Abstract
Introduction
Hyperhomocysteineamia (HHcy) has long been suggested as a risk factor for atherosclerosis. However the association between HHcy and peripheral arterial disease (PAD) is still controversial. There is a lack of research on this topic in the Chinese population. This study aims to provide further results.
Methods
240 PAD patients and 240 control subjects were evaluated for both serum total homocysteine levels and ankle brachial indexes (ABIs). Multivariable logistic regression models were used to estimate the association between HHcy and the risk of developing PAD. Interaction and stratified analyses were conducted according to age, sex, smoking status, drinking status, and histories of chronic disease.
Results
The multivariate logistic analyses revealed that the risk of PAD was significantly associated with serum homocysteine levels. The interaction analysis showed no interactive role in the association between HHcy and PAD, indicating that homocysteine was associated with PAD independent of classical vascular risk factors.
Conclusion
In conclusion, HHcy is an independent risk factor for PAD in the Chinese Han population. A prospective and randomized clinical trial of homocysteine lowering therapy in the Chinese population is needed to assess the causal nature of the relationship.
Keywords: Peripheral arterial disease, Hyperhomocysteineamia, Homocysteine, Case-control study
Introduction
Peripheral arterial disease (PAD) refers to the various diseases that affect noncardiac and nonintracranial arteries [1]. More than 200 millon people worldwide are estimated to have PAD [2], and these statistics are expected to rise as the population grows and ages. Nevertheless, only 22% of PAD are symptomatic [3]. The most common cause of PAD is atherosclerosis, implying that the risk factors for PAD are similar to those for other atherosclerotic vascular diseases, with smoking and diabetes being the strongest two risk factors [2]. This means that more than 50% of the people who are diagnosed with PAD have also suffered from either coronary heart disease, cerebrovascular disease, or both [4]. Additionally, the incidence of adverse limb outcomes in PAD patients such as worsening of symptoms, peripheral revascularization and ischemic amputations was 26.2% in 4 years [5].
An elevated plasma homocysteine concentration is known as hyperhomocysteinaemia (HHcy). The association between HHcy and atherosclerosis was discovered in 1969 when McCully studied the vascular pathology of children with inherited disorders of homocysteine metabolism [6]. HHcy occurs in about 5 to 7 percent of the general population [7, 8], which is typically caused either by genetic defects in the enzymes involved in homocysteine metabolism or by nutritional deficiencies in vitamin cofactors (folate, vitamin B12, and vitamin B6) [9]. Several disease states also influence homocysteine metabolism, including chronic renal failure [10], hypothyroidism [8], pernicious anemia [11], and some types of tumor [12]. Moreover, HHcy has been suggested as a potent independent risk factor for arteriosclerosis for a long time [13].
Since McCully discovered that an elevated plasma homocysteine concentration could lead to atherosclerosis, abundant epidemiologic studies have been performed to validate the hypothesis in the western population [14–16]. In 1995, Boushey et al. [13] reviewed 27 studies on homocysteine and atherosclerotic disease and performed a meta-analysis of 5 studies revealing an association between elevated plasma homocysteine and PAD. In addition an updated meta-analysis [17] of 14 studies was performed in 2009 confirming the hypothesis that patients with PAD have significantly higher levels of homocysteine. Few similar studies [18, 19] were performed to investigate the association between HHcy and presence of PAD within the Chinese population, which compared to the western population are very different in their lifestyle, nutrition, and metabolism. In addition, the interactions of other cardiovascular risk factors such as age, smoking, and diabetes with HHcy in the development of PAD are still unknown. Therefore, this study aimed to assess the association between HHcy and PAD within the Chinese Han population, the main Chinese nationality with a population of up to 1.2 billion people. The influence of other classical cardiovascular risk factors on the association was also evaluated by using interaction and stratified analyses.
Methods
Study design and study population
In accordance with STROBE statement, we performed this case-control study with the purpose of estimating the potential relationship between HHcy and PAD. Data was collected between July 2011 and December 2012 in China. 240 patients diagnosed with PAD by photoplethysmography and computed tomography angiography (CTA) at the Vascular and Endovascular Department of the Chinese PLA General Hospital in Beijing and 240 control healthy subjects that underwent a medical examination in the same hospital were enrolled in this study. Participants were excluded based on the following criteria: (1) an ethnic origin other than Han; (2) a history of vitamin supplement intake; (3) a history of serum homocysteine related drug intake; (4) a history of serum homocysteine related disease (renal impairment, malignant tumor or hypothyroidism); (4) a history of mental disorder; (5) pregnancy; (6) an announcement of declining to participate. This study protocol was approved by the ethics committee of Chinese PLA General Hospital, and all subjects provided informed consent. All experiments were performed in accordance with relevant guidelines and protocols.
