Abstract
Objective
This study investigates the relationship between electrophysiological and psychophysical measures of amplitude modulation (AM) detection. Prior studies have reported both measures of AM detection recorded separately from cochlear implant (CI) users and acutely deafened animals, but no study has made both measures in the same CI users. Animal studies suggest a progressive loss of high frequency encoding as one ascends the auditory pathway from the auditory nerve to the cortex. Since the CI speech processor uses the envelope of an ongoing acoustic signal to modulate pulse trains that are subsequently delivered to the intracochlear electrodes, it is of interest to explore auditory nerve responses to modulated stimuli. In addition, psychophysical AM detection abilities have been correlated with speech perception outcomes. Thus, our goal was to explore how the auditory nerve responds to AM stimuli and to relate those physiologic measures to perception.
Design
Eight patients using Cochlear Ltd. Implants participated in this study. Electrically evoked compound action potentials (ECAPs) were recorded using a 4000 pps pulse train that was sinusoidally amplitude modulated at 125, 250, 500, and 1000 Hz. Responses were measured for each pulse over at least one modulation cycle for an apical, medial, and basal electrode. Psychophysical modulation detection thresholds (MDTs) were also measured via a 3-alternative-forced-choice, 2-down, 1-up adaptive procedure using the same modulation frequencies and electrodes.
Results
ECAPs were recorded from individual pulses in the AM pulse train. ECAP amplitudes varied sinusoidally, reflecting the sinusoidal variation in the stimulus. A modulated response amplitude (MRA) metric was calculated as the difference in the maximal and minimum ECAP amplitudes over the modulation cycles. MRA increased as modulation frequency increased, with no apparent cutoff (up to 1000 Hz). In contrast, MDTs increased as the modulation frequency increased. This trend is inconsistent with the physiologic measures. For a fixed modulation frequency, correlations were observed between MDTs and MRAs; this trend was evident at all frequencies except 1000 Hz (though only statistically significant for 250 and 500 Hz AM rates), possibly an indication of central limitations in processing of high modulation frequencies. Finally, peripheral responses were larger and psychophysical thresholds were lower in the apical electrodes relative to basal and medial electrodes, which may reflect better cochlear health and neural survival evidenced by lower pre-operative low-frequency audiometric thresholds and steeper growth of neural responses in ECAP amplitude growth functions for apical electrodes.
Conclusions
Robust ECAPs were recorded for all modulation frequencies tested. ECAP amplitudes varied sinusoidally, reflecting the periodicity of the modulated stimuli. MRAs increased as the modulation frequency increased, a trend we attribute to neural adaptation. For low modulation frequencies, there are multiple current steps between the peak and valley of the modulation cycle, which means successive stimuli are more similar to one another and neural responses are more likely to adapt. Higher MRAs were correlated with lower psychophysical thresholds at low modulation frequencies but not at 1000 Hz, implying a central limitation to processing of modulated stimuli.
Keywords: Neural Response Telemetry, Electrically Evoked Compound Action Potential, Temporal Modulation Transfer Function, Amplitude Modulation
SHORT SUMMARY
Electrically evoked compound action potentials (ECAPs) and temporal modulation transfer functions (TMTFs) were measured in cochlear implant users using pulse trains amplitude modulated at 125, 250, 500, and 1000 Hz. ECAP amplitudes varied sinusoidally over the pulse trains. Both modulated ECAP amplitudes and psychophysical modulation detection thresholds increased with increasing modulation frequency. ECAP amplitude modulation was positively correlated with psychophysical measures for 125–500 Hz modulation frequencies (statistically significant for 250 and 500 Hz) but not for 1000 Hz. This suggested central limitations in processing stimuli modulated at higher rates despite robust ECAPs at all modulation rates.
INTRODUCTION
Since their FDA approval in the 1980s, cochlear implants (CIs) have become a common surgical option for severely deafened patients who do not benefit from hearing aids. The conversion of acoustic energy into electrical pulse trains is done via a series of transduction processes taking place in the CI sound processor and internal receiver/stimulator. The temporal envelope of the acoustic signal is extracted and used to modulate a train of biphasic current pulses that are delivered to the appropriate intracochlear electrodes. Therefore, it is of interest to study how the auditory nerve responds to modulated electrical stimuli, especially since impoverished spectral information delivered by the CI forces listeners to rely on temporal information for relevant speech cues (Fu et al., 2004; Xu et al., 2005; Xu & Zheng, 2007; Luo et al., 2007; Xu & Pfingst, 2008).
Electrically evoked compound action potentials (ECAPs) are measures of the synchronized response of a large number of auditory nerve fibers to the presentation of an electrical stimulus. An intracochlear electrode is used to record the ECAP. Reverse telemetry is used to transmit that information to the analysis software. The typical ECAP recording consists of a negative peak (N1) followed by a smaller positive peak (P2), with typical latencies of 200–400 µs and 600–800 µs, respectively (Brown et al., 1990, 1998; Abbas et al., 1999).
Temporal properties of neurons – specifically adaptation and recovery – can be investigated via such electrophysiological measures of action potentials, and these concepts are relevant for the current study. Upon sustained stimulation of an auditory nerve fiber, there is an instantaneous rise in neural spiking. Eventually, the spike rate decreases to a steady state value until the stimulus is turned off. This decrease in spike rate is termed adaptation. Adaptation to acoustic hearing may be attributed to depletion of neurotransmitters at the synapse between the hair cell and the auditory nerve fiber during the stimulation period, indicating a pre-synaptic origin (Kiang et al., 1965; Smith & Brachman, 1982). However, electrical stimulation bypasses this synapse but can still show evidence of adaptation. Thus, it has also been suggested that nerve fibers can show evidence of adaptation. The propagation of action potentials may be affected by refractory and recovery characteristics of the nerve fiber (Chimento and Schreiner, 1991). Upon cessation of a stimulus, there is a refractory period in which the auditory nerve cannot produce action potentials. This refractory period is about 330 µs, as evidenced by single auditory nerve fiber recordings in deafened cats in response to single pulses (Miller et al., 2001). Several single fiber studies in normal hearing animals using acoustic stimuli have shown adaptation plays several roles in neural encoding of speech stimuli (Delgutte, 1997 for review). For example, adaptation enhances spectral contrasts between successive speech stimuli. A speech segment with similar frequency content activates and eventually adapts fibers of similar center frequencies; when a successive speech segment has different spectral content, differing fibers respond, thus enhancing the differences between the current and previous speech stimulus (Delgutte, 1997). Electrical stimulation of deafened ears show differing adaptation properties compared to acoustic stimulation of normal ears. Deeper discussion is beyond the scope of this manuscript, but these differences have been shown via single fiber studies (van den Honert & Stypulkowski, 1987; Parkins, 1989; Zheng et al., 2007), ECAP recordings in animals (Haenggeli et al., 1998; Matsuoka et al., 2000a, b), and ECAP recordings in CI users (Wilson et al., 1994, 1997a; Hay-McCutcheon et al., 2005; Hughes et al., 2012, 2014). It is not currently known specifically how differing adaptation properties in electrical hearing affect speech encoding.
ECAPs evoked by each pulse in a constant amplitude pulse train have been recorded from both humans and animals. Depending on the stimulation rate used, these studies have shown that the ECAP amplitude is largest for the first pulse in the pulse train, but then generally declines for successive pulses, due to adaptation. An alternating pattern has also been reported. That is, ECAP amplitudes are largest for the first pulse. The amplitude of the second pulse is relatively small. The response to the third pulse is larger (but generally not as large as the first pulse) and this alternation in amplitude can persist across time, despite the fact that the stimulus is not varying (Wilson et al., 1994, 1997a,b; Abbas et al., 1997; Matsuoka et al., 2000a,b; Hay-McCutcheon et al., 2005, Hughes et al., 2012). This pattern of amplitude alternation occurs for rates > 500 pps but diminishes for higher stimulation rates (> 1500 pps, Wilson et al, 1997a; > 2400 pps, Hughes et al., 2012). The alternating pattern is likely a reflection of an interaction between the refractory and recovery properties of the stimulated neurons, in that the ECAP response is smaller for the 2nd pulse in the sequence because many neurons may be in refraction after firing to the 1st pulse. By the time the 3rd pulse occurs, neurons previously in refraction from the 1st pulse will show greater excitability, resulting in an increased ECAP amplitude. In some cases, an increase in ECAP amplitude across the pulse train has been observed (He et al., 2016). This increase in amplitude has been attributed to integration effects.
While neural responses to constant amplitude pulse trains have been well documented, less is known about the response of the auditory nerve to modulated pulse trains, which may have more relevant applications in CI speech processors. Early studies of Ineraid CI users (Wilson et al., 1994, 1997b) using sinusoidally amplitude modulated (SAM) pulse trains showed that the amplitude of the ECAP tended to follow the amplitude of the stimulus across time. However, those responses were distorted when insufficiently high carrier rates were used. Animal studies have expanded on these observations, examining the effects of modulation rate, modulation depth, carrier rate, and stimulus level on ECAP amplitude (Abbas et al., 1997, 2003; Jeng et al., 2009). With other stimulus parameters fixed, greater modulation depths showed increased modulation of the response but also more distortion of the ECAP responses and a phase shift (lead) in ECAP responses relative to the stimulus waveform. These studies quantified the extent of modulation in the neural response using a metric that they referred to as the modulated response amplitude (MRA). The MRA is calculated as the difference between maximal and minimal ECAP response amplitude for each modulation cycle. For a constant modulation depth, MRA increased as modulation frequency increased up to a cutoff frequency, after which it decreased (Abbas et al., 2003; Jeng et al., 2009). The Jeng et al. guinea pig data demonstrated increases in MRA until a 300 Hz AM rate for a 1000 pps carrier and 400–800 Hz rate for a 5000 pps carrier.
In single auditory nerve fiber recordings, the effect of stimulus modulation on synchrony of neural spiking has been assessed by measuring vector strength (Goldberg & Brown, 1969; Hu et al., 2008, 2010). Vector strength is a measure of the degree to which nerve fibers phase lock to the stimulus (or to the periodic modulation of the stimulus amplitude), and thus is a method of quantifying temporal distribution of neural responses relative to the modulation frequency. Generally, vector strength is weaker at the onset of the modulated pulse train, attributed to refractory effects. Vector strength improves over the time course of the AM pulse train (Hu et al., 2008, 2010). Similar to the ECAP MRA data, vector strength increases with modulation frequency up to a cutoff; beyond that, vector strength decreases at higher modulation frequencies (Hu et al., 2008).
Processing of modulated stimuli (and speech sounds in general) can be quite different at higher levels of the auditory pathway, i.e. they exhibit progressively lower low-pass filter cutoffs to modulated frequency or, in some cases, exhibit band-pass characteristics (Langer, 1992; Delgutte, 1997; Fitch et al., 1997 for reviews; Middlebrooks, 2008). Thus, one may expect to see differences in peripheral and central responses to amplitude modulated signals delivered through a cochlear implant.
Similarly, psychophysical measures may show different characteristics if central processing of the modulated signal affects perception. Psychophysical temporal modulation transfer functions (TMTFs) are measures of amplitude modulation detection threshold (MDT) as a function of modulation frequency, and provide insight into the individual’s temporal resolution. These measures in cochlear implant users have low-pass filter characteristics with a cut-off frequency of approximately 100 Hz (e.g. Shannon, 1992; Busby et al., 1993). The ability to detect modulation of a signal is critical in cochlear implant users; cut-off frequencies and slopes of TMTFs have been shown to correlate with speech understanding in cochlear implant users (Cazals et al., 1994; Fu, 2002; Won et al., 2011).
The peripheral physiological measures in animals are inconsistent with psychophysical measures in human CI users. Psychophysical TMTFs recorded from CI users generally show an increased threshold with increasing modulation frequency (Shannon, 1992; Busby et al., 1993). Animal studies of peripheral physiological responses generally show an increase in response amplitude across frequencies followed by a decrease at high modulation frequencies beyond a cutoff (Abbas et al., 1997, 2003; Jeng et al., 2009). No studies have investigated both peripheral and psychophysical measures in the same CI users, which could help resolve some of the inconsistencies seen in human and animal data. Thus, the present study compares physiologic measures of how amplitude modulation is coded at the level of the auditory nerve and contrasts that with psychophysical measures of modulation detection obtained using nearly identical stimuli. Our goal was to characterize the differences in these measures across individuals as well as across electrodes and to assess the relationship between the peripheral physiological response and psychophysical perception.
METHODS
This study was approved by the University of Iowa Institutional Review Board. Experimental protocols and potential benefits / risks were explained to the participants prior to data collection. All participants signed an informed consent document.
Participants
Eight users of Cochlear Ltd. implants from our clinic participated in this study. They ranged in age from 39 to 77 years old at the time of testing, and seven of the eight were postlingually deafened. Table 1 shows details about individual participants, including audiological history and demographic information.
Table 1.
