Abstract
Background and aims
Inflammation, particularly innate immunity, play an important role in cardiovascular diseases. The aim of this study was to investigate whether atherogenic determinants such as oxidized LDL modulate the phenotype of eosinophils.
Methods
Cultured eosinophils were treated with oxidized LDL and the expression of selective inflammatory and anti-inflammatory cytokines was determined. In addition, the eosinophil receptor and signaling that mediates these events were identified.
Results
Treatment of cultured eosinophils with oxidized LDL (Ox-LDL) specifically induced the expression of IFNα and IFNβ without affecting expression of other proinflammatory cytokines, such as TNFα, IL-1β, and IL-6. In macrophages, Ox-LDL downregulated expression of both IFNα and IFNβ, suggesting that the effect of Ox-LDL on the expression of type I interferons is specific to eosinophils. Furthermore, we noted that eosinophils constitutively expressed IL-4 and IL-13, and Ox-LDL markedly downregulated their expression. Analysis of Ox-LDL signaling revealed that eosinophils constitutively expressed SRB2, CD36, and CD68 scavenger receptors, and Ox-LDL markedly induced the expression of CD36. Further analysis of CD36 signaling by siRNA and neutralizing antibodies showed that the induction of type I IFN by Ox-LDL is mediated by CD36 signaling whereas downregulation of IL-4 is independent of CD36 activation. We further showed that peritoneal macrophages treated with condition medium collected from Ox-LDL treated eosinophils markedly induced the expression of M1 markers such as iNOS, IL6, SOSC3 and TNFα; whereas the condition medium from non-treated eosinophils significantly induced expression of M2 markers like ARG1 and CCL24.
Conclusions
Our data suggest that an atherogenic condition could activate eosinophils and modulate the phenotype of macrophages (from M2 to M1 phenotype), in part, through the CD36 receptor signaling.
Introduction
Lipid accumulation and the ensuing inflammation have been recognized as a major cause of the initiation and progression of atherosclerosis 1. The clinical events resulting from atherosclerosis are directly related to the oxidation of lipids in low-density lipoproteins (LDLs) that become trapped in the extracellular matrix of the subendothelial space 2. These oxidized lipids activate inflammatory signaling pathways that lead to the formation of fatty streak, and ultimately the development of atherosclerotic plaques. While the signaling pathways that are activated by oxidized LDL (Ox-LDL) in macrophages are under intensive investigation, little is known about the effect of this compound on the activation of other inflammatory cells, including eosinophils.
Several lines of evidence suggest that eosinophils may be involved in the pathogenesis of atherosclerosis. For example, prospective studies have consistently shown an association between eosinophil count and an increased risk for future cardiovascular events 3, 4. Eosinophil cationic protein (ECP), a sensitive marker of eosinophil activation, is associated with coronary atherosclerosis 5. Eotaxin, a potent eosinophil chemoattractant and activator 6, 7, is overexpressed in human atherosclerotic lesions 8. Patients with coronary artery disease show higher circulating levels of eotaxin than healthy controls 9, 10. An association between the number of diseased coronary arteries and circulating levels of eotaxin was reported as an indicator of a possible involvement of eosinophils in determining coronary atherosclerotic burden 10. Accordingly, a nonconservative polymorphism in the eotaxin gene 11, together with sequence variants affecting eosinophil count 12, has been associated with an increased risk of myocardial infarction. We have reported that the level of atherosclerosis highly correlates with the level of eotaxin in a murine model of atherosclerosis 13.
To further understand the involvement of eosinophils in atherosclerosis, we investigated the effect of hyperlipidemia, specifically oxidatively modified LDL, on the phenotype of eosinophils and subsequent impact on macrophages. Our data suggest that hyperlipidemia directly modulates the phenotype of eosinophils and subsequently imposes a significant impact on macrophage polarization.
Materials and methods
Mice
All mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). All animal protocols were approved by the Institutional Animal Care and Use Committee at Cedars-Sinai Medical Center. C57BL/6 mice (4–8 weeks old) were used as donors for the culture of bone marrow-derived eosinophils. C57BL/6 mice (10 weeks old) were used for peritoneal macrophage preparation. Mice were euthanized by an isoflurane overdose followed by cervical dislocation.
Culture cells
Eosinophils
Murine eosinophils were cultured from bone marrow harvested from C57BL/6 mice as essentially described 14. Briefly, bone marrow cells were obtained by flushing femurs and tibiae from C57BL/6 mice and they were cultured in IMDM (Gibco, Life Technologies, Grand Island, NY) supplemented with 10% FBS (Hyclone, GE Healthcare Life Sciences, Logan, UT) and 50 μM of 2-ME (Invitrogen, Life Technologies, Grand Island, NY). Stem cell factor and FLT3 ligand (Peprotech, Rocky Hill, NJ) were added to the medium (100 ng/ml each) for the first 4 days. Following this, cells were cultured in IL-5 containing medium (10 ng/ml, Peprotech, Rocky Hill, NJ) for additional 8 days. Every other day, one-half of the medium was replaced with fresh IL-5 containing medium and the cells were transferred to a new plate. Cell density was maintained at 1 X106/ml throughout the culture. At day 10, more than 95% of the cells were Siglec F positive based on flow cytometric analysis (Supplementary Fig. 1). Cells differentiated between 10 to 12 days were used for oxidized LDL (indicated concentration or 75 μg/ml otherwise, Alfa Aesar, Ward Hill, MA) treatment. For the CD36 blocking experiments, cultured eosinophils were incubated with anti-CD36 MAb (10 μg/ml, LifeSpan BioSciences, Inc., Seattle, WA) for 1h followed by Ox-LDL treatment (75 μg/ml). Cells were further incubated for 24 h and harvested for gene expression analysis. To prepare eosinophil conditioned medium for the macrophage polarization experiment, cultured eosinophils (1×107/ml) were first treated with or without Ox-LDL (75 μg/ml) for 24 h, cells were collected, centrifuged, and subsequently suspended with fresh eosinophil medium in order to remove Ox-LDL. Cells were further incubated for 24 h. After 24 h incubation, medium was collected, filtered through a 0.45 μm filter, and used in the macrophage polarization experiment.
