expression and signaling, among other regions (Navarro and Mandyam, 2015; Vendruscolo et al., 2012). Further, these studies do not reveal whether these changes persist into extended ethanol abstinence (24 h and longer). Therefore, analysis of the adaptations in expression and function of GR in the mPFC of rats during acute withdrawal (2-8 h) from CIE and abstinence (1 d-28 d) from CIE may reveal whether CORT action at the level of mPFC is associated with altered stress-related behaviors and or relapse in these animals.
Thus, the objective of the current research was to investigate the diurnal variations of plasma CORT from CIE through protracted abstinence. Unlike voluntary abstinence in humans seeking to quit drinking, abstinence in animal models is experimenter-imposed, and thus henceforth will be referred to as forced abstinence. Anxiety-like behavior and aggression-like, irritable behavior were evaluated using the elevated plus maze (EPM) and bottle brush irritability tests (BBIT), respectively (Bourin, 2015; Lagerspetz and Portin, 1968; Lagerspetz et al., 1968; Riittinen et al., 1986; Scott, 1966) at various intervals into forced ethanol abstinence along with evaluation of GR in the mPFC. Further, to parse out the differences between escalated alcohol intake (dependent-like drinking in rats with CIE; CIE-ED) and low alcohol intake (nondependent-like or “recreational” drinking in CIE-naïve rats; ED), CORT levels and mPFC GR levels were evaluated in rats self-administering ethanol (both in CIE and CIE naïve rats). Furthermore, to understand whether mPFC GR adaptations are differentially modulated in CIE-ED and ED rats, mPFC GR levels were evaluated in following chronic mifepristone treatment that suppresses escalation of ethanol intake in CIE-ED rats without altering ethanol consumption in ED rats (Vendruscolo et al., 2012).
2. Methods
2.1 Animals
Two hundred nine adult, male Wistar rats (Charles River, Raleigh, NC) completed the study. Rats were 8 weeks old at the beginning of the study, and weighed approximately 220-250 g. The rats were group housed (2-3/cage) and maintained in reverse 12 h light -12 h dark cycle rooms with lights ON from 8 pm to 8 am. Food and water were available ad libitum. All experimental procedures were carried out in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication number 85–23, revised 1996), and were approved by the Institutional Animal Care and Use Committee at The Scripps Research Institute.
2.2 Chronic Intermittent Ethanol Vapor (CIE) Procedure
Rat cages were housed in specialized airtight chambers with regulated airflow (La Jolla Alcohol Research Inc, La Jolla, CA) and exposed to a 14 h ON/10 h OFF schedule for ethanol vapors. Specifically, ethanol vapor was switched on at 1800 h for 14 h per day and switched off at 0800 h for 10 h per day. Ethanol vapor delivery was initiated two hours before the onset of the light cycle and was terminated simultaneously with the end of the light cycle. Using a peristaltic pump (model QG-6, FMI Laboratory, Fluid Metering), 95% ethanol from a large reservoir was delivered to a heated flask at a regulated flow rate. The drops of ethanol in the flask were immediately vaporized and carried by controlled airflow (regulated by a pressure gauge) to the vapor chambers housing the rat cages. The air and ethanol flow rates were optimized to result in blood ethanol concentration (BEC) between 125 and 250 mg/dl or 27.2 and 54.4 mM (Gilpin et al., 2008a); these BECs are 2-3 times the BEC observed in binge drinking, but not high enough to abolish righting reflex (Courtney and Polich, 2009; Ernst et al., 1976). This CIE procedure was continued for seven weeks. Although CIE utilizes a non-human route-of-administration (inhalation and not oral) to the induce dependence, none-the-less several features of the CIE model make it a valuable paradigm for understanding ethanol dependence and alcohol use disorder. For example, it models a cyclic intoxication-withdrawal repeated over days, a feature mimicking human drinking behavior. More importantly, CIE has been shown previously to generate escalated voluntary ethanol intake (via self-administration) and induced somatic (stereotypy) and motivational signs of ethanol withdrawal (Gilpin et al., 2008b; Somkuwar et al., 2016a; Somkuwar et al., 2016b; Vendruscolo and Roberts, 2014).
2.3 Blood Ethanol Concentration (BEC) Assay
For measuring BECs, tail bleeding was performed once a week (Mondays) at 0700 h, the time of day corresponding to the last hour of ethanol exposure of CIE rats (Gilpin et al., 2008b). Rats were gently restrained while the tip of the tail was pricked with a clean 18G needle. Tail blood (0.2 ml) was collected in specialized anticoagulant-coated microcentrifuge tubes (Fischer Scientific, Hannover Park, IL) and centrifuged. Plasma (5 μL) was used for measurement of BEC using an Analox AM1 analyzer (Analox Instruments USA Inc., MA). Single-point calibrations were performed for each set of samples using reagents provided by Analox Instruments (25–400 mg/dl or 5.4– 87.0 mM). Because CIE naïve rats would be expected to have no ethanol in their system, any apparent BEC from these controls was considered ‘instrument/technical noise,’ and the mean of this error value (3.9 ± 0.92) was deducted from the BEC of rats experiencing CIE to obtain actual BEC. When BECs were outside the target range (125–250 mg/dl), vapor levels were adjusted accordingly. The mean BECs during the 7 weeks of CIE exposure for the rats in the various experiments are reported in Supplementary Materials.
2.4 Experiment 1: Effects of CIE on circadian fluctuations of plasma corticosterone
Plasma corticosterone (CORT) was measured in behaviorally naïve rats experiencing CIE in their home cages (n = 8). Once per week, tail blood (100-150 μl) was collected from rats one hour before the end of the light cycle (typically Monday), and again two-and-half hours before the end of the dark cycle (typically Wednesday). These time points coincide with the highest and lowest concentrations of CORT (peak CORT and nadir CORT, respectively) during a circadian cycle (Malisch et al., 2008). After the first week, the rats were subjected to seven weeks of CIE followed by four weeks of forced ethanol abstinence, with continued bi-weekly tail-blood collection. BECs also were determined from the ‘peak CORT’ blood sample using the procedure described above. Other than standard husbandry (3×/week food and water changes), no other procedures were conducted on these rats. Half of the rats were euthanized 72 h (3 day; 3 d) after the end of the 7-week CIE while the other half was euthanized at the end of the 4-week (28 days; 28 d) withdrawal from CIE. Euthanasia was performed using rapid decapitation at a timeoint corresponding to plasma nadir CORT levels. The rat brains were isolated and stored at -80°C for subsequent immunoblotting experiments as detailed in Experiment 2.
2.5 Experiment 2: Effect of CIE on the expression and activation of GR in the medial prefrontal cortex across protracted abstinence
Temporal changes in GR function in mPFC at various intervals (2 h-28 d) of forced abstinence from CIE were investigated. Brains from rats in Experiment 1 (3d and 28d abstinence time point) were used. In addition, 33 behaviorally-naïve rats were subjected to seven weeks of CIE, and 30 age-matched ethanol-, behaviorally-naïve control rats were air-exposed for the same duration. These CIE and control rats were decapitated at 2 h, 1 d (24 h), 7 d and 21 d after the cessation of CIE (4-12/group); their brains were frozen and stored at -80°C. A separate cohort of ethanol-naïve rats were housed under standard conditions for the same duration as CIE rats were used as age-matched, ethanol naïve controls (n=8/time point). Note the brains of all rats used herein were collected at a time point corresponding to nadir CORT levels in their circadian cycle. Western blot analysis was conducted using mPFC tissue homogenate (see below for details) to evaluate GR expression and function. Function of GR was evaluated indirectly via immunoquantification of GR phosphorylated at the Serine 211 residue (pGRSer211) (Wang et al., 2002). This particular serine residue is located in a GR region associated with transactivation (ligand binding, dimerization and translocation into the nucleus of the cell) of the receptor (Iniguez-Lluhi et al., 1997). An increase in the proportion of pGRSer211 to total GR (tGR) expression enhanced receptor-dependent transcription (Wang et al., 2002). Therefore, alterations in pGRSer211/tGR observed here were interpreted as a change in transactivation of GR.
2.6 Experiment 3: Effect of forced abstinence on agonistic behaviors: Irritability Test
Aggression was considered herein to be a type of agonistic behavior, i.e. behavioral patterns having the common function of adaptation to situations involving physical conflict between members of the same species (Brain and Haug, 1992; Scott, 1966). Aggressive and defensive behaviors were observed in the rat's home-cage environment following the presentation of a previously neutral mechanical stimulation (moving bottlebrush). This method, called the bottle brush irritability test (BBIT), has advantages over other behavioral paradigms that measure aggressive/defensive behaviors, such as the popular social dominance/subordination paradigms (or resident/intruder confrontation paradigm), because the experimenter has greater control over the mechanical stimulus, and therefore has better precision for ensuring uniform provocation. Furthermore, the ‘social’ factor in eliciting agonistic behavior, and the risk of physical injury in an agonistic encounter are both removed (Scott, 1966). The mechanical stimulus of the moving bottlebrush were found to be more effective at eliciting these behaviors compared to dead or stuffed animals (Lagerspetz, 1965). Rats were tested individually in their home cages. In the procedure, the rat was “attacked” by moving a bottlebrush (16” long steel wire round 1-1/2” diameter tube cleaning brush, referred to as the “target”) using the following standardized steps (Riittinen et al., 1986):
Brush (in rotation) approaching the rat from the opposite end (starting position) of the cage.
Brush (in rotation) touching the whiskers of the rat.
Brush (in rotation) returning to the starting position in the opposite end of the cage.
Brush (in rotation) at the starting position.
Brush (no rotation) at the starting position.
Each step of the attack lasted about 1.5 sec; this sequence of attacks (collectively referred to as a trial) was repeated 10 times per test session, with a 10-15 sec interval between each trial. Basic agonistic behavior patterns (ethograms) elicited during the BBIT have been well-documented in rodent literature (Lagerspetz and Portin, 1968; Lagerspetz et al., 1968; Scott, 1966). The following behaviors were specifically monitored and scored by an independent observer:
Escape: the rat moves in front of the brush or avoids it.
Digging: the rat digs around and/or throws bedding.
Climbing: the rat mounts the cage wall while standing on its rear limbs, i.e., rearing while using the wall as a support.
Jumping: the rat jumps onto the edge of the cage or out of the cage.
Boxing: the rat drums with its front paws against the cage.
Biting: the rat bites the brush.
Following: the rat follows the brush at stage 3.
Grooming: the rat uses its forelimbs of hindlimbs to brush its coat and/or whiskers.
Vocalization: Any noise in response to the brush.
Exploration: the rat sniffs around the cage and outside the walls in a non-defined way (i.e., without paying attention to the brush).
Freezing: the rat becomes still (almost motionless) and stiffens its body, i.e., all body movements, except breathing, are absent.
Boxing, biting and following were summed as aggressive-like behaviors; escape, digging, climbing, jumping and grooming were summed together as defensive-like behaviors. The scores for individual ethograms were summed over the 10 trials for each test session. The test was repeated at five intervals (8 h-14 d) after removal from ethanol vapor.
2.7 Experiment 4: Effect of CIE on anxiety-like behavior during early protracted abstinence: Elevated Plus Maze
Previous studies have shown increased anxiety-like behavior using the elevated plus-maze (EPM), a popular behavioral paradigm, during acute withdrawal (2-10 h) as well as following several weeks (3-6 weeks) of forced abstinence from ethanol (Doremus et al., 2003; Gehlert et al., 2007; Rasmussen et al., 2000; Valdez et al., 2002; Zhao et al., 2007). However, anxiety-like behavior have not been reported for an early-intermediate abstinence time point in rats withdrawn from CIE (2 -14 d). To address this gap in literature, the current study tested EPM at eight days (8 d) after cessation of ethanol vapor exposure in CIE (n=12) and age-matched control (n=15) rats. In a separate set of behaviorally-naive rats with approximately seven-weeks of CIE experience (n=7, and age-matched controls n=5), EPM test was conducted 8 h after cessation of CIE. The purpose of the 8 h acute withdrawal time-point was to serve as a positive control, which are expected to exhibit anxiogenic-like behavior (Baldwin et al., 1991; Zhao et al., 2007).
