NEUROMYELITIS OPTICA: A NOVEL AUTOIMMUNE CENTRAL NERVOUS SYSTEM DISORDER
In 1894, Eugene Devic (1) and Fernand Gault summarized multiple cases of acute, concurrent optic neuritis (ON) and transverse myelitis (TM), which they termed “neuromyélite optique aiguë.” Over the ensuing decades, the publication of multiple additional cases of neuromyelitis optica (NMO) documented that this relatively rare condition had both relapsing and monophasic presentations. Central nervous system (CNS) injury was usually dramatic: severe myelitis that was accompanied, preceded, or followed by severe vision loss progressing to complete blindness (2). Some early clinicopathologic studies argued for a distinct nosology, whereas others suggested classification with disseminated sclerosis (multiple sclerosis [MS]) or diffuse sclerosis (Schilder disease). During this time, it is likely that definitive categorization of NMO was hampered by both the overlapping clinical presentations and histopathology of demyelinating disorders.
Although evidence for humoral-mediated disease pathology was observed in active NMO lesions (3), it was the identification of an autoantibody against the aquaporin-4 (AQP4) water channel in NMO patients that dramatically changed perspectives on the pathophysiology of disease and the relationship between NMO and other inflammatory CNS disorders (4,5). However, several fundamental questions needed to be addressed. First, are AQP4 autoantibodies (AQP4-IgG) causing pathology or are there additional NMO-specific immune targets? Second, how does an immune response against AQP4, the major astrocytic CNS water channel, result in myelin destruction? Third, why are the optic nerves and spinal cord predisposed to injury while AQP4-expressing tissues such as lung, stomach, kidney, and skeletal muscle spared? And finally, how can we use our understanding of disease mechanisms in NMO to preserve vision and neurologic function? In this review, we will assess progress in the field toward answering these important questions and examine how information from the bench is leading to the generation of novel therapeutic strategies at the bedside.
AQP4-IgG: A SPECIFIC AND PATHOGENIC HUMORAL AUTOANTIBODY
In 2004, Lennon et al (5) identified an autoantibody in NMO patient sera, termed NMO-IgG, that stained CNS microvessels, pia, subpia, and Virchow–Robin spaces in mouse midbrain and spinal cord by indirect immunofluorescence (IIF). Serum samples from NMO (5), MS, Japanese optic–spinal multiple sclerosis (O-S-MS), recurrent ON or TM, and various immune, vascular, nutritional, neoplastic, paraneoplastic, and idiopathic conditions were subsequently assayed for this immunoreactivity. NMO-IgG showed high sensitivity (73%; confidence interval [CI]: 60%–86%) and specificity (91%; CI: 79%–100%) for NMO patients. Interestingly, O-S-MS demonstrated similar sensitivity (58%; CI: 30%–86%) and specificity (100%; CI: 66%–100%), and 46% of cases of recurrent ON and TM cases were also positive for NMO-IgG. None of the classical MS or miscellaneous control samples were positive. The similar frequency of positive samples among NMO and Japanese O-S-MS patients also suggested that these 2 disorders were identical and that a significant fraction of individuals with recurrent ON and TM at high risk for NMO could be identified by IIF testing. The target of the serum autoantibody was rapidly identified as the AQP4 water channel (4), the unique CNS staining pattern on IIF resulting from localized expression of AQP4 on astrocyte foot processes on the abluminal face of CNS microvessels and pia mater.
The identification of AQP4 as a specific immune target in NMO allowed the further development of specific quantitative and semiquantitative immunoassays for detection of AQP4-IgG in body fluids. Enzyme-linked immunosorbent assay (ELISA), fluorescence immunoprecipitation assay (FI-PA) and radioimmunoprecipitation assay, and cell binding assays (CBA) with fluorescence microscopy or fluorescence-activated cell sorting (FACS) detection were subsequently developed and used to independently verify the sensitivity and specificity of AQP4-IgG for NMO (6,7). Although the number of studies and subjects varied considerably, they consistently demonstrated that AQP4-IgG was sensitive (range: 49%–77%) and specific (range: 96%–99%) for NMO (7). A multicenter comparison of IIF, ELISA, FIPA, CBA, and FACS assays demonstrated that CBA and FACS assays were most sensitive; IIF and FIPA assays lacked sensitivity (8). Therefore, multiple independent studies confirmed AQP4-IgG as a disease-specific biomarker of NMO and indicated that cell binding assays offer optimal sensitivity and specificity.
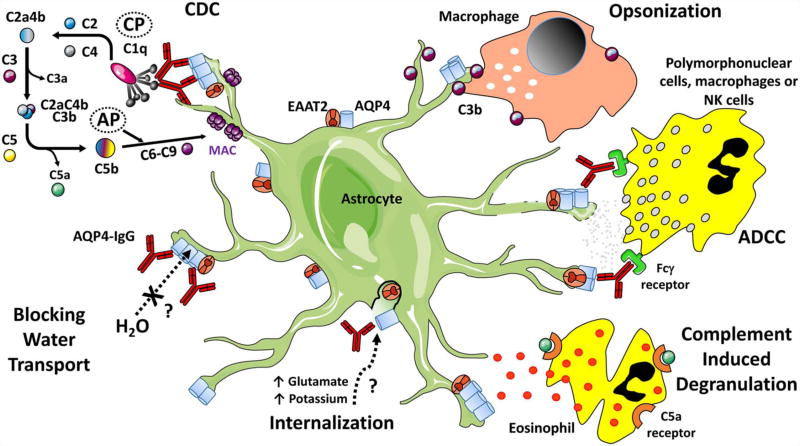
Disease-specific autoantibodies, however, are not necessarily pathogenic. In NMO, the pathogenecity of AQP4-IgG was examined using multiple experimental strategies. Serum NMO-IgG, patient-derived AQP4-specific monoclonal recombinant antibodies (rAbs), or control human IgG were administered to rats with experimental autoimmune encephalomyelitis (EAE), and the CNS was examined for NMO-specific histopathology (9–11). NMO-IgG or patient-derived AQP4-specific rAb, but not control serum or control rAb, caused NMO-like pathology in the background of myelin-targeted EAE. CNS lesions demonstrated perivascular AQP4 and astrocyte loss, IgG and complement deposition, granulocytic and lymphocytic infiltrates, macrophage influx, and myelinolysis. NMO pathology was specific to NMO-IgG or AQP4-specific rAb. Using a distinct model of direct NMO-IgG and human complement (HC) intracerebral injection (ICI), Saadoun et al (12) were able to recapitulate the seminal histopathologic features of NMO lesions independent of a systemic immune response. Identical histopathology could be produced in the ICI model using AQP4-specific rAb and HC (13), indicating that intracerebral complement activation by AQP4-specific IgG is sufficient to induce the seminal features of NMO histopathology (Fig. 1).
FIG. 1.
Inflammatory and noninflammatory mechanisms contributing to astrocyte injury in neuromyelitis optica. Inflammatory mechanisms include complement-dependent cytotoxicity (CDC), antibody-dependent cell-mediated cytotoxicity (ADCC), opsonization, and complement-induced degranulation. Potential noninflammatory mechanisms contributing to injury include aquaporin-4 (AQP4) and glutamate transporter (EAAT2) internalization and direct inhibition of AQP4-mediated water transport. AP, alternative pathway; C2aC4bC3b, C5 convertase; C4bC2a, C3 convertase; CP, classical pathway; MAC, membrane attack complex; NK, natural killer.
