Abstract
Potential long term effects on brain development are a concern when drugs are used to treat depression and anxiety in childhood. In this study, male juvenile rhesus monkeys (three-four years of age) were dosed with fluoxetine or vehicle (N = 16/group) for two years. Histomorphometric examination of cortical dendritic spines conducted after euthanasia at one year postdosing (N = 8/group) suggested a trend toward greater dendritic spine synapse density in prefrontal cortex of the fluoxetine-treated monkeys. During dosing, subjects were trained for automated cognitive testing, and evaluated with a test of sustained attention. After dosing was discontinued, sustained attention, recognition memory and cognitive flexibility were evaluated. Sustained attention was affected by fluoxetine, both during and after dosing, as indexed by omission errors. Response accuracy was not affected by fluoxetine in post-dosing recognition memory and cognitive flexibility tests, but formerly fluoxetine-treated monkeys compared to vehicle controls had more missed trial initiations and choices during testing. Drug treatment also interacted with genetic and environmental variables: MAOA genotype (high- and low transcription rate polymorphisms) and testing location (upper or lower tier of cages). Altered development of top-down cortical regulation of effortful attention may be relevant to this pattern of cognitive test performance after juvenile fluoxetine treatment.
Keywords: Fluoxetine, Juvenile, Cognitive, Nonhuman primate, Serotonin, Dendritic spine synapses
1. Introduction
When psychoactive drugs are used during development, there are concerns for interference with brain function and development (Oberlander et al., 2009, Shrestha et al., 2014). The selective serotonin reuptake inhibitor (SSRI) fluoxetine was approved by FDA in 2003 for treating major depression (MDD) and obsessive-compulsive disorder (OCD) in children, and continues to maintain a favorable profile for efficacy and safety for these disorders (Hetrick et al., 2007, Henry et al., 2012, Cipriani et al., 2016). Additionally, use has extended to therapy for anxiety disorders, autism, attention deficit hyperactivity disorder (ADHD), and conduct disorders (Strawn et al., 2015, Riggs et al., 2007, Quintana et al., 2007, Williams et al., 2013, Hollander et al., 2005).
Due to ethical and practical considerations, there are no human studies of long term effects of childhood fluoxetine use on brain development. The rodent literature with fluoxetine administration at a corresponding lifestage is very limited (Olivier et al., 2011). Using a nonhuman primate model, the rhesus monkey, Shrestha et al. (2014) found that fluoxetine dosing during juvenile brain development, a lifestage parallel to childhood in humans, led to long-term effects on brain serotonin systems when assessed in adulthood. Specifically, serotonin transporter, the primary therapeutic target of SSRIs, was increased in hippocampus and neocortex. In addition, some long-term effects on social behavior were reported. These findings point to the value of a more extensive evaluation of long-term effects of juvenile fluoxetine on brain function in the nonhuman primate model.
Several characteristics of nonhuman primates contribute to study designs and outcome measures with translational potential. Nonhuman primates have prolonged cognitive development similar to humans (Goldman-Rakic, 1987, Bachevalier and Vargha-Khadem, 2005, Alvarado et al., 2016, Blue et al., 2013, Zeamer et al., 2010), and also have many genetic polymorphisms parallel to those seen in humans (Miller and Madras, 2005). In addition, the complex life histories of nonhuman primates are subject to gene*environment interactions relevant to brain function that are encountered in humans (Kinnally et al., 2009, Kinnally et al., 2010, Duncan et al., 2014).
The hypothesis for the present study was that fluoxetine could influence cognitive processes that mature during juvenile brain development. Specifically, sustained attention, recognition memory, and cognitive flexibility, cognitive abilities with a clear developmental trajectory in primates, were evaluated with automated touch screen testing like that used in children (Green et al., 2009, Luciana and Nelson, 2002, Syvaoja et al., 2015). Sustained attention was also assessed during dosing because this cognitive domain has been shown to be impaired during fluoxetine dosing in adult humans (Ramaekers et al., 1995, Riedel et al., 2005, Riedel et al., 1999, Schmitt et al., 2002, Wingen et al., 2008).
This report is from a larger, broadly targeted nonhuman primate study meant to help fill gaps in our knowledge of the developmental effects of fluoxetine in children. Genetic and environmental variables were also considered in the study. A pharmacokinetic study with multiple doses identified a dose in juvenile monkeys in the therapeutic range for children based on serum concentrations (Golub and Hogrefe, 2014a). We have previously reported that growth was not generally compromised by the treatment (Golub et al., 2015a). Fluoxetine effects on sleep (Golub and Hogrefe, 2016), social interaction (Golub et al., 2015b), delay impulsivity (He et al., 2014), and emotional response (Golub et al., 2016), have also been reported from this study along with metabolomic biomarkers of fluoxetine action (He et al., 2014, Su et al., 2016).
After completion of post-dosing cognitive testing, measurement of spine synapse density was conducted in brains of a subset of the subjects. Synaptic pruning is an important brain maturation process active in prefrontal cortex during the pre-pubertal stage of development in primates (Anderson et al., 1995, Bianchi et al., 2013, Bourgeois et al., 1994, Petanjek et al., 2011, Rakic et al., 1986). Dendritic synaptic remodeling is one of the aspects of neuronal plasticity under investigation as a mechanism of fluoxetine therapeutic action (Ampuero et al., 2013, Chen et al., 2016, Guidi et al., 2013, Hajszan et al., 2005, Kobayashi et al., 2010, Stagni et al., 2013). Hippocampus and prefrontal cortex were selected for study because they show histomorphometric changes in depressed patients (Licznerski and Duman, 2013) and because hippocampal spine density responds to fluoxetine in rodents (Hajszan et al., 2005). Thus, the impact of fluoxetine on spine synapses was an additional important goal of the study.
2. Methods and materials
2.1. Study design and animal selection
Thirty-two male rhesus macaques were selected for the study from a single birthing season in the outdoor colony at the California National Primate Research Center (CNPRC). They were divided into two treatment groups of 16 each (fluoxetine, vehicle). These groups were further subdivided (n = 8/subgroup) for low and high transcription rate polymorphisms of the serotonin metabolizing enzyme monoamine oxidase A (MAOA) gene, a second factor in the two-factor design of the study. The male-only cohort allowed inclusion of the two MAOA polymorphism subgroups for this X-linked gene.
Subjects entered the study at 12.23 ± 0.03 months of age (range 11.8–12.8 months). The large colony and electronic databases at CNPRC allowed use of detailed selection criteria for the study. Colony infants were excluded from consideration for low birth weight, poor health or inappropriate ages. All infants had been screened for temperament and response to stress with the BioBehavioral Assessment (BBA) (Capitanio et al., 2006), a protocol applied colony-wide to assist in individual care and selection of subjects for research studies. BBA emotionality scores greater or less than 2 standard deviations from the colony mean were also used to eliminate potential subjects for this study (Golub et al., 2016). Finally, the monkeys had been genotyped for the HTTLPR polymorphisms of the serotonin transporter (SERT) gene, as well as for monoamine oxidase A polymorphisms (Karere et al., 2012). The experimental groups were balanced for HTTLPR polymorphism genotypes (high or low transcription rates) as well as other background variables (Table S1). Of note, the representation of low transcription HTTLPR genotype polymorphism (SS) subjects was not adequate to assess HTTLPR effects on cognition. The SS polymorphism is most commonly associated with impaired cognition in human and nonhuman primates (Enge et al., 2011, Izquierdo et al., 2007, Jedema et al., 2010).
2.2. Animal care
The monkeys were transferred indoors and pair-housed in double-cages with a compatible cagemate, all in the same cageroom restricted to this study. Both members of each pair were assigned to the same treatment group. Cage location (left/right side of room, top/bottom tier, front/back of room) was balanced between groups (Table S1).
Housing and care followed standard CNPRC protocols (Golub et al., 2015a). Commercial monkey diet (Lab Diet #5047, St. Louis, MO) was provided ad libitum twice per day. Health and behavior of the monkeys were monitored daily by the husbandry staff, and animals of concern were reported to the veterinary staff. All reported concerns were relatively minor and all animals completed the study protocol. Weight gain during and after dosing was not significantly affected by fluoxetine (Golub et al., 2015a), or by cage tier or MAOA genotype, two covariates that influenced behavior (Table S2).
Experimental procedures followed the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011) and protocols were approved prior to implementation by the UC Davis Institutional Animal Care and Use Committee. UC Davis is accredited by AAALAC.
2.3. Fluoxetine dosing
Fluoxetine hydrochloride, obtained as a pediatric solution (20 mg/5 ml, Patterson Veterinary Supply, Inc., Devens, MA), was diluted 1:1 with commercial flavored syrup or baby food for administration via an oral dosing syringe. The dose was based on a preliminary pharmacokinetic study (Golub and Hogrefe, 2014a).
Monkeys were dosed for 24 months, from one to three years of age. Dosing was administered with blinding for treatment group. Dosing began at 1.6 mg/kg/day, and was adjusted to 2.4 mg/kg/day to be at the pediatric therapeutic mid-range (serum fluoxetine + norfluoxetine 237 ± 31 ng/mL) after 11 months when serum assay data became available. Dosing ended with a taper period, during which the dose was reduced by 25% per week, followed by a 2-week washout period before post-dosing testing commenced. The median weekly dose acceptance was 99.7% (range 85.3–99.9%).
