Abstract
Verbal-spatial discrepancies are common in healthy individuals and in those with neurodevelopmental disorders associated with cognitive control deficits including: Autism Spectrum Disorder, Non-Verbal Learning Disability, Fragile X, 22q11 deletion, and Turner Syndrome. Previous data from healthy individuals suggest that the magnitude of the difference between verbal IQ (VIQ) and performance IQ (PIQ) scores (the VIQ>PIQ discrepancy) is associated with reduced thickness in frontal and parietal cortices (inferior frontal, anterior cingulate, inferior parietal lobule, and supramarginal gyrus) that support cognitive control. Unknown is whether the VIQ>PIQ discrepancy is associated with functional deficits in these areas in healthy or ill children and adolescents. We assessed the effects of the VIQ>PIQ discrepancy on fMRI BOLD response during the resolution of cognitive conflict in 55 healthy children and adolescents during performance of a Simon Spatial Incompatibility task. As the magnitude of the VIQ>PIQ discrepancy increased, activation of fronto-striatal, limbic, and temporal regions decreased during conflict resolution (p < 0.05, corrected). In exploratory analyses, the VIQ>PIQ discrepancy was associated with reduced functional connectivity from right inferior frontal gyrus to right thalamus and increased functional connectivity to right supramarginal gyrus (ps < 0.03, uncorrected). The VIQ>PIQ discrepancy may be an important aspect of an individual’s cognitive profile and likely contributes to, or is associated with, deficient cognitive control processes characteristic of many childhood disorders.
Introduction
The capacity for cognitive control is critical for the successful development of social competence and school readiness (Diamond, Barnett, Thomas, & Munro, 2007). This capacity develops gradually over childhood and adolescence, paralleling structural and functional changes in fronto-striatal and fronto-parietal circuits (Rubia et al., 2006). Unknown is how cognitive control processes are impacted by individual variation in the discrepancy between verbal and spatial abilities, a well-known but under-studied source of variance within and between individuals. Since children learn to use language to regulate their behavior (Vygotsky, 2004), verbal-spatial discrepancies may lead to under- or over-regulation of behavior and subsequent cognitive control problems. Such discrepancies are characteristic of a number of neurodevelopmental and genetic disorders in which cognitive control deficits are present (Davis & Broitman, 2011; Hong et al., 2009; Hooper et al., 2008; Woodin et al., 2001). Whereas spatial abilities are typically greater than verbal abilities in Autism Spectrum Disorder (ASD, Charman et al., 2011), Dyslexia (Diehl et al., 2014), and Language Disorder (Rice, Warren, & Betz, 2005), verbal are typically greater than spatial abilities in 22q11 deletion syndrome (Woodin et al., 2001), Turner Syndrome (Hong et al., 2009), Fragile X (Hooper et al., 2008), and Non-Verbal Learning Disability (Davis & Broitman, 2011). Thus, verbal and spatial abilities are dissociable, and the discrepancy between these abilities may arise developmentally, possibly contributing to the cognitive control deficits associated with these disorders.
Although the neural correlates of verbal-spatial discrepancies are understudied, previous data from healthy individuals suggest that decreases in verbal and spatial abilities are associated with thinning in left frontal cortices (Burgaleta et al., 2014) and that the magnitude of the verbal-spatial discrepancy, as measured by differences between verbal IQ (VIQ) and performance IQ (PIQ) scores, is associated with cortical thickness (CT) in bilateral frontal (inferior frontal gyrus and anterior cingulate cortex) and parietal (inferior parietal lobule and supramarginal gyrus) regions, as well as temporal and occipital regions (Margolis et al. 2013; Yokota et al. 2015). These frontal and parietal regions support cognitive control processes (Luna, Padmanabhan, & O’Hearn, 2010), but unknown is whether functional deficits within these regions are associated with the VIQ-PIQ discrepancy in healthy or ill individuals. As a first step towards understanding whether the VIQ-PIQ discrepancy might contribute to cognitive control deficits in neurodevelopmental disorders characterized by the VIQ-PIQ discrepancy, we used a spatial task to study the effects of this discrepancy on brain function during the engagement of cognitive control.
The Simon Spatial Incompatibility task (Craft & Simon, 1970) requires participants to engage cognitive control to ignore a salient, but task-irrelevant feature of a stimulus (the side of the screen on which an arrow appears) when it conflicts with a more task-relevant one (the direction the arrow points). When responding correctly on incongruent trials (a left-facing arrow appears on the right side of the screen), healthy individuals resolve cognitive conflict by activating frontal (dorsolateral/dorsomedial prefrontal, anterior cingulate, and supplementary motor), parietal (Luna et al., 2010), and striatal (Rubia et al., 2006) regions. Healthy individuals experience the most cognitive conflict and show more activation within these regions when responding to incongruent trials that are preceded by congruent trials (‘post-congruent conflict effect’) (Horga et al., 2011). We hypothesized that a VIQ>PIQ discrepancy would be associated with reduced activation of frontal and parietal regions during the resolution of conflict and post-congruent conflict, consistent with previous structural findings that a greater VIQ>PIQ discrepancy was associated with decreased thickness in frontal and parietal cortices. We also evaluated age effects and explored effects of the VIQ>PIQ discrepancy on task-related functional connectivity within the fronto-striatal and fronto-parietal circuits involved in conflict resolution.
Methods
Participants
FMRI scans were acquired from 55 healthy individuals ranging from 7 to 22 years old recruited for case control studies (Horga et al., 2011; Marsh et al., 2014; Marsh et al., 2011). The Institutional Review Board of the New York State Psychiatric Institute approved the study. Participants and their parents provided assent/consent.
Details of exclusion criteria, MRI pulse sequence, image processing, and behavioral analyses are described in the online Supporting Information (SI).
VIQ/PIQ Discrepancy
The verbal/spatial discrepancy was operationalized using VIQ and PIQ scores obtained from the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). The VIQ score derives from the Vocabulary and Similarities subtests, and PIQ from Block Design and Matrix Reasoning. Each participant’s VIQ score was regressed onto his/her PIQ score with the intercept set to zero, resulting in residuals that were the VIQ-regressed-on-PIQ (VIQ>PIQ) discrepancy scores (Margolis et al., 2013). These VIQ-regressed-on-PIQ residual ‘discrepancy’ scores were normally distributed.
fMRI Paradigm: Simon Task
Stimuli were presented with EPRIME software (Psychology Software Tools, Inc., Sharpsburg, Pennsylvania) and back-projected onto a screen in the scanner. White arrows pointing left or right were displayed against a black background to the left or right of a white gaze fixation cross-hair positioned at midline. Stimuli subtended 1 vertical and 3.92 horizontal degrees of the visual field. Stimuli were “congruent” (pointing in the same direction as their on-screen position), “incongruent” (pointing opposite their on-screen position), or “blank” (a cross-hair at midline). Participants were instructed to respond with their right hand to the direction of the arrow by pressing a button on a response box, the index finger for a left-pointing, and the middle finger for a right-pointing arrow. The button press recorded responses and reaction times (RTs) for each trial containing congruent (C) or incongruent (I) stimuli. Stimulus duration was 1300 msec, with a jittered interstimulus interval (mean = 5352 msec, SD = 842 msec, range = 4009 – 6857 msec). Each run contained 55 stimuli (5 min, 7 sec), with 22 congruent stimuli (11 left-pointing arrows presented to the left of midline; 11 right-pointing arrows presented to the right of midline), 22 incongruent stimuli (11 left-pointing arrows presented to the right of midline; 11 right-pointing arrows presented to the left of midline), and 11 blank stimuli (longer periods of fixation). Stimuli were arranged and presented in pseudorandom order (see SI).
