Abstract
We review the apparent discrepancies between studies that report anti-inflammatory effects of cerium oxide nanoparticles (CeO2 NPs) through their reactive oxygen species-chelating properties and immunological studies highlighting their toxicity. We observe that several underappreciated parameters, such as aggregation size and degree of impurity, are critical determinants that need to be carefully addressed to better understand the NP biological effects in order to unleash their potential clinical benefits. This is because NPs can evolve toward different states, depending on the environment where they have been dispersed and how they have been dispersed. As a consequence, final characteristics of NPs can be very different from what was initially designed and produced in the laboratory. Thus, aggregation, corrosion, and interaction with extracellular matrix proteins critically modify NP features and fate. These modifications depend to a large extent on the characteristics of the biological media in which the NPs are dispersed. As a consequence, when reviewing the scientific literature, it seems that the aggregation state of NPs, which depends on the characteristics of the dispersing media, may be more significant than the composition or original size of the NPs. In this work, we focus on CeO2 NPs, which are reported sometimes to be protective and anti-inflammatory, and sometimes toxic and pro-inflammatory.
Keywords: nanoparticles, cerium oxide, nanoparticle evolution, nanoparticle agglomeration, ion leaching, antioxidant activity, inflammation, immune response
Introduction
Nanotechnology has already qualified as the industrial revolution of the twenty-first century. Although its development is a logical continuation of the development of microelectronics and colloid chemistry, the beginning of the nano era corresponds, for most people, with Smalley’s synthesis of fullerene (C60) (1). Since then, organic nanomaterials (e.g., C60, carbon nanotubes, graphene) have garnered much interest, but have also generated concerns regarding toxicity (2–4). Meanwhile, the development of inorganic nanomaterials has caused far less controversy, and it is only in the past few years that some of these materials (e.g., TiO2, Ag, Fe3O4) have come under closer scrutiny to address human and environmental toxicity issues (5–7). It has also become increasingly common to examine the effects of a nanocomposite or nano-enabled products instead of the pristine nanoparticle (NP) alone. Indeed, the effects of the “active ingredient” can be (and actually often are) deeply modified by the formulation of the final product and the properties of the media in which it is dispersed. This highlights the complexity of addressing the fate of a nanomaterial through its life cycle in a meaningful manner.
Cerium oxide nanoparticles (CeO2 NPs) have recently received much attention because of their excellent catalytic redox properties (8). In addition to being a rather chemically inert ceramic, a CeO2 nanocrystal has a fluorite-like structure where the unfilled 4f electronic orbital confers it a variety of relevant catalytic properties when it reaches the nanoscale. Consequently, nanoceria has been used in the petrochemical industry and in catalytic exhaust converters for decades. CeO2 NPs have high capacity to buffer electrons in redox environments due to the ease of oxidation and reduction from Ce3+ to Ce4+ and vice versa (9, 10), followed by the capture or release of oxygen. As a consequence, they act as electron sponges in the presence of free radicals degrading thus reactive oxygen species (ROS) (11). In detail, inflammation and oxidative stress are interconnected processes that contribute decisively to the pathogenesis of many diseases, including highly prevalent, age-related disorders, such as obesity, cardiovascular disease, diabetes mellitus, cancer, chronic respiratory diseases, and neurological diseases. Mutual stimulation between oxidative stress and inflammation contributes decisively to the chronic nature of these diseases. Oxidative stress involves elevated intracellular levels of ROS, such as peroxides, superoxides, hydroxyl radicals, and singlet oxygen, which have critical roles in physiological processes through the regulation of cell signaling cascades. Prolonged exposure to high ROS concentrations damages proteins, lipids, and nucleic acids, causing various metabolic complications.
Thus, CeO2 NPs in the size range of 3–50 nm have recently received increased attention for their participation in biochemical redox reactions, providing sites for free radical scavenging and reducing inflammation (12–14). Thus, CeO2 NPs have been reported to confer cellular protection, especially in the reduction of oxidative and nitrosative stress in living organisms, and are considered an alternative approach offering new opportunities for the treatment of physiopathological processes leading to chronic inflammation (15).