2.4. Automated cognitive testing
2.4.1. Scheduling
Automated cognitive testing was part of an extensive evaluation program (Table S3). The cognitive test schedule was based on consideration of the maturity of the subjects and the time required to train and test within the developmental window. The commercial monkey CANTAB software contains the memory test (Delayed Nonmatch to Sample, DNMS) and the cognitive flexibility test (Intra Dimensional Extra Dimensional shift, IDED); the sustained attention test (Continuous Performance Test, CPT) was added specifically for this study because of its extensive use in children and known sensitivity to fluoxetine. The CPT attention task was conducted during dosing as well as after dosing because sustained attention is known to be affected by fluoxetine in adult humans. DNMS and IDED tasks were tested post-dosing to examine potential long-term effects on more mature cognitive abilities. We have been able to train and test automated CPT between one and two years of age (Golub et al., 2005). Delayed recognition memory (DNMS) emerges around 18 months of age (Zeamer et al., 2010), while attentional set shifting (IDED) develops later and is still immature at two years of age (Zeamer et al., 2010, Weed et al., 2008). Training to stable performance with the CANTAB operant equipment required 6–8 months. Thus, CANTAB training was conducted at 1–2 years of age, CPT testing at 2–3 years (dosing) and 4 years of age (post-dosing), and DNMS and IDED at 3–4 years of age (post-dosing).
2.4.2. Training and testing
Shortly after initiation of dosing, training began with the Monkey CANTAB apparatus (Lafayette Instruments, Lafayette, IN) and Whisker software (Cardinal and Aitken, 2010). Cagemates were separated and the equipment cart was placed in front of the home cage providing access to the touchscreen and blocking the view from the front of the cage. In the lower tier, the upper cages further isolated the occupant and limited lighting during testing, whereas upper tier cages were open at the top. Subjects were not identified by treatment during testing.
Shaping with successive approximations was used to establish screen touching, and the association between touch and reinforcement. The CANTAB software provided software to train the touching of small targets displayed for short durations in different location (see Table S4). After a training criterion was reached, maintenance sessions were given until the appropriate age to initiate specific tests.
Four subjects were tested simultaneously and all subjects completed daily testing between 0800 and 1200 h. The software administered stimuli, recorded responses and dispensed rewards (45 mg sugar pellets). Behavior was motivated by this food incentive. Monkeys were not food deprived; they were fed ad lib twice a day with the morning feeding and food-based enrichment delayed until after testing.
Fluoxetine did not influence the number of sessions required for touchscreen training. After training, CPT testing was initiated. Parameters and testing conditions were adjusted and a final 21-session CPT test was conducted at 32 months of age. After drug taper and wash out, the animals were evaluated sequentially over a 10-month period with DNMS and IDED and another CPT test.
2.4.3. Test details
The task parameters for the CANTAB programs are provided in Table S4. Briefly, the CPT attention test developed for monkeys (Golub and Hogrefe, 2014b, Golub et al., 1999) requires differentiation between three different colored squares (white, correct; red, green, incorrect) presented in random sequence. Sustained attention is reflected in consistent responding to the correct stimulus (which is rewarded) and lack of behavioral inhibition is reflected in responding to the incorrect stimuli (which results in a “time-out”). Some Individual CPT session data were omitted from statistical analyses due to technical malfunction or disruptive events in the test environment.
CANTAB DNMS and IDED testing protocols were modified from previous work in juvenile rhesus (Weed et al., 1999). Briefly, the DNMS task used trial-unique stimuli to assess recognition memory. A trial is initiated by touching a sample stimulus on the touchscreen. After a delay, the original sample stimulus is presented along with a new stimulus. A correct response is touching the new stimulus, or “non-match”, and is rewarded with a pellet. Failure to touch the sample and initiate the trial is recorded as a “sample miss” and the trial is terminated. Failure to touch one of the two choices after the delay is recorded as a “choice miss” and also terminates the trial. Initial training to a performance criteria was conducted at 0 and 1 s delays. Then five sessions at each test delay (2, 4, 8, 16, 32 and 64 s) were administered sequentially.
IDED is a test of cognitive flexibility including discrimination reversal and attentional set-shifting. It is comprised of four two-item visual discrimination problems increasing in complexity: a simple discrimination (one dimension), compound discrimination (two dimensions), intra-dimensional (ID) shift, and extra-dimensional (ED) shift (Weed et al., 2008). The problems were presented in order of complexity, and each task was followed by a reversal (previously correct choice now incorrect). Test sessions were 60 min long. When a criterion of 12 correct out of 15 successive trials was reached for given a problem or reversal, the next problem/reversal was immediately initiated.
2.5. Spine synapse methods
At four years of age, eight fluoxetine-treated and eight vehicle animals with complete behavioral datasets were selected for spine synapse evaluation after balance for potential covariates (Table S1). Sample sizes of three to eight have previously been found adequate to detect effects of doses of drugs and toxicants relevant to human exposure on spine density in nonhuman primates (Elsworth et al., 2015a, Elsworth et al., 2011, Elsworth et al., 2015b, Elsworth et al., 2013a, Elsworth et al., 2013b), and effects of fluoxetine on spine density in rodents have been detected with a sample size of 3 (Hajszan et al., 2005). After ketamine anesthesia (10 mg/kg i.m.), intravenous pentobarbital overdose (100 mg/kg i.v.), and perfusion with ice-cold saline, brains were quickly removed and chilled in ice-cold phosphate buffer. The right hemisphere was cut in 5 mm coronal slabs and fixed in increasing concentrations of paraformaldehyde and glutaraldehyde (Elsworth et al., 2013b). Two perfusion-fixed brains were examined to confirm the quality of the post-fixation method. No differences between perfusion and post-perfusion fixation were noted and data from one of the perfusion fixed brains (control) was added to the dataset.
Volumetric spine synapse calculations for CA1 stratum radiatum and Layers 2 and 3 of the dorsolateral prefrontal cortex (DLPFC) were performed as previously described (Leranth et al., 2008, MacLusky et al., 2006). Serial sections (200 μm) were cut throughout the entire hippocampal formation and prefrontal cortex, and sorted into 10 groups, one of which was randomly selected and post-fixed for ultrasectioning at 75-nm. Twenty sampling sites with a total volume of 118.8 μm2 were taken in each sampling area with a systematic, random approach. Digital electron micrographs were taken from identical location on two consecutive serial sections for a total of 40 images per brain used for spine synapse recognition and counting (Elsworth et al., 2013b). Synapses present in only one of the two sections were counted for the CA1 stratum radiatum and DLPFC Layers II and II. Axo-dendritic and axo-axonic synapses were not included. Image acquisition and analysis were conducted blind to treatment. The volume of the CA1 and DLPFC areas was calculated with Cavalieri’s method (Leranth et al., 2008) for estimation of total synapses/area.
2.6. Statistical analysis
Preliminary steps were taken in analysis of the behavioral data to reduce error variability, limit the number of animals needed for the study, and prevent potential confounding.
(1.) Genetic and environmental factors. As described above, treatment groups were balanced as much as possible prior to treatment initiation for categorical variables with potential to influence cognitive performance (Table S1). This approach prevented potential confounding and allowed inclusion of identified covariates in ANOVA analysis to evaluate treatment modification. In screening background, genetic and environmental factors, only cage Tier and MAOA genotype were found to contribute significant variability to behavioral performance. (2.) Group assignment bias. After random group assignment, treatment groups were screened for possible assignment bias using data from a Biobehavioral Assessment (BBA) conducted at CNPRC when infants were 3–4 months of age (Capitanio et al., 2005, Golub et al., 2009, Capitanio et al., 2006). Treatment groups did not differ on these variables prior to initiation of treatment (Table S1). Infants could not be given the cognitive tests prior to dosing to establish “baselines” because of their immaturity.
ANOVA, RMANOVA and ANCOVA (JMP, SAS Institute, Carey, NC) with limited post-hoc planned comparisons were used to analyze preselected apical endpoints from each test including response accuracy (e.g. percent correct, error rate), as well as test participation and performance (trial initiation, trial choice misses, latency, rewards obtained). Endpoints were screened for outliers and normality prior to analysis. Because cage tier was identified early in testing as a significant covariate, planned comparisons between tiers were conducted in the fluoxetine treated group upon identification of Treatment*Tier interactions. At the conclusion of the study, principal components analysis was used to identify associations between fluoxetine-affected endpoints across tests.
3. Results
3.1. Overview
In tests of sustained attention, recognition memory, and cognitive flexibility, only sustained attention demonstrated fluoxetine effects on the targeted domain. For recognition memory and cognitive flexibility tests, response accuracy was not influenced by fluoxetine. However, a pattern was seen in the fluoxetine group of increased missed responses to stimuli initiating trials and requiring choices. Many fluoxetine effects emerged in interactions with cage location (Tier). The statistical analysis is detailed below.
