Abstract
Background and aims
There is strong evidence that fat accumulating in non-adipose sites, “ectopic fat”, is associated with cardiovascular disease (CVD), including vascular calcification. Most previous studies of this association have assessed only a single ectopic fat depot. Therefore, our aim was to assess the association of total, regional, and ectopic fat with abdominal aortoilliac calcification (AAC) and coronary artery calcification (CAC) in 798 African ancestry men.
Methods
Participants (mean age 62) were from the Tobago Bone Health Study cohort. Adiposity was assessed via clinical examination, dual x-ray absorptiometry, and computed tomography (CT). Ectopic fat depots included: abdominal visceral adipose tissue (VAT), liver attenuation, and calf intermuscular adipose tissue (IMAT). Vascular calcification was assessed by CT and quantified as present versus absent. Associations were tested using multiple logistic regression adjusted for traditional cardiovascular risk factors. Models of ectopic fat were additionally adjusted for total body fat and standing height.
Results
All adiposity measures, except VAT, were associated with AAC. Lower liver attenuation or greater calf IMAT was associated with 1.2–1.3-fold increased odds of AAC (p < 0.03 for both), though calf IMAT was a stronger predictor than liver attenuation (p < 0.001) when entered in a single model. No ectopic fat measure was associated with CAC.
Conclusions
Greater adiposity in the skeletal muscle and liver, but not in the visceral compartment, was associated with increased odds of AAC in African ancestry men. These results highlight the potential importance of both quantity and location of adiposity accumulation throughout the body.
Keywords: Ectopic fat, Vascular calcification, African ancestry, Computed tomography
1. Introduction
There is strong evidence that fat accumulating in non-adipose sites, “ectopic fat”, is associated with cardiometabolic disease, including cardiovascular disease (CVD; [1]). While there have been previous studies of the association between ectopic adipose tissue and vascular calcification [2–23], a marker of subclinical CVD, many studies assessed only a single depot. The existing research has also primarily focused on Caucasians and may not be applicable to other races because of known racial/ethnic differences in body composition [24,25] and cardiovascular risk [26]. Specifically, African ancestry men are known to be generally lean, but have higher cardiovascular disease risk compared to Caucasians [26]. We hypothesize that differences in ectopic fat distribution may be one explanation for this apparent paradox. Indeed, we recently reported that intermuscular adipose tissue (IMAT) in the calf muscle, is associated with increased risk of diabetes [27], hypertension [28], and mortality [29], independent of total and central adiposity in African ancestry men. Therefore, we hypothesize that IMAT may be an ectopic fat depot of particular importance for vascular calcification, as well, in African ancestry men. In the current study, we tested if whole body, regional, and ectopic fat measures were associated with vascular calcification in the coronary and abdominal aortoilliac arteries in a sample of 798 African ancestry men.
2. Materials and methods
2.1. Tobago Health Study
All men in this analysis were from the Tobago Bone Health Study (TBHS), a population-based, prospective cohort study of community-dwelling men aged 40 years and older, residing on the Caribbean island of Tobago [30]. Participants in the TBHS were recruited without regard to health status and men were eligible if they were ambulatory, not terminally ill and without a bilateral hip replacement. Men from Tobago are of homogeneous African ancestry with low European admixture (<6%) [31]. The 798 men for the current analysis were recruited consecutively at follow-up visits completed between 2011 and 2016, which consisted of chest, abdominal and calf computed tomography (CT) scans, total body dual x-ray absorptiometry (DXA), clinical examination and interviewer-administered health history questionnaires. Written informed consent was obtained from each participant using forms and procedures approved by the University of Pittsburgh Institutional Review Board, the U.S. Surgeon General’s Human Use Review Board, and the Tobago Division of Health and Social Services Institutional Review Board.
2.2. Vascular calcification
Vascular calcification was assessed by chest and central CT using a dual slice, high-speed NX/I scanner (GE Medical Systems, Waukesha, WI). The scans were obtained using the axial, two-slice scan mode (2i) and a segmented (a.k.a “half-scan”) reconstruction to provide an effective temporal resolution of approximately 350 msec for each 3 mm thick slice without cardiac gating. Coronary artery calcification (CAC) values were obtained from cross-sectional slices through the chest from the carina through the entire inferior aspect of the heart and measurements made by vessel for each of the major epicardial coronary arteries. For the abdominal scan series, a helical scan mode (120 KVp, 250 mA, 3 mm slice thickness and pitch of 1.5:1) was utilized since the higher temporal resolution for the coronary arteries was not required. Aortic artery calcification (AAC) values were obtained from cross-sectional slices through the abdomen from L3 to S1 and included the summation of calcification in the abdominal aorta and common iliac arteries. Measurements were performed by experienced analysts using an FDA approved computer workstation and software (Calcium, Aquarius workstation, TeraRecon San Mateo, CA) that accounts for slice thickness and scan field of view. The Agatston method [32] was used to report scores of calcified plaque. The lead reader for this study also led the Coronary Artery Risk Development In young Adults (CARDIA) Study CT analyses, and a blinded re-read of 153 CARDIA scans found intra-reader technical error of 6.6%.
For the current analysis, an Agatston score >10 defined presence of CAC or AAC to further reduce false positive classification [33]. We also calculated the Agatston to volume ratio (AVR), which is a measure of calcified plaque density that takes into account both the total volume of plaque and the amount of calcification, using the recent methods described by Bellasi et al. [34].
2.3. Adiposity assessment
Adiposity was measured using clinical examination (body weight, body mass index (BMI), and waist circumference), whole body DXA (total percent fat and percent fat in the trunk), abdominal CT [abdominal subcutaneous adipose tissue (SAT), abdominal visceral adipose tissue (VAT) and liver attenuation], and peripheral quantitative CT [calf intermuscular adipose tissue (IMAT)]. Body weight was measured to the nearest 0.1 kg on a balance beam scale and standing height was measured to the nearest 0.1 cm using a wall-mounted stadiometer, both without participants wearing shoes. Body mass index was calculated as body weight in kilograms divided by standing height in meters squared. Waist circumference was measured at the level of the umbilicus or greatest circumference using a flexible tape measure. Total body fat percent and percent fat in the trunk were measured via DXA using a Hologic QDR 4500 W densitometer (Hologic, Bedford, MA).