In this regard, most therapeutic CeO2 NPs applications are proposed based on their ability to reduce ROS levels and consequently, the levels of most inflammatory mediators, such as inducible nitric oxide synthase, nuclear factor κβ, tumor necrosis factor-α, and interleukins (16–19). Indeed, CeO2 NPs were recently found to have multi-enzyme mimetic properties, including those related to superoxide dismutase (SOD), catalase, and oxidase (8). In this context, CeO2 NPs have potential applications in many different medical fields. For example, in cardiology, intravenously administered CeO2 NPs in a transgenic murine model of cardiomyopathy were proved to reduce the myocardial oxidative stress, the endoplasmic reticulum stress, and suppress the inflammatory process, ensuring protection against progression of cardiac dysfunction (20). In oncology, antioxidant properties of CeO2 NPs were successfully tested to protect cells from radiation-induced damage (21). In another study, CRL8798 cells (immortalized normal human breast epithelial cell line) and MCF-7 (a breast carcinoma cell line), were exposed to radiation and CeO2 NPs were reported to confer radioprotection to the normal human breast line but not to the tumoral one (22). In hepatology, CeO2 NPs were shown to display hepatoprotective effects against steatosis in rats with diet-induced non-alcoholic steatohepatitis (23) and to reduce steatosis, portal pressure, and ameliorate systemic inflammatory biomarkers, attenuating the intensity of the inflammatory response in a model of rats with induced liver fibrosis. In ophtalmology, CeO2 NPs are being tested to treat ocular diseases such as macular degeneration and glaucoma. The ability of CeO2 NPs to protect retinal neurons was shown for primary cell cultures of dissociated rat retinas injecting the suspension of CeO2 NPs into the vitreous of both eyes (9). Similarly, beneficial effects of the use of CeO2 NPs have been found in the case of neurodegenerative diseases (24). In this studies, CeO2 NPs are shown to display SOD mimetic activity (25, 26), catalase mimetic activity (11, 27), and/or nitric oxide (⋅NO) scavenging abilities (17). Last, CeO2 NPs are also amenable to local targeting and delivery, as shown in the works of Li et al., (28) and Xu et al (29).
Positive and Negative Effects of NPs
Obviously, the safe and effective use of these promising therapeutic NPs requires the precise assessment of their potential risks and unwanted side effects. Despite the vast range of publications that address the toxicity and safety of nanomaterials, results are still controversial, with different observed effects for similar NPs ranging from severely toxic effects—as in the study of Kovriznych et al. (30), which assess and compare the acute toxicity of 31 different nanomaterials to fish mature individuals of Danio rerio—to innocuous [e.g., Ref. (31)] or beneficial [e.g., Ref. (32, 33)]. CeO2 NPs are no exception. While they have been reported many times to be safe and beneficial, protecting against oxidative stress (9, 13, 21, 22, 34), other studies, mainly related to the toxicity of CeO2 nanopowders employed in industry, reported in vitro and in vivo toxicity (35, 36). In addition, while some studies report CeO2 NP uptake by hepatocytes and anti-inflammatory effects in the liver (14, 37), others report macrophage (Kupffer cell) uptake and pro-inflammatory effects (38).
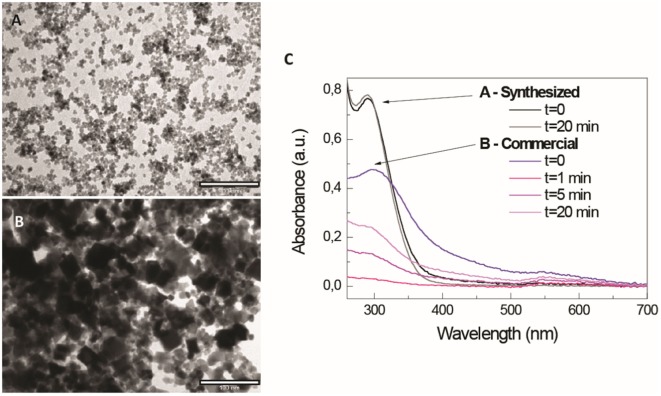
At the source of these discrepancies, one can observe the diversity of the materials actually employed in the different studies, which are presented under the same name. For instance, most research regarding CeO2 NP toxicity has been performed with commercially available NPs (often supplied in dry aggregated form) in order to assess the consequences of occupational and environmental exposure. These are different materials from those produced by wet chemistry routes in the laboratory, where the NPs are always kept isolated and well dispersed. In addition, for these types of studies, administered doses are usually higher than those proposed in nanomedicine (Figures 1A,B). In addition to their different initial characteristics, these materials are often prone to aggregation when dispersed into biological fluids, such as complete cell culture medium or serum (5, 39). For instance, He et al. (39) showed how intratracheally instilled CeO2 NPs into Wistar rats agglomerate and form sediments in the bronchoalveolar medium. Consequently, the actual objects that cells encounter may behave very differently from the initially designed and produced NPs (Figure 1C).
Figure 1.
Different aspect and stability of commercial and designed CeO2 nanoparticles (NPs). Different morphologies and sedimentation behavior of CeO2 nanopowders (commercial, nominal size <25 nm) and CeO2 NPs synthesized in the laboratory after dispersion in TMAOH 1 mM, a good stabilizer of metal oxide NPs. (A,B) Representative TEM images CeO2 NPs and CeO2 nanopowders, respectively (scale bar = 100 nm); (C) UV-VIS spectroscopy measurements over time of both samples after resuspension in TMAOH 1 mM and at the same NP concentration.