3.2. CPT: dosing and post-dosing testing
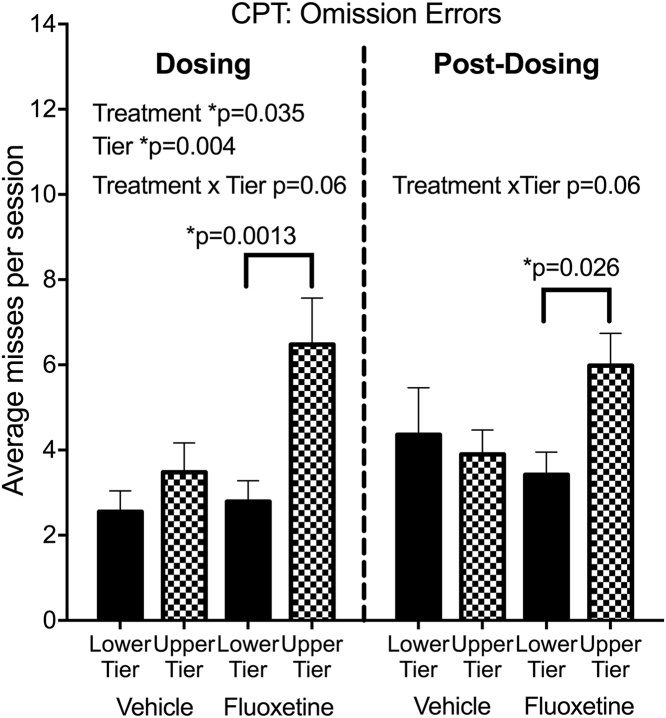
For testing during dosing, fluoxetine Treatment led to increased omission errors (signal misses) (F(1,28) = 4.88, p = 0.035, Cohen’s d = 0.92) (Fig. 1, see also individual data in Fig. S1) indicating an effect on sustained attention. There was no effect on commission errors (false alarms). A strong main effect of cage Tier on omission errors during dosing (F(1,28) = 9.95, p = 0.004) was also seen, with more omission errors in the upper tier, as well as a trend for the Treatment*Tier interaction (F(1,28) = 3.57, p = 0.069). Planned comparisons showed that the Tier effect was significant in the fluoxetine-treated group (p = 0.001) but not the vehicle controls.
Fig. 1.
Continuous Performance Test (CPT). Omission errors. N = 8 subjects in each of the 4 subgroups. The dosing test was conducted at 32 months of age, 20 months after initiation of dosing. The post dosing test was conducted 6–10 months after discontinuation of dosing at 42–46 months of age. Mean of 14–16 CPT sessions (dosing) or 16–20 sessions (post-dosing). Group mean ± s.e.m. are shown. See text for details of statistical analysis.
Response accuracy for CPT was computed by the software as the signal detection parameter d’. Signal detection theory is often used with sustained attention tests to calculate parameters that reflect whether performance is affected by ability to discriminate the stimuli (d’) or by a general non-response bias (c) (Macmillan and Creelman, 2005). Response accuracy was not influenced by Treatment, Tier or their interaction. Rewards obtained (correct responses) were inversely related to omission errors and showed the same statistical effects. Fluoxetine subjects in the upper tier received 2 fewer sugar pellets per session (out of 28 possible) compared to those in the lower tier.
To better understand the Tier effect on omission errors, we investigated other performance parameters. Animals on the upper tier had a bias toward not responding (signal detection parameter c) (Macmillan and Creelman, 2005) (F1,28) = 12.82, p = 0.001), longer response latency averages (F(1,28) = 4.90, p = 0.035) and fewer touches of the screen when no stimuli were being presented (blank screen touches) (F(1,28) = 7.2, p = 0.013). Notably, no Treatment effects or interactions were seen for response bias, latency or blank screen touches, suggesting that fluoxetine and upper tier increases in omission errors had different behavioral origins.
In post-dosing testing (Fig. 1), analysis did not demonstrate the main effects of Treatment or Tier on omission errors that were seen during dosing. However, interaction analyses demonstrated that fluoxetine-treated subjects in the upper tier continued to have higher omission errors than those in the lower tier Treatment*Tier (F(1,27) = 3.83, p = 0.06, Cohen’s d = 0.74, planned comparison p = 0.026). Additionally a main effect of Tier continued to be shown for non-response bias (c”) (Tier, F(1,27) = 4.79, p = 0.037) and longer latencies (Tier, F(1,27) = 6.49, p = 0.017) than those in the lower tier (data not shown). As in CPT during dosing, non-response bias and latencies were not influenced by fluoxetine.
In summary, during dosing fluoxetine increased CPT omission errors and subjects in the upper cage tier made more omission errors than those in the lower tier, with fluoxetine-treated subjects in the upper tier showing the most omission errors (Treatment*Tier interaction). Auxiliary measures (non-response bias index, blank screen touches, response latencies) suggested that reduced tendency to respond was involved in the omission errors of subjects in the upper tier, but not the fluoxetine-treated subjects. After dosing was discontinued, there were no main effects of Treatment or Tier on omission errors, but the greater omission errors in upper tier fluoxetine subjects persisted.
3.3. DNMS: post-dosing testing
Five subjects, three fluoxetine-treated monkeys and two vehicle-treated monkeys were not included in the data set for repeated measures analysis across delays. One subject failed to meet the training criteria and four failed to reach a participation criterion of 25% trial initiation (<75% sample misses) at a delay of 16 s and were not tested at the longer intervals (32 and 64 s). Endpoints analyzed were sample misses, choice misses, response accuracy and rewards obtained. Analyses were conducted for all intervals (N = 27) and for intervals ≤16 s (N = 31).
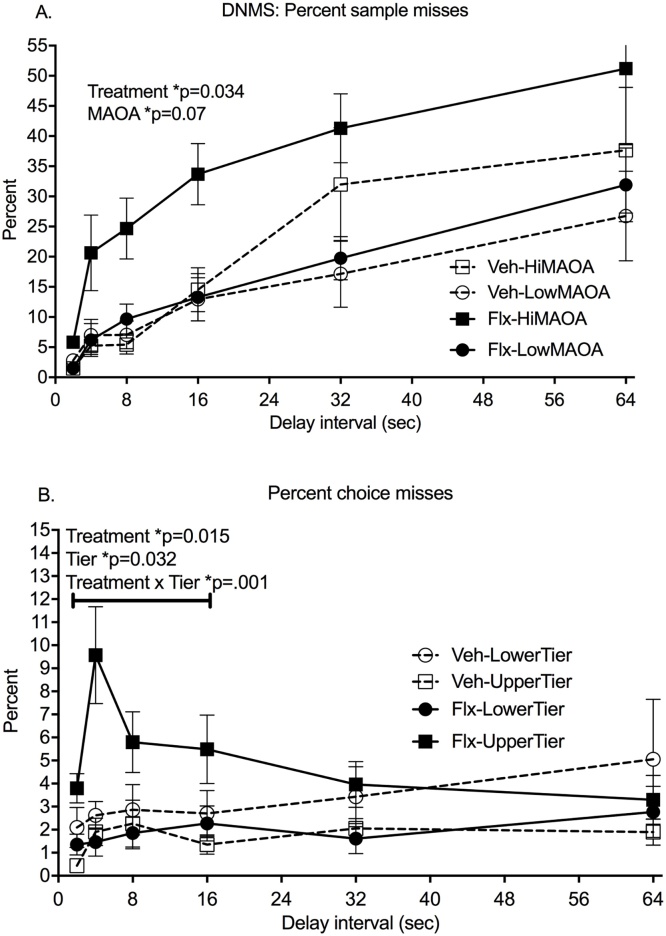
Sample misses (failure to initiate a trial by touching the sample) increased from 3% to 37% across delays in the 27 subjects that completed all testing (RMANOVA, delay: F(5,19) = 15.56, p < 0.0001) (Fig. 2A). Main effects of both Treatment (F(1,23) = 5.05, p = 0.034, Cohen’s d = 1.05) and MAOA genotype (F(1,23) = 8.70, p = 0.007) were seen in the RMANOVA for this measure. Fluoxetine-treated monkeys and monkeys with the hi-MAOA polymorphism genotype had more sample misses. Tier did not influence this measure. The Treatment*MAOA interaction was not significant across all delays, but RMANOVA across the shorter delays (2,4,8,16 s), completed by all but one subject, showed a highly significant interaction (F(1,23) = 9.27, p = 0.0006) and a significant fluoxetine effect in the hi-MAOA subgroup (post hoc comparison, p = 0.0003).
Fig. 2.
Delayed Non Match to Sample test (DNMS). All data are from the post-dosing phase. A. Percent sample misses, failure to start the trial by touching the sample. B. Percent choice misses, failure to make a choice after the delay. Mean ± s.e.m. are shown. N = 13 fluoxetine subjects; N = 14 vehicle controls. See text for details of statistical analysis.
Once trials were initiated, choice misses (failure to choose between the match and nonmatch stimuli after the delay) were low (averaging 3% in all subjects) and did not increase across delays (Fig. 2B). A marginal effect of delay in the RMANOVA (F(5.19) = 2.82, p = 0.046) was seen. There were no main effects of fluoxetine treatment on this measure, although RMANOVA across the shorter delays (2,4,8,16 s) showed a significant Treatment main effect (F(1,22) = 7.30, p = 0.034, Cohen’s d = 1.56). The Treatment*Tier interaction was significant across all delays (F(1,23) = 10.12, p = 0.004) and also for the shorter delays (F(1,22) = 15.23, p = 0.0008). As was the case for omission errors in the CPT, more choice misses were seen in the upper tier than the lower tier in the fluoxetine-treated group (p = 0.005) but not in the vehicle group.
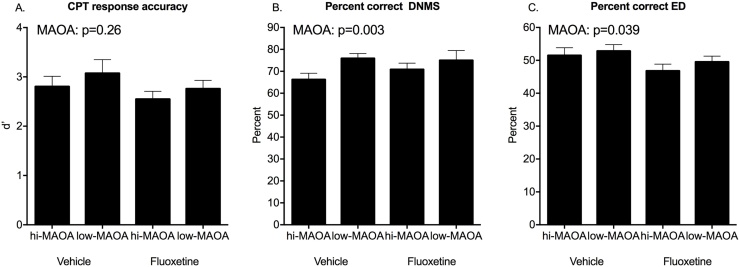
Response accuracy, reflected in percent correct choices, did not decrease significantly across these delay intervals in agreement with other studies in rhesus (Weed et al., 1999, Taffe et al., 2001). No Treatment main effects or interactions were seen for accuracy. Percent correct choice, the direct measure of recognition memory, showed a strong MAOA genotype effect (F(1,23) = 11.29, p = 0.003) with poorer performance in the hi-MAOA subjects (Fig. 3B).