Abdominal adiposity was assessed using the CT scans collected to measure vascular calcification as discussed above. Measures of abdominal SAT and VAT were obtained from the same cross-sectional images that were used for AAC assessment from L3 to S1. Liver attenuation was assessed in Hounsfield units (HU) from 3 contiguous scan slices taken in the T12 to L1 space. Calf IMAT was measured via peripheral quantitative CT performed with a Stratec XCT-2000 scanner (Orthometrix, Inc.; White Plains, NY). A site at 66% of the calf length, proximal to the terminal end of the tibia was scanned, since it has the largest circumference and the lowest variability in composition between individuals [35]. All images were analyzed with STRATEC analysis software version 5.5D (Orthometrix, Inc., White Plains, NY) and performed by a trained investigator who was unaware of the participant’s vascular calcification status. For this analysis, ectopic fat was assessed using abdominal VAT, liver attenuation and calf IMAT; whereas, all other measures were considered to be measures of general or regional adiposity.
2.4. Other characteristics
Demographic, health history and anthropomorphic characteristics were assessed by trained staff via interview and clinical exams. Blood pressure was measured three times while seated and the average of the 2nd and 3rd reading were used in this analysis. Hypertension was defined as a systolic blood pressure (SBP) ≥ 140 mmHg, diastolic blood pressure (DBP) ≥ 90 mmHg, current self-reported use of antihypertensive medication or an affirmative to the question “has a doctor ever told you that you have hypertension or high blood pressure?” Fasting blood was drawn at the time of interview and was spun into serum samples and aliquoted and frozen at −80 °C on the day of blood draw. Fasting serum glucose was measured using an enzymatic procedure and fasting serum insulin was measured using a radioimmunoassay procedure developed by Linco Research, Inc. Glucose is presented in mg/dL units that can be converted to SI units by dividing by 18. Insulin is presented in μU/mL units that can be converted to SI units by multiplying by 6.945. The degree of insulin resistance was estimated by homeostasis model assessment (HOMA-IR) according to the method described by Matthews et al. [36]. Diabetes was defined as a fasting serum glucose level ≥ 126 mg/dL when fasting measures were available (N = 711), and/or current self-reported use of diabetes medication, or an affirmative response to the question “has a doctor ever told you that you have diabetes?” Serum high-density lipoprotein cholesterol (HDL-c) was determined using the selective heparin/manganese chloride precipitation method. Serum low-density lipoprotein cholesterol (LDL-c) was calculated by means of the Friedewald equation. Triglycerides were determined enzymatically using the procedure of Bucolo and David [37]. Dyslipidemia was defined as having HDL-c <40 mg/dL or triglycerides >150 mg/dL when fasting measures were available (N = 686), and/ or current self-reported use of statins or other lipid-lowering medications.
Smoking status was classified both as either current or not, or ever or not wherein participants reporting smoking <100 cigarettes in their lifetime were considered never-smokers. Alcohol consumption is very limited in this cohort and was coded as consuming ≥4 drinks per week (yes/no) to identify individuals with greater than average cohort alcohol intake. As typical modes of physical activity and inactivity are not easy to capture using standardized questionnaires in this cohort, we have determined that using TV watching as a surrogate for sedentary behavior is most powerful in distinguishing active versus inactive participants in the TBHS. Therefore, we modeled sedentary behavior as watching <14 h of TV per week versus <14 h of TV per week.
2.5. Statistical analysis
Differences in means or frequencies of covariates and adiposity measures by CAC or AAC presence were tested by chi-squared test or T-test, as appropriate. Multiple logistic regression was used to evaluate the association of adiposity (independent variable) with prevalent CAC or AAC (dependent variables) after adjustment for traditional cardiovascular risk factors including age, hypertension, diabetes, dyslipidemia, smoking, alcohol intake and sedentary lifestyle. Models of ectopic fat, including abdominal VAT, liver attenuation and calf IMAT, were also adjusted for total body percent fat and standing height. In a secondary analysis of men with all biochemical measures available (N = 686), we included adjustment for HOMA-IR instead of diabetes, and LDL-c, HDL-c, and triglycerides instead of dyslipidemia. We expressed odds ratios from all models per 1 standard deviation increase in adiposity measure. Liver attenuation was modeled per 1 SD decrease because a lower level of this measure indicates greater fat infiltration in the liver. In order to handle the skewed distribution and high proportion of zeros in models of AAC and CAC AVR, we used multiple tobit regression with a lower bound of 0 on the natural logarithm of AVR. All tobit models were adjusted for the same factors as the logistic models of prevalent calcification.
In order to assess the combined association of liver attenuation and calf IMAT, we categorized men by tertile of each measure and then created groups as follows: highest tertile of liver attenuation and lowest tertile of calf IMAT (N = 113; AAC cases = 55; CAC cases = 29), lowest tertile of liver attenuation and lowest tertile of calf IMAT (N = 54; AAC cases = 30; CAC cases = 13), highest tertile of liver attenuation and highest tertile of calf IMAT (N = 64; AAC cases = 48; CAC cases = 29), and lowest tertile of liver attenuation and highest tertile of calf IMAT (N = 109; AAC cases = 85; CAC cases = 37). Then we tested the odds of having prevalent AAC or CAC for each group using the group of high liver attenuation and low calf IMAT, i.e. those with the least fat in the liver and calf, as the reference group in multiple logistic regression adjusting for covariates as previously described. We also constructed a single variable to test for linear trend based on these tertile groupings: highest liver, lowest calf = 0; lowest liver, lowest calf = 1; highest liver, highest calf = 2; and lowest liver, highest calf = 3. We then tested if this constructed variable was linearly associated with the odds of prevalent AAC or CAC using multiple logistic regression adjusting for covariates as before.
