Abstract
Background and Objectives
Loxapine for inhalation is a drug-device combination product approved in adults for the acute treatment of agitation associated with schizophrenia or bipolar I disorder. The primary objective of this study was to develop a clinical trial protocol to support a phase I pharmacokinetics (PK) study in children aged 10 years and older. In addition, this report details the results of the clinical study in relation to predicted likelihood of achieving target exposure associated with therapeutic effect in adults.
Methods
A nonlinear mixed-effects population PK model was developed using adult data and was adjusted for the targeted pediatric age groups by applying allometric scaling to account for body size effects. Based on this pediatric model, age-appropriate regimens to achieve loxapine exposures similar to the ones associated with therapeutic effect in the adult studies were identified via trial simulation. D-optimal design and power analysis were conducted to identify optimal PK sampling times and sample size, respectively.
Results and Conclusion
The developed clinical trial design formed the basis of a phase I study to assess the safety and pharmacokinetics of loxapine for inhalation in children aged 10 years and older (ClinicalTrials.gov ID: NCT02184767). The results of the study indicated that overall loxapine exposures were consistent with what had been predicted by the trial simulations. The presented approach illustrates how modeling and simulation can assist in the design of informative clinical trials to identify safe and effective doses and dose ranges in children and adolescents.
Keywords: children, clinical trial simulation, loxapine, pediatrics, pharmacometrics, population pharmacokinetics
1. INTRODUCTION
Loxapine is a dibenzoxazepine compound, a subclass of tricyclic antipsychotic agents, chemically distinct from the phenothiazines, butyrophenones, and thioxanthenes [1, 2]. It may reduce agitation via antagonism of central dopamine (D1, D2, D3, and D4) receptors and serotonin 5-HT (2a) receptors. Loxapine 10 mg for inhalation (Adasuve®; manufactured by Alexza Pharmaceuticals, Inc., Mountain View, CA, for Teva Select Brands, a division of Teva Pharmaceuticals USA, Horsham, PA) is a drug-device combination product using the Staccato® technology that delivers the antipsychotic loxapine by oral inhalation [3]. Staccato loxapine represents a novel orally inhaled medication and has been approved for the acute treatment of agitation associated with schizophrenia or bipolar I disorder in adults [4–6]. Despite a very low incidence of bipolar I disorder diagnosed prior to age 10 or schizophrenia diagnosed prior to age 13, data suggest that the prevalence of these psychotic disorders during the adolescent years can be as high as that of the adult population [7, 8]. A recent clinical trial and case report indicate that loxapine (5–15 mg; formulation unidentified) can successfully treat irritable adolescents with good efficacy and tolerability [9, 10]. These findings have prompted an evaluation of the safety, efficacy, and pharmacokinetics of loxapine for inhalation in children and adolescents.
In adults, administration of loxapine for inhalation results in rapid absorption, with a median time to maximum plasma concentration (tmax) of 2 minutes and a mean terminal half-life (t1/2) of approximately 6 hours [11]. Loxapine is metabolized extensively by cytochrome P450 enzyme isoforms including CYP1A2, CYP2D6, and CYP3A4 in the liver with multiple metabolites formed [12]. The major metabolite, 8-OH-loxapine, is not pharmacologically active. Other metabolites include 7-OH-loxapine (active) and N-oxides of loxapine (inactive) [12, 13]. Loxapine is also metabolized to amoxapine, a tricyclic antidepressant [13]. A substantial proportion of loxapine and its metabolites is excreted within the first 24 hours after drug administration, mainly through the urine (56%–70%) and, to a lesser extent, via the feces (15%–22%). Loxapine exposure was shown to be dose proportional over the evaluated dosing range from 0.625 mg to 10 mg [11].
The objective of the current study was to develop a pediatric pharmacokinetic (PK) population model for loxapine to be used for clinical trial simulations in support of dose selection, sampling strategy, and sample size determination for a phase I study in pediatric patients aged 10 to 17 years, inclusive. The actual data observed in the pediatric phase I PK study were compared with the predicted exposures from the modeling and simulation analysis.
2. METHODS
2.1. Pediatric Population PK Model Development
2.1.1. Adult Population PK Model
In the first step, a population PK model was constructed on the basis of loxapine concentration data in healthy adults after administration of loxapine for inhalation as part of two phase I studies [11, 14]. Subject characteristics and study information are summarized in Online Resource 1. A total of 884 plasma concentrations collected at multiple time points over 24 hours in 71 healthy adult subjects was available for population PK modeling. Plasma samples were assayed for loxapine and its metabolites using a validated liquid chromatography tandem mass spectrometry assay [15]. Population PK analysis was conducted by nonlinear mixed effects modeling with NONMEM software, version 7.2.0 (ICON, Ellicott City, MD). The first-order conditional estimation with interaction (FOCE-I) method was employed throughout [16]. Shrinkage for inter-individual variability (IIV) and residual errors was estimated as part of diagnostic assessments [17]. Visualization of NONMEM output was implemented with Xpose4 package in R (v 3.0.3) [18].
The IIV model was described as:
| (1) |
where θi represents the value of the PK parameter θ for the ith subject; θTV is the typical value of parameter θ. The deviation of θ from the mean θTV was approximated with ηi, which was assumed to follow a normal distribution with a mean of 0 and a variance of ω2 (ie, ηi ~N[0, ω2]) [19].
The residual variability was described by a combined additive and proportional model, but other residual error models, such as exponential, additive, or proportional, were also examined. The combined additive and proportional residual model was described as:
| (2) |
where Cij represents the jth observed concentration in the ith individual, ij is the jth model predicted concentration in the ith individual, and εpij and εaij are the proportional and additive residual random errors, respectively. εpij and εaij are assumed to be independently normally distributed with a mean of 0 and a variance of σ2 (ie, εpij~N[0, σp2] and εaij ~N[0, σa2]) [19].
A variety of possible compartmental PK models was explored. Model selection was based on various goodness-of-fit indicators, including visual inspection of diagnostic scatter plots, comparisons based on the minimum objective function value (OFV), and evaluation of the estimates of population fixed and random effect parameters [16].