Individual rats were allowed to explore the maze with four arms (each 50 cm long and 10 cm wide, two open and two enclosed by 40 cm walls) that are arranged to form a plus shape and placed at an elevation of 80 cm off the floor. Behavior in this task reflects a conflict between the rodent's preference for protected areas and their innate motivation to explore novel environments, and hence is a simple procedure for assessing anxiety-like behavior in rodents (Pellow et al., 1985). Briefly, the rats were placed in the intersection of the four arms, facing an open arm, and allowed to explore the maze for 5 min. Time spent and entries made on the open and closed arms were recorded using a video-tracking system (Any-Maze, Stoelting Inc.). Anxiety-like behavior (decreased open arm time) was determined as a percent of (open arm)/(open+closed arm) time. The number of closed arm entries and distance travelled in the EPM were used to measure general activity/locomotion. The test was not repeated in individual rats because several studies have reported the unsuitability of retesting in the EPM (Almeida et al., 1993; Bertoglio and Carobrez, 2000, 2002; File, 1993).
2.8 Experiment 5: Effect of chronic GR inhibition on ethanol self-administration and on GR expression and function in the mPFC in CIE-ED and ED rats
Rats (n=36) were trained to self-administer 10% w/v ethanol via lever-responding on a fixed-ratio 1 schedule of reinforcement in operant conditioning boxes (Vendruscolo et al., 2012; Vendruscolo et al., 2015). Subsequently, the rats were divided into two groups; one group experienced CIE (n=17) and ethanol drinking (ED) was monitored during the 24 days of CIE, henceforth, called ‘CIE-ED’; the second group were exposed to air (n=19) and was monitored for 24 days, henceforth termed ‘ED’ rats. Prior to CIE initiation, the rats were subcutaneously implanted with a sustained release mifepristone pellet (200 mg, 24-d sustained release pellet, Innovative Research of America; CIE-ED-Mif, n=9; ED-Mif, n=9) or a placebo pellet (CIE-ED, n=8; ED, n=10). The mifepristone dose chosen was greater and longer-lasting than one that has previously been shown to suppress escalation of ethanol intake in CIE-ED rats without altering ethanol consumption in ED rats (Vendruscolo et al., 2012). Ethanol intake (in g/kg/session) for these rats are presented (Figure 3A). On the 24th day, the rats were euthanized 8 h after ethanol vapors were turned off. The 8 h post-CIE time-point was chosen as this is the time when the rats underwent the ethanol self-administration sessions; BECs are minimal and somatic and motivational withdrawal signs are maximal in CIE rats (Richardson et al., 2008a; Vendruscolo and Roberts, 2014). Their brains were removed and stored at -80°C till further use.
Figure 3. Mifepristone (Mif) inhibited escalated ethanol self-administration and reversed ethanol exposure mediated decrease in glucocorticoid receptor (GR) signaling in the medial prefrontal cortex (mPFC).
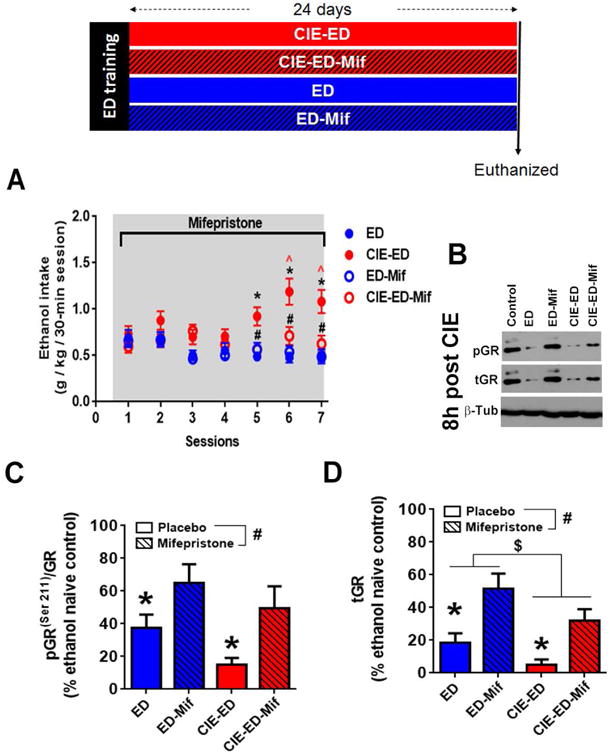
Schematic representation of the experimental design is presented. Rats were trained to self-administer ethanol (10%w/v; ethanol drinking, ED) and then divided into four groups. Two groups received chronic intermittent ethanol vapor (CIE) treatment (CIE-ED rats), the other group continued to be maintained under standard housing conditions (ED rats). One CIE-ED and one ED group was implanted with mifepristone sustained release pellet (CIE-ED-Mif and ED-Mif) and the others were implanted with placebo pellets (CIE-ED and ED). All groups were tested for seven sessions of ethanol self-administration across the 24 days of the mifepristone release. (A) Mif suppressed escalation of ethanol self-administration in CIE-ED rats such that CIE-ED-Mif were not different from ED and ED-Mif rats. Also, mifepristone did not alter ethanol self-administration in ED rats. Values are mean ± S.E.M. for ethanol intake in g/kg/30-min ethanol self-administration session. n=8-10/group, * p<0.05 increase in CIE-ED compared to ED rats at an equivalent time; ˆ p<0.05 increase in CIE-ED compared to intake during session 1; # p<0.05 decrease in CIE-ED-Mif compared to CIE-ED at an equivalent time. (B) Representative immunoblots of pGR(ser211), tGR, with corresponding blots for b-tubulin (β-Tub) from ethanol naïve control, ED, CIE-ED, ED-Mif and CIE-ED-Mif at 8 h withdrawal from CIE treatment. Densitometric analysis of (C) GR function (pGR(ser211)/tGR) and (D) total GR expression (tGR) showed that both decreased during acute withdrawal, an effect that was reversed by chronic Mif treatment. Values are mean ± S.E.M. for expression of pGR(ser211)/tGR and GR in the medial prefrontal cortex presented as a percent of age-matched, ethanol and behaviorally naïve controls. n=8-10/group, * p<0.05 decreased compared to age-matched ethanol and behaviorally naïve controls; # p<0.05 main effect of Mif, i.e., increased by Mif compared to placebo, $ p<0.05 main effect of CIE, i.e. decreased in CIE treated rats compared to ED. For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.
2.9 Experiment 6: Effect of ethanol self-administration on peak CORT levels in CIE-ED and ED rats
Effects of voluntary ethanol intake (in CIE-ED and ED rats) on peak CORT levels during the weeks of ethanol exposure were determined in a separate cohort of rats. Rats (n=16) were trained to lever press for 10% v/v ethanol using a previously published procedure (Somkuwar et al., 2016a). In this cohort ED was monitored during 7 weeks of CIE or air experience. The behavior data for these animals have been recently published (Somkuwar et al., 2016a). Briefly, rats responded on a fixed-ratio 1 schedule for ethanol reinforcement during 30-min daily sessions. Correct lever responses resulted in the presentation of a cue-light above the correct lever and 100 μl ethanol solution dispensed in a receptacle fitted in the operant conditioning boxes (Med Associates Inc, VT), and was followed by a 4 s time-out during which lever responses were recorded but did not have a programmed consequence. After the ethanol self-administered drinking behavior was established, the rats were divided into two groups; CIE-ED (n=8) and ED (n=8) that received CIE (procedure detailed above) or air exposure for 7 weeks and continued to lever press for ethanol twice weekly (Tuesdays and Thursdays) about 0800 h after ethanol vapor was turned off. By the first week of CIE, these rats already had 8-10 days of ethanol self-administration experience. Detailed lever responding data for these rats during CIE exposure as well as lever responses during extinction and reinstatement are available elsewhere (Somkuwar et al., 2016a). Ethanol intake (in g/kg/session) for these rats is presented. Peak plasma CORT was evaluated weekly during the seven-week CIE procedure and these data have never been published previously. Brain tissue from these animals were used to measure other markers of oligodendrogenesis and have been published elsewhere (Somkuwar et al., 2016a). Separate aliquots of mPFC tissue was used in this study to measure tGR and pGRser211 levels and have never been published previously (Experiment 8). An additional cohort of ethanol naïve rats were trained to self-administer saline (n=4) in identical operant conditioning paradigm (behavior data presented in (Galinato et al., 2017)); plasma from these rats were collected prior to abstinence from self-administration to determine peak CORT levels in ethanol naïve but behaviorally trained rats.
2.10 Experiment 7: Effect of forced abstinence from ethanol on peak CORT levels in CIE-ED and ED rats
At the end of 7 weeks of ethanol self-administration, all rats from Experiment 6 were withdrawn from CIE as well as ethanol self-administration and maintained under standard housing conditions for 28 d. Peak CORT was determined at three different time points during this forced ethanol abstinence, i.e. at day 9, day 17 and day 27 post cessation of CIE. During the last week of withdrawal, the rats were subjected to one session of ethanol self-administration (drinking after protracted abstinence; day 21), six sessions of extinction training (in a novel context, day 22-27), and one session of cued-context induced reinstatement of ethanol-seeking behavior (without ethanol delivery; day 28). Detailed methods and lever responding data for these sessions are available elsewhere (Somkuwar et al., 2016a). Lever responses during last extinction session and reinstatement sessions have been included here. At 2 h after the end of the reinstatement session, the rats were killed by rapid decapitation, and their brains were removed and stored at -80°C. The CORT levels in CIE-ED and ED rats were also compared to that obtained by the saline self-administering rats mentioned in Experiment 6.
2.11 Experiment 8: Effect of ethanol self-administration on the expression and activation of GR in the mPFC in CIE and CIE-naïve rats
To evaluate the effect of 8 h of forced abstinence from ethanol, the mPFC of CIE-ED and ED rats with placebo and with mifepristone treatment from Experiment 5, and age matched ethanol- and behaviorally-naïve controls (n=6) were evaluated for GR expression and function (detailed below). To evaluate the effects of protracted abstinence from ethanol, mPFC of CIE-ED and ED rats from Experiment 7, and from age matched ethanol- and behaviorally-naïve controls (n=6) were evaluated for GR expression and function 2 h after completion of contextual cued reinstatement. To address whether behavioral self-administration training alters GR function in the absence of ethanol (self-administration and/or CIE), GR expression and function were also evaluated in the saline self-administering rats from Experiment 6. These rats were also subjected to forced abstinence from saline self-administration and followed by extinction and cue-induced reinstatement. The self-administration data for these saline rats are presented elsewhere (Galinato et al., 2017). Thus, these rats were behaviorally trained, but ethanol-naïve. GR expression and function in the mPFC from these rats were compared to the above ethanol- and behaviorally-naïve rats.
2.12 Plasma Corticosterone Quantification
Plasma corticosterone (CORT) was measured using the DetectX ® Corticosterone Enzyme Immunoassay Kit (Arbor Assays, Ann Arbor, MI) following manufacturer instructions. Briefly, 100-150 ul tail blood was collected in specialized anticoagulant-coated microcentrifuge tubes (Fischer Scientific, Hannover Park, IL). Plasma was separated by centrifugation (Centrifuge 5415C, Eppendorf, Hauppauge, NY) of the blood samples at 3000 rpm for 15 min at 4°C, and stored at − 80°C. On the day of the assay, the plasma was allowed to thaw on ice and the reagents of the Enzyme Immunoassay Kit were allowed to warm to room temperature prior to use. Samples were prepared by mixing plasma with the Dissociation Reagent in a 1:1 ratio, and then diluting the mixture with Assay Buffer to get a final dilution of 1:100 for the plasma. The manufacturer provided corticosterone standard (100 ng/ml) was serially diluted to generate an 8-point standard curve ranging from 78.125 pg/ml to 10,000 pg/ml. Standards and the diluted samples were added to microtiter plate coated with secondary antibody against sheep. A sheep polyclonal antibody against corticosterone and corticosterone-peroxidase conjugate were added to sample and standard wells. Following an hour of incubation, binding of the corticosterone and corticosterone-peroxidase conjugate to the plate was stopped by washing the wells. Then a peroxidase substrate was added to the wells that produced a colorimetric reaction with the bound corticosterone-peroxidase conjugate. This reaction was stopped and the intensity of the generated color (or optical density) was measured at 450 nm using a microtiter plate reader (Biotek Synergy, Winooski, VT). The concentration of corticosterone in the samples was calculated from the 4-parameter logistic non-linear regression obtained from concentration-optical density plot generated by the 8 known standard dilutions using Prism 6 (GraphPad Software, Inc). Eleven plates were utilized to complete the assay for all the samples presented here. The inter-assay 95% confidence interval for the mean IC50 values ranges from 504.2 to 833.9 ng/L, and the coefficient of variation is 13.7% for the assays conducted.