Participants’ audiological history and demographic information. Note that hearing loss durations are patient-reported measures.
| Participant | Implant | Age (yr) | CI Use (mo) | Onset of Hearing Loss |
Duration of Hearing Loss (yr) |
Duration of Severe to Profound Loss (yr) |
Etiology | CNC (words / phonemes) |
AZ Bio (Quiet / + 10 dB SNR) |
Notes |
|---|---|---|---|---|---|---|---|---|---|---|
| E18 | 24RE | 77 | 108 | Postlingual | 18 | 7 | Unknown | 70 / 84 | 85 / 29 | |
| E55R | 24RE | 64 | 90 | Postlingual | 16 | 2 | Hereditary | 95 / 98 | 98 / 72 | |
| E96L | 24RE | 71 | 72 | Postlingual | 10 | 8 | Noise Induced | 61 / 80 | 75 / 25 | Partial Insertion (E1-E3 out) |
| F10L | 512 | 61 | 37 | Postlingual | 3 | 1 | Unknown | 68 / 86 | 95 | Single Sided Deafness |
| F26L | 512 | 54 | 34 | Postlingual | 22 | 3 | Unknown | 98 / 98 | 97 / 57 | |
| S13R | 422 | 67 | 5 | Postlingual | 14 | 3 | Unknown | 64 / 79 | 74 | Reimplanted Device; 45 Months CI Use with Old Implant |
| S19L | 422 | 39 | 12 | Pre or Perilingual | 33 | 0 | Unknown | 94 / 21 | ||
| E97L | 24RE | 70 | 36 | Postlingual | 27 | 7 | Noise Induced | 72 / 89 | 90 / 19 |
The intracochlear array of the Nucleus CI has 22 electrodes. Electrode 1 is the most basal and electrode 22 is the most apical. In this study, electrophysiological testing was completed before psychophysical testing. Table 2 summarizes the test conditions used. Three electrodes were tested if possible; however, in some cases only two were included in data analysis. The most common reason for excluding an electrode was excessive artifacts found in ECAP recordings. Electrodes excluded based upon ECAP analyses were also not included in the psychophysical portion of this study. Electrodes 4, 11, and 19 were generally tested to sample across the electrode array. In some cases an adjacent electrode was substituted if ECAPs recorded from that electrode were more robust.
Table 2.
Test conditions for peripheral and psychophysical measures. “CNT” indicates that the electrode could not be tested for reasons stated in the table. “Removed” means that the electrode was tested, but later excluded from analysis and results.
| Electrode Tested (ECAP) | Electrode Tested (TMTF) | |||||||
|---|---|---|---|---|---|---|---|---|
| Participant | Implant | Basal | Medial | Apical | Basal | Medial | Apical | Issues |
| E18 | 24RE | 4 | 11 | CNT | 4 | 11 | CNT | Apical: Artifacts in ECAP |
| E55R | 24RE | 4 | 11 | 19 | 4 | 11 | 19 | |
| E96L | 24RE | CNT | 11 | 19 | CNT | 11 | 19 | Basal: Partial Insertion |
| F10L | 512 | 5 | 10 | 19 | 5 | 10 | 19 | |
| F26L | 512 | 4 | 10 | 19 | 4 | 10 | 19 | |
| S13R | 422 | 4 | 11 | 19 | 4 | 11 | CNT | Apical: Time Constraints for TMTF |
| S19L | 422 | 4 | Removed | 18 | 4 | 11 | 18 | Medial: Artifacts in ECAP |
| E97L | 24RE | CNT | 11 | 19 | CNT | 11 | 19 | Basal: Artifacts in ECAP |
Electrophysiologic Measures
ECAPs were recorded using Custom Sound EP. This software is commercially available from Cochlear Ltd. It allows user control of the Neural Response Telemetry (NRT) system. Prior to making experimental measures, the stimulus threshold (T) and uncomfortable listening (C) level were determined for each test electrode. T and C levels were determined using default settings in the “Stimulate Only” mode in Custom Sound EP. Stimuli were 500 ms in duration, presented at 80 pps in incremental steps of 5 clinical levels (CL), until the patient verbally indicated when the sound was just noticeable. Stimulus level again increased in steps of 5 CL until the uncomfortable level was reached. This procedure was repeated 2–3 times to establish a consistent T and C level. The individual pulses were cathodic-leading and biphasic. They had an interphase gap (IPG) of 7 µs and pulse widths of 25, 37, or 50 µs. For some individuals whose electrode impedances were higher, the use of the longer pulse widths was necessary to overcome voltage compliance limits (Table 3). This pulse width was used for the remainder of the experiment.
Table 3.
Dynamic range of modulation and pulse durations used for peripheral and psychophysical measures. Some electrodes were not tested / excluded (see Table 2).
| Range of Modulation (CL) (ECAP) | Range of Modulation (CL) (TMTF) | Pulse Width (µs) |
||||||
|---|---|---|---|---|---|---|---|---|
| Participant | Implant | Basal | Medial | Apical | Basal | Medial | Apical | |
| E18 | 24RE | 50 | 15 | CNT | 33 | 35 | CNT | 25 |
| E55R | 24RE | 20 | 10 | 25 | 38 | 38 | 45 | 25 |
| E96L | 24RE | CNT | 55 | 20 | CNT | 30 | 40 | 25 |
| F10L | 512 | 20 | 30 | 60 | 58 | 55 | 45 | 37 |
| F26L | 512 | 30 | 30 | 50 | 45 | 45 | 43 | 25 |
| S13R | 422 | 30 | 45 | 30 | 48 | 53 | CNT | 37 |
| S19L | 422 | 40 | Removed | 55 | 25 | 15 | 18 | 50 |
| E97L | 24RE | CNT | 20 | 15 | CNT | 48 | 45 | 37 |
ECAP Threshold Measures
ECAP amplitude growth functions (AGF) were recorded using a two-pulse masker-probe paradigm that has been described previously in the literature (Brown et al., 1990, 1998; Abbas et al., 1999). AGFs were measured in order to establish the threshold level at which an ECAP is recorded. This threshold measure was used later in the experiment. To record an ECAP, an 80 pps stimulus was presented. Two stimulating pulses (the “masker,” followed by the “probe”) were presented to a single intracochlear electrode just below C level. The masker level was fixed 10 CL higher than the probe, and the masker-probe interval was fixed at 400 µs. (The use of a high-level masker was necessary for measuring the stimulus artifact, which can later be accounted for when measuring the response to the probe. For a detailed description of this paradigm, see Brown et al., 1990, 1998, and Abbas et al., 1999). If an ECAP response to the probe was recorded, the masker and probe levels were systematically decreased in 2 CL intervals until the ECAP clearly could no longer be identified in the recorded response during online analysis. Each ECAP recording was based on an average of 50–100 sweeps. The electrode used for recording ECAP responses was located two electrodes apical to the probe (i.e. when electrode 4 was stimulated, the electrode used for recording the ECAP was number 6.). Recordings were exported for analysis via custom-written MATLAB scripts. N1 and P2 peaks for each ECAP waveform were manually selected. The voltage difference between the two peaks was the ECAP amplitude. The lowest probe level at which an ECAP was visually observed was considered the ECAP threshold. Additionally, ECAP amplitudes were plotted as a function of stimulus level to generate the amplitude growth function. The slope of the growth function was calculated via a linear regression fit to all non-zero amplitudes, and represents the rate of change in ECAP amplitude as stimulus level varies (µV / CL). The noise floor of the NRT system is approximately 5 µV (Patrick et al., 2006), thus all ECAP amplitudes were above this noise floor.
SAM Stimuli
Custom Sound EP allows the user to select a masker that is either a single pulse or a pulse train. The number of pulses in the pulse train can be specified. After a user-selected masker probe interval (MPI), a single probe pulse is delivered. In this study we adjusted the masker-probe interval so that it was equal to the interpulse interval in the preceding pulse train, so that the probe pulse became the last pulse of the AM pulse train.
A research patch was provided by Cochlear Ltd. that allowed the masker pulse train to be amplitude modulated. A 4000 pps biphasic pulse train was sinusoidally amplitude modulated at 125, 250, 500, and 1000 Hz. The masker train was 15 ms long and consisted of 60 biphasic current pulses presented at a rate of 4000 pps. The pulse train was designed to be long enough so that when modulated at the slowest rate (125 Hz), at least one full cycle of modulation would be presented. When higher modulation frequencies were used, more cycles of modulation were presented.
To measure ECAP responses evoked by a pulse train, the NRT system delivers a series of masker pulses followed by a probe pulse and measures the response to that probe pulse. Our goal was to measure ECAP amplitudes across the duration of the pulse train, requiring us to record a series of ECAPs for each pulse in the train. Initially, we used the full series of 60 pulses as the masker. A series of recordings were then made using one less pulse in the masker pulse train, where the final pulse in the masker train preceding the probe was removed. The removal of the final pulse effectively shortens the duration of the modulated pulse train. This process was continued until ECAP responses were obtained for each pulse across one to two full cycles of amplitude modulation. The probe rate (rate at which each successive masker-probe stimulus was presented) was set to 10 pps, resulting in a period of 100 ms between the onset of successive stimulus presentations. This relatively slow stimulation rate was used to minimize the impact of long-term adaptation of neural responses between recordings. Fifty sweeps per recording were used to obtain ECAP responses.
Pulse amplitudes in the masker train were modulated from slightly above ECAP threshold (10 CL above) to slightly below the uncomfortable level (5 CL below). This range of amplitude modulation was large but the stimuli were still comfortable for the listener (Table 3). We set the lowest level in the train 10 CL above ECAP threshold to attempt to record a response even at the lowest level of the AM train. Masker levels were set automatically by the NRT software, while the probe level had to be calculated individually based on the modulation frequency and stimulus levels. The probe level was set to continue the amplitude modulation pattern of the masker pulse train. This level was calculated as:
where CLprobe is the clinical level of the probe, C Level is the uncomfortable level, m is the modulation depth (in clinical levels), fm is modulating frequency, and t is the time when the last masker pulse was presented. As previously mentioned, the modulation depth was set to span the ECAP dynamic range for each electrode:
Neural responses were measured in response to each pulse in the SAM pulse train. Normally, ECAPs are measured using a two-pulse paradigm, where a high level masker pulse is presented and followed by a lower level probe pulse (typically a 10 CL difference; Abbas et al., 1999; Brown et al., 1990, 1998). In this standard method, four stimulus traces are used, as illustrated in the schematic on the left: a probe pulse (A), masker pulse + probe pulse (B), masker pulse (C), and a no stimulus condition (D). Responses are recorded after each, and the ECAP is mathematically resolved as A-(B-C)-D. The masker preceding the probe can be set to be a single pulse (Figure 1, left panel) or a train of pulses (Figure 1, right panel). In any ECAP recording obtained using the NRT system, the probe pulse is always the last pulse. The masker (whether it is a single pulse or a pulse train) always precedes the probe pulse.
Figure 1.
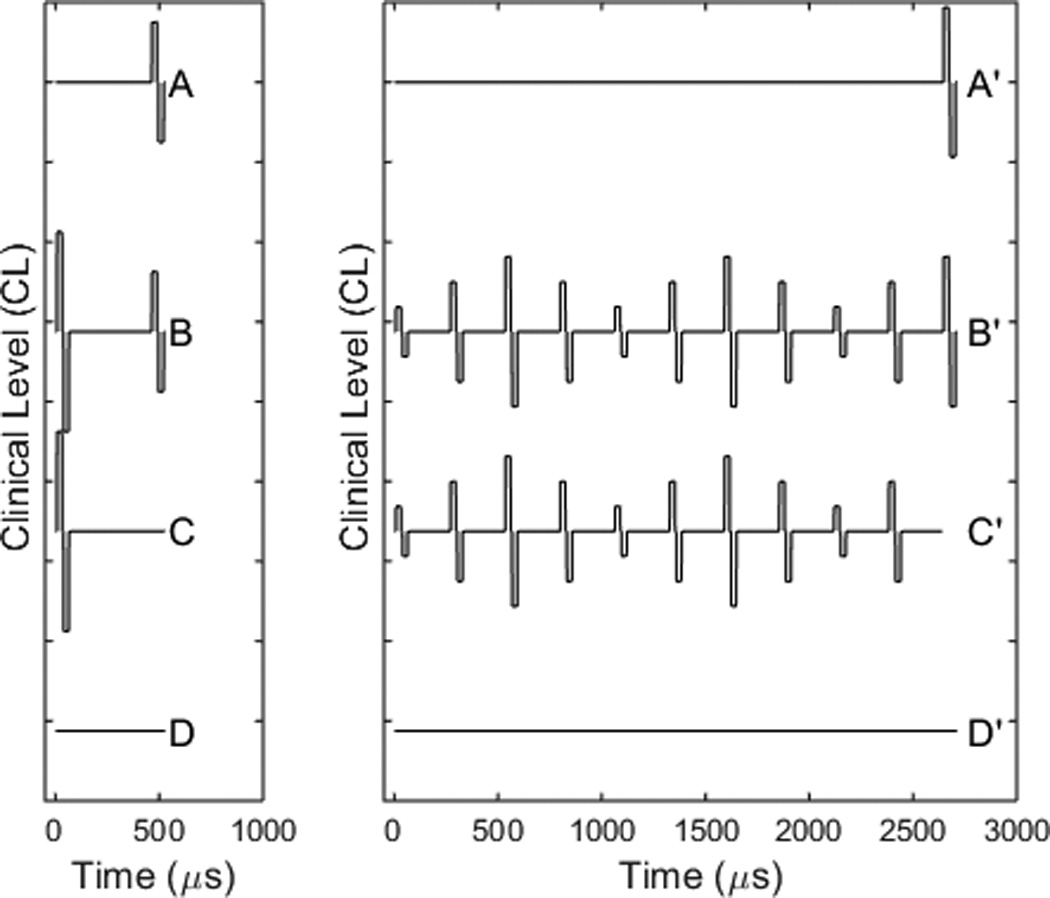
Schematic of the two-pulse masker-probe recording (left) and pulse train recording (right). The masker, whether a single biphasic pulse or a pulse train, always precedes the probe. The two-pulse recording is done separately from the masker train recording. The B and C traces from each recording then are subtracted from one another to obtain the true probe response that follows the masker train.
