Abstract
Objective
The Vascular Registry (VR) on carotid procedures collects long-term outcomes on carotid artery stenting (CAS) and carotid endarterectomy (CEA) patients. The purpose of this report is to describe in-hospital and 30-day CAS outcomes in patients with atherosclerotic carotid artery disease (CAD; atherosclerosis [ATH]) compared to recurrent carotid stenosis (RES) and radiation-induced stenosis (RAD).
Methods
The VR collects provider-reported data on CAS using a Web-based data management system. For this report, data were analyzed at the preprocedure, procedure, predischarge, and 30-day intervals.
Results
As of November 20, 2008, there were 4017 patients with CAS with discharge data, of which 72% were due to ATH. A total of 2321 patients were available for 30-day outcomes analysis (1623 ATH, 529 restenosis, 119 radiation, 17 dissection, 3 trauma, and 30 other). Baseline demographics showed that ATH occurred in older patients (72-years-old), had the greatest history of coronary artery disease (CAD; 62%), myocardial infarction (MI; 24%), valvular heart disease (8%), arrhythmia (16%), congestive heart failure (CHF; 16%), diabetes mellitus (DM; 35%), and chronic obstructive pulmonary disease (COPD; 20%). RES had a higher degree of baseline stenosis (87.0 vs 85.8 ATH; P = .010), were less likely to be symptomatic (35.5% vs 46.3% ATH; P < .001), but had a greater history of hypertension, peripheral vascular disease (PVD), and smoking. RAD was seen in younger patients (66.6 vs 71.7 ATH; P < .001), were more likely to be male (78.2% vs 60.9% ATH; P < .001), and had less comorbidities overall, with the exception of amaurosis fugax, smoking, and cancer. The only statistically significant difference in perioperative rates was in transient ischemic attack (TIA; 2.7% ATH vs 0.9% RES; P = .02). There were no statistically significant differences in in-hospital death/ stroke/MI (ATH 5.4%, RES 3.8%, RAD 4.2%) or at 30 days (ATH 7.1%, RES 5.1%, RAD 5.0%). Even after adjusting for age, gender, symptomatology, CHF, and renal failure, the only statistically significant difference at 30 days was amaurosis fugax between ATH and RAD (odds ratio [OR] 0.13; P = .01).
Conclusion
Although patients with ATH have statistically significant comorbidities, they did not have statistically significant increased rates of death/stroke/MI during hospitalization or within 30 days after discharge when compared to RES or RAD. The CAS event rates for ATH vs RES and RAD are similar, despite prior published reports. Symptomatic ATH have statistically significant higher rates of death/stroke/MI compared to asymptomatic cohort. Finally, consistent and accurate entry of long-term data beyond initial hospitalization is essential to fully assess CAS outcomes since a significant number of adverse events occur in the interval from hospital discharge to 30 days.
In the United States, stroke is the leading cause of serious long-term disability and the third leading cause of death.1 Stroke is the most devastating complication of carotid artery stenosis, and atherosclerosis (ATH) is the leading etiology of carotid artery disease. Other etiologies include recurrent carotid stenosis (restenosis [RES]) and radiation-induced (RAD) or accelerated carotid ATH, which are considered high-risk factors for carotid endarterectomy (CEA).
It has been reported that recurrent carotid stenosis (or restenosis) occurs in approximately 20% of patients having an interventional carotid procedure (prior carotid artery stenting [CAS] or CEA), mostly asymptomatic.2, 3 Given the reported high event rates for carotid revascularization with redo CEA, CAS is frequently the preferred alternative to operative management in these patients.4
Radiation-induced or accelerated stenosis is a well-defined entity and can occur up to 20 years after treatment.5 Because stenotic lesions in this cohort are reported to be multiple, long, surgically less accessible, consisting primarily of fibrotic tissue, and at high risk for cranial nerve palsy, CAS is felt by many to be the preferred intervention compared to CEA.4, 6
In 2008, the Society for Vascular Surgery (SVS) issued clinical practice guidelines suggesting CAS for the treatment of symptomatic patients with moderate-to-severe carotid stenosis (≥50%) and high perioperative risk, which includes recurrent stenosis or radiation therapy to the neck.7
As part of the rapid evolution in vascular interventional techniques, there is a need for methods to assess efficacy of CAS and compare these results in routine clinical practice to conventional surgical procedures. Continued improvement in surgical interventions and the rapid development of CAS interventional devices and methods makes ongoing comparison of CEA and CAS imperative to ensure quality improvement.
In response to this need, the Vascular Registry (VR) on carotid procedures was developed to collect long-term outcomes on patients with CAS and CEA.8 As the first societal registry to enroll patients with CAS and CEA, the VR is the largest published database of CAS procedures in the United States. As the most representative sample of carotid artery procedures in the United States, the VR is a wealth of information for studying the indications for CAS. Whereas much is known about CAS procedures for the primary indication of ATH, less is known about the total experience with stenting including, for example, radiation-induced stenosis.
The purpose of this report is to describe in-hospital (procedural and predischarge) and 30-day outcomes in patients treated with CAS comparing atherosclerotic CAD (ATH) with nonatherosclerotic CAD (mainly restenosis [RES] and radiation-induced stenosis [RAD]).
METHODS
VR data are reported by providers through Web-based electronic data capture. The measurement schedule includes baseline (preoperative) information such as demographics, medical history, carotid symptom status, preprocedural diagnostic imaging and laboratory, procedural information including clinical utility, procedural and predischarge complications; and follow-up information such as postprocedure mortality, stroke, myocardial infarction (MI), and other morbidity. All data entered into the VR are fully compliant with the Health Insurance Portability and Accountability Act (HIPAA) regulations and are auditable. All data reports and analyses performed include only deindentified and aggregated data.
The New England Research Institutes, Inc (NERI, Watertown, Mass) maintains the online database. Funding for the administration and database management of the VR has been provided by the Society of Vascular Surgery (SVS, Chicago, Ill).