3. Results
3.1. Participant characteristics
Men were aged 62 years on average, 67% were hypertensive, 20% were diabetic, 36% were dyslipidemic, and 9% were smokers (Table 1). AAC and CAC were present in 63% and 29% of men, respectively. Presence of AAC or CAC was associated with increasing age, hypertension, and diabetes, but only AAC was associated with smoking (all p < 0.05). On average, men were overweight (mean BMI = 27.7 kg/m2) and had 24% total body fat (Table 2). In age-adjusted analyses, all general, regional and ectopic fat measures were associated with AAC (all p < 0.05). However, presence of CAC was only associated with BMI, waist circumference, and trunk percent fat (all p < 0.05).
Table 1.
Characteristics of the African ancestry men by calcification status.
| Characteristics | Overall (N = 798) | AAC absent (N = 292) | AAC present (N = 506) | CAC absent (N = 567) | CAC present (N = 231) |
|---|---|---|---|---|---|
| Age (years) | 62.0 ± 8.6 | 57.7 ± 6.3 | 64.1 ± 8.7 | 60.0 ± 9.6 | 66.7 ± 9.6 |
| Hypertension (%) | 66.7 | 54.0 | 73.4 | 61.4 | 80.0 |
| Diabetes (%) | 20.1 | 11.1 | 25.2 | 17.6 | 26.1 |
| Fasting glucose (mg/dL)a | 103.5 ± 41.2 | 96.2 ± 34.3 | 107.6 ± 44.3 | 100.1 ± 40.0 | 111.5 ± 43.0 |
| Fasting insulin (μU/mL)a | 15.0 ± 10.6 | 13.8 ± 7.7 | 15.8 ± 11.9 | 14.8 ± 10.8 | 15.7 ± 10.0 |
| HOMA-IRa | 4.1 ± 4.7 | 3.4 ± 3.0 | 4.5 ± 5.4 | 3.9 ± 5.1 | 4.4 ± 3.5 |
| Dyslipidemia (%) | 36.0 | 32.9 | 38.3 | 34.5 | 40.1 |
| LDL-cholesterol (mg/dL)b | 130.1 ± 38.6 | 125.5 ± 32.3 | 132.8 ± 40.2 | 129.1 ± 38.5 | 132.5 ± 38.7 |
| HDL-cholesterol (mg/dL)b | 46.2 ± 11.4 | 47.9 ± 11.6 | 45.2 ± 11.1 | 46.6 ± 10.9 | 45.3 ± 12.4 |
| Triglycerides (mg/dL)b | 109.0 ± 61.3 | 106.1 ± 62.3 | 110.8 ± 58.9 | 106.3 ± 59.6 | 116.7 ± 65.0 |
| Current smoking (%) | 8.9 | 4.5 | 11.5 | 8.5 | 10.0 |
| ≥4 drinks/week (%) | 12.5 | 11.5 | 13.5 | 11.6 | 14.4 |
| ≥14 h TV/week (%) | 51.3 | 52.5 | 50.5 | 50.5 | 52.9 |
Characteristics shown as mean ± SD or percent, as appropriate.
Bold indicates significant difference by calcification status (p < 0.05). A log transformation was used to assess significance for glucose, insulin, HOMA-IR, and triglycerides models.
AAC, abdominal aortoilliac artery calcification; CAC, coronary artery calcification.
Measures of glucose, insulin, and HOMA-IR were only available in a subset of N = 711 (AAC no/yes: 261/450; CAC no/yes: 503/208).
Measures of lipoprotein cholesterols and triglycerides were only available in a subset of N = 686 (AAC no/yes: 255/431; CAC no/yes: 485/201).
Table 2.
Adiposity measures in the African ancestry men by calcification status.
| Adiposity measure | Unadjusted | Age-adjusted | |||
|---|---|---|---|---|---|
|
|
|
||||
| Overall | AAC absent | AAC present | CAC absent | CAC present | |
| Body weight (kg) | 85.8 ± 14.9 | 82.8 (0.9) | 87.7 (0.7) | 85.3 (0.6) | 86.9 (1.0) |
| BMI (kg/m2) | 27.7 ± 4.4 | 26.6 (0.3) | 28.3 (0.2) | 27.4 (0.2) | 28.4 (0.3) |
| Total body fat (%) | 24.0 ± 6.5 | 23.2 (0.4) | 24.4 (0.3) | 23.8 (0.3) | 24.4 (0.4) |
| Waist circumference (cm) | 98.3 ± 12.5 | 95.9 (0.8) | 99.7 (0.6) | 97.4 (0.5) | 100.4 (0.9) |
| Trunk fat (%) | 50.5 ± 5.1 | 49.6 (0.3) | 51.1 (0.2) | 50.2 (0.2) | 51.1 (0.4) |
| Abdominal SAT (cm3) | 198.8 ± 96.6 | 181.4 (5.9) | 209.1 (4.4) | 195.1 (4.2) | 207.9 (6.8) |
| Abdominal VAT (cm3) | 104.1 ± 59.1 | 95.4 (3.6) | 109.5 (2.7) | 101.8 (2.5) | 109.9 (4.1) |
| Liver attenuation (HU) | 57.7 ± 7.9 | 59.0 (0.5) | 56.8 (0.4) | 57.7 (0.3) | 57.6 (0.5) |
| Calf IMAT (mm2) | 300.1 ± 324.8 | 255.3 (20.2) | 324.1 (15.1) | 286.9 (14.1) | 331.3 (22.4) |
Characteristics are shown as unadjusted mean ± SD for overall measures, and as age-adjusted mean (SE) by prevalent AAC or CAC group.
Bold indicates significant difference by calcification status after adjustment for age (p < 0.05).
SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; IMAT, intermuscular adipose tissue.
3.2. Association of adiposity measures with vascular calcification
After adjusting for traditional CVD risk factors, all general and regional adiposity measures were associated with odds of prevalent AAC (all p < 0.05; Table 3). For instance, each 1 SD (4.4 kg/m2) increase in BMI was associated with 1.5-fold increased odds of AAC (95% CI: 1.3–1.9). In addition, after adjusting for total body fat and standing height, ectopic fat measures including liver attenuation (OR (95% CI) per 1 SD (8 HU) decrease: 1.2 (1.0–1.5)) and calf IMAT (OR (95% CI) per 1 SD (325 mm2) increase: 1.3 (1.1–1.6)) were associated with increased odds of AAC. However, only BMI and waist circumference were associated with increased odds of CAC (OR (95% CI) per 1 SD increase: 1.2 (1.0–1.5) for both). Abdominal VAT was not associated with odds of AAC or CAC. Results for ectopic fat measures were similar in secondary analyses adjusting for HOMA-IR instead of diabetes, and continuous measures of LDL-c, HDL-c, and triglycerides instead of dyslipidemia (subset of men with blood biomarkers available N = 686; Supplemental Table 1).
Table 3.
Multivariablea adjusted odds of prevalent calcification per 1 SD increase in adiposity measure.
| Adiposity measure | AAC | CAC | ||
|---|---|---|---|---|
|
|
|
|||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Body weight (kg) | 1.41 (1.17–1.69) | <0.001 | 1.05 (0.88–1.26) | 0.596 |
| BMI (kg/m2) | 1.54 (1.27–1.87) | <0.001 | 1.21 (1.01–1.45) | 0.041 |
| Total body fat (%) | 1.23 (1.03–1.48) | 0.026 | 1.08 (0.90–1.30) | 0.413 |
| Waist circumference (cm) | 1.34 (1.12–1.61) | 0.002 | 1.24 (1.03–1.48) | 0.022 |
| Trunk fat (%) | 1.25 (1.04–1.50) | 0.019 | 1.09 (0.91–1.31) | 0.345 |
| Abdominal SAT (cm3) | 1.28 (1.08–1.53) | 0.006 | 1.09 (0.91–1.32) | 0.355 |
| Abdominal VAT (cm3) | 1.17 (0.90–1.52) | 0.245 | 1.07 (0.84–1.38) | 0.582 |
| Liver attenuation (HU)b | 1.24 (1.02–1.50) | 0.029 | 0.98 (0.82–1.19) | 0.868 |
| Calf IMAT (mm2) | 1.32 (1.09–1.60) | 0.005 | 1.11 (0.92–1.35) | 0.284 |
Bold indicates p < 0.05.
All adiposity models were run independently from each other and adjusted for age, hypertension, diabetes, dyslipidemia, smoking, alcohol intake, and sedentary lifestyle. In addition, ectopic fat models for abdominal VAT, liver attenuation and calf IMAT were also adjusted for total percent body fat and standing height.
Per 1 SD decrease in liver attenuation.
Similar associations were seen when modeling the effect of adiposity measures on linear vascular calcification AVR in fully adjusted analyses (data not shown). For example, a 1 SD increase in BMI was associated with 13% greater AAC AVR (p < 0.001) and a 1 SD increase in calf IMAT was associated with 11% greater AAC AVR (p < 0.001). The only measure not associated with AAC AVR was abdominal VAT. Only waist circumference was significantly associated with CAC AVR (1 SD = 13% greater AVR; p = 0.03).
3.3. Association of low versus high liver and skeletal muscle fat with vascular calcification
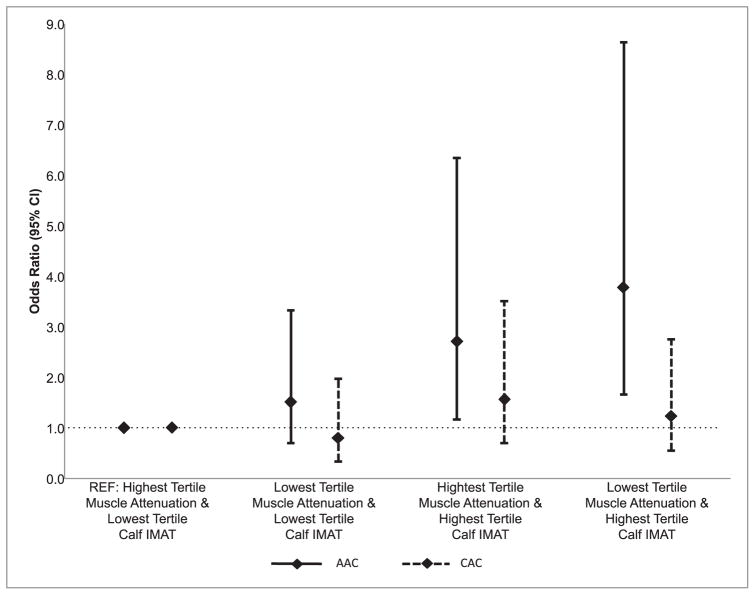
In fully adjusted analyses, men in the highest tertile of calf IMAT had significantly greater odds of AAC regardless of if they were in the highest or lowest tertile of liver attenuation (p < 0.05 for both; Figure). However, men in the lowest tertile for liver attenuation, i.e. those with the most liver fat, only had significantly greater odds of AAC when they were also in the highest tertile for calf IMAT (p < 0.05). Importantly, men had nearly 4-fold increased odds of AAC (OR (95% CI): 3.77 (1.65–8.63)) if they were in the highest tertile of calf IMAT and the lowest tertile of liver attenuation compared to the reference group (highest tertile of liver attenuation and lowest tertile of calf IMAT; Figure). The effect sizes of these relationships were consistent with a linear trend (p = 0.001; Fig. 1), but there was no evidence of a statistical interaction between liver attenuation and calf IMAT (p = 0.78). Ectopic fat tertile groupings were neither individually associated, nor was there evidence for a linear trend, with CAC.