The schematic at the right panel of Figure 1 illustrates the measurement of the response to a modulated pulse train. The masker is set to be multiple pulses modulated in amplitude and the masker probe interval is set to be equal to the interval between masker pulses. A modified artifact reduction scheme was used to recover the ECAPs from the measured responses, similar to that used previously for constant amplitude pulse trains (Wilson et al., 1997; Hay-McCutcheon et al., 2005; Hughes et al., 2012, 2014). The response to the probe at the end of the masker pulse train can be calculated by subtracting B’-C’. This eliminates the effect of the masker during the recording interval after the probe and leaves both the probe response as well as the probe artifact. The probe artifact can be determined using the two-pulse paradigm illustrated in the left panel. If the probe in the left frame is set to the same level the probe level in the right frame and the masker-probe interval is short enough (400 µs) in the left frame, then the neurons will respond to the masker and be refractory to the probe. Thus the B-C subtraction will provide a template of the probe artifact without probe response. The ECAP response to the pulse train then is calculated as: (B’-C’)-(B–C). A series of two-pulse masker-probe recordings were obtained using the same current levels as in the modulated train. These recordings allowed us to obtain the probe artifact (B and C traces) needed to recover the ECAP responses to the SAM train.
ECAP traces were exported from Custom Sound EP and analyzed with custom-written MATLAB scripts. The scripts performed the modified subtraction procedure previously outlined, resulting in a series of ECAPs whose amplitudes were used in subsequent analyses.
For each subject, up to 12 stimulus conditions were tested (4 modulation frequencies × 3 test electrodes; see Table 2). The order of testing was randomized across subjects.
Psychophysical Measures
Psychophysical measures were assessed behaviorally using direct stimulation. The speech processor was bypassed and stimulation of the individual electrodes in the implant was controlled via a Nucleus Implant Communicator (NIC) routine, version 2. The electrodes used for ECAP testing were also used for psychophysical testing. The stimulus parameters were as similar as possible to the parameters used for evoked potential testing. The stimulus used for psychophysical testing was a 300 ms pulse train consisting of cathodic leading, biphasic current pulses presented at 4000 pps. Each current pulse in the train had an IPG of 7 µs and the pulse width was the same as that used for electrophysiologic testing (i.e. 25, 37, or 50 µs/phase). The pulse train was amplitude modulated at 125, 250, 500, and 1000 Hz rates.
Threshold and Comfort Levels
Measures of T and C levels were obtained using a 300 ms constant amplitude pulse train stimulus presented at a 4000 pps rate in steps of 5 CL. Participants indicated when they first heard the stimulus and when it was uncomfortably loud. This procedure was repeated 2–3 times to ensure consistency in T and C level measures. A 50% dynamic range was calculated from these levels. Custom Sound EP was used for T and C level measurements; the remainder of psychophysical measures was done via NIC routines.
Loudness Balancing
Prior to measurement of modulation detection thresholds, we performed loudness balancing between SAM and constant amplitude pulse trains. As pointed out by McKay and colleagues (McKay & Henshall, 2010; Fraizer & McKay, 2012), modulated stimuli are generally perceived as louder than constant amplitude pulse trains when the same carrier level is used; these differing loudness percepts can be used as a cue for AM detection tasks. Thus, performance in AM detection tasks using stimuli that are not loudness-balanced may reflect the use of loudness cues rather than the listener’s ability to perceive changes in the temporal envelope of the signal. Potential loudness cues were not accounted for by older psychophysical studies that did not incorporate level roving (e.g. Shannon, 1992; Busby et al, 1993, as discussed in McKay & Henshall, 2010).
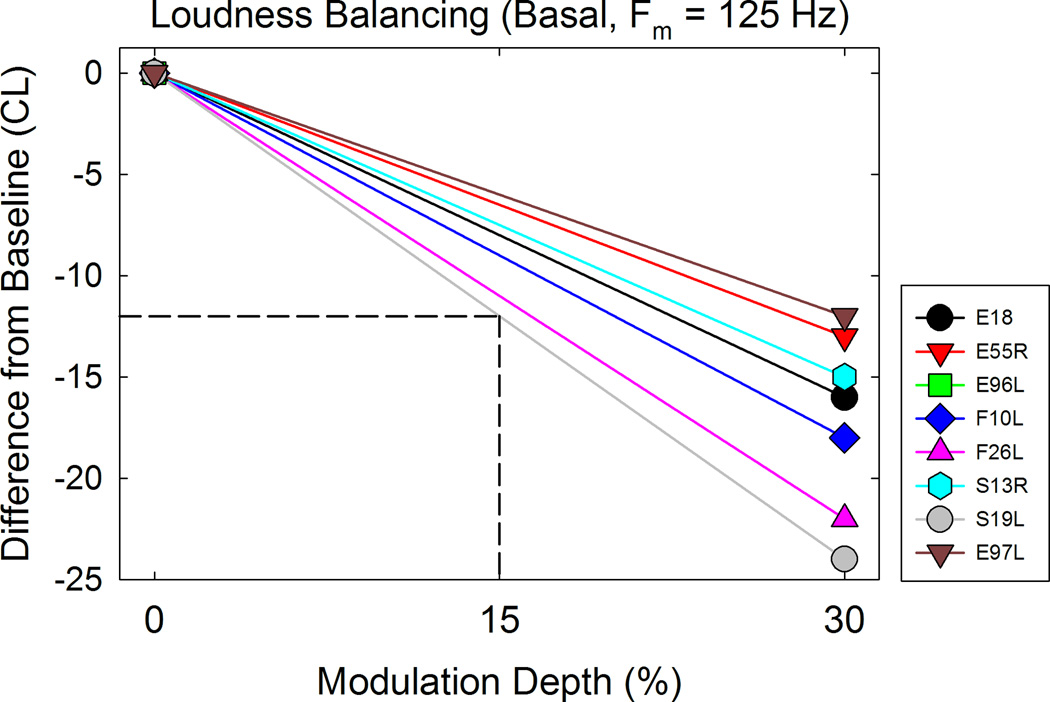
We incorporated dynamic loudness balancing to reduce loudness cues (Galvin et al, 2014). Briefly, Galvin et al used an adaptive loudness balancing task between non-AM stimuli and stimuli that were amplitude modulated at different modulation depths (5 – 30%). They calculated the difference in peak levels between loudness balanced AM and non-AM stimuli for each modulation depth and observed greater differences in levels as modulated depth increased. In this study, we performed loudness balancing between non-AM and stimuli amplitude modulated only at a depth of 30% in the interest of time. A two alternative-forced-choice (AFC) task was performed, where the reference stimulus was the non-AM pulse train presented at 50% dynamic range. The experimental stimulus was an AM pulse train. Modulation depth was fixed at 30%. Participants were presented with an AM and a non-AM stimulus and were asked to indicate which sounded louder. The carrier level of the non-AM stimuli remained the same, while the carrier level of the AM stimuli was modified according to an adaptive procedure to determine the point of equal loudness. The procedure was terminated after ten reversals and the final level was based on an average of the last six reversals. The procedure was repeated 2–3 times, and results were averaged to give a loudness balanced level for a pulse train modulated at 30% depth. Generally, the carrier level of the modulated stimuli is reduced relative to the unmodulated stimuli in order for a balanced loudness perception. This difference in current level is plotted as Figure 2 from the eight study participants for the basal electrode at a 125 Hz AM rate. Interpolation was used to estimate equal loudness levels for modulation depths between 0% and 30% for each participant as illustrated in the figure. These level adjustments were utilized during measurement of modulation detection thresholds. Loudness balancing was performed for each modulation frequency and each electrode.
Figure 2.
Loudness balancing performed on the basal electrode for one modulation frequency. The difference from baseline represents the required decrease in peak carrier level of the modulated stimuli in order for the non-AM and AM stimuli to sound equally loud. These plots were used to determine levels for intermediate depths. For example, at 30% depth, the peak carrier level was decreased by 24 CL for participant S19L. If the stimulus was modulated at 15% depth, the peak carrier level would be decreased by 12 CL to sound equally loud as the non-AM stimulus.
Our dynamic balancing technique slightly differed from the Galvin et al study in that we used a linear interpolation technique to determine the loudness adjustment needed for stimuli modulated at depths other than 30%. CI patients in the Galvin et al study performed an adaptive loudness balancing task at different modulation depths (5 – 30%) to determine a similar level adjustment function as in Figure 2, but they performed an exponential fit (see Fig 1 of Galvin et al, 2014) rather than a linear fit as we did. One might argue that loudness does not necessarily grow linearly with modulation depth based on prior data (e.g. McKay & Henshall, 2010; Chatterjee & Oberzut, 2011; Fraiser & McKay, 2012; Galvin et al, 2014). Galvin et al, 2014 provided the individual logarithm equations use to fit individual subject data. Thus, we replotted their equations, and could see that the data was sufficiently modeled by a linear fit, at least over the range of modulation depths that we were interested in. Based on this, it appeared justified to use a linear fit and an interpolation technique. In addition, given the four modulation frequencies and three test electrodes, loudness balancing at multiple modulation depths would not be feasible to perform within the time constraints of the participants.
Modulation Detection Thresholds
MDTs were measured via 3 AFC, 2-down 1-up adaptive procedure (Levitt, 1971). The reference intervals were an unmodulated pulse train presented at 50% DR, while the experimental interval was a loudness-balanced SAM train. Modulation depth (m) was varied adaptively. The carrier level of the AM train was adjusted so as to control for loudness cues (as in Figure 2). Additionally, a ±3 CL rove was used to further minimize the chances that the AM signal was selected due to differences in perceived loudness between the modulated and constant amplitude stimuli. The initial step size for modulation depth was 0.1 (or 10% modulation), which was reduced by a factor of two for subsequent reversals. The procedure terminated after ten reversals, and the MDT was based on an average of the last six reversals. The MDT task was repeated 2–3 times.
Though our dynamic loudness balancing technique should minimize loudness cues, our rove was implemented as a secondary method of further removing loudness cues. The rove used here (± 3 CL) is also in line with previous psychophysical studies of AM detection (e.g ± 4 CL in Fraizer & McKay, 2012 and Galvin et al, 2014). We were cautious not to use larger roves than necessary, as large roves can distract from the AM task, although rather large roves (up to ± 3 dB, or ± 17.1 CL) have small (though sometimes significant) effects on psychophysical AM detection thresholds (Chatterjee & Oberzut, 2011).
RESULTS
Peripheral ECAPs
Figure 3 shows a series of ECAPs recorded from two participants (F26L and S19L). The stimulus was a pulse train modulated at 250 Hz and presented to the medial electrode. The series of ECAP recordings shown span one modulation cycle. The ECAP typically consists of a single negative peak followed by a positive peak. The clinical levels for each pulse in the pulse train are shown at the bottom of the graph. For F26L, ECAP amplitudes tend to follow changes in clinical level for the individual pulses in the modulated pulse train. At high stimulus levels the responses show correspondingly high amplitudes and at low levels smaller responses are evident.
Figure 3.
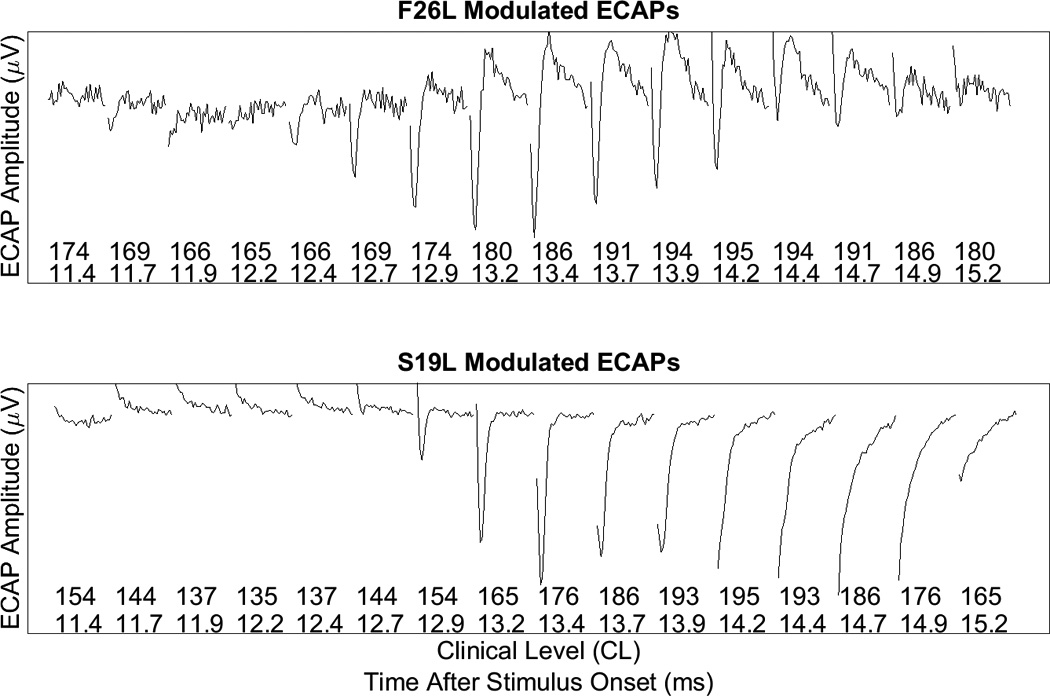
A series of ECAPs recorded from each pulse in the AM train for participants F26L and S19L. The corresponding level of each pulse in the train, as well as the time after stimulus onset, is represented by the x-axis. Normal ECAP responses (as illustrated for F26L) show a clear negative peak followed by a smaller positive peak. For S19L, negative peaks were not clear in some traces and there tends to be a rising tilt in the waveforms which masks the positive peak.
Recordings obtained from participant S19L were abnormal, which appeared to reflect an incomplete removal of stimulus artifact from the ECAP response when using the modified subtraction procedure. No clear negative or positive peak is apparent for many of the pulses in the SAM pulse train. He et al. (2016) reported similar results when the modified subtraction procedure was used to minimize artifact contamination. Data such as that shown in the lower panel of Figure 3 were excluded from further analysis.