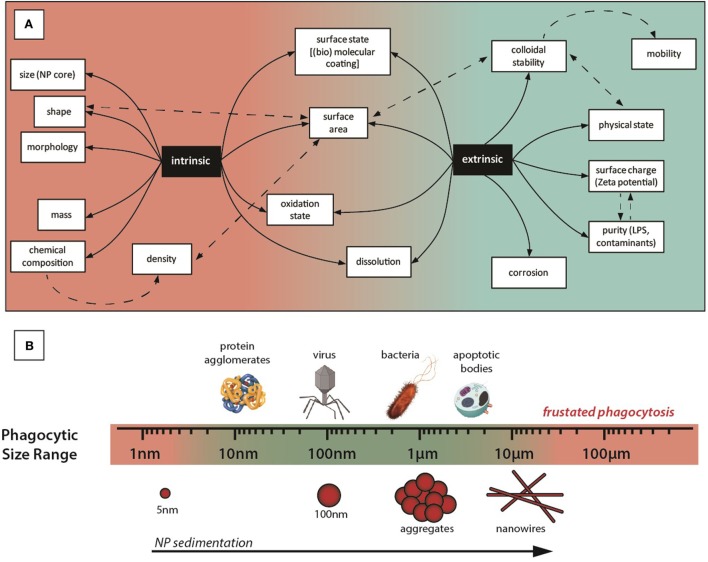
Comparing studies regarding nanomedicine and nanosafety, it seems that often the differently observed biological effects of NPs are related not only to its parental composition and purity but also to its final aggregation state (40), which is independent of the employed material and can be reproduced with other NPs. For instance, aggregates of TiO2 (41), Al2O3 (42), and Fe2O3 (43) NPs show similar toxicity to CeO2 aggregates (37, 44), as well as CeO2 (45) or Au NPs (46) carrying cationic amphipathic molecules on their surfaces have been observed to be similarly toxic. Regarding aggregates, in the case of CeO2, Rogers et al. (44) evaluate how exposure to different concentrations of aggregated CeO2 NPs affects indices of whole animal stress and survivability in Caenorhabditis elegans. Results showed that CeO2 aggregates promoted strain-dependent decreases in animal fertility, a decline in stress resistance as measured by thermotolerance and shortened worm length. Moreover, chronic exposure of CeO2 NP aggregates was found to be associated with increased levels of ROS and heat shock stress response (HSP-4). Regarding surface state, Dowding et al. (45) prepared different samples of CeO2 NPs using identical precursor (Cerium nitrate hexahydrate) through similar wet chemical process but using different oxidizer/reducer: H2O2, NH4OH, or hexamethylenetetramine (HMT). Results showed that unlike the other CeO2 NPs preparations, HMT-CeO2 NPs were readily taken into endothelial cells and reduced cell viability at a 10-fold lower concentration than the others. This indicates that the biological effects of NPs depend not only on intrinsic but also extrinsic features, aspects related to the NP itself and to its history and environment. Thus, colloidal stability, which determines the agglomeration and sedimentation, depends on the concentration and nature of ions and molecules present in the media at a certain temperature. This affects the hydrodynamic radius, which depends on temperature and viscosity; NP corrosion, which depends on the combined redox potential of the species present in the environment; and speciation of leached ions, which depends on the nature of the dispersing media (Figure 2A). The NP concentration will affect the kinetics of the previously coexisting phenomena.
Figure 2.
(A) Intrinsic and extrinsic properties of nanoparticles (NPs). Different properties of the NP, related to the NP itself (intrinsic) or to the NP behavior in the exposure media (extrinsic). For instance, we can design CeO2 NPs with specific sizes and shapes, but agglomeration in the exposure media leads to specific surfaces, concentrations, mobilities, etc., very different from the initially prepared NPs. As agglomerated NPs behave as a large particle, this makes the NP more immunogenic and affects the concentration of NPs in different parts of the body, where they are accumulated in organs of the MPS system. Importantly, for the (immuno)toxicity aspects, agglomerates of NPs are no longer on the nanometric regime of sizes and may have similar consequences as the incidental inorganic microparticles, extensively investigated during the last century: burning oil residues, silica from mining or asbestos have been found stacked in affected tissues, causing pathologies such as silicosis, asbestosis, and/or inflammatory reactions. Thus, in this example, even if CeO2 NPs are not toxic (and therapeutically beneficial) by themselves, they may be risky because they could be a source of toxic aggregates. (B) Graphical representative sizes of key entities capable of generating immune response and different NP morphologies and NP aggregates.
In this context, interactions between NPs and the immune system are of particular interest for both their efficient use and their safety in biomedical applications. NPs are foreign objects, sized within the range of that detected and managed by the immune system, which has a responsibility for categorizing invasion and providing an appropriate response (Figure 2B). For example, NPs may exacerbate immune responses by ordering and repetition of ligands (47–49), as well as by altering redox status, both increasing (50) and decreasing ROS and inflammatory mediator levels (14).