Are There Other Target Antigens in Neuromyelitis Optica?
Approximately 25% of patients meeting the 2006 Wingerchuk diagnostic criteria (14) for NMO are seronegative for AQP4-IgG (8). A small fraction of these seronegative patients have serum myelin oligodendrocyte glycoprotein autoantibodies (MOG-IgG) (15). AQP4-IgG and MOG-IgG are rarely observed in the same patient (16,17). Despite overlapping clinical presentations, multiple lines of data indicate that AQP4-IgG and MOG-IgG seropositive patients are distinct demyelinating conditions (18,19). Although both MOG-IgG and AQP4-IgG seropositive patients have relapsing clinical courses with significant disability (20), a lower female bias and higher predominance of monophasic disease are reported in multiple MOG-IgG–positive cohorts (18,19,21). In addition, radiographic features often distinguish MOG-IgG seropositive TM and ON from their AQP4-IgG counterparts: inflammation of the conus and cauda equina, perineuritic enhancement of the optic nerve, and shorter optic nerve lesion length (20,22). Moreover, functional recovery and steroid responsivity are more frequently reported for MOG-IgG seropositive ON (18,19,21,23,24). Importantly, the histopathology of brain lesions from MOG-IgG seropositive patients reveal Type 2 MS pathology (25), and MOG-IgG does not produce NMO lesion pathology in the ICI animal model (26,27).
In recognition of the broadening clinical presentation of NMO, the International Panel for NMO Diagnosis recently established new criteria for the diagnosis of neuromyelitis optica spectrum disorder (NMOSD) (28). Novel clinical and radiologic criteria for seronegative NMOSD were formulated, and in a recent analysis of a large cohort of NMOSD patients, the fraction of AQP4-IgG seronegative patients fell from 15% to 10% (29). The remaining small fraction of AQP4-IgG seronegative NMOSD patients may be due to limited assay sensitivity (6–8), AQP4-IgG seroconversion (29), and clinically overlapping demyelinating conditions (15,19,30). The possibility of a pathogenic T-cell–restricted adaptive immune response against AQP4 is unlikely given the inability to reproduce complete AQP4-targeted pathology following the adoptive transfer of AQP4-specific T cells in experimental systems (31,32). Although additional autoantibodies are frequently observed in AQP4-IgG seropositive and seronegative NMOSD patients (33,34), candidate autoantigen targets, such as aquaporin-1, have failed to show reproducible disease association (35,36).
NMO LESION PATHOLOGY: LINKING ASTROCYTE DESTRUCTION TO DEMYELINATION
Myelinolysis Precedes Demyelination in NMO Lesions
Understanding the link between AQP4-IgG–mediated astrocyte injury and downstream oligodendrocyte loss, myelin destruction, and neuronal injury may reveal critical stages for interventions to prevent or ameliorate CNS injury (Fig. 2). Early NMO lesions exhibit regions of intact myelin associated with either complete astrocyte loss or significantly reduced AQP4 and glial fibrillary acidic protein (GFAP) staining (37,38). The remaining astrocytes appear fragmented with surrounding GFAP-positive debris and perivascular GFAP-laden macrophages (38). Perivascular immunoglobulin and terminal complement complex (C5b-9; C9neo) deposition are evident, consistent with the primary humoral immune-mediated destruction of perivascular astrocytes (38,39). Myelin in these regions show numerous intracytoplasmic vacuoles or widening of the extracellular space between myelinated fibers, and immunohistochemical stains reveal early loss of myelin-associated glycoprotein (38,39). Oligodendrocytes are either reduced in number or absent, and many remaining oligodendrocytes demonstrate seminal features of apoptotic cell death including nuclear chromatin condensation (38), immunoreactivity for activated caspase-3 (38), and DNA fragmentation (39). Regions of demyelination in active NMO lesions are marked by the presence of lipid- and complement-laden macrophages (38,39). In contrast to still-myelinated areas, active demyelinating regions displayed more abundant infiltrating immune subsets, such as granulocytes, macrophages, eosinophils, lymphocytes, and plasma cells (38,40–42).
FIG. 2.
Myelinolysis and progressive axonal swelling following astrocyte injury in neuromyelitis optica lesions. Astrocyte destruction mediated by AQP4-IgG results in early myelinolysis and progressive axonal swelling. The potential mechanisms driving these pathologies include inflammatory, metabolic, ionic, and excitotoxic mechanisms. Progressive axonal swellings identified in intravital microscopy may represent periaxonal swelling or myelin injury (splitting or focal bulging). Adapted from (27). Ca2+, calcium.
Regions of asynchronous glial injury in human NMO lesions provide evidence that targeted destruction of CNS astrocytes leads to secondary oligodendrocyte loss and demyelination. Interestingly, spongiform demyelination is also evident in leukodystrophies associated with astrocyte dysfunction, such as Alexander disease and vanishing white matter disease (43). And, in a rat model of osmotic demyelination, astrocyte death occurs rapidly following correction of hyponatremia and outlines regions of future myelin loss. These observations suggest that the link between astrocyte damage and myelinolysis in NMO are independent of the mechanism of AQP4-IgG–mediated cytotoxicity. Possibilities include impaired potassium and cell volume regulation (44,45), excitotoxicity (46,47), altered astrocyte–oligodendroglial communication (48,49), release of inflammatory mediators (50), or deranged oligodendroglial metabolism (51) (Fig. 2).
Experimental models of NMO lesion formation are uniquely positioned to decipher the complex pathophysiology linking astrocyte loss and myelinolysis. In EAE (9,13), passive transfer (52) and ICI (12,13,27) NMO models, oligodendrocyte apoptosis and myelin vacuolization are apparent in regions of isolated astrocyte loss before inflammatory cell infiltration. Oligodendroglial cells are relatively preserved 1 hour after ICI of an AQP4 rAb and HC despite significant astrocyte depletion; 3 hours after injection, oligodendrocyte loss is readily apparent but myelin sheaths are spared (13). Within 12 hours of ICI, there is significant myelin edema and vacuolation (12), and by 18 hours, there is significant myelin fragmentation and vesiculated membrane profiles at the innermost layers of the sheath (27). Mechanistically, pathophysiology appears linked to increased intracellular calcium. Ionomycin treatment of acute brain slices results in myelin vesiculation and fragmentation identical to NMO lesions, and calcium chelation lessens myelin fragmentation in the NMO ICI model (27).
Recently, Herwerth et al (53) used intravital imaging to monitor the early phases of CNS lesion formation following the application of AQP4-IgG and HC to murine spinal cord in situ. Although oligodendrocytes remained relatively intact soon after the onset of astrocyte destruction, progressive axonal swellings were prominent (Fig. 2). Axonal swellings were not evident in the absence of the co-application of AQP-IgG and HC, and the number of axonal swellings correlated with AQP4-Ig titer and the degree of astrocyte loss. Previous research has shown that axonal swellings demonstrate complex ultrastructural changes in the subaxolemmal cytoskeleton that may result from a variety of extrinsic stimuli (54). Potential pathogenic mechanisms are similar to those linking astrocyte destruction and myelinolysis: excitotoxicity (55), altered ion homeostasis (45), increased oxidative stress (56), and reduced axonal metabolic support (57,58). Experimental manipulation of neuronal signaling and metabolism in NMO animal models should help to clarify the relationship of progressive axonal swelling, oligodendrocyte apoptosis, and myelinolysis in subacute NMO lesions. The results may identify novel therapeutic avenues for the treatment of acute attacks.