Fig. 3.
Response accuracy in post-dosing testing. A. CPT discrimination accuracy (d'). B. DNMS percent correct. C. ED percent correct. Mean ± s.e.m. are shown. See text for details of statistical analysis.
There were fewer rewards obtained as the delays lengthened (RMANOVA, F(5,23) = 158, p < 0.0001). Fluoxetine-treated monkeys did not differ significantly from the vehicle group on this measure. The low-MAOA subjects, with fewer sample misses and greater accuracy, obtained significantly more rewards than the high-MAOA subjects (F(1,23) = 10.88, p = 0.003).
In summary, prior exposure to fluoxetine led to reduced initiation of DNMS trials (sample misses) and reduced the number of choices made once the trial was initiated (choice misses). If a choice was made, the accuracy was not influenced by fluoxetine. Interestingly, Treatment*Tier interactions seen in CPT testing emerged for DNMS choice misses, while Treatment*Genotype interactions were seen for DNMS sample misses. Additionally, an unanticipated main effect of MAOA genotype on response accuracy was seen.
3.4. IDED: post-dosing testing
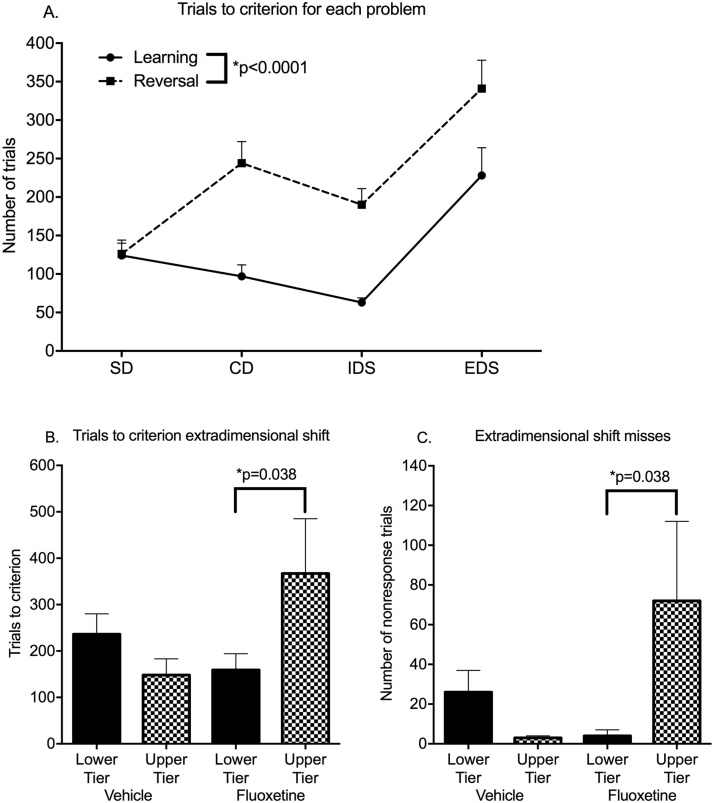
All subjects reached criterion on training and all problems of the IDED series with the exception of two subjects who failed to complete the last problem, ED reversal. The endpoints for each stage of the IDED series were trials to criterion, number of choice misses to criterion, and percent correct of choices.
Trials to criterion for learning and reversal of the first three problems were not influenced by fluoxetine. Trials to criterion declined across the first three stages (simple discrimination, complex discrimination, intra-dimensional shift) but increased for extra-dimensional shift (ED) (Fig. 4A). ED learning was the only problem showing fluoxetine influences.
Fig. 4.
Intradimensional Extradimensional Shift (IDED). All data are from the post-dosing phase of the study. A. Trials to criterion for learning and reversal of each of the problems in the series. B. Trials to criterion for ED learning. C. Choice misses for ED learning. Mean ± s.e.m. are shown. N = 16 fluoxetine-treated and 16 vehicle-treated controls. See text for details of statistical analysis.
For ED learning, there was a Treatment*Tier interaction for trials to criterion (F(1,28) = 4.77, p = 0.037, Cohen’s d=0.90). A Tier effect (poorer performance in the upper tier) occurred only in the fluoxetine-treated group (planned comparison, p = 0.038) (Fig. 4B). The Treatment*Tier interaction was also significant for choice misses (F(1,28) = 4.891, p = 0.035, Cohen’s d=0.92, planned comparison p = 0.027)(Fig. 4C). Further analysis suggested that the delay in reaching the criterion of 12/15 correct trials was due to failure to complete trials (choice misses) rather than incorrect choices. In multivariate analysis, choice misses were strongly associated with trials to criterion (r = 0.97), while incorrect choices were not (r = 0.18).
Response accuracy (percent correct of choices) for ED learning was not influenced by fluoxetine treatment. There was a significant effect of MAOA genotype (F(1, 28) = 4.77, p = 0.039) with poorer accuracy in the hi-MAOA group (Fig. 3C). Notably, the same MAOA effect on response accuracy was also seen for DNMS sample misses, (Fig. 3B), but not for CPT omission errors (Fig. 3A). There were no effects of Treatment or Tier on response accuracy.
In terms of rewards obtained during ED learning, the fluoxetine-treated subjects in the upper tier obtained more rewards than their lower tier counterparts, but the difference was not significant (p = 0.08).
In summary, subjects previously treated with fluoxetine showed normal learning and reversal on the progressively more difficult problems in the IDED series, but had slower learning and less responding on ED learning in interaction with different testing conditions in the upper and lower cage tier. In the upper tier, the vehicle-treated group failed to respond on 2% of the choice trials, while the fluoxetine-treated subjects failed to respond on 24%. Genotype influenced response accuracy in the ED task. The Treatment*Tier effects on choice misses and the MAOA Genotype effects on accuracy of responding in learning the ED problem paralleled those seen in the DNMS test.
3.5. Associations between fluoxetine sensitive endpoints across tests
Principle components analyses were conducted to determine whether sustained attention deficits in the CPT were associated with sample and choice misses in the DNMS and ED tests across subjects. The analysis examined the association between endpoints sensitive to fluoxetine in the DNMS test (sample misses, choice misses, all intervals and first four intervals) and the ED test (trials to criterion, choice misses) with CPT endpoints (omission errors, commission errors, “d”', “c”, latency). For DNMS (N = 27), the first principle component, accounting for 47% of variance, had a loading matrix with a correlation of 0.89 for CPT omission errors, 0.77 for DNMS choice misses (all delays) and 0.77 for DNMS choice misses (4 delays). For ED (N = 32), the first principle component accounting for 45% of variance demonstrated a correlation of 0.79 for CPT omission errors, 0.55 for ED trials to criterion, and 0.63 for ED choice misses. This analysis suggests that CPT sustained attention endpoint sensitive to fluoxetine (omission errors) was associated with the endpoints sensitive to fluoxetine in the DNMS and ED tests.
3.6. Spine synapse density
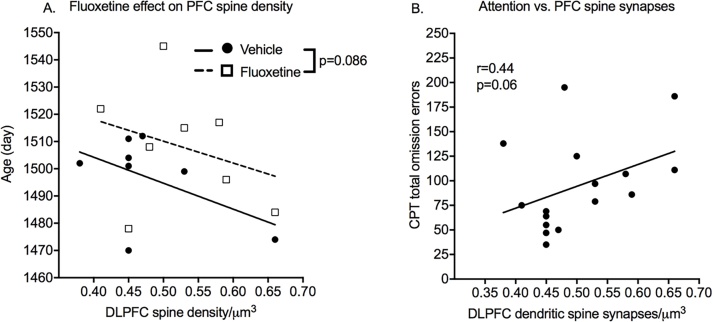
Cage Tier and MAOA genotype did not affect these measures. Age at necropsy correlated with DLPFC spine synapse density and was used as a covariate in the analysis. Mean DLPFC spine density was greater in the fluoxetine group, but the effect of prior exposure to fluoxetine on DLPFC was not significant with a two-tailed test (p = 0.086, Cohen’s d = 0.77) (Fig. 5). There was no indication of an effect of previous treatment with fluoxetine on CA1 hippocampal spine density. DLPFC spine synapse density was then examined as a predictor of cognitive performance using endpoints sensitive to fluoxetine (CPT omission errors, DNMS sample and choice misses, ED choice misses). There was a trend (r = 0.44, p = 0.06) for prediction of post-dosing attention performance (omission errors) by DLPFC spine density.
Fig. 5.
Spine synapses. Dendritic spine synapses were quantified from brain tissue obtained at necropsy at 4 years of age after completion of testing. A. Scatter plot of dendritic spine synapses in dorsolateral prefrontal cortex (DLPFC) as a function of age and treatment group. B. Correlation between sustained attention measure and DLPFC spine synapses. N = 9 vehicle-treated and 8 fluoxetine-treated brains.