Demographic data including age, body weight, height, sex, race, body mass index, body surface area, and smoking status were evaluated as part of the covariate analysis, which used a stepwise forward addition and backward elimination approach. The difference in the OFVs from nested models was assumed to be χ2 distributed. A drop in OFV of greater than or equal to 3.84 (P<0.05) in the forward selection and greater than or equal to 6.63 (P 0.01) in the backward elimination were used as selection criteria. The formulation of covariate analysis was described as:
| (3) |
The typical value of a model parameter (TVP) was described as a function of m individual continuous covariates (covmi) and p individual categorical (0–1) covariates (covpi) such that θTVP is an estimated parameter describing the typical PK parameter value for an individual with covariates equal to the reference covariate values (covmi = refm, covpi = 0). θm and θ(p+m) are estimated parameters describing the magnitude of the covariate–parameter relationships.
2.1.2. Model Validation
Non-parametric bootstrap analysis was performed with 1000 resampled datasets, and the estimated means and 95% confidence intervals (CIs) of parameter estimates were compared to the final model estimates. Models with a >10% difference in the estimated means or with a 95% CI containing zero were rejected [20, 21]. The final model was also evaluated by visual predictive check (VPC), where the final model was used to simulate 1,000 datasets and the real data observations were compared with the distribution of the simulated concentrations [22, 23].
2.1.3. Pediatric Population PK Model
To account for the effects of growth, body weight (WT) was included in the pediatric model, which used an allometric scaling component on clearance (CL) and volume of distribution (V), with respective power coefficients of 0.75 and 1.0 [24, 25], as presented below [26]:
| (4) |
| (5) |
This methodology is considered reasonable in children aged 6 years and older, as indicated by recent systematic assessments [27–29]; however, its theoretical basis is still under discussion [30–32] and, in younger children, in addition to the body size effect, specific drug properties, such as protein binding and metabolism, as well as maturation, should be taken into consideration for PK parameter prediction [27]. A maturation function was not included in the model, as the PK study was to be performed in children aged 10 years when maturation of metabolism is assumed to be complete [24, 25].
2.2. Clinical Trial Simulations
The primary purpose of this trial simulation was to select an age-appropriate dose for a phase I trial in children aged 10 years and older. The study was designed on the premise that it was reasonable to assume a comparable exposure–response relationship in children 10 years and older and in adults [33]. Using the pediatric population PK model, predicted pharmacokinetic profiles after inhalation of different loxapine dose levels (1.25, 2.5, 5, and 10 mg) were generated in a virtual pediatric population. Allometric scaling was included in the model by default to take body size effects into account. Inter-patient variability and residual errors were assumed to be the same as in the adult model. A total of 8000 pediatric patients aged 10 to 17 years, inclusive, were randomly sampled (age-matched weights) from the US Centers for Disease Control and Prevention National Health and Nutrition Examination Survey (CDC-NHANES) database and comprised the simulated population dataset. External data that were not part of the database (kindly provided by the Division of Child and Adolescent Psychiatry at Cincinnati Children’s Hospital Medical Center, Cincinnati, OH) were plotted alongside the model predictions to assess the model’s applicability to external data sources. For the definition of the target exposure in pediatric patients, an exposure-response relationship similar to that observed in adults was assumed. Loxapine target exposure parameters were the peak concentration (Cmax) and area under the concentration-time curve (AUC) data at 0 to 2 hours (AUC0-2h) and at 0 to 24 hours (AUC0-24h) after a single inhaled dose as observed in the adult healthy volunteers studies at the 5 mg and 10 mg loxapine dose levels. These exposure targets were chosen because of their association with good efficacy and minimal adverse events in the adult studies. AUC0-2h and AUC0-24h were estimated based on the trapezoidal rule for each individual with the R package metrumrg [34]. Cmax was defined as the highest plasma concentration in the observed concentration-time profile. Four age cohorts were used for summary purposes covering the age range of 10 to 17 years, inclusive: 10 to <12 years, 12 to <14 years, 14 to <16 years, and 16 to <18 years. Twelve body weight cohorts (10-kg weight increase per group) were defined to cover the weight range of 20 to 130 kg, with the last cohort representing a body weight range of 130 to 215 kg.
The distributions of AUC0-2h, AUC0-24h and Cmax values were compared with the target exposures associated with loxapine efficacy and safety in adult patients in order to choose the optimal dose and appropriate weight cutoffs. The reference values for AUCs and Cmax were based on what was observed in the adult studies and are summarized in Table 1. For each age and body weight cohort, doses and related exposures were targeted to have more than 95% of patients with predicted exposures within the defined minimum and maximum AUC ranges. In addition, more than 2% of patients with exposures above the maximum AUC levels and peak concentration (Cmax) were defined as not acceptable.
Table 1.
Reference values for AUC0-24h, AUC0-2h, and Cmax based on adult studies
| AUC0-24h (ng h/mL) | AUC0-2h (ng h/mL) | Cmax (ng/mL) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| 5 mg | 10 mg | 5 mg | 10 mg | 5 mg | 10 mg | |
|
| ||||||
| Maximum | 144 | 288 | 55 | 110 | 312 | 624 |
| 75 percentile | 99 | 198 | 36 | 72 | 100 | 200 |
| Mean | 87 | 174 | 31 | 61 | 71 | 142 |
| Median | 84 | 169 | 31 | 62 | 48 | 96 |
| 25 percentile | 74 | 147 | 24 | 49 | 24 | 48 |
| Minimum | 33 | 67 | 7.1 | 14.2 | 6 | 12 |
AUC0-2h, area under the concentration-time curve at 0 to 2 hours; AUC0-24h, area under the concentration-time curve from 0 to 24 hours; Cmax, maximum observed drug concentration.
2.3. Optimal Sampling Design and Sample Size Estimation
To identify the most informative sampling times and number of samples required to robustly estimate individual PK parameters, a D-optimal design analysis was performed using WinPOPT version 1.2 (WinPOPT Development Team, School of Pharmacy, University of Otago, New Zealand) [35]. D-optimal design was conducted with the following constraints: all samplings were to be collected within 12 hours after drug administration, with additional samples drawn 24 and/or 48 hours postdose. In addition, the earliest possible sampling time was 1 minute after drug administration, with only one sample allowed from 1 to 3 minutes. In order to be able to estimate all of the primary PK parameters similar to those in the adult PK model, the number of sampling points was set at 7. Feasibility of the resulting sampling schedules was evaluated with input from the clinical team.