Complement-Mediated Cytotoxicity: NMO Lesion Initiation and Propagation
Although noninflammatory mechanisms are likely to drive oligodendrocyte injury after astrocyte loss, NMO animal models consistently indicate that AQP4-IgG alone is insufficient to initiate demyelination. NMO lesions in the ICI model require the addition of HC (13,59–61) (Fig. 1). In the absence of HC, in the presence of complement inhibitors, or CDC-deficient AQP4 rAb, there is no evidence of astrocyte or oligodendrocyte loss. Similarly, in the rat where there is a robust endogenous complement system, CNS lesion formation by peripherally administered AQP4-IgG is abrogated by the administration of cobra venom factor, an inhibitor of complement function (52). Similarly, NMO lesion formation is greatly reduced after inhibiting antibody-dependent cell-mediated cytotoxicity (ADCC) using mutated AQP4 rAb, FcγRIII receptor–deficient mice, or a Fcγ receptor blocking antibody (61). Interestingly, CNS lesions initiated by ADCC cause astrocyte destruction but limited myelin loss (62). Therefore, astrocyte destruction by AQP4-IgG requires antibody effector function (CDC or ADCC), but only CDC is sufficient to initiate secondary demyelination.
CDC could be critical for demyelination in NMO lesions due to the combined production of anaphylatoxins (C3a, C4a, and C5a) and opsonins (C3b, C4b, and C1q) that recruit inflammatory cells (63), enhance polymorphonuclear-mediated ADCC (64), and facilitate phagocytosis (65) (Fig. 1). Anaphylatoxins are chemoattractants for polymorphonuclear cells (66). Indeed, experimental lesions initiated in the absence of complement show significantly reduced immune cell infiltration (62). After attracting granulocytes to the lesion site, C3a, C5a, and C1q promote further tissue injury by mediating eosinophil (67,68) and neutrophil degranulation (69) and free radical production (70). In NMO lesion models, inhibition of Fc receptor (FcR) binding or signaling significantly lessens tissue damage (62,68). Finally, opsonins (C3b and C4b) are important for phagocytic clearance of apoptotic cells (71,72). Macrophages are abundant in active demyelinating NMO lesions and constitute the major phagocytic population removing myelin debris (37,38,42). Opsonin production in NMO lesions may be critical for subsequent inflammatory demyelination by targeting apoptotic oligodendrocytes for phagocytosis (62).
Potential Noninflammatory Mechanisms of CNS Injury
In NMO, AQP4-IgG binding to CNS astrocytes has been reported to alter surface expression of AQP4 and diminish water channel function (Fig. 1). On the plasma membrane, AQP4 forms supramolecular assemblies, termed orthogonal arrays of particles (OAPs), that directly impact AQP4-IgG binding (7). In tissue culture cells, AQP4-IgG causes internalization of surface AQP4 (46,73–75). Hinson et al (73) reported differential internalization of M1-AQP4 tetramers resulting in increased OAP size and potentially enhanced CDC. Other investigators, however, have failed to reproduce these findings (74–76). The effects of AQP4-IgG on surface AQP4 expressed by primary murine astrocytes and mixed glial cultures have been equally inconsistent (47,74,75,77,78). In mouse models, systemic or intracerebral administration of AQP-IgG failed to cause internalization of astrocyte AQP4 or tissue injury (10,12,74). However, in rats, chronic intrathecal infusion of large amounts of AQP4-IgG over 3 weeks resulted in local depletion of AQP4 in the adjacent spinal cord and reversible myelopathy (79). Interestingly, similar histopathology has been described in the cortex of cognitively impaired NMO patients (80) and in the vicinity of active NMO lesions (81).
The astrocyte glutamate transporter EAAT2 is complexed with AQP4 in astrocyte membranes and downregulated in AQP4-deficient mice (82). In human NMO spinal cord lesions, there is reduced EAAT2 expression in AQP4-deficient regions (77), suggesting that impaired glutamate reuptake and secondary excitotoxicity may contribute to CNS tissue injury. However, like observations regarding AQP4 internalization in transfected cells and primary cultures, there are conflicting reports on changes in EAAT2 expression and glutamate transport after exposure to AQP4-IgG (46,47,74,79). In mixed glial cultures, treatment with AQP-IgG in the absence of complement causes oligodendrocyte injury that is reduced with N-methyl-D-aspartate receptor antagonism (47). EAAT2 expression is also reduced following chronic intrathecal infusion of AQP4-IgG; however, the contribution of excitotoxicity to tissue injury in this model was not examined (79).
AQP4-IgG may also directly inhibit water channel function. In Xenopus oocytes expressing AQP4 water channels, AQP4-IgG was observed to delay cell lysis under hypotonic conditions (73). In contrast, neither AQP4-IgG nor AQP4-specific rAb was found to inhibit AQP4 water permeability in reconstituted M1-AQP4 proteoliposomes or M23-AQP4 plasma vesicles (75). The differential effect on oocyte water permeability is likely due to an artifact of experimental design as neither astrocyte nor myelin edema is noted in AQP4-deficient mice (83) or after administration of large amounts of intrathecal AQP4-IgG (12,79).
CENTRAL NERVOUS SYSTEM SPECIFICITY IN NEUROMYELITIS OPTICA
Although AQP4 is expressed in multiple tissues, NMO pathology is remarkably limited to the CNS. Transient serum elevation in creatine kinase is the most common observation of extra-CNS pathology (84,85); less frequently, muscle pathology with immune infiltrates, AQP4 loss, and complement deposition is evident (86–88).
CNS-specific injury in NMO is not determined by access to AQP4 in peripheral tissue. Serum AQP4-IgG or AQP4-specific rAb injected into rodents binds readily to AQP4 in kidney, stomach, lung, retina, and muscle in the periphery and the circumventricular organs and area postrema in the CNS (52,89). NMO pathology and complement deposition are not observed in peripheral tissues in the absence or presence of inflammation (10,52,89), indicating that CDC is not effectively activated in these tissues. Plasma membrane complement regulatory proteins CD46, CD55, and CD59 are co-expressed with AQP4 in kidney, stomach, and skeletal muscle, whereas CD59 seems to be excluded from AQP4-positive astrocyte processes contacting endothelial cells (90). Indeed, intrathecal injection of NMO-IgG and HC in CD59 knockout mice produces longitudinally extensive spinal cord lesions and exacerbates ON in passive transfer models (91,92).