Outcomes
The primary outcome measures are combined death, stroke, and MI. Stroke is defined as any nonconvulsive, focal neurologic deficit of abrupt onset persisting more than 24 hours. The ischemic event must correspond to a vascular territory. An MI is classified as either Q wave MI in which one of the following criteria is required: (1) chest pain or other acute symptoms consistent with myocardial ischemia and new pathologic Q waves in two or more contiguous electrocardiogram (ECG) leads; or (2) new pathologic Q waves in two or more contiguous ECG leads and elevation of cardiac enzymes; or non-Q wave MI, which is defined as CK ratio >2 and CK-MB >1 in the absence of new, pathologic Q waves. In addition, although not considered specific outcomes but of interest, transient ischemic attack (TIA) and amaurosis fugax (or transient monocular blindness [TMB]) are also reported. Analysis of the 30-day outcomes were based on only those patients who had at least a 30-day follow-up visit (≥15 days) or experienced an endpoint (death, stroke, or MI) within 30 days of treatment.
Procedural success data were also collected. A CAS procedure is deemed successful when all of its components are completed without the need of conversion to CEA, or its abandonment before completion, and <30% residual stenosis is achieved postprocedure.
Statistical methods
Tests of statistical significance were conducted with χ2 or Fisher’s exact tests for categoric variables and analysis of variance (ANOVA) for continuous variables. Descriptive statistics are listed as mean ± SD for continuous variables and percent (frequency) for categoric variables. Subset analyses were performed using the two-tailed t test for continuous variables and the χ2 or Fisher’s exact test, as necessary, for discrete/categoric data. Unadjusted and adjusted odds ratios were used to compare the primary outcomes across treatment groups. Odds ratios were adjusted for age and any significant baseline factors that were kept after using backward elimination methods. Differences were considered significant if P < .05. All statistical analyses were performed by NERI using SAS Statistical Software (Cary, NC).
RESULTS
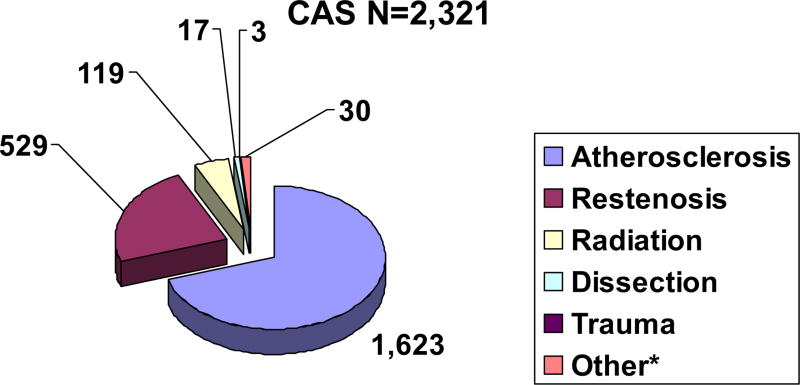
For the purpose of this report, data collected in the VR from the beginning of electronic data entry on July 11, 2005, to November 20, 2008, were analyzed. There were 4017 patients with CAS with procedural and discharge data, of which 72% underwent CAS for ATH. At 30 days, there were 2321 patients with CAS in-hospital and 30-day outcome data available for analysis (1623 ATH, 529 restenosis, 119 radiation, 17 dissection, 3 trauma, and 30 other), as illustrated in Fig 1. For purposes of this report, comparisons will be made between ATH vs RES and radiation (RAD).
Fig 1.
Carotid artery disease etiology for all carotid artery stenting (CAS) patients. *Other includes aneurysm, pseudoaneurysm, fibromuscular dysplasia, multiple and unknown etiologies.
Baseline demographics, seen in Table I, showed that patients with ATH were the oldest (71.7-years-old), had the greatest history of CAD (62.1%), MI (23.8%), valvular heart disease (7.9%), cardiac arrhythmia (15.7%), congestive heart failure (CHF; 16.1%), diabetes mellitus (DM; 34.6%), chronic obstructive pulmonary disease (COPD; 19.9%), and were the most likely to have a NYHA Class >2 (13.5%). Recurrent stenosis patients had a higher degree of baseline stenosis than patients with ATH (87.0 vs 85.8; P = .010), were less likely to be symptomatic (35.5% vs 46.3%; P < .001), but had a greater history of hypertension, peripheral vascular disease (PVD), and smoking. Radiation-induced patients were younger than patients with ATH (66.6 vs 71.7; P < .001), were more likely to be male (78.2% vs 60.9%; P < .001), and had less comorbidities overall, with the exception of amaurosis fugax, smoking, and cancer.
Table I.