A power analysis was performed to determine the appropriate number of patients required for the pediatric PK study [36]. We used the recently proposed 80% power criterion to be able to estimate the 95% CI within 60% and 140% of the geometric mean estimates of the PK parameters of interest (i.e., clearance and volume of distribution) in pediatric patients [25]. To satisfy the quality criterion, different candidate sample-size scenarios were tested using a simulation and fitting procedure after the appropriate dose and optimal PK sampling schedule were identified. Five hundred replicate trials in pediatric subjects were simulated using the developed pediatric PK model with the proposed design and candidate sample-size scenarios. Simulated plasma concentration profiles were fit using the FOCE-I method in NONMEM 7.2, and the 95% CIs for loxapine clearance (CL/F) were obtained using the standard error estimates for each replicate. The power was calculated as the proportion of replicates with a 95% CI that did not fall outside the referenced 60% and 140% of the mean estimates.
2.4. Comparison of Modeling and Simulation Results With the Pediatric Phase I Clinical Trial Data
The phase I study in pediatric patients (ClinicalTrials.gov registration number NCT02184767) was completed under a study protocol supported by the described modeling and simulation work. The complete results of this study will be reported separately elsewhere. Briefly, 30 children and adolescents with any condition warranting chronic use of an antipsychotic medication were enrolled in the study. Patients were administered 2.5, 5, or 10 mg of loxapine depending on dose allocation by body weight subgroup. In order to better understand the early loxapine exposure, sampling times suggested by D-optimal design were modified and blood samples were collected predose, at 2, 5, 15, and 45 minutes, and at 2, 4, 8, 24, and 48 hours after administration of loxapine. These phase I pediatric pharmacokinetic data were analyzed with NONMEM, in a similar fashion as described in section 2.1. A total of 300 plasma concentrations from 30 pediatric patients were available for analysis. The pediatric PK parameter estimates obtained from the PK analysis were evaluated and compared with the typical predicted pediatric parameter estimates obtained in the initial clinical trial simulations. In addition, the individual exposure estimates, including AUC0-2h, AUC0-t (where t=48h), and Cmax, were compared by graphically superimposing the data onto the parameter distributions predicted by the initial trial simulations.
3. RESULTS
3.1. Adult and Pediatric PK Model Development
A 3-compartment model with first-order absorption best described the adult data. Adding a lag time to the absorption phase did not significantly improve the model fit. A schematic representation of the base model is shown in Online Resource 2. Because intravenous data were not available, the absolute bioavailability (F) of loxapine was not defined and the model was parameterized as apparent clearance (CL/F), apparent intercompartment clearance (Q3/F, Q4/F), and apparent volume of distribution (Vc/F, V3/F, V4/F) with a first-order absorption rate-constant Ka. None of the tested covariates (age, body weight, height, sex, race, body mass index, body surface area, and smoking status) significantly decreased variability in CL/F or Vc/F. The correlation between CL/F and V2/F was low with a covariance of 0.139.
Goodness-of-fit criteria revealed that the final model provided a good description of the loxapine PK data. Model validation results further demonstrated that the final model adequately described the observed data without any bias. The performance results of the population PK model are presented in Online Resource 3. Observed concentrations versus population predicted concentrations and individual predicted concentrations were tightly grouped along the line of identity. Conditional weighted residuals (CWRES) were homogeneously distributed around the zero line with only one concentration that had a |CWRES| 4. The VPC also demonstrated the validity of the final model (Online Resource 4). The final population PK parameters of loxapine in adult patients are presented in Table 2. The pediatric model used in the clinical trial simulations to support the phase I study design had a structure and parameters similar to those of the adult model but included allometrically scaled loxapine clearance and volume of distribution to account for differences in body size.
Table 2.
Loxapine population pharmacokinetic parameter estimates in adult subjects and pediatric patients
| Adult subjects | Pediatric patients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Parameter | Estimate | RSE, % | Bootstrap analysis | Estimate | RSE, % | Bootstrap analysis | ||||
| Mean | Bias, % | 95% CI | Mean | Bias, % | 95% CI | |||||
| CL/F (L/h/70 kg) | 54.1 | 4.1 | 54.0 | 0.1 | 50.3–58.0 | 78.0 | 4.6 | 78.0 | 0.0 | 71–85 |
| Vc/F (L/70 kg) | 83.1 | 11.6 | 81.2 | 2.3 | 63.8–102.4 | 195.0 | 19.1 | 203.0 | 4.1 | 116–274 |
| Q3/F (L/h/70 kg) | 703 | 8 | 716.2 | 1.9 | 571.3–834.5 | 878.0 | 8.1 | 795.0 | 9.5 | 364–1391 |
| V3/F (L/70 kg) | 149 | 5 | 150.5 | 1.0 | 132.5–165.5 | 214.0 | 11.3 | 220.0 | 2.8 | 168–259 |
| Q4/F (L/h/70 kg) | 27.6 | 4.9 | 27.8 | 0.8 | 24.8–30.4 | 39.1 | 16.5 | 39.2 | 0.3 | 32.6–45.7 |
| V4/F (L/70 kg) | 335 | 10.1 | 341.2 | 1.8 | 264.4–405.6 | 866.0 | 10.2 | 894.0 | 3.2 | 667–1064 |
| Ka (hr−1) | 58.2 | 12.7 | 57.5 | 1.2 | 43.5–72.9 | 58.0 Fixed | - | - | - | - |
|
| ||||||||||
| Between-subject variability | ||||||||||
|
| ||||||||||
| ω2CL/F | 0.053 | 11.8 | 0.053 | 0.4 | 0.026–0.080 | 0.032 | 14.7 | 0.031 | 3.1 | 0.013–0.051 |
| ω2V2/F | 0.992 | 8.8 | 0.994 | 0.3 | 0.662–1.32 | 0.981 | 9.6 | 0.893 | 9.0 | 0.593–1.368 |
| ω2Ka | 0.631 | 12.6 | 0.626 | 0.8 | 0.287–0.975 | -- | - | - | - | - |
|
| ||||||||||
| Residual error | ||||||||||
|
| ||||||||||
| σ2Prop | 0.105 | 6.4 | 0.105 | 0.1 | 0.09–0.12 | 0.083 | 14.7 | 0.080 | 3.6 | 0.065–0.102 |
CI, confidence interval; CL/F, apparent clearance of loxapine; Ka, rate constant of absorption of loxapine; prop, proportional; Q3-4/F, apparent intercompartmental clearance; RSE, relative standard error; Vc,3-4/F, apparent volume of distribution; ω2, variance of between-subject variability.