Image Analysis
First-level parametric analyses were completed for each individual with a modified version of the general linear model function in SPM8 with a weighted least-squares algorithm (Wellcome Department of Imaging Neuroscience, http://www.fil.ion.ucl.ac.uk/spm/). Preprocessed blood oxygen level–dependent time series data at each voxel, concatenated from all three runs of the task (420 volumes), were modeled using a general linear model with the following regressors corresponding to each trial type: 1) congruent preceded by congruent (cC); 2) congruent preceded by incongruent (iC); 3) incongruent preceded by congruent (cI); 4) incongruent preceded by incongruent (iI); 5) blank trials preceded by C; 6) blank trials preceded by I; 7) fixation trials; 8) all correct trials; and 9) incorrect trials. These events were convolved with the canonical hemodynamic response function (Henson, Price, Rugg, Turner, & Friston, 2002). A first-order autoregression with restricted maximum likelihood algorithm estimated parameters for each independent variable and removed serial correlations in the fMRI time series. Parameter estimates for the three runs were averaged to produce beta maps for each trial type for each participant.
Resulting beta maps were entered into a second-level analysis in SPM8. General linear models assessed effects of VIQ>PIQ discrepancy score on brain activations during correct responses to I versus C trials (conflict resolution), and to cI versus cC trials (post-congruent conflict). Monte Carlo simulations (10,000 iterations) implemented in AFNI (v.16.0.01, Jan 27 2016) 3dClustSim generated a cluster extent threshold (K=38, 3 × 3 x 3 mm voxels) that was applied with an a priori significance threshold of p< 0.0125 to correct for multiple comparisons (p < 0.05, corrected). We applied parametric inference and report findings identified on contrast maps with this corrected p-value.
Hypothesis Testing
We tested the effects of the VIQ>PIQ discrepancy on brain activation during the resolution of conflict (I–C) and post-congruent conflict (cI-cC), controlling for age and sex. We additionally tested the effects of age and its interaction with the discrepancy on conflict-related activations, controlling for sex. We hypothesized that a greater magnitude of the VIQ>PIQ discrepancy would be associated with reduced activation of frontal and parietal regions, consistent with prior structural findings (Margolis et al., 2013). Using a psychophysiological interaction analysis (PPI, O’Reilly, Woolrich, Behrens, Smith, & Johansen-Berg, 2012), we explored whether the magnitude of the VIQ>PIQ discrepancy was associated with task-related functional connectivity within fronto-striatal and fronto-parietal circuits involved in conflict resolution. We further assessed whether PIQ was uniquely associated with neural activity during the resolution of conflict (I–C) on this spatial task.
Results
Participants
Fifty-five healthy participants ranging from 7 to 22 years old completed the Simon task. Table 1 summarizes participant characteristics and demographic variables. No participants were excluded from analyses due to excessive motion (greater than 3 mm, 1 voxel, in any direction) in the scanner or inability to perform the task.
Table 1.
Demographic data for all participants. FSIQ, Full-Scale Intelligence Quotient; PIQ, Performance Intelligence Quotient; VIQ, Verbal Intelligence Quotient.
| Characteristic | Male (N=12) | Female (N=43) | Total (N=55) |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Age (years) | 15.8 (4.7) | 16.2 (3.6) | 16.1 (3.8) |
| Age range (years) | 8–22 | 7–22 | 7–22 |
| VIQ | 110.3 (18.1) | 114.4 (18.1) | 113.5 (18.0) |
| PIQ | 110.3 (15.4) | 106.4 (17.0) | 107.3 (16.6) |
| FSIQ | 111.3 (17.6) | 111.8 (18.5) | 111.7 (18.2) |
Behavioral Performance
Analysis of errors revealed significant effects of sequence-by-gender (p < 0.01) and sequence (mean errors for repeated > alternated stimuli, p < 0.003), but no other main effects of, or interactions with, age or gender (ps > 0.11). Including the VIQ>PIQ discrepancy in the model also revealed significant sequence-by-gender (p < 0.022) and sequence (p < 0.003) effects, but no significant effect of, or interactions with, the VIQ>PIQ discrepancy (ps > 0.29). The sequence-by-gender effect was driven by a significant sequence effect (repeated > alternated) in girls (p < 0.017), but not boys (p = 0.314).
Analysis of RTs revealed significant effects of conflict-by-sequence-by-gender (p < 0.004), conflict (incongruent > congruent, p < 0.001), sequence (repeated > alternated, p < 0.01), age (p < 0.001) and gender (p < 0.023). Including VIQ>PIQ discrepancy in the model also revealed significant effects of conflict-by-sequence-by-gender (p < 0.005), conflict (p < 0.001), sequence (p < 0.019) and age (p < 0.001), but no significant effect of, or interactions with, the VIQ>PIQ discrepancy (ps > 0.19). Girls, but not boys, showed significant conflict and sequence effects (ps = 0.05, see SI and Table S1). Advancing age was associated with less post-congruent conflict (p < 0.02).