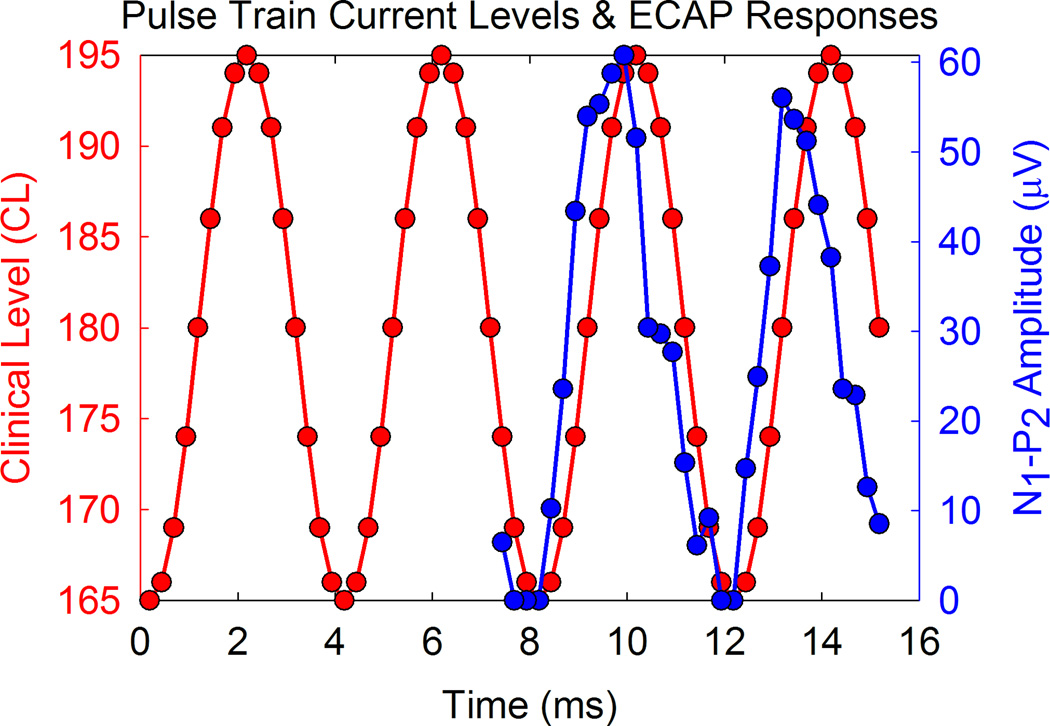
Figure 4 shows a comparison of the variation in the amplitude of the ECAP and the clinical level of the individual pulses in the SAM pulse train. The left y-axis (in red) represents the clinical levels of each pulse in the modulated train, while the right y-axis (in blue) represents the ECAP amplitude evoked by each pulse for two cycles of the pulse train. Clearly there is periodic variation in the ECAP amplitude measures, albeit with some distortion. This example also shows a phase difference in the peak of the modulated response compared to the peak of the input SAM train, though this phase difference did not necessarily occur for all CI users. The ECAP responses appear to lead relative to the stimulus waveform. We attribute the differences in phase as well as distortion in response waveforms to differences in neural refractory and recovery as each pulse of the stimulus is presented.
Figure 4.
Stimulus level (red) and ECAP amplitudes (blue) are plotted as a function of time after stimulus onset for each pulse in the SAM pulse train for electrode 10 of participant F26L. Note that response amplitudes approximately follow the sinusoidal modulation of the stimulus (250 Hz) but show some phase lead and distortion.
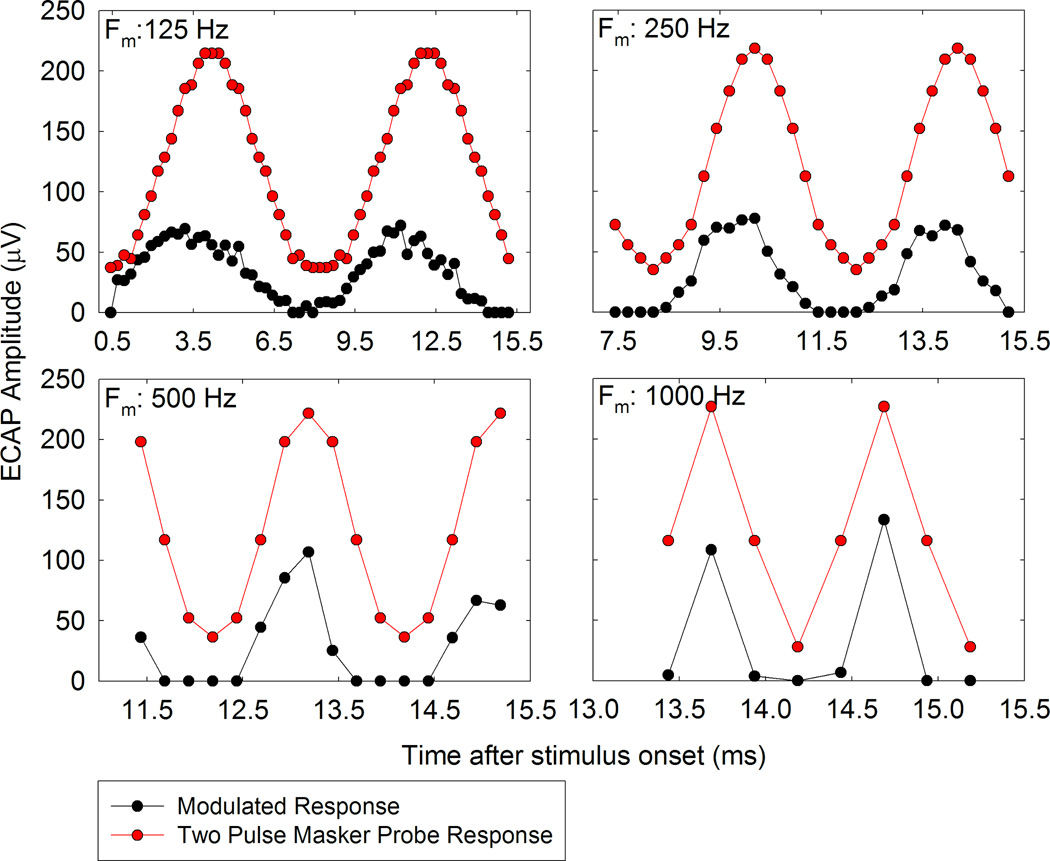
Figure 5 plots an example of ECAP amplitudes for the four modulation frequencies recorded from one participant (F26L) and one electrode (basal). In addition to measuring ECAPs for the SAM pulse trains, we measured a series of ECAPs using the standard two-pulse masker-probe paradigm, where the probe levels correspond to the levels of each pulse in the AM train. These individual responses are superimposed (red symbols) with the corresponding current levels in the modulated train. There is clearly some adaptation of the SAM ECAPs in that neural responses evoked by the SAM train are reduced compared to the neural responses evoked by a two pulse masker-probe stimulus. We also note that the amount of adaptation is not as pronounced as the modulation frequency increases. For example, the highest ECAP amplitude for the pulse train amplitude modulated at 125 Hz is around 75 µV while the highest ECAP amplitude for the 1000 Hz amplitude modulated pulse is approximately 100 µV. This effect is seen consistently across electrodes and modulation frequencies for all of our CI users. We attribute this effect to the fact that at low modulation frequencies there are more current steps between maximal and minimal current levels; multiple stimulation pulses could result in greater accumulated adaptation effects, which reduces neural responses. We also note that the SAM stimulus varies in starting and ending phases, but should not impact our results since the Modulated Response Amplitude (MRA) was based on the difference in the peak ECAP and valley ECAP.
Figure 5.
Responses to amplitude modulated pulse trains (black symbols) presented to electrode 4 of participant F26L are plotted as a function of time after stimulus onset for four different modulation frequencies indicated on each plot. ECAP amplitudes to a single pulse (recorded using the standard two-pulse subtraction paradigm) at each of the levels used in the amplitude modulated stimulus are plotted on the same time axis (red symbols).
As a result of the accumulated adaptation effects at the lower modulation frequencies, the difference in ECAP amplitudes evoked by the SAM train (Figure 5, black data points) and the ECAP amplitudes evoked by the two-pulse masker-probe stimulus (red data points) is greater at lower modulation frequencies. These differences become smaller as modulation frequency increases. This trend is similar across most of our CI users, though we notice some variation that can be attributed to both sparse sampling at higher modulating frequencies and the no response conditions (0 ECAP amplitudes) at the valley of the modulated stimulus.
No ECAPs could be measured at the valley of the modulated stimulus for many of our participants, which could reflect neural adaptation occurring from prior stimulation. The modulation range varied from 10 CL above ECAP threshold to 5 CL below C level. To measure responses at the valley of the stimuli, we may need to start stimulation at higher levels (e.g. 15–20 CL above ECAP threshold).
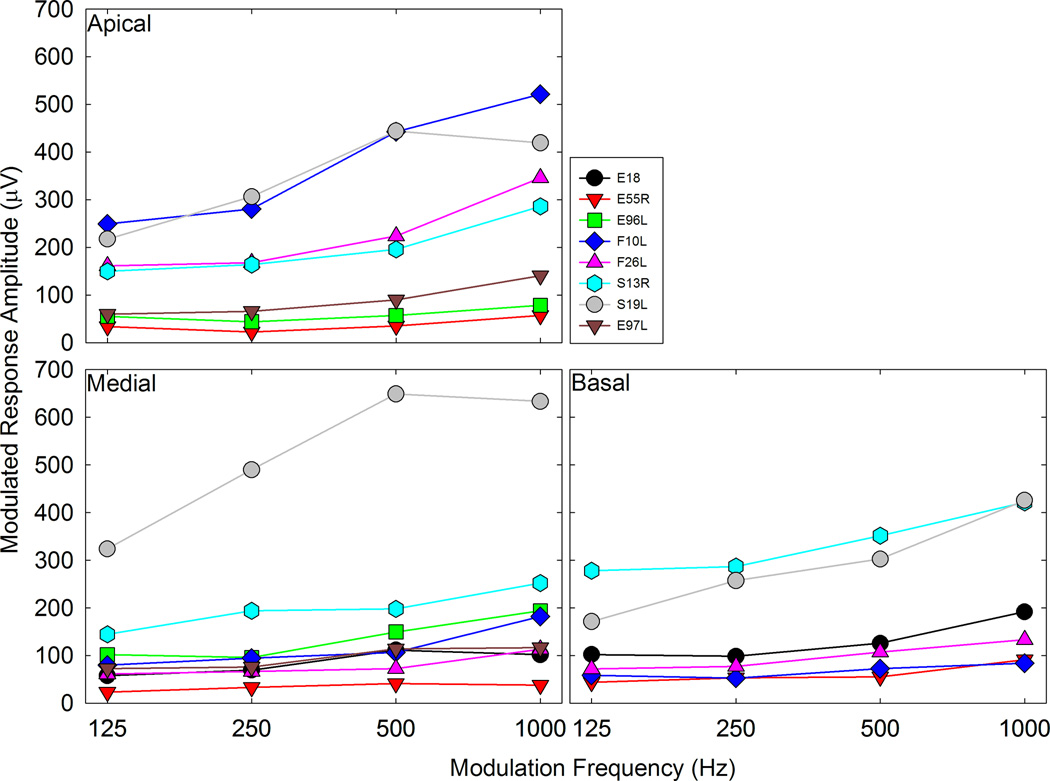
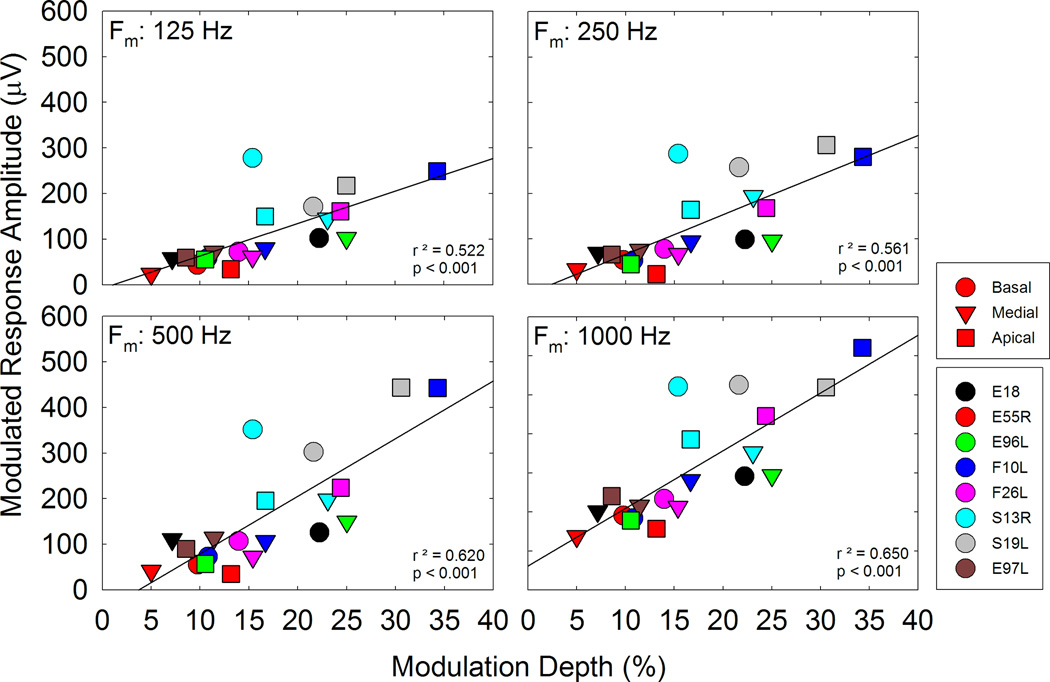
From Figure 5, the Modulated Response Amplitude (MRA) was calculated. Figure 6 shows how MRA changes as a function of modulating frequency and test electrode for all the CI users. For the majority of participants, MRA tends to increase as the modulation frequency increased. The impact of modulating frequency on MRA was evaluated via a Linear Mixed Effects (LME) model, where electrode location and frequency were fixed effects and subjects were random effects. MRAs at 1000 Hz were significantly higher than MRAs at 125 and 250 Hz (p ≤ 0.001 in both cases) (also see Figure 8). Although mean MRA at 1000 Hz was higher than the mean MRA at 500 Hz, the difference did not reach statistical significance.