3.2. Clinical Trial Simulations
The NHANES pediatric age-body weight distribution used for the clinical trial simulations in the virtual pediatric population are presented in Figure 1. To verify the age-matched body weight distribution for this group of patients aged 10 to 17 years, inclusive, we compared the NHANES data with age-weight pairs obtained from 159 psychiatric inpatients at our institution (Figure 1, psychiatric patient data). No obvious deviations were observed except that there are several patients with extremely low body weight in the psychiatric patient cohort.
Fig. 1.
Age-matched body weight values for boys and girls aged 10 to 17 years, inclusive, from the CDC-NHANES database. The external data from psychiatric patients (n=159; admission period, June–November 2013; red triangles) and pediatric patients participating in the phase I loxapine pharmacokinetic study (n=30; blue triangles) are shown separately. CDC, Centers for Disease Control and Prevention; NHANES, National Health and Nutrition Examination Survey
Predicted loxapine exposures were generated across the 10- to 17-year, inclusive, age groups, with body weights ranging from 20 to 215 kg. The predicted values of Cmax, AUC0-2h, and AUC0-24h and their association with age and weight were evaluated. Cmax was not well predicted by age or weight (R2 < 0.10). AUC0-2h and AUC0-24h showed an association with age, but were better predicted by body weight (R2 of 0.19 and 0.21 vs. R2 of 0.46 and 0.51, respectively). Body weight, therefore, was chosen as the primary metric in the simulation analysis to define loxapine dose requirements. In patients weighing less than 50 kg, the predicted drug exposure after a 2.5-mg or 5-mg dose was comparable with that observed in adults after a 5-mg or 10-mg loxapine dose, respectively. In both scenarios, >95% of patients had predicted drug exposures within the defined target AUC range, with <0.1% of the pediatric patients having predicted exposures above the maximum AUC values. In patients weighing more than 50 kg, the 5-mg and 10-mg doses provided loxapine exposures comparable with those observed in the adult studies after 5-mg and 10-mg doses (>95% of the patients had predicted AUC levels within the AUC reference ranges). Figure 2 shows the distribution of predicted AUC0-24h values at the 4 selected dose levels (columns D–G) compared with the adult reference values (columns A, B, and C). The reference ranges for the AUC0-24h and AUC0-2h levels overlap with the AUCs in the 2.5-mg and 5-mg dose groups. The predicted AUC0-24h and AUC0-2h values with the 1.25-mg dose were mostly below the reference ranges, whereas the 10-mg loxapine dose led to higher-than-desired predicted AUC0-24h and AUC0-2h levels in patients weighing <50 kg.
Fig. 2.
Distribution of predicted AUC0-24h (a) and AUC0-2h (b) values at the 4 candidate dose levels from the trial simulations (columns D–G) compared with the observed adult data as reference (columns A, B, and C). Simulation was conducted using the developed PK model with virtual pediatric patients sampled from the CDC-NHANES database as described in the Methods. AUC0-2h, area under the concentration-time curve from time 0 to 2 hours; AUC0-24h, area under the concentration-time curve from 0 to 24 hours; CDC, Centers for Disease Control and Prevention; NHANES, National Health and Nutrition Examination Survey; PK, pharmacokinetic
Based on predicted loxapine exposures that were shown to be safe and effective in the adult studies, a loxapine dose of 2.5 mg or 5 mg was suggested for patients with a body weight in the range of 20 to 50 kg, and a dose of 5 mg or 10 mg was suggested for patients weighing >50 kg.
The results of the clinical trial simulations of loxapine exposure (expressed as AUC0-24h and Cmax) using the proposed doses in pediatric patients compared with the observations in adult subjects are summarized in Figure 3.
Fig. 3.
Predicted drug exposure (expressed as AUC0-24h [a] and Cmax [b]) in pediatric patients aged 10 to 17 years, inclusive, for loxapine compared with values in adult subjects. The clinical trial simulations identified the pediatric doses that would result in comparable drug exposures as observed in adult studies. A virtual population of 8000 pediatric patients aged 10 to 17 years, inclusive, was randomly sampled from the CDC-NHANES database. Red, orange, and green lines represent the 1st quartile, median, and 3rd quartile of the observations in the adult studies. AUC0-24h, area under the concentration-time curve from 0 to 24 hours; CDC, Centers for Disease Control and Prevention; Cmax, maximum observed drug concentration (peak); NHANES, National Health and Nutrition Examination Survey; Obs: observed exposure (AUC0-24h) and Cmax level in the adult study at the listed dose level; Sim: simulated exposure (AUC0-24h) and Cmax in the pediatric study at the listed dose level
3.3. Optimal Sampling Design and Sample Size
Taking feasibility into consideration, the proposed sampling schedule was 1 to 3, 10, and 40 minutes and 4, 8, 24, and 48 hours postdose. Power analysis suggested that enrollment of at least 20 pediatric patients would result in CL/F estimation with >80% power. A much larger number of patients (>100) would be required to satisfy the criterion for estimation of volume of distribution (Vc/F) unless several early time points were to be included in the sampling design. This would have hampered study feasibility and given that total drug exposure (AUC) is determined in large part by clearance, sample size justification in the proposed design was based primarily on estimation of clearance.