The Apparent Contradiction
Lack of understanding NP characteristics and their evolution inside biological media is recognized as one of the key points underpinning the abovementioned controversies (40). Thus, as with many other inorganic NPs employed in nanomedical research, CeO2 NPs evolve when in contact with physiological media (5, 51). This evolution may entail the loss of intended catalytic activity, transforming beneficial NPs into deleterious ones. The most significant alterations affecting the biological fate and effects of NPs when dispersed in biological media are: (i) agglomeration and aggregation of the NPs (5, 52, 53), (ii) formation of the NP protein corona as a result of the adsorption of proteins onto the inorganic surface (54, 55), and (iii) NP corrosion and/or dissolution into ionic species (56–59). Indeed, it has been proposed that the higher toxicity of unstable preparations of NPs may not be due to the material per se but to its rapid aggregation into final micro- or macrometric sizes (5, 51) and the leaching of toxic ionic species into the solution (57). For instance, in the work of Kirchner et al. (57), the release of toxic Cd2+ ions from CdSe and CdSe/ZnS NPs and their stability toward aggregation were demonstrated to play an important role for the observed cytotoxic effects. Similarly, aggregation of NPs has been shown to clearly determine the exposure of NPs to cells. Xia et al. (50), comparing the toxicity induced by different ambient and manufactured NPs, showed a dramatic change in their state of aggregation, dispersibility, and charge during transfer from a buffered aqueous solution to cell culture medium and how it affects the observed cellular responses. Cho et al., (60) studied how sedimentation affected the cellular uptake of gold NPs in in vitro experiments, dramatically altering their exposure and biological effects. Typically, in vitro experiments measure the uptake of NPs by exposing cells at the bottom of a culture plate to a suspension of NPs, and it is generally assumed that the suspension is well dispersed. But, if NPs sediment, their concentration on the cell surface may be higher than the initial bulk concentration, and this could lead to increased uptake by cells. Indeed, results showed that cellular uptake of gold NPs mostly depended on the sedimentation and the diffusion velocities of the NPs.
Other NP transformations can also alter biological responses, leading to unexpected results. For example, Xue et al. (61) reported that CeO2 NPs can protect DNA from damage in Tris–HCl and sulfate buffers, but not in phosphate-buffered saline. A mechanism of action was proposed: cerium phosphate is formed on the surface of the NPs, which interferes with redox cycling between Ce3+ and Ce4+. As a result, the antioxidant activity of CeO2 NPs is greatly affected by the external environment. Similarly, Perez et al. (62) observed that the antioxidant properties of CeO2 NPs were pH-dependent. They suggested that a high concentration of H+ interferes with the regeneration of Ce3+, resulting in a loss of antioxidant activity. However, disintegration of CeO2 in acidic media could also account for the observed effects, similar to NP disintegration observed in different media (57, 63).
Given these effects, when conducting studies involving NPs for safety or medicine, it is essential to understand the changes that take place with their insertion into biological media, from complete cell culture media, to full blood, or lymph, to the intracellular cytoplasm. This includes NP colloidal stability, vicinity interactions, chemical transformations, association with plasma proteins, interaction with components of the immune system, and traditional absorption, distribution, metabolism, and excretion studies adapted to the unique specifications of NPs. Additionally, NPs can be complex and composed of different entities, all of which can have different fates. As an example, in the work of Feliu et al. (64), the authors review a vast collection of recent scientific literature indicating that NPs in vivo should no longer be considered as homogeneous entities. They conceptually divide a NP into the inorganic core, the engineered surface coating, comprising of the ligand shell and optionally also bio-conjugates, and the corona of adsorbed biological molecules. The authors found empirical evidence showing that all of these three described components may degrade individually in vivo. Due to this, the life cycle and biodistribution of the whole heterostructure is drastically modified.
Concluding Remarks
There is an increasing number of conflicting reports on the impact of CeO2 NPs on oxidative stress and inflammation, with some studies reporting the promotion of oxidative stress induced by immune system activation, and others reporting protective effects against inflammatory processes. To overcome this apparent contradiction, understanding the physicochemical transformations and evolution of the NPs in biological systems is imperative. Understanding these mechanisms will enable the design of nanomaterials that work more precisely in medicine and safely in society.
The majority of negative immune effects reported in the scientific literature are related to NP aggregation and contamination, which cause biological effects independent of the composition, size, and shape of individual NPs. Generally, isolated, non-contaminated NPs show no toxicity, while contaminated and aggregated NPs are often described as immunotoxic (65, 66). This is especially dramatic in the case of CeO2 NPs, which have been reported many times as anti-inflammatory or pro-inflammatory, often without a proper description of the material used or its purity (40).