Data collection
Demographic characteristics, history of chronic disease, long term medication use, and lifestyle factors were recorded using a standardized questionnaire for subjects. Standard physical and biochemical examinations were completed in all participants. PAD was defined as a decreased ABI below 0.9 according to previous studies reporting over 90% sensitivity and specificity to detect PAD compared with angiography [20]. The subjects in the control group underwent a peripheral arterial photoplethysmographic[21] examination by two experienced specialists in order to exclude patients with PAD. Biochemical parameters were analyzed at the clinical laboratory of the Chinese PLA Hospital by using the Roche Modular chemistry analyzer (Roche Diagnostics, Basel, Switzerland). All blood samples were withdrawn after overnight fasting and were placed in freezer at 4°C immediately after phlebotomy. Centrifugation for separation of serum were carried out within 1 hour of collection and stored at ‒80°C until analysis.
Before undergoing peripheral arterial photoplethysmography, all recruited subjects were evaluated for the presence of risk factors for PAD. On the basis of self-reported histories, clinical examinations and biochemical tests, data on the following variables were recorded on a standard form: smoking habits, defined by a consumption of more than 100 cigarettes over a lifetime; drinking habits, defined by weekly white spirits consumption of more than 50 ml for a period over six months; hypertension, defined by a systolic blood pressure of more than 140 mm Hg or a diastolic blood pressure of more than 90 mm Hg (or both) on at least two occasions or by the use of hypotensive drugs; hyperlipidemia, defined by a fasting venous cholesterol level exceeding 5.7 mmol per liter, a fasting venous triglyceride level exceeding 1.7 mmol per liter, a fasting venous low-density lipoprotein (LDL) cholesterol level exceeding 3.4 mmol per liter, a fasting venous high-density lipoprotein (HDL) cholesterol level below 1.0 mmol per liter, or by the use of lipid-lowering drugs. Histories of diabetes, coronary artery disease (CAD), and ischemic stroke were self-reported. HHcy was defined as a serum total homocysteine concentration exceeding 15 μmol/L, as determined based on the laboratory reference range.
Statistical analysis
Multiple inputs were used for missing data on smoking, drinking, LDL, HDL, hypertension, and diabetes. In total, 5 data sets were imputed, and results were pooled according to Rubin’s rules [22]. Categorical variables were presented as frequencies and percentages, and continuous variables as means and standard deviations. The clinical characteristics of patients and control subjects were compared with the use of the Mann-Whitney test for continuous variables and the chi-square test for categorical variables. The association of HHcy and PAD was assessed with the use of multivariable logistic regression models. In another separate analysis, homocysteine was included as a continuous variable in the model. Unadjusted and adjusted odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. We adjusted multivariable logistic regression models for age, sex, and other classical risk factors of vascular disease: smoking status, drinking status, coronary artery disease, ischemic stroke, diabetes, hypertension and hyperlipidemia. Interaction and stratified analyses were conducted according to age grouping (above and below 60 years), sex, smoking status, drinking status, and histories of chronic disease previously described. All of the analyses were performed with the statistical software packages R (http://www.R-project.org, The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solution, Inc., Boston, MA).
Results
Characteristics of the subjects
There were 240 patients and 240 controls enrolled in this study. Table 1 shows the main characteristics of the study subjects as well as the prevalence of risk factors for PAD. Patients with PAD were slightly older (median age, 64 vs 59 years), smokers (119[49.6%] vs 46[19.2%]), had a diagnosis of hypertension (142[59.2%] vs 74[30.8%]), hyperlipidemia (168[70.0%] vs 123[51.2%]), diabetes (83[34.6%] vs 17[7.1%]), CAD (78[32.5%] vs 21[8.8%]), or ischemic stroke (62[25.8%] vs 13[5.4%]). Additionally, patients with PAD had a significantly higher (20.4±11.5 vs 17.2±8.7) serum total homocysteine than the control subjects.