AQP4 OAPs are essential for AQP4-IgG–mediated CDC (76). Therefore, the abundance and size of AQP4 OAPs may contribute to the observed distribution of pathology in NMO. The relative expression of AQP4 mRNA and protein is higher in optic nerve and spinal cord than peripheral tissues (93), and optic nerve tissue demonstrates the highest ratio of large to small AQP4 supramolecular aggregates (93). CNS injury may be further polarized due to the cellular distribution of larger AQP4 supramolecular aggregates. Spinal cord white matter astrocyte processes display diffuse expression of AQP4 OAPs, whereas AQP4 OAP expression in grey matter astrocytes is polarized (94). Super resolution microscopy of spinal cord sections demonstrates large AQP4 clusters in both perivascular and parenchymal white matter but biased localization to perivascular grey matter (95). The diffuse expression of AQP4 OAPs in spinal cord and optic nerve white matter may provide an anatomic explanation for the longitudinal extension of optic nerve and spinal cord lesions in affected patients (14,28).
NOVEL APPROACHES TO THE TREATMENT OF ACUTE NEUROMYELITIS OPTICA LESIONS
Data from NMO experimental models provide an outline for the development of novel therapeutic approaches for the treatment of acute exacerbations. Both inflammatory and noninflammatory pathologic mechanisms may be addressed through various strategies. Small molecules and synthetic peptoid ligands that block AQP4-IgG binding have been identified in high-throughput screens (96–98). With suitable potency, half-life, and CNS penetration, these agents may offer a fundamental mechanism for limiting CNS lesion propagation during relapse. Alternatively, an AQP4-specific blocking antibody devoid of effector activity (aquaporumab) could be used to inhibit serum AQP4-IgG binding (60). As reviewed earlier, there is no definitive evidence for noninflammatory AQP4-IgG–mediated lesion pathology in experimental systems (10,12,61,99), and there is no evidence that AQP4-IgG directly inhibits AQP4 water channel function (75).
Both CDC and ADCC are necessary for complete lesion formation in the ICI animal model (61), and ADCC seems to play a critical role in facilitating macrophage-mediated demyelination at the outer rim of developing lesions (100). A C1-esterase inhibitor was recently shown to be safe in a small open-label treatment trial of acute NMO TM (101); however, high-dose therapy of acute lesions in an animal model was ineffective (102). In contrast, a C1q-targeted monoclonal antibody demonstrated effective inhibition of AQP4-IgG–mediated CDC in vitro, ex vivo, and in vivo (103). Treatment of acute relapses in NMOSD patients with complement inhibitors, however, may face unique therapeutic challenges due to limited CNS penetration, abundant target proteins, and intrathecal production of complement components.
Inhibition of eosinophil degranulation and neutrophil elastase with Silevestat (69,104) and cetirizine (68) has shown promise in animal models, and clinical trials with an alpha-1-antitrypsin inhibitor (clinicaltrials.gov: NCT02087813) and cetirizine (clinicaltrials.gov: NCT02865018) are currently underway. Since IVIg may interfere with ADCC through the upregulation of inhibitory FcR expression (105), administration of IVIg may offer another strategy for acute NMOSD relapses unresponsive to corticosteroids (clinicaltrials.gov: NCT01845584).
Recent histologic analysis of mitochondria in affected optic nerves from NMOSD patients suggest that disordered mitochondrial dynamics may contribute to retinal ganglion cell injury and loss (106). Lesioned optic nerve showed increased staining for transient receptor potential melastatin 4 (TRPM4) cation channels that may contribute to axonal injury. TRPM4 channels are directly inhibited by the antidiabetic drug glibenclamide, offering a novel avenue for mitigating axonal injury in ON (107).
In summary, multiple avenues of investigation in the laboratory have significantly advanced our understanding of NMO pathophysiology. The result is a framework for understanding immunologic and nonimmunologic mechanisms that lead from a targeted antibody response against astrocytes to demyelination and neuronal injury. Consequently, the therapeutics pipeline is rich with agents that target a wide range of pathways. Many offer significant appeal for acute therapy by selectively targeting pathways that propagate and amplify CNS lesions. Moving these agents from the bench to the bedside offers the opportunity to identify safe and effective therapies that limit CNS injury and preserve visual function.
Acknowledgments
Supported by the National Institutes of Health NEI EY022936 (J.L.B.), UM1AI110498 (J.L.B.), NINDS NS072141 (G.P.O.), the Guthy-Jackson Charitable Foundation (J.L.B.), and a Collaborative Research Grant from the National Multiple Sclerosis Society (J.L.B.).
Footnotes
The authors report no conflicts of interest.
References
- 1.Devic E. Myelite Aigue compliquee de nevrite optique. Bull Med (Paris) 1894;8:1033–1034. [Google Scholar]
- 2.Balser BH. Neuromyelitis optica. Brain. 1936;59:353–365. [Google Scholar]
- 3.Lucchinetti CF, Mandler RN, McGavern D, Bruck W, Gleich G, Ransohoff RM, Trebst C, Weinshenker B, Wingerchuk D, Parisi JE, Lassmann H. A role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis optica. Brain. 2002;125:1450–1461. doi: 10.1093/brain/awf151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202:473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, Weinshenker BG. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 6.Jarius S, Wildemann B. Aquaporin-4 antibodies (NMO-IgG) as a serological marker of neuromyelitis optica: a critical review of the literature. Brain Pathol. 2013;23:661–683. doi: 10.1111/bpa.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waters PJ, Pittock SJ, Bennett JL, Jarius S, Weinshenker BG, Wingerchuk DM. Evaluation of aquaporin-4 antibody assays. Clin Exp Neuroimmunol. 2014;5:290–303. doi: 10.1111/cen3.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waters PJ, McKeon A, Leite MI, Rajasekharan S, Lennon VA, Villalobos A, Palace J, Mandrekar JN, Vincent A, Bar-Or A, Pittock SJ. Serologic diagnosis of NMO: a multicenter comparison of aquaporin-4-IgG assays. Neurology. 2012;78:665–671. doi: 10.1212/WNL.0b013e318248dec1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett J, Lam C, Kalluri S, Saikali P, Bautista K, Dupree C, Glogowska M, Case D, Antel J, Owens G, Gilden D, Nessler S, Stadelmann C, Hemmer B. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann Neurol. 2009;66:617–629. doi: 10.1002/ana.21802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradl M, Misu T, Takahashi T, Watanabe M, Mader S, Reindl M, Adzemovic M, Bauer J, Berger T, Fujihara K, Itoyama Y, Lassmann H. Neuromyelitis optica: pathogenicity of patient immunoglobulin in vivo. Ann Neurol. 2009;66:630–643. doi: 10.1002/ana.21837. [DOI] [PubMed] [Google Scholar]