Based on these results, the clinical protocol was projected to enroll up to 30 patients with the intent that at least 20 patients would complete the study. Above-mentioned D-optimal sampling time points were later revised, and blood samples for PK analysis were to be obtained before administration of inhaled loxapine, and then at 2, 5, 15, and 45 minutes, and at 2, 4, 8, 24, and 48 hours following administration of inhaled loxapine. Study patients would be randomized into two cohorts. Each cohort would consist of 15 patients who would receive a loxapine for inhalation dose of 2.5 mg, 5 mg, or 10 mg, depending on body weight.
3.4. Loxapine Pharmacokinetics in Pediatric Patients
The phase I study to assess the safety and pharmacokinetics of loxapine for inhalation was conducted in pediatric patients according to the design recommendations resulting from the modeling work. A complete description of this study will be reported elsewhere. Here we present the PK data in order to compare the actual pediatric results with what was predicted by the earlier modeling and simulation analysis. A total of 30 patients with a median age of 13 years (range, 10–17 years) and median body weight of 50.2 kg were enrolled. Study patients comprised 17 females (56.7%) and 13 males (43.3%). A summary of the demographic data of the pediatric patients in this study is listed in Online Resource 5. Figure 1 presents the age-weight data of the pediatric patients participating in the PK study in comparison with the NHANES data used in the clinical trial simulations.
The pediatric PK data were well described by a similar three-compartment model (Online Resource 2). Due to the relatively sparse sampling during the absorption phase in the pediatric study, the first-order absorption rate constant Ka was fixed to the adult value. Of the covariate factors tested, none significantly decreased variability in clearance (CL/F) and volume of distribution (Vc/F). Goodness-of-fit criteria revealed that the final model provided a good description of the loxapine PK data (Online Resource 6). The final population PK parameter estimates of loxapine in pediatric patients and results from the bootstrap analysis are listed in Table 2. Model validation results, including the bootstrapping analysis and VPC, indicated that the final model appropriately described the observed data and that no bias remained (Online Resource 7).
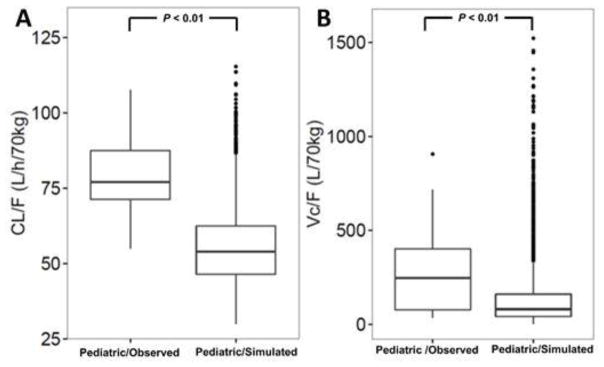
In both lower-dose groups (Cohort 1), several patients had AUC0-2h, AUC0-t, and Cmax values slightly lower than the target ranges as observed in the adult studies (Figure 4). However, most of the values were in good agreement with the predictions in the clinical trial simulations. In both higher-dose groups (Cohort 2), nearly all patients had AUC0-2h, AUC0-t, and Cmax values within the adult target range and the observed PK data coincided with the clinical trial predictions (Figure 4). Overall, both cohorts had loxapine exposures around the lower range of the observed values in the adult studies. The population estimate of loxapine clearance (CL/F) in the pediatric PK study was 78.0 L/h/70 kg and was significantly higher than that observed in the adult studies (54.1 L/h/70 kg; P<0.01; Figure 5) and in the pediatric clinical trial simulations. The population estimate of volume of distribution (Vc/F) in pediatric patients was also significantly higher compared with results in the adult studies (195.0 L/70 kg vs. 83.1 L/70 kg; P<0.01) and pediatric simulations.
Fig. 4.
Predicted AUC0-2h, AUC0-t, and Cmax values for Cohort 1 at dose levels 2.5 mg (<50 kg) and 5 mg (>50 kg; [a, c, e]) and for Cohort 2 at dose levels 5 mg (<50 kg) and 10 mg (>50 kg; [b, d, f]). The observations from the pediatric pharmacokinetic study are shown separately (blue circles). The black dots represent the model prediction for pediatric patients. The solid lines present the upper and lower ranges as observed in the adult studies; the dotted line represents the mean. AUC0-2h, area under the concentration-time curve from time 0 to 2 hours; AUC0-t, area under the concentration vs. time curve from time 0 to time t; Cmax, maximum observed drug concentration; WT, weight. Note: Log scale for the Y-axis in E and F
Fig. 5.
Box-whisker plots representing individual post-hoc Bayesian estimates of clearance (CL/F; [a]) and volume of distribution (Vc/F; [b]) of loxapine in pediatric patients participating in the phase I study compared with the model-based predictions in the clinical trial simulations as part of the phase I study design. CL/F, apparent clearance of loxapine; Vc/F, apparent volume of distribution; pediatric, pediatric phase I study data; pediatric/simulation, pediatric PK model-based predictions in the clinical trial simulations as part of the phase I study design
Retrospective power analysis showed that the data collected in 30 pediatric patients participating in this phase I trial resulted in precise estimation of CL/F and Vc/F, as indicated by respective power estimates of 100% and 78%. These parameter estimates, which largely determine total systemic exposure (AUC), exceeded or were in line with the precision criterion of 80% as suggested recently [36].
4. DISCUSSION
This study describes the application of population modeling and clinical trial simulation as part of the design of a clinical trial of loxapine for inhalation in children and adolescents. Included in the rationale for the study design was an assumption that there was a comparable exposure-response relationship for inhaled loxapine in children 10 years of age and older to that observed in adults. Results of the simulations were then verified with the actual pediatric PK data observed in a phase I study in children and adolescent with psychiatric disorders. The pediatric population PK model of loxapine was based on adult data and included an allometric component to account for the effects of differences in body size on PK parameter estimates in the targeted pediatric patient population. In addition, the simulations used realistic age-matched body weight data specific to the children and adolescents with psychiatric disorders. The clinical trial simulations with the pediatric model were considered instrumental in the evaluation of to-be-expected loxapine exposures at different dose levels as part of the phase I study design. Different dose levels were evaluated, and, based on the exposure levels associated with a 5-mg or 10-mg loxapine dose as observed in the adult studies, loxapine doses of 2.5 mg or 5 mg in patients weighing 20 to 50 kg and loxapine doses of 5 mg or 10 mg in patients weighing more than 50 kg were selected for the clinical study.