Analyses of Neural Activity
Cognitive conflict
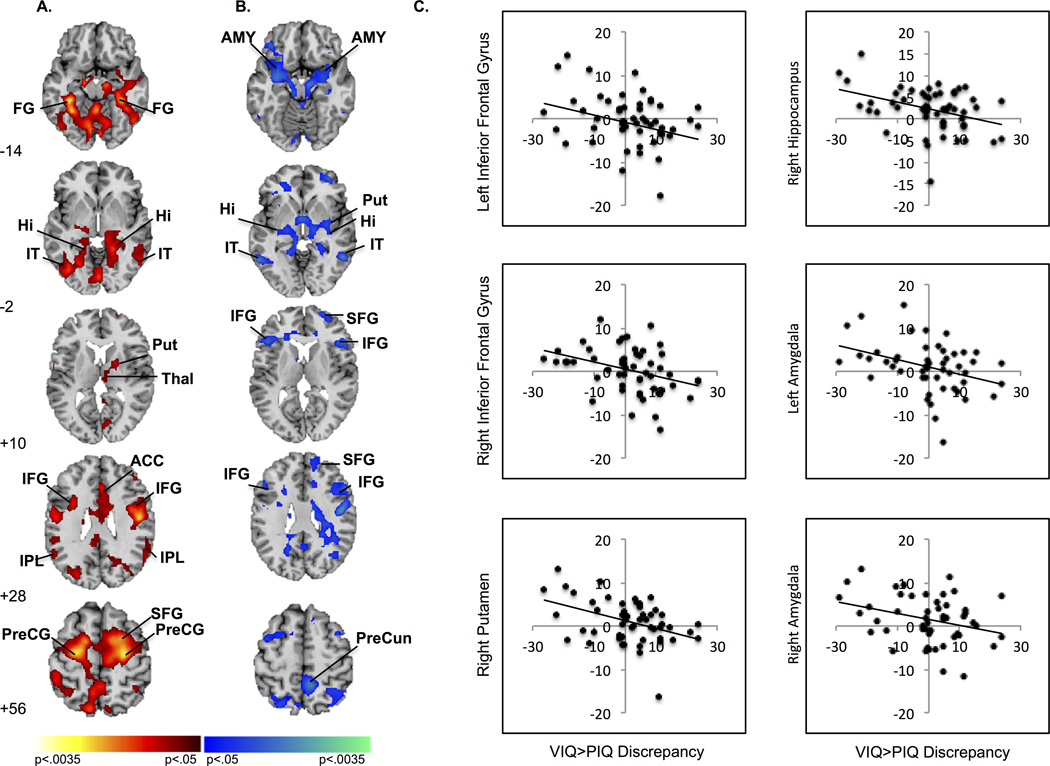
Activation associated with conflict resolution (I>C) was detected in one cluster comprising bilateral inferior frontal, anterior cingulate, and parietal regions, right frontal regions (superior, mid frontal, precentral gyri) and right putamen and a second cluster comprising bilateral temporal areas (inferior temporal, hippocampus, and fusiform gyri, Table 2, Figure 1a). The magnitude of the VIQ>PIQ discrepancy was inversely associated with activation in four clusters comprising: (1) bilateral inferior frontal, precentral gyri, amygdala and hippocampus, right precuneus, superior, and middle frontal gyri; (2) right middle orbital gyrus; (3) bilateral inferior temporal gyri; and (4) right putamen and middle temporal gyrus (Table 2, Figure 1b). As the magnitude of the VIQ>PIQ discrepancy increased, activation of these regions decreased (Figure 1c). Plots of the mean parameter estimates for congruent and incongruent stimuli show that as the VIQ>PIQ discrepancy increased, activation of right inferior frontal gyrus (IFG) decreased in response to incongruent stimuli while activation of left IFG and right putamen increased in response to both congruent and incongruent stimuli (Figure S1). These plots also reveal that as the discrepancy increased, deactivations (relative to baseline) in hippocampus and amygdala became less prominent in response to both types of stimuli (Figure S1). Activation in regions associated with the VIQ>PIQ discrepancy was neither associated with PIQ scores (Figure S2) nor with the age-by-discrepancy interaction.
Table 2.
Functional Brain Activity During Conflict Resolution. Global peak and total voxels reported for each cluster. Location of significant voxel reported for each region within each cluster.
| Activated Region | Side | Brodmann’s Area |
Number of Voxels |
Location (MNI) |
t |
|---|---|---|---|---|---|
| Activation during conflict resolution (Fig. 1a) | |||||
| Cluster 1 | 6819 | 24, −7, 61 | >2.31 | ||
| Superior frontal gyrus | R | 6 | 125 | 24, −7, 61 | 5.38 |
| recentral gyrus | R | 6 | 125 | 27, −13, 58 | 4.93 |
| Middle Frontal gyrus | R | 44 | 105 | 27, 2, 49 | 3.84 |
| Inferior parietal lobule | L | 40 | 105 | −39, −40, 37 | 4.25 |
| R | 40 | 78 | 56, −51, 23 | 3.52 | |
| Anterior cingulate cortex | L | 24 | 60 | 1, 25, 23 | 3.2 |
| R | 24 | 60 | 6, 25, 23 | 2.87 | |
| Inferior frontal gyrus | R | 45 | 65 | 44, 5, 25 | 2.72 |
| L | 45 | 39 | −35, 5, 25 | 2.15 | |
| Putamen | R | 47 | 23, 2, 7 | 2.77 | |
| Cluster 2 | 3752 | 6, −67, −29 | >2.31 | ||
| Fusiform gyrus | R | 37 | 109 | 33, −37, −14 | 4.31 |
| L | 37 | 95 | −33, −49, −14 | 4.35 | |
| Hippocampus | R | 28 | 104 | 15, −33, −7 | 3.79 |
| L | 28 | 68 | −30, −31, −7 | 2.55 | |
| Inferior temporal gyrus | L | 20 | 89 | −36, −37, −14 | 4.7 |
| R | 20 | 66 | 57, −55, −14 | 2.86 | |
| Association of VIQ>PIQ discrepancy with conflict resolution (Fig. 1b) | |||||
| Cluster 1 | 6997 | 9, −53, 62 | >2.31 | ||
| Middle frontal gyrus | R | 44 | 124 | 39, 8, 40 | 4.33 |
| Superior frontal gyrus | R | 9 | 111 | 21, 20, 37 | 4.43 |
| Amygdala | R | 25 | 103 | 27, −2, −17 | 2.91 |
| L | 25 | 53 | −21, −7, −23 | 4.16 | |
| Precentral Gyrus | R | 4 | 97 | 54, 23, 13 | 4.23 |
| L | 4 | 112 | −36, −1, 43 | 4.16 | |
| Precuneus | R | 7 | 99 | 9, −49, 58 | 4.44 |
| Inferior frontal gyrus | R | 45 | 95 | 48, 23, 13 | 4.36 |
| L | 45 | 60 | −38, 38, −8 | 2.71 | |
| Hippocampus | R | 28 | 49 | 34, −25, −10 | 2.77 |
| L | 28 | 53 | 21, −7, −23 | 4.16 | |
| Cluster 2 | 179 | 36, 46, 0 | >2.31 | ||
| Middle orbital gyrus | R | 11 | 47 | 36, 46, 0 | 3.65 |
| Cluster 3 | 154 | −60, −59, 0 | >2.31 | ||
| Inferior temporal gyrus | R | 20 | 79 | 53, −50, −5 | 3.34 |
| L | 20 | 61 | −60, −55, −5 | 5.88 | |
| Cluster 4 | 100 | 54, −56, 3 | >2.31 | ||
| Putamen | R | 90 | 34, −7, −5 | 3.49 | |
| Middle temporal gyrus | R | 21 | 82 | 54, −52, −2 | 4.03 |
Figure 1.