2.13 GR expression and activation in the medial prefrontal cortex
Tissue punches from 500-um thick sections of mPFC were sonicated on ice in freshly prepared homogenization buffer (320 mM sucrose, 5 mM HEPES, 1 mM EGTA, 1 mM EDTA, 1% SDS, with Protease Inhibitor Cocktail and Phosphatase Inhibitor Cocktails II and III diluted 1:100; Sigma). Samples were heated at 100°C for five minutes and total protein concentration was determined using a detergent-compatible Lowry method (Bio-Rad, Hercules, CA). tGR and Ser 211 phosphorylated GR (pGRser211) protein levels were determined using gel electrophoresis, western blotting and immunodensity quantification. Briefly, 20 μg protein samples mixed (1:1) with a Laemmli sample buffer containing β-mercaptoethanol were subjected to SDS-PAGE (8% acrylamide) using Tris-Glycine-SDS buffer (Bio-Rad), followed by overnight electrophoretic transfer to PVDF membranes (pore size 0.2 μm) in Tris-Glycine-Methanol buffer at 4°C. The membranes were blocked with 5% w/v non-fat dried milk in Tris-buffer saline with Tween (TBS-T; 25 mM Tris-HCl, 150 mM NaCl and 0.1% v/v Tween-20; pH 7.4) for 1 h at room temperature, with shaking. The membranes were incubated with appropriate primary antibody for 16-20 h at 4 °C: tGR (1:1000, Cell Signaling Technologies #3660 in 5% milk in TBS-T buffer), pGRser211 (1:200, Cell Signaling Technologies #4161 in 1% milk in TBS-T buffer), β-Tubulin (1:8000 SCBT sc-53140, in 5% milk in TBS-T buffer). Blots were washed three times for 5 min in TBS-T, and then incubated for 1 h at room temperature (24°C) with the appropriate horseradish peroxide–conjugated secondary antibody (goat anti-rabbit IgG, 1:1000-500, BioRad or goat anti-mouse IgG1, 1:5000, BioRad; in TBS-T). After another set of three 5-min washes with TBS-T, immunoreactivity was detected using SuperSignal West Dura chemiluminescence detection reagent (Thermo Scientific) and collected using HyBlot CL Autoradiography film (Denville Scientific) and a Kodak film processor. Net intensity values were determined using the ImageStudio Lite software (Li-Cor Biosciences). Total protein levels of β-Tubulin was used for normalization purposes. Protein expression is presented as percent of age-matched ethanol and behaviorally-naïve control samples on the same blot to normalize for blot-to-blot variability.
2.14 Statistical analyses
The number of subjects that completed the various time points for each of the experiments have been presented in Table 1 (Supplementary materials). For Experiment 1, CORT was evaluated using linear mixed model analysis with time of day (peak and nadir, two levels) and time in weeks (twelve levels) as within subject-factors; the degree of freedom and the p values are reported for the effects. For Experiment 2, protein expression, quantified as % respective age-matched ethanol naïve control, was compared using 1-way ANOVA with time points (six levels) as the between-subjects factors. Further, one-sample t-tests were conducted to identify deviation from 100% value of protein expression in ethanol naïve controls. For Experiment 3, agonistic ethograms during BBIT, such as aggressive- and defensive-like behavior as well as freezing were evaluated using 2-way ANOVA with duration of withdrawal (6 levels, i.e. 8 h, 1 d, 3 d, 7 d, 14 d) and ethanol treatment condition (two levels, CIE and Ethanol naive) as the between-subjects factors. Newman-Kewls post-hoc analyses was used for further investigating main effects and interactions. For Experiment 4, time spent in open arms, calculated as (open arm time)/([open + closed] arm time)*100, as well as number of entries into the closed arms, and total distance traveled in the maze were each evaluated using one-way ANOVAs with ethanol treatment condition (three levels) as the between-subjects factor. For Experiment 5, ethanol intake (g/kg body weight/session) was compared using repeated measures 2-way ANOVA with session as the within-subjects factor (seven levels) and treatment as the between-subjects factor (four levels, ED, CIE-ED, ED-Mif, CIE-ED-Mif). Protein expressions, quantified as % age-matched ethanol and behaviorally naïve controls, were compared using 2-way ANOVA with CIE experience (two levels) and mifepristone treatment (two levels) as between-subjects factors. Separate 1-way ANOVAs were conducted to compare the effect of ethanol experience (three levels, naïve, ED and CIE-ED) on protein expression. For Experiment 6, peak CORT was evaluated using repeated measures 2-way ANOVA with time (seven levels during CIE and three levels during abstinence) as the within-subjects factor and treatment (ED and CIE-ED, two levels) as the between-subjects factor. Furthermore, separate 1-way ANOVAs were conducted to compare the effect of ethanol experience (three levels, saline, ED and CIE-ED) on CORT during CIE and abstinence. Also, ethanol intake during CIE was compared using repeated measures 2-way ANOVA with time in weeks (eight levels) as the within-subjects factor and treatment (ED and CIE-ED, two levels) as the between-subjects factor. Further, active lever responding during extinction and reinstatement were compared as repeated measures 2-way ANOVA with session (extinction and reinstatement, two levels) as the within-subjects factor and treatment (ED and CIE-ED, two levels) as the between subjects factor. Separate paired t-tests were conducted for CIE-ED and ED rats to compare differences in responding on the active and inactive levers during reinstatement. For Experiment 7, GR expression and function were compared between groups using separate 1-way ANOVAs for each time point, with treatment (ED, CIE-ED and ethanol- and behaviorally-naïve control, three levels) as between-subjects factors. A separate set of unpaired t-tests were used to compare GR expression and function between ethanol-naïve behaviorally-trained saline-self-administering rats and ethanol-naïve behaviorally-naïve control rats. Tukey's post-hoc analyses were used to further probe significant main effects and interactions in ANOVAs. Significance was determined at p<0.05. All statistical analyses were conducted using Prism 6 (GraphPad Software, Inc) and SPSS Statistics Version 19 (SPSS Inc., IBM Company, Armonk, NY).
Table 1.
Distance travelled in the EPM did not differ between groups. Values are mean ± S.E.M. of distance moved within the 5-min session.
| Ethanol Naive | CIE withdrawn | ||
|---|---|---|---|
| 8 h | 8 d | ||
|
| |||
| Distance travelled in the EPM | 11.2 ± 0.980 | 10.5 ± 1.34 | 13.7 ± 0.800 |
3. Results
3.1 Experiment 1: CIE and abstinence alters diurnal plasma corticosterone levels
BECs are presented in Supplementary Table 2. Plasma corticosterone (CORT) levels are presented for two time points during the circadian cycle, peak CORT and nadir CORT (Figure 1A). Significant fixed effects of time of day when CORT was measured (Χ2(1)=61.6, p<0.0001), of weeks (Χ2(11)=10.7, p=0.001) as well as an interaction of the two (Χ2(11)=9.05, p=0.006) were obtained. Consistent with previously published findings, CORT levels prior to the onset of the dark cycle (i.e., peak CORT) was greater than that towards the end of the dark cycle (nadir CORT, p<0.0001). Separate mixed model analysis of nadir CORT revealed no effect of weeks (Χ2(11)=3.39, p=0.23). In contrast, significant effect of week was obtained on peak CORT (Χ2(11)=13.0, p=0.0001); peak CORT was increased at week 5 and 6 of CIE and at day 24 of abstinence compared to week 0 (ps <0.05), and was increased at week 5 and 6 compared to week 2 of CIE (ps < 0.05). Although, peak CORT at week 7 was comparable to week 5 and week 6, statistical significance was not obtained (p=0.066). Peak CORT was decreased on day 1 and 8 (early abstinence) compared to week 5 and 6 (ps < 0.05), and was increased back on day 24 of abstinence (ps < 0.05) such that they were no longer different from pre-abstinence levels. Additional results for power analysis of plasma CORT data during abstinence is provided in supplementary results section.
Figure 1. Plasma corticosterone (CORT) concentration and glucocorticoid receptors (GR) expression in the medial prefrontal cortex are modulated by chronic intermittent ethanol experience (CIE) and abstinence from CIE.
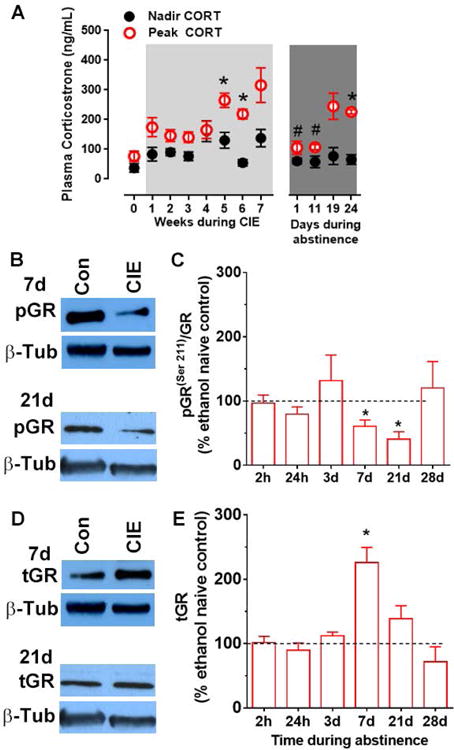
Rats were housed in reverse light-cycle 12h light/12h dark rooms and were exposed to 7 weeks of CIE followed by 4 weeks of abstinence. (A) For Experiment 1, plasma CORT was measured one hour before the end of the light cycle (peak CORT) and again two hours before the end of the dark cycle (nadir CORT). Peak CORT increased at week 5 and 6 of CIE compared to pre-CIE levels (week 0). Peak CORT levels decreased transiently during early-intermediate abstinence (day 1-11) compared to pre-abstinence (week 5-6) and then increased back to levels seen during CIE. No differences were revealed in nadir CORT between weeks. Values are mean ± S.E.M of plasma CORT (in ng/mL plasma). n=8 during CIE and 4 during abstinence. * p<0.05 compared to CORT before the onset of CIE (Week 0); # p<0.05 compared to peak CORT before the cessation of CIE (Week 5-6). (B-E). For Experiment 2, protein expression of total GR (tGR) and GR phosphorylated at Ser211 (pGR(Ser211)) was evaluated during various intervals into ethanol abstinence. Representative immunoblots of pGR(Ser211) (B), tGR (D), with corresponding blots for beta-tubulin (β-Tub) from one control and one CIE rat from 7 d and 21 d time points. Densitometric analysis revealed that GR function (pGR(Ser211)/tGR) transiently decreased during intermediate abstinence (7-21 d) from CIE (C). In contrast, tGR transiently increased during early-intermediate abstinence (7 d). Values are mean ± S.E.M. for pGR(ser211)/tGR and tGR in the prefrontal cortex of rats withdrawn from CIE presented as a percent of ethanol naïve controls (100 ± 11.8 and 114 ± 13.0, respectively, represented by the dotted line). n=4-12/group, * p<0.05 compared to the 100% expression in the ethanol naïve controls. For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.
3.2 Experiment 2: Expression and activation of mPFC GR is altered during early and protracted abstinence from CIE
Changes in the expression and function of GR in the mPFC were evaluated over six time-points during forced abstinence from CIE, and expressed as a percentage of the ethanol naïve controls (Figures 1B-E). Activation of GR, expressed as the ratio of GR phosphorylated at Ser211 and total GR (pGRser211/tGR) was altered during forced abstinence (F[5,39]=3.63, p=0.009, Figure 1C), with significant reductions at 7d (t3=4.05, p=0.027) and 21d (t3=3.08, p=0.006) of ethanol abstinence compared to their controls (100 ± 11.8 arbitrary densitometric units). Expression of total GR (tGR) in the mPFC was altered transiently during forced abstinence (F[5,16]=4.13, p=0.013, Figure 1E), with a significant increase at 7d of ethanol abstinence (t3=5.35, p=0.013) compared to their controls (114 ± 13.0 arbitrary densitometric units).