Table 1.
Main Characteristics of the Study Population.a
| Variable | Patients with PAD (N=240) |
Control subjects (N=240) |
P value |
|---|---|---|---|
| Age — yr | 63.6±12.0 | 59.2±7.8 | <0.001 |
| Male sex — no.(%) | 203(84.6) | 195(80.0) | 0.332 |
| Smoking habits — no.(%) | 119(49.6) | 46(19.2) | <0.001 |
| Drinking habits — no.(%) | 63(26.2) | 44(18.3) | 0.037 |
| Hypertension — no.(%) | 142(59.2) | 74(30.8) | <0.001 |
| Hyperlipidemia — no.(%) | 168(70.0) | 123(51.2) | <0.001 |
| Diabetes — no.(%) | 83(34.6) | 17(7.1) | <0.001 |
| CAD — no.(%) | 78(32.5) | 21(8.8) | <0.001 |
| Ischemic stroke— no.(%) | 62(25.8) | 13(5.4) | <0.001 |
| tHcy — μmol/L | 20.4±11.5 | 17.2±8.7 | <0.001 |
Plus-minus values are means ±SD. P values were calculated by comparing characteristics between two groups.
HHcy and homocysteine level
HHcy was defined as serum total homocysteine levels>15μmol/L and was detected in 159 (66.2%) of the 240 PAD patients and in 121 (50.2%) of the 240 control subjects. The mean serum total homocysteine level in the PAD group was significantly higher than that in the control group (20.4±11.5μmol/L vs 17.2±8.7μmol/L; Figure 1).
Figure 1.
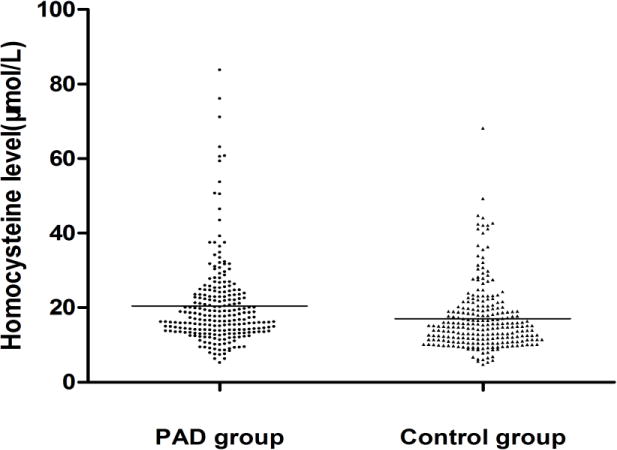
Distribution of serum homocysteine levels in patients with PAD and controls.
Relationship between HHcy and PAD
The relationship between HHcy and PAD is presented in Table 2. The univariate logistic regression analysis revealed a significant association between HHcy and PAD (odds ratio [OR] =1.93; 95% confidence interval [CI], 1.34–2.79). After adjusting for confounding variables, the risk of PAD was decreased in subjects with HHcy (OR=1.63; 95% CI, 1.01–2.63). When included as a continuous variable, homocysteine was also associated with an increased risk of PAD (adjusted OR per 1-unit increase in homocysteine, 1.03; 95% CI, 1.00–1.05).
Table 2.
Multivariable logistic regression models evaluating the association between tHcy and PAD.
| No.(%) of study participants | OR(95%CI) | |||
|---|---|---|---|---|
|
|
||||
| Variable | Cases | Controls | Crude | Adjusteda |
| tHcy,1μmol/L | 240 | 240 | 1.03 (1.01, 1.05) | 1.03 (1.00, 1.05) |
| tHcy<15μmol/L | 81(33.8) | 119(49.6) | Ref | Ref |
| tHcy≥15μmol/L | 159(66.2) | 121(50.2) | 1.93 (1.34, 2.79) | 1.63 (1.01, 2.63) |
Adjusted for Age, Sex, Smoking status, Drinking status, Coronary Artery Disease, Ischemic stroke, Diabetes, Hypertension and Hyperlipidemia.