Average brain activations associated with conflict resolution. (A) Increased activation during conflict resolution was detected in frontal, temporal, parietal, and striatal regions. Increases in signal are in red and decreases in blue. (B) Activation during conflict resolution in frontostriatal regions, hippocampus, and amygdala inversely associated with VIQ>PIQ discrepancy. Positive correlations are in red and inverse in blue. (C) Scatterplots demonstrate decreasing activation in left (−38,38,−8) and right (48,23,13) IFG, left (−21, −7, −23) and right (27, −2, −18) amygdala, and right Put (34,−7,−5 ) and Hippocampus (−34, −25, −10) with increasing magnitude of VIQ>PIQ discrepancy. Maps (generated with MRIcroN; McCausland Center for Brain Imaging, Columbia, South Carolina) are thresholded at p < .0125, with cluster filter 38. ACC, anterior cingulate cortex; AMY, amygdala; FG, fusiform gyrus; Hi, hippocampus; IFG, inferior frontal gyrus; IPL, inferior parietal lobule; IT, inferior temporal gyrus; PreCG, precentral gyrus; PreCun, precuneus; Put, putamen; Thal, thalamus; SFG, superior frontal gyrus.
Post-congruent conflict
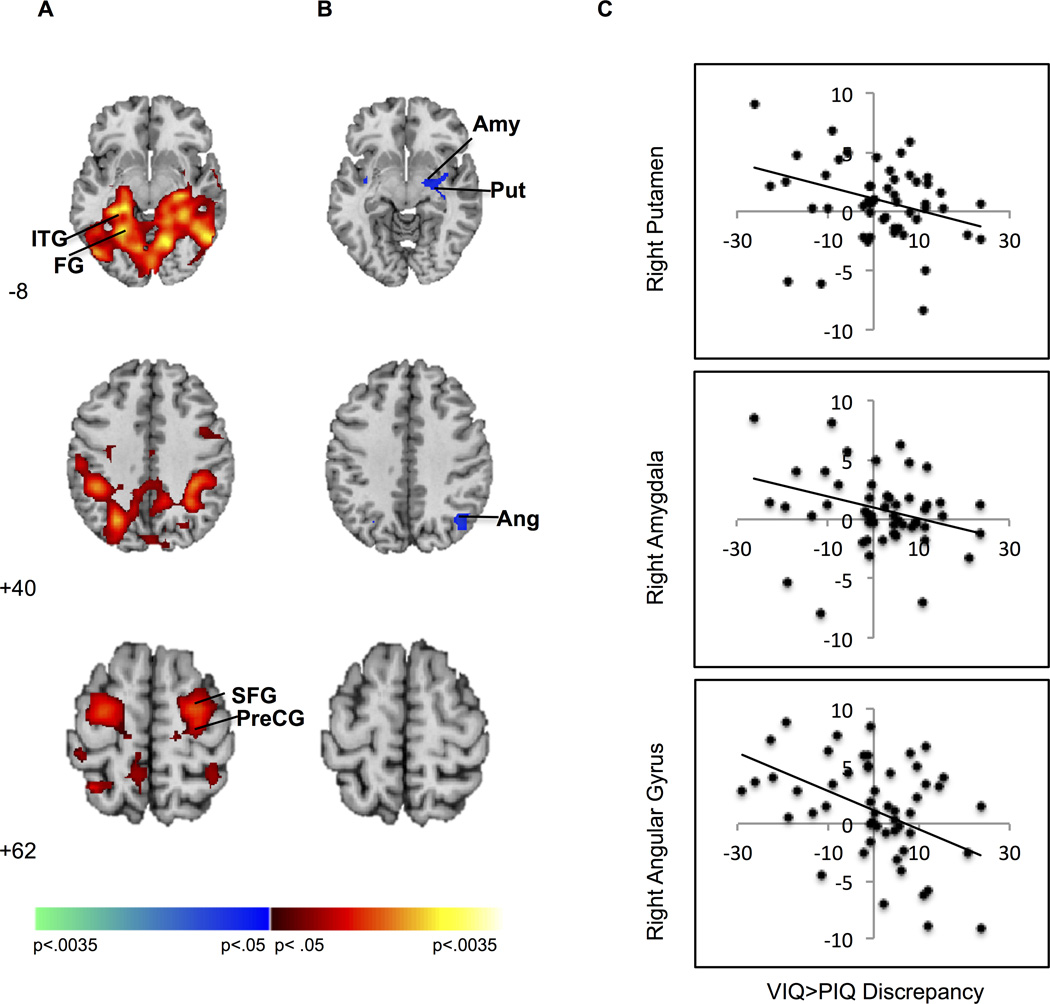
Activation associated with post-congruent conflict resolution (cI>cC) was detected in one cluster comprising bilateral fusiform and lingual gyri and left inferior temporal gyrus, and a second comprising right precentral and superior frontal gyrus (Fig 2a). The magnitude of the VIQ>PIQ discrepancy was inversely associated with activation of right hemisphere regions: one subcortical cluster comprised putamen and amygdala and a smaller cluster comprised angular gyrus (Figure 2b, Table 3). Activation of these regions decreased as the magnitude of the VIQ>PIQ discrepancy increased (Figure 2c). To explore whether this effect was associated with conflict or trial sequence, parameter estimates from the putamen, amygdala, and angular gyrus (corresponding to each trial type -- cI, cC, iC, iI -- for each participant) were entered as dependent variables in separate repeated measures linear models with congruence and sequence as within-subjects factors and the VIQ>PIQ discrepancy and age as between-subjects factors. Significant interactions of the discrepancy with congruence (ps < 0.002) but not sequence (ps > 0.75) were detected in amygdala and putamen, indicating that decreased activation associated with the discrepancy represented reduced conflict (rather than sequence) processing. A significant interaction of the discrepancy with both congruence and sequence (p < 0.001) in right angular gyrus suggests that decreased activation associated with the discrepancy in this region represented both reduced conflict and sequence processing.
Figure 2.
Post-congruent conflict effects. (A) Increased activation during post-congruent conflict was detected in frontal and temporal regions. Increases in signal are in red and decreases in blue. (B) The VIQ>PIQ discrepancy inversely associated with activation during post-congruent conflict resolution in amygdala, putamen, and angular gyrus. Positive correlations are in red and inverse in blue. (C) Scatterplots demonstrate decreased activation in right Put (33, −9, −8), AMY (30, −7, −11) and angular gyrus (39, −64, 37) with increasing magnitude of the VIQ>PIQ discrepancy. Maps are thresholded at p < .0125, with cluster filter 38. AMY, amygdala; Ang, angular gyrus; FG, fusiform gyrus; ITG, inferior temporal gyrus; PreCG, precentral gyrus; Put, putamen; SFG, superior frontal gyrus.