Author Contributions
All authors listed have made substantial, direct, and intellectual contributions to the work and approved it for publication. VP suggested the topic and provided the concept and design of the work. EC and VP retrieved the relevant literature, compiled all information on the topic, and wrote the mini-review. MG contributed to the design of graphical information. JP contributed to the cerium oxide reactivity sections, retrieving the relevant literature, and participating in the discussion and writing. GC and WJ contributed to the oxidative stress, inflammatory processes, and antioxidant activity sections by retrieving the literature and participating in the discussion and writing.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. GC was supported by a grant from Ministerio de Economía y Competitividad, Instituto de Salud Carlos III PI15/00777. WJ was supported by grants from Ministerio de Economia y Competitividad (SAF2015-6412-R). Financial support from the FutureNanoNeeds Project (GA: 604602) financed by the European Community under the FP7 Programme (FP7-NMP-2013-LARGE-7) is gratefully acknowledged.
References
- 1.Kroto HW, Heath JR, O’Brien SC, Curl RF, Smalley RE. C60: buckminsterfullerene. Nature (1985) 318(6042):162–3. 10.1038/318162a0 [DOI] [Google Scholar]
- 2.Huczko A, Lange H, Calko E, Grubek-Jaworska H. Physiological testing of carbon nanotubes: are they asbestos-like? Fullerene Sci Technol (2001) 9:251–4. 10.1081/FST-100102973 [DOI] [Google Scholar]
- 3.Hughes LS, Cass GR, Gone J, Ames M, Olmez I. Physical and chemical characterization of atmospheric ultrafine particles in the Los Angeles area. Environ Sci Technol (1998) 32:1153–61. 10.1021/es970280r [DOI] [Google Scholar]
- 4.Li X, Brown D, Smith S, MacNee W, Donaldson K. Short-term inflammatory responses following intratracheal instillation of fine and ultrafine carbon black in rats. Inhal Toxicol (1999) 11:709–31. 10.1080/089583799196826 [DOI] [PubMed] [Google Scholar]
- 5.Casals E, Vázquez-Campos S, Bastús N, Puntes V. Distribution and potential toxicity of engineered inorganic nanoparticles and carbon nanostructures in biological systems. Trends Anal Chem (2008) 27(8):672–83. 10.1016/j.trac.2008.06.004 [DOI] [Google Scholar]
- 6.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science (2006) 311(5761):622–7. 10.1126/science.1114397 [DOI] [PubMed] [Google Scholar]
- 7.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect (2005) 113(7):823–39. 10.1289/ehp.7339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu C, Qu X. Cerium oxide nanoparticle: a remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater (2014) 6:e90. 10.1038/am.2013.88 [DOI] [Google Scholar]
- 9.Chen JP, Patil S, Seal S, McGinnis JF. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat Nanotechnol (2006) 1(2):142–50. 10.1038/nnano.2006.91 [DOI] [PubMed] [Google Scholar]
- 10.Esch F, Fabris S, Zhou L, Montini T, Africh C, Fornasiero P, et al. Electron localization determines defect formation on ceria substrates. Science (2005) 309(5735):752–5. 10.1126/science.1111568 [DOI] [PubMed] [Google Scholar]
- 11.Cafun JD, Kvashnina KO, Casals E, Puntes VF, Glatzel P. Absence of Ce3+ sites in chemically active colloidal ceria nanoparticles. ACS Nano (2013) 7(12):10726–32. 10.1021/nn403542p [DOI] [PubMed] [Google Scholar]
- 12.DeCoteau W, Heckman K, Estevez A, Reed K, Costanzo W, Sandford D, et al. Cerium oxide nanoparticles with antioxidant properties ameliorate strength and prolong life in mouse model of amyotrophic lateral sclerosis. Nanomedicine (2016) 12(8):2311–20. 10.1016/j.nano.2016.06.009 [DOI] [PubMed] [Google Scholar]
- 13.Hirst S, Karakoti A, Tyler R, Sriranganathan N, Seal S, Reilly C. Anti-inflammatory properties of cerium oxide nanoparticles. Small (2009) 5(24):2848–56. 10.1002/smll.200901048 [DOI] [PubMed] [Google Scholar]
- 14.Oro D, Yudina T, Fernandez-Varo G, Casals E, Reichenbach V, Casals G, et al. Cerium oxide nanoparticles reduce steatosis, portal hypertension and display anti-inflammatory properties in rats with liver fibrosis. J Hepatol (2016) 64(3):691–8. 10.1016/j.jhep.2015.10.020 [DOI] [PubMed] [Google Scholar]