Macrophages
Peritoneal macrophages were cultured as described 15, 16. Briefly, thioglycollate medium was injected into C57BL/6 mouse (Jackson labs) peritoneum. After 72 h, cells were harvested, washed, and cultured in the presence of RPMI medium supplemented with 10% bovine serum. For Ox-LDL treatment, the cultured cells were treated with Ox-LDL (100 μg/ml) in the presence of RPM/10% serum for 24 h. For the macrophage polarization experiment, peritoneal macrophages were treated with eosinophil conditioned medium (mixed with fresh macrophage medium at 1:1 ratio) for 24 h along with medium control. After 24 h incubation, macrophage cells were harvested, total RNA extracted and quantitative realtime PCR performed to access M1 and M2 marker’s expression profile.
Flow cytometry analysis
Cultured bone marrow-derived eosinophils from C57 mice were analyzed by flow cytometry. Red blood cells were removed by adding 1X red blood cell lysis buffer to the whole blood sample. Cells were washed once (1XPBS), centrifuged, and re-suspended in staining buffer (1X PBS with 0.5% FBS). Cells were counted using a Beckman – Coulter Z1 Automated Cell Counter. For staining, one million cells were first incubated with CD16/32 FC blocker (BioLegend, San Diego, CA) for 30 min in the dark to block FcγRII/III receptors. Cells were then single stained with Violet 421 conjugated Siglec F antibody (BD biosciences, San Diego, CA) and subjected for flow cytometric analysis. Cells were analyzed with the Cedar Sinai Flow Cytometry Core Facility instrument (Bechman-Coulter, Dako Cytomation CyAn ADP). The results were analyzed using Summit v4.3 software. Information about the antibodies used in this study can be found in Supplementary Table 1.
CD36 knockdown
Cultured bone marrow-derived eosinophils were transfected with scrambled control siRNA and siRNA specific for CD36 (OriGene Technologies, Inc., Rockville, MD) using INTERFERin™ siRNA transfection reagent (Polyplus-transfection SA, Illkirch, France) according to the manufacturer’s instructions. Briefly, siRNA was first added to 200 μl of Opti-MEM serum free medium (Gibco, Life Technologies, Grand Island, NY) and then mixed with 10 μl transfection reagent. The complex was briefly vortexed, incubated at room temperature for 15 min and then added to the cells (2×106 of cultured eosinophil cells in 2 ml complete medium). The final concentration for both control and CD36 siRNA was 20 nM. After 24 h post siRNA transfection, oxidized LDL was added to the cells (75 μg/ml) and incubated for an additional 24 h. Cells were then collected and the total RNA extracted. Subsequently, reverse transcription and real time PCR were performed. Knockdown effect was assessed by measuring CD36 and type I IFN gene expression using quantitative real time PCR.
ELISA assay
To quantitatively measure the concentration of IL-4 and IFNα in Ox-LDL treated or non-treated eosinophil conditioned media, two Elisa assays were performed. IL-4 Elisa was done using IL-4 Mouse ELISA Kit (Abcam, Cambridge, MA) and IFNα ELISA was done using VeriKine™ Mouse IFN Alpha ELISA Kit (PBL Assay Science, Piscataway, NJ). Both assays were performed exactly following the instructions provided by the manufactures.
RNA preparation and quantitative PCR
Total RNA was isolated from cultured cells using RNeasy mini kit (Qiagen, Valencia, CA). First strand cDNA was transcribed from 500 ng of RNA using Superscript RT (Bio-Rad Laboratories, Hercules, CA) following the protocol provided by the supplier. mRNA expression was determined by Eva green–based real time PCR reactions according to the manufacturer’s protocol (Bio-Rad Laboratories, Hercules, CA). The results were calculated using ΔCT method normalized against GAPDH and are presented either in normalized expression or fold change in comparison to control. The primers used in the present study are listed in Supplementary Table 2.
Statistical analysis
Statistical analysis was performed with GraphPad Prism Software (version 6.0.4, GraphPad, La Jolla, California). Data are presented as means ± SD from triplicate data unless otherwise stated.
Results
Ox-LDL selectively and specifically upregulates expression of proinflammatory molecules in eosinophils
Immune cells respond to Ox-LDL by expressing inflammatory cytokines and phenotypic modulation. While there is extensive literature on the effect of Ox-LDL on the regulation of activity of macrophages and resident vascular cells 17, little is known about the role of Ox-LDL on activation of eosinophils. To explore this, we investigated the effect of Ox-LDL on the expression of several cytokines implicated in atherosclerosis. To accomplish this, we cultured eosinophils and determined the effect of Ox-LDL on the expression of a wide array of cytokines using qPCR. We initially evaluated the purity of bone marrow-derived cultured eosinophils by flow cytometry. This showed that >97% of the total cultured cells expressed Siglec F, demonstrating these cells are eosinophils (Supplemental Fig. 1). Immunostaining analysis confirmed this data (not shown).