Table 3.
Association of VIQ>PIQ discrepancy with post-congruent conflict resolution. Global peak and total voxels reported for each cluster. Location of significant voxel reported for each region within each cluster.
| Activated Region | Side | Brodmann’s Area |
Number of Voxels |
Peak Location (MNI) |
t |
|---|---|---|---|---|---|
| Activation during post-congruent conflict resolution (Fig. 2a) | |||||
| Cluster 1 | 10505 | −36,−37,−14 | >2.31 | ||
| Inferior temporal gyrus | L | 20 | 121 | −36,−37,−14 | 5.87 |
| Fusiform gyrus | L | 37 | 125 | −27, −55, −11 | 5.19 |
| R | 37 | 125 | 33, −40, −11 | 4.89 | |
| Lingual gyrus | L | 19 | 125 | −24, −64, −2 | 5.05 |
| R | 19 | 120 | 21, −46, −2 | 4.92 | |
| Cluster 2 | 623 | 30, −7, 58 | >2.31 | ||
| Superior frontal gyrus | R | 6 | 125 | 30, −7, 58 | 3.82 |
| Precentral gyrus | R | 4 | 97 | 27, −19, 67 | 3.61 |
| Association of VIQ>PIQ discrepancy with post-congruent conflict resolution (Fig. 2b) | |||||
| Cluster 1 | 126 | 30, −7, −11 | >2.31 | ||
| Amygdala | R | 25 | 53 | 30, −7, −11 | 2.78 |
| Putamen | R | 41 | 33, −9, −8 | 2.46 | |
| Cluster 2 | 55 | 39, −64, 37 | >2.31 | ||
| Angular gyrus | R | 39 | 49 | 39, −64, 37 | 2.98 |
Age effects
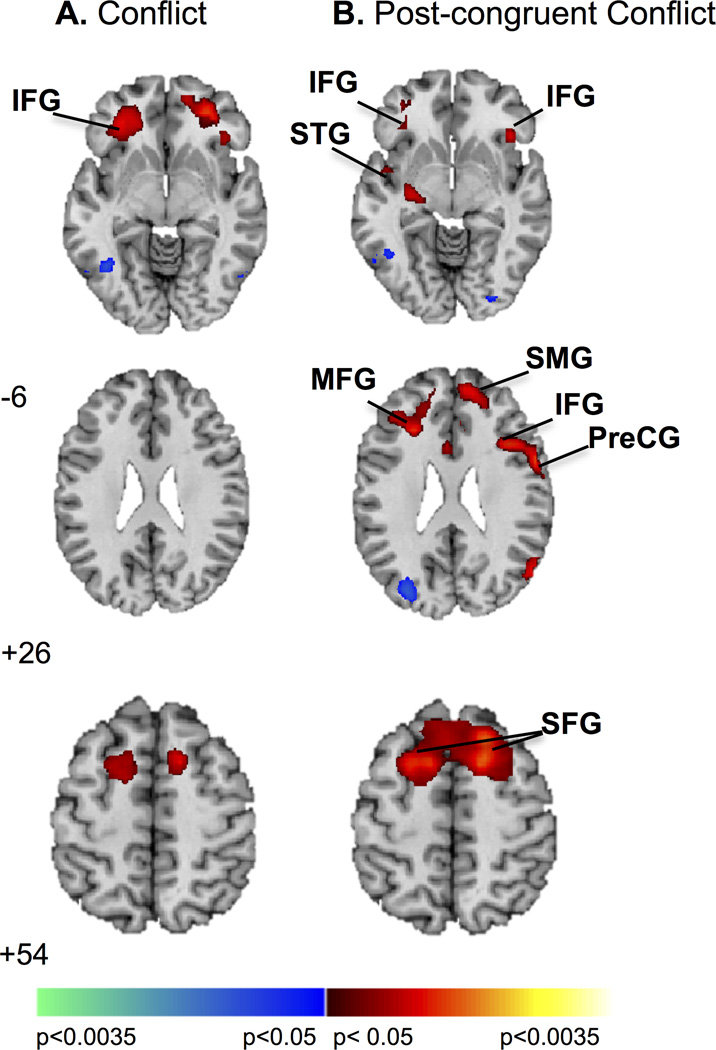
Age was positively associated with activation of left inferior frontal gyrus during conflict (I>C), and with activation in four clusters during post-congruent conflict (cI>cC) comprising: (1) bilateral superior frontal and right superior medial; (2) left middle frontal; (3) right precentral and inferior frontal; and (4) left superior temporal and inferior frontal gyri (Table S2, Figure 3). With advancing age, activation of left inferior frontal gyrus increased during conflict, while activation of these bilateral regions increased during post-congruent conflict.
Figure 3.
Age effects. (A) Age was positively associated with activation in left IFG during conflict resolution. Increases in signal are in red and decreases in blue. (B) During resolution of post-congruent conflict increased activation in bilateral inferior and superior frontal gyri, right superior medial and precentral gyri, and left middle frontal and superior temporal gyri associated with increasing age. Increases in signal are in red. Maps are thresholded at p < .0125, with a cluster filter of 38. IFG, inferior frontal gyrus; MFG, middle frontal gyrus; PreCG, precentral gyrus; SFG, superior frontal gyrus; SMG, superior medial gyrus; STG, superior temporal gyrus.