Baseline demographics, medical history, and evaluation of carotid stenosis of patients with carotid artery stenting (CAS) by disease etiology
| ATH (n= 1623) n (%) |
RES (n = 529) n (%) |
RAD (n = 119) n (%) |
P value ATH-RES |
P value ATH-RAD |
|
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 71.7 ± 10.01 | 71.1 ± 8.68 | 66.6 ± 10.02 | .24 | <.001 |
| Gender (male, %) | 989 (60.9%) | 277 (52.4%) | 93 (78.2%) | <.001 | <.001 |
| Race (white, %) | 1496 (92.2%) | 511 (96.6%) | 111 (93.3%) | <.001 | .66 |
| Ethnicity (Hispanic, %) | 92 (5.7%) | 10 (1.9%) | 3 (2.5%) | <.001 | .21 |
| Medical history | |||||
| CAD | 1008 (62.1%) | 310 (58.6%) | 33 (27.7%) | .15 | <.001 |
| MI | 386 (23.8%) | 122 (23.1%) | 15 (12.6%) | .77 | .005 |
| VHD | 128 (7.9%) | 21 (4.0%) | 1 (0.8%) | .002 | .002 |
| CA | 255 (15.7%) | 45 (8.5%) | 13 (10.9%) | <.001 | .19 |
| CHF | 262 (16.1%) | 49 (9.3%) | 1 (0.8%) | <.001 | <.001 |
| HTN | 1331 (82.0%) | 446 (84.3%) | 84 (70.6%) | .23 | .002 |
| DM | 562 (34.6%) | 154 (29.1%) | 23 (19.3%) | .02 | <.001 |
| Stroke | 404 (24.9%) | 134 (25.3%) | 24 (20.2%) | .84 | .25 |
| TIA | 373 (23.0%) | 128 (24.2%) | 28 (23.5%) | .57 | .89 |
| Amaurosis fugax | 107 (6.6%) | 48 (9.1%) | 19 (16.0%) | .07 | <.001 |
| COPD | 323 (19.9%) | 82 (15.5%) | 19 (16.0%) | .02 | .30 |
| CRF | 57 (3.5%) | 14 (2.6%) | 0 (0.0%) | .40 | .03 |
| PVD | 586 (36.1%) | 229 (43.3%) | 27 (22.7%) | .003 | .003 |
| GI ulcer/bleeding | 70 (4.3%) | 31 (5.9%) | 5 (4.2%) | .16 | >.999 |
| Current or past smoker | 914 (56.3%) | 341 (64.5%) | 82 (68.9%) | <.001 | .007 |
| Cancer | 248 (15.3%) | 59 (11.2%) | 110 (92.4%) | .02 | <.001 |
| Coagulopathy | 13 (0.8%) | 7 (1.3%) | 3 (2.5%) | .30 | .09 |
| ASA grade >3 | 122 (7.5%) | 34 (6.4%) | 8 (6.7%) | .44 | .86 |
| NYHA >2 | 219 (13.5%) | 43 (8.1%) | 10 (8.4%) | <.001 | .12 |
| Carotid evaluation | |||||
| Symptomatic | 752 (46.3%) | 188 (35.5%) | 60 (50.4%) | <.001 | .39 |
| Baseline stenosis (%) | 85.8 ± 9.12 | 87.0 ± 8.85 | 83.2 ± 13.35 | .010 | .03 |
| Stenosis >80% | 1016 (63.1%) | 360 (68.4%) | 67 (56.3%) | .03 | .14 |
| Baseline ultrasound >80% stenosis | 1080 (66.5%) | 399 (75.4%) | 84 (70.6%) | <.001 | .37 |
| Contralateral stenosis | 405 (25.0%) | 131 (24.8%) | 29 (24.4%) | .95 | >.999 |
| Stents deployed | 1.1 ± 0.33 | 1.1 ± 0.27 | 1.2 ± 0.42 | .07 | .02 |
| Embolic protection Stent type | 1549 (95.4%) | 512 (96.8%) | 106 (89.1%) | .18 | .002 |
| Open | 1080 (79.4%) | 321 (75.9%) | 62 (78.5%) | .12 | .84 |
| Closed | 280 (20.6%) | 102 (24.1%) | 17 (21.5%) | ||
| Anti-platelet use | 1588 (97.8%) | 520 (98.3%) | 111 (93.3%) | .60 | .007 |
ATH, Atherosclerosis; RES, restenosis; RAD, radiation-induced; CAD, coronary artery disease; MI, myocardial infarction; VHD, valvular heart disease; CA, cardiac arrhythmia; CHF, congestive heart failure; HTN, hypertension; DM, diabetes mellitus; TIA, transient ischemic attack; COPD, chronic obstructive pulmonary disease; CRF, chronic renal failure; PVD, peripheral vascular disease; GI, gastrointestinal; ASA, American Society of Anesthesiology; NYHA, New York Heart Association.
P values are based on χ2 tests for categoric variables and t tests for continuous variables.
In-hospital outcomes
The only statistically significant difference in in-hospital (includes procedure and pre-discharge on initial hospitalization) adverse event rates comparing ATH to RES or RAD was in TIA (2.7% ATH vs 0.9% RES; P = .02; Table II, A). When comparing both disease etiology and presentation (eg, symptomatology), as expected, there were some statistically significant differences, with symptomatic patients having higher in-hospital event rates in general, compared with asymptomatic patients (Table II, B). In the ATH cohort, symptomatic patients had a higher combined death/stroke/MI rate (7.2% vs 3.8% asymptomatic [ASYMP]; P = .003) and a higher stroke rate (5.5% vs 3.0% ASYMP; P = .02). In the recurrent stenosis cohort (RES), symptomatic patients had a higher in-hospital rate of stroke (4.8% vs 1.5% ASYMP; P = .04) and TIA (2.7% vs 0% ASYMP; P = .005). The differences between symptomatology in in-hospital event rates in the radiation-induced cohort did not reach statistical significance, however, the numbers were small. When comparing in-hospital outcomes of symptomatic patients receiving CAS by disease etiology (Table III, A), there were no statistically significant differences. However, it is interesting to note that patients with ATH had higher rates of MI and TIA, and patients with RAD had high rates of mortality, stroke, and amaurosis fugax. In asymptomatic patients (Table III, B), patients with ATH had a statistically significant higher in-hospital rate of TIA compared with patients with RES (2.4% vs 0%; P = .001). Of note, although not statistically significant, the asymptomatic RES cohort had a higher mortality rate (1.5% vs 0.8% ATH vs 0% RAD).
Table II.