The results of the stratified and interaction analyses of the association between HHcy and the risk of PAD are presented in Table 3 and Figure 2. The association between HHcy and PAD in the stratified analysis was consistent with that in the multivariable logistic regression analysis. The stratified analysis demonstrated a statistically significant association between HHcy and PAD in male subjects (adjusted OR, 1.5; 95% CI, 1.0–2.5), subjects younger than 60 years (adjusted OR, 1.9; 95% CI, 1.0–3.7), subjects without smoking habits (adjusted OR, 1.8; 95% CI, 1.0–3.2), without hypertension (adjusted OR, 2.0; 95% CI, 1.0–4.1), without diabetes (adjusted OR, 1.8; 95% CI, 1.0–3.1), without CAD (adjusted OR, 1.8; 95% CI, 1.0–3.1), without ischemic stroke (adjusted OR, 1.7; 95% CI, 1.0–2.9), and without hyperlipidemia (adjusted OR, 2.2; 95% CI, 1.0–5.1). The interaction analysis revealed no interactive role in the association between HHcy and PAD.
Table 3.
Association between hyperhomocystenaemia (HHcy) and PAD according to baseline characteristics.
| No.(%) of study participants | OR(95%CI) | P Value for interaction |
|||||
|---|---|---|---|---|---|---|---|
| Cases(N=240) | Controls(N=240) | Mutually | |||||
| Subgroup | HHcy | nHcy | HHcy | nHcy | Crude | Adjusteda | |
| Age | 0.25 | ||||||
| <60 | 56 | 33 | 59 | 74 | 2.1 (1.2, 3.7) | 1.9(1.0, 3.7) | |
| ≥60 | 103 | 48 | 62 | 45 | 1.6 (0.9, 2.7) | 1.3(0.6, 2.7) | |
| Sex | 0.55 | ||||||
| male | 139 | 64 | 112 | 83 | 1.6 (1.1, 2.4) | 1.5 (1.0, 2.5) | |
| female | 20 | 17 | 9 | 36 | 4.7 (1.8, 12.5) | 2.3 (0.6, 8.6) | |
| Smoking habits | 0.91 | ||||||
| No | 75 | 46 | 92 | 102 | 1.8(1.1, 2.9) | 1.8 (1.0, 3.2) | |
| Yes | 84 | 35 | 29 | 17 | 1.4(0.7, 2.9) | 1.4 (0.6, 3.2) | |
| Drinking habits | 0.88 | ||||||
| No | 115 | 62 | 97 | 99 | 1.9(1.2, 2.9) | 1.6 (0.9, 2.9) | |
| Yes | 44 | 19 | 24 | 20 | 1.9(0.9, 4.3) | 1.8 (0.6, 5.1) | |
| Hypertension | 0.73 | ||||||
| No | 62 | 36 | 78 | 88 | 1.9 (1.2, 3.2) | 2.0 (1.0, 4.1) | |
| Yes | 97 | 45 | 43 | 31 | 1.6 (0.9, 2.8) | 1.6 (0.8, 3.3) | |
| Diabetes | 0.27 | ||||||
| No | 115 | 42 | 113 | 110 | 2.7 (1.7, 4.1) | 1.8 (1.0, 3.1) | |
| Yes | 44 | 39 | 8 | 9 | 1.3 (0.4, 3.6) | 0.8 (0.2, 2.6) | |
| CAD | 0.52 | ||||||
| No | 108 | 54 | 108 | 111 | 2.1 (1.3, 3.1) | 1.8 (1.0, 3.1) | |
| Yes | 51 | 27 | 13 | 8 | 1.2 (0.4, 3.1) | 1.1 (0.3, 3.6) | |
| Ischemic stroke | 0.79 | ||||||
| No | 117 | 61 | 114 | 113 | 1.9 (1.3, 2.8) | 1.7 (1.0, 2.9) | |
| Yes | 42 | 20 | 7 | 6 | 1.8 (0.5, 6.1) | 2.0 (0.4, 11.4) | |
| Hyperlipidaemia | 0.31 | ||||||
| No | 44 | 28 | 51 | 66 | 2.0 (1.1, 3.7) | 2.2 (1.0, 5.1) | |
| Yes | 115 | 53 | 70 | 53 | 1.6 (1.0, 2.7) | 1.4 (0.8, 2.5) | |
Each stratification adjusted for all the factors(Age, Sex, Smoking status, Drinking status, Coronary Artery Disease, Ischemic stroke, Diabetes, Hypertension and Hyperlipidemia) except the stratification factor itself.