- 15.Karakoti AS, Monteiro-Riviere NA, Aggarwal R, Davis JP, Narayan RJ, Self WT, et al. Nanoceria as antioxidant: synthesis and biomedical applications. JOM (2008) 60(3):33–7. 10.1007/s11837-008-0029-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colon J, Hsieh N, Ferguson A, Kupelian P, Seal S, Jenkins DW, et al. Cerium oxide nanoparticles protect gastrointestinal epithelium from radiation-induced damage by reduction of reactive oxygen species and upregulation of superoxide dismutase 2. Nanomedicine (2010) 6(5):698–705. 10.1016/j.nano.2010.01.010 [DOI] [PubMed] [Google Scholar]
- 17.Dowding J, Dosani T, Kumar A, Seal S, Self W. Cerium oxide nanoparticles scavenge nitric oxide radical ((NO)-N-center dot). Chem Commun (2012) 48(40):4896–8. 10.1039/c2cc30485f [DOI] [PubMed] [Google Scholar]
- 18.Estevez AY, Pritchard S, Harper K, Aston JW, Lynch A, Lucky JJ, et al. Neuroprotective mechanisms of cerium oxide nanoparticles in a mouse hippocampal brain slice model of ischemia. Free Radic Biol Med (2011) 51(6):1155–63. 10.1016/j.freeradbiomed.2011.06.006 [DOI] [PubMed] [Google Scholar]
- 19.Selvaraj V, Nepal N, Rogers S, Manne ND, Arvapalli R, Rice KM, et al. Inhibition of MAP kinase/NF-kB mediated signaling and attenuation of lipopolysaccharide induced severe sepsis by cerium oxide nanoparticles. Biomaterials (2015) 59:160–71. 10.1016/j.biomaterials.2015.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niu JL, Azfer A, Rogers LM, Wang XH, Kolattukudy PE. Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovasc Res (2007) 73(3):549–59. 10.1016/j.cardiores.2006.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asati A, Santra S, Kaittanis C, Perez J. Surface-charge-dependent cell localization and cytotoxicity of cerium oxide nanoparticles. ACS Nano (2010) 4(9):5321–31. 10.1021/nn100816s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarnuzzer RW, Colon J, Patil S, Seal S. Vacancy engineered ceria nanostructures for protection from radiation-induced cellular damage. Nano Lett (2005) 5(12):2573–7. 10.1021/nl052024f [DOI] [PubMed] [Google Scholar]
- 23.Carvajal S, Oró D, Fernández-Varo G, Yudina T, Perramón M, Oller L, et al. Therapeutic effect of cerium oxide nanoparticles (CeO2NPs) in rats with diet-induced non-alcoholic steatohepatitis. J Hepatol (2017) 66(1):S608. 10.1016/S0168-8278(17)31652-5 [DOI] [Google Scholar]
- 24.Schubert D, Dargusch R, Raitano J, Chan SW. Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem Biophys Res Commun (2006) 342(1):86–91. 10.1016/j.bbrc.2006.01.129 [DOI] [PubMed] [Google Scholar]
- 25.Heckert E, Karakoti A, Seal S, Self W. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials (2008) 29(18):2705–9. 10.1016/j.biomaterials.2008.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korsvik C, Patil S, Seal S, Self W. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem Commun (2007) 10:1056–8. 10.1039/b615134e [DOI] [PubMed] [Google Scholar]
- 27.Pirmohamed T, Dowding J, Singh S, Wasserman B, Heckert E, Karakoti A, et al. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem Commun (2010) 46(16):2736–8. 10.1039/b922024k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li M, Shi P, Xu C, Ren J, Qu X. Cerium oxide caged metal chelator: anti-aggregation and anti-oxidation integrated H2O2-responsive controlled drug release for potential Alzheimer’s disease treatment. Chem Sci (2013) 4:2536–42. 10.1039/C3SC50697E [DOI] [Google Scholar]
- 29.Xu C, Lin Y, Wang J, Wu L, Wei W, Ren J, et al. Nanoceria-triggered synergetic drug release based on CeO(2)-capped mesoporous silica host-guest interactions and switchable enzymatic activity and cellular effects of CeO(2). Adv Healthc Mater (2013) 2(12):1591–9. 10.1002/adhm.201200464 [DOI] [PubMed] [Google Scholar]
- 30.Kovriznych JA, Sotnikova R, Zeljenkova D, Rollerova E, Szabova E, Wimmerova S. Acute toxicity of 31 different nanoparticles to zebrafish (Danio rerio) tested in adulthood and in early life stages – comparative study. Interdiscip Toxicol (2013) 6(2):67–73. 10.2478/intox-2013-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Connor E, Mwamuka J, Gole A, Murphy C, Wyatt M. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small (2005) 1(3):325–7. 10.1002/smll.200400093 [DOI] [PubMed] [Google Scholar]