4. Discussion
4.1. Fluoxetine and sustained attention
Juvenile fluoxetine led to a greater incidence of omission errors in the CPT sustained attention task both during and after dosing. Juvenile fluoxetine treatment was not found to have post-dosing effects on recognition memory (DNMS), or cognitive flexibility (IDED) as reflected in response accuracy in the tests administered here. However, performance of the DNMS and ED cognitive tasks was impaired in fluoxetine-treated monkeys by failure to respond to task stimuli as reflected in DNMS trial misses and choice misses, and ED choice misses. Principle components analysis showed associations between the CPT omission errors and the DNMS and IDED endpoints sensitive to fluoxetine. This suggests that impaired attention could underlie the missed responding seen in DNMS and ED performance. Both tasks require attention to the computer screen in order to detect the brief stimulus presentations and respond within designated time limits.
In people, fluoxetine and other SSRIs have been shown to affect sustained attention by increasing omission errors in healthy adults (Ramaekers et al., 1995, Riedel et al., 2005, Riedel et al., 1999, Schmitt et al., 2002, Wingen et al., 2008). This effect was the basis of our focus on attention testing during dosing. The findings from the young monkeys performing CPT during dosing agrees with these human studies, and the post-dosing performance suggests persistence of the effect on sustained attention. Notably response accuracy in the IDED task, sometimes referred to as “attentional set shifting”, was not affected by fluoxetine, although missed choice responses were more frequent.
The present study suggests that sustained attention may be a domain of interest in connection with fluoxetine cognitive effects in children. Regulation of visual attention emerges early in infancy (Sheese et al., 2008), but development of sustained attention is a distinctive and important characteristic of childhood. Sustained attention can be measured by CPT in children beginning at about 4.5 years of age (Levy, 1980) and performance in terms of omission and commission errors improves through early puberty (Lin et al., 1999), with commission errors rising again in adolescence. Omission errors in the CPT are characteristic of children diagnosed with ADHD and respond to ADHD therapies (Peskin et al., 2016, Kempton et al., 1999). Some of these children have comorbidities that result in concurrent fluoxetine therapy (Gunther et al., 2011).
Several considerations suggest that the concept of effortful attention may be particularly relevant to translation of these fluoxetine findings to children. Effortful attention, attention that must be maintained in the presence of distractors, is recognized as a cognitive skill that develops during childhood (Rothbart and Rueda, 2005). This high-level, late maturing, executive function emerges in situations where additional cortical networks need to be accessed to guide attention (Rothbart and Rueda, 2005, Rueda et al., 2005). These top-down cortical networks have been characterized in monkeys (Buschman and Miller, 2007), as well as humans (Sarter et al., 2001). Failure to respond to task stimuli under conditions of increased cognitive load was reported in a study of development of sustained attention in children (Betts et al., 2006). In the DNMS and IDED testing, choice misses appeared at particular stages of the task; longer delays for DNMS, and ED learning for IDED suggesting the emergence of attention deficits under more challenging conditions.
Fluoxetine could also have impaired cognitive performance through pathways that mediate reward processing or incentive motivation. Reward processing directs performance of cognitive tasks (Arnsten and Rubia, 2012, Helie et al., 2017) and can contribute to mood-related psychopathology. Response to reward is well known to influence sustained attention performance in adults (Nusslock and Alloy, 2017) and children (Chantiluke et al., 2012) with depression, and children with ADHD (Bubnik et al., 2015). SSRIs influence reward processing in normal adults (McCabe et al., 2010, Scholl et al., 2017). In adolescent ADHD patients, fluoxetine effects on reward processing have been demonstrated using a temporal discounting task (Carlisi et al., 2016). Experiments that specifically assess aspects of reward processing like reward salience and reward valence would be needed to pursue this interpretation. Incentive motivation could also be involved. However, fluoxetine did not interfere with incentive-based learning of the cognitive tasks and did not decrease the number of reinforcements obtained during testing. This indicates that the fluoxetine treated monkeys were sufficiently motivated to obtain the reward incentives.
4.2. Fluoxetine interactions
An interesting and unique aspect of the study was the demonstration of fluoxetine interactions with genetic and environmental conditions. Specifically, the location during testing (upper or lower tier in the cage room) interacted with fluoxetine in determining CPT omission errors, DNMS choice misses and ED choice misses. Also, MAOA genotype interacted with fluoxetine in determining DNMS trial misses. While these interactions seem complex, they may be an appropriate representation of the genetic and environmental heterogeneity of children requiring psychoactive drug treatment.
Although cage tier has long been recognized as a significant variable in NHP research (Reinhardt and Reinhardt, 2000, Schapiro and Bloomsmith, 2001) the basis for the Tier effect in our study is difficult to define without further research. When the test equipment was in use, lighting was greater in the upper tier cages due to the open tops, reducing the salience of the computer monitor. Also some visual access to the room was available to the top tier animals, although it was limited to the 30 cm space between the cage top and ceiling. The pretesting experience of living and being dosed in the upper tier is, however, an alternate explanation to differences in the test environment. An interesting study in mice suggested that fluoxetine treatment made behavior more open to influences of the “living environment” (Branchi et al., 2013, Alboni et al., 2015). However, the behavioral tests not conducted with CANTAB in the homecage did not show Tier effects. Whatever the underlying basis of the Tier effects, their appearance in the fluoxetine group, but not the vehicle group, post-dosing suggests a greater sensitivity to performance disruption after fluoxetine treatment. This idea could be tested by adding distractors to the CPT.
This report reflects the value of considering effect modification in studies of fluoxetine. While main effects of fluoxetine were seen on task responsiveness, the interactions illustrate how genetic and environmental factors add another layer of value in formulating and interpreting research in this area: (1) False negative and false positive findings can emerge if relevant genetic and environmental influences are not controlled in design and analysis; (2) Genetic and environmental modifiers of fluoxetine effects may be relevant to variability in efficacy and safety of therapeutic use in children.
4.3. MAOA effects on response accuracy
Poorer response accuracy of subjects with high-transcription MAOA VNTR polymorphisms in DNMS and ED testing was a clear and striking finding. The subjects with hi-MAOA genotypes were also more prone to miss trial-initiating stimuli in the DNMS test. Research on MAOA polymorphisms in humans has a social-emotional focus with little study of cognition. One study suggested an interaction between MAOA and COMT genotypes in influencing recognition memory in boys (Barnett et al., 2011), and another found that the high-MAOA transcription polymorphism positively influenced alerting but negatively influenced executive control of attention in adults (Fossella et al., 2002). The current findings indicate that MAOA polymorphism genotype deserves further study for its influence on cognition during childhood. It is important to note that the MAOA and fluoxetine effects observed here could be specific to the juvenile ages and may not be seen later in maturation.
4.4. Dendritic spine synapse density
We examined spine synapse density as a maturational process that is active at the ages studied and that is known to be influenced by fluoxetine and other antidepressants. We found a nonsignificant trend toward greater spine density in the fluoxetine group in the prefrontal cortex (DLPFC), but not in hippocampus (CA1), and also a nonsignificant trend for the association of greater DLPFC spine density with CPT omission errors. These results are suggestive but clearly requires confirmation. A possible mechanism mediating fluoxetine effects on dendritic spine plasticity is upregulation of BDNF, discovered shortly after the introduction of SSRI therapy and currently being actively explored (Huang et al., 2012, Rubio et al., 2013, Orefice et al., 2016, Jia et al., 2013). While increased spine density is associated with fluoxetine in adult rodents, studies specific to the period of late cortical synaptic pruning have not been undertaken. A few rodent studies of early postnatal fluoxetine exposure have reported lower spine density in adulthood (Ko et al., 2014, Norrholm and Ouimet, 2000, Zheng et al., 2011).
4.5. Limitations
The population of monkeys used in this study was not selected to represent a model of human psychopathology. This limits translation to pharmacotherapy but allows a straightforward interpretation that is difficult to achieve when drugs are given to patients with psychopathology. Research in adults, adolescents and children with MDD shows a suite of cognitive impairments in domains including sustained attention, cognitive flexibility and recognition memory (Shehab et al., 2016, Rock et al., 2014, Vilgis et al., 2015, Wagner et al., 2015), making it difficult to study drug-induced cognitive effects in patient populations.
A second, very serious, limitation requiring redress in future research is lack of female subjects. Obstacles to a complete design with males and females include the multiple female genotypes for the X-linked gene MAOA, the lower availability of young females in colonies with breeding programs, and cost. A third limitation was the use of just one test for each domain. Other tests of recognition memory and attentional set shift accuracy may have proven sensitive to fluoxetine.
Financial interests and conflicts of interest
None of the authors report biomedical financial interests or professional conflicts of interest.
Conflict of Interest:
None.