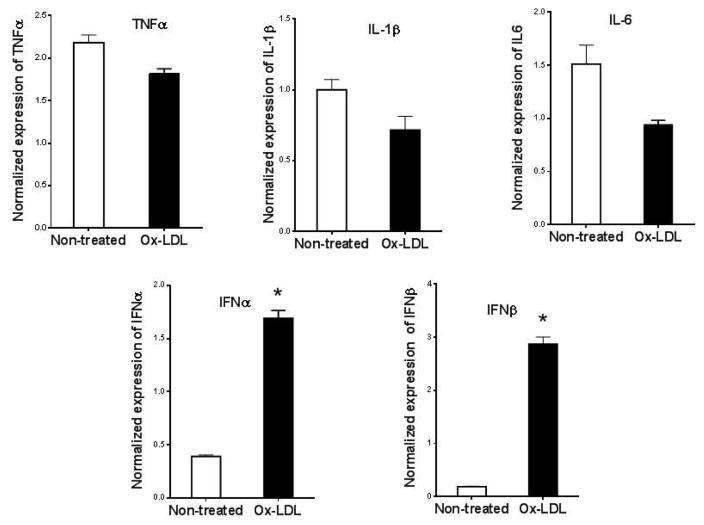
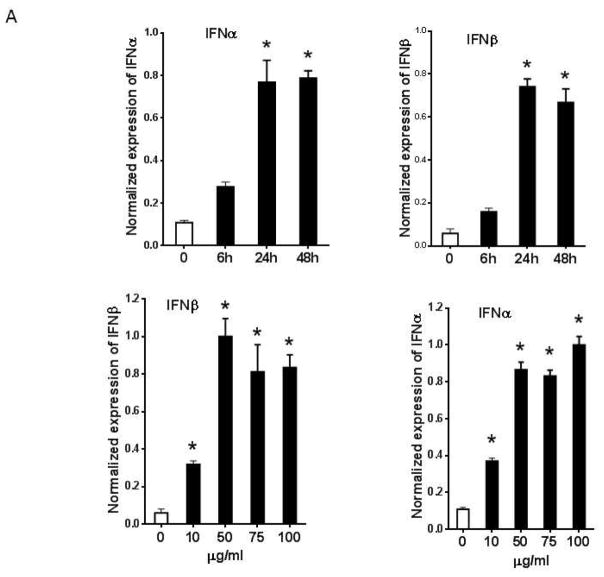
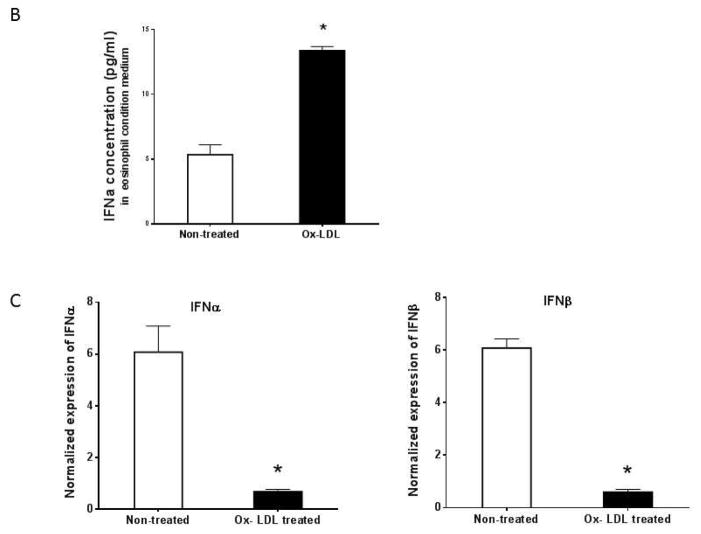
It has previously been shown that oxidized LDL induces proinflammatory gene expression in macrophages, including TNFα, IL-1β, and IL-6 18, 19. We found that cultured eosinophils constitutively expressed low levels of proinflammatory cytokines such as TNFα, IL-1β, IL-6, IFNα, and IFNβ, and addition of Ox-LDL selectively upregulated expression of IFNα and IFNβ (Fig. 1). Further analysis showed that maximum induction of IFNα and IFNβ mRNAs was seen after 24 hr and the level of mRNAs remained elevated for 48 hr following the addition of Ox-LDL (Fig. 2A). Dose-response showed that 50 μg/ml Ox-LDL induced maximum response and the addition of higher concentrations of Ox-LDL did not significantly affect the expression levels of these mRNAs (Fig. 2A). ELISA analysis of cultured eosinophil media showed that IFNα protein is significantly upregulated in response to treatment of eosinophils with Ox-LDL (Fig. 2B).
Fig. 1. Oxidized LDL induces expression of proinflammatory cytokines in eosinophils.
Cultured eosinophils were treated with 75 μg/ml Ox-LDL for 24 h and the expression of indicated cytokines was determined by qPCR using specific primers (Table 1). Representative data are shown from 3 independent experiments. (*) Significant difference.
Fig. 2. Ox-LDL specifically induced expression of type I interferons in eosinophils.
(A) Cultured eosinophils were treated with indicated times and concentrations of Ox-LDL and the expression of IFNα and IFNβ was determined by qPCR. (B) IFNα concentration in Ox-LDL (100 μg/ml for 24 h) treated eosinophil conditioned media was measured by an ELISA assay. (C) Peritoneal macrophage was treated with Ox-LDL (100 μg/ml) for 24 h and the expression of IFNα and IFNβ was evaluated by qPCR. Representative data are shown from 3 independent experiments. (*) Significant difference.
To determine the specificity of Ox-LDL mediated expression of type I interferons in eosinophils, we investigated the expression of the interferons in macrophages in response to Ox-LDL. Cultured peritoneal macrophages were treated with 100 μg/ml of Ox-LDL for 24 h and the expression of type I interferons was evaluated by qPCR. We noted that peritoneal macrophages constitutively expressed high level of IFNα and IFNβ and addition of Ox-LDL markedly suppressed expressions of these interferons (Fig. 1C). These data suggest that the effect of Ox-LDL on the expression of type I interferons in macrophages is opposite to those of eosinophils.
Ox-LDL downregulates expression of anti-inflammatory cytokines
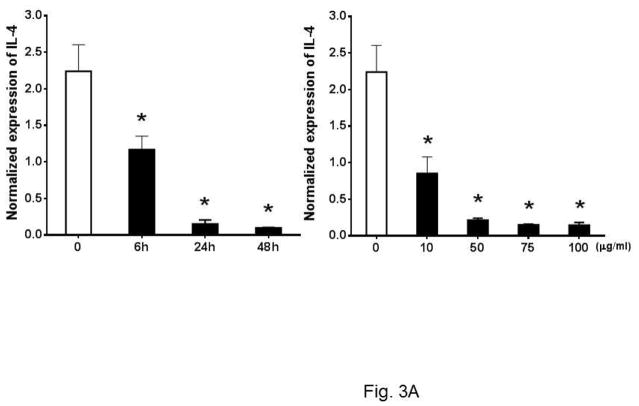
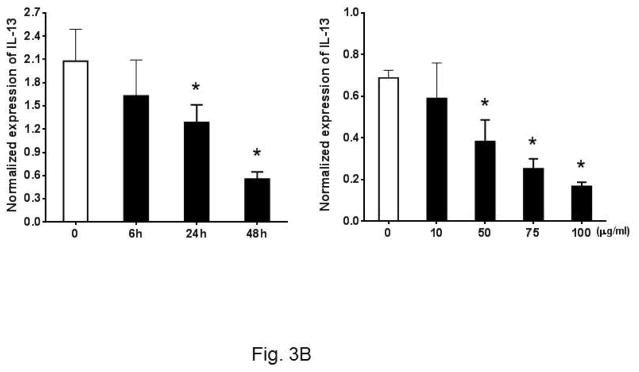
Past studies have shown that eosinophils control the Th2 cell-mediated immune response through expression of IL-4 20, 21. As Th2 response is critical to atherosclerosis, we asked whether Ox-LDL influences the Th2 response of eosinophils. To explore this, we investigated the expression of anti-inflammatory cytokines such as IL-4 and IL-13 in cultured eosinophils in response to Ox-LDL treatment. Under basal conditions, eosinophils constitutively expressed IL-4 and addition of Ox-LDL suppressed the expression of IL-4 in a time and dose-dependent manner (Fig. 3A). In addition, dose response experiments showed that Ox-LDL (10 μg/ml) reduced expression of IL-4 mRNA by more than 50%, indicating that eosinophil expression of IL-4 is highly sensitive to the presence of Ox-LDL (Fig. 3A). Similarly, we noted that eosinophils constitutively expressed IL-13 and the level of gene expression was reduced in response to treatment of cells with Ox-LDL in a time and dose-dependent manner (Fig. 3B). This effect of Ox-LDL in secreted IL-4 reduction was promptly confirmed by an IL-4 ELISA assay. Comparing with none treated control, after 24 h Ox-LDL treatment, IL-4 concentration in eosinophil conditioned medium dropped approximately 4-fold (Fig. 3C). Collectively, these data suggest that Ox-LDL downregulates Th2 response in eosinophils.