Exploratory analysis of functional connectivity
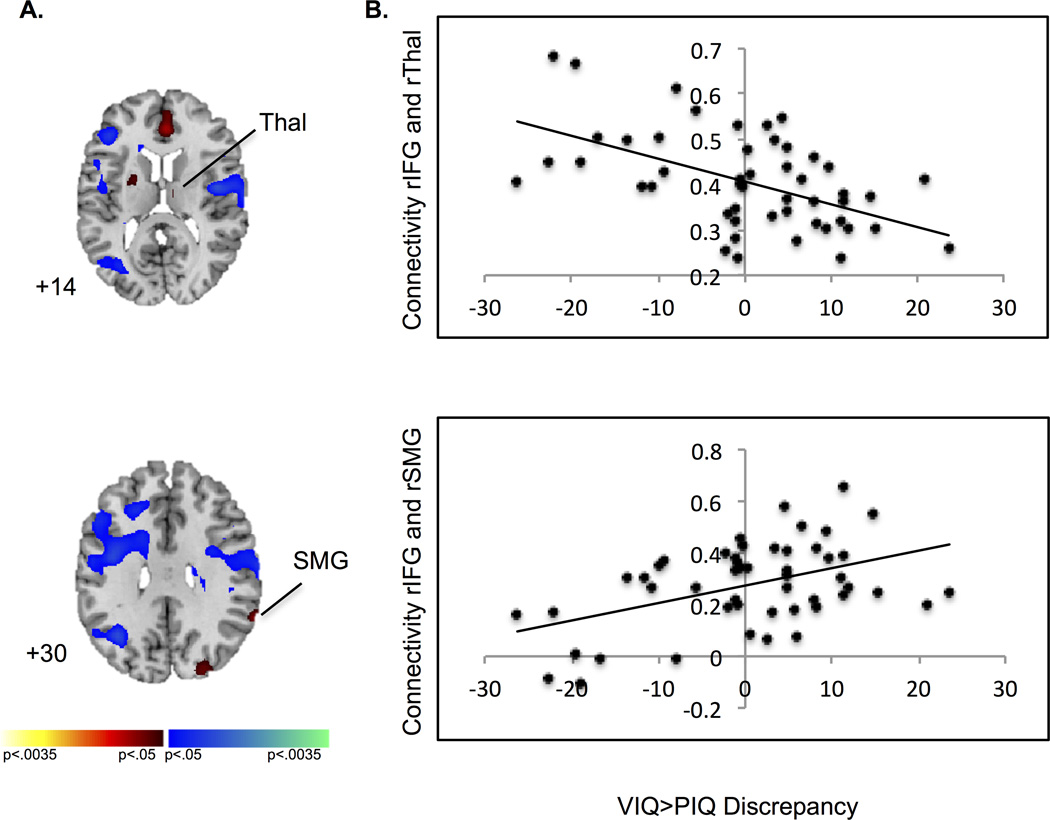
A PPI analysis explored effects of the VIQ>PIQ discrepancy on conflict-related connectivity within fronto-striatal and fronto-parietal circuits. A seed was placed in the right IFG (48, 23, 13, with a 1-mm radius sphere), corresponding to the peak-level of significance in which activation during conflict resolution was associated with the VIQ>PIQ discrepancy in our a priori hypothesis test. We narrowed this analysis to this cluster because the right IFG is commonly implicated in control processes (Hampshire, Chamberlain, Monti, Duncan, & Owen, 2010), and since activation of right IFG during correct responses to incongruent stimuli decreased with the increasing magnitude of the VIQ>PIQ discrepancy. Connectivity from right IFG to bilateral fronto-parietal, temporal, and occipital regions, right thalamus, and left putamen was detected during conflict (p < 0.05, k=38, uncorrected, Table S3, Figure 4). Task-dependent changes in connectivity from right IFG to right SMG and thalamus varied with the magnitude of the VIQ>PIQ discrepancy (ps < 0.03) such that connectivity to SMG increased and connectivity to thalamus decreased as the magnitude of the VIQ>PIQ discrepancy increased (Figure 4b).
Figure 4.
Psychophysiological interaction analysis. (A) During conflict resolution, connectivity from right IFG (48, 23, 13 with a 1-mm radius sphere) to left putamen (−15, 5, −2), right thalamus (9, −13, 13), and right SMG (63, −49, 31) was detected. Increases in connectivity during task conditions are in red and decreases in blue. (B) Scatterplots demonstrate as the VIQ>PIQ discrepancy increased, task-dependent functional connectivity decreased from right IFG to right THAL, and increased from rIFG to right SMG during conflict resolution (ps < 0.03). Maps are thresholded at p < .05, uncorrected. IFG, inferior frontal gyrus; SMG, supramarginal gyrus; THAL, thalamus.
Discussion
This is the first study to show that a verbal-spatial IQ discrepancy is associated with specific patterns of brain activation during conflict resolution in healthy children and adolescents. As the magnitude of the VIQ>PIQ discrepancy increased, conflict-related activations decreased in fronto-striatal, temporal, and limbic regions. A greater VIQ>PIQ discrepancy was also associated with reduced functional connectivity from right IFG to right thalamus, and increased connectivity from right IFG to right SMG. Thus, individuals with greater verbal compared to spatial abilities may rely less on fronto-striatal circuits to resolve cognitive conflict, consistent with the phenomenological difficulty with cognitive control functions reported in neurodevelopmental disorders characterized by this discrepancy (Davis & Broitman, 2011; Hong et al., 2009; Hooper et al., 2008; Woodin et al., 2001).
Activation of fronto-striatal, parietal, and temporal regions (inferior temporal and fusiform gyri and hippocampus) increased during conflict resolution, but conflict-related activation of right IFG and putamen decreased with increasing magnitude of the VIQ>PIQ discrepancy, perhaps reflecting reduced conflict processing within these regions in individuals with greater verbal compared to spatial abilities. A greater VIQ>PIQ discrepancy was also associated with decreased activation of limbic regions (hippocampus and amygdala) that derived from deactivations becoming less prominent in response to all stimuli. Less prominent deactivations within hippocampus in participants with greater verbal than spatial abilities may suggest difficulty allocating attentional resources during the Simon task, consistent with evidence that the hippocampus is part of the default mode network (Buckner, Andrews-Hanna, & Schacter, 2008). Alternatively, this finding may reflect attempts to use hippocampal-based, overt memory strategies to resolve conflict. Amygdala deactivation during conflict resolution is unusual, however, especially in the absence of emotional stimuli.
Exploratory analyses suggested task-dependent changes in connectivity from right IFG to right thalamus and right supramarginal gyrus during conflict resolution, but the VIQ>PIQ discrepancy was associated with less connectivity to right thalamus and greater connectivity to right SMG. Given the influence of the thalamus over the functioning of cortico-striato-thalamo-cortical circuits via its projections to cortex and striatum (Haber & Calzavara, 2009), reduced connectivity between right IFG and thalamus may further suggest reduced conflict processing within these circuits in individuals with greater verbal compared to spatial abilities. Increased connectivity between right IFG and SMG may reflect increased processing within fronto-parietal circuits to attend to and select trial specific information (Sheffield et al., 2015). The combination of reduced conflict processing and difficulty selecting relevant stimuli may contribute to the cognitive control deficits observed in individuals with disorders characterized by VIQ>PIQ discrepancies, such as Fragile X (Hooper et al., 2008), Non-Verbal Learning Disability (Davis & Broitman, 2011), 22q11 deletion (Woodin et al., 2001), and Turner Syndrome (Hong et al., 2009). However, neither the behavioral nor neural effects of the VIQ>PIQ discrepancy have been studied in these populations.