Figure 6.
The Modulated Response Amplitude (MRA) is the difference in µV between the maximum and minimum ECAP amplitude recorded at each modulation frequency. Response amplitudes vary among subjects and electrodes, but in all cases it increases as modulation frequency increases.
Figure 8.
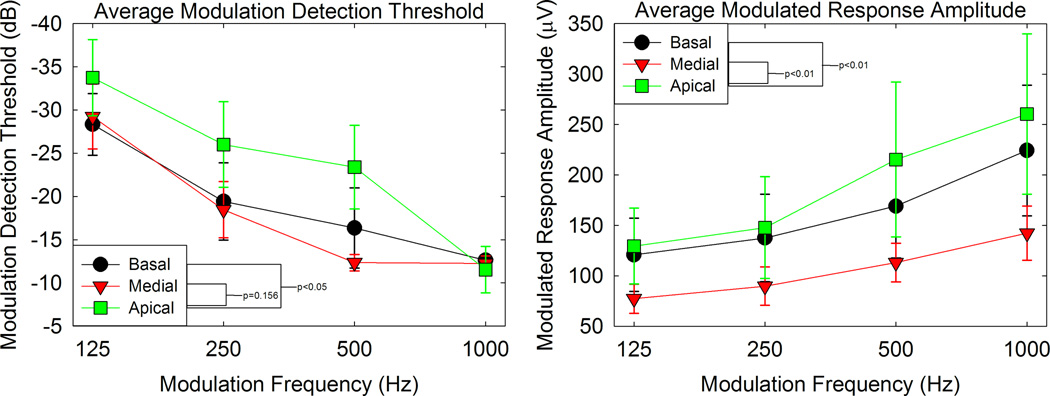
Average (± SEM) MDTs and MRAs are plotted as a function of modulation frequency with stimulating electrode as the parameter.
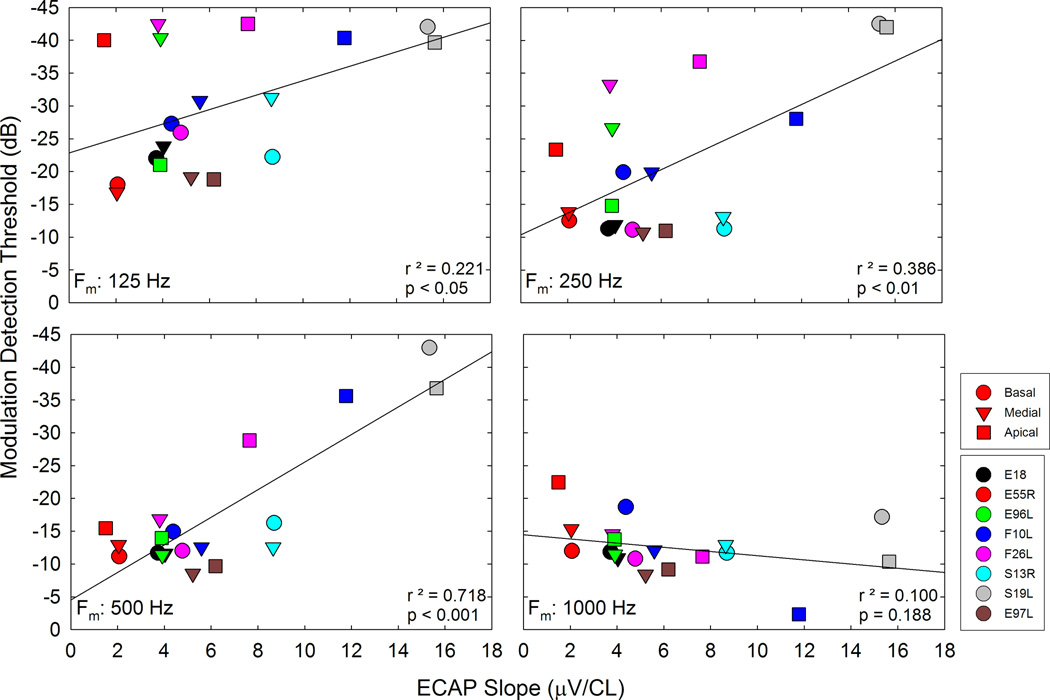
Psychophysical Temporal Modulation Transfer Functions
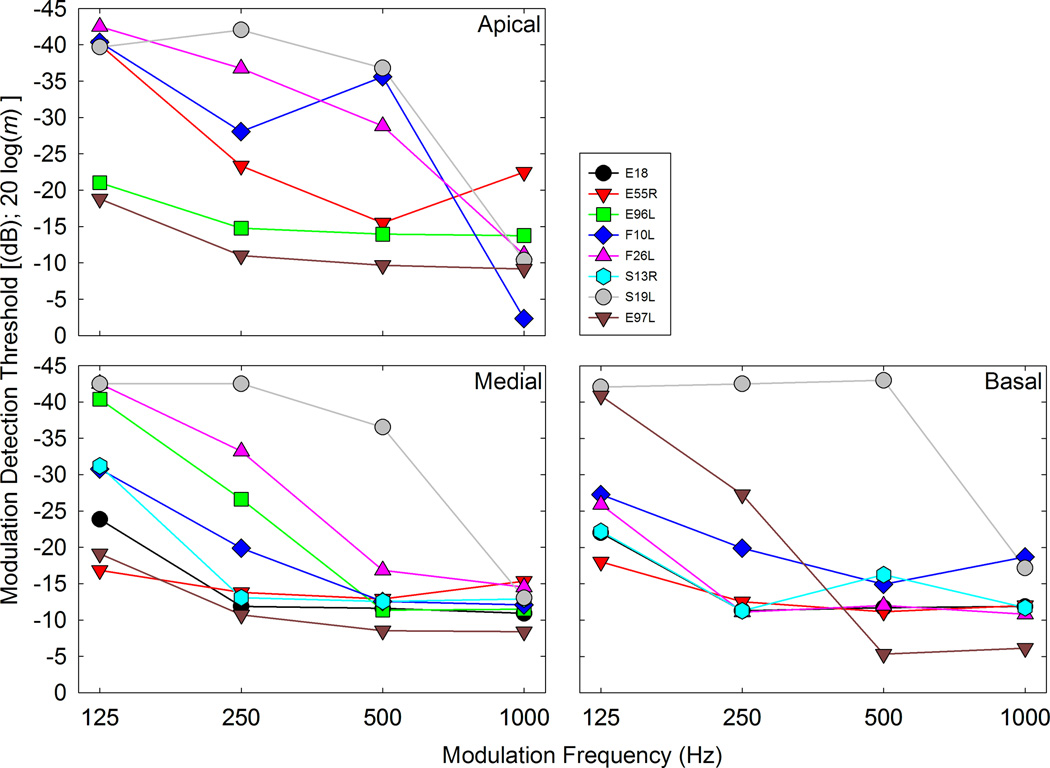
Figure 7 shows the effect that modulation frequency had on MDTs. MDTs were measured in terms of modulation depth, converted into dB (re: 100% modulation), averaged across trials, and plotted as a function of modulation frequency. Thus, 0 dB represents a fully modulated signal and a threshold of 0 dB represents the highest AM detection threshold that can be achieved. Increasingly negative numbers represented lower thresholds. Previous studies measuring psychophysical temporal modulation transfer functions in CI users have demonstrated low-pass characteristics with cutoff frequencies of approximately 100 Hz (Shannon 1992, Busby et al., 1993). Our results are consistent with previous work in that across all patients and electrodes, the MDTs tend to increase as the modulation frequency increases. However, with relatively few modulation frequencies tested and none of them being below the 100 Hz lowest test frequency commonly encountered in the literature, we were not able to determine a clear cutoff frequency. Note that the psychophysical data clearly shows trends opposite that of the ECAP amplitude data shown in Figure 6. The ECAPs show an increase in MRA as modulation frequency is increased for the same stimuli used in the psychophysical measures shown in Figure 7.
Figure 7.
Modulation depth (m) thresholds in dB re: 100% modulation (0 dB = 100% modulation) is plotted as a function of modulation frequency for all participants. Each panel shows data from an apical, medial, or basal electrode as indicated on the graph.
Effects of Stimulating Electrode
We replotted the data in Figures 6 and 7 in Figure 8 to examine variations with stimulus electrode. Averaged data across participants for MRAs and MDTs are plotted for individual electrodes. As in the previous figures, the physiological responses and psychophysical data show opposite trends across modulation frequency, but for both, apical electrodes showed lower psychophysical thresholds and higher MRAs than medial and basal electrodes. The impact of electrode location on peripheral and psychophysical responses was assessed via LME modeling. In the LME model, electrode location and frequency were fixed effects and subjects were random effects (same analysis as discussed earlier for the “Peripheral ECAPs” section of the results). For the psychophysical responses, thresholds were significantly lower for the apical electrodes compared to the basal electrodes (p < 0.05) but not significantly lower when compared to the medial electrodes (p = 0.156). For the peripheral responses, amplitudes were significantly higher for apical electrodes compared the medial and basal electrodes (p < 0.01 in both cases). In both analyses, the psychophysical and peripheral data were averaged across frequencies.
We predicted that the low psychophysical threshold and increased MRA on the apical electrodes might be related to better neural survival in the more apical areas of the cochlea relative to the basal areas. Consequently, we also calculated the slope of the ECAP amplitude growth function (recorded using the two-pulse masker probe paradigm), where steeper slopes suggest greater recruitment of neurons as stimulus levels are increased. The averaged data shows slope of the ECAP growth function tended to be steeper for the apical electrodes. However, when slopes were subjected to an LME analysis, with electrode location as the fixed effect and subject as the random effect, the slopes for apical electrodes were greater than the medial and basal electrodes but those differences did not reach significance (p = 0.07 and 0.09, respectively).
Comparison of Peripheral and Psychophysical Measures within Modulation Frequency
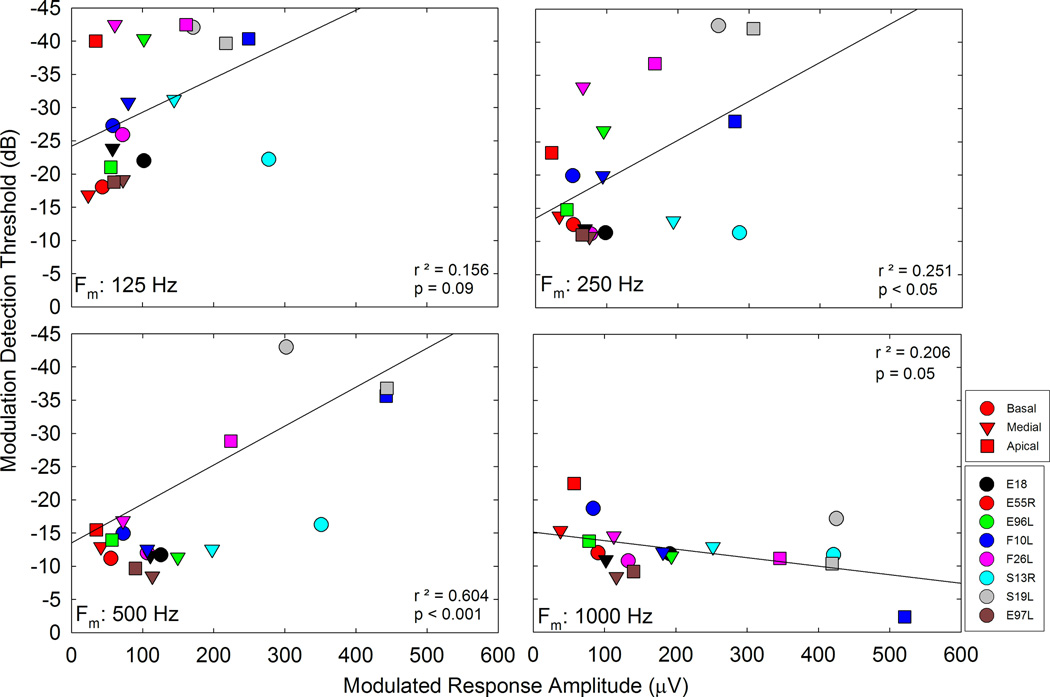
The trends demonstrated in Figures 6, 7, and 8 show differences between peripheral physiological and psychophysical measures. We also investigated trends across subjects and electrodes at each modulation frequency to compare ECAP and TMTF results. Figure 9 shows scatterplots of MDT vs MRA; each panel shows data for a different modulation frequency. In general, larger peripheral responses were correlated with lower MDTs for the lower modulation frequencies, though the correlation was significant for only 250 Hz and 500 Hz modulation. The trend was present for 125 Hz, but did not reach statistical significance. Despite the robust peripheral response with a 1000 Hz SAM pulse train, psychophysical MDTs are high and not correlated with the peripheral response, which may indicate limitations in central processing of signals modulated at high rates.
Figure 9.
MDTs are plotted relative to MRAs for all participants and 2–3 electrodes/participant. Each panel represents data for a different modulation rate as indicated on the panel. Linear regression was performed on each and r2 and p values are indicated on each panel.