3.3 Experiment 3: Effect of abstinence from CIE on agonistic behaviors using BBIT
Change in agonistic ethogram was evaluated over six time-points during forced abstinence from ethanol in CIE rats and in age-matched ethanol naïve controls using BBIT (Figures 2A-B, Supplementary Table 3). For aggression-like behavior, 2-way ANOVA did not show an interaction, but revealed significant main effects of timepoint of abstinence and CIE history (Ftime × treatment[4,111] = 1.03, p = 0.4; Ftime[4,111] = 3.47, p = 0.01; Ftreatment[1,111] = 6.33, p = 0.013; Figure 2A). Thus, rats with a history of CIE showed greater aggression-like behavior compared to ethanol naïve controls. Separate one-way ANOVA revealed no effect of time in CIE withdrawn rats (F[4,77] = 2.27, p = 0.069), which may be due to the aberrant decreased score at day 7 (not statistically significant) compared to day 3. Closer inspection of the data revealed that six out of the twelve rats with CIE history exhibited reduced or unchanged aggressive-like behavior on day 7 compared to both day 3 and day 14, despite all experimental conditions remaining identical. In contrast to the CIE group, one-way ANOVA revealed a significant effect of time in ethanol naïve controls (F[4,34]=5.22, p=0.002). Specifically, aggressionlike behavior increased at day 14 compared to 8h-3d time-points (ps < 0.05).
Figure 2. Aggression-like behavior and anxiety-like behavior were altered during abstinence from chronic intermittent ethanol exposure (CIE).
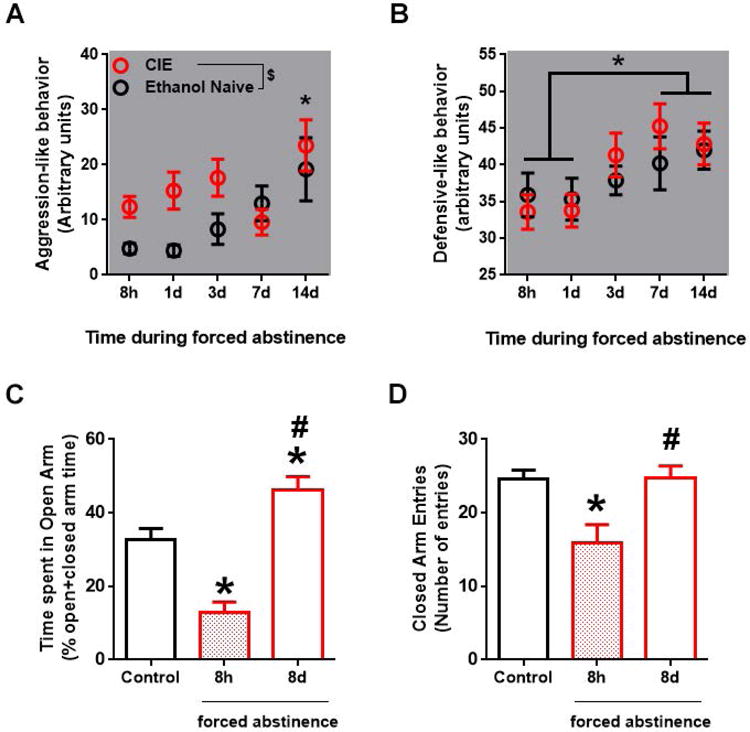
(A, B) Agonistic behavior in rats withdrawn from ethanol after 7 weeks of chronic intermittent ethanol exposure (CIE) and in age-matched ethanol naïve controls was measured using bottlebrush irritability test (BBIT). Values are mean ± S.E.M of aggressive score and defensive score at various time points during ethanol abstinence; n=5-24/group. (A) CIE withdrawn rats show greater aggression-like behavior compared to control, $p<0.05 indicates main effect of CIE; *p<0.05 main effect of time, with score at 14 d being greater than 8 h -3 d time-points. (B) Defensive-like behavior was increased over repeated BBIT across treatment groups, but no effect of ethanol abstinence was revealed. *p<0.05 main effect of time such that 7 d and 14 d was greater than 8 h and 1 d. (C, D) Anxiety-like behavior, measured using elevated plus maze (EPM), was increased at 8 h of abstinence and was decreased at 8 d of abstinence. (C) Time spent in the open arm (% open arm time/(open+closed) arm time) was decreased after 8 h, and increased after 8 d of forced abstinence from ethanol. (C) Number of closed arm entries was decreased at only at 8 h of forced abstinence from ethanol. Values are mean ± S.E.M; n=7-12/group, * p<0.05 compared to corresponding value in ethanol naïve control rats, # p<0.05 compared to corresponding value after 8 h of forced abstinence. For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.
For defensive-like behaviors, 2-way ANOVA revealed no interaction or main effect of treatment (Ftime × treatment[4,113] = 0.50, p = 0.7; Ftreatment[1,113] = 0.27, p = 0.6). Main effect of duration of abstinence was revealed (Ftime[4,113] = 3.00, p = 0.021; Figure 2B), such that defensive-score (averaged across treatment groups) was increased on day 7 and 14 compared to both 8 h and 1 d into forced abstinence (ps < 0.05). No group differences were revealed in other agonistic behavior observed using the BBIT (Supplementary Table 3).
3.4 Experiment 4: Effect of abstinence from CIE on anxiety-like behaviors in EPM
Anxiety-like behavior was evaluated only once per rat at either the acute withdrawal time point (8 h into forced abstinence) or during early-intermediate time point (8 d into forced abstinence). The 8 d data was collected from the same rats used for BBIT; the 8 h data was collected from a separate cohort of EPM naïve rats. Abstinence duration modulated time spent in the open arms of the EPM (F[2,31]=18.1, p=0.0001; Figure 2C), such that open arm time was decreased in acutely withdrawn rats and increased in early-intermediate abstinent rats compared to age-matched controls, and was increased in early-intermediate abstinent rats compared to acutely withdrawn rats (ps < 0.01). Abstinence duration also affected number of closed arm entries (F[2,31]=7.45, p=0.005; Figure 2D), such that closed arm entries were reduced during acute withdrawal compared to controls, and increased back to control levels in the early-intermediate abstinent rats compared to acutely withdrawn rats (ps < 0.005). No group differences were found in the total distance travelled in the EPM (Table 1). Taken together, these results suggest that anxiety-like behavior was differentially affected by the length of abstinence from ethanol, with anxiogenic-like effect observed during acute ethanol withdrawal but an anxiolytic-like effect during the early-intermediate protracted abstinence.
3.5 Experiment 5: Mifepristone suppressed ethanol intake in CIE-ED rats only, but reversed ethanol-induced suppression of mPFC GR in both CIE-ED and ED rats
Effect of sustained release mifepristone (200 mg – 24 day formulation) or placebo treatment on rats self-administering ethanol with and without CIE exposure (CIE-ED and ED, respectively) was measured during seven sessions over a period of 24 days. For amount of ethanol consumed per unit bodyweight, a significant interaction (Fsession × treatment[18,192]=4.10, p<0.0001) and main effects of treatment (Ftreatment[3,32]=8.16, p=0.0004) and session (Fsession[6,192]=3.05, p=0.007) were revealed. As expected, ethanol intake was escalated in CIE-ED rats, but not in ED rats, at sessions 6 and 7 compared to session 1 (ps<0.05; Figure 3A); ethanol intake was greater in CIE-ED compared to ED rats at sessions 5-7. Mifepristone treatment did not alter ethanol self-administration in ED rats. In contrast, mifepristone decreased ethanol self-administration in the CIE-ED rats at sessions 5-7 (ps<0.05), such that ethanol intake in CIE-ED-Mif was not different from ED-Mif and ED rats.
Function and expression of GR in the mPFC were evaluated in CIE-ED and ED rats 8 h after cessation of CIE (Figures 3B-D). Function of GR receptor, expressed as pGRser211/tGR, decreased in both CIE-ED and ED rats compared to age-matched ethanol and behaviorally-naïve control rats (99.8 ± 6.06) 8 h after cessation of CIE (F[2,15]=5.55, p=0.016, Figure 3C), and was not different between CIE-ED and ED rats. A separate 2-way ANOVA revealed a significant main effect of mifepristone (FMif[1,31]=14.7, p=0.0006), but not an interaction (FCIE×Mif[1,31]=0.571, p=0.456) or an effect of CIE (Fcie[1,31]=3.23, p=0.082), showing that mifepristone increased GR function irrespective of CIE experience (Figure 3C).
Expression of tGR was decreased in both CIE-ED and ED rats compared to age-matched ethanol and behaviorally naïve control rats (100 ± 11.8; F[2,19]=5.62, p=0.012, Figures 3B,D), but was not different between CIE-ED and ED rats. Separate 2-way ANOVA revealed a significant main effects of mifepristone (FMif[1,31]=20.2, p<0.0001) and CIE (FCIE[1,31]=6.09, p=0.019), but not an interaction (FCIE×Mif[1,31]=0.226, p=0.638), showing that mifepristone increased GR expression (irrespective of CIE experience), and CIE rats have lower GR expression in the mPFC compared to CIE-naïve ED rats (Figure 3D).
3.6 Experiment 6: Plasma CORT levels are altered by ethanol self-administration in both CIE-ED and ED rats
Ethanol intake was different between CIE-ED and ED groups (Ftime × treatment[7,112]=4.28, p=0.0003; Ftreatment[1,16]=11.2, p=0.004), such that CIE-ED rats escalated their ethanol intake in week 4-7 of CIE experience (ps <0.05), while ED rats maintained ethanol intake levels attained at self-administration training (i.e. at Week 0; Figure 4A). The detailed lever responses for ethanol for CIE-ED and ED rats have been published previously (Somkuwar et al., 2016a).
Figure 4. Abstinence in rats with ethanol vapor history and those without it demonstrate differential effects on plasma CORT levels but not in glucocorticoid receptor (GR) signaling in the medial prefrontal cortex.
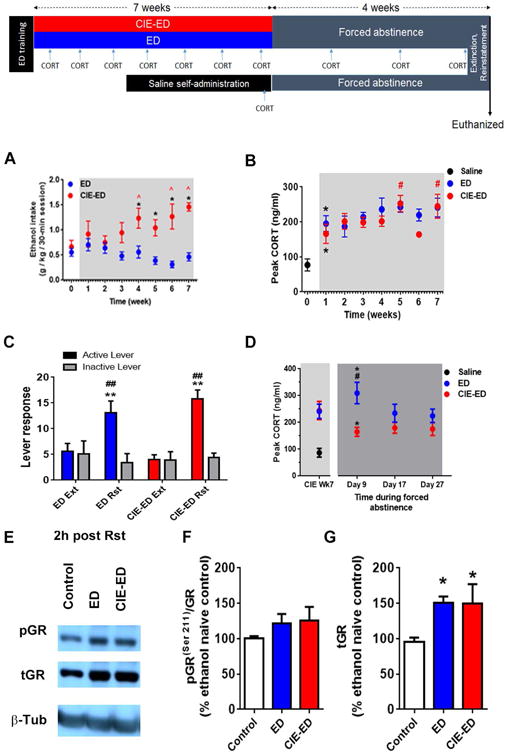
Schematic representation of the experimental design is presented. Rats were trained to self-administer ethanol (10%w/v; ethanol drinking, ED) and then divided into two groups. Plasma CORT was evaluated during alcohol experience and again during experimenter imposed abstinence. In the saline self-administering group, plasma CORT levels were determined only once during abstinence. GR expression and function in the mPFC were evaluated in postmortem tissue obtained after four weeks of abstinence. (A) Peak plasma CORT increased in rats self-administering ethanol (ED) and those exposed to chronic intermittent ethanol exposure (CIE) with ethanol self-administration (CIE-ED) at week 1 of CIE compared to ethanol naïve controls that experienced saline self-administration. In CIE-ED rats, peak CORT increased further during weeks 5 and 7 of vapor experience compared to week 1. Values are mean ± S.E.M of peak CORT levels. n=8-10/group; * p<0.05 compared to ethanol naïve saline self-administering rats; # p<0.05 compared to CIE-ED rats at Week 1. (B) Ethanol intake increases over weeks of CIE in CIE-ED compared to ED. Values are mean ± S.E.M. for ethanol intake in g/kg/30-min ethanol self-administration session. n=8-10/group, * p<0.05 compared to ED rats at an equivalent time; ˆ p<0.05 compared to intake before onset of CIE (Wk 0). (C) Ethanol paired cued-context induced reinstatement (Rst) of ethanol seeking in rats self-administering ethanol with and without CIE (CIE-ED and ED, respectively). Values are mean ± S.E.M. for lever presses. ** p<0.001, increased compared to responding during extinction (Ext); ## p<0.001, increased compared to responding on the inactive lever. (D) Peak plasma CORT was greater in ED compared to CIE-ED after 9 days of forced abstinence from ethanol, although peak CORT was increased in both CIE-ED and ED compared to ethanol naïve saline self-administering rats. Values are mean ± S.E.M of peak CORT levels. n=8-10/group; * p<0.05 compared to ethanol naïve saline self-administering rats; # p<0.05 compared to CIE-ED rats at 9 days of forced abstinence. (E) Representative immunoblots of pGR, tGR, with corresponding blots for b-tubulin (β-Tub) from control, ED and CIE-ED rats 2h after cue-induced reinstatement of ethanol seeking. Densitometric analysis revealed that GR function (pGR(ser211)/tGR, F) was not different between groups, and GR expression (tGR, G) was increased in both CIE-ED and ED compared to ethanol and behaviorally naïve controls. Values are mean ± S.E.M. for expression of GR and pGR(ser211)/tGR in the medial prefrontal cortex of CIE-ED and ED rats presented as a percent of age-matched, ethanol and behaviorally naïve controls. n=8-10/group, * p<0.05 compared to age-matched ethanol and behaviorally naïve controls. For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.