Fig. 1.
Multivariable adjusted odds of prevalent calcification by low versus high liver and skelet al muscle fat groups.
Men were separated into groups based on tertile distributions of liver attenuation and calf IMAT, and categorized as either lowest or highest tertiles (middle tertiles were excluded). Shown are the OR and 95% confidence intervals for prevalent AAC (solid line) or CAC (dashed line) for each group, in reference to men in the tertile with the lowest amount of fat in both the liver and calf (OR = 1). The test for linear trend was constructed as: highest tertile of liver attenuation and lowest tertile of calf IMAT = 0a (reference group); lowest tertile of liver attenuation and lowest tertile of calf IMAT = 1b; highest tertile of liver attenuation and highest tertile of calf IMAT = 2c; lowest tertile of liver attenuation and highest tertile of calf IMAT = 3d. All analyses were adjusted for age, hypertension, dyslipidemia, diabetes, smoking, alcohol intake, sedentary lifestyle, total percent body fat, and standing height.
aN = 113; AAC cases = 55; CAC cases = 29. bN = 54; AAC cases = 30; CAC cases = 13. cN = 64; AAC cases = 48; CAC cases = 29. dN = 109; AAC cases = 85; CAC cases = 37.
4. Discussion
This is the first study to test the association of ectopic fat in multiple anatomical depots with vascular calcification. In this sample of African ancestry men, we found that fat accumulating in the skeletal muscle or liver is associated with greater prevalence of abdominal aorto-illiac artery calcification independent of total body adiposity and other CVD risk factors, including insulin resistance and serum lipoprotein cholesterol levels. This is in sharp contrast to results for abdominal VAT, the most commonly studied ectopic fat depot [2,9,11,12,14], which was not associated with calcification in our analyses. In looking at the combined effect of calf IMAT and liver attenuation, having more calf IMAT was associated with greater odds of AAC regardless of liver attenuation level, suggesting that IMAT is the stronger ectopic fat predictor of prevalent AAC. Measures of central (BMI and total body fat) and regional (waist circumference, total body fat, and abdominal SAT) adiposity were also associated with vascular calcification, highlighting the link between adiposity and cardiovascular disease, as has long been cited [38]. However, the novel findings from this study suggest that the location of fat, in addition to the quantity, may be an important consideration when determining the cardiovascular impact of adiposity.
While this is the first study to compare the association of these specific ectopic fat depots with vascular calcification, there are two previous similar studies conducted in African ancestry participants [3,6]. The first study was conducted in 422 men and women from the Diabetes Heart Study [3]. In fully adjusted models, they found that while pericardial fat was associated with CAC, abdominal IMAT and VAT were not. In the same cohort, they later reported that liver attenuation was not significantly associated with vascular calcification [7]. A similar report from the Jackson Heart Study included 2884 men and women with measures of abdominal VAT and liver attenuation only [6]. They found that liver attenuation, but not VAT, was associated with coronary, but not aortic, artery calcification. These studies, and others [12,16–19], agree with our current findings that other measures of adiposity may be more strongly associated with vascular calcification than abdominal VAT or total adiposity. However, there is some inconsistency with other published reports, which have found significant association between abdominal VAT and vascular calcification [2,4,11,13,14]. Future studies will be needed to tease out the joint association of total body ectopic fat stores on vascular disease.
Our current study suggests that skeletal muscle adiposity may be a novel ectopic fat depot associated with vascular disease measures. To our knowledge, there are only two previous reports that tested the association of IMAT with vascular calcification [3,4] and found no association independent of total adiposity. However, both studies used measures of abdominal muscle IMAT, rather than skeletal muscle IMAT. It is possible that fat found in skeletal muscle represents a distinct physiologic environment from fat found in the abdominal muscles. In addition, African ancestry men appear to have greater stores of skeletal muscle adiposity than Caucasian men even though they are leaner and have less abdominal VAT [39,40]. The mechanisms underlying these differences are unknown, but have been hypothesized to be due to differences in fat oxidation [41] and lipolysis [42], differences in fatty acid uptake and transporting proteins in adipocytes [43], and/or differences in the expression of type II muscle fibers [44,45]. Regardless of the cause, the higher level of skeletal muscle fat in African ancestry men may partly explain the strength of the calf IMAT results in the current study.
Greater liver attenuation was also associated with increased odds of AAC, independent of total adiposity and other CVD risk factors. There are inconsistent results from previous studies of fatty infiltration in the liver and vascular calcification [8,10,13,15,23], which examined liver attenuation with or without consideration of abdominal VAT. Some studies found greater fat accumulation in the liver to be significantly associated with greater vascular calcification [8,15], which aligns with our results. However, others found that this association was attenuated by the addition of obesity into the models [10,13,23]. No previous study of liver fat included consideration for skeletal muscle adiposity; therefore, this is the first study to test and show that, while liver fat is associated with vascular calcification, calf IMAT may be a stronger predictor of vascular calcification. It is important to note, however, that there was low variation in liver attenuation in our sample, and only 3.5% of men had fatty liver as defined by an attenuation ≤ 40HU [46]. It is has been shown that African ancestry men have less fat infiltration in the liver than Caucasians [10], although the mechanisms underlying these differences are unknown [47]. While our findings suggest that liver attenuation may not be as important of a cardiovascular risk factor as calf IMAT in African ancestry men, others have shown that even small increases within the typically ‘normal’ range of liver fat are indicators of metabolic risk in African ancestry compared to other racial/ethnic groups [48]. Therefore, future studies of liver attenuation and vascular calcification should use diverse population samples to determine the veracity of this association and to determine the potential modifying role of race/ethnicity.