- 11.Kinoshita M, Nakatsuji Y, Kimura T, Moriya M, Takata K, Okuno T, Kumanogoh A, Kajiyama K, Yoshikawa H, Sakoda S. Neuromyelitis optica: passive transfer to rats by human immunoglobulin. Biochem Biophys Res Commun. 2009;386:623–627. doi: 10.1016/j.bbrc.2009.06.085. [DOI] [PubMed] [Google Scholar]
- 12.Saadoun S, Waters P, Bell BA, Vincent A, Verkman AS, Papadopoulos MC. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain. 2010;133:349–361. doi: 10.1093/brain/awp309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wrzos C, Winkler A, Metz I, Kayser DM, Thal DR, Wegner C, Bruck W, Nessler S, Bennett JL, Stadelmann C. Early loss of oligodendrocytes in human and experimental neuromyelitis optica lesions. Acta Neuropathol. 2014;127:523–538. doi: 10.1007/s00401-013-1220-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wingerchuk D, Lennon V, Pittock S, Lucchinetti C, Weinshenker B. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–1489. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 15.Kitley J, Woodhall M, Waters P, Leite MI, Devenney E, Craig J, Palace J, Vincent A. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology. 2012;79:1273–1277. doi: 10.1212/WNL.0b013e31826aac4e. [DOI] [PubMed] [Google Scholar]
- 16.Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, Pache F, Stich O, Beume LA, Hummert MW, Trebst C, Ringelstein M, Aktas O, Winkelmann A, Buttmann M, Schwarz A, Zimmermann H, Brandt AU, Franciotta D, Capobianco M, Kuchling J, Haas J, Korporal-Kuhnke M, Lillevang ST, Fechner K, Schanda K, Paul F, Wildemann B, Reindl M cooperation with the Neuromyelitis Optica Study G. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 1: frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin. J Neuroinflammation. 2016;13:279. doi: 10.1186/s12974-016-0717-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoftberger R, Sepulveda M, Armangue T, Blanco Y, Rostasy K, Cobo Calvo A, Olascoaga J, Ramio-Torrenta L, Reindl M, Benito-Leon J, Casanova B, Arrambide G, Sabater L, Graus F, Dalmau J, Saiz A. Antibodies to MOG and AQP4 in adults with neuromyelitis optica and suspected limited forms of the disease. Mult Scler. 2015;21:866–874. doi: 10.1177/1352458514555785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitley J, Waters P, Vincent A, Palace J. Features of neuromyelitis optica spectrum disorders and aquaporin-4 with myelin-oligodendrocyte glycoprotein antibodies-reply. JAMA Neurol. 2014;71:924. doi: 10.1001/jamaneurol.2014.940. [DOI] [PubMed] [Google Scholar]
- 19.Sato DK, Callegaro D, Lana-Peixoto MA, Waters PJ, de Haidar Jorge FM, Takahashi T, Nakashima I, Apostolos-Pereira SL, Talim N, Simm RF, Lino AM, Misu T, Leite MI, Aoki M, Fujihara K. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology. 2014;82:474–481. doi: 10.1212/WNL.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, Pache F, Stich O, Beume LA, Hummert MW, Ringelstein M, Trebst C, Winkelmann A, Schwarz A, Buttmann M, Zimmermann H, Kuchling J, Franciotta D, Capobianco M, Siebert E, Lukas C, Korporal-Kuhnke M, Haas J, Fechner K, Brandt AU, Schanda K, Aktas O, Paul F, Reindl M, Wildemann B in cooperation with the Neuromyelitis Optica Study G. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016;13:280. doi: 10.1186/s12974-016-0718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Pelt ED, Wong YY, Ketelslegers IA, Hamann D, Hintzen RQ. Neuromyelitis optica spectrum disorders: comparison of clinical and magnetic resonance imaging characteristics of AQP4-IgG versus MOG-IgG seropositive cases in The Netherlands. Eur J Neurol. 2016;23:580–587. doi: 10.1111/ene.12898. [DOI] [PubMed] [Google Scholar]
- 22.Akaishi T, Nakashima I, Takeshita T, Mugikura S, Sato DK, Takahashi T, Nishiyama S, Kurosawa K, Misu T, Nakazawa T, Aoki M, Fujihara K. Lesion length of optic neuritis impacts visual prognosis in neuromyelitis optica. J Neuroimmunol. 2016;293:28–33. doi: 10.1016/j.jneuroim.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Chalmoukou K, Alexopoulos H, Akrivou S, Stathopoulos P, Reindl M, Dalakas MC. Anti-MOG antibodies are frequently associated with steroid-sensitive recurrent optic neuritis. Neurol Neuroimmunol Neuroinflamm. 2015;2:e131. doi: 10.1212/NXI.0000000000000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pache F, Zimmermann H, Mikolajczak J, Schumacher S, Lacheta A, Oertel FC, Bellmann-Strobl J, Jarius S, Wildemann B, Reindl M, Waldman A, Soelberg K, Asgari N, Ringelstein M, Aktas O, Gross N, Buttmann M, Ach T, Ruprecht K, Paul F, Brandt AU in cooperation with the Neuromyelitis Optica Study G. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 4: afferent visual system damage after optic neuritis in MOG-IgG-seropositive versus AQP4-IgG-seropositive patients. J Neuroinflammation. 2016;13:282. doi: 10.1186/s12974-016-0720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spadaro M, Gerdes LA, Mayer MC, Ertl-Wagner B, Laurent S, Krumbholz M, Breithaupt C, Hogen T, Straube A, Giese A, Hohlfeld R, Lassmann H, Meinl E, Kumpfel T. Histopathology and clinical course of MOG-antibody-associated encephalomyelitis. Ann Clin Transl Neurol. 2015;2:295–301. doi: 10.1002/acn3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saadoun S, Waters P, Owens GP, Bennett JL, Vincent A, Papadopoulos MC. Neuromyelitis optica MOG-IgG causes reversible lesions in mouse brain. Acta Neuropathol Commun. 2014;2:35. doi: 10.1186/2051-5960-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weil MT, Mobius W, Winkler A, Ruhwedel T, Wrzos C, Romanelli E, Bennett JL, Enz L, Goebels N, Nave KA, Kerschensteiner M, Schaeren-Wiemers N, Stadelmann C, Simons M. Loss of myelin basic protein function triggers myelin breakdown in models of demyelinating diseases. Cell Rep. 2016;16:314–322. doi: 10.1016/j.celrep.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, de Seze J, Fujihara K, Greenberg B, Jacob A, Jarius S, Lana-Peixoto M, Levy M, Simon JH, Tenembaum S, Traboulsee AL, Waters P, Wellik KE, Weinshenker BG International Panel for NMOD. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyun JW, Jeong IH, Joung A, Kim SH, Kim HJ. Evaluation of the 2015 diagnostic criteria for neuromyelitis optica spectrum disorder. Neurology. 2016;86:1772–1779. doi: 10.1212/WNL.0000000000002655. [DOI] [PubMed] [Google Scholar]