Relationship between Modulated Responses and other ECAP Measures
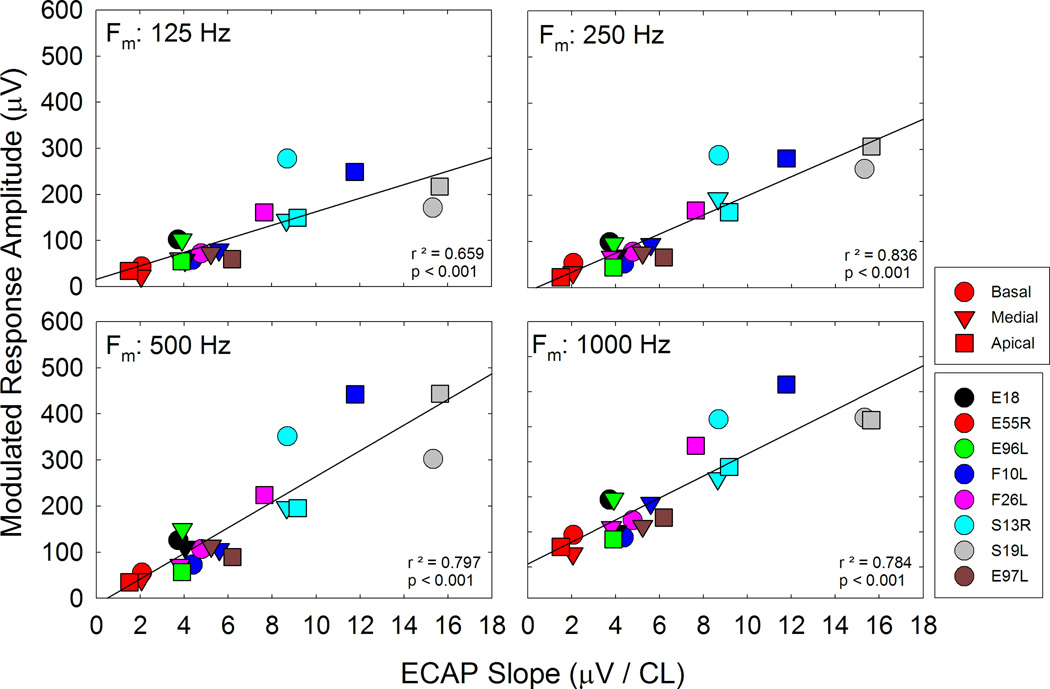
We predicted that sensitivity to modulation may be related to neural health and consequently may be related to the growth of response to single pulses. For instance, if the amplitude growth function is steep, then MRA might be expected to be large and corresponding psychophysical sensitivity might be expected to be greater. Figure 10 shows a scatterplot of MRA vs. slope of the growth function for individual subjects/electrodes. Data for each modulation frequency are plotted on separate panels. The steeper AGF slopes indicate greater change in ECAP amplitude for a given change in current level. Thus, for the modulated stimuli, greater MRAs, which reflect amplitude differences between the peak and valley of modulated responses, may be related to greater changes in ECAP amplitudes as reflected by steeper AGF slopes. The correlations were significant across all modulation frequencies.
Figure 10.
MRA is plotted as a function of ECAP slope for individual participants/electrodes. Each panel plots data for different modulation frequency as indicated on the panel. Linear regression was performed on each data set; r2 and p values are indicated on each panel.
Figure 11 shows the comparisons between MDT and AGF slope. In general, steeper slopes were significantly correlated with lower psychophysical thresholds at 125, 250, and 500 Hz. No correlation was found for 1000 Hz, implying once again a possible central limitation to processing modulated signals despite the fact that steeper slopes suggested better neural health.
Figure 11.
MDT is plotted as a function of ECAP slope for individual subjects/electrodes. Each panel plots data for different modulation frequency as indicated on the panel. Linear regression was performed on each data set; r2 and p values are indicated on each panel.
Regarding the individual data in Figures 9 and 11, participant S19L may be an outlier given his low psychophysical modulation detection thresholds and high ECAP responses. Performing the correlations again with S19L excluded, the only significant correlations are for 500 and 1000 Hz for the data in Figure 9. For Figure 11, the correlation is only significant for 500 Hz when S19L is excluded, while the negative trend observed for 1000 Hz has now become significant. Nevertheless, the trends remain the same despite the removal of S19L for the data presented in Figures 9 and 11. Given that 8 patients were in the study (and that CI data can be noisy), it may be difficult to see statistically significant results. We also performed the correlations separately for basal, medial, and apical electrodes to assess any effects of electrode location. For the psychophysical vs MRA data (Figure 9), the correlation between both measures was significant only for the basal electrode at 500 Hz modulation frequency. For the psychophysical vs ECAP slope data (Figure 11), there were significant correlations for the basal conditions (125, 250, and 500 Hz AM rates) and apical conditions (500 Hz AM rate).
Effects of Modulation Depth on Modulated ECAP Responses
Based on animal recordings, we expect that modulated response amplitudes will be dependent on the modulation depth of the stimulus (Jeng et al., 2009). The data shown in Figure 12 are based on a particular modulation depth used for the peripheral recordings, held constant across modulation frequency, but chosen on the basis of ECAP threshold and behavioral uncomfortable listening level for each electrode, presumably tailoring the range to the individual’s dynamic range for that electrode. Thus, while the methodology for determining modulation depth is the same for each participant and each electrode, the actual modulation depth varies based upon the ECAP threshold and C level. For instance, modulation depth is small for patients with higher ECAP thresholds and lower C levels, and vice-versa.
Figure 12.
MRA is plotted as a function of modulation depth (m). Each plot includes data from the 8 participants and 3 electrodes; each plot shows data for a different modulation frequency as indicated on the plot. Linear regression lines show significant correlations between modulation depth and MRA for all frequencies.
Figure 12 plots MRA as a function of modulation depth for each modulation frequency as indicated on each graph. In each plot, data are combined for the eight participants and the 2–3 stimulating electrodes. The plots show a significant relationship between MRA and modulation depth in that higher depths led to a greater response. This is evident across all participants and electrodes for each modulation frequency tested.
DISCUSSION
ECAP responses to amplitude modulated pulse trains reflect the periodicity of the stimulus, albeit somewhat distorted and occasionally with a phase difference between stimulus and response (Fig 4). This is consistent with previous reports in Ineraid listeners (Wilson et al., 1994, 1997b) and animal data (Abbas et al., 1997, 2003; Jeng et al., 2009). As previously mentioned, the differences in phase and the distortion in response may be due to refractory and recovery properties of the neurons as they respond to each pulse in the pulse train. Similar to the ECAP alternation phenomenon that occurs with the use of constant amplitude pulse train (outlined in the introduction), subsets of neurons may be available for stimulation while other subsets may be in differing degrees of refractory states due to previous stimulation in the AM train. The degree of refractoriness can be variable since previous pulses in the train may be lower in level (as in the rising portion of the AM stimulus) or higher in level (as in the falling portion of the AM stimulus). In addition, there may be threshold / saturation effects that can cause further distortion (e.g. Jeng et al., 2009), in that the valleys of the AM train may be below neural thresholds while neural responses may saturate near or at the peak of the AM train.
The MRA increases with increasing modulation frequency, consistent with prior data in guinea pigs (Fig 6; Abbas et al., 1997, 2003; Jeng et al., 2009). The increased response amplitudes may reflect neural adaptation mechanisms. With a low modulating frequency, there are multiple current steps between the peak and valley of the modulation cycle, which means successive stimuli are more similar to one another and neural responses are more likely to adapt. However, with higher modulation frequencies, there are fewer current steps between the peak and valley of modulation and there are greater differences in current levels with each successive pulse in the pulse train. These larger current steps decrease the likelihood of adaptation and would consequently increase the modulated response amplitude. Additionally, auditory nerve single fiber data indicate that vector strength tended to increase with modulation frequency when a pulse train was sinusoidally amplitude modulated over a range of 20–500 Hz (Hu et al., 2008). Vector strength quantifies the degree at which nerve fibers phase lock to the stimulus. In addition, the MRA quantifies the degree of neural responsiveness to the modulations of the stimulus. Thus, both MRA and vector strength may be interpreted as similar measures of neural synchrony to modulated stimuli.
There is also a possibility of facilitative or conditioning effects contributing to the increased MRAs with increasing modulation frequency. Facilitation refers to an increased excitation of nerve fibers due to prior sub-threshold stimulation, causing decreases in response thresholds and increases in suprathreshold amplitudes (Dynes, 1996; Heffer et al., 2010). As demonstrated by the black data points in Figure 5, there are peaks and valleys in the modulated response amplitudes. Pulses in the valleys are essentially sub-threshold stimuli, as evidenced by a lack of ECAP responses. These pulses may act as a conditioner and lead to increased responses for the next supra-threshold stimulus on the rising portion of the AM pulse train. Conditioning could play a bigger role at higher modulation frequencies. For example, at a 1000 Hz AM rate, there are more subthreshold stimuli in the valleys compared to the singular peak. This peak may by enhanced by facilitative effects. For lower modulation frequencies, the peaks are unlikely affected by facilitation since prior stimulation is well above threshold. Those peaks likely are more affected by adaptation from prior stimulation, as previously discussed.
One difference between the present CI data and animal ECAP data of Jeng et al., 2009 and Abbas et al., 2003 is that the modulated responses demonstrate a decrease at high frequencies in the animal data. The Jeng et al., 2009 study measured MRAs for different modulating frequencies, and showed increases in MRA until a cutoff of approximately 300 Hz modulating frequency for a 1000 pps carrier and 400–800 Hz for a 5000 pps carrier. In the present study, despite using a high modulating frequency (1000 Hz) with a high carrier (4000 pps), the response amplitude continues to increase across all of our CI users and across all test electrodes (Fig 6). In comparing these data there is certainly a species differences. In addition, animals were acutely deafened while the human implant population has a variety of etiologies, typically with more long-term pathology. Also, the animal data were collected using long-duration stimuli (200–400 ms), while the human data reported here used shorter duration stimuli (15 ms) primarily as a means of limiting data collection time due to limitations inherent in the NRT system. All of these factors may affect the degree of adaptation and consequently may affect the modulated responses. In prior animal studies (Hu et al., 2008, 2010), ANF responses to SAM pulse trains indicate large spike rate adaptation as well as low vector strength within the first 50 ms. After this initial 50 ms, adaptation still occurs, but the rate of adaptation is reduced. In addition, vector strength of ANF responses improves over time. Thus, there may be differences in the state of temporal synchrony and neural adaptation given the short stimulus duration in the present study, complicating comparisons to animal data.
Another similarity between the current study and prior animal data is the increase in modulated responses as the modulation depth increases (Fig 12). Prior animal data indicated that with increasing modulation depths, the evoked modulated response amplitudes increase, but also become increasingly distorted (Jeng et al., 2009). In addition, the vector strength also increases with increasing modulation depth (Hu et al., 2008). Though we only tested one modulation depth for any given electrode in each CI participant, a wide range of modulation depths were tested across all users and electrodes. CI users with wider dynamic ranges and/or lower ECAP thresholds also had greater modulation depths to be tested, resulting in a greater range of current levels that could be tested, and finally greater modulated responses.
The differences between peripheral measures and psychophysical measures reported here likely are related to temporal processing at different levels of the auditory pathway. In general, at each level going from auditory nerve, through the brainstem, midbrain, and cortex, there is decreased sensitivity to high modulation frequencies, i.e., the cutoff tends to decrease at higher levels of the auditory pathway. The comparison of effects of modulation frequency for both peripheral and psychophysical measures (Fig 9) are consistent with an assumption of limited processing of higher modulation frequencies in the auditory pathway, though there are some caveats. Our psychophysical measures were made using a 300 ms stimulus, while our physiological measures were made using a 15 ms stimulus. It would be extremely time consuming to measure ECAPs evoked by a 300 ms pulse train for various electrodes and modulating frequencies. Many psychophysical studies have used 200–300 ms stimuli for AM detection tasks, likely to ensure perceptual salience of the stimulus to the implant user, as stimulus duration affects its loudness percept. Thus the levels used for psychophysical and peripheral recordings differ in our study. A future study could compromise between experimental time and the need for similar stimuli for psychophysical and electrophysiologic measures – such as using a 100 ms stimulus for both measures and alternative means to optimize the experimental time need to collect both measures. In addition, as we previously mentioned, adaptation to AM stimuli can still occur beyond 50 ms of stimulation as shown by animal data (Hu et al, 2008, 2010). Although adaptation to longer duration pulse train stimuli have been studied in human CI users (Hay-McCutcheon et al., 2005; Hughes et al, 2012, 2014), the time course of adaptation was not a focus of these studies. While there are these caveats, the peripheral data using short duration stimuli still show increasing modulated responses as modulation frequency increases, similar to the animal data collected using long duration stimuli (Abbas et al., 1997, 2003; Jeng et al., 2009), albeit without an apparent cutoff. There is also a stimulus encoding / sampling issue involved when performing psychophysical tasks, in that there are fewer samples per modulation cycle as modulation frequency increases. In the current study, a 4000 pps carrier was modulated at 125, 250, 500, and 1000 Hz rates. Thus, 32, 16, 8, and 4 samples per modulation cycle were available for their respective modulation frequencies. Thus, at low frequencies, there are more responses per modulation cycle and consequently better sampling of the modulated response. Also, stimulus encoding is affected at the periphery, as shown by the increasing distortion of the modulated response waveform as modulating frequency increases (Fig 5). Despite the robust peripheral response at higher modulation frequencies, the lack of finer detail in stimulation waveform with higher frequencies combined with reduced temporal precision at higher auditory pathways may impact psychophysical tasks and account for the decline in psychophysical sensitivity to higher amplitude modulations (Fig 7).