A, In-hospital (intraprocedural and predischarge) outcomes of carotid artery stenting (CAS) patients by disease etiology
| Peri-op AEs | ATH (n = 1623) n (%) |
RES (n = 529) n (%) |
RAD (n = 119) n (%) |
P value ATH-RES |
P value ATH-RAD |
|---|---|---|---|---|---|
| Death/stroke/MI | 87 (5.4%) | 20 (3.8%) | 5 (4.2%) | .17 | .83 |
| Death/stroke | 74 (4.6%) | 18 (3.4%) | 5 (4.2%) | .32 | >.999 |
| Mortality | 14 (0.9%) | 6 (1.1%) | 1 (0.8%) | .60 | >.999 |
| Stroke | 67 (4.1%) | 14 (2.6%) | 5 (4.2%) | .15 | >.999 |
| MI | 20 (1.2%) | 3 (0.6%) | 0 (0.0%) | .23 | .39 |
| TIA | 44 (2.7%) | 5 (0.9%) | 1 (0.8%) | .02 | .36 |
| Amaurosis fugax | 5 (0.3%) | 1 (0.2%) | 2 (1.7%) | >.999 | .08 |
| B, In-hospital (intraprocedural and predischarge) outcomes of patients with carotid artery stenting (CAS) by disease etiology and symptomatology | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
|
ATH
|
RES
|
RAD
|
|||||||
| Peri-op AEs | SYMPT (n = 752) n (%) |
ASYMP (n = 871) n (%) |
P value | SYMPT (n = 188) n (%) |
ASYMP (n = 341) n (%) |
P value | SYMPT (n = 60) n (%) |
ASYMP (n = 59) n (%) |
P value |
| Death/stroke/MI | 54 (7.2%) | 33 (3.8%) | .003 | 10 (5.3%) | 10 (2.9%) | .23 | 4 (6.7%) | 1 (1.7%) | .36 |
| Death/stroke | 43 (5.7%) | 31 (3.6%) | .04 | 9 (4.8%) | 9 (2.6%) | .21 | 4 (6.7%) | 1 (1.7%) | .36 |
| Mortality | 7 (0.9%) | 7 (0.8%) | .79 | 1 (0.5%) | 5 (1.5%) | .43 | 1 (1.7%) | 0 (0.0%) | >.999 |
| Stroke | 41 (5.5%) | 26 (3.0%) | .02 | 9 (4.8%) | 5 (1.5%) | .04 | 4 (6.7%) | 1 (1.7%) | .36 |
| MI | 12 (1.6%) | 8 (0.9%) | .26 | 1 (0.5%) | 2 (0.6%) | >.999 | 0 (0.0%) | 0 (0.0%) | .36 |
| TIA | 23 (3.1%) | 21 (2.4%) | .45 | 5 (2.7%) | 0 (0.0%) | .005 | 0 (0.0%) | 1 (1.7%) | .50 |
| Amaurosis fugax | 1 (0.1%) | 4 (0.5%) | .38 | 1 (0.5%) | 0 (0.0%) | .36 | 1 (1.7%) | 1 (1.7%) | >.999 |
ATH, Atherosclerosis; RES, restenosis; RAD, radiation-induced; AE, adverse event; MI, myocardial infarction; TIA, transient ischemic attack.
P values are based on Fisher’s exact test.
Events are defined as any AE occurring intraprocedural or predischarge.
Event rates are reported per-patient.
ATH, Atherosclerosis; RES, restenosis; RAD, radiation-induced; AE, adverse event; SYMPT, symptomatic; ASYMP, asymptomatic; MI, myocardial infarction; TIA, transient ischemic attack.
Table III.
A, In-hospital (intraprocedural and predischarge) outcomes of symptomatic patients with carotid artery stenting (CAS) by disease etiology
| Symptomatic
|
|||||
|---|---|---|---|---|---|
| Peri-op AEs | ATH (n = 752) n (%) |
RES (n = 188) n (%) |
RAD (n = 60) n (%) |
P value ATH-RES |
P value ATH-RAD |
| Death/stroke/MI | 54 (7.2%) | 10 (5.3%) | 4 (6.7%) | .42 | >.999 |
| Death/stroke | 43 (5.7%) | 9 (4.8%) | 4 (6.7%) | .72 | .77 |
| Mortality | 7 (0.9%) | 1 (0.5%) | 1 (1.7%) | >.999 | .46 |
| Stroke | 41 (5.5%) | 9 (4.8%) | 4 (6.7%) | .86 | .57 |
| MI | 12 (1.6%) | 1 (0.5%) | 0 (0.0%) | .48 | >.999 |
| TIA | 23 (3.1%) | 5 (2.7%) | 0 (0.0%) | >.999 | .40 |
| Amaurosis fugax | 1 (0.1%) | 1 (0.5%) | 1 (1.7%) | .36 | .14 |
| B, In-hospital (intraprocedural and predischarge) outcomes of asymptomatic patients with carotid artery stenting (CAS) by disease etiology | |||||
|---|---|---|---|---|---|
| Asymptomatic
|
|||||
| Peri-op AEs | ATH (n = 871) n (%) |
RES (n = 341) n (%) |
RAD (n = 59) n (%) |
P value ATH-RES |
P value ATH-RAD |
| Death/stroke/MI | 33 (3.8%) | 10 (2.9%) | 1 (1.7%) | .60 | .72 |
| Death/stroke | 31 (3.6%) | 9 (2.6%) | 1 (1.7%) | .48 | .72 |
| Mortality | 7 (0.8%) | 5 (1.5%) | 0 (0.0%) | .33 | >.999 |
| Stroke | 26 (3.0%) | 5 (1.5%) | 1 (1.7%) | .16 | >.999 |
| MI | 8 (0.9%) | 2 (0.6%) | 0 (0.0%) | .73 | >.999 |
| TIA | 21 (2.4%) | 0 (0.0%) | 1 (1.7%) | .001 | >.999 |
| Amaurosis fugax | 4 (0.5%) | 0 (0.0%) | 1 (1.7%) | .58 | .28 |
AE, Adverse event; ATH, atherosclerosis; RES, restenosis; RAD, radiation-induced; MI, myocardial infarction; TIA, transient ischemic attack.
Thirty-day outcomes
There were no statistically significant differences in 30-day (includes in-hospital through 30 days) adverse event rates between ATH and RES or RAD in the primary outcome of combined stroke/ death/or MI, or the individual outcomes of death, stroke, MI, TIA, or amaurosis fugax (or TMB; Table IV, A).
Table IV.