- 30.Kitley J, Waters P, Woodhall M, Leite MI, Murchison A, George J, Kuker W, Chandratre S, Vincent A, Palace J. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study. JAMA Neurol. 2014;71:276–283. doi: 10.1001/jamaneurol.2013.5857. [DOI] [PubMed] [Google Scholar]
- 31.Pohl M, Fischer M-T, Mader S, Schanda K, Kitic M, Sharma R, Wimmer I, Misu T, Fujihara K, Reindl M, Lassmann H, Bradl M. Pathogenic T cell responses against aquaporin 4. Acta Neuropathol. 2011;122:21–34. doi: 10.1007/s00401-011-0824-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones MV, Huang H, Calabresi PA, Levy M. Pathogenic aquaporin-4 reactive T cells are sufficient to induce mouse model of neuromyelitis optica. Acta Neuropathol Commun. 2015;3:28. doi: 10.1186/s40478-015-0207-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pittock SJ, Lennon VA, de Seze J, Vermersch P, Homburger HA, Wingerchuk DM, Lucchinetti CF, Zéphir H, Moder K, Weinshenker BG. Neuromyelitis optica and non organ-specific autoimmunity. Arch Neurol. 2008;65:78–83. doi: 10.1001/archneurol.2007.17. [DOI] [PubMed] [Google Scholar]
- 34.McKeon A, Lennon VA, Jacob A, Matiello M, Lucchinetti CF, Kale N, Chan KH, Weinshenker BG, Apiwattinakul M, Wingerchuk DM, Pittock SJ. Coexistence of myasthenia gravis and serological markers of neurological autoimmunity in neuromyelitis optica. Muscle Nerve. 2009;39:87–90. doi: 10.1002/mus.21197. [DOI] [PubMed] [Google Scholar]
- 35.Schanda K, Waters P, Holzer H, Aboulenein-Djamshidian F, Leite MI, Palace J, Vukusic S, Marignier R, Berger T, Reindl M. Antibodies to aquaporin-1 are not present in neuromyelitis optica. Neurol Neuroimmunol Neuroinflamm. 2015;2:e160. doi: 10.1212/NXI.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tzartos JS, Stergiou C, Kilidireas K, Zisimopoulou P, Thomaidis T, Tzartos SJ. Anti-aquaporin-1 autoantibodies in patients with neuromyelitis optica spectrum disorders. PLoS One. 2013;8:e74773. doi: 10.1371/journal.pone.0074773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Misu T, Fujihara K, Kakita A, Konno H, Nakamura M, Watanabe S, Takahashi T, Nakashima I, Takahashi H, Itoyama Y. Loss of aquaporin 4 in lesions of neuromyelitis optica: distinction from multiple sclerosis. Brain. 2007;130:1224–1234. doi: 10.1093/brain/awm047. [DOI] [PubMed] [Google Scholar]
- 38.Parratt JDE, Prineas JW. Neuromyelitis optica: a demyelinating disease characterized by acute destruction and regeneration of perivascular astrocytes. Mult Scler. 2010;16:1156–1172. doi: 10.1177/1352458510382324. [DOI] [PubMed] [Google Scholar]
- 39.Bruck W, Popescu B, Lucchinetti CF, Markovic-Plese S, Gold R, Thal DR, Metz I. Neuromyelitis optica lesions may inform multiple sclerosis heterogeneity debate. Ann Neurol. 2012;72:385–394. doi: 10.1002/ana.23621. [DOI] [PubMed] [Google Scholar]
- 40.Lucchinetti C, Bruck W, Parisis J, Schiethauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 41.Popescu BFG, Lennon VA, Parisi JE, Howe CL, Weigand SD, Cabrera-Gómez JA, Newell K, Mandler RN, Pittock SJ, Weinshenker BG, Lucchinetti CF. Neuromyelitis optica unique area postrema lesions: nausea, vomiting, and pathogenic implications. Neurology. 2011;76:1229–1237. doi: 10.1212/WNL.0b013e318214332c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roemer SF, Parisi JE, Lennon VA, Benarroch EE, Lassmann H, Bruck W, Mandler RN, Weinshenker BG, Pittock SJ, Wingerchuk DM, Lucchinetti CF. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007;130:1194–1205. doi: 10.1093/brain/awl371. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez D. Leukodystrophies with astrocytic dysfunction. Handb Clin Neurol. 2013;113:1619–1628. doi: 10.1016/B978-0-444-59565-2.00030-7. [DOI] [PubMed] [Google Scholar]
- 44.Illarionova NB, Gunnarson E, Li Y, Brismar H, Bondar A, Zelenin S, Aperia A. Functional and molecular interactions between aquaporins and Na,K-ATPase. Neuroscience. 2010;168:915–925. doi: 10.1016/j.neuroscience.2009.11.062. [DOI] [PubMed] [Google Scholar]
- 45.Yang X, Ransom BR, Ma JF. The role of AQP4 in neuromyelitis optica: more answers, more questions. J Neuroimmunol. 2016;298:63–70. doi: 10.1016/j.jneuroim.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Hinson SR, Roemer SF, Lucchinetti C, Fryer JP, Kryzer TJ, Chamberlain JL, Howe CL, Pittock SJ, Lennon VA. Aquaporin-4-binding autoantibodies in patients with neuromyelitis optica impair glutamate transport by down-regulating EAAT2. J Exp Med. 2008;205:2473–2481. doi: 10.1084/jem.20081241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marignier R, Nicolle A, Watrin C, Touret M, Cavagna S, Varrin-Doyer M, Cavillon G, Rogemond V, Confavreux C, Honnorat J, Giraudon P. Oligodendrocytes are damaged by neuromyelitis optica immunoglobulin G via astrocyte injury. Brain. 2010;133:2578–2591. doi: 10.1093/brain/awq177. [DOI] [PubMed] [Google Scholar]
- 48.Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, Fields RD. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49:823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watkins TA, Emery B, Mulinyawe S, Barres BA. Distinct stages of myelination regulated by gamma-secretase and astrocytes in a rapidly myelinating CNS coculture system. Neuron. 2008;60:555–569. doi: 10.1016/j.neuron.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benn T, Halfpenny C, Scolding N. Glial cells as targets for cytotoxic immune mediators. Glia. 2001;36:200–211. doi: 10.1002/glia.1109. [DOI] [PubMed] [Google Scholar]
- 51.Rinholm JE, Hamilton NB, Kessaris N, Richardson WD, Bergersen LH, Attwell D. Regulation of oligodendrocyte development and myelination by glucose and lactate. J Neurosci. 2011;31:538–548. doi: 10.1523/JNEUROSCI.3516-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Asavapanumas N, Verkman AS. Neuromyelitis optica pathology in rats following intraperitoneal injection of NMO-IgG and intracerebral needle injury. Acta Neuropathol Commun. 2014;2:48. doi: 10.1186/2051-5960-2-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herwerth M, Kalluri SR, Srivastava R, Kleele T, Kenet S, Illes Z, Merkler D, Bennett JL, Misgeld T, Hemmer B. In vivo imaging reveals rapid astrocyte depletion and axon damage in a model of neuromyelitis optica-related pathology. Ann Neurol. 2016;79:794–805. doi: 10.1002/ana.24630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ochs S, Pourmand R, Jersild RA, Jr, Friedman RN. The origin and nature of beading: a reversible transformation of the shape of nerve fibers. Prog Neurobiol. 1997;52:391–426. doi: 10.1016/s0301-0082(97)00022-1. [DOI] [PubMed] [Google Scholar]
- 55.Pitt D, Werner P, Raine CS. Glutamate excitotoxicity in a model of multiple sclerosis. Nat Med. 2000;6:67–70. doi: 10.1038/71555. [DOI] [PubMed] [Google Scholar]