Figure 2.
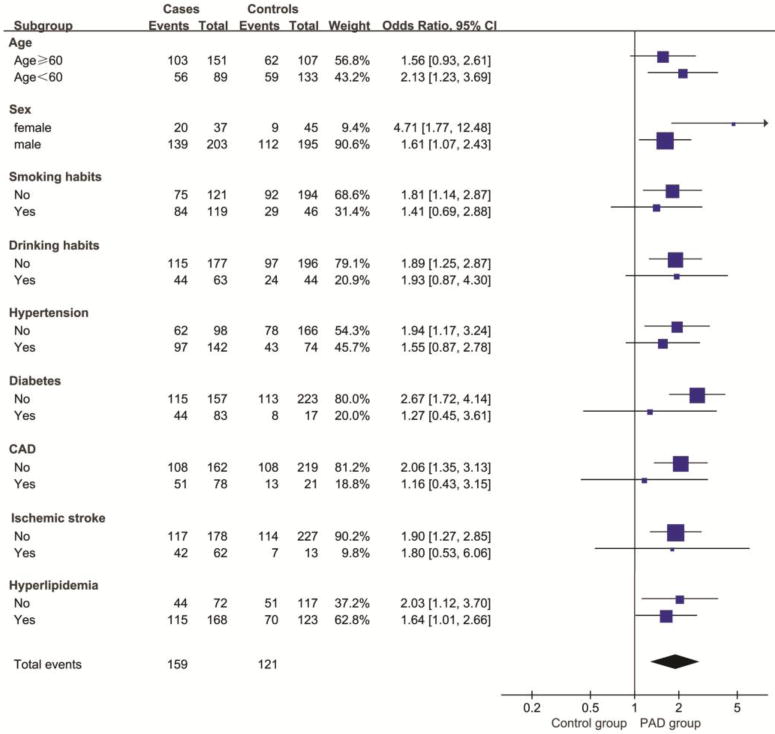
Odds ratios for peripheral arterial disease, according to baseline characteristics.
Discussion
In this case-control study, serum homocysteine levels were significantly associated with risk of PAD in the Chinese Han population. Subjects with HHcy had a 1.64-fold higher risk of PAD than those with normal serum total homocysteine levels. A 3% higher risk of PAD associated with a 1μmol/L increase in serum total homocysteine level further confirmed the relationship between homocysteine and PAD. Although participants with PAD were slightly older and had a greater prevalence of risk factors than controls, the association remained significant after adjustment for these risk factors. Moreover, we found no interactive role in the association between HHcy and PAD, suggesting that homocysteine is associated with the incidence of PAD independent of classical vascular risk factors.
An up to date meta-analysis revealed PAD to be significantly associated with elevated homocysteine levels which is consistent with our study result [17], however previous studies of PAD have yielded inconsistent outcomes. Ridker et al. [23] reported no association between homocysteine and PAD within the Physicians’ Health Study (PHS, 1997–2007) cohort of male physicians. Aboyans et al. [24] and Taylor et al.[25] examined homocysteine levels and PAD progression, no association was discovered between homocysteine levels and PAD progression in both studies.
The decreased female sample size after stratification may have resulted in biases, but our study finding that homocysteine levels were associated with PAD in men is consistent with previous studies. Pradhan et al. [26] reported no association between homocysteine levels and the risk of PAD in the Women’s Health Study (WHS, 1993–2004). Monica et al. [27] also reported no association between homocysteine levels and PAD within the Nurses’ Health Study (NHS, 1990–2010) of female subjects. There may be gender differences in homocysteine metabolism as homocysteine levels are higher in men compared to women. One hypothesis has been postulated that these differences may be due to estradiol which lowers homocysteine levels in women [27,28]. More specifically, estrogns can lower plasma homocysteine levels by formating of estrogen-homocysteine conjugates [29]. Additionally, catechol-O-methyltransferase (COMT) is responsible for both converting S-adenosyl-homocysteine to homocysteine and metabolizing catecholestrogens to methoxyestrogens and catabolize catecholamines [30,31]. Estrogens can also lower plasma homocysteine levels by competing with S-adenosyl-homocysteine for COMT. Other mechanisms contributing to gender differences of plasma homocysteine levels may exist simultaneously.