Fig. 3. Oxidized LDL downregulates expression of anti-inflammatory cytokines.
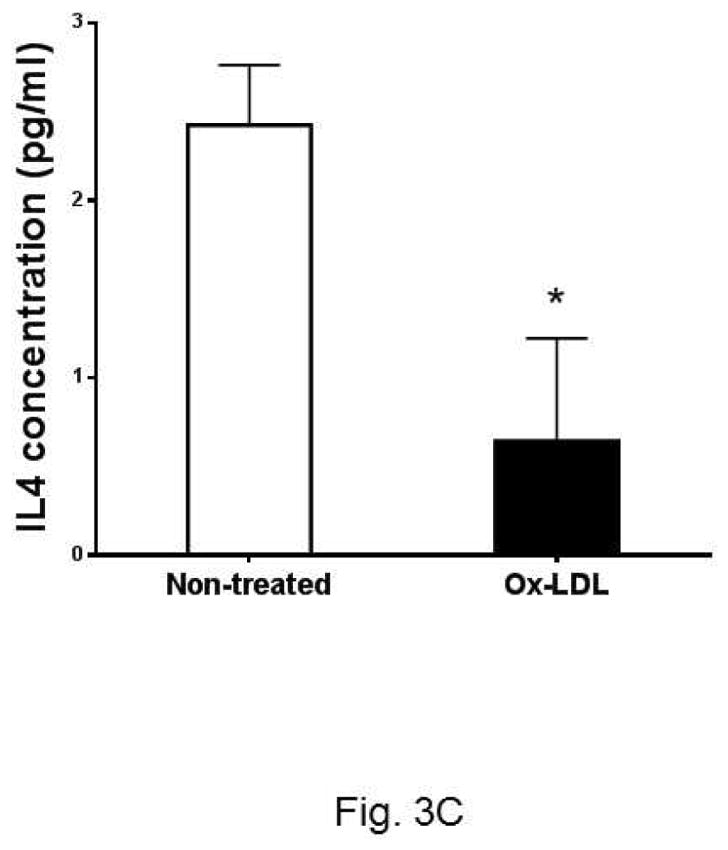
Cultured eosinophils were treated with indicated times and concentrations of Ox-LDL and then harvested at the indicated times for qPCR analysis of (A) IL-4 or (B) IL-13. For the dose response experiment, cultured cells were treated with indicated concentrations of Ox-LDL and then harvested after 24 h for qPCR analysis. (C) An ELISA assay was performed to determine IL-4 concentration in Ox-LDL (100 μg/ml for 24 h) eosinophil conditioned medium. Representative data are shown from 3 (A and B) or 2 (C) independent experiments. (*) Significant difference.
Ox-LDL activates eosinophils through scavenger receptor CD36
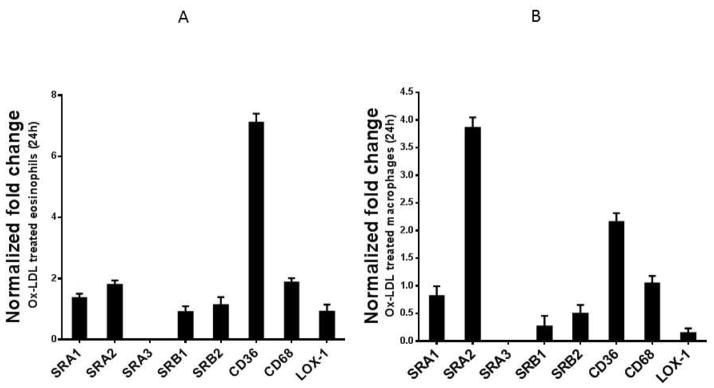
To understand the underlying mechanism by which Ox-LDL modulate the cytokine response in eosinophils, we investigated the expression patterns of scavenger receptors in these cells. Cells interact with modified LDL through multiple scavenger receptors such as SRA1, SRA2, SRB1, CD36, CD68, and Lox-1 2. Little is known about the expression of scavenger receptors in eosinophils. Therefore, we first determined the expression patterns of scavenger receptors in eosinophils under basal conditions. We noted that cultured eosinophils constitutively express high levels of SRB2, CD36, and CD68 receptors, while the expression of SRA1, SRA3, SRB1, and LOX-1 receptors is barely detectable (not shown). When these expression patterns were compared to those of mouse peritoneal macrophages, we noted similarities and differences. The two major macrophage scavenger receptors CD68 and SRB2, constitutively expressed in macrophages, were also expressed in eosinophils (not shown). Treatment of cultured eosinophils with Ox-LDL showed that CD36 is highly and selectively upregulated compared to the control non-treated cells (Fig. 4A). In contrast, analysis of scavenger receptors in macrophages showed that the SRA2 scavenger receptor was selectively and highly upregulated by Ox-LDL (Fig. 4B). Thus, although the expression levels of scavenger receptors in eosinophils were generally less prominent compared with those in macrophages, eosinophil CD36 expression is strongly impacted by Ox-LDL, suggesting that modified LDL may be a natural ligand for CD36 in eosinophils.