- 56.Desagher S, Glowinski J, Premont J. Astrocytes protect neurons from hydrogen peroxide toxicity. J Neurosci. 1996;16:2553–2562. doi: 10.1523/JNEUROSCI.16-08-02553.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 58.Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27:219–249. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- 59.Saadoun S, Bridges LR, Verkman AS, Papadopoulos MC. Paucity of natural killer and cytotoxic T cells in human neuromyelitis optica lesions. Neuroreport. 2012;23:1044–1047. doi: 10.1097/WNR.0b013e32835ab480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tradtrantip L, Zhang H, Saadoun S, Phuan PW, Lam C, Papadopoulos MC, Bennett JL, Verkman AS. Anti-aquaporin-4 monoclonal antibody blocker therapy for neuromyelitis optica. Ann Neurol. 2012;71:314–322. doi: 10.1002/ana.22657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ratelade J, Asavapanumas N, Ritchie AM, Wemlinger S, Bennett JL, Verkman AS. Involvement of antibody-dependent cell-mediated cytotoxicity in inflammatory demyelination in a mouse model of neuromyelitis optica. Acta Neuropathol. 2013;126:699–709. doi: 10.1007/s00401-013-1172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ratelade J, Zhang H, Saadoun S, Bennett JL, Papadopoulos MC, Verkman AS. Neuromyelitis optica IgG and natural killer cells produce NMO lesions in mice without myelin loss. Acta Neuropathol. 2012;123:861–872. doi: 10.1007/s00401-012-0986-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boos L, Campbell IL, Ames R, Wetsel RA, Barnum SR. Deletion of the complement anaphylatoxin C3a receptor attenuates, whereas ectopic expression of C3a in the brain exacerbates, experimental autoimmune encephalomyelitis. J Immunol. 2004;173:4708–4714. doi: 10.4049/jimmunol.173.7.4708. [DOI] [PubMed] [Google Scholar]
- 64.Derer S, Cossham M, Rosner T, Kellner C, Beurskens FJ, Schwanbeck R, Lohse S, Sina C, Peipp M, Valerius TA. Complement-optimized EGFR antibody improves cytotoxic functions of polymorphonuclear cells against tumor cells. J Immunol. 2015;195:5077–5087. doi: 10.4049/jimmunol.1501458. [DOI] [PubMed] [Google Scholar]
- 65.Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- 66.Sewell DL, Nacewicz B, Liu F, Macvilay S, Erdei A, Lambris JD, Sandor M, Fabry Z. Complement C3 and C5 play critical roles in traumatic brain cryoinjury: blocking effects on neutrophil extravasation by C5a receptor antagonist. J Neuroimmunol. 2004;155:55–63. doi: 10.1016/j.jneuroim.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giembycz MA, Lindsay MA. Pharmacology of the eosinophil. Pharmacol Rev. 1999;51:213–340. [PubMed] [Google Scholar]
- 68.Zhang H, Verkman AS. Eosinophil pathogenicity mechanisms and therapeutics in neuromyelitis optica. J Clin Invest. 2013;123:2306–2316. doi: 10.1172/JCI67554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saadoun S, Waters P, MacDonald C, Bell BA, Vincent A, Verkman AS, Papadopoulos MC. Neutrophil protease inhibition reduces neuromyelitis optica-immunoglobulin G-induced damage in mouse brain. Ann Neurol. 2012;71:323–333. doi: 10.1002/ana.22686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goodman EB, Tenner AJ. Signal transduction mechanisms of C1q–mediated superoxide production. Evidence for the involvement of temporally distinct staurosporine-insensitive and sensitive pathways. J Immunol. 1992;148:3920–3928. [PubMed] [Google Scholar]
- 71.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 72.Taylor PR, Carugati A, Fadok VA, Cook HT, Andrews M, Carroll MC, Savill JS, Henson PM, Botto M, Walport MJ. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med. 2000;192:359–366. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hinson SR, Romero MF, Popescu BFG, Lucchinetti CF, Fryer JP, Wolburg H, Fallier-Becker P, Noell S, Lennon VA. Molecular outcomes of neuromyelitis optica (NMO)-IgG binding to aquaporin-4 in astrocytes. Proc Natl Acad Sci U S A. 2011;109:11245–11250. doi: 10.1073/pnas.1109980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ratelade J, Bennett JL, Verkman AS. Evidence against cellular internalization in vivo of NMO-IgG, Aquaporin-4, and excitatory amino acid transporter 2 in neuromyelitis optica. J Biol Chem. 2011;286:45156–45164. doi: 10.1074/jbc.M111.297275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rossi A, Ratelade J, Papadopoulos MC, Bennett JL, Verkman AS. Neuromyelitis optica IgG does not alter aquaporin-4 water permeability, plasma membrane M1/M23 isoform content, or supramolecular assembly. Glia. 2012;60:2027–2039. doi: 10.1002/glia.22417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Phuan PW, Ratelade J, Rossi A, Tradtrantip L, Verkman AS. Complement-dependent cytotoxicity in neuromyelitis optica requires aquaporin-4 protein assembly in orthogonal arrays. J Biol Chem. 2012;287:13829–13839. doi: 10.1074/jbc.M112.344325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hinson S, Roemer S, Lucchinetti C, Fryer J, Kryzer T, Chamberlain J, Howe C, Pittock S, Lennon V. Aquaporin-4-binding autoantibodies in patients with neuromyelitis optica impair glutamate transport by down-regulating EAAT2. J Exp Med. 2008;205:2473–2481. doi: 10.1084/jem.20081241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rossi A, Ratelade J, Papadopoulos MC, Bennett JL, Verkman AS. Consequences of NMO-IgG binding to aquaporin-4 in neuromyelitis optica. Proc Natl Acad Sci U S A. 2012;109:1511. doi: 10.1073/pnas.1203463109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Geis C, Ritter C, Ruschil C, Weishaupt A, Grunewald B, Stoll G, Holmoy T, Misu T, Fujihara K, Hemmer B, Stadelmann C, Bennett JL, Sommer C, Toyka KV. The intrinsic pathogenic role of autoantibodies to aquaporin 4 mediating spinal cord disease in a rat passive-transfer model. Exp Neurol. 2015;265:8–21. doi: 10.1016/j.expneurol.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saji E, Arakawa M, Yanagawa K, Toyoshima Y, Yokoseki A, Okamoto K, Otsuki M, Akazawa K, Kakita A, Takahashi H, Nishizawa M, Kawachi I. Cognitive impairment and cortical degeneration in neuromyelitis optica. Ann Neurol. 2013;73:65–76. doi: 10.1002/ana.23721. [DOI] [PubMed] [Google Scholar]