The data in later figures demonstrating correlations between MRA and performance at low modulation but not at high modulation frequencies is also consistent with this assumption. Figure 9 and Figure 11 described correlations between psychophysical performance and peripheral measures. These correlations were similar for 125, 250, and 500 Hz modulation frequencies; however, correlations reached much higher levels of statistical significance for the 250 and 500 Hz conditions when compared to the 125 Hz conditions. CI users can have MDTs as low as −30 to −40 dB (1–3% modulation depth) for frequencies below a modulating frequency of 100 Hz (Shannon, 1992; Busby et al 1993; Cazals et al, 1994; Chatterjee and Obzerzut, 2011), which appears to be in line with our data, though with some variations in CI performance (Fig 7, Fig 8). Since TMTF functions tend to roll off around 100 Hz, the use of 125 Hz modulating frequency may lead to ceiling effects in psychophysics and the spread of scores may not necessarily reflect the true range in performance. Additionally, the use of 1000 Hz modulating frequency leads to a floor effect as it is an extremely difficult test condition. Consequently, the spread in scores for 125 Hz and 1000 Hz condition is smaller than the 250 Hz and 500 Hz condition (Fig 9, 11). Nevertheless, there appears to be a relationship between stimulus encoding at the periphery and the ability of CI users to perform the psychophysical temporal modulation detection task. The lack of a correlation between the peripheral response and psychophysical response at 1000 Hz modulation (Fig 9, 11) also implies that limitations in central processing of modulated signals at high modulation frequencies are a factor. This may be consistent with progressive lowering of the low-pass cutoff as one ascends the auditory pathway. Our findings based on Figures 9 and 11 are tempered by the possibility that participant S19L is an outlier; his removal from the dataset reduces/removes the statistical significance of the correlations. Conservatively, we may say that there are trends in the perceptual and physiological data that may imply positive correlations between physiologic responses and psychophysical performance; larger datasets may reveal more robust and statistically significant trends.
Though not a focus of the current study, speech perception data were compared to psychophysical and peripheral responses. There were no correlations between MDTs at each frequency/electrode and speech performance, as well as MDTs averaged across electrodes for each frequency. In addition, the slopes of the TMTFs were not correlated with speech performance. These results contrast previous studies (Cazals et al., 1994; Fu, 2002; Won et al., 2011), but are not surprising given the few modulation frequencies tested in this study. For the peripheral data, animal electrophysiology studies have shown AGF slopes are positively correlated with neural survival (e.g. Smith & Simmons, 1989; Miller et al, 1994). Thus, one might presume steeper slopes would indicate better speech outcomes. No correlations were seen in our data, which is in contrast to Kim et al (2010). This null outcome has also been seen in literature comparing post-mortem SGN counts to speech outcomes (e.g. Nadol et al 2001; Khan et al 2005; Fayad & Linthicum, 2006; however, see Seyyedi et al, 2014). These mixed results are not surprising, given that the ECAP is a peripheral response and speech perception involves integration of information from peripheral and central processes, as well as cognitive resources.
Our CI participants are fairly homogeneous with respect to age, with the exception of S19L, who was 39 years old at the time of testing. He is relatively young compared to the remaining participants, which may have influenced the psychophysical results. Age related declines in temporal processing have been well established (Gordon-Salant et al., 2010), and although our intent was not to evaluate potential age effects on AM detection abilities, we see that S19L exhibits lower psychophysical AM detection thresholds compared to the rest of the patients, at least for the 250 and 500 Hz conditions. Thresholds for the remaining participants tended to cluster, which appears sensible given the similarity in ages. We don’t see this trend for the 125Hz and 1000 Hz conditions, which may reflect ceiling and floor effects, as previously discussed. We caution against making strong conclusions regarding age and AM detection thresholds given the sample size and the singular young CI user, and focus rather on the within subject comparisons of the psychophysical and peripheral results.
Interestingly, both psychophysical thresholds were lower and peripheral responses were higher in the apical electrodes compared to the medial and basal electrodes (Fig 8). ECAP growth function slopes were steeper for the apical electrode, suggesting possible better neural survival for the apical portion of the cochlea. A plausible explanation may be inferred from pre-operative audiograms of these patients – low frequency hearing tended to be better than high frequency hearing – thus underlying hair cell and neural health may be better for the more apical portions of the cochlea. One confound is that the Modulation Response Amplitude was measured in response to different modulation depths across electrodes / subjects. The average modulation depth applied for the basal, medial, and apical electrodes were 31.6, 23.3, and 36.4%, respectively, and we had also demonstrated that greater modulation depths lead to greater MRAs (Fig 12). However, our ECAP growth function slope measures are independent of our modulated response measures (and therefore independent of modulation depth). Yet, the pattern in our slope measures was consistent with the pattern in our psychophysical and peripheral measures, in that the steepest slopes on average were obtained from the apical electrodes.
Another minor source of variation in the ECAP data is the use of differing pulse widths. A default of 25 µs / phase was used if possible; however, 37 and 50 µs was used if necessary to overcome voltage compliance limits during ECAP recordings. Given that more charge is delivered for the longer duration stimuli presented at the same current level, higher pulse durations lead to a reduction in thresholds for electrophysiologic measures (van dan Honert & Stypulkowski, 1984; Parkins & Colombo, 1987; Abbas and Brown, 1991; Miller et al., 1995, Shephard et al., 2001) and a shift towards lower current levels for amplitude growth functions (Shephard et al., 2001). Threshold vs. pulse duration functions are commonly referred to as strength-duration functions, where the slope of the function is indicative of the integrative properties of the cell membrane. Slope data from these studies have implied that the cell membrane is not a perfect integrator – that is, electrical charge delivered over a longer period will not be as effective in eliciting a response. In addition, the animal data of Shephard et al. (2001) appears to indicate that longer pulse durations lead to shallower growth functions, possibly because charge is not delivered as efficiently as the pulse duration increases, leading to a less efficient recruitment of neurons. However, given that individual peripheral and psychophysical data were collected with the same pulse duration, and that the focus of the study was on the relationship between both, we do not foresee any significant confounds that can be caused by the differing pulse durations used, though we cannot be completely sure. Future experiments can avoid potential confounds by using the same pulse duration for all participants. Longer pulse durations such as 50 µs would be best to ensure stimulation is within voltage compliance limits. This is especially an issue for newer generation electrode arrays (e.g. CI 422) which have smaller electrode contacts, leading to higher impedances, higher current requirements, and increased chances of exceeding voltage compliance limits unless longer pulse durations are used.
One of the challenges in analyzing our ECAP data was removing stimulus artifact. As previously mentioned, we had used a method of artifact removal used in previous studies of ECAPs evoked by constant amplitude pulse trains (Wilson et al., 1997; Hay-McCutcheon et al., 2005, Hughes et al., 2012, 2014). In some cases (S19L as plotted in Figure 3), artifact removal was incomplete and such data was discarded. However, the waveforms for F26L may not be completely free of stimulus artifact, given subtle changes in waveform morphology (e.g. differences in N1 and P2 latencies, possible trailing contamination beyond the P2 peak). However, for any given electrode, the range of current levels used is the same across modulating frequencies (e.g. Fig 5). Thus, any artifact contamination may be similar across frequencies and not likely a major confound given that we were interested in comparing modulated responses at different frequencies. In addition, while we cannot guarantee complete artifact removal, waveforms such as those measured from F26L still show clear N1 and P2 peaks and were still used in data analysis. As previously explained, the probe artifact is calculated from the two-pulse masker-probe recording, with the assumption that this artifact is the same for each pulse in the pulse train. However, over the course of a pulse train, the stimulus artifact may differ (He et al, 2016). Thus, the artifact template obtained from a single masker-probe pulse recording may not be sufficient to remove artifacts from ECAPs evoked by a pulse train. The source of the residual artifact is not clear. He et al (2016) hypothesized that demyelination of auditory nerve fibers could either lead to (1) greater spread of excitation amongst nerve fibers with longer pulse trains or (2) altered cell membrane temporal integration properties, leading to an accumulation of charge over the course of the pulse train. Both could affect stimulation artifacts and response properties of the nerve when electrically stimulated. Regardless of the source of the artifact, a more effective means of artifact removal may be to obtain an artifact recording for different points in time of the pulse train. One can then derive an ECAP response for each point in the pulse train based on the corresponding artifact.
Results of studies looking at neural and psychophysical responses to modulated pulsatile stimuli are relevant to the design of signal processing algorithms, given that speech processors deliver such stimuli to the internal electrodes. There may be optimal parameters of the stimuli (e.g. stimulation rate, pulse duration) being delivered to the auditory nerve, so that the stimuli can be processed appropriately by higher order centers. Here, we focused on one stimulation rate (4000 Hz) and logarithmically spaced modulation frequencies over large intervals (125 to 1000 Hz). Such high stimulation rates have been proposed to reintroduce stochastic activity in the auditory nerve to improve temporal representations in cochlear implants (Rubinstein et al, 1999; Litvak, 2002). Stochastic activity may help with psychophysical AM detection tasks as well (Chatterjee & Robert, 2001). Psychophysical and physiological improvements in AM detection / encoding are relevant since CI users rely on temporal cues for speech perception. Yet, many prior studies of neural responses to electric pulsatile stimuli are based on animal work – for a variety of methodological reasons – while there are fewer studies using human CI users. In addition, there is conflicting evidence regarding the optimal stimulation rate for speech perception in CI users (e.g. Loizou et al, 2000; Friesen et al, 2005; Nie et al, 2006; Weber et al, 2007; Shannon et al, 2011). Thus, understanding how pulsatile stimuli are processed from the nerve to higher order centers may yield insight into the variability in speech perception seen in previous literature.
CONCLUSIONS
In general, auditory nerve responses to amplitude modulated stimuli represent the periodicity of the stimuli. Despite robust peripheral responses, there may be limitations in central processing of stimuli modulated at high frequencies. The current study has demonstrated how those limitations may affect psychophysical performance by exploring the effects of stimulus properties (e.g., modulation depth, modulation frequency) on peripheral and psychophysical responses in CI users. Despite differences in effects of modulation frequency, psychophysical performance showed a significant correlation to peripheral responses for low modulation frequencies (250 and 500 Hz). Having a better understanding of the source of limitations in temporal processing and assessing such effects in individuals may be relevant in the design of speech processing algorithms and rehabilitative strategies.
Acknowledgments
Wenjun Wang provided NIC programming and software support. Jacob Oleson was consulted on statistical analyses. Cochlear Ltd. provided the research patch allowing for Custom Sound EP to output modulated pulse trains. Finally, we thank the patients for their efforts.
Funded by NIH/NIDCD P50 DC000242 and R01 DC012082. No conflicts of interests are reported.