Conflict effects are greatest when incongruent trials are preceded by congruent trials (Gratton, Coles, & Donchin, 1992). Post-congruent conflict resolution was accompanied by activation of right precentral and superior frontal gyri, consistent with prior findings (Horga et al., 2011; Marsh et al., 2014). A greater magnitude of the VIQ>PIQ discrepancy was associated with decreased post-congruent conflict-related activation of a right hemisphere subcortical cluster comprised of putamen and amygdala, and a cluster including right angular gyrus. Decreased conflict-related activation of putamen may suggest under-engagement of an automatic response tendency based within this region (Packard & Knowlton, 2002). Decreased activation of angular gyrus represented reduced processing of both conflict and sequence in individuals with greater verbal than spatial abilities, consistent with the role of right angular gyrus in orienting attention (Rushworth, Ellison, & Walsh, 2001). Application of single pulse transcranial magnetic stimulation to this region following stimulus onset decreases response times to incongruent stimuli, thereby reducing the Simon conflict effect in healthy adults (Schiff, Bardi, Basso, & Mapelli, 2011). Thus, decreased activation of right angular gyrus in individuals with greater verbal than spatial abilities likely reflects overall difficulty processing spatial stimuli.
Our data suggest developmental shifts in activation patterns associated with conflict and post-congruent conflict resolution. With increasing age, participants demonstrated faster responses, less cognitive conflict, and increased activation of bilateral inferior and superior frontal cortices. Previous data suggest age-related increases in activation of inferior frontal cortices during the engagement of cognitive control (Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002), and age-related increases in activation of bilateral inferior frontal, posterior cingulate, and right anterior cingulate cortices during post-congruent conflict resolution (Durston et al., 2002). Our results suggest that with increasing age, increased activation of bilateral frontal cortices facilitates improved conflict resolution. Increased right hemisphere involvement during the more complex task of resolving post-congruent conflict is consistent with right hemisphere dominance over attentional control (Corbetta & Shulman, 2002). Age-related increases in activation of bilateral superior and anterior frontal cortices are also consistent with the temporal sequence of brain development from ventral to dorsal and posterior to anterior regions (Gogtay et al., 2004). Thus, with increasing age, improved processing within more dorsal and anterior frontal regions likely allows for improved ability to resolve the greatest cognitive conflict.
Few studies have examined the structural or functional correlates of IQ discrepancies in health or illness. Prior data suggest that CT in bilateral IFG varies with the magnitude of the VIQ-PIQ discrepancy, decreasing with increases in VIQ>PIQ and increasing with increases in PIQ>VIQ (Margolis et al., 2013). Thus, decreased IFG activation in association with VIQ>PIQ (or increased activation with PIQ>VIQ) may represent a structure-function relationship within this region such that decreased/increased CT is associated with deficient/heightened processing in individuals with greater verbal/spatial abilities. Relative to healthy individuals, those with 22q11 deletion syndrome (Montojo et al., 2015) and Fragile X (Menon, Leroux, White, & Reiss, 2004) demonstrate decreased activation of fronto-striatal regions and hippocampus during response inhibition. Conversely, those with ASD (PIQ>VIQ) demonstrate increased activation of these regions during inhibition (Schmitz et al. 2006). Thus, the cognitive discrepancy (VIQ>PIQ or PIQ>VIQ) may be a dimensional, behavioral marker for functional impairments in cognitive control processes that are magnified in clinical samples. As the magnitude of the discrepancy increases in either direction, conflict-related activation of control circuits increases or decreases, perhaps underlying individual differences in cognitive control. Future studies should assess the association of the VIQ-PIQ discrepancy with functional neural correlates of cognitive control deficits in patient populations characterized by these discrepancies, as well as with measures of psychosocial function and other cognitive processes.
Our study has some limitations. The relatively small number of participants limits our ability to stratify the sample and assess differences in brain activation between participants with large versus small verbal-spatial discrepancies. Our sample comprises more females than males, however we controlled for sex in all analyses. We did not assess effects of the VIQ>PIQ discrepancy on brain function in adults who may demonstrate different patterns of conflict-related activations. However, our study adds to the body of literature on the development of cognitive control capacities from childhood through adolescence.
Conclusion
Children and adolescents with greater verbal relative to spatial abilities under-engage fronto-striatal regions and deactivate limbic regions during conflict resolution. Reduced fronto-thalamic connectivity may further reflect reduced processing within the fronto-striatal components of cortico-striato-thalamo-cortical circuits, and increased fronto-parietal connectivity may reflect a need for greater activation of these regions in order to attend to and select relevant trial stimuli. Thus individuals with greater verbal than spatial abilities may have a combination of reduced conflict processing and difficulty selecting and attending to relevant stimuli, which may in turn underlie the cognitive control problems associated with neurodevelopmental disorders characterized by the discrepancy. Together, these findings suggest the VIQ>PIQ discrepancy may be an important aspect of an individual’s cognitive profile that may contribute to deficient cognitive control processes characteristic of many childhood disorders. Future studies should examine whether this brain-behavior relationship is magnified in children with neurodevelopmental disorders characterized by verbal-spatial discrepancies.
Supplementary Material
Research Highlights.
Cognitive control deficits are present in childhood disorders characterized by verbal-spatial discrepancies, such as Autism Spectrum Disorders, Non-Verbal Learning Disability, Fragile X, 22q11 deletion, and Turner Syndrome.
Previous data suggest the magnitude of the verbal-spatial discrepancy is associated with reduced thickness in frontal and parietal cortices that support cognitive control.
Current findings suggest children and adolescents with greater verbal relative to spatial abilities under-engage fronto-striatal regions and deactivate limbic regions during conflict resolution, one aspect of cognitive control.
The VIQ>PIQ discrepancy is likely an important aspect of an individual’s cognitive profile that may contribute to deficient cognitive control processes characteristic of many childhood disorders.
Acknowledgments
This work was supported by The NVLD Project, Promise Project at Columbia, NIMH grant R01MH090062, NIDA-AACAP 2K12DA000357-11, and the Klingenstein Foundation.