Female subjects are suppose to have a lower odds ratio for PAD since female subjects have a lower plasma homocysteine levels. However, the odds ratio for PAD of female subjects is significantly higher (p=0.042, not shown in results) than male subjects in figure 2. The power calculated by sample size and effect is 0.906, demonstrating that the female sample size meets the analysis requirement. We included homocysteine as a continuous variable in stratification analysis by gender, results showed that female subjects (OR=1.14; 95% CI, 1.04–1.25) have a higher risk of PAD than male subjects (OR=1.03; 95% CI, 1.00–1.05), females suffer a higher increased risk of PAD than males per 1-unit increase in homocysteine. Considering the results above, we hypothesize that females are more sensitive to plasma homocysteine. Fundamental experiment is needed to validate our hypothsis.
Although the association between HHcy and PAD in the stratified analysis was consistent with that in the multivariable logistic regression analysis, we found that it wasn’t statistically significant in subjects older than 60 years, smokers and in subjects who were diagnosed with hypertension, diabetes, coronary artery disease, ischemic stroke or hyperlipidemia. CAD and ischemic stroke are also atherosclerotic vascular diseases which share similar risk factors with PAD; subjects with CAD and ischemic stroke are supposed to have higher homocysteine levels in both the case group and the control group. While aging, smoking, hypertension, diabetes, and hyperlipidemia are risk factors for PAD, subjects with these risk factors are also supposed to have higher homocysteine levels in both the case group and the control group. Consequently, residual confoundings were inevitable when we analyzed association of HHcy and prevalence of PAD in subjects with these factors. Additionally, the decreased sample size after stratification may also have resulted in biases.
Whether elevated homocysteine levels are a cause of atherosclerosis or a marker for atherosclerosis has yet to be confirmed [9]. The crude molecular mechanism of adverse vascular effects of homocyst(e)ine has been previously postulated. Oxidative metabolism of homocysteine to homocystine and homocysteine thiolactone would bring oxidative damage to vascular endothelial cells and increase the proliferation of vascular smooth-muscle cells [9]. B vitamins can lower homocysteine levels by promoting homocysteine metabolism [32]. Monica et al.[27] revealed an inverse association between higher folate intake (including supplements) and the risk of developing PAD in men. Moreover, a randomised trial showed that there was a significant improvement in ABI in participants who received either folic acid or 5-methyltetrahydrofolate compared with placebo [33]. The results of the China Stroke Primary Prevention Trial (CSPPT) also showed that participants in the enalaprol group have a higher risk of ischemic stroke than those in the enalapril-folic acid group [34]. However, the results of the Heart Outcomes Prevention Evaluation (HOPE) 2 study showed that daily administration of the combination of folic acid, vitamin B6, and vitamin B12 lowered homocysteine levels significantly but did not reduce the incidence of death from cardiovascular causes, myocardial infarction, and stroke during a mean follow-up period of five years in a population with a history of vascular disease [35]. A meta-analysis of 8 randomized trials involving 37485 individuals found that although folic acid allocation reduced homocysteine levels by 25% on average, no significant effects on vascular outcomes was revealed during a median follow-up of 5 years[36]. Therefore, homocysteine could be a marker, but not a cause of vascular disease. Also homocysteine may play an important role in the initiation of atherosclerosis but does not affect its outcomes. Whether homocysteine contributes to the initiation and/or progression of atherosclerosis requires further investigation.
Further limitations of our study included: (1) recall bias, which couldn’t be excluded because of the self-reported histories of chronic diseases, that affected the accuracy of estimation of disease prevalence; (2) the severity of PAD was neglected and only occurrence was recorded, thus the role of homocysteine in progression of PAD cannot be estimated(3) information of the MTHFR C677T polymorphism is lacking, therefore, we did not evaluate the roles of MTHFR C677T polymorphism in the association between HHcy and PAD; (4) vitamin B12 and folic acid levels were not measured, therefore, we can not evaluate the interaction between vitamins and homocysteine. These limitations should be considered in future studies.
In conclusion, HHcy is an independent risk factor for PAD in the Chinese Han population. Considering the limitations mentioned above, a prospective and randomized clinical trial of homocysteine lowering therapy in the Chinese population is needed.
Acknowledgments
We thank all the participants of the study.