A, Thirty-day outcomes of patients with carotid artery stenting (CAS) by disease etiology
| 30-day AEs | ATH (n= 1623) n (%) |
RES (n = 529) n (%) |
RAD (n = 119) n (%) |
P value ATH vs RES |
P value ATH vs RAD |
|---|---|---|---|---|---|
| Death/stroke/MI | 116 (7.1%) | 27 (5.1%) | 6 (5.0%) | .11 | .46 |
| Death/stroke | 98 (6.0%) | 24 (4.5%) | 6 (5.0%) | .23 | .84 |
| Mortality | 23 (1.4%) | 9 (1.7%) | 2 (1.7%) | .68 | .69 |
| Stroke | 82 (5.1%) | 17 (3.2%) | 5 (4.2%) | .09 | .83 |
| MI | 25 (1.5%) | 4 (0.8%) | 0 (0.0%) | .20 | .41 |
| TIA | 52 (3.2%) | 9 (1.7%) | 1 (0.8%) | .07 | .26 |
| Amaurosis fugax | 6 (0.4%) | 1 (0.2%) | 2 (1.7%) | >.999 | .10 |
| B, Thirty-day outcomes of patients with carotid artery stenting (CAS) by disease etiology and symptomatology | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| ATH
|
RES
|
RAD
|
|||||||
| 30-day AEs | SYMPT (n = 752) n (%) |
ASYMP (n = 871) n (%) |
P value | SYMPT (n = 188) n (%) |
ASYMP (n = 341) n (%) |
P value | SYMPT (n = 60) n (%) |
ASYMP (n = 59) n (%) |
P value |
| Death/stroke/MI | 67 (8.9%) | 49 (5.6%) | .01 | 12 (6.4%) | 15 (4.4%) | .41 | 4 (6.7%) | 2 (3.4%) | .68 |
| Death/stroke | 54 (7.2%) | 44 (5.1%) | .08 | 11 (5.9%) | 13 (3.8%) | .28 | 4 (6.7%) | 2 (3.4%) | .68 |
| Mortality | 10 (1.3%) | 13 (1.5%) | .84 | 1 (0.5%) | 8 (2.3%) | .17 | 1 (1.7%) | 1 (1.7%) | >.999 |
| Stroke | 49 (6.5%) | 33 (3.8%) | .02 | 11 (5.9%) | 6 (1.8%) | .02 | 4 (6.7%) | 1 (1.7%) | .36 |
| MI | 14 (1.9%) | 11 (1.3%) | .42 | 1 (0.5%) | 3 (0.9%) | >.999 | 0 (0.0%) | 0 (0.0%) | .36 |
| TIA | 28 (3.7%) | 24 (2.8%) | .32 | 8 (4.3%) | 1 (0.3%) | .001 | 0 (0.0%) | 1 (1.7%) | .50 |
| Amaurosis fugax | 1 (0.1%) | 5 (0.6%) | .23 | 1 (0.5%) | 0 (0.0%) | .36 | 1 (1.7%) | 1 (1.7%) | >.999 |
AE, Adverse event; ATH, atherosclerosis; RES, restenosis; RAD, radiation-induced; MI, myocardial infarction; TIA, transient ischemic attack.
P values are based on Fisher’s exact test.
Events are defined as any AE occurring intraprocedure, predischarge, or after discharge to 30 days.
Event rates are reported per-patient.
ATH, Atherosclerosis; RES, restenosis; RAD, radiation-induced; AE, adverse event; SYMPT, symptomatic; ASYMP, asymptomatic; MI, myocardial infarction; TIA, transient ischemic attack.
When comparing both disease etiology and presentation (eg, symptomatology), as expected there were some statistically significant differences, with symptomatic patients, in general, having higher 30-day event rates compared with asymptomatic patients (Table IV, B). Ofnote, in the ATH cohort, symptomatic patients had a higher combined death/stroke/MI rate (8.9% vs 5.6% ASYMP; P = .01) and a higher stroke rate (6.5% vs 3.8% ASYMP; P = .02), which was similar to what was observed perioperatively. In the recurrent stenosis cohort (RES), symptomatic patients had a higher 30-day rate of stroke (5.9% vs 1.8% ASYMP; P = .02) and TIA (4.3% vs 0.3% ASYMP; P = .001), again similar to what was observed with the in-hospital rates. There were no statistically significant differences between symptomatology in 30-day event rates in the radiation-induced cohort.
When comparing 30-day outcomes of symptomatic patients receiving CAS by disease etiology (Table V, A), there were no statistically significant differences, which is similar to what was observed in-hospital. However, it is interesting to note that patients with ATH had higher combined event rates (death, stroke, MI) and high rates of MI, and patients with RAD had high rates of mortality, stroke, and amaurosis fugax. In asymptomatic patients (Table V, B), patients with ATH had a statistically significant higher 30-day rate ofTIA compared with patients with RES (2.8% vs 0.3%; P = .005).
Table V.