An optimal sampling strategy was developed to minimize the number of blood samples collected while maximizing the robustness of PK parameters of loxapine in pediatric patients. A sampling strategy suggested by D-optimal design involving 7 blood samples over a 48-hour period in 20 pediatric patients was found to be required for precise estimation of loxapine clearance. In order to better describe early exposure of loxapine, 2 additional sampling times were added during clinical trial protocol development. The adequacy of this design was subsequently confirmed by the findings from the clinical PK study in children and adolescents with psychiatric disorders. The PK parameters in the modeling were precise and unbiased. The total drug exposure of inhaled loxapine in the pediatric phase I study was slightly lower but within a range similar to that observed in the adult studies. The estimates for loxapine clearance and volume of distribution in the pediatric study patients, however, were higher than what was predicted by the simulations. One possible explanation is that this is the result of differences in the total amount of loxapine delivered to the lungs, especially in the younger patients. Drug delivery to the lung can be reduced in younger patients as a result of differences in physiology and coordination [37]. In addition, motor skill disabilities could also be related to psychiatric disorders and hence caused reduced absorption. A recent review highlighted a high prevalence of impaired motor coordination in children and adolescents with psychiatric disorders [38].
Despite the differences in pediatric PK parameter estimates, loxapine peak concentrations (Cmax) and early and overall exposures (expressed as AUC0-2h and AUC0-24h) in this pediatric study group were all well within the ranges observed in the adult studies (Figures 4–5). As such, the findings of this study are confirming what was predicted by the clinical trial simulations and will be used to further inform the design of future loxapine phase III studies in children. One consideration for dosing selection in future pediatric studies is related to potential safety issues (eg, unexpected higher sensitivity of children to adverse events or unexpected differences in true clearance). If the higher loxapine clearance in the current phase I trial suggests the need for a higher dose or a different body weight cut-off for dose selection, this should be evaluated based on loxapine exposure (PK) and, most importantly, on safety and clinical response data in children.
In conclusion, the presented population PK modeling and simulation analysis applied to inhaled loxapine represents an attractive approach in support of pediatric clinical trial design in children and adolescents, and will help to optimize dosing strategies to achieve the desired efficacy of a given treatment in pediatric patients.
Supplementary Material
Online Resource 1. Subject characteristics of adult pharmacokinetic studies. BMI, body mass index.
Online Resource 2. A schematic representation of the structural pharmacokinetic model used in the population modeling. CL/F, apparent clearance of loxapine; Ka, rate constant of absorption of loxapine; Q3-4/F, apparent intercompartmental clearance; Vc,3-4/F, apparent volume of distribution
Online Resource 3. Goodness-of-fit plots for the final pharmacokinetics model. (A) Population predictions versus observed concentration. (B) Individual predictions versus observed concentration. (C) Conditional weighted residuals versus population predictions. (D) Conditional weighted residuals versus time after dose. Red line: a locally weighted least-squares regression; black line: line of identity.
Online Resource 4. Visual predictive check of the final pharmacokinetics of loxapine in adults. Black circles: observed plasma concentrations; red dashed lines: predicted 90% interval; red solid line: predicted median; blue and red areas: 95% confidence interval of predicted percentiles.
Online Resource 5. Demographics of pediatric patients participating in the loxapine phase I pharmacokinetic study. BMI, body mass index.
Online Resource 6. Goodness-of-fit plots for data analysis of the pediatric pharmacokinetic study. (A) Population predictions versus observed concentration. (B) Individual predictions versus observed concentration. (C) Conditional weighted residuals versus population predictions. (D) Conditional weighted residuals versus time after dose. Red line: a locally weighted least-squares regression; black line: line of identity.
Online Resource 7. Visual predictive check of PK model developed from pediatric data. Black circles: observed plasma concentrations; red dashed lines: predicted 90% interval; red solid line: predicted median; blue and red areas: 95% confidence interval of predicted percentiles. Insert shows the same data with both x- and y-axes in logarithmic scale.
Key Points.
This report details the development of a pediatric trial design for a clinical pharmacokinetics (PK) study of loxapine for inhalation in children aged 10 years and older using available adult PK data.
The study design formed the basis for a phase I clinical trial, and we report the PK results in relation to the predicted likelihood of achieving target loxapine exposure associated with therapeutic effect in adults.
This study represents a proof of concept of modeling and simulation in support of PK study design in children and adolescents, which has merit as a generalizable approach for the design and analysis of similar pediatric studies.
Acknowledgments
We are grateful to Drs. Michael Sorter and Drew Barzman of the Division of Child and Adolescent Psychiatry at Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, for sharing the age-weight data that were used in the external model validation.
Funding: This study was sponsored by Teva Branded Pharmaceutical Products R & D, Inc. and Alexza Pharmaceuticals. Both companies reviewed and approved the manuscript. Editorial assistance was provided by Peloton Advantage and was funded by Teva Branded Pharmaceutical Products R & D, Inc. (Frazer, PA).
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
Conflicts of interest
Min Dong has received a National Institute of Child Health and Human Development training grant (5T32HD069054) at and is an employee of Cincinnati Children’s Hospital Medical Center, Cincinnati, OH.
Tsuyoshi Fukuda declares that he has no conflict of interest.
Sally Selim and Laura Rabinovich-Guilatt are employees of Teva Pharmaceuticals and may hold stock/stock options in that company.
Mark A. Smith was an employee of Teva Pharmaceuticals at the time of study conduct.
James V. Cassella was an employee of Alexza Pharmaceuticals at the time of study design and conduct, and received consulting fees from Alexza Pharmaceuticals during manuscript review.