Acknowledgements
The authors thank Alicia Bulleri and Ashley Schnider for technical support, Michael Weed for CANTAB advice, CNPRC animal care staff and veterinarians. Supported by NIH grants R01 HD065862 (Mari Golub, PI), OD010962 (John Capitanio, PI) and OD011107 (Harris Lewin, PI). NIH had no role in conduct, analysis or interpretation of the study. Portions of the cognitive assessment data were presented at the 2014 Annual Meeting of the Society for Toxicology and the 2016 Gatlinburg Annual Meeting.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2017.04.008.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Alboni S., van Dijk R.M., Poggini S., Milior G., Perrotta M., Drenth T. Fluoxetine effects on molecular, cecellular and behavioral endophenotypes of depression are driven by the living environment. Mol. Psychiatry. 2015;22:552–561. doi: 10.1038/mp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado M.C., Malkova L., Bachevalier J. Development of relational memory processes in monkeys. Dev. Cogn. Neurosci. 2016;22:27–35. doi: 10.1016/j.dcn.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ampuero E., Stehberg J., Gonzalez D., Besser N., Ferrero M., Diaz-Veliz G. Repetitive fluoxetine treatment affects long-term memories but not learning. Behav. Brain Res. 2013;247:92–100. doi: 10.1016/j.bbr.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Anderson S.A., Classey J.D., Conde F., Lund J.S., Lewis D.A. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience. 1995;67(1):7–22. doi: 10.1016/0306-4522(95)00051-j. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F., Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51(4):356–367. doi: 10.1016/j.jaac.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Bachevalier J., Vargha-Khadem F. The primate hippocampus: ontogeny, early insult and memory. Curr. Opin. Neurobiol. 2005;15(2):168–174. doi: 10.1016/j.conb.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Barnett J.H., Xu K., Heron J., Goldman D., Jones P.B. Cognitive effects of genetic variation in monoamine neurotransmitter systems: a population-based study of COMT, MAOA, and 5HTTLPR. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011;156(2):158–167. doi: 10.1002/ajmg.b.31150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts J., McKay J., Maruff P., Anderson V. The development of sustained attention in children: the effect of age and task load. Child Neuropsychol. 2006;12(3):205–221. doi: 10.1080/09297040500488522. [DOI] [PubMed] [Google Scholar]
- Bianchi S., Stimpson C.D., Bauernfeind A.L., Schapiro S.J., Baze W.B., McArthur M.J. Dendritic morphology of pyramidal neurons in the chimpanzee neocortex: regional specializations and comparison to humans. Cereb. Cortex. 2013;23(10):2429–2436. doi: 10.1093/cercor/bhs239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blue S.N., Kazama A.M., Bachevalier J. Development of memory for spatial locations and object/place associations in infant rhesus macaques with and without neonatal hippocampal lesions. J. Int. Neuropsychol. Soc. 2013;19(10):1053–1064. doi: 10.1017/S1355617713000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois J.P., Goldman-Rakic P.S., Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb. Cortex. 1994;4(1):78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Branchi I., Santarelli S., Capoccia S., Poggini S., D'Andrea I., Cirulli F. Antidepressant treatment outcome depends on the quality of the living environment: a pre-clinical investigation in mice. PLoS One. 2013;8(4):e62226. doi: 10.1371/journal.pone.0062226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubnik M.G., Hawk L.W., Jr., Pelham W.E., Jr., Waxmonsky J.G., Rosch K.S. Reinforcement enhances vigilance among children with ADHD: comparisons to typically developing children and to the effects of methylphenidate. J. Abnorm. Child Psychol. 2015;43(1):149–161. doi: 10.1007/s10802-014-9891-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman T.J., Miller E.K. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315(5820):1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Capitanio J.P., Mendoza S.P., Mason W.A., Maninger N. Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys (Macaca mulatta) Dev. Psychobiol. 2005;46(4):318–330. doi: 10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- Capitanio J., Mason W.A., Mendoza S.P., DelRosso L., Roberts J. Nursery rearing and biobehavioral organization. In: Sackett G., Ruppenthal G., Elias K., editors. Nursery Rearing of Nonhuman Primates in the 21st Century. Springer; US: 2006. pp. 191–214. [Google Scholar]
- Cardinal R.N., Aitken M.R.F. Whisker: a client—server high-performance multimedia research control system. Behav. Res. Methods. 2010;42(4):1059–1071. doi: 10.3758/BRM.42.4.1059. [DOI] [PubMed] [Google Scholar]
- Carlisi C.O., Chantiluke K., Norman L., Christakou A., Barrett N., Giampietro V. The effects of acute fluoxetine administration on temporal discounting in youth with ADHD. Psychol. Med. 2016;46(6):1197–1209. doi: 10.1017/S0033291715002731. [DOI] [PubMed] [Google Scholar]
- Chantiluke K., Halari R., Simic M., Pariante C.M., Papadopoulos A., Giampietro C. Fronto-striato-cerebellar dysregulation in adolescents with depression during motivated attention. Biol. Psychiatry. 2012;71(1):59–67. doi: 10.1016/j.biopsych.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Chen F., du Jardin K.G., Waller J.A., Sanchez C., Nyengaard J.R., Wegener G. Vortioxetine promotes early changes in dendritic morphology compared to fluoxetine in rat hippocampus. Eur. Neuropsychopharmacol. 2016;26(2):234–245. doi: 10.1016/j.euroneuro.2015.12.018. [DOI] [PubMed] [Google Scholar]
- Cipriani A., Zhou X., Del Giovane C., Hetrick S.E., Qin B., Whittington C. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016;388(10047):881–890. doi: 10.1016/S0140-6736(16)30385-3. [DOI] [PubMed] [Google Scholar]
- Duncan L.E., Pollastri A.R., Smoller J.W. Mind the gap: why many geneticists and psychological scientists have discrepant views about gene-environment interaction (GxE) research. Am. Psychol. 2014;69(3):249–268. doi: 10.1037/a0036320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsworth J.D., Hajszan T., Leranth C., Roth R.H. Loss of asymmetric spine synapses in dorsolateral prefrontal cortex of cognitively impaired phencyclidine-treated monkeys. Int. J. Neuropsychopharmacol. 2011;14(10):1411–1415. doi: 10.1017/S1461145711000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsworth J.D., Jentsch J.D., Vandevoort C.A., Roth R.H., Redmond D.E., Jr., Leranth C. Prenatal exposure to bisphenol A impacts midbrain dopamine neurons and hippocampal spine synapses in non-human primates. Neurotoxicology. 2013;35:113–120. doi: 10.1016/j.neuro.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsworth J.D., Leranth C., Redmond D.E., Jr., Roth R.H. Loss of asymmetric spine synapses in prefrontal cortex of motor-asymptomatic: dopamine-depleted, cognitively impaired MPTP-treated monkeys. Int. J. Neuropsychopharmacol. 2013;16(4):905–912. doi: 10.1017/S1461145712000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsworth J.D., Groman S.M., Jentsch J.D., Leranth C., Redmond D.E., Jr., Kim J.D. Primate phencyclidine model of schizophrenia: sex-specific effects on cognition, brain derived neurotrophic factor, spine synapses, and dopamine turnover in prefrontal cortex. Int. J. Neuropsychopharmacol. 2015;18(6):1–10. doi: 10.1093/ijnp/pyu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsworth J.D., Jentsch J.D., Groman S.M., Roth R.H., Redmond E.D., Jr., Leranth C. Low circulating levels of bisphenol-A induce cognitive deficits and loss of asymmetric spine synapses in dorsolateral prefrontal cortex and hippocampus of adult male monkeys. J. Comp. Neurol. 2015;523(8):1248–1257. doi: 10.1002/cne.23735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enge S., Fleischhauer M., Lesch K.P., Strobel A. On the role of serotonin and effort in voluntary attention: evidence of genetic variation in N1 modulation. Behav. Brain Res. 2011;216(1):122–128. doi: 10.1016/j.bbr.2010.07.021. [DOI] [PubMed] [Google Scholar]
- Fossella J., Sommer T., Fan J., Wu Y., Swanson J.M., Pfaff D.W. Assessing the molecular genetics of attention networks. BMC Neurosci. 2002;3:14. doi: 10.1186/1471-2202-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic P.S. Development of cortical circuitry and cognitive function. Child Dev. 1987;58(3):601–622. [PubMed] [Google Scholar]
- Golub M.S., Hogrefe C.E. Fluoxetine: juvenile pharmacokinetics in a nonhuman primate model. Psychopharmacology (Berl.) 2014;231(20):4041–4047. doi: 10.1007/s00213-014-3537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub M., Hogrefe C. Prenatal iron deficiency and monoamine oxidase A (MAOA) polymorphisms: combined risk for later cognitive performance in rhesus monkeys. Genes Nutr. 2014;9(2):381. doi: 10.1007/s12263-013-0381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub M.S., Hogrefe C.E. Sleep disturbance as detected by actigraphy in juvenile monkeys receiving therapeutic doses of fluoxetine. Neurotoxicol. Teratol. 2016;5(55):1–7. doi: 10.1016/j.ntt.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub M.S., Keen C.L., Gershwin M.E. Behavioral and hematologic consequences of marginal iron-zinc nutrition in adolescent monkeys and the effect of a powdered beef supplement. Am. J. Clin. Nutr. 1999;70(6):1059–1068. doi: 10.1093/ajcn/70.6.1059. [DOI] [PubMed] [Google Scholar]