REFERENCES
- Abbas PJ, Brown CJ. Electrically evoked auditory brainstem response: Refractory properties and strength-duration functions. Hearing Research. 1991;50:139–148. doi: 10.1016/0378-5955(91)90012-x. [DOI] [PubMed] [Google Scholar]
- Abbas PJ, Brown CJ, Shallop JK, Firszt JB, Hughes ML, Hong SH, Staller SJ. Summary of results using the Nucleus CI24M implant to record the electrically evoked compound action potential. Ear and Hearing. 1999;20:45–59. doi: 10.1097/00003446-199902000-00005. [DOI] [PubMed] [Google Scholar]
- Abbas PJ, Miller CA, Matsuoka AJ, Rubinstein JT. The neurophysiological effects of simulated auditory prosthesis stimulation. Fourth quarterly progress report. NIH contract N01-DC-6-2111. 1997 [Google Scholar]
- Abbas PJ, Miller CA, Rubinstein JT, Robinson BK, Mino H, Hu N, Nourski KV, Jeng F-C, Runge Samuelson CL. Neurophysiological effects of simulated auditory prosthesis stimulation. Final report. NIH contract N01-DC-9-2107. 2003 [Google Scholar]
- Busby PA, Tong YC, Clark GM. The perception of temporal modulations by cochlear implant patients. Journal of the Acoustic Society of America. 1993;94(1):124–131. doi: 10.1121/1.408212. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Abbas PJ, Gantz BJ. Electrically evoked whole-nerve action potentials: Data from human cochlear implant users. Journal of the Acoustic Society of America. 1990;88(3):320–327. doi: 10.1121/1.399716. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Abbas PJ, Gantz BJ. Preliminary experience with Neural Response Telemetry in the Nucleus CI24M cochlear implant. American Journal of Otology. 1998;19:320–327. [PubMed] [Google Scholar]
- Cazals Y, Pelizzione M, Saudan O, Boex C. Low-pass filtering in amplitude modulation detection associated with vowel and consonant identification in subjects with cochlear implants. Journal of the Acoustical Society of America. 1994;94(4):2048–2054. doi: 10.1121/1.410146. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Oberzut C. Detection and rate discrimination of amplitude modulation in electrical hearing. Journal of the Acoustical Society of America. 2011;130(3):1567–1580. doi: 10.1121/1.3621445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M, Robert ME. Noise enhances modulation sensitivity in cochlear implant listeners: Stochastic resonance in a prosthetic sensory system? Journal of the Association for Research in Otolaryngology. 2001;2:159–171. doi: 10.1007/s101620010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimento TC, Schreiner CE. Adaptation and recovery from adaptation in single fiber responses of the cat auditory nerve. Journal of the Acoustical Society of America. 1991;90(1):263–273. doi: 10.1121/1.401296. [DOI] [PubMed] [Google Scholar]
- Delgutte B. Auditory neural processing of speech. In: Hardcastle WJ, Laver J, editors. The handbook of phonetic science. Oxford: Blackwell; 1997. pp. 507–538. [Google Scholar]
- Dynes SBC. Ph.D. dissertation. Cambridge, MA: Massachusetts Institute of Technology; 1996. Discharge characteristics of auditory nerve fibers for pulsatile electrical stimuli. [Google Scholar]
- Fayad JN, Linthicum FH., Jr Multichannel cochlear implants: relation of histopathology to performance. The Laryngoscope. 2006;116:1310–1320. doi: 10.1097/01.mlg.0000227176.09500.28. [DOI] [PubMed] [Google Scholar]
- Fitch R, Miller S, Tallal P. Neurobiology of speech perception. Annual Review of Neuroscience. 1997;20:331–353. doi: 10.1146/annurev.neuro.20.1.331. [DOI] [PubMed] [Google Scholar]
- Fraizer M, McKay CM. Temporal modulation transfer functions in cochlear implantees using a method that limits overall loudness cues. Hearing Research. 2012;283:59–69. doi: 10.1016/j.heares.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen LM, Shannon RV, Cruz RJ. Effects of stimulation rate on speech recognition with cochlear implants. Audiology & Neurotology. 2005;10:169–184. doi: 10.1159/000084027. [DOI] [PubMed] [Google Scholar]
- Fu Q-J. Temporal processing and speech recognition in cochlear implant users. NeuroReport. 2002;13(13):1635–1639. doi: 10.1097/00001756-200209160-00013. [DOI] [PubMed] [Google Scholar]
- Fu Q-J, Chinchilla S, Galvin JJ. The role of spectral and temporal cues in voice gender discrimination by normal-hearing and cochlear implant users. Journal of the Association for Research in Otolaryngology. 2004;5:253–260. doi: 10.1007/s10162-004-4046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin JJ, Fu Q-J, Oba S, Başkent D. A method to dynamically control unwanted loudness cues when measuring amplitude modulation detection in cochlear implant users. Journal of Neuroscience Methods. 2014;222:207–212. doi: 10.1016/j.jneumeth.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM, Brown PB. Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: Some physiological mechanisms of sound localization. Journal of Neurophysiology. 1969;32(4):613–636. doi: 10.1152/jn.1969.32.4.613. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Frisina RD, Popper AN, et al. The Aging Auditory System. New York, NY: Springer; 2010. [Google Scholar]
- Haenggeli A, Zhang JS, Fischer MW, Pelizzone M, Rouiller EM. Electrically evoked compound action potential (ECAP) of the cochlear nerve in response to pulsatile electrical stimulation of the cochlea in the rat: effects of stimulation at high rates. Audiology. 1998;37(6):353–371. doi: 10.3109/00206099809072989. [DOI] [PubMed] [Google Scholar]
- Hay-McCutcheon MJ, Brown CJ, Abbas PJ. An analysis of the impact of auditory-nerve adaptation on behavioral measures of temporal integration in cochlear implant recipients. Journal of the Acoustical Society of America. 2005;118(4):2444–2457. doi: 10.1121/1.2035593. [DOI] [PubMed] [Google Scholar]
- He S, Abbas PJ, Doyle DV, McFayden TC, Mulherin S. Temporal response properties of the auditory nerve in implanted children with auditory neuropathy spectrum disorder and implanted children with sensorineural hearing loss. Ear and Hearing. 2016;37:397–411. doi: 10.1097/AUD.0000000000000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffer LF, Sly DJ, Fallon JB, White MW, Shephard RK, O’Leary SJ. Examining the auditory nerve fiber response to high rate cochlear implant stimulation: Chronic sensorineural hearing loss and facilitation. Journal of Neurophysiology. 2010;104:3124–3135. doi: 10.1152/jn.00500.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N, Miller CA, Abbas PJ, Robinson BK, Woo J. Temporal response properties of auditory nerve fibers to modulated high-frequency electric pulse trains; Poster presented at the Association for Research in Otolaryngology midwinter meeting; Phoenix, AZ. 2008. [Google Scholar]
- Hu N, Miller CA, Abbas PJ, Robinson BK, Woo J. Changes in auditory nerve responses across the duration of sinusoidally amplitude-modulated electric pulse-train stimuli. Journal of the Association for Research in Otolaryngology. 2010;11:641–656. doi: 10.1007/s10162-010-0225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ML, Castioni EE, Goehring JL, Baudhuin JL. Temporal response properties of the auditory nerve: Data from human cochlear-implant recipients. Hearing Research. 2012;285:46–57. doi: 10.1016/j.heares.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ML, Baudhuin JL, Goehring JL. The relation between auditory-nerve temporal responses and perceptual rate integration in cochlear implants. Hearing Research. 2014;316:44–56. doi: 10.1016/j.heares.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng F-C, Abbas PJ, Hu N, Miller CA, Nourski KV, Robinson BK. Effects of temporal properties on compound action potentials in response to amplitude-modulated electric pulse trains in guinea pigs. Hearing Research. 2009;247:47–59. doi: 10.1016/j.heares.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Khan AM, Handzel O, Burgess BJ, et al. Is word recognition correlated with the number of surviving spiral ganglion cells and electrode insertion depth in human subjects with cochlear implants? Laryngoscope. 2005;115(4):672–677. doi: 10.1097/01.mlg.0000161335.62139.80. [DOI] [PubMed] [Google Scholar]
- Kiang NYS, Watanabe T, Thomas EC, et al. Discharge patterns of single fibers in the cat’s auditory nerve. Cambridge: MIT Press; 1965. [Google Scholar]
- Kim J-R, Abbas PJ, Brown CJ, Etler CP, O’Brien S, Kim L-S. The relationship between electrically evoked compound action potential and speech perception: a study in cochlear implant users with short electrode array. Otology & Neurotology. 2010;31(7):1041–1048. doi: 10.1097/MAO.0b013e3181ec1d92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer G. Periodicity coding in the auditory system. Hearing Research. 1992;60:115–142. doi: 10.1016/0378-5955(92)90015-f. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. Journal of the Acoustical Society of America. 1971;49(2):467–477. [PubMed] [Google Scholar]
- Litvak L. Ph.D. dissertation. Cambridge, MA: Massachusetts Institute of Technology; 2002. Towards a better speech processor for cochlear implants: Auditory-nerve responses to high-rate electric pulse trains. [Google Scholar]
- Loizou PC, Poroy O, Dorman M. The effects of parametric variations of cochlea implant processors on speech understanding. Journal of the Acoustical Society of America. 2000;108(2):790–802. doi: 10.1121/1.429612. [DOI] [PubMed] [Google Scholar]
- Luo X, Fu Q-J, Galvin JJ. Vocal emotion recognition by normal-hearing listeners and cochlear implants users. Trends in Amplification. 2007;11(4):301–315. doi: 10.1177/1084713807305301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka AJ, Abbas PJ, Rubinstein JT, Miller CA. The neuronal response to electrical constant-amplitude pulse train stimulation: Evoked compound action potential recordings. Hearing Research. 2000a;149:115–128. doi: 10.1016/s0378-5955(00)00172-6. [DOI] [PubMed] [Google Scholar]
- Matsuoka AJ, Abbas PJ, Rubinstein JT, Miller CA. The neuronal response to electrical constant-amplitude pulse train stimulation: additive Gaussian noises. Hearing Research. 2000b;149:129–137. doi: 10.1016/s0378-5955(00)00173-8. [DOI] [PubMed] [Google Scholar]
- McKay CM, Henshall KR. Amplitude modulation and loudness in cochlear implantees. Journal of the Association for Research in Otolaryngology. 2010;11:101–111. doi: 10.1007/s10162-009-0188-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrooks JC. Auditory cortex phase locking to amplitude-modulated cochlear implant pulse trains. Journal of Neurophysiology. 2008;100:76–91. doi: 10.1152/jn.01109.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Abbas PJ, Robinson BK. The use of long-duration current pulses to assess nerve survival. Hearing Research. 1994;78(1):11–26. doi: 10.1016/0378-5955(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Miller CA, Abbas PJ, Robinson BK. Response properties of the refractory auditory nerve fiber. Journal of the Association for Research in Otolaryngology. 2001;2:216–232. doi: 10.1007/s101620010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Woodruff KE, Pfingst Bryan BE. Functional responses from guinea pigs with cochlear implants. I. Electrophysiological and psychophysical measures, Hearing Research. 1995;92:85–99. doi: 10.1016/0378-5955(95)00204-9. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Jr, Shiao JY, Burgess BJ, et al. Histopathology of cochlear implants in humans. The Annals of Otology, Rhinology, and Laryngology. 2001;110(9):883–891. doi: 10.1177/000348940111000914. [DOI] [PubMed] [Google Scholar]
- Nie K, Barco A, Zeng F-G. Spectral and temporal cues in cochlear implant speech perception. Ear & Hearing. 2006;27(2):208–217. doi: 10.1097/01.aud.0000202312.31837.25. [DOI] [PubMed] [Google Scholar]
- Parkins CW. Temporal response patterns of auditory nerve fibers to electrical stimulation in deafened squirrel monkeys. Hearing Research. 1989;41:137–168. doi: 10.1016/0378-5955(89)90007-5. [DOI] [PubMed] [Google Scholar]
- Parkins CW, Colombo J. Auditory-nerve single-neuron thresholds to electrical stimulation from scala tympani electrodes. Hearing Research. 1987;31:267–286. doi: 10.1016/0378-5955(87)90196-1. [DOI] [PubMed] [Google Scholar]
- Patrick JF, Busby PA, Gibson PJ. The development of the Nucleus Freedom cochlear implant system. Trends in Amplification. 2006;10(4):175–200. doi: 10.1177/1084713806296386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JT, Wilson BS, Finley CC, et al. Pseudospontaneous activity: stochastic independence of auditory nerve fibers with electrical stimulation. Hearing Research. 1999;127:108–118. doi: 10.1016/s0378-5955(98)00185-3. [DOI] [PubMed] [Google Scholar]
- Seyyedi M, Viana LM, Nadol JB., Jr Within-subject comparison of word recognition and spiral ganglion cell count in bilateral cochlear implant recipients. Otology & Neurotology. 2014;35(8):1446–1450. doi: 10.1097/MAO.0000000000000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon RV. Temporal modulation transfer functions in patients with cochlear implants. Journal of the Acoustical Society of America. 1992;91(4):2158–2164. doi: 10.1121/1.403807. [DOI] [PubMed] [Google Scholar]
- Shannon RV, Cruz RJ, Galvin III JJ. Effects of stimulation rate on cochlear implant users’ phoneme, word and sentence recognition in quiet and noise. Audiology & Neurotology. 2011;16:113–123. doi: 10.1159/000315115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Hardie NA, Baxi JH. Electrical stimulation of the auditory nerve: single neuron strength-duration functions in deafened animals. Annals of Biomedical Engineering. 2001;29:195–201. doi: 10.1114/1.1355276. [DOI] [PubMed] [Google Scholar]
- Smith RL, Brachman ML. Adaptation in auditory-nerve fibers: A revised model. Biological Cybernetics. 1982;44:107–120. doi: 10.1007/BF00317970. [DOI] [PubMed] [Google Scholar]
- Smith L, Simmons FB. Estimating eighth nerve survival by electrical stimulation. The Annals of Otology, Rhinology, and Laryngology. 1983;92(1 Pt 1):19–23. doi: 10.1177/000348948309200105. [DOI] [PubMed] [Google Scholar]
- Stypulkowski PH, van den Honert C. Physiological properties of the electrically stimulated auditory nerve. I. Compound action potential recordings. Hearing Research. 1984;14:205–223. doi: 10.1016/0378-5955(84)90051-0. [DOI] [PubMed] [Google Scholar]
- van den Honert C, Stypulkowski PH. Physiological properties of the electrically stimulated auditory nerve. II. Single fiber recordings. Hearing Research. 1984;14:225–243. doi: 10.1016/0378-5955(84)90052-2. [DOI] [PubMed] [Google Scholar]
- van den Honert C, Stypulkowski PH. Temporal response patterns of single auditory nerve fibers elicited by periodic electrical stimuli. Hearing Research. 1987;29(207–222) doi: 10.1016/0378-5955(87)90168-7. [DOI] [PubMed] [Google Scholar]
- Weber BP, Lai WK, Dillier N, et al. Performance and preference for ACE stimulation rates obtained with Nucleus RP8 and Freedom system. Ear & Hearing. 2007;28(2):46S–48S. doi: 10.1097/AUD.0b013e3180315442. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Finley C, Zerbi M, Lawson DT. Speech processors for auditory prosthesis. Seventh quarterly progress report. NIH contract N01-DC-2-2401. 1994 [Google Scholar]
- Wilson BS, Finley CC, Lawson DT, Zerbi M. Temporal representations with cochlear implants. American Journal of Otology. 1997a;18(6 Suppl):S30–S34. [PubMed] [Google Scholar]
- Wilson BS, Zerbi M, Finley C, Lawson D, van den Honert C. Speech processors for auditory prosthesis. Eighth quarterly progress report. NIH contract N01-DC-5-2103. 1997b [Google Scholar]
- Won JH, Drennen WR, Nie K, Jameyson EM, Rubinstein JT. Acoustic temporal modulation detection and speech perception in cochlear implant listeners. Journal of the Acoustical Society of America. 2011;130(1):376–388. doi: 10.1121/1.3592521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Thompson CS, Pfingst BE. Relative contributions of spectral and temporal cues for phoneme recognition. Journal of the Acoustical Society of America. 2005;117(5):3255–3267. doi: 10.1121/1.1886405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Zheng Y. Spectral and temporal cues for phoneme recognition in noise. Journal of the Acoustical Society of America. 2007;122(35):1758–1764. doi: 10.1121/1.2767000. [DOI] [PubMed] [Google Scholar]
- Xu L, Pfingst BE. Spectral and temporal cues for speech recognition: Implications for auditory prostheses. Hearing Research. 2008;242:132–140. doi: 10.1016/j.heares.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Miller CA, Robinson BK, Abbas PJ, Hu N. Changes across time in spike rate and spike amplitude of auditory nerve fibers stimulated by electric pulse trains. Journal of the Association for Research in Otolaryngology. 2007;8:356–372. doi: 10.1007/s10162-007-0086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]