Peak CORT was measured once in saline controls, and in CIE-ED and ED rats each week during the 7-week CIE treatment (Figure 4B). Compared to saline controls, peak CORT was elevated in ED as well as CIE-ED with only one week of CIE experience (Fethanol[2,22]=7.54, p=0.003). Over the seven weeks of ethanol self-administration, in CIE and CIE-naïve rats, repeated measures two-way ANOVA revealed a significant main effects of time (Ftime[6,96]=2.83, p=0.014), but not of CIE (Ftreatment[1,12]=0.379) or an interaction (Ftime × treatment[6,96]=0.774, p=0.592). Post hoc comparisons revealed that peak CORT increased in CIE-ED rats at week 5 and 7 of CIE compared with respective week 1, while no statistically significant differences were revealed in ED rats during the seven weeks of ethanol self-administration (Figure 4B). No differences were revealed between CIE-ED vs. ED rats during the 7 weeks of exposure.
3.7 Experiment 7: Ethanol abstinence produced differential effects on peak CORT between CIE-ED and ED rats
Both CIE-ED and ED rats extinguished ethanol seeking within 6 sessions such that the number of active lever responses was not different from the respective inactive lever responses (Figure 4C, replotted from (Somkuwar et al., 2016a), also see Supplementary Table 5). Active lever presses were increased during contextual-cued reinstatement (context and cue-lights were identical to that used during maintenance of ethanol self- administration) compared to extinction (Fsession[1,14]=68.4, p=0.0001) in both CIE-ED (average - 459%; range 83-667% of respective extinction; p<0.05) and ED (average – 303%; range 125-650% of respective extinction; p<0.05). No main effect of CIE or interaction were observed (Fsession × treatment[1,14]=3.33, p=0.089; Ftreatment[1,14]=0.058, p=0.81). Active-inactive lever discrimination was significant during the reinstatement sessions, but not during the last extinction session (ps < 0.0001, Figure 4C).
Peak CORT was measured at three different time-points during the 4-week protracted abstinence and has never been published elsewhere. Statistical significance was revealed for main effect of treatment (Ftreatment[1,14]=10.8, p=0.005), but not for time or interaction (Ftime × treatment[2,28]=2.15, p=0.136; Ftime[2,28]=1.15, p=0.332 Figure 4D) such that peak CORT was higher in ED compared to CIE-ED. However, this effect appears to be due to the CORT differences on the 9th day of forced abstinence. An additional 2-way repeated measures ANOVA comparing CORT levels between week 7 of CIE and day 9 (only) of abstinence revealed significant interaction of treatment and time and main effect of treatment (Ftime × treatment[1,14]=7.01, p=0.018, Ftreatment[1,14]=6.40, p=0.024), but not of time (Ftime[1,14]=0.244, p=0.64), thus suggesting differential effects of early-intermediate abstinence on peak CORT between CIE-ED and ED groups. Interestingly, compared to ethanol naïve saline self-administering rats tested during abstinence from self-administration, peak CORT levels on the 9th day of forced abstinence were significantly greater in both ED and CIE-ED groups (F[2,20]=19.2, p<0.0001, Figure 4D), and was greater in ED compared to CIE-ED (p < 0.005).
3.8 Experiment 8: Suppressed expression and activation of mPFC GR observed during acute withdrawal were reversed following protracted abstinence
Function and expression of GR in the mPFC were evaluated in CIE-ED and ED rats 2 h after reinstatement of ethanol seeking triggered by ethanol contextual cues (Figures 4E-G). Function of GR receptor, expressed as pGRser211/tGR, was not different between saline-self-administering rats and ethanol- and behaviorally-naïve controls (Supplementary Table 6). pGRser211/tGR levels in both CIE-ED and ED rats 2 h after reinstatement testing were comparable to those of ethanol- and behaviorally- naïve controls (F[2,19]=0.691, p=0.513; Figures 4E-F), suggesting that GR function was increased and normalized following protracted ethanol abstinence compared to acute withdrawal.
Expression of tGR was increased 2 h after reinstatement in both CIE-ED and ED compared to age-matched ethanol- and behaviorally-naïve controls (F[2,23]=4.45, p=0.023 Figures 4E,G). However, tGR expression was not different between CIE-ED and ED rats. Finally, tGR (but not pGRser211/tGR) was found to be decreased in saline-self-administering rats compared to ethanol- and behaviorally-naïve controls (p < 0.05; Supplementary Table 6). Taken together, these results suggest that the suppressed GR expression during acute withdrawal was reversed following protracted abstinence and these effects are specific to forced abstinence from alcohol and not merely abstinence from lever responding.
4. Discussion
The findings reported herein demonstrate that peak CORT increased in CIE rats, nadir CORT levels did not change, suggesting therefore that CIE experience increased amplitude of diurnal fluctuation of CORT. One limitation is the absence of CORT data from an ethanol naïve control group across all the time-points tested, since age, among other factors, may alter CORT levels independent of CIE experience. However, increase in peak CORT with age is typically observed in >18 month old male rats (Goya et al., 1989; Lo et al., 2000), while the diurnal variation in CORT in rats of the age-range comparable to the current study (3-6 months) were not different than that observed here (week 0) in the absence of ethanol (Allen and Kendall, 1967; Guo et al., 2016; Meaney et al., 1992). Therefore, the CORT effects reported herein are more likely attributable to ethanol. The lack of effect on nadir CORT observed here was comparable to previously published negative findings with nadir CORT in ethanol preferring rats, in rats administered ethanol via liquid diet, and with plasma ACTH, upstream of CORT, in the CIE model (Apter and Eriksson, 2006; Little et al., 2008; Richardson et al., 2008a). Lastly, no tolerance was observed in peak or nadir CORT following extended ethanol experience, supporting prior observations of a lack of neuroendocrine tolerance in animals with increasing BEC (Ogilvie et al., 1997). These effects are in contrast with studies in primates that revealed a mild attenuation of diurnal cortisol fluctuation, and a greater attenuation of diurnal ACTH rhythm following ethanol access (Cuzon Carlson et al., 2011; Helms et al., 2012). A major point of methodological difference is that in the primate studies, glucocorticoids were measured during 22 h access to voluntary ethanol intake; therefore longer duration of access and voluntary nature of ethanol experience may underlie the differences in plasma glucocorticoid changes. Relative to CIE, peak CORT levels were reduced transiently during early to intermediate abstinence. At the level of mPFC, transactivation of GR was decreased significantly at 7-21 days of abstinence, after which GR activity returned to control levels. Total GR expression in the mPFC was transiently increased at 7 days of abstinence, suggesting that adaptations in receptor expression were associated with (compensatory mechanism) reduced transactivation of the receptor. However, the association was not all-or-none, since the opposite relation between tGR and GR activation did not persist into 21 days. GR activation in mPFC exerts negative feedback on the HPA response (Akana et al., 2001; Diorio et al., 1993), in part, via modulation of downstream subcortical structures, for example the hypothalamus (Herman, 2012; Li et al., 2011). Therefore, the observed transition of dampened CORT during early-intermediate abstinence to elevated CORT during prolonged abstinence may be mediated by the observed transient decrease in GR function in the mPFC. Alternatively, other mechanisms, such as endocannabinoid signaling (Hill et al., 2011; Rimondini et al., 2002) and CRF receptor signaling (Myers et al., 2014; Watts, 2005) in the mPFC may also modulate plasma CORT during abstinence. Furthermore, our findings in adult male rats could be extended to adult female rats to determine whether CIE and abstinence from CIE produces alterations in GR activation in the mPFC and diurnal CORT, as few studies have demonstrated that chronic ethanol experience produces long-lasting changes in HPA axis responsiveness in a sex-specific manner (Karanikas et al., 2013; Logrip et al., 2013).
In a different group of rats, ethanol self-administration was tested biweekly during acute withdrawal through the CIE treatment. ED rats maintained low ethanol intake during their self-administration sessions, whereas the CIE-ED rats escalated their ethanol intake over weeks of vapor exposure, as expected based on previous studies (O'Dell et al., 2004; Richardson et al., 2008b). Peak CORT levels in rats self-administering saline (Fig 4B) were comparable to that in ethanol naïve rats (Fig 1A), suggesting that self-administration training per se does not alter peak CORT. Ethanol self-administration training was sufficient to increase peak CORT levels (by ∼110%), supporting previous research that ethanol has stressor-like effects to activate the HPA axis (Lee and Rivier, 2003; Ogilvie et al., 1997; Richardson et al., 2008a; Rivier, 1993; Willey et al., 2012). This effect on peak CORT was comparable to that observed with CIE. Following self-administration training, additional exposure to ethanol via vapor inhalation did not further increase (or decrease) these peak CORT levels. Taken together, these results suggest that plasma CORT does not correlate with the magnitude or extent of ethanol exposure.
During acute withdrawal, ethanol (CIE and/or self-administration) suppressed GR expression and activation in the mPFC. mPFC GR suppression was also reported following non-alcohol-related chronic stress (McKlveen et al., 2016), suggesting that the mPFC GR suppression reported herein may be a manifestation of chronic HPA-axis activation, perhaps engaging opponent (e.g., desensitization, tolerance) mechanisms in the mPFC. Mifepristone, a GR antagonist, may inhibit the effects of chronically elevated CORT on such compensatory processes in the mPFC, leading to a relative increase in GR expression and function compared to placebo groups. Note though that GR expression and function following chronic mifepristone still remained lower than ethanol naïve controls (100% level in Fig 3). Alternatively, perhaps mifepristone increased GR levels in the mPFC via its effects on the progesterone receptors (Cadepond et al., 1997; Spitz and Bardin, 1993). Indeed, mifepristone-mediated GR increase has been observed in other parts of the body with high progesterone receptor expression (Narvekar et al., 2006). Interestingly, the absence of similar mPFC-GR effects in the CIE rats (self-administration naïve) during acute withdrawal suggests that ethanol self-administration produced more profound effects on mPFC GR activation and expression compared to CIE experience alone.
Although chronic inhibition of CORT action (via mifepristone) selectively suppressed self-administration in CIE-ED rats (here and others (Repunte-Canonigo et al., 2015; Vendruscolo et al., 2012; Vendruscolo et al., 2015), its reversal of ethanol-mediated mPFC-GR suppression was seen in both CIE-ED and ED rats. Similarly, the current study and others found that mPFC GR expression was not different between CIE-ED and ED groups following prolonged abstinence (Vendruscolo et al., 2012), despite showing quite different ethanol self-administration. These mismatches suggests that the GR-mediated effects on ethanol self-administration (also) involve regions other than the mPFC, perhaps the nucleus accumbens and ventral tegmental area (Repunte-Canonigo et al., 2015). Instead, mPFC GR changes may contribute to other stress-related behaviors (McKlveen et al., 2016; Myers et al., 2014). Our study also shows similar changes in plasma peak CORT levels in CIE-ED and ED rats. Previous research suggests that CORT changes (in rate and magnitude) produced by chronic alcohol experience and abstinence differ between plasma and different parts of the brain (Croft et al., 2008; Little et al., 2008). For example, nadir CORT in the PFC was increased in rats with 8 months (but not one month) of abstinence of liquid ethanol diet, even though plasma nadir CORT was not different from controls (Little et al., 2008). CORT in other brain regions was found to be more sensitive to manipulations such as ethanol (Croft et al., 2008; Little et al., 2008). The disconnect between plasma CORT and brain CORT may be due to differences in diffusion across the blood-brain barrier (Droste et al., 2008) or intrinsic signaling mechanisms within these brain regions. Therefore, it is possible that peak CORT levels in the regions other than the mPFC may be different from plasma CORT thereby producing the selective effect with escalated ethanol drinking and differential response to mifepristone. These effects may also differ between liquid ethanol diet and CIE. Additional experiments measuring brain CORT measures (from post-mortem tissue or via microdialysis) may reveal greater insights into stress-alcohol interaction in mediating escalated drinking and increased relapse vulnerability.