- Golub M.S., Hogrefe C.E., Germann S.L., Tran T.T., Beard J.L., Crinella F.M. Neurobehavioral evaluation of rhesus monkey infants fed cow's milk formula: soy formula, or soy formula with added manganese. Neurotoxicol. Teratol. 2005;27(4):615–627. doi: 10.1016/j.ntt.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Golub M.S., Hogrefe C.E., Widaman K.F., Capitanio J.P. Iron deficiency anemia and affective response in rhesus monkey infants. Dev. Psychobiol. 2009;51(1):47–59. doi: 10.1002/dev.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub M.S., Bulleri A.M., Hogrefe C.E., Sherwood R.J. Bone growth in juvenile rhesus monkeys is influenced by 5HTTLPR polymorphisms and interactions between 5HTTLPR polymorphisms and fluoxetine. Bone. 2015;79:162–169. doi: 10.1016/j.bone.2015.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub M.S., Hogrefe C.E., Bulleri A.M. Peer social interaction in rhesus monkeys treated with fluoxetine during juvenile development. Neuropharmacology. 2015;105:553–560. doi: 10.1016/j.neuropharm.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub M.S., Hogrefe-Phi C.E., Bulleri A.M. Regulation of emotional response in juvenile monkeys treated with fluoxetine: MAOA interactions. Eur. Neuropsychopharm. 2016;26(12):1920–1929. doi: 10.1016/j.euroneuro.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C.R., Mihic A.M., Nikkel S.M., Stade B.C., Rasmussen C., Munoz D.P. Executive function deficits in children with fetal alcohol spectrum disorders (FASD) measured using the Cambridge Neuropsychological Tests Automated Battery (CANTAB) J. Child Psychol. Psychiatry. 2009;50(6):688–697. doi: 10.1111/j.1469-7610.2008.01990.x. [DOI] [PubMed] [Google Scholar]
- Guidi S., Stagni F., Bianchi P., Ciani E., Ragazzi E., Trazzi S. Early pharmacotherapy with fluoxetine rescues dendritic pathology in the Ts65Dn mouse model of down syndrome. Brain Pathol. 2013;23(2):129–143. doi: 10.1111/j.1750-3639.2012.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther T., Konrad K., De Brito S.A., Herpertz-Dahlmann B., Vloet T.D. Attentional functions in children and adolescents with ADHD: depressive disorders, and the comorbid condition. J. Child Psychol. Psychiatry. 2011;52(3):324–331. doi: 10.1111/j.1469-7610.2010.02320.x. [DOI] [PubMed] [Google Scholar]
- Hajszan T., MacLusky N.J., Leranth C. Short-term treatment with the antidepressant fluoxetine triggers pyramidal dendritic spine synapse formation in rat hippocampus. Eur. J. Neurosci. 2005;21(5):1299–1303. doi: 10.1111/j.1460-9568.2005.03968.x. [DOI] [PubMed] [Google Scholar]
- He Y., Hogrefe C.E., Grapov D., Palazoglu M., Fiehn O., Turck C.W. Identifying individual differences of fluoxetine response in juvenile rhesus monkeys by metabolite profiling. Transl. Psychiatry. 2014;4:e478. doi: 10.1038/tp.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helie S., Shamloo F., Novak K., Foti D. The roles of valuation and reward processing in cognitive function and psychiatric disorders. Ann. N. Y. Acad. Sci. 2017 doi: 10.1111/nyas.13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry A., Kisicki M.D., Varley C. Efficacy and safety of antidepressant drug treatment in children and adolescents. Mol. Psychiatry. 2012;17(12):1186–1193. doi: 10.1038/mp.2011.150. [DOI] [PubMed] [Google Scholar]
- Hetrick S., Merry S., McKenzie J., Sindahl P., Proctor M. Selective serotonin reuptake inhibitors (SSRIs) for depressive disorders in children and adolescents. Cochrane Database Syst. Rev. 2007 doi: 10.1002/14651858.CD004851.pub2. CD004851. pub2(3): CD004851. [DOI] [PubMed] [Google Scholar]
- Hollander E., Phillips A., Chaplin W., Zagursky K., Novotny S., Wasserman S. A placebo controlled crossover trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology. 2005;30(3):582–589. doi: 10.1038/sj.npp.1300627. [DOI] [PubMed] [Google Scholar]
- Huang G.J., Ben-David E., Tort Piella A., Edwards A., Flint J., Shifman S. Neurogenomic evidence for a shared mechanism of the antidepressant effects of exercise and chronic fluoxetine in mice. PLoS One. 2012;7(4):e35901. doi: 10.1371/journal.pone.0035901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A., Newman T.K., Higley J.D., Murray E.A. Genetic modulation of cognitive flexibility and socioemotional behavior in rhesus monkeys. Proc. Natl. Acad. Sci. U. S. A. 2007;104(35):14128–14133. doi: 10.1073/pnas.0706583104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedema H.P., Gianaros P.J., Greer P.J., Kerr D.D., Liu S., Higley J.D. Cognitive impact of genetic variation of the serotonin transporter in primates is associated with differences in brain morphology rather than serotonin neurotransmission. Mol. Psychiatry. 2010;15(5):512–522. doi: 10.1038/mp.2009.90. 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J.M., Zhao J., Hu Z., Lindberg D., Li Z. Age-dependent regulation of synaptic connections by dopamine D2 receptors. Nat. Neurosci. 2013;16(11):1627–1636. doi: 10.1038/nn.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karere G.M., Sullivan E., Kinnally E.L., Capitanio J.P., Lyons L.A. Enhancing genotyping of MAOA-LPR and 5-HTT-LPR in rhesus macaques (Macaca mulatta) J. Med. Primatol. 2012;41(6):407–411. doi: 10.1111/jmp.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempton S., Vance A., Maruff P., Luk E., Costin J., Pantelis C. Executive function and attention deficit hyperactivity disorder: stimulant medication and better executive function performance in children. Psychol. Med. 1999;29(3):527–538. doi: 10.1017/s0033291799008338. [DOI] [PubMed] [Google Scholar]
- Kinnally E.L., Huang Y.Y., Haverly R., Burke A.K., Galfalvy H., Brent D.P. Parental care moderates the influence of MAOA-uVNTR genotype and childhood stressors on trait impulsivity and aggression in adult women. Psychiatr. Genet. 2009;19(3):126–133. doi: 10.1097/YPG.0b013e32832a50a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnally E.L., Karere G.M., Lyons L.A., Mendoza S.P., Mason W.A., Capitanio J.P. Serotonin pathway gene–gene and gene-environment interactions influence behavioral stress response in infant rhesus macaques. Dev. Psychopathol. 2010;22(1):35–44. doi: 10.1017/S0954579409990241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko M.C., Lee L.J., Li Y., Lee L.J. Long-term consequences of neonatal fluoxetine exposure in adult rats. Dev. Neurobiol. 2014;74(10):1038–1051. doi: 10.1002/dneu.22185. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Ikeda Y., Sakai A., Yamasaki N., Haneda E., Miyakawa T. Reversal of hippocampal neuronal maturation by serotonergic antidepressants. Proc. Natl. Acad. Sci. U. S. A. 2010;107(18):8434–8439. doi: 10.1073/pnas.0912690107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C., Hajszan T., Szigeti-Buck K., Bober J., MacLusky N.J., Bisphenol A. prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. Proc. Natl. Acad. Sci. U. S. A. 2008;105(37):14187–14191. doi: 10.1073/pnas.0806139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy F. The development of sustained attention (vigilance) and inhibition in children: some normative data. J. Child Psychol. Psychiatry. 1980;21(1):77–84. doi: 10.1111/j.1469-7610.1980.tb00018.x. [DOI] [PubMed] [Google Scholar]
- Licznerski P., Duman R.S. Remodeling of axo-spinous synapses in the pathophysiology and treatment of depression. Neuroscience. 2013;251:33–50. doi: 10.1016/j.neuroscience.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.C., Hsiao C.K., Chen W.J. Development of sustained attention assessed using the continuous performance test among children 6–15 years of age. J. Abnorm. Child Psychol. 1999;27(5):403–412. doi: 10.1023/a:1021932119311. [DOI] [PubMed] [Google Scholar]
- Luciana M., Nelson C.A. Assessment of neuropsychological function through use of the Cambridge Neuropsychological Testing Automated Battery: performance in 4- to 12-year-old children. Dev. Neuropsychol. 2002;22(3):595–624. doi: 10.1207/S15326942DN2203_3. [DOI] [PubMed] [Google Scholar]
- MacLusky N.J., Hajszan T., Johansen J.A., Jordan C.L., Androgen Leranth C. effects on hippocampal CA1 spine synapse numbers are retained in Tfm male rats with defective androgen receptors. Endocrinology. 2006;147(5):2392–2398. doi: 10.1210/en.2005-0673. [DOI] [PubMed] [Google Scholar]
- Macmillan N., Creelman C. 2nd edn. Lawrence Erlbaum Associates, Inc.; Mahwah, New Jersey: 2005. Signal Detection: A User's Guide. [Google Scholar]
- McCabe C., Mishor Z., Cowen P.J., Harmer C.J. Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol. Psychiatry. 2010;67(5):439–445. doi: 10.1016/j.biopsych.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Madras B. Similarities of non-human primates to humans: genetic variations and phenotypic associations common to rhesus monkeys and humans. In: Wolfe-Coote S., editor. The Laboratory Primate. Academic Press; San Diego, CA: 2005. pp. 17–26. [Google Scholar]
- National Research Council . eighth edn. 2011. Guide for the Care and Use of Laboratory Animals. 248 pp. [Google Scholar]
- Norrholm S.D., Ouimet C.C. Chronic fluoxetine administration to juvenile rats prevents age-associated dendritic spine proliferation in hippocampus. Brain Res. 2000;883(2):205–215. doi: 10.1016/s0006-8993(00)02909-7. [DOI] [PubMed] [Google Scholar]