- 32.Schwenk M. Ferumoxytol: a new intravenous iron preparation for the treatment of iron deficiency anemia in patients with chronic kidney disease. Pharmacotherapy (2010) 30(1):70–9. 10.1592/phco.30.1.70 [DOI] [PubMed] [Google Scholar]
- 33.Weissleder R, Stark D, Engelstad B, Bacon B, Compton C, White D, et al. Superparamagnetic iron-oxide – pharmocokinetics and toxicity. Am J Roentgenol (1989) 152(1):167–73. 10.2214/ajr.152.1.167 [DOI] [PubMed] [Google Scholar]
- 34.Minarchick VC, Stapleton PA, Sabolsky EM, Nurkiewicz TR. Cerium dioxide nanoparticle exposure improves microvascular dysfunction and reduces oxidative stress in spontaneously hypertensive rats. Front Physiol (2015) 6:339. 10.3389/fphys.2015.00339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisichella M, Berenguer F, Steinmetz G, Auffan M, Rose J, Prat O. Toxicity evaluation of manufactured CeO2 nanoparticles before and after alteration: combined physicochemical and whole-genome expression analysis in Caco-2 cells. BMC Genomics (2014) 15:700. 10.1186/1471-2164-15-700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mittal S, Pandey A. Cerium oxide nanoparticles induced toxicity in human lung cells: role of ROS mediated DNA damage and apoptosis. Biomed Res Int (2014) 891934. 10.1155/2014/891934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oro D, Fernandez-Varo G, Reichenbach V, Yudina T, Casals E, Casals G, et al. Cerium oxide nanoparticles reduce portal hypertension and show antiinflammatory properties in CCl4-treated rats. Hepatology (2014) 60:1175A. 10.1002/hep.27535 [DOI] [PubMed] [Google Scholar]
- 38.Nalabotu S, Kolli M, Triest W, Ma J, Manne N, Katta A, et al. Intratracheal instillation of cerium oxide nanoparticles induces hepatic toxicity in male Sprague-Dawley rats. Int J Nanomedicine (2011) 6:2327–35. 10.2147/IJN.S25119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He X, Zhang H, Ma Y, Bai W, Zhang Z, Lu K, et al. Lung deposition and extrapulmonary translocation of nano-ceria after intratracheal instillation. Nanotechnology (2010) 21(28):285103. 10.1088/0957-4484/21/28/285103 [DOI] [PubMed] [Google Scholar]
- 40.Krug H. Nanosafety research—are we on the right track? Angewandte Chemie (2014) 53(46):12304–19. 10.1002/anie.201403367 [DOI] [PubMed] [Google Scholar]
- 41.Noel A, Maghni K, Cloutier Y, Dion C, Wilkinson K, Halle S, et al. Effects of inhaled nano-TiO2 aerosols showing two distinct agglomeration states on rat lungs. Toxicol Lett (2012) 214(2):109–19. 10.1016/j.toxlet.2012.08.019 [DOI] [PubMed] [Google Scholar]
- 42.Yoon D, Woo D, Kim J, Kim M, Kim T, Hwang E, et al. Agglomeration, sedimentation, and cellular toxicity of alumina nanoparticles in cell culture medium. J Nanopart Res (2011) 13(6):2543–51. 10.1007/s11051-010-0147-4 [DOI] [Google Scholar]
- 43.Zhu X, Tian S, Cai Z. Toxicity assessment of iron oxide nanoparticles in zebrafish (Danio rerio) early life stages. PLoS One (2012) 7(9):e46286. 10.1371/journal.pone.0046286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rogers S, Rice KM, Manne ND, Shokuhfar T, He K, Selvaraj V, et al. Cerium oxide nanoparticle aggregates affect stress response and function in Caenorhabditis elegans. SAGE Open Med (2015) 3:2050312115575387. 10.1177/2050312115575387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dowding J, Das S, Kumar A, Dosani T, McCormack R, Gupta A, et al. Cellular interaction and toxicity depend on physicochemical properties and surface modification of redox-active nanomaterials. ACS Nano (2013) 7(6):4855–68. 10.1021/nn305872d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alkilany A, Nagaria P, Hexel C, Shaw T, Murphy C, Wyatt M. Cellular uptake and cytotoxicity of gold nanorods: molecular origin of cytotoxicity and surface effects. Small (2009) 5(6):701–8. 10.1002/smll.200801546 [DOI] [PubMed] [Google Scholar]
- 47.Bachmann MF, Rohrer UH, Kundig TM, Burki K, Hengartner H, Zinkernagel RM. The influence of antigen organization on B cell responsiveness. Science (1993) 262(5138):1448–51. 10.1126/science.8248784 [DOI] [PubMed] [Google Scholar]
- 48.Bastus N, Sanchez-Tillo E, Pujals S, Farrera C, Kogan M, Giralt E, et al. Peptides conjugated to gold nanoparticles induce macrophage activation. Mol Immunol (2009) 46(4):743–8. 10.1016/j.molimm.2008.08.277 [DOI] [PubMed] [Google Scholar]