- 81.Misu T, Höftberger R, Fujihara K, Wimmer I, Takai Y, Nishiyama S, Nakashima I, Konno H, Bradl M, Garzuly F, Itoyama Y, Aoki M, Lassmann H. Presence of six different lesion types suggests diverse mechanisms of tissue injury in neuromyelitis optica. Acta Neuropathol. 2013;125:815–827. doi: 10.1007/s00401-013-1116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zeng XN, Sun XL, Gao L, Fan Y, Ding JH, Hu G. Aquaporin-4 deficiency down-regulates glutamate uptake and GLT-1 expression in astrocytes. Mol Cell Neurosci. 2007;34:34–39. doi: 10.1016/j.mcn.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 83.Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- 84.Di Filippo M, Franciotta D, Massa R, Di Gregorio M, Zardini E, Gastaldi M, Terracciano C, Rastelli E, Gaetani L, Iannone A, Menduno P, Floridi P, Sarchielli P, Calabresi P. Recurrent hyperCKemia with normal muscle biopsy in a pediatric patient with neuromyelitis optica. Neurology. 2012;79:1182–1184. doi: 10.1212/WNL.0b013e3182698d39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Suzuki N, Takahashi T, Aoki M, Misu T, Konohana S, Okumura T, Takahashi H, Kameya S, Yamaki K, Kumagai T, Fujihara K, Itoyama Y. Neuromyelitis optica preceded by hyperCKemia episode. Neurology. 2010;74:1543–1545. doi: 10.1212/WNL.0b013e3181dd445b. [DOI] [PubMed] [Google Scholar]
- 86.Guo Y, Lennon VA, Popescu BF, Grouse CK, Topel J, Milone M, Lassmann H, Parisi JE, Pittock SJ, Stefoski D, Balabanov R, Lucchinetti CF. Autoimmune aquaporin-4 myopathy in neuromyelitis optica spectrum. JAMA Neurol. 2014;71:1025–1029. doi: 10.1001/jamaneurol.2014.775. [DOI] [PubMed] [Google Scholar]
- 87.He D, Li Y, Dai Q, Zhang Y, Xu Z, Li Y, Cai G, Chu L. Myopathy associated with neuromyelitis optica spectrum disorders. Int J Neurosci. 2016;126:863–866. doi: 10.3109/00207454.2015.1113175. [DOI] [PubMed] [Google Scholar]
- 88.Malik R, Lewis A, Cree BA, Ratelade J, Rossi A, Verkman AS, Bollen AW, Ralph JW. Transient hyperckemia in the setting of neuromyelitis optica (NMO) Muscle Nerve. 2014;50:859–862. doi: 10.1002/mus.24298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ratelade J, Bennett JL, Verkman AS. Intravenous neuromyelitis optica autoantibody in mice targets aquaporin-4 in peripheral organs and area postrema. PLoS One. 2011;6:e27412. doi: 10.1371/journal.pone.0027412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saadoun S, Papadopoulos MC. Role of membrane complement regulators in neuromyelitis optica. Mult Scler. 2015;21:1644–1654. doi: 10.1177/1352458515571446. [DOI] [PubMed] [Google Scholar]
- 91.Asavapanumas N, Ratelade J, Papadopoulos MC, Bennett JL, Levin MH, Verkman AS. Experimental mouse model of optic neuritis with inflammatory demyelination produced by passive transfer of neuromyelitis optica-immunoglobulin G. J Neuroinflammation. 2014;11:16. doi: 10.1186/1742-2094-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang H, Verkman AS. Longitudinally extensive NMO spinal cord pathology produced by passive transfer of NMO-IgG in mice lacking complement inhibitor CD59. J Autoimmun. 2014;53:67–77. doi: 10.1016/j.jaut.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Matiello M, Schaefer-Klein J, Sun D, Weinshenker BG. Aquaporin 4 expression and tissue susceptibility to neuromyelitis optica. JAMA Neurol. 2013;70:1118–1125. doi: 10.1001/jamaneurol.2013.3124. [DOI] [PubMed] [Google Scholar]
- 94.Oklinski MK, Lim JS, Choi HJ, Oklinska P, Skowronski MT, Kwon TH. Immunolocalization of water channel proteins AQP1 and AQP4 in rat spinal cord. J Histochem Cytochem. 2014;62:598–611. doi: 10.1369/0022155414537495. [DOI] [PubMed] [Google Scholar]
- 95.Smith AJ, Verkman AS. Superresolution imaging of Aquaporin-4 cluster size in antibody-stained Paraffin brain sections. Biophys J. 2015;109:2511–2522. doi: 10.1016/j.bpj.2015.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun M, Wang J, Zhou Y, Wang Z, Jiang Y, Li M. Isotetrandrine reduces astrocyte cytotoxicity in neuromyelitis optica by blocking the binding of NMO-IgG to aquaporin 4. Neuroimmunomodulation. 2016;23:98–108. doi: 10.1159/000444530. [DOI] [PubMed] [Google Scholar]
- 97.Tradtrantip L, Zhang H, Anderson MO, Saadoun S, Phuan PW, Papadopoulos MC, Bennett JL, Verkman AS. Small-molecule inhibitors of NMO-IgG binding to aquaporin-4 reduce astrocyte cytotoxicity in neuromyelitis optica. FASEB J. 2012;26:2197–2208. doi: 10.1096/fj.11-201608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Raveendra BL, Wu H, Baccala R, Reddy MM, Schilke J, Bennett JL, Theofilopoulos AN, Kodadek T. Discovery of peptoid ligands for anti-aquaporin 4 antibodies. Chem Biol. 2013;20:351–359. doi: 10.1016/j.chembiol.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang H, Bennett JL, Verkman AS. Ex vivo spinal cord slice model of neuromyelitis optica reveals novel immunopathogenic mechanisms. Ann Neurol. 2011;70:943–954. doi: 10.1002/ana.22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Asavapanumas N, Ratelade J, Verkman AS. Unique neuromyelitis optica pathology produced in naive rats by intracerebral administration of NMO-IgG. Acta Neuropathol. 2014;127:539–551. doi: 10.1007/s00401-013-1204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Levy M, Mealy MA. Purified human C1-esterase inhibitor is safe in acute relapses of neuromyelitis optica. Neurol Neuroimmunol Neuroinflamm. 2014;1:e5. doi: 10.1212/NXI.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tradtrantip L, Asavapanumas N, Phuan PW, Verkman AS. Potential therapeutic benefit of c1-esterase inhibitor in neuromyelitis optica evaluated in vitro and in an experimental rat model. PLoS One. 2014;9:e106824. doi: 10.1371/journal.pone.0106824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Phuan PW, Zhang H, Asavapanumas N, Leviten M, Rosenthal A, Tradtrantip L, Verkman AS. C1q–targeted monoclonal antibody prevents complement-dependent cytotoxicity and neuropathology in in vitro and mouse models of neuromyelitis optica. Acta Neuropathol. 2013;125:829–840. doi: 10.1007/s00401-013-1128-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Herges K, de Jong BA, Kolkowitz I, Dunn C, Mandelbaum G, Ko RM, Maini A, Han MH, Killestein J, Polman C, Goodyear AL, Dunn J, Steinman L, Axtell RC. Protective effect of an elastase inhibitor in a neuromyelitis optica-like disease driven by a peptide of myelin oligodendroglial glycoprotein. Mult Scler. 2012;18:398–408. doi: 10.1177/1352458512440060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci U S A. 2008;105:19571–19578. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Archer SL. Mitochondrial dynamics–mitochondrial fission and fusion in human diseases. N Engl J Med. 2013;369:2236–2251. doi: 10.1056/NEJMra1215233. [DOI] [PubMed] [Google Scholar]
- 107.Schattling B, Steinbach K, Thies E, Kruse M, Menigoz A, Ufer F, Flockerzi V, Bruck W, Pongs O, Vennekens R, Kneussel M, Freichel M, Merkler D, Friese MA. TRPM4 cation channel mediates axonal and neuronal degeneration in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med. 2012;18:1805–1811. doi: 10.1038/nm.3015. [DOI] [PubMed] [Google Scholar]