Interestingly, the extent of ethanol exposure differentiated the directionality of CORT response to forced ethanol abstinence, albeit transiently. For example, ED rats showed enhanced peak CORT during early-intermediate abstinence, while those of CIE-experienced rats, while still elevated, had decreased relative to the elevated levels seen during alcohol availability. These differences did not persist; peak CORT had returned to pre-abstinence levels in both CIE-ED and ED rats before cue-induced reinstatement was evaluated. Importantly, the current results in CIE rats are consistent with those observed in clinical studies that salivary cortisol awakening response is increased in longer abstaining patients compared to subjects with shorter abstinence periods (Junghanns et al., 2007).
With respect to adaptations in mPFC, saline self-administering rats showed suppressed GR expression following cue-induced reinstatement tested after prolonged self-administration abstinence, suggesting suppression of mPFC GR expression during acute withdrawal may be, in part, an effect of operant training experience. They did not show altered GR transactivation however. Taken together, GR transactivation during acute withdrawal and GR overexpression during protracted abstinence may result from the history of ethanol experience (in ED and CIE-ED rats). Interestingly, although relative GR activation in ED and CIE-ED rats were not different from controls during protracted abstinence, the increased GR expression (both in CIE-ED and ED) may underlie the increase in GR-mediated gene expression in the mPFC observed at a comparable (3 week) time during protracted abstinence (Repunte-Canonigo et al., 2015). Future studies should test whether CORT suppression (or mPFC GR inhibition) may protect against ethanol-cue mediated relapse.
One important inference from the current study is that the changes in peak CORT following ethanol exposure is not identical between rats with high (CIE and CIE-ED) and low (ED) ethanol exposure. This was evidenced by the opposite effect elicited in peak plasma CORT during early-intermediate abstinence (∼first two weeks of forced abstinence). Specifically, while CIE animals showed attenuated peak CORT levels compared to levels measured at the end of CIE exposure, ED rats exhibit potentiated peak CORT levels. These changes in peak CORT in CIE rats during early-intermediate abstinence mirrors effects observed in ACTH responses in comparable vapor model (Richardson et al., 2008a), as well as with CORT and ACTH levels in rats withdrawn from ethanol liquid diet (Rasmussen et al., 2000; Zorrilla et al., 2001). The directional differences in peak CORT changes between CIE-ED vs. ED rats may relate to the previously reported dampened HPA response to ethanol challenge in CIE withdrawn rats vs. the sensitized HPA response in non-dependent ED-like rats compared to controls (Richardson et al., 2008a). Perhaps low ethanol intake sensitizes HPA response, while high ethanol exposure induces neuroendocrine tolerance during early-intermediate abstinence (Edwards et al., 2015; Lu and Richardson, 2014).
The current study evaluated anxiety and aggression as glucocorticoid-modulated behaviors in rats with history of high ethanol experience (CIE). Previous studies have reported that alcohol intoxication following acute (single dose) as well as chronic ethanol (multiple experimenter-administered, p.o.) increased aggression-like behavior in (intoxicated) rodents in a resident-intruder confrontation paradigm (de Almeida et al., 2010; Peterson et al., 1989). High aggression in this paradigm was associated with increased anxiety and increased plasma CORT (Patki et al., 2015; Peterson et al., 1989). Here, instead, we report effects during acute withdrawal and over various intervals of ethanol abstinence. Acute withdrawal (8 h post-CIE) increased anxiety-like behavior, which is consistent with previous publications (Valdez et al., 2002; Zhao et al., 2007). In contrast, during early-intermediate abstinence (concurrently with the dip in peak CORT), CIE rats exhibited increased aggression-like behavior and reduced anxiety-like behavior compared to ethanol-naïve controls. The causal relations among the reduced peak CORT on the one hand and altered aggression- and anxiety-like behavior on the other remains to be determined, but the results are consistent with clinically observed co-occurrence of increased irritability and hypocortisolism (Bender et al., 2013; Hahner et al., 2007; Tiemensma et al., 2014).
Previous studies have implicated the mPFC in intoxication-induced aggression (Hwa et al., 2015; Patki et al., 2015; Peterson et al., 1989), for review see (Miczek et al., 2015b)). GR expression is 4-fold greater than MR expression in the mPFC, a disproportionately greater ratio than in many other regions associated with the stress response (Cintra et al., 1994; Diorio et al., 1993), and, thus, the mPFC may play a differential role in stress-induced regulation of CORT or behavior, since the GR preferentially responds to elevated glucocorticoid levels. Yet, few studies have investigated the relation between mPFC GR and anxiety- and aggression-like behaviors (Lin et al., 2016; Scotti et al., 2015). Firstly, mPFC GR can modulate physiological stress through negative feedback on the HPA response (Akana et al., 2001; Diorio et al., 1993). For example, CORT in mPFC can inhibit plasma insulin and ACTH secretion (Akana et al., 2001); therefore we can speculate that suppressed mPFC GR function, as observed in the current study, may increase insulin and ACTH. Following, a month of abstinence, alcohol dependent subjects with glucose intolerance exhibited reduced fasting glucose levels (Kim et al., 2013), supporting an increase in insulin function. As a corollary, the decreased mPFC GR function at the 7-day time-point may be related to disinhibition of the biological stress response in an EPM, resulting in reduced anxietylike behavior. Furthermore, mPFC GR activation modulate response of amygdala and hippocampus to psychogenic stress (Figueiredo et al., 2003). Glutamatergic inputs from the mPFC dampen amygdala output (Likhtik et al., 2005), and deficient GR responses in the mPFC could contribute to amplified stress responses (McEwen, 2012). Furthermore, chronic stress increases local GABA release in mPFC and inhibits glutamatergic projections from mPFC (McKlveen et al., 2016). Therefore, chronic alcohol intake-mediated HPA dysregulation (and perhaps the associated elevated CORT) may disinhibit the regions regulated by mPFC and thereby exacerbate stress-related behavior.
Aggression-like behavior increased with repeated testing in ethanol naïve controls, but not in CIE abstinent rats, perhaps due to a ceiling effect. In contrast, defensive-like behavior increased over repeated exposure in both the groups. Anxietylike behavior on an EPM task has been reported to be increased following protracted abstinence from ethanol experience (∼4-12 weeks), in several models of alcohol use, including CIE (Morales et al., 2015; Rasmussen et al., 2001; Zhao et al., 2007). However, only one other study investigated an intermediate time-point during early abstinence (2 week), and found that time spent in the open arms of the maze were not different in CIE abstinent rats compared to controls (Zhao et al., 2007). Our results advance our understanding of this relatively understudied early abstinence period, to show that about a week (8d) following CIE cessation, anxiety-like behavior was even decreased compared to ethanol naïve controls. The transient decrease in anxiety-like behavior may be related to the time course of some clinical studies that describe hyperactivity periods during early abstinence followed by depressive periods during protracted abstinence (Voltaire-Carlsson et al., 1996). This apparent “improvement” might lead to premature cessation of treatment seeking, compliance or care, leaving patients vulnerable for future relapse (Mossberg et al., 1985; Pickens et al., 1985; Voltaire-Carlsson et al., 1996). Finally, the transiently reduced anxiety coupled with increased aggression may be associated with increased sensation seeking (Parente, 2000; Wilson and Scarpa, 2011), a known risk factor for relapse.
Supplementary Material
Highlights.
Chronic ethanol enhances peak CORT and does not alter nadir CORT
Acute withdrawal from chronic ethanol enhances irritability-like behavior
Withdrawal and abstinence from chronic ethanol alters GR signaling in the mPFC
Systemic mifepristone prevents withdrawal-induced alterations in GR signaling in the mPFC
Acknowledgments
The study was supported by funds from the National Institute on Alcoholism and Alcohol Abuse and National Institute on Drug Abuse (NIDA; AA020098, AA06420 and DA034140 to CDM) and the Veterans Medical Research Foundation start-up funds to CDM. Tran Bao Nguyen was supported by funds for summer research at the Skaggs School of Pharmacy and Pharmaceutical Sciences (SSPPS), UCSD, and Jasmin Guevara was supported by funds from the summer research internship with NIDA. We appreciate the assistance of In Kyung Kim (SSPPS, UCSD) and Khush Kharidia (Reagents Scholars program, UCSD) with data collection and data entry. We thank Dr. Gery Schulteis from VA San Diego for the EPM.
Footnotes
Conflict of Interest: The authors report no conflict of interests.
Disclosure Statement: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adinoff B, Iranmanesh A, Veldhuis J, Fisher L. Disturbances of the stress response: the role of the HPA axis during alcohol withdrawal and abstinence. Alcohol Health Res World. 1998;22:67–72. [PMC free article] [PubMed] [Google Scholar]
- Akana SF, Chu A, Soriano L, Dallman MF. Corticosterone exerts site-specific and state-dependent effects in prefrontal cortex and amygdala on regulation of adrenocorticotropic hormone, insulin and fat depots. J Neuroendocrinol. 2001;13:625–637. doi: 10.1046/j.1365-2826.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- Allen C, Kendall JW. Maturation of the circadian rhythm of plasma corticosterone in the rat. Endocrinology. 1967;80:926–930. doi: 10.1210/endo-80-5-926. [DOI] [PubMed] [Google Scholar]
- Almeida SS, Garcia RA, de Oliveira LM. Effects of early protein malnutrition and repeated testing upon locomotor and exploratory behaviors in the elevated plus-maze. Physiol Behav. 1993;54:749–752. doi: 10.1016/0031-9384(93)90086-u. [DOI] [PubMed] [Google Scholar]
- Apter SJ, Eriksson CJP. The role of social isolation in the effects of alcohol on corticosterone and testosterone levels of alcohol-preferring and non-preferring rats. Alcohol Alcoholism. 2006;41:33–38. doi: 10.1093/alcalc/agh220. [DOI] [PubMed] [Google Scholar]
- Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology (Berl) 1991;103:227–232. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- Bender SL, Sherry NA, Masia R. Case records of the Massachusetts General Hospital. Case 16-2013. A 12-year-old girl with irritability, hypersomnia, and somatic symptoms. N Engl J Med. 2013;368:2015–2024. doi: 10.1056/NEJMcpc1208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman B, Brismar B. Hormone levels and personality traits in abusive and suicidal male alcoholics. Alcohol Clin Exp Res. 1994;18:311–316. doi: 10.1111/j.1530-0277.1994.tb00019.x. [DOI] [PubMed] [Google Scholar]
- Bertoglio LJ, Carobrez AP. Previous maze experience required to increase open arms avoidance in rats submitted to the elevated plus-maze model of anxiety. Behav Brain Res. 2000;108:197–203. doi: 10.1016/s0166-4328(99)00148-5. [DOI] [PubMed] [Google Scholar]
- Bertoglio LJ, Carobrez AP. Anxiolytic effects of ethanol and phenobarbital are abolished in test-experienced rats submitted to the elevated plus maze. Pharmacol Biochem Behav. 2002;73:963–969. doi: 10.1016/s0091-3057(02)00958-9. [DOI] [PubMed] [Google Scholar]
- Bourin M. Animal models for screening anxiolytic-like drugs: a perspective. Dialogues in clinical neuroscience. 2015;17:295–303. doi: 10.31887/DCNS.2015.17.3/mbourin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain PF, Haug M. Hormonal and neurochemical correlates of various forms of animal “aggression”. Psychoneuroendocrinology. 1992;17:537–551. doi: 10.1016/0306-4530(92)90014-x. [DOI] [PubMed] [Google Scholar]