Fig. 4. Ox-LDL induces expression of CD36 and CD68 in eosinophils.
Cultured eosinophils were treated with 75 μg/ml Ox-LDL for 24 h and the expression of indicated scavenger receptors was determined by qPCR. For comparison, mouse peritoneal macrophages were cultured, treated with Ox-LDL (100 μg/ml), and the expression of the same scavenger receptor was determined by qPCR. Representative data are shown as the fold change compared with the non-treated control from 3 independent experiments.
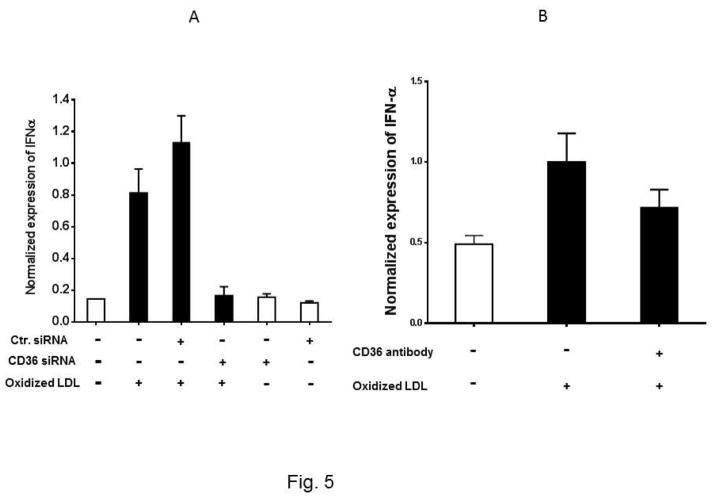
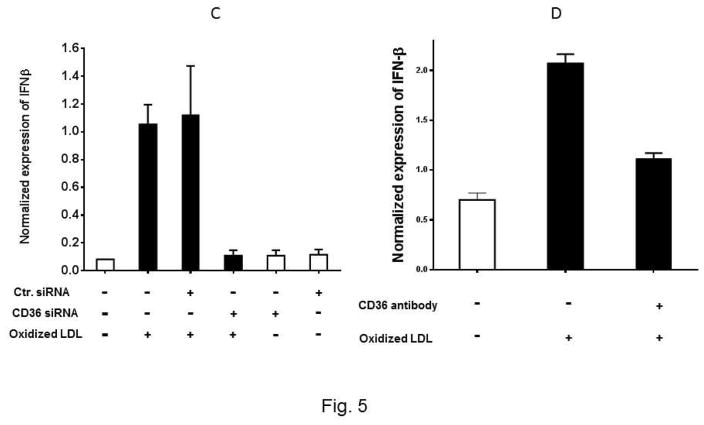
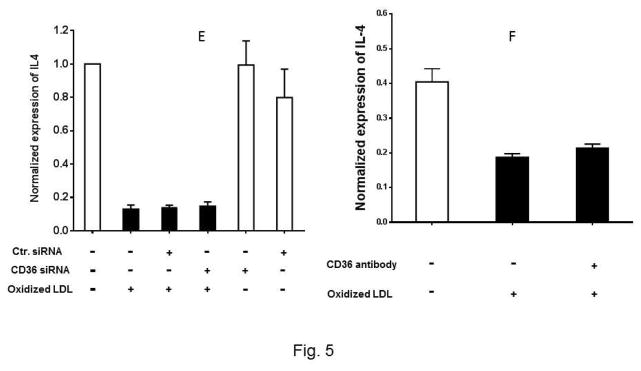
To determine whether the interaction between Ox-LDL and CD36 receptor activates signaling pathways in eosinophils, we first asked whether the expression of Type I interferon is mediated by the Ox-LDL/CD36 axis. We used two approaches to address this question. In the first approach, CD36 gene expression was knocked down using siRNA and then the expression of IFNα, IFNβ, and IL-4 in response to treatment with Ox-LDL was determined. We found that CD36 siRNA markedly and specifically downregulated expression of CD36 in eosinophils by approximately 100% (Supplemental Fig. 2). Treatment of eosinophils with control siRNA prior to addition of Ox-LDL had no significant impact on the markedly increased expression level of IFNα and IFNβ (Fig. 5A and 5C). Treatment of eosinophils with CD36 siRNA completely reduced induction of IFNα and IFNβ by Ox-LDL (Fig. 5A and C). Similar results were obtained with an antibody neutralizing experiment where we noted that while control antibody did not affect expression of IFNα and IFNβ by Ox-LDL, anti-CD36 antibody reduced the effect of Ox-LDL (Fig. 5B and D). In contrast to IFNα and IFNβ, siRNA (Fig. 5E) and antibody (Fig. 5F) studies showed that downregulation of IL-4 by Ox-LDL in eosinophils is independent of CD36 signaling (Fig. 5E and F). Collectively, the presence of scavenger receptors in eosinophils and their activation/signaling by Ox-LDL implies that eosinophils are not only involved in an allergic reaction, but also mediate chronic inflammation through activation of scavenger receptors.
Fig. 5. CD36 signaling mediates expression of IFNα and IFNβ in eosinophils.
Cultured eosinophils were transfected with the indicated control siRNA (Ctr siRNA) or CD36 siRNA for 24 h and then treated with Ox-LDL for an additional 24 h. The qPCR was performed using specific primers to (A) IFNα, (C) IFNβ, (E) IL-4. For the antibody experiments, the cultured cells were treated with anti-CD36 antibody for 1 h prior to the addition of Ox-LDL. The treated cells were harvested after 24 h and the expression of (B) IFNα, (D) IFNβ, and (F) IL-4 was determined by qPCR. Representative data are shown from 3 independent experiments.