Financial support
This study was supported by scholarships from the Chinese Scholarship Council (CSC) awarded to J.L., the Eleventh Five-year Plan in Health Care Foundation of PLA (09BJZ04) and the National Natural Science Foundation of China (30940070). Dr. RA Khalil was partly supported by grants from National Heart, Lung, and Blood Institute (HL-65998, HL-111775).
Abbreviations
- HHcy
hyperhomocysteineamia
- PAD
peripheral arterial disease
- ABI
ankle brachial index
- PLA
people’s liberation army
- LDL
low-density lipoprotein
- HDL
high-density lipoprotein
- CAD
coronary artery disease
- OR
odds ratio
- CI
confidence interval
- PHS
Physicians’ Health Study
- WHS
Women’s Health Study
- NHS
Nurses’ Health Study
- 5-MTHF
5-methyltetrahydrofolate
- HOPE
Heart Outcomes Prevention Evaluation
Footnotes
Conflicts of interest
There are no conflicts of interest associated with this study.
Author Contributions Statement
W.G. designed the study; J.L., G.S., H.L., and S.J. collected the data; D.R., J.L., and W.L. analysed the data; D.R., J.L., Y.L., H.Z., X.J., J.X., X.M., and X.L. analysed and interpreted the results; D.R., J.L., R.A.K., and D.N. wrote the article. All the authors have read and reviewed this manuscript.
References
- 1.Kullo IJ, Rooke TW. CLINICAL PRACTICE. Peripheral Artery Disease. N Engl J Med. 2016;374(9):861–71. doi: 10.1056/NEJMcp1507631. [DOI] [PubMed] [Google Scholar]
- 2.Fowkes FG, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329–40. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 3.Stoffers HE, et al. The prevalence of asymptomatic and unrecognized peripheral arterial occlusive disease. Int J Epidemiol. 1996;25(2):282–90. doi: 10.1093/ije/25.2.282. [DOI] [PubMed] [Google Scholar]
- 4.Bhatt DL, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295(2):180–9. doi: 10.1001/jama.295.2.180. [DOI] [PubMed] [Google Scholar]
- 5.Kumbhani DJ, et al. Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J. 2014;35(41):2864–72. doi: 10.1093/eurheartj/ehu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56(1):111–28. [PMC free article] [PubMed] [Google Scholar]
- 7.Ueland PM, Refsum H. Plasma homocysteine, a risk factor for vascular disease: plasma levels in health, disease, and drug therapy. J Lab Clin Med. 1989;114(5):473–501. [PubMed] [Google Scholar]
- 8.McCully KS. Homocysteine and vascular disease. Nat Med. 1996;2(4):386–9. doi: 10.1038/nm0496-386. [DOI] [PubMed] [Google Scholar]
- 9.Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med. 1998;338(15):1042–50. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- 10.Chauveau P, et al. Hyperhomocysteinemia, a risk factor for atherosclerosis in chronic uremic patients. Kidney Int Suppl. 1993;41:S72–7. [PubMed] [Google Scholar]
- 11.Savage DG, et al. Sensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficiencies. Am J Med. 1994;96(3):239–46. doi: 10.1016/0002-9343(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 12.Mayer EL, Jacobsen DW, Robinson K. Homocysteine and coronary atherosclerosis. J Am Coll Cardiol. 1996;27(3):517–27. doi: 10.1016/0735-1097(95)00508-0. [DOI] [PubMed] [Google Scholar]
- 13.Boushey CJ, et al. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995;274(13):1049–57. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 14.Clarke R, et al. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991;324(17):1149–55. doi: 10.1056/NEJM199104253241701. [DOI] [PubMed] [Google Scholar]
- 15.Brattstrom L, et al. Impaired homocysteine metabolism in early-onset cerebral and peripheral occlusive arterial disease. Effects of pyridoxine and folic acid treatment. Atherosclerosis. 1990;81(1):51–60. doi: 10.1016/0021-9150(90)90058-q. [DOI] [PubMed] [Google Scholar]
- 16.Bergmark C, et al. Hyperhomocysteinemia in patients operated for lower extremity ischaemia below the age of 50–effect of smoking and extent of disease. Eur J Vasc Surg. 1993;7(4):391–6. doi: 10.1016/s0950-821x(05)80255-5. [DOI] [PubMed] [Google Scholar]