A, Thirty-day outcomes of symptomatic patients with carotid artery stenting (CAS) by disease etiology
| Symptomatic
|
|||||
|---|---|---|---|---|---|
| 30-day AEs | ATH (n = 752) n (%) |
RES (n = 188) n (%) |
RAD (n = 60) n (%) |
P value ATH-RES |
P value ATH-RAD |
| Death/stroke/MI | 67 (8.9%) | 12 (6.4%) | 4 (6.7%) | .31 | .81 |
| Death/stroke | 54 (7.2%) | 11 (5.9%) | 4 (6.7%) | .63 | >.999 |
| Mortality | 10 (1.3%) | 1 (0.5%) | 1 (1.7%) | .70 | .57 |
| Stroke | 49 (6.5%) | 11 (5.9%) | 4 (6.7%) | .87 | >.999 |
| MI | 14 (1.9%) | 1 (0.5%) | 0 (0.0%) | .33 | .62 |
| TIA | 28 (3.7%) | 8 (4.3%) | 0 (0.0%) | .68 | .26 |
| Amaurosis fugax | 1 (0.1%) | 1 (0.5%) | 1 (1.7%) | .36 | .14 |
| B, Thirty-day outcomes of asymptomatic patients with carotid artery stenting (CAS) by disease etiology | |||||
|---|---|---|---|---|---|
|
| |||||
| Asymptomatic
|
|||||
| 30-day AEs | ATH (n = 871) n (%) |
RES (n = 341) n (%) |
RAD (n = 59) n (%) |
P value ATH-RES |
P value ATH-RAD |
| Death/stroke/MI | 49 (5.6%) | 15 (4.4%) | 2 (3.4%) | .48 | .77 |
| Death/stroke | 44 (5.1%) | 13 (3.8%) | 2 (3.4%) | .45 | .76 |
| Mortality | 13 (1.5%) | 8 (2.3%) | 1 (1.7%) | .33 | .60 |
| Stroke | 33 (3.8%) | 6 (1.8%) | 1 (1.7%) | .10 | .72 |
| MI | 11 (1.3%) | 3 (0.9%) | 0 (0.0%) | .77 | >.999 |
| TIA | 24 (2.8%) | 1 (0.3%) | 1 (1.7%) | .005 | >.999 |
| Amaurosis fugax | 5 (0.6%) | 0 (0.0%) | 1 (1.7%) | .33 | .33 |
AE, Adverse event; ATH, atherosclerosis; RES, restenosis; RAD, radiation-induced; MI, myocardial infarction; TIA, transient ischemic attack.
Even after adjusting for age, gender, symptomatology, CHF, and chronic renal failure (Table VI), the only statistically significant difference at 30 days was in amaurosis fugax between ATH and radiation cohorts (odds ratio [OR] 0.13; P = .01).
Table VI.
Risk-adjusted 30-day outcomes of patients with carotid artery stenting (CAS) by disease etiology
| Unadjusted
|
Adjusted1
|
|||||||
|---|---|---|---|---|---|---|---|---|
| ATH vs RES
|
ATH vs RAD
|
ATH vs RES
|
ATH vs RAD
|
|||||
| OR (P value) |
95% CI | OR (P value) |
95% CI | OR (P value) |
95% CI | OR (P value) |
95% CI | |
| Death/stroke/MI | 1.43 (0.55) | 0.93–2.20 | 1.45 (0.66) | 0.62–3.37 | 1.31 (0.47) | 0.85–2.03 | 1.12 (0.96) | 0.47–2.63 |
| Death/stroke | 1.35 (0.49) | 0.86–2.14 | 1.21 (0.93) | 0.52–2.82 | 1.25 (0.43) | 0.79–1.99 | 0.96 (0.74) | 0.41–2.28 |
| Mortality | 0.83 (0.84) | 0.38–1.81 | 0.84 (0.91) | 0.20–3.61 | 0.78 (0.84) | 0.35–1.71 | 0.49 (0.44) | 0.11–2.20 |
| Stroke | 1.60 (0.27) | 0.94–2.73 | 1.21 (0.93) | 0.48–3.05 | 1.46 (0.29) | 0.85–2.50 | 1.03 (0.75) | 0.40–2.64 |
| MI | 2.05 (0.96) | 0.71–5.93 | N/A | N/A | 1.92 (0.96) | 0.66–5.58 | N/A | N/A |
| TIA | 1.91 (0.96) | 0.94–3.91 | 3.91 (0.31) | 0.54–28.50 | 1.84 (0.97) | 0.89–3.77 | 3.23 (0.40) | 0.44–23.87 |
| Amaurosis fugax | 1.96 (0.18) | 0.24–16.31 | 0.22 (0.04) | 0.04–1.09 | 1.90 (0.13) | 0.22–16.00 | 0.13 (0.01) | 0.02–0.72 |
ATH, Atherosclerosis; RES, restenosis; OR, odds ratio; CI, confidence interval; MI, myocardial infarction; N/A, not applicable; TIA, transient ischemic attack.
Adjusted for age, gender, symptomatology, congestive heart failure, and chronic renal failure.
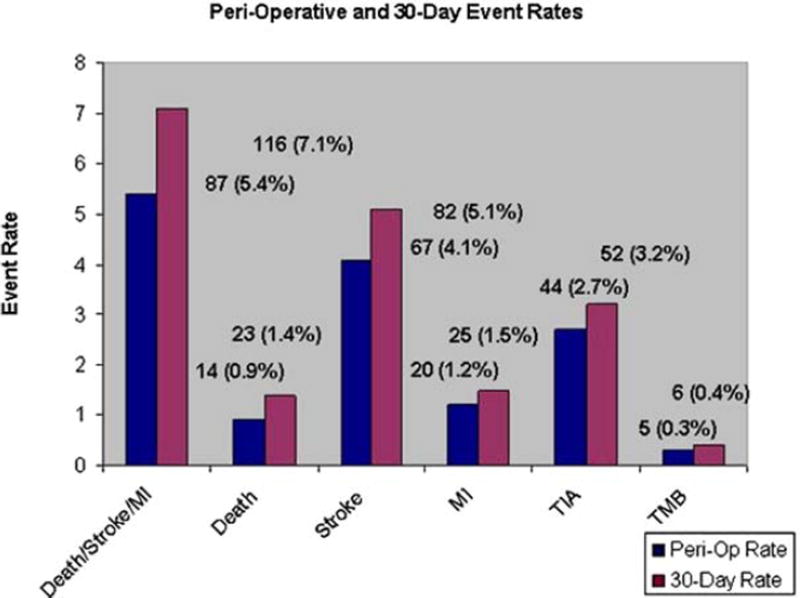
It is important to note that although there were few statistically significant differences for combined events between the cohorts, 25% of atherosclerotic events and 24% of the nonatherosclerotic events occurred after the in-hospital period, as illustrated in Fig 2. Approximately 40% of the atherosclerotic deaths and 36% of the nonatherosclerotic deaths occurred after the in-hospital period, which stresses the importance of patient follow-up.