- 49.Bastus N, Sanchez-Tillo E, Pujals S, Farrera C, Lopez C, Giralt E, et al. Homogeneous conjugation of peptides onto gold nanoparticles enhances macrophage response. ACS Nano (2009) 3(6):1335–44. 10.1021/nn8008273 [DOI] [PubMed] [Google Scholar]
- 50.Xia T, Kovochich M, Brant J, Hotze M, Sempf J, Oberley T, et al. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett (2006) 6(8):1794–807. 10.1021/nl061025k [DOI] [PubMed] [Google Scholar]
- 51.Casals E, Gonzalez E, Puntes V. Reactivity of inorganic nanoparticles in biological environments: insights into nanotoxicity mechanisms. J Phys D: Appl. Phys. (2012) 45(44):443001. 10.1088/0022-3727/45/44/443001 [DOI] [Google Scholar]
- 52.Bastus N, Casals E, Vázquez-Campos S, Puntes V. Reactivity of engineered inorganic nanoparticles and carbon nanostructures in biological media. Nanotoxicology (2008) 2(3):99–112. 10.1080/17435390802217830 [DOI] [Google Scholar]
- 53.Moore TL, Rodriguez-Lorenzo L, Hirsch V, Balog S, Urban D, Jud C, et al. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem Soc Rev (2015) 44(17):6287–305. 10.1039/c4cs00487f [DOI] [PubMed] [Google Scholar]
- 54.Casals E, Pfaller T, Duschl A, Oostingh GJ, Puntes V. Time evolution of the nanoparticle protein corona. ACS Nano (2010) 4(7):3623–32. 10.1021/nn901372t [DOI] [PubMed] [Google Scholar]
- 55.Casals E, Pfaller T, Duschl A, Oostingh GJ, Puntes VF. Hardening of the nanoparticle-protein corona in metal (Au, Ag) and oxide (Fe3O4, CoO, and CeO2) nanoparticles. Small (2011) 7(24):3479–86. 10.1002/smll.201101511 [DOI] [PubMed] [Google Scholar]
- 56.Chithrani B, Chan W. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett (2007) 7(6):1542–50. 10.1021/nl070363y [DOI] [PubMed] [Google Scholar]
- 57.Kirchner C, Liedl T, Kudera S, Pellegrino T, Javier A, Gaub H, et al. Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Lett (2005) 5(2):331–8. 10.1021/nl047996m [DOI] [PubMed] [Google Scholar]
- 58.Muhammad F, Wang A, Qi W, Zhang S, Zhu G. Intracellular antioxidants dissolve man-made antioxidant nanoparticles: using redox vulnerability of nanoceria to develop a responsive drug delivery system. ACS Appl Mater Interfaces (2014) 6(21):19424–33. 10.1021/am5055367 [DOI] [PubMed] [Google Scholar]
- 59.Wang X, Ji Z, Chang C, Zhang H, Wang M, Liao Y, et al. Use of coated silver nanoparticles to understand the relationship of particle dissolution and bioavailability to cell and lung toxicological potential. Small (2014) 10(2):385–98. 10.1002/smll.201301597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cho E, Zhang Q, Xia Y. The effect of sedimentation and diffusion on cellular uptake of gold nanoparticles. Nat Nanotechnol (2011) 6(6):385–91. 10.1038/nnano.2011.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xue Y, Zhai Y, Zhou K, Wang L, Tan H, Luan Q, et al. The vital role of buffer anions in the antioxidant activity of CeO2 nanoparticles. Chemistry (2012) 18(35):11115–22. 10.1002/chem.201200983 [DOI] [PubMed] [Google Scholar]
- 62.Perez J, Asati A, Nath S, Kaittanis C. Synthesis of biocompatible dextran-coated nanoceria with pH-dependent antioxidant properties. Small (2008) 4(5):552–6. 10.1002/smll.200700824 [DOI] [PubMed] [Google Scholar]
- 63.Casals E, Barrena R, Garcia A, Gonzalez E, Delgado L, Busquets-Fite M, et al. Programmed iron oxide nanoparticles disintegration in anaerobic digesters boosts biogas production. Small (2014) 10(14):2801–8. 10.1002/smll.201303703 [DOI] [PubMed] [Google Scholar]
- 64.Feliu N, Docter D, Heine M, Del Pino P, Ashraf S, Kolosnjaj-Tabi J, et al. In vivo degeneration and the fate of inorganic nanoparticles. Chem Soc Rev (2016) 45(9):2440–57. 10.1039/c5cs00699f [DOI] [PubMed] [Google Scholar]
- 65.Braydich-Stolle L, Hussain S, Schlager J, Hofmann M. In vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxicol Sci (2005) 88(2):412–9. 10.1093/toxsci/kfi256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pfaller T, Colognato R, Nelissen I, Favilli F, Casals E, Ooms D, et al. The suitability of different cellular in vitro immunotoxicity and genotoxicity methods for the analysis of nanoparticle-induced events. Nanotoxicology (2010) 4(1):52–72. 10.3109/17435390903374001 [DOI] [PubMed] [Google Scholar]