Abstract
Objectives:
This study aimed to evaluate the effectiveness of supervised exercise among nurses conducting shift work for health promotion.
Methods:
A total of 30 healthy female nurses conducting shift work participated in this study and they were randomly assigned to one of the following 2 groups: The supervised exercise group (SG; participants exercised under the supervision of a physical therapist (PT)) and the voluntary exercise group (VG; participants exercised without supervision). The study participants were asked to exercise twice/week for 12 weeks for 24 sessions. The primary outcome was aerobic fitness, and the secondary outcomes were muscle strength, anthropometric data, biochemical parameters, and mental health. We compared all the outcomes before and after the intervention within each group and between both groups at follow-up.
Results:
Aerobic fitness increased in the SG whereas it decreased in the VG, but these changes were not statistically significant (p=0.053 and 0.073, respectively). However, the between-group difference was significant in the intervention effect (p=0.010). Muscle strength, high-density lipoprotein cholesterol and metabolic profile (high-molecular weight adiponectin), and depressive symptom significantly improved in the SG over time, even though the SG exercised less as compared with the VG. Moreover, significant differences in muscle strength, and low-density lipoprotein cholesterol and reactive oxygen metabolite levels were observed between both groups, and these parameters were better in the SG than in the VG.
Conclusions:
Our data-suggest the effectiveness of exercise supervised by a PT at the workplace of nurses conducting shift work for health promotion.
Keywords: Exercise, Nurses, Physical therapist, Shift work, Workplace
Introduction
Occupational physical therapy (OPT) is an established field of physical therapy in which physical therapists (PT) are employed to achieve its major aims of workplace disease prevention and health promotion. Recent studies have demonstrated that OPT was effective for improving musculoskeletal symptoms, physical health outcomes, psychological well-being, sickness absence, and work ability in workers1,2).
Nurses have a high incidence of musculoskeletal problem, including low back pain3-5). Moreover, it was shown that nurse's shift work is associated with the prevalence of obesity6) and depressive symptom7). Thus, exercise intervention for health promotion as a primary prevention is necessary for nurses conducting shift work.
Takano et al.8) reported that nurses and nursing care workers have a lower rate of exercise adherence (17%) as compared with other professionals after receiving exercise instructions. This finding suggests that voluntary exercise after receiving instructions is not effective for improving exercise adherence. Moreover, supervised exercise at the workplace is necessary to improve exercise adherence.
This study aimed to evaluate the effectiveness of workplace exercise supervised by a PT on physical fitness, anthropometric data, biochemical data, and mental health status among nurses conducting shift work for health promotion.
Methods
Study design and participants
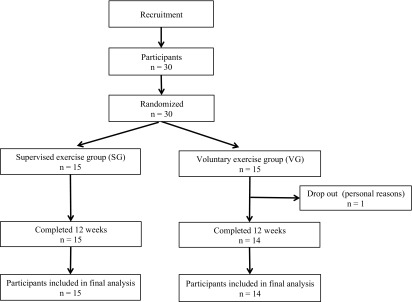
This study was a non-blinded, randomized, controlled trial. A total of 30 healthy female nurses was recruited through the nurses' network, University of Occupational and Environmental Health, Japan using convenience sampling. The inclusion criteria were 20 to 40 years of age, conducting shift work, and working full time. Subjects who were undergoing ambulatory treatment due to medical problems were excluded from this study. Study participants were then randomly assigned to the following 2 groups: The supervised exercise group (SG; participants exercised under the supervision of a PT) and the voluntary exercise group (VG; participants exercised without supervision). The assignment of participants was performed after baseline data collection in a 1:1 ratio using a computerized random number function in Microsoft Excel by the co-author. There were 15 participants in each group, and 1 participant in the VG dropped out before completing the study (Fig. 1).
Fig. 1.
Flow chart of the study design. Thirty healthy female nurses conducting shift work were recruited and randomly assigned to 2 groups: the supervised exercise group (SG) and the voluntary exercise group (VG). One participant of the VG did not complete the 12-week exercise program because of personal reasons. Therefore, data from a total of 29 participants were analyzed in this study.
Ethics statement
Our study was conducted in accordance with the recommendations outlined in the Declaration of Helsinki 2013. All participants provided written informed consent. The study design was approved by the ethics committee of the University of Occupational and Environmental Health, Japan (H26-052).
Exercise intervention protocol
All participants were asked to perform exercise intervention, including resistance and aerobic training, in an individual setting for 2 sessions/week for 12 weeks (August-November 2014). Exercise was conducted in the training room of the hospital where the participants were working.
Resistance training included squats, calf raises, back extensions, and sit-ups. Each exercise was repeated 10 times for 1 set, and 1 session included 3 sets. The study participants were instructed to take a break for 2 minutes between the sets.
Study participants were asked to perform aerobic exercise for 20 minutes without rest by aerobic ergometer (Ergometer STB-3300, OG wellness, Okayama, Japan). The exercise intensity for aerobic exercise was determined by the heart rate reserve (target heart rate [HR] = (HR max -HR rest) × 0.6 + HR rest). HR max was calculated by 220 subtracted by age. All participants were instructed to exercise at ±10 bpm of their target HR.
Participants of the SG exercised under the supervision of a PT and were permitted to ask for PT advice if necessary. VG participants received instruction from a PT only during the first session. An exercise manual for this intervention and biweekly e-mails (such as a "do not give up" ) to encourage exercise were provided to all participants.
Clinical assessments
The primary outcome measure was aerobic capacity as a physical fitness. The secondary outcome measures were muscle strength, anthropometric measurements, biochemical data, and mental health status. These outcome measures were assessed before and after 12 weeks of exercise.
The body mass index (BMI) was calculated by dividing the weight (kg) by height squared (m2). Body fat, muscle mass, and basal metabolic rate were measured with a body composition analyzer (D045-1; TANITA, Tokyo, Japan). Resting blood pressure and pulse rate were measured in the sitting position with an automated sphygmomanometer (HEM-7430; OMRON, Kyoto, Japan) after 5 minutes of rest. Blood pressure and pulse rate measurements were conducted 3 times between 8:00 and 10:00 AM, and then averaged.
Maximum oxygen uptake (VO2 max) was estimated using a multistep submaximal exercise test with a cycle ergometer (ML-3600, Fukuda Denshi, Tokyo, Japan). The load was increased by 30 W in 3-minute intervals under medical evaluation (i.e., 6 inductive electrocardiograms and blood pressure monitoring using an automated sphygmomanometer); the rating of perceived exertion was also monitored with the Borg scale. The endpoint of the multistep submaximal exercise test has to reach 85% of the maximum HR or a Borg scale of 17 (i.e., very hard).
The isokinetic strength of the knee extensor muscle was measured as an indicator of lower muscle strength with a Biodex system 3 dynamometer (Biodex Medical Systems Inc., New York, USA). Participants sat on a stationary seat, their trunk and femur were fixated with stabilizing belts on the measured side, and their knee was attached proximal to the medial malleoli during the measurements. The dynamometer was set to isokinetic mode, and the angular velocities were set to 180 deg/s and 60 deg/s. Muscle strength was measured at each angular velocity and repeated 5 times for both knee extension and flexion. Both lower extremities were measured once, and the average of the lower extremities' peak torque (Nm/kg) was calculated.