- Breese GR, Sinha R, Heilig M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacol Therapeut. 2011;129:149–171. doi: 10.1016/j.pharmthera.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts KA, Phillips AG. Glucocorticoid receptors in the prefrontal cortex regulate dopamine efflux to stress via descending glutamatergic feedback to the ventral tegmental area. Int J Neuropsychopharmacol. 2013;16:1799–1807. doi: 10.1017/S1461145713000187. [DOI] [PubMed] [Google Scholar]
- Buydens-Branchey L, Branchey MH. Cortisol in alcoholics with a disordered aggression control. Psychoneuroendocrinology. 1992;17:45–54. doi: 10.1016/0306-4530(92)90075-i. [DOI] [PubMed] [Google Scholar]
- Cadepond F, Ulmann A, Baulieu EE. RU486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med. 1997;48:129–156. doi: 10.1146/annurev.med.48.1.129. [DOI] [PubMed] [Google Scholar]
- Cintra A, Zoli M, Rosen L, Agnati LF, Okret S, Wikstrom AC, Gustaffsson JA, Fuxe K. Mapping and computer assisted morphometry and microdensitometry of glucocorticoid receptor immunoreactive neurons and glial cells in the rat central nervous system. Neuroscience. 1994;62:843–897. doi: 10.1016/0306-4522(94)90481-2. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Polich J. Binge drinking in young adults: Data, definitions, and determinants. Psychol Bull. 2009;135:142–156. doi: 10.1037/a0014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft AP, O'Callaghan MJ, Shaw SG, Connolly G, Jacquot C, Little HJ. Effects of minor laboratory procedures, adrenalectomy, social defeat or acute alcohol on regional brain concentrations of corticosterone. Brain Res. 2008;1238:12–22. doi: 10.1016/j.brainres.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Cuzon Carlson VC, Seabold GK, Helms CM, Garg N, Odagiri M, Rau AR, Daunais J, Alvarez VA, Lovinger DM, Grant KA. Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharmacology. 2011;36:2513–2528. doi: 10.1038/npp.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, Santella S, Gianoulakis C. Response of the HPA-axis to alcohol and stress as a function of alcohol dependence and family history of alcoholism. Psychoneuroendocrinology. 2007;32:293–305. doi: 10.1016/j.psyneuen.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Daskalakis NP, Lehrner A, Yehuda R. Endocrine aspects of post-traumatic stress disorder and implications for diagnosis and treatment. Endocrinol Metab Clin North Am. 2013;42:503–513. doi: 10.1016/j.ecl.2013.05.004. [DOI] [PubMed] [Google Scholar]
- de Almeida RM, Saft DM, Rosa MM, Miczek KA. Flunitrazepam in combination with alcohol engenders high levels of aggression in mice and rats. Pharmacol Biochem Behav. 2010;95:292–297. doi: 10.1016/j.pbb.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75:411–418. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Driessen M, Meier S, Hill A, Wetterling T, Lange W, Junghanns K. The course of anxiety, depression and drinking behaviours after completed detoxification in alcoholics with and without comorbid anxiety and depressive disorders. Alcohol Alcoholism. 2001;36:249–255. doi: 10.1093/alcalc/36.3.249. [DOI] [PubMed] [Google Scholar]
- Droste SK, de Groote L, Atkinson HC, Lightman SL, Reul JMHM, Linthorst ACE. Corticosterone levels in the brain show a distinct ultradian rhythm but a delayed response to forced swim stress. Endocrinology. 2008;149:3244–3253. doi: 10.1210/en.2008-0103. [DOI] [PubMed] [Google Scholar]
- Edwards S, Little HJ, Richardson HN, Vendruscolo LF. Divergent regulation of distinct glucocorticoid systems in alcohol dependence. Alcohol. 2015;49:811–816. doi: 10.1016/j.alcohol.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst AJ, Dempster JP, Yee R, Dennis C, Nakano L. Alcohol toxicity, blood alcohol concentration and body water in young and adult rats. J Stud Alcohol. 1976;37:347–356. doi: 10.15288/jsa.1976.37.347. [DOI] [PubMed] [Google Scholar]
- Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur J Neurosci. 2003;18:2357–2364. doi: 10.1046/j.1460-9568.2003.02932.x. [DOI] [PubMed] [Google Scholar]
- File SE. The interplay of learning and anxiety in the elevated plus-maze. Behav Brain Res. 1993;58:199–202. doi: 10.1016/0166-4328(93)90103-w. [DOI] [PubMed] [Google Scholar]
- Finsterwald C, Alberini CM. Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: from adaptive responses to psychopathologies. Neurobiol Learn Mem. 2014;112:17–29. doi: 10.1016/j.nlm.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinato MH, Lockner JW, Fannon-Pavlich MJ, Sobieraj JC, Staples MC, Somkuwar SS, Ghofranian A, Chaing S, Navarro AI, Joea A, Luikart BW, Janda KD, Heyser C, Koob GF, Mandyam CD. A synthetic small-molecule Isoxazole-9 protects against methamphetamine relapse. Mol Psychiatry. 2017 doi: 10.1038/mp.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Ciccocioppo R, Heilig M. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo [1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, Crawford E, Mandyam CD, Koob GF. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc Natl Acad Sci U S A. 2012;109:18156–18161. doi: 10.1073/pnas.1116523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF. Vapor inhalation of alcohol in rats. Curr Protoc Neurosci. 2008a;Chapter 9 doi: 10.1002/0471142301.ns0929s44. Unit 9 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Lumeng L, Koob GF. Dependence-induced alcohol drinking by alcohol-preferring (P) rats and outbred Wistar rats. Alcohol Clin Exp Res. 2008b;32:1688–1696. doi: 10.1111/j.1530-0277.2008.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Swanson AM, Jacobs AM, Howell JL, Mo M, Dileone RJ, Koleske AJ, Taylor JR. Action control is mediated by prefrontal BDNF and glucocorticoid receptor binding. Proc Natl Acad Sci U S A. 2012;109:20714–20719. doi: 10.1073/pnas.1208342109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goya RG, Castro MG, Sosa YE. Diminished diurnal secretion of corticosterone in aging female but not male rats. Gerontology. 1989;35:181–187. doi: 10.1159/000213020. [DOI] [PubMed] [Google Scholar]
- Guo R, Simasko SM, Jansen HT. Chronic Alcohol Consumption in Rats Leads to Desynchrony in Diurnal Rhythms and Molecular Clocks. Alcohol Clin Exp Res. 2016;40:291–300. doi: 10.1111/acer.12944. [DOI] [PubMed] [Google Scholar]
- Hahner S, Loeffler M, Fassnacht M, Weismann D, Koschker AC, Quinkler M, Decker O, Arlt W, Allolio B. Impaired subjective health status in 256 patients with adrenal insufficiency on standard therapy based on cross-sectional analysis. J Clin Endocrinol Metab. 2007;92:3912–3922. doi: 10.1210/jc.2007-0685. [DOI] [PubMed] [Google Scholar]
- Haller J, van de Schraaf J, Kruk MR. Deviant forms of aggression in glucocorticoid hyporeactive rats: a model for ‘pathological’ aggression? J Neuroendocrinol. 2001;13:102–107. doi: 10.1046/j.1365-2826.2001.00600.x. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, McClintick MN, Grant KA. Social rank, chronic ethanol self-administration, and diurnal pituitary-adrenal activity in cynomolgus monkeys. Psychopharmacology (Berl) 2012;224:133–143. doi: 10.1007/s00213-012-2707-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP. Neural pathways of stress integration: relevance to alcohol abuse. Alcohol research : current reviews. 2012;34:441–447. [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Pan B, Fitzgerald ML, Roberts CJ, Lee TT, Karatsoreos IN, Mackie K, Viau V, Pickel VM, McEwen BS, Liu QS, Gorzalka BB, Hillard CJ. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci. 2011;31:10506–10515. doi: 10.1523/JNEUROSCI.0496-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Nathanson AJ, Shimamoto A, Tayeh JK, Wilens AR, Holly EN, Newman EL, DeBold JF, Miczek KA. Aggression and increased glutamate in the mPFC during withdrawal from intermittent alcohol in outbred mice. Psychopharmacology (Berl) 2015;232:2889–2902. doi: 10.1007/s00213-015-3925-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniguez-Lluhi JA, Lou DY, Yamamoto KR. Three amino acid substitutions selectively disrupt the activation but not the repression function of the glucocorticoid receptor N terminus. The Journal of biological chemistry. 1997;272:4149–4156. doi: 10.1074/jbc.272.7.4149. [DOI] [PubMed] [Google Scholar]
- Junghanns K, Backhaus J, Tietz U, Lange W, Bernzen J, Wetterling T, Rink L, Driessen M. Impaired serum cortisol stress response is a predictor of early relapse. Alcohol Alcoholism. 2003;38:189–193. doi: 10.1093/alcalc/agg052. [DOI] [PubMed] [Google Scholar]
- Junghanns K, Horbach R, Ehrenthal D, Blank S, Backhaus J. Cortisol awakening response in abstinent alcohol-dependent patients as a marker of HPA-axis dysfunction. Psychoneuroendocrinology. 2007;32:1133–1137. doi: 10.1016/j.psyneuen.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Junghanns K, Tietz U, Dibbelt L, Kuether M, Jurth R, Ehrenthal D, Blank S, Backhaus J. Attenuated salivary cortisol secretion under cue exposure is associated with early relapse. Alcohol Alcohol. 2005;40:80–85. doi: 10.1093/alcalc/agh107. [DOI] [PubMed] [Google Scholar]
- Karanikas CA, Lu YL, Richardson HN. Adolescent drinking targets corticotropin-releasing factor peptide-labeled cells in the central amygdala of male and female rats. Neuroscience. 2013;249:98–105. doi: 10.1016/j.neuroscience.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, Zamora-Martinez ER, Edwards S, Mandyam CD. Structural reorganization of pyramidal neurons in the medial prefrontal cortex of alcohol dependent rats is associated with altered glial plasticity. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim SJ, Lee WY, Cheon YH, Lee SS, Ju A, K M, Kim DJ. The effects of alcohol abstinence on BDNF, ghrelin, and leptin secretions in alcohol-dependent patients with glucose intolerance. Alcohol Clin Exp Res. 2013;37(1):E52–58. doi: 10.1111/j.1530-0277.2012.01921.x. [DOI] [PubMed] [Google Scholar]
- Lagerspetz K, Portin R. Simulation of cues eliciting aggressive responses in mice at two age levels. The Journal of genetic psychology. 1968;113:53–63. doi: 10.1080/00221325.1968.10533808. [DOI] [PubMed] [Google Scholar]
- Lagerspetz KM, R Simulation experiments on stimuli eliciting aggressive behavior in mice. Rep Psychol Inst Univ Turku. 1965;13:1–9. [Google Scholar]
- Lagerspetz KY, Tirri R, Lagerspetz KM. Neurochemical and endocrinological studies of mice selectively bred for aggressiveness. Scandinavian journal of psychology. 1968;9:157–160. doi: 10.1111/j.1467-9450.1968.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Lee S, Rivier C. Long-term influence of an initial exposure to alcohol on the rat hypothalamic-pituitary axis. Alcohol Clin Exp Res. 2003;27:1463–1470. doi: 10.1097/01.ALC.0000086065.06203.DD. [DOI] [PubMed] [Google Scholar]
- Li J, Bian W, Dave V, Ye JH. Blockade of GABA(A) receptors in the paraventricular nucleus of the hypothalamus attenuates voluntary ethanol intake and activates the hypothalamic-pituitary-adrenocortical axis. Addict Biol. 2011;16:600–614. doi: 10.1111/j.1369-1600.2011.00344.x. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Paz R, Pare D. Prefrontal control of the amygdala. J Neurosci. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YT, Liu TY, Yang CY, Yu YL, Chen TC, Day YJ, Chang CC, Huang GJ, Chen JC. Chronic activation of NPFFR2 stimulates the stress-related depressive behaviors through HPA axis modulation. Psychoneuroendocrinology. 2016;71:73–85. doi: 10.1016/j.psyneuen.2016.05.014. [DOI] [PubMed] [Google Scholar]
- Little HJ, Croft AP, O'Callaghan MJ, Brooks SP, Wang G, Shaw SG. Selective increases in regional brain glucocorticoid: a novel effect of chronic alcohol. Neuroscience. 2008;156:1017–1027. doi: 10.1016/j.neuroscience.2008.08.029. [DOI] [PubMed] [Google Scholar]
- Lo MJ, Kau MM, Cho WL, Wang PS. Aging effects on the secretion of corticosterone in male rats. J Investig Med. 2000;48:335–342. [PubMed] [Google Scholar]