Alexander A. Vinks has been a consultant for the study sponsor through a consulting agreement between Teva
Pharmaceuticals and Cincinnati Children’s Hospital Medical Center, Cincinnati, OH.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Adasuve [package insert] Horsham, PA: Teva Pharmaceuticals; 2013. [Google Scholar]
- 2.Heel RC, Brogden RN, Speight TM, Avery GS. Loxapine: a review of its pharmacological properties and therapeutic efficacy as an antipsychotic agent. Drugs. 1978 Mar;15(3):198–217. doi: 10.2165/00003495-197815030-00002. [DOI] [PubMed] [Google Scholar]
- 3.Dinh K, Myers DJ, Glazer M, Shmidt T, Devereaux C, Simis K, et al. In vitro aerosol characterization of Staccato((R)) Loxapine. Int J Pharm. 2011 Jan 17;403(1–2):101–8. doi: 10.1016/j.ijpharm.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 4.Allen MH, Feifel D, Lesem MD, Zimbroff DL, Ross R, Munzar P, et al. Efficacy and safety of loxapine for inhalation in the treatment of agitation in patients with schizophrenia: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2011 Oct;72(10):1313–21. doi: 10.4088/JCP.10m06011yel. [DOI] [PubMed] [Google Scholar]
- 5.Lesem MD, Tran-Johnson TK, Riesenberg RA, Feifel D, Allen MH, Fishman R, et al. Rapid acute treatment of agitation in individuals with schizophrenia: multicentre, randomised, placebo-controlled study of inhaled loxapine. Br J Psychiatry. 2011 Jan;198(1):51–8. doi: 10.1192/bjp.bp.110.081513. [DOI] [PubMed] [Google Scholar]
- 6.Kwentus J, Riesenberg RA, Marandi M, Manning RA, Allen MH, Fishman RS, et al. Rapid acute treatment of agitation in patients with bipolar I disorder: a multicenter, randomized, placebo-controlled clinical trial with inhaled loxapine. Bipolar Disord. 2012 Feb;14(1):31–40. doi: 10.1111/j.1399-5618.2011.00975.x. [DOI] [PubMed] [Google Scholar]
- 7.Geller B, Luby J. Child and adolescent bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 1997 Sep;36(9):1168–76. doi: 10.1097/00004583-199709000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Hafner H, Nowotny B. Epidemiology of early-onset schizophrenia. Eur Arch Psychiatry Clin Neurosci. 1995;245(2):80–92. doi: 10.1007/BF02190734. [DOI] [PubMed] [Google Scholar]
- 9.Hellings JA, Reed G, Cain SE, Zhou X, Barth FX, Aman MG, et al. Loxapine add-on for adolescents and adults with autism spectrum disorders and irritability. J Child Adolesc Psychopharmacol. 2015 Mar;25(2):150–9. doi: 10.1089/cap.2014.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reinblatt SP, Abanilla PK, Jummani R, Coffey B. Loxapine treatment in an autistic child with aggressive behavior: therapeutic challenges. J Child Adolesc Psychopharmacol. 2006 Oct;16(5):639–43. doi: 10.1089/cap.2006.16.639. [DOI] [PubMed] [Google Scholar]
- 11.Spyker DA, Munzar P, Cassella JV. Pharmacokinetics of loxapine following inhalation of a thermally generated aerosol in healthy volunteers. J Clin Pharmacol. 2010 Feb;50(2):169–79. doi: 10.1177/0091270009347866. [DOI] [PubMed] [Google Scholar]
- 12.Luo JP, Vashishtha SC, Hawes EM, McKay G, Midha KK, Fang J. In vitro identification of the human cytochrome p450 enzymes involved in the oxidative metabolism of loxapine. Biopharm Drug Dispos. 2011 Oct;32(7):398–407. doi: 10.1002/bdd.768. [DOI] [PubMed] [Google Scholar]
- 13.Midha KK, Hubbard JW, McKay G, Hawes EM, Hsia D. The role of metabolites in a bioequivalence study 1: loxapine, 7-hydroxyloxapine and 8-hydroxyloxapine. Int J Clin Pharmacol Ther Toxicol. 1993 Apr;31(4):177–83. [PubMed] [Google Scholar]
- 14.Takahashi LH, Huie K, Spyker DA, Fishman RS, Cassella JV. Effect of smoking on the pharmacokinetics of inhaled loxapine. Ther Drug Monit. 2014 Oct;36(5):618–23. doi: 10.1097/FTD.0000000000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmer JS, Needham SR, Christianson CD, Piekarski CM, Sheaff CN, Huie K, et al. Validation of HPLC-MS/MS methods for analysis of loxapine, amoxapine, 7-OH-loxapine, 8-OH-loxapine and loxapine N-oxide in human plasma. Bioanalysis. 2010 Dec;2(12):1989–2000. doi: 10.4155/bio.10.156. [DOI] [PubMed] [Google Scholar]
- 16.Beal S, Sheiner LB, Boeckmann A, Bauer RJ. NONMEM User's Guide (1989–2009) Ellicott City, MO: Icon Development Solutions; 2009. [Google Scholar]
- 17.Savic RM, Karlsson MO. Importance of shrinkage in empirical bayes estimates for diagnostics: problems and solutions. AAPS J. 2009 Sep;11(3):558–69. doi: 10.1208/s12248-009-9133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonsson EN, Karlsson MO. Xpose--an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed. 1999 Jan;58(1):51–64. doi: 10.1016/s0169-2607(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 19.Dong M, McGann PT, Mizuno T, Ware RE, Vinks AA. Development of a pharmacokinetic-guided dose individualization strategy for hydroxyurea treatment in children with sickle cell anaemia. Br J Clin Pharmacol. 2016 Apr;81(4):742–52. doi: 10.1111/bcp.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mould DR, Upton RN. Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacometrics Syst Pharmacol. 2013;2:e38. doi: 10.1038/psp.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Punyawudho B, Ramsay ER, Brundage RC, Macias FM, Collins JF, Birnbaum AK. Population pharmacokinetics of carbamazepine in elderly patients. Ther Drug Monit. 2012 Apr;34(2):176–81. doi: 10.1097/FTD.0b013e31824d6a4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holford N. The visual predictive check - superiority to standard diagnostic (Rorschach) plots [abstract 738]. June 16–17, 2005; Pamplona, Spain. Annual Meeting of the Population Approach Group in Europe; 2005. [Google Scholar]