In this study, ectopic fat was associated with AAC, but not CAC. Calcification in the coronary arteries is thought to directly represent CVD risk given its proximity to the heart, while calcification in the abdominal aorta appears to be a stronger marker of aging and exposure to cigarette smoke [49,50]. However, calcification begins in the larger arteries and then progresses to the smaller arteries [49,50], so our findings may largely reflect the earlier stage of vascular disease present in our population sample. Indeed, the prevalence of vascular calcification in these African ancestry men is lower than those seen in similarly aged US Caucasian and African American samples [51]. This is also the probable explanation for why BMI and waist circumference were the only measures associated with CAC in fully adjusted models. Out of all adiposity measures, these two had the strongest effects in AAC models and were likely the only measures with power to show an association with CAC given the low burden of vascular disease. It is possible that in African ancestry men with more advanced coronary disease, ectopic fat depots including calf IMAT and liver attenuation may also be significant predictors of CAC. However, future studies will be needed to evaluate these associations in population samples of older individuals and/or in those with more advanced vascular disease.
The current study is not without limitations. First, pericardial fat is a widely studied ectopic fat depot, especially with respect to CAC, given its proximity to the carotid arteries [16–22]. However, we only assessed it in a subset of men from this study (N = 484; data not shown), and, thus, we could not include it in our primary analyses. In fully adjusted models in this subset, pericardial fat volume was not associated with AAC or CAC prevalence (p = 0.20 and 0.91, respectively). The lack of an association between pericardial fat and AAC may be a function of the decreased statistical power for this subset analysis; however, calf IMAT remained significantly associated with AAC (p = 0.004) in models restricted to the subset of men with pericardial fat measured. While we cannot directly quantify the significance of the association between pericardial fat volume and vascular calcification in our study, it is unlikely that pericardial fat is a stronger predictor of AAC than calf IMAT. Therefore, our conclusion that greater calf IMAT is a novel marker of prevalent AAC in African ancestry men is not likely to be confounded by the absence of pericardial fat in the full sample.
In addition, there are other potential confounding factors that we could not take into account in our study, including inflammation, diet, and objectively measured physical activity. These factors are known to be important predictors of obesity and body composition, and are may play a role in the adiposity-vascular disease relationship. We are currently measuring these in the TBHS with the goal to be able to tease apart their impact on both ectopic fat and vascular disease in future studies. Lastly, given the TBHS study design, we cannot generalize our findings to populations other than African ancestry men. Future studies will need to replicate the association of calf IMAT and liver attenuation with vascular calcification in diverse population samples including women. These studies should also determine the potential clinical utility of calf IMAT as a marker of vascular health and/or as a target for intervention in patients at risk for vascular disease or clinical events.
In conclusion, we report that calf IMAT and liver attenuation, but not abdominal VAT, were significant ectopic fat predictors of prevalent aorto-illiac artery calcification independent of total body fat and other CVD risk factors in African ancestry men. Our results suggest that the adiposity-vascular disease relationship may vary by ectopic fat quantity and location and by vascular bed location. Further studies are needed to determine the physiological basis of these associations, including assessing the potential for causality, in diverse population samples.
Supplementary Material
Acknowledgments
Financial support
This work was supported by grant K01-NL125658 (PI: Kuipers) from the National Heart, Lung and Blood Institute, by grants R03-DK092348 and R01-DK097084 (PI: Miljkovic) from the National Institute of Diabetes and Digestive and Kidney Diseases, and by grant R01-AR049747 (PI: Zmuda) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
The authors would like to thank all supporting staff from the Tobago Health Study Office and the Calder Hall Medical Clinic, as well as all TBHS participants.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.atherosclerosis.2017.06.030.
Footnotes
Conflict of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Author contributions
Study concept and design: Kuipers, Zmuda, Carr, Wassel, Miljkovic. Acquisition, analysis, or interpretation of data: Kuipers, Zmuda, Carr, Terry, Nair, Cvejkus, Wassel, Miljkovic. Drafting of the manuscript: Kuipers, Zmuda, Miljkovic. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Kuipers. Obtained funding: Kuipers, Zmuda, Bunker, Miljkovic. Study supervision: Kuipers, Zmuda, Bunker, Patrick, Miljkovic.
References
- 1.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 2.Ditomasso D, Carnethon MR, Wright CM, Allison MA. The associations between visceral fat and calcified atherosclerosis are stronger in women than men. Atherosclerosis. 2010;208(2):531–536. doi: 10.1016/j.atherosclerosis.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Divers J, Wagenknecht LE, Bowden DW, Carr JJ, Hightower RC, Ding J, et al. Regional adipose tissue associations with calcified atherosclerotic plaque: African American-diabetes heart study. Obes (Silver Spring) 2010;18(10):2004–2009. doi: 10.1038/oby.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensky NE, Criqui MH, Wright CM, Wassel CL, Alcaraz JE, Allison MA. The association between abdominal body composition and vascular calcification. Obes (Silver Spring) 2011;19(12):2418–2424. doi: 10.1038/oby.2011.70. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Fox CS, Hickson D, Sarpong D, Ekunwe L, May WD, et al. Pericardial adipose tissue, atherosclerosis, and cardiovascular disease risk factors: the Jackson heart study. Diabetes Care. 2010;33(7):1635–1639. doi: 10.2337/dc10-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Musani SK, Bidulescu A, Carr JJ, Wilson JG, Taylor HA, et al. Fatty liver, abdominal adipose tissue and atherosclerotic calcification in African Americans: the Jackson Heart Study. Atherosclerosis. 2012;224(2):521–525. doi: 10.1016/j.atherosclerosis.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKimmie RL, Daniel KR, Carr JJ, Bowden DW, Freedman BI, Register TC, et al. Hepatic steatosis and subclinical cardiovascular disease in a cohort enriched for type 2 diabetes: the Diabetes Heart Study. Am J Gastroenterol. 2008;103(12):3029–3035. doi: 10.1111/j.1572-0241.2008.02188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mellinger JL, Pencina KM, Massaro JM, Hoffmann U, Seshadri S, Fox CS, et al. Hepatic steatosis and cardiovascular disease outcomes: an analysis of the Framingham Heart Study. J Hepatol. 2015;63(2):470–476. doi: 10.1016/j.jhep.2015.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ong KL, McClelland RL, Rye KA, Cheung BM, Post WS, Vaidya D, et al. The relationship between insulin resistance and vascular calcification in coronary arteries, and the thoracic and abdominal aorta: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2014;236(2):257–262. doi: 10.1016/j.atherosclerosis.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.VanWagner LB, Ning H, Lewis CE, Shay CM, Wilkins J, Carr JJ, et al. Associations between nonalcoholic fatty liver disease and subclinical atherosclerosis in middle-aged adults: the Coronary Artery Risk Development in Young Adults Study. Atherosclerosis. 2014;235(2):599–605. doi: 10.1016/j.atherosclerosis.2014.05.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golledge J, Jayalath R, Oliver L, Parr A, Schurgers L, Clancy P. Relationship between CT anthropometric measurements, adipokines and abdominal aortic calcification. Atherosclerosis. 2008;197(1):428–434. doi: 10.1016/j.atherosclerosis.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho JS, Cannaday JJ, Barlow CE, Willis B, Haskell WL, FitzGerald SJ. Comparative relation of general, central, and visceral adiposity measures for coronary artery calcium in subjects without previous coronary events. Am J Cardiol. 2009;104(7):943–946. doi: 10.1016/j.amjcard.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs K, Brouha S, Bettencourt R, Barrett-Connor E, Sirlin C, Loomba R. Association of nonalcoholic fatty liver disease with visceral adiposity but not coronary artery calcification in the elderly. Clin Gastroenterol Hepatol. 2016;14(9):1337–1344. e1333. doi: 10.1016/j.cgh.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Kramer CK, von Muhlen D, Gross JL, Barrett-Connor E. A prospective study of abdominal obesity and coronary artery calcium progression in older adults. J Clin Endocrinol Metab. 2009;94(12):5039–5044. doi: 10.1210/jc.2009-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patil HR, Patil NT, King SI, O’Keefe E, Chhabra R, Ansari S, et al. Increased intrathoracic and hepatic visceral adipose tissue independently correlates with coronary artery calcification in asymptomatic patients. J Nucl Cardiol. 2014;21(5):880–889. doi: 10.1007/s12350-014-9946-9. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Post WS, McLenithan J, Terrin M, Magder L, Zeb I, et al. Determinants of intrathoracic adipose tissue volume and associations with cardiovascular disease risk factors in Amish. Nutr Metab Cardiovasc Dis. 2014;24(3):286–293. doi: 10.1016/j.numecd.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okada K, Ohshima S, Isobe S, Harada K, Hirashiki A, Funahashi H, et al. Epicardial fat volume correlates with severity of coronary artery disease in nonobese patients. J Cardiovasc Med Hagerst. 2014;15(5):384–390. doi: 10.2459/JCM.0b013e32836094da. [DOI] [PubMed] [Google Scholar]
- 18.Wassel CL, Laughlin GA, Araneta MR, Kang E, Morgan CM, Barrett-Connor E, et al. Associations of pericardial and intrathoracic fat with coronary calcium presence and progression in a multiethnic study. Obes (Silver Spring) 2013;21(8):1704–1712. doi: 10.1002/oby.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yerramasu A, Dey D, Venuraju S, Anand DV, Atwal S, Corder R, et al. Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis. 2012;220(1):223–230. doi: 10.1016/j.atherosclerosis.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 20.Shields KJ, Barinas-Mitchell E, Gingo MR, Tepper P, Goodpaster BH, Kao AH, et al. Perivascular adipose tissue of the descending thoracic aorta is associated with systemic lupus erythematosus and vascular calcification in women. Atherosclerosis. 2013;231(1):129–135. doi: 10.1016/j.atherosclerosis.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bettencourt N, Toschke AM, Leite D, Rocha J, Carvalho M, Sampaio F, et al. Epicardial adipose tissue is an independent predictor of coronary atherosclerotic burden. Int J Cardiol. 2012;158(1):26–32. doi: 10.1016/j.ijcard.2010.12.085. [DOI] [PubMed] [Google Scholar]
- 22.Oka T, Yamamoto H, Ohashi N, Kitagawa T, Kunita E, Utsunomiya H, et al. Association between epicardial adipose tissue volume and characteristics of non-calcified plaques assessed by coronary computed tomographic angiography. Int J Cardiol. 2012;161(1):45–49. doi: 10.1016/j.ijcard.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 23.Al Rifai M, Silverman MG, Nasir K, Budoff MJ, Blankstein R, Szklo M, et al. The association of nonalcoholic fatty liver disease, obesity, and metabolic syndrome, with systemic inflammation and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2015;239(2):629–633. doi: 10.1016/j.atherosclerosis.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flegal KM, Shepherd JA, Looker AC, Graubard BI, Borrud LG, Ogden CL, et al. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. 2009;89(2):500–508. doi: 10.3945/ajcn.2008.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL, Jr, Ravussin E, et al. Racial differences in abdominal depot-specific adiposity in white and African American adults. Am J Clin Nutr. 2010;91(1):7–15. doi: 10.3945/ajcn.2009.