- 17.Khandanpour N, et al. Homocysteine and Peripheral Arterial Disease: Systematic Review and Meta-analysis. European Journal of Vascular and Endovascular Surgery. 2009;38(3):316–322. doi: 10.1016/j.ejvs.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Cheng SW, Ting AC, Wong J. Fasting total plasma homocysteine and atherosclerotic peripheral vascular disease. Ann Vasc Surg. 1997;11(3):217–23. doi: 10.1007/s100169900037. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, et al. Hyperhomocysteinaemia is an independent risk factor of abdominal aortic aneurysm in a Chinese Han population. Sci Rep. 2016;6:17966. doi: 10.1038/srep17966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aboyans V, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126(24):2890–909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 21.Beutner F, et al. Automated photoplethysmography-based determination of ankle-brachial index: a validation study against Doppler sonography. Clin Res Cardiol. 2012;101(11):875–83. doi: 10.1007/s00392-012-0471-z. [DOI] [PubMed] [Google Scholar]
- 22.Donders AR, et al. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59(10):1087–91. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285(19):2481–5. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- 24.Aboyans V, et al. Risk factors for progression of peripheral arterial disease in large and small vessels. Circulation. 2006;113(22):2623–9. doi: 10.1161/CIRCULATIONAHA.105.608679. [DOI] [PubMed] [Google Scholar]
- 25.Taylor LJ, et al. The association of elevated plasma homocyst(e)ine with progression of symptomatic peripheral arterial disease. J Vasc Surg. 1991;13(1):128–36. doi: 10.1067/mva.1991.24913. [DOI] [PubMed] [Google Scholar]
- 26.Pradhan AD, et al. Symptomatic peripheral arterial disease in women: nontraditional biomarkers of elevated risk. Circulation. 2008;117(6):823–31. doi: 10.1161/CIRCULATIONAHA.107.719369. [DOI] [PubMed] [Google Scholar]
- 27.Bertoia ML, et al. Plasma homocysteine, dietary B vitamins, betaine, and choline and risk of peripheral artery disease. Atherosclerosis. 2014;235(1):94–101. doi: 10.1016/j.atherosclerosis.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dierkes J, et al. Factors explaining the difference of total homocysteine between men and women in the European Investigation Into Cancer and Nutrition Potsdam study. Metabolism. 2001;50(6):640–5. doi: 10.1053/meta.2001.23286. [DOI] [PubMed] [Google Scholar]
- 29.Gaikwad NW. Mass spectrometry evidence for formation of estrogen–homocysteine conjugates: Estrogens can regulate homocysteine levels. Free Radical Biology and Medicine. 2013;65:1447–1454. doi: 10.1016/j.freeradbiomed.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 30.Goodman JE, et al. Characterization of human soluble high and low activity catechol-O-methyltransferase catalyzed catechol estrogen methylation. Pharmacogenetics. 2002;12(7):517–28. doi: 10.1097/00008571-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Brown LJ, et al. Using S-adenosyl-L-homocysteine capture compounds to characterize S-adenosyl-L-methionine and S-adenosyl-L-homocysteine binding proteins. Anal Biochem. 2014;467:14–21. doi: 10.1016/j.ab.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNulty H, et al. Homocysteine, B-vitamins and CVD. Proc Nutr Soc. 2008;67(2):232–7. doi: 10.1017/S0029665108007076. [DOI] [PubMed] [Google Scholar]
- 33.Khandanpour N, et al. Peripheral arterial disease and methylenetetrahydrofolate reductase (MTHFR) C677T mutations: A case-control study and meta-analysis. J Vasc Surg. 2009;49(3):711–8. doi: 10.1016/j.jvs.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Huo Y, et al. Efficacy of Folic Acid Therapy in Primary Prevention of Stroke Among Adults With Hypertension in China. JAMA. 2015;313(13):1325. doi: 10.1001/jama.2015.2274. [DOI] [PubMed] [Google Scholar]
- 35.Lonn E, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354(15):1567–77. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 36.Clarke R, et al. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: Meta-analysis of 8 randomized trials involving 37485 individuals. Arch Intern Med. 2010;170(18):1622–31. doi: 10.1001/archinternmed.2010.348. [DOI] [PubMed] [Google Scholar]