- Logrip ML, Rivier C, Lau C, Im S, Vaughan J, Lee S. Adolescent alcohol exposure alters the rat adult hypothalamic-pituitary-adrenal axis responsiveness in a sex-specific manner. Neuroscience. 2013;235:174–186. doi: 10.1016/j.neuroscience.2012.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YL, Richardson HN. Alcohol, stress hormones, and the prefrontal cortex: a proposed pathway to the dark side of addiction. Neuroscience. 2014;277:139–151. doi: 10.1016/j.neuroscience.2014.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malisch JL, Breuner CW, Gomes FR, Chappell MA, Garland T., Jr Circadian pattern of total and free corticosterone concentrations, corticosteroid-binding globulin, and physical activity in mice selectively bred for high voluntary wheel-running behavior. Gen Comp Endocrinol. 2008;156:210–217. doi: 10.1016/j.ygcen.2008.01.020. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The ever-changing brain: cellular and molecular mechanisms for the effects of stressful experiences. Dev Neurobiol. 2012;72:878–890. doi: 10.1002/dneu.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKlveen JM, Morano RL, Fitzgerald M, Zoubovsky S, Cassella SN, Scheimann JR, Ghosal S, Mahbod P, Packard BA, Myers B, Baccei ML, Herman JP. Chronic Stress Increases Prefrontal Inhibition: A Mechanism for Stress-Induced Prefrontal Dysfunction. Biol Psychiatry. 2016;80:754–764. doi: 10.1016/j.biopsych.2016.03.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, Sharma S, Viau V. Basal ACTH, corticosterone and corticosterone-binding globulin levels over the diurnal cycle, and age-related changes in hippocampal type I and type II corticosteroid receptor binding capacity in young and aged, handled and nonhandled rats. Neuroendocrinology. 1992;55:204–213. doi: 10.1159/000126116. [DOI] [PubMed] [Google Scholar]
- Miczek KA, DeBold JF, Hwa LS, Newman EL, de Almeida RM. Alcohol and violence: neuropeptidergic modulation of monoamine systems. Ann N Y Acad Sci. 2015a;1349:96–118. doi: 10.1111/nyas.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Takahashi A, Gobrogge KL, Hwa LS, de Almeida RM. Escalated Aggression in Animal Models: Shedding New Light on Mesocorticolimbic Circuits. Current opinion in behavioral sciences. 2015b;3:90–95. doi: 10.1016/j.cobeha.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, McGinnis MM, McCool BA. Chronic ethanol exposure increases voluntary home cage intake in adult male, but not female, Long-Evans rats. Pharmacol Biochem Behav. 2015;139:67–76. doi: 10.1016/j.pbb.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossberg D, Liljeberg P, Borg S. Clinical conditions in alcoholics during long-term abstinence: a descriptive, longitudinal treatment study. Alcohol. 1985;2:551–553. doi: 10.1016/0741-8329(85)90133-8. [DOI] [PubMed] [Google Scholar]
- Myers B, McKlveen JM, Herman JP. Glucocorticoid actions on synapses, circuits, and behavior: implications for the energetics of stress. Front Neuroendocrinol. 2014;35:180–196. doi: 10.1016/j.yfrne.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narvekar N, Critchley HO, Cheng L, Baird DT. Mifepristone-induced amenorrhoea is associated with an increase in microvessel density and glucocorticoid receptor and a decrease in stromal vascular endothelial growth factor. Hum Reprod. 2006;21:2312–2318. doi: 10.1093/humrep/del182. [DOI] [PubMed] [Google Scholar]
- Navarro AI, Mandyam CD. Protracted abstinence from chronic ethanol exposure alters the structure of neurons and expression of oligodendrocytes and myelin in the medial prefrontal cortex. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Ogilvie K, Lee S, Rivier C. Effect of three different modes of alcohol administration on the activity of the rat hypothalamic-pituitary-adrenal axis. Alcohol Clin Exp Res. 1997;21:467–476. doi: 10.1111/j.1530-0277.1997.tb03792.x. [DOI] [PubMed] [Google Scholar]
- Parente D. Influence of aerobic and stretching exercise on anxiety and sensation-seeking mood state. Percept Mot Skills. 2000;90:347–348. doi: 10.2466/pms.2000.90.1.347. [DOI] [PubMed] [Google Scholar]
- Patki G, Atrooz F, Alkadhi I, Solanki N, Salim S. High aggression in rats is associated with elevated stress, anxiety-like behavior, and altered catecholamine content in the brain. Neurosci Lett. 2015;584:308–313. doi: 10.1016/j.neulet.2014.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Peterson JT, Pohorecky LA, Hamm MW. Neuroendocrine and beta-adrenoceptor response to chronic ethanol and aggression in rats. Pharmacol Biochem Behav. 1989;34:247–253. doi: 10.1016/0091-3057(89)90307-9. [DOI] [PubMed] [Google Scholar]
- Pickens RW, Hatsukami DK, Spicer JW, Svikis DS. Relapse by alcohol abusers. Alcohol Clin Exp Res. 1985;9:244–247. doi: 10.1111/j.1530-0277.1985.tb05744.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Boldt BM, Bryant CA, Mitton DR, Larsen SA, Wilkinson CW. Chronic daily ethanol and withdrawal: 1. Long-term changes in the hypothalamo-pituitary-adrenal axis. Alcohol Clin Exp Res. 2000;24:1836–1849. [PubMed] [Google Scholar]
- Rasmussen DD, Mitton DR, Green J, Puchalski S. Chronic daily ethanol and withdrawal: 2. Behavioral changes during prolonged abstinence. Alcohol Clin Exp Res. 2001;25:999–1005. [PubMed] [Google Scholar]
- Repunte-Canonigo V, Shin W, Vendruscolo LF, Lefebvre C, van der Stap L, Kawamura T, Schlosburg JE, Alvarez M, Koob GF, Califano A, Sanna PP. Identifying candidate drivers of alcohol dependence-induced excessive drinking by assembly and interrogation of brain-specific regulatory networks. Genome Biol. 2015;16:68. doi: 10.1186/s13059-015-0593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O'Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008a;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Zhao Y, Fekete EM, Funk CK, Wirsching P, Janda KD, Zorrilla EP, Koob GF. MPZP: a novel small molecule corticotropin-releasing factor type 1 receptor (CRF1) antagonist. Pharmacol Biochem Behav. 2008b;88:497–510. doi: 10.1016/j.pbb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riittinen ML, Lindroos F, Kimanen A, Pieninkeroinen E, Pieninkeroinen I, Sippola J, Veilahti J, Bergstrom M, Johansson G. Impoverished rearing conditions increase stress-induced irritability in mice. Dev Psychobiol. 1986;19:105–111. doi: 10.1002/dev.420190203. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Rivier C. Female Rats Release More Corticosterone Than Males in Response to Alcohol - Influence of Circulating Sex Steroids and Possible Consequences for Blood-Alcohol Levels. Alcoholism-Clinical and Experimental Research. 1993;17:854–859. doi: 10.1111/j.1530-0277.1993.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Rivier C. Role of hypothalamic corticotropin-releasing factor in mediating alcohol-induced activation of the rat hypothalamic-pituitary-adrenal axis. Front Neuroendocrinol. 2014;35:221–233. doi: 10.1016/j.yfrne.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Scott JP. Agonistic behavior of mice and rats: a review. American zoologist. 1966;6:683–701. doi: 10.1093/icb/6.4.683. [DOI] [PubMed] [Google Scholar]
- Scotti MA, Rendon NM, Greives TJ, Romeo RD, Demas GE. Short-day aggression is independent of changes in cortisol or glucocorticoid receptors in male Siberian hamsters (Phodopus sungorus). Journal of experimental zoology. Part A, Ecological genetics and physiology. 2015;323:331–341. doi: 10.1002/jez.1922. [DOI] [PubMed] [Google Scholar]
- Somkuwar SS, Fannon-Pavlich MJ, Ghofranian A, Quigley JA, Dutta RR, Galinato MH, Mandyam CD. Wheel running reduces ethanol seeking by increasing neuronal activation and reducing oligodendroglial/neuroinflammatory factors in the medial prefrontal cortex. Brain Behav Immun. 2016a;58:357–368. doi: 10.1016/j.bbi.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somkuwar SS, Fannon MJ, Staples MC, Zamora-Martinez ER, Navarro AI, Kim A, Quigley JA, Edwards S, Mandyam CD. Alcohol dependence-induced regulation of the proliferation and survival of adult brain progenitors is associated with altered BDNF-TrkB signaling. Brain Struct Funct. 2016b;221:4319–4335. doi: 10.1007/s00429-015-1163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz IM, Bardin CW. Mifepristone (RU 486)--a modulator of progestin and glucocorticoid action. N Engl J Med. 1993;329:404–412. doi: 10.1056/NEJM199308053290607. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Nagayasu K, Nishitani N, Kaneko S, Koide T. Control of intermale aggression by medial prefrontal cortex activation in the mouse. PLoS One. 2014;9:e94657. doi: 10.1371/journal.pone.0094657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE, Blackson T, Brigham J, Moss H, Caprara GV. The association between childhood irritability and liability to substance use in early adolescence: a 2-year follow-up study of boys at risk for substance abuse. Drug Alcohol Depend. 1995;39:253–261. doi: 10.1016/0376-8716(95)01175-6. [DOI] [PubMed] [Google Scholar]
- Tennison LR, Rodgers LS, Beker D, Vorobjeva KI, Creed ET, Simonenko A. Cortisol and symptoms of psychopathology in Russian and American college students. International journal of psychology : Journal international de psychologie. 2010;45:165–173. doi: 10.1080/00207590903452309. [DOI] [PubMed] [Google Scholar]
- Tiemensma J, Andela CD, Kaptein AA, Romijn JA, van der Mast RC, Biermasz NR, Pereira AM. Psychological morbidity and impaired quality of life in patients with stable treatment for primary adrenal insufficiency: cross-sectional study and review of the literature. Eur J Endocrinol. 2014;171:171–182. doi: 10.1530/EJE-14-0023. [DOI] [PubMed] [Google Scholar]
- Timmermans W, Xiong H, Hoogenraad CC, Krugers HJ. Stress and excitatory synapses: from health to disease. Neuroscience. 2013;248:626–636. doi: 10.1016/j.neuroscience.2013.05.043. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Estey D, Goodell V, Macshane LG, Logrip ML, Schlosburg JE, McGinn MA, Zamora-Martinez ER, Belanoff JK, Hunt HJ, Sanna PP, George O, Koob GF, Edwards S, Mason BJ. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Invest. 2015;125:3193–3197. doi: 10.1172/JCI79828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Roberts AJ. Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol. 2014;48:277–286. doi: 10.1016/j.alcohol.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voltaire-Carlsson A, Hiltunen AJ, Koechling UM, Borg S. Effects of long-term abstinence on psychological functioning: a prospective longitudinal analysis comparing alcohol-dependent patients and healthy volunteers. Alcohol. 1996;13:415–421. doi: 10.1016/0741-8329(96)81678-8. [DOI] [PubMed] [Google Scholar]
- von der PB, Sarkola T, Seppa K, Eriksson CJ. Testosterone, 5 alpha-dihydrotestosterone and cortisol in men with and without alcohol-related aggression. J Stud Alcohol. 2002;63:518–526. doi: 10.15288/jsa.2002.63.518. [DOI] [PubMed] [Google Scholar]
- Wang Z, Frederick J, Garabedian MJ. Deciphering the phosphorylation “code” of the glucocorticoid receptor in vivo. The Journal of biological chemistry. 2002;277:26573–26580. doi: 10.1074/jbc.M110530200. [DOI] [PubMed] [Google Scholar]
- Warmuz-Stangierska I, Baszko-Blaszyk D, Sowinski J. Emotions and features of temperament in patients with Addison's disease. Endokrynol Pol. 2010;61:90–92. [PubMed] [Google Scholar]
- Watts AG. Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: a complexity beyond negative feedback. Front Neuroendocrinol. 2005;26:109–130. doi: 10.1016/j.yfrne.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Willey AR, Anderson RI, Morales M, Ramirez RL, Spear LP. Effects of ethanol administration on corticosterone levels in adolescent and adult rats. Alcohol. 2012;46:29–36. doi: 10.1016/j.alcohol.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LC, Scarpa A. The link between sensation seeking and aggression: a meta-analytic review. Aggress Behav. 2011;37:81–90. doi: 10.1002/ab.20369. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Weiss F, Zorrilla EP. Remission and resurgence of anxiety-like behavior across protracted withdrawal stages in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31:1505–1515. doi: 10.1111/j.1530-0277.2007.00456.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann U, Spring K, Kunz-Ebrecht SR, Uhr M, Wittchen HU, Holsboer F. Effect of ethanol on hypothalamic-pituitary-adrenal system response to psychosocial stress in sons of alcohol-dependent fathers. Neuropsychopharmacology. 2004;29:1156–1165. doi: 10.1038/sj.npp.1300395. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Logrip ML, Koob GF. Corticotropin releasing factor: a key role in the neurobiology of addiction. Front Neuroendocrinol. 2014;35:234–244. doi: 10.1016/j.yfrne.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