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke Statistics-2016 update: a report from the american heart association. Circulation. 2016;133(4):e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 27.Miljkovic I, Kuipers AL, Cvejkus R, Bunker CH, Patrick AL, Gordon CL, et al. Myosteatosis increases with aging and is associated with incident diabetes in African ancestry men. Obes (Silver Spring) 2016;24(2):476–482. doi: 10.1002/oby.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Q, Zmuda JM, Kuipers AL, Bunker CH, Patrick AL, Youk AO, et al. Muscle attenuation is associated with newly developed hypertension in men of african ancestry. Hypertension. 2017;69(5):957–963. doi: 10.1161/HYPERTENSIONAHA.116.08415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Q, Zmuda JM, Kuipers AL, Jonnalagadda P, Bunker CH, Patrick AL, et al. Greater skeletal muscle fat infiltration is associated with higher all-cause mortality among men of African ancestry. Age Ageing. 2016;45(4):529–534. doi: 10.1093/ageing/afw062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill DD, Cauley JA, Sheu Y, Bunker CH, Patrick AL, Baker CE, et al. Correlates of bone mineral density in men of African ancestry: the Tobago bone health study. Osteoporos Int. 2008;19(2):227–234. doi: 10.1007/s00198-007-0450-9. [DOI] [PubMed] [Google Scholar]
- 31.Miljkovic-Gacic I, Ferrell RE, Patrick AL, Kammerer CM, Bunker CH. Estimates of african, european and native american ancestry in afro-caribbean men on the island of Tobago. Hum Hered. 2005;60(3):129–133. doi: 10.1159/000089553. [DOI] [PubMed] [Google Scholar]
- 32.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 33.Oudkerk M, Stillman AE, Halliburton SS, Kalender WA, Mohlenkamp S, McCollough CH, et al. Coronary artery calcium screening: current status and recommendations from the european society of cardiac radiology and north american society for cardiovascular imaging. Int J Cardiovasc Imaging. 2008;24(6):645–671. doi: 10.1007/s10554-008-9319-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bellasi A, Ferramosca E, Ratti C, Block G, Raggi P. The density of calcified plaques and the volume of calcium predict mortality in hemodialysis patients. Atherosclerosis. 2016;250:166–171. doi: 10.1016/j.atherosclerosis.2016.03.034. [DOI] [PubMed] [Google Scholar]
- 35.Simonsick EM, Maffeo CE, Rogers SK, Skinner EA, Davis D, Guralnik JM, et al. Methodology and feasibility of a home-based examination in disabled older women: the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 1997;52(5):M264–M274. doi: 10.1093/gerona/52a.5.m264. [DOI] [PubMed] [Google Scholar]
- 36.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 37.Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973;19(5):476–482. [PubMed] [Google Scholar]
- 38.Larsson B. Obesity, fat distribution and cardiovascular disease. Int J Obes. 1991;15(Suppl 2):53–57. [PubMed] [Google Scholar]
- 39.Gallagher D, Kuznia P, Heshka S, Albu J, Heymsfield SB, Goodpaster B, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81(4):903–910. doi: 10.1093/ajcn/81.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miljkovic I, Cauley JA, Petit MA, Ensrud KE, Strotmeyer E, Sheu Y, et al. Greater adipose tissue infiltration in skeletal muscle among older men of African ancestry. J Clin Endocrinol Metab. 2009;94(8):2735–2742. doi: 10.1210/jc.2008-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hickner RC, Privette J, McIver K, Barakat H. Fatty acid oxidation in African-American and Caucasian women during physical activity. J Appl Physiol. 1985;90(6):2319–2324. doi: 10.1152/jappl.2001.90.6.2319. (2001) [DOI] [PubMed] [Google Scholar]
- 42.Barakat H, Hickner RC, Privette J, Bower J, Hao E, Udupi V, et al. Differences in the lipolytic function of adipose tissue preparations from Black American and Caucasian women. Metabolism. 2002;51(11):1514–1518. doi: 10.1053/meta.2002.35589. [DOI] [PubMed] [Google Scholar]
- 43.Bower JF, Davis JM, Hao E, Barakat HA. Differences in transport of fatty acids and expression of fatty acid transporting proteins in adipose tissue of obese black and white women. Am J Physiol Endocrinol Metab. 2006;290(1):E87–e91. doi: 10.1152/ajpendo.00194.2005. [DOI] [PubMed] [Google Scholar]
- 44.Ama PF, Simoneau JA, Boulay MR, Serresse O, Theriault G, Bouchard C. Skeletal muscle characteristics in sedentary black and Caucasian males. J Appl Physiol. 1985;61(5):1758–1761. doi: 10.1152/jappl.1986.61.5.1758. (1986) [DOI] [PubMed] [Google Scholar]
- 45.Simoneau JA, Kelley DE. Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J Appl Physiol. 1985;83(1):166–171. doi: 10.1152/jappl.1997.83.1.166. (1997) [DOI] [PubMed] [Google Scholar]
- 46.Hamer OW, Aguirre DA, Casola G, Sirlin CB. Imaging features of peri-vascular fatty infiltration of the liver initial observations. Radiology. 2005;237(1):159–169. doi: 10.1148/radiol.2371041580. [DOI] [PubMed] [Google Scholar]
- 47.Goran MI, Walker R, Allayee H. Genetic-related and carbohydrate-related factors affecting liver fat accumulation. Curr Opin Clin Nutr Metab Care. 2012;15(4):392–396. doi: 10.1097/MCO.0b013e3283544477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alderete TL, Toledo-Corral CM, Desai P, Weigensberg MJ, Goran MI. Liver fat has a stronger association with risk factors for type 2 diabetes in African-American compared with Hispanic adolescents. J Clin Endocrinol Metab. 2013;98(9):3748–3754. doi: 10.1210/jc.2013-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24(2):331–336. doi: 10.1161/01.ATV.0000110786.02097.0c. [DOI] [PubMed] [Google Scholar]
- 50.Wagenknecht LE, Langefeld CD, Freedman BI, Carr JJ, Bowden DW. A comparison of risk factors for calcified atherosclerotic plaque in the coronary, carotid, and abdominal aortic arteries: the diabetes heart study. Am J Epidemiol. 2007;166(3):340–347. doi: 10.1093/aje/kwm091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuipers AL, Zmuda JM, Carr JJ, Terry JG, Patrick AL, Ge Y, et al. Association of volumetric bone mineral density with abdominal aortic calcification in African ancestry men. Osteoporos Int. 2014;25(3):1063–1069. doi: 10.1007/s00198-013-2486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.