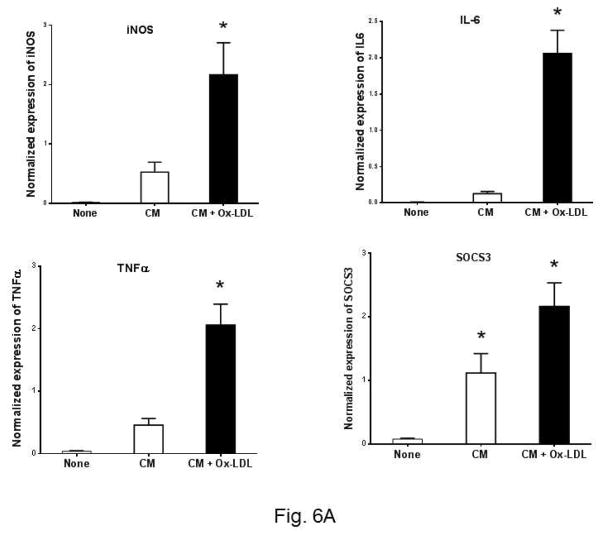
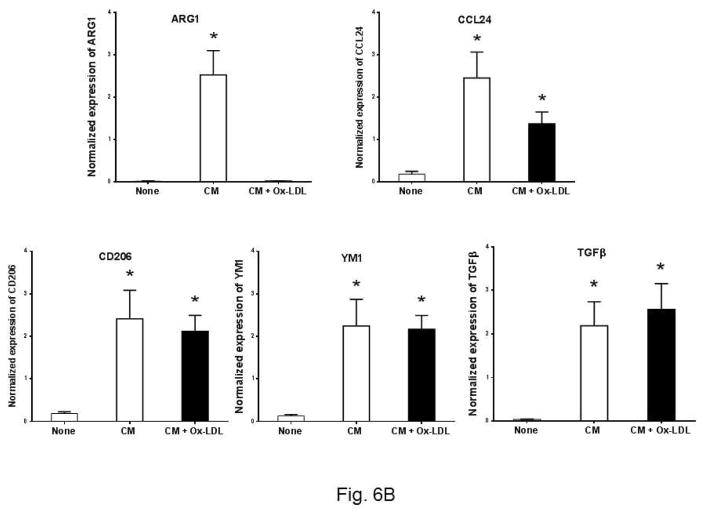
Ox-LDL treated eosinophil conditioned media polarize peritoneal macrophage towards M1 phenotype
The phenotype of macrophages is a critical determinant of atherosclerosis 22. We asked whether Ox-LDL activated eosinophils influence the phenotype of macrophages. To explore this, we used Ox-LDL treated eosinophil condition medium to determine M1/M2 polarization of macrophages. We used expression of iNOs, IL6, SOCS3, and TNFα as a M1 markers, and ARG1, CCL24, CD206, YM1, and TGFβ as M2 markers. Cultured peritoneal macrophages were treated with the eosinophil conditioned media and the expression of the markers was determined by qPCR. Two control conditions were employed. In the first control, cultured media alone were used (Fig. 6A, None). The second control is consisting of the eosinophil conditioned media under basal condition, i.e., in the absence of Ox-LDL (Fig. 6A, CM column). Under basal condition, the expression of the M1 markers was low, if any (Fig. 6A, None column). Addition of eosinophil conditioned media in the absence of Ox-LDL treatment induced low levels of M1 markers (Fig. 6A, blank column). Significant upregulation of M1 markers was detected when cultured macrophages were treated with the conditioned media from Ox-LDL activated eosinophils (Fig. 6A, black column). In contrast, analysis of the M2 markers of macrophage polarization revealed that addition of conditioned media from Ox-LDL activated eosinophils leads to the significant downregulation of M2 polarization of macrophages compared to the control conditioned media (Fig. 6B). The expression of ARG1 is specifically sensitive to the conditioned media treatment as it is completely downregulated (Fig. 6B). Collectively, these data show that exposure of eosinophils to Ox-LDL influence macrophage polarization, promoting a M1 phenotype in the expense of M2 polarization.
Fig. 6. Ox-LDL treated eosinophil conditioned media polarize peritoneal macrophage toward the M1 phenotype.
Peritoneal macrophages were treated for 24 h with condition medium, generated from Ox-LDL treated eosinophils, along with macrophage medium as control. The expression of M1 and M2 markers was determined by qPCR with a panel of marker genes. (A) M1 markers, (B) M2 markers. Representative data are shown from 2 independent experiments. (*) Significant difference.
Discussion
Atherosclerosis is a chronic inflammatory disease triggered by an accumulation of liproproteins, specifically LDL, in the arteries 23. The accumulated lipids undergo oxidation which leads to the activation of inflammatory cells such as macrophages, T cells, and neutrophils 2. Little is known about the role of eosinophils in atherogenesis. We found that treatment of cultured eosinophils with Ox-LDL significantly fostered expression of type I interferons (IFNα and IFNβ). The expression of other proinflammatory cytokines such as TNFα, IL-β, and IL-6 in eosinophils remained unaffected by Ox-LDL suggesting that the expression of IFNα and IFNβ in eosinophils by Ox-LDL is a specific event and is not the result of global activation of proinflammatory cytokines. Moreover, we noted that upregulation of type I interferon by Ox-LDL is specific to eosinophils as macrophages remained unresponsive to this effect of Ox-LDL. In addition, we noted that the expression of IL-4 and IL-13, anti-inflammatory cytokines, is markedly downregulated by Ox-LDL. Together, these data suggest that Ox-LDL treated eosinophil triggered an event that can promote proinflammatory diseases such as atherosclerosis.
This notion is further enforced by the expression pattern of scavenger receptors in eosinophils and the influence of Ox-LDL activated eosinophils on macrophage polarization. We noted that the expression of CD36 scavenger receptors in eosinophils is exquisitely upregulated by Ox-LDL. This receptor was first classified as a scavenger receptor and best studied for its role in sterile inflammation and atherosclerosis 24, 25. It is a highly glycosylated transmembrane protein belonging to the class B scavenger receptor family, and is expressed by several cell types such as adipocytes, erythrocytes, platelets, and endothelial and myeloid cells. It binds to a broad range of endogenous ligands, including proteins like amyloid-β 26, collagen 27, thrombospondin 28, and lipids that include Ox-LDL 29 intact and oxidized phosphatidylserine 30, 31 and long-chain fatty acids 32. Absence of CD36 was found to protect against atherosclerosis in apo E knockout mice 33. Whether the downstream effects of CD36 signaling are pro or anti-inflammatory depends on the nature of the ligand and the accompanying coreceptors. In conjunction with TLR4 and TLR6, CD36-mediated uptake of Ox-LDL results in the formation of intracellular cholesterol crystals, which drive activation of the NLRP3 inflammasome and secretion of the proinflammatory cytokine IL-1β 34, 35.