Fig 2.
Comparison of perioperative and 30-day event rates.
Secondary outcomes
Secondary outcomes are shown by etiology in Table VII. Patients with restenosis had shorter hospital stays on average (1.6 days) than patients with ATH (2.2 days; P < .001) and were less likely to experience hypotension requiring treatment (1.3% vs 4.7% ATH; P < .001). Patients who received radiation were more likely to experience puncture site complications (2.5% vs 0.2% ATH; P = .009).
Table VII.
Secondary outcomes for patients with carotid artery stenting (CAS) by etiology
| Secondary outcomes | ATH (n= 1623) n (%) |
RES (n = 529) n (%) |
RAD (n = 119) n (%) |
P value ATH-RES |
P value ATH-RAD |
|---|---|---|---|---|---|
| Hospital length of stay (days) | 2.2 ± 3.59 | 1.6 ± 3.08 | 2.1 ± 3.75 | <.001 | .77 |
| Procedural/technical success | 1612 (99.3%) | 523 (98.9%) | 118 (99.2%) | .39 | .57 |
| Intraprocedural events | |||||
| Abrupt closure | 1 (0.1%) | 2 (0.4%) | 0 (0.0%) | .15 | >.999 |
| Spasm requiring treatment | 24 (1.5%) | 1 (0.2%) | 2 (1.7%) | .02 | .70 |
| Loss of external carotid | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | N/A | .07 |
| Embolization (systemic) | 1 (0.1%) | 0 (0.0%) | 0 (0.0%) | >.999 | >.999 |
| Embolization (carotid) | 7 (0.4%) | 0 (0.0%) | 0 (0.0%) | .20 | >.999 |
| Thrombosis | 6 (0.4%) | 2 (0.4%) | 1 (0.8%) | >.999 | .39 |
| Occlusive untreated dissection | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | N/A | N/A |
| Arrhythmia (treated) | 35 (2.2%) | 4 (0.8%) | 2 (1.7%) | .04 | >.999 |
| Hypotension (treated) | 77 (4.7%) | 7 (1.3%) | 2 (1.7%) | <.001 | .17 |
| Seizure | 2 (0.1%) | 2 (0.4%) | 0 (0.0%) | .25 | >.999 |
| Puncture site complication | 4 (0.2%) | 2 (0.4%) | 3 (2.5%) | .64 | .009 |
ATH, Atherosclerosis; RES, restenosis; RAD, radiation-induced; N/A, not applicable.
P values are based on Fisher’s exact test.
Event rates are reported per-patient.
DISCUSSION
The introduction of CAS to clinical practice has changed the selection criterion of patients with extracranial CAD for surgical intervention. Although there was early enthusiasm that CAS would be favorable for all patients, recent reports are mixed regarding the utility of CAS for asymptomatic patients and for patients with symptomatic lesions with good surgical anatomy.8, 9 In response to the favorable results of CAS from ARCHER and SAPPHIRE for patients who are at high surgical risk or who have unfavorable anatomy for surgery, the Center for Medicare and Medicaid Services (CMS) has offered payment for these patients but has waited for additional data to support broader application.10, 11 Thereafter, randomized controlled trials such as SPACE and EVA-3S demonstrated less favorable results.12, 13
In response to the need to provide ongoing assessment for the performance of new devices and techniques compared to conventional surgical options, the VR was developed to provide longitudinal data entry. This not only addresses the science needed to answer critical clinical questions but also addresses means to provide quality assurance and fulfill recertification requirements of federal agencies.
In this registry, although patients with ATH appear to be sicker due to statistically significant comorbidities, they did not have statistically significant increased rates of death/stroke/MI in-hospital or 30 days after discharge when compared to other etiologies. Although the VR 30-day results of death/stroke in ATH vs RES are not statistically significant (6% ATH vs 4.5% RES; P = .84), AbuRahma et al14 reported a statistically significant increase in 30-day rates of death/stroke in patients undergoing primary CAS compared with those undergoing CAS for post-CEArestenosis (7.4% primary CAS vs 0.9% restenosis; P= .03).14 However, Cuadra et al15 reported lower 30-day rates of death/stroke in ATH compared to RES (3.0% ATH vs 5.1% RES; P = .51).15 With continued enrollment and follow-up, analysis of VR will supplement randomized trials by providing CAS outcomes in current clinical practice with sufficient numbers to serve as an outcome assessment tool of important patient subsets.
The current report compares the short-term efficacy of carotid stents in the management of atherosclerotic, recurrent, and radiation-induced carotid lesions.
In a prior report, the VR demonstrated that CEA had better 30-day event rates than CAS, with the 30-day data being valuable in making this determination, as there was a demonstrated increase in event rates from the in-hospital to 30-day interval.8 A similar increase was noted in this analysis, with the increase in ATH being greater than in the patients with restenosis. An important conclusion of this analysis is the need for a 30-day reporting interval, as the in-hospital event rate in both groups did not reflect the 30-day results.
It is also noteworthy that given the current CMS reporting requirement, only 87 death/stroke/MI events in a little less than 4017 discharges or just over 2.2% events would be reported, compared to 30-day data. One could contend that 30-day event rates with less than 100% follow-up may underestimate, overestimate, or correctly estimate the true 30-day rates depending on whether the presence of follow-up is related to events or the lack thereof. Nevertheless, this discrepancy (2.2% vs 5.4% vs 7.1%) clearly supports a more thorough 30-day analysis.
Finally, it is important to discuss the limitations of VR and the analyses presented. The main weakness of these results is the VR reliance on self-reporting with its biases inherent to any registry-based study, as reported previously.8 Furthermore, some facilities participating in VR entered either CAS or CEA data only; some institutions do not perform CAS and elected to participate in the registry and enter only CEA data, and others entered only CAS data. In addition, although VR is designed for long-term entry of follow-up, current CMS requirements for CAS facility certification are limited to the initial hospitalization and do not include follow-up. Thus, some facilities are not motivated to enter follow-up information. However, the concurrent entry of all patients treated for CAD in independent and verifiable registries provides valuable information about current clinical practice patterns.