- Nusslock R., Alloy L.B. Reward processing and mood-related symptoms: an RDoC and translational neuroscience perspective. J. Affect. Disord. 2017 doi: 10.1016/j.jad.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander T.F., Gingrich J.A., Ansorge M.S. Sustained neurobehavioral effects of exposure to SSRI antidepressants during development: molecular to clinical evidence. Clin. Pharmacol. Ther. 2009;86(6):672–677. doi: 10.1038/clpt.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier J.D., Blom T., Arentsen T., Homberg J.R. The age-dependent effects of selective serotonin reuptake inhibitors in humans and rodents: a review. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35(6):1400–1408. doi: 10.1016/j.pnpbp.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Orefice L.L., Shih C.C., Xu H., Waterhouse E.G., Xu B. Control of spine maturation and pruning through proBDNF synthesized and released in dendrites. Mol. Cell. Neurosci. 2016;71:66–79. doi: 10.1016/j.mcn.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskin M., Sommerfeld E., Basford Y., Rozen S., Zalsman G., Weizman A. Continuous performance test is sensitive to a single methylphenidate challenge in preschool children with ADHD. J. Atten. Disord. 2016 doi: 10.1177/1087054716680075. [DOI] [PubMed] [Google Scholar]
- Petanjek Z., Judas M., Simic G., Rasin M.R., Uylings H.B., Rakic P. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2011;108(32):13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana H., Butterbaugh G.J., Purnell W., Layman A.K. Fluoxetine monotherapy in attention-deficit/hyperactivity disorder and comorbid non-bipolar mood disorders in children and adolescents. Child Psychiatry Hum. Dev. 2007;37(3):241–253. doi: 10.1007/s10578-006-0032-7. [DOI] [PubMed] [Google Scholar]
- Rakic P., Bourgeois J.P., Eckenhoff M.F., Zecevic N., Goldman-Rakic P.S. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232(4747):232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- Ramaekers J.G., Muntjewerff N.D., O'Hanlon J.F. A comparative study of acute and subchronic effects of dothiepin, fluoxetine and placebo on psychomotor and actual driving performance. Br. J. Clin. Pharmacol. 1995;39(4):397–404. doi: 10.1111/j.1365-2125.1995.tb04468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt V., Reinhardt A. The lower row monkey cage: an overlooked variable in biomedical research. J. Appl. Anim. Welf. Sci. 2000;3(2):141–149. [Google Scholar]
- Riedel W.J., Klaassen T., Deutz N.E., van Someren A., van Praag H.M. Tryptophan depletion in normal volunteers produces selective impairment in memory consolidation. Psychopharmacology (Berl.) 1999;141(4):362–369. doi: 10.1007/s002130050845. [DOI] [PubMed] [Google Scholar]
- Riedel W.J., Eikmans K., Heldens A., Schmitt J.A. Specific serotonergic reuptake inhibition impairs vigilance performance acutely and after subchronic treatment. J. Psychopharmacol. 2005;19(1):12–20. doi: 10.1177/0269881105048887. [DOI] [PubMed] [Google Scholar]
- Riggs P.D., Mikulich-Gilbertson S.K., Davies R.D., Lohman M., Klein C., Stover S.K. A randomized controlled trial of fluoxetine and cognitive behavioral therapy in adolescents with major depression: behavior problems, and substance use disorders. Arch. Pediatr. Adolesc. Med. 2007;161(11):1026–1034. doi: 10.1001/archpedi.161.11.1026. [DOI] [PubMed] [Google Scholar]
- Rock P.L., Roiser J.P., Riedel W.J., Blackwell A.D. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol. Med. 2014;44(10):2029–2040. doi: 10.1017/S0033291713002535. [DOI] [PubMed] [Google Scholar]
- Rothbart M., Rueda M. The development of effortful control. In: Mayr U., Keele S., editors. Developing Individuality in the Human Brain: A Tribute to Michael I. Posner. American Psychological Association; Washington, D.C: 2005. pp. 167–188. [Google Scholar]
- Rubio F.J., Ampuero E., Sandoval R., Toledo J., Pancetti F., Wyneken U. Long-term fluoxetine treatment induces input-specific LTP and LTD impairment and structural plasticity in the CA1 hippocampal subfield. Front. Cell. Neurosci. 2013;7:66. doi: 10.3389/fncel.2013.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda M.R., Rothbart M.K., McCandliss B.D., Saccomanno L., Posner M.I. Training: maturation, and genetic influences on the development of executive attention. Proc. Natl. Acad. Sci. U. S. A. 2005;102(41):14931–14936. doi: 10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M., Givens B., Bruno J.P. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res. Brain Res. Rev. 2001;35(2):146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Schapiro S., Bloomsmith M. Lower-row caging in a two-tiered housing system does not affect the behaviour of young, singly housed rhesus macaques. Anim. Welf. 2001;10(4):387–394. [Google Scholar]
- Schmitt J.A., Ramaekers J.G., Kruizinga M.J., van Boxtel M.P., Vuurman E.F., Riedel W.J. Additional dopamine reuptake inhibition attenuates vigilance impairment induced by serotonin reuptake inhibition in man. J. Psychopharmacol. 2002;16(3):207–214. doi: 10.1177/026988110201600303. [DOI] [PubMed] [Google Scholar]
- Scholl J., Kolling N., Nelissen N., Browning M., Rushworth M.F., Harmer C.J. Beyond negative valence: 2-week administration of a serotonergic antidepressant enhances both reward and effort learning signals. PLoS Biol. 2017;15(2):e2000756. doi: 10.1371/journal.pbio.2000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheese B.E., Rothbart M.K., Posner M.I., White L.K., Fraundorf S.H. Executive attention and self-regulation in infancy. Infant Behav. Dev. 2008;31(3):501–510. doi: 10.1016/j.infbeh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Shehab A.A., Brent D., Maalouf F.T. Neurocognitive changes in selective serotonin reuptake inhibitors-treated adolescents with depression. J. Child Adolesc. Psychopharmacol. 2016;26(8):713–720. doi: 10.1089/cap.2015.0190. [DOI] [PubMed] [Google Scholar]
- Shrestha S.S., Nelson E.E., Liow J.S., Gladding R., Lyoo C.H., Noble P.L. Fluoxetine administered to juvenile monkeys: effects on the serotonin transporter and behavior. Am. J. Psychiatry. 2014;171(3):323–331. doi: 10.1176/appi.ajp.2013.13020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagni F., Magistretti J., Guidi S., Ciani E., Mangano C., Calza L. Pharmacotherapy with fluoxetine restores functional connectivity from the dentate gyrus to field CA3 in the Ts65Dn mouse model of down syndrome. PLoS One. 2013;8(4):e61689. doi: 10.1371/journal.pone.0061689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn J.R., Welge J.A., Wehry A.M., Keeshin B., Rynn M.A. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis. Depress. Anxiety. 2015;32(3):149–157. doi: 10.1002/da.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S.Y., Hogrefe-Phi C.E., Asara J.M., Turck C.W., Golub M.S. Peripheral fibroblast metabolic pathway alterations in juvenile rhesus monkeys undergoing long-term fluoxetine administration. Eur. Neuropsychopharmacol. 2016;26(7):1110–1118. doi: 10.1016/j.euroneuro.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syvaoja H.J., Tammelin T.H., Ahonen T., Rasanen P., Tolvanen A., Kankaanpaa A. Internal consistency and stability of the CANTAB neuropsychological test battery in children. Psychol. Assess. 2015;27(2):698–709. doi: 10.1037/a0038485. [DOI] [PubMed] [Google Scholar]
- Taffe M.A., Weed M.R., Davis S., Huitron-Resendiz S., Schroeder R., Parsons L.H. Functional consequences of repeated (+/−)3,4-methylenedioxymethamphetamine (MDMA) treatment in rhesus monkeys. Neuropsychopharmacology. 2001;24(3):230–239. doi: 10.1016/S0893-133X(00)00185-8. [DOI] [PubMed] [Google Scholar]
- Vilgis V., Silk T.J., Vance A. Executive function and attention in children and adolescents with depressive disorders: a systematic review. Eur. Child Adolesc. Psychiatry. 2015;24(4):365–384. doi: 10.1007/s00787-015-0675-7. [DOI] [PubMed] [Google Scholar]
- Wagner S., Muller C., Helmreich I., Huss M., Tadic A. A meta-analysis of cognitive functions in children and adolescents with major depressive disorder. Eur. Child Adolesc. Psychiatry. 2015;24(1):5–19. doi: 10.1007/s00787-014-0559-2. [DOI] [PubMed] [Google Scholar]
- Weed M.R., Taffe M.A., Polis I., Roberts A.C., Robbins T.W., Koob G.F. Performance norms for a rhesus monkey neuropsychological testing battery: acquisition and long-term performance. Brain Res. Cogn. Brain Res. 1999;8(3):185–201. doi: 10.1016/s0926-6410(99)00020-8. [DOI] [PubMed] [Google Scholar]
- Weed M.R., Bryant R., Perry S. Cognitive development in macaques: attentional set-shifting in juvenile and adult rhesus monkeys. Neuroscience. 2008;157(1):22–28. doi: 10.1016/j.neuroscience.2008.08.047. [DOI] [PubMed] [Google Scholar]
- Williams K., Brignell A., Randall M., Silove N., Hazell P. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD) Cochrane Database Syst. Rev. 2013 doi: 10.1002/14651858.CD004677.pub3. CD004677. pub3(8): Cd004677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingen M., Kuypers K.P., van de Ven V., Formisano E., Ramaekers J.G. Sustained attention and serotonin: a pharmaco-fMRI study. Hum. Psychopharmacol. 2008;23(3):221–230. doi: 10.1002/hup.923. [DOI] [PubMed] [Google Scholar]
- Zeamer A., Heuer E., Bachevalier J. Developmental trajectory of object recognition memory in infant rhesus macaques with and without neonatal hippocampal lesions. J. Neurosci. 2010;30(27):9157–9165. doi: 10.1523/JNEUROSCI.0022-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Xu D.F., Li K., Wang H.T., Shen P.C., Lin M. Neonatal exposure to fluoxetine and fluvoxamine alters spine density in mouse hippocampal CA1 pyramidal neurons. Int. J. Clin. Exp. Pathol. 2011;4(2):162–168. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.