We noted that expression of IL-1β remained unaffected by Ox-LDL in eosinophils, suggesting that the downstream signaling pathways activated by CD36 in eosinophils are likely different than those of monocytes/macrophages. We have previously shown that toll-like receptor 4 (TLR4) is highly expressed in murine macrophages and its expression is upregulated by Ox-LDL 36. Although both TLR4 and TLR6 are expressed in eosinophils, their expression level is markedly lower in comparison with macrophages where TLR4 is the dominant TLR 37. Thus, the signaling pathways activated by CD36 are dependent on the cell context and are likely different in the eosinophils compared to those in monocytes/macrophages.
We used two approaches to determine whether CD36 is activated in eosinophils in response to Ox-LDL treatment. We found that upregulation of IFNα and IFNβ by Ox-LDL in eosinophils is mediated by CD36 signaling whereas this signaling pathway seems not to be involved in the downregulation of IL-4 by Ox-LDL. Signaling pathways that are activated by CD36 are complex and they involve at least 36 proteins that are consistently tyrosine-phosphorylated in response to CD36 crosslinking 38.
The activation of eosinophils by Ox-LDL may contribute to the pathogenesis of several diseases. For example, type I interferons have been suggested as a marker of cardiovascular disease associated with atherosclerosis development in systemic lupus erythematosus patients 39. IFNβ was found to promote atherosclerosis by enhancing macrophage adhesion to sites of atherosclerotic plaque formation in a chemokine-dependent manner 40. IFNα priming has been shown to foster lipid uptake by macrophages and formation of foam cells 41. We reported that the level of atherosclerosis highly correlates with the level of eosinophil attracting chemokine eotaxin in a murine model of atherosclerosis 13. These data combined with the other studies that correlate eosinophil activation with atherosclerosis described above suggest a link between atherosclerosis and eosinophils.
In conclusion, we found that Ox-LDL is a potent activator of eosinophils. The activation involves selective upregulation of proinflammatory cytokines while downregulating the expression of anti-inflammatory cytokines, such as IL-4. These activities are in part mediated by the CD36 scavenger receptors signaling pathway. In addition, we noted that Ox-LDL activated eosinophils can promote M1 polarization of macrophages while downregulating M2 macrophage phenotype. This indicates that Ox-LDL can activate multiple immune cells that contribute to the pathogenesis of atherosclerosis. The knowledge of molecular pathways that are regulated by Ox-LDL in eosinophils allows a better understanding of the role of leukocytes in atherosclerosis and other chronic inflammatory diseases.
Supplementary Material
Highlights.
Inflammation is associated with atherosclerosis
Eosinophils are activated by hyperlipidemia
CD36 scavenger receptor mediates uptake of ox-LDL by eosinophils
Acknowledgments
The authors gratefully acknowledge the support of the Heart Foundation, The Eisner Foundation, the Spielberg Foundation, Corday Foundation and the Skirball Foundation. The evolution of this project was supported by NHLBI grants R01 HL104068 and NIH R37 A1045898.
Footnotes
Conflict of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg D, Witztum JL. Oxidized Low-Density Lipoprotein and Atherosclerosis. Arteriosclerosis, Thrombosis and Vascular Biology. 2010;30:2311–2316. doi: 10.1161/ATVBAHA.108.179697. [DOI] [PubMed] [Google Scholar]
- 3.Prentice RL, Szatrowski TP, Fujikura T, et al. Leukocyte counts and coronary heart disease in a Japanese cohort. American journal of epidemiology. 1982;116:496–509. doi: 10.1093/oxfordjournals.aje.a113434. [DOI] [PubMed] [Google Scholar]
- 4.Lee CD, Folsom AR, Nieto FJ, et al. White blood cell count and incidence of coronary heart disease and ischemic stroke and mortality from cardiovascular disease in African-American and White men and women: atherosclerosis risk in communities study. American journal of epidemiology. 2001;154:758–764. doi: 10.1093/aje/154.8.758. [DOI] [PubMed] [Google Scholar]
- 5.Niccoli G, Ferrante G, Cosentino N, et al. Eosinophil cationic protein: A new biomarker of coronary atherosclerosis. Atherosclerosis. 2010;211:606–611. doi: 10.1016/j.atherosclerosis.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 6.Graziano FM, Cook EB, Stahl JL. Cytokines, Chemokines, RANTES, and Eotaxin. Allergy and Asthma Proceedings. 1999;20:141–146. doi: 10.2500/108854199778553055. [DOI] [PubMed] [Google Scholar]
- 7.Van Coillie E, Van Damme J, Opdenakker G. The MCP/eotaxin subfamily of CC chemokines. Cytokine & Growth Factor Reviews. 1999;10:61–86. doi: 10.1016/s1359-6101(99)00005-2. [DOI] [PubMed] [Google Scholar]
- 8.Haley KJ, Lilly CM, Yang JH, et al. Overexpression of eotaxin and the CCR3 receptor in human atherosclerosis: using genomic technology to identify a potential novel pathway of vascular inflammation. Circulation. 2000;102:2185–2189. doi: 10.1161/01.cir.102.18.2185. [DOI] [PubMed] [Google Scholar]
- 9.Economou E. Chemokines in patients with ischaemic heart disease and the effect of coronary angioplasty. International journal of cardiology. 2001;80:55–60. doi: 10.1016/s0167-5273(01)00454-5. [DOI] [PubMed] [Google Scholar]
- 10.Emanuele E, Falcone C, D’Angelo A, et al. Association of plasma eotaxin levels with the presence and extent of angiographic coronary artery disease. Atherosclerosis. 2006;186:140–145. doi: 10.1016/j.atherosclerosis.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Zee RYL, Cook NR, Cheng S, et al. Threonine for alanine substitution in the eotaxin (CCL11) gene and the risk of incident myocardial infarction. Atherosclerosis. 2004;175:91–94. doi: 10.1016/j.atherosclerosis.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 12.Gudbjartsson DF, Bjornsdottir US, Halapi E, et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet. 2009;41:342–347. doi: 10.1038/ng.323. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Shah PK, Wang W, et al. Tenascin-C deficiency in apo E−/− mouse increases eotaxin levels: Implications for atherosclerosis. Atherosclerosis. 2013;227:267–274. doi: 10.1016/j.atherosclerosis.2013.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu TX, Lim E-J, Besse JA, et al. miR-223 Deficiency Increases Eosinophil Progenitor Proliferation. The Journal of Immunology. 2013;190:1576–1582. doi: 10.4049/jimmunol.1202897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang l, Song L, Shah PK, et al. Deletion of tenascin gene in apo E mice promotes destabilization of atherosclerotic plaques: The potential role of TN in accumulation of mast cells and over-expression of eotaxin. Circulation. 2007;116:34. [Google Scholar]