CONCLUSION
Three major conclusions can be drawn from the analyses of registry data. First, that event rates for carotid stent treatment of ATH vs restenosis and radiation therapy patients are similar. This finding differs from results of prior published reports. Next, symptomatic patients with ATH etiology had statistically significant higher rates of death/ stroke/MI both periprocedurally and at 30 days compared with the asymptomatic patients with atherosclerotic etiology. And finally, consistent entry of patient data beyond in-hospital (ie, intraprocedural and predischarge) event rates, as currently mandated by CMS, is required to determine the true risks. This finding supports the importance of continued data reporting in the postprocedure intervals to enable accurate assessment of procedural success.
Society for Vascular Surgery (SVS) Outcomes Committee
Gregorio A. Sicard (Chair), Ruth L. Bush, Patrick J. Geraghty, Thomas S. Huber, Gregg S. Landis, Louis L. Nguyen, Marc L. Schermerhorn, Rebecca J. Shackelton (Ad Hoc), Flora S. Siami (Ad Hoc), Anton N. Sidawy, Rodney A. White, and Robert M. Zwolak.
Acknowledgments
The study was exclusively funded by the Society for Vascular Surgery (SVS).
Footnotes
Competition of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a competition of interest.
AUTHOR CONTRIBUTIONS
Conception and design: RW, GS, RZ, AS, MS, RS, FS
Analysis and interpretation: RW, GS, RZ, AS, MS, RS, FS
Data collection: RS, FS
Writing the article: RW, GS, RZ, AS, MS, RS, FS
Critical revision of the article: RW, GS, RZ, AS, MS, RS, FS
Final approval of the article: RW, GS, RZ, AS, MS, FS
Statistical analysis: RS, FS
Obtained funding: GS
Overall responsibility: RW, FS
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics-2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Liapis CD, Paraskevas KI. Factors affecting recurrent carotid stenosis. Vasc Endovascular Surg. 2005;39:83–95. doi: 10.1177/153857440503900108. [DOI] [PubMed] [Google Scholar]
- 3.McCabe DJ, Pereira AC, Clifton A, Bland JM, Brown MM, CAVATAS Investigators. Restenosis after carotid angioplasty, stenting, or endar-terectomy in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS) Stroke. 2005;36:281–6. doi: 10.1161/01.STR.0000152333.75932.fe. [DOI] [PubMed] [Google Scholar]
- 4.Hobson RW, 2nd, Lal BK, Chakhtoura E, Goldstein J, Haser PB, Kabicka R, et al. Carotid artery stenting: Analysis of data for 105 patients at high risk. J Vasc Surg. 2003;37:1234–9. doi: 10.1016/s0741-5214(02)75448-7. [DOI] [PubMed] [Google Scholar]
- 5.Elerding SC, Fernandez RN, Grotta JC, Lindberg RD, Causay LC, McMurtrey MJ. Carotid artery disease following external cervical irradiation. Ann Surg. 1981;194:609–15. doi: 10.1097/00000658-198111000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Younis GA, Gupta K, Mortazavi A, Strickman NE, Krajcer Z, Perin E, Achari A. Predictors of carotid stent restenosis. Catheter Cardiovasc Interv. 2007;69:673–82. doi: 10.1002/ccd.20809. [DOI] [PubMed] [Google Scholar]
- 7.Hobson RW, 2nd, Mackey WC, Ascher E, Murad MH, Calligaro KD, Comerota AJ, et al. Management of atherosclerotic carotid artery disease: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2008;48:480–6. doi: 10.1016/j.jvs.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 8.Sidawy AN, Zwolak RM, White RA, Siami FS, Schermerhorn ML, Sicard GA Outcomes Committee for the Society for Vascular Surgery. Risk-adjusted 30-day outcomes of carotid stenting and endarterectomy: results from the SVS Vascular Registry. J Vasc Surg. 2009;49:71–9. doi: 10.1016/j.jvs.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 9.Zarins CK, White RA, Diethrich EB, Shackelton RJ, Siami FS CaRESS Steering Committee CaRESS Investigators. Carotid revascularization using endarterectomy or stenting systems (CaRESS): 4-year outcomes. J Endovasc Ther. 2009;16:397–409. doi: 10.1583/08-2685.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray WA, Hopkins LN, Yadav S, Davis T, Wholey M, Atkinson R, et al. Protected carotid stenting in high-surgical-risk patients: the ARCHeR results. J Vasc Surg. 2006;44:258–68. doi: 10.1016/j.jvs.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 11.Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493–501. doi: 10.1056/NEJMoa040127. [DOI] [PubMed] [Google Scholar]
- 12.SPACE Collaborative Group. Ringleb PA, Allenberg J, Brückmann H, Eckstein HH, Fraedrich G, et al. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239–47. doi: 10.1016/S0140-6736(06)69122-8. [DOI] [PubMed] [Google Scholar]
- 13.Mas JL, Chatellier G, Beyssen B, Brancherau A, Moulin T, Becquemin JP, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660–71. doi: 10.1056/NEJMoa061752. [DOI] [PubMed] [Google Scholar]
- 14.AbuRahma AF, AbuHalimah S, Besenhaver J, Nanjundappa A, Stone PA, Dean LS, et al. Primary carotid artery stenting versus carotid artery stenting for postcarotid endarterectomy stenosis. J Vasc Surg. 2009;50:1031–9. doi: 10.1016/j.jvs.2009.06.051. [DOI] [PubMed] [Google Scholar]
- 15.Cuadra S, Hobson RW, Lal BK, Goldstein J, Chakhtoura E, Jamil Z. Outcome of carotid artery stenting for primary versus restenotic lesions. Ann Vasc Surg. 2009;23:330–4. doi: 10.1016/j.avsg.2008.05.013. [DOI] [PubMed] [Google Scholar]