- 23.Keizer RJ, Karlsson MO, Hooker A. Modeling and Simulation Workbench for NONMEM: Tutorial on Pirana, PsN, and Xpose. CPT Pharmacometrics Syst Pharmacol. 2013;2:e50. doi: 10.1038/psp.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson BJ, Holford NH. Tips and traps analyzing pediatric PK data. Paediatr Anaesth. 2011 Mar;21(3):222–37. doi: 10.1111/j.1460-9592.2011.03536.x. [DOI] [PubMed] [Google Scholar]
- 25.Vinks AA, Emoto C, Fukuda T. Modeling and simulation in pediatric drug therapy: Application of pharmacometrics to define the right dose for children. Clin Pharmacol Ther. 2015 Sep;98(3):298–308. doi: 10.1002/cpt.169. [DOI] [PubMed] [Google Scholar]
- 26.Anderson BJ, Allegaert K, Holford NH. Population clinical pharmacology of children: modelling covariate effects. Eur J Pediatr. 2006 Dec;165(12):819–29. doi: 10.1007/s00431-006-0189-x. [DOI] [PubMed] [Google Scholar]
- 27.Calvier EA, Krekels EH, Valitalo PA, Rostami-Hodjegan A, Tibboel D, Danhof M, et al. Allometric Scaling of Clearance in Paediatric Patients: When Does the Magic of 0.75 Fade? Clin Pharmacokinet. 2016 Aug 10; doi: 10.1007/s40262-016-0436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu T, Ghafoori P, Gobburu JV. Allometry is a reasonable choice in pediatric drug development. J Clin Pharmacol. 2016 Sep 21; doi: 10.1002/jcph.831. [DOI] [PubMed] [Google Scholar]
- 29.Edginton AN, Shah B, Sevestre M, Momper JD. The integration of allometry and virtual populations to predict clearance and clearance variability in pediatric populations over the age of 6 years. Clin Pharmacokinet. 2013 Aug;52(8):693–703. doi: 10.1007/s40262-013-0065-6. [DOI] [PubMed] [Google Scholar]
- 30.Kolokotrones T, Van S, Deeds EJ, Fontana W. Curvature in metabolic scaling. Nature. 2010 Apr 1;464(7289):753–6. doi: 10.1038/nature08920. [DOI] [PubMed] [Google Scholar]
- 31.Krekels EH, van Hasselt JG, Tibboel D, Danhof M, Knibbe CA. Systematic evaluation of the descriptive and predictive performance of paediatric morphine population models. Pharm Res. 2011 Apr;28(4):797–811. doi: 10.1007/s11095-010-0333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahmood I. Pharmacokinetic allometric scaling of antibodies: application to the first-in-human dose estimation. J Pharm Sci. 2009 Oct;98(10):3850–61. doi: 10.1002/jps.21682. [DOI] [PubMed] [Google Scholar]
- 33.Dunne J, Rodriguez WJ, Murphy MD, Beasley BN, Burckart GJ, Filie JD, et al. Extrapolation of adult data and other data in pediatric drug-development programs. Pediatrics. 2011 Nov;128(5):e1242–9. doi: 10.1542/peds.2010-3487. [DOI] [PubMed] [Google Scholar]
- 34.Bergsma TT, Knebel W, Fisher J, Gillespie WR, Riggs MM, Gibiansky L, et al. Facilitating pharmacometric workflow with the metrumrg package for R. Comput Methods Programs Biomed. 2013 Jan;109(1):77–85. doi: 10.1016/j.cmpb.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Duffull S, Denman N, Eccleston J, Kimko H. WinPOPT (Version 1.2) User Guide. Dunedin, New Zealand: School of Pharmacy, University of Otago; 2008. [Google Scholar]
- 36.Wang Y, Jadhav PR, Lala M, Gobburu JV. Clarification on precision criteria to derive sample size when designing pediatric pharmacokinetic studies. J Clin Pharmacol. 2012 Oct;52(10):1601–6. doi: 10.1177/0091270011422812. [DOI] [PubMed] [Google Scholar]
- 37.D'Hondt E, Deforche B, Gentier I, De Bourdeaudhuij I, Vaeyens R, Philippaerts R, et al. A longitudinal analysis of gross motor coordination in overweight and obese children versus normal-weight peers. Int J Obes (Lond) 2013 Jan;37(1):61–7. doi: 10.1038/ijo.2012.55. [DOI] [PubMed] [Google Scholar]
- 38.Damme TV, Simons J, Sabbe B, van West D. Motor abilities of children and adolescents with a psychiatric condition: A systematic literature review. World J Psychiatry. 2015 Sep 22;5(3):315–29. doi: 10.5498/wjp.v5.i3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Resource 1. Subject characteristics of adult pharmacokinetic studies. BMI, body mass index.
Online Resource 2. A schematic representation of the structural pharmacokinetic model used in the population modeling. CL/F, apparent clearance of loxapine; Ka, rate constant of absorption of loxapine; Q3-4/F, apparent intercompartmental clearance; Vc,3-4/F, apparent volume of distribution
Online Resource 3. Goodness-of-fit plots for the final pharmacokinetics model. (A) Population predictions versus observed concentration. (B) Individual predictions versus observed concentration. (C) Conditional weighted residuals versus population predictions. (D) Conditional weighted residuals versus time after dose. Red line: a locally weighted least-squares regression; black line: line of identity.
Online Resource 4. Visual predictive check of the final pharmacokinetics of loxapine in adults. Black circles: observed plasma concentrations; red dashed lines: predicted 90% interval; red solid line: predicted median; blue and red areas: 95% confidence interval of predicted percentiles.
Online Resource 5. Demographics of pediatric patients participating in the loxapine phase I pharmacokinetic study. BMI, body mass index.
Online Resource 6. Goodness-of-fit plots for data analysis of the pediatric pharmacokinetic study. (A) Population predictions versus observed concentration. (B) Individual predictions versus observed concentration. (C) Conditional weighted residuals versus population predictions. (D) Conditional weighted residuals versus time after dose. Red line: a locally weighted least-squares regression; black line: line of identity.
Online Resource 7. Visual predictive check of PK model developed from pediatric data. Black circles: observed plasma concentrations; red dashed lines: predicted 90% interval; red solid line: predicted median; blue and red areas: 95% confidence interval of predicted percentiles. Insert shows the same data with both x- and y-axes in logarithmic scale.