- 16.Wang L, Yang M, Arias A, et al. Splenocytes Seed Bone Marrow of Myeloablated Mice: Implication for Atherosclerosis. PLoS ONE. 2015;10:e0125961. doi: 10.1371/journal.pone.0125961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ait-Oufella H, Taleb S, Mallat Z, et al. Recent Advances on the Role of Cytokines in Atherosclerosis. Arteriosclerosis, Thrombosis and Vascular Biology. 2011;31:969–979. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton TA. Oxidized low density lipoprotein suppresses the expression of tumor necrosis factor-alpha mRNA in stimulated murine peritoneal macrophages. The Journal of immunology (1950) 1990;144:2343. [PubMed] [Google Scholar]
- 19.Malden LT. The influence of oxidatively modified low density lipoproteins on expression of platelet-derived growth factor by human monocyte-derived macrophages. The Journal of biological chemistry. 1991;266:13901. [PubMed] [Google Scholar]
- 20.Bjerke T, Gaustadnes M, Nielsen S, et al. Human blood eosinophils produce and secrete interleukin 4. Respiratory Medicine. 1996;90:271–277. doi: 10.1016/s0954-6111(96)90098-0. [DOI] [PubMed] [Google Scholar]
- 21.Piehler D, Stenzel W, Grahnert A, et al. Eosinophils Contribute to IL-4 Production and Shape the T-Helper Cytokine Profile and Inflammatory Response in Pulmonary Cryptococcosis. The American Journal of Pathology. 2011;179:733–744. doi: 10.1016/j.ajpath.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chinetti-Gbaguidi G, Colin S, Staels B. Macrophage subsets in atherosclerosis. Nat Rev Cardiol. 2015;12:10–17. doi: 10.1038/nrcardio.2014.173. [DOI] [PubMed] [Google Scholar]
- 23.Steinberg D. Atherogenesis in perspective: Hypercholesterolemia and inflammation as partners in crime. Nat Med. 2002;8:1211–1217. doi: 10.1038/nm1102-1211. [DOI] [PubMed] [Google Scholar]
- 24.Collot-Teixeira S, Martin J, McDermott-Roe C, et al. CD36 and macrophages in atherosclerosis. 2007:468–477. doi: 10.1016/j.cardiores.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Park YM. CD36, a scavenger receptor implicated in atherosclerosis. Experimental & Molecular Medicine. 2014;46:e99. doi: 10.1038/emm.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coraci IS, Husemann J, Berman JW, et al. CD36, a Class B Scavenger Receptor, Is Expressed on Microglia in Alzheimer’s Disease Brains and Can Mediate Production of Reactive Oxygen Species in Response to β-Amyloid Fibrils. The American Journal of Pathology. 2002;160:101–112. doi: 10.1016/s0002-9440(10)64354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tandon NN, Kralisz U, Jamieson GA. Identification of glycoprotein IV (CD36) as a primary receptor for platelet-collagen adhesion. Journal of Biological Chemistry. 1989;264:7576–7583. [PubMed] [Google Scholar]
- 28.Asch AS, Barnwell J, Silverstein RL, et al. Isolation of the thrombospondin membrane receptor. The Journal of Clinical Investigation. 1987;79:1054–1061. doi: 10.1172/JCI112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Endemann G, Stanton LW, Madden KS, et al. CD36 is a receptor for oxidized low density lipoprotein. Journal of Biological Chemistry. 1993;268:11811–11816. [PubMed] [Google Scholar]
- 30.Rigotti A, Acton SL, Krieger M. The Class B Scavenger Receptors SR-BI and CD36 Are Receptors for Anionic Phospholipids. Journal of Biological Chemistry. 1995;270:16221–16224. doi: 10.1074/jbc.270.27.16221. [DOI] [PubMed] [Google Scholar]
- 31.Greenberg ME, Sun M, Zhang R, et al. Oxidized phosphatidylserine–CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. The Journal of Experimental Medicine. 2006;203:2613–2625. doi: 10.1084/jem.20060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abumrad NA, el-Maghrabi MR, Amri EZ, et al. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. Journal of Biological Chemistry. 1993;268:17665–17668. [PubMed] [Google Scholar]
- 33.Kuchibhotla S, Vanegas D, Kennedy DJ, et al. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. 2008:185–196. doi: 10.1093/cvr/cvm093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheedy FJ, Grebe A, Rayner KJ, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14:812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart CR, Stuart LM, Wilkinson K, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu XH, Shah PK, Faure E, et al. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001;104:3103–3108. doi: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- 37.Nagase H, Okugawa S, Ota Y, et al. Expression and Function of Toll-Like Receptors in Eosinophils: Activation by Toll-Like Receptor 7 Ligand. The Journal of Immunology. 2003;171:3977–3982. doi: 10.4049/jimmunol.171.8.3977. [DOI] [PubMed] [Google Scholar]
- 38.Heit B, Kim H, Cosío G, et al. Multimolecular Signaling Complexes Enable Syk-Mediated Signaling of CD36 Internalization. Developmental Cell. 2013;24:372–383. doi: 10.1016/j.devcel.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Somers EC, Zhao W, Lewis EE, et al. Type I Interferons Are Associated with Subclinical Markers of Cardiovascular Disease in a Cohort of Systemic Lupus Erythematosus Patients. PLoS ONE. 2012;7:e37000. doi: 10.1371/journal.pone.0037000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goossens P, Gijbels MJJ, Zernecke A, et al. Myeloid Type I Interferon Signaling Promotes Atherosclerosis by Stimulating Macrophage Recruitment to Lesions. Cell Metabolism. 2010;12:142–153. doi: 10.1016/j.cmet.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Fu Q, Cui H, et al. Interferon-α priming promotes lipid uptake and macrophage-derived foam cell formation: A novel link between interferon-α and atherosclerosis in lupus. Arthritis & Rheumatism. 2011;63:492–502. doi: 10.1002/art.30165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.