References
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33(2):301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgaleta M, Johnson W, Waber DP, Colom R, Karama S. Cognitive ability changes and dynamics of cortical thickness development in healthy children and adolescents. Neuroimage. 2014;84:810–819. doi: 10.1016/j.neuroimage.2013.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman T, Pickles A, Simonoff E, Chandler S, Loucas T, Baird G. IQ in children with autism spectrum disorders: data from the Special Needs and Autism Project (SNAP) Psychological Medicine. 2011;41(3):619–627. doi: 10.1017/S0033291710000991. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews: Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Craft JL, Simon JR. Processing symbolic information from a visual display: interference from an irrelevant directional cue. Journal of Experimental Psychology. 1970;83(3):415–420. doi: 10.1037/h0028843. [DOI] [PubMed] [Google Scholar]
- Davis JM, Broitman J. Nonverbal Learning Disability in Children: Bridging the gap between science and practice. New York, N.Y: Springer; 2011. [Google Scholar]
- Diamond A, Barnett WS, Thomas J, Munro S. Preschool program improves cognitive control. Science. 2007;318(5855):1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl JJ, Frost SJ, Sherman G, Mencl WE, Kurian A, Molfese P, Pugh KR. Neural correlates of language and non-language visuospatial processing in adolescents with reading disability. Neuroimage. 2014;101:653–666. doi: 10.1016/j.neuroimage.2014.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang Y, Ulug AM, Zimmerman RD, Casey BJ. A neural basis for the development of inhibitory control. Developmental Science. 2002;5(4):F9–F16. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Science U S A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. Optimizing the use of information: strategic control of activation of responses. Journal of Experimental Psychology: General. 1992;121(4):480–506. doi: 10.1037//0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- Haber SN, Calzavara R. The cortico-basal ganglia integrative network: the role of the thalamus. Brain Research Bulletin. 2009;78(2–3):69–74. doi: 10.1016/j.brainresbull.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50(3):1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Price CJ, Rugg MD, Turner R, Friston KJ. Detecting latency differences in event-related BOLD responses: application to words versus nonwords and initial versus repeated face presentations. Neuroimage. 2002;15(1):83–97. doi: 10.1006/nimg.2001.0940. [DOI] [PubMed] [Google Scholar]
- Hong D, Scaletta Kent J, Kesler S. Cognitive profile of Turner syndrome. Dev Disabil Res Rev. 2009;15(4):270–278. doi: 10.1002/ddrr.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper SR, Hatton D, Sideris J, Sullivan K, Hammer J, Schaaf J, Bailey DP., Jr Executive functions in young males with fragile X syndrome in comparison to mental age-matched controls: baseline findings from a longitudinal study. Neuropsychology. 2008;22(1):36–47. doi: 10.1037/0894-4105.22.1.36. [DOI] [PubMed] [Google Scholar]
- Horga G, Maia TV, Wang P, Wang Z, Marsh R, Peterson BS. Adaptation to conflict via context-driven anticipatory signals in the dorsomedial prefrontal cortex. Journal of Neuroscience. 2011;31(45):16208–16216. doi: 10.1523/JNEUROSCI.2783-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain and Cognition. 2010;72(1):101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis A, Bansal R, Hao X, Algermissen M, Erickson C, Klahr KW, Peterson BS. Using IQ Discrepancy Scores To Examine the Neural Correlates of Specific Cognitive Abilities. Journal of Neuroscience. 2013;33(35):14135–14145. doi: 10.1523/JNEUROSCI.0775-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R, Horga G, Parashar N, Wang Z, Peterson BS, Simpson HB. Altered activation in fronto-striatal circuits during sequential processing of conflict in unmedicated adults with obsessive-compulsive disorder. Biological Psychiatry. 2014;75(8):615–622. doi: 10.1016/j.biopsych.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R, Horga G, Wang Z, Wang P, Klahr KW, Berner LA, Peterson BS. An FMRI study of self-regulatory control and conflict resolution in adolescents with bulimia nervosa. American Journal of Psychiatry. 2011;168(11):1210–1220. doi: 10.1176/appi.ajp.2011.11010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Leroux J, White CD, Reiss AL. Frontostriatal deficits in fragile X syndrome: relation to FMR1 gene expression. Proceedings of the National Academy of Science U S A. 2004;101(10):3615–3620. doi: 10.1073/pnas.0304544101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montojo CA, Jalbrzikowski M, Congdon E, Domicoli S, Chow C, Dawson C, Bearden CE. Neural substrates of inhibitory control deficits in 22q11.2 deletion syndrome. Cerebral Cortex. 2015;25(4):1069–1079. doi: 10.1093/cercor/bht304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly JX, Woolrich MW, Behrens TE, Smith SM, Johansen-Berg H. Tools of the trade: psychophysiological interactions and functional connectivity. Social Cognitive and Affective Neuroscience. 2012;7(5):604–609. doi: 10.1093/scan/nss055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annual Review of Neuroscience. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Rice, Mabel L, Warren, Steven F, Betz, Stacy K. Language symptoms of developmental language disorders: An overview of autism, Down syndrome, fragile X, specific language impairment, and Williams syndrome. Applied Psycholinguistics. 2005;26(1):7–27. [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Human Brain Mapping. 2006;27(12):973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Ellison A, Walsh V. Complementary localization and lateralization of orienting and motor attention. Nature Neuroscience. 2001;4(6):656–661. doi: 10.1038/88492. [DOI] [PubMed] [Google Scholar]
- Schiff S, Bardi L, Basso D, Mapelli D. Timing spatial conflict within the parietal cortex: a TMS study. Journal of Cognitive Neuroscience. 2011;23(12):3998–4007. doi: 10.1162/jocn_a_00080. [DOI] [PubMed] [Google Scholar]
- Schmitz N, Rubia K, Daly E, Smith A, Williams S, Murphy DG. Neural correlates of executive function in autistic spectrum disorders. Biol Psychiatry. 2006;59:7–16. doi: 10.1016/j.biopsych.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Schnack HG, van Haren NE, Brouwer RM, Evans A, Durston S, Boomsma DI, Hulshoff Pol HE. Changes in thickness and surface area of the human cortex and their relationship with intelligence. Cerebral Cortex. 2015;25(6):1608–1617. doi: 10.1093/cercor/bht357. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(7084):676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Sheffield JM, Repovs G, Harms MP, Carter CS, Gold JM, MacDonald AW, 3rd, Barch DM. Fronto-parietal and cingulo-opercular network integrity and cognition in health and schizophrenia. Neuropsychologia. 2015;73:82–93. doi: 10.1016/j.neuropsychologia.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vygotsky LS. In: The Essential Vygotsky. Rieber RW, Robinson KD, editors. New York, NY: Kluwer Academic/Plenum Publishers; 2004. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. New York, NY: The Psychological Corporation: Harcourt Brace & Company; 1999. [Google Scholar]
- Woodin M, Wang PP, Aleman D, McDonald-McGinn D, Zackai E, Moss E. Neuropsychological profile of children and adolescents with the 22q11.2 microdeletion. Genetics in Medicine. 2001;3(1):34–39. doi: 10.1097/00125817-200101000-00008. [DOI] [PubMed] [Google Scholar]
- Yokota S, Takeuchi H, Hashimoto T, Hashizume H, Asano K, Asano M, Sassa Y, Taki Y, Kawashima R. Individual differences in cognitive performance and brain structure in typically developing children. Dev Cogn Neurosci. 2015;14:1–7. doi: 10.1016/j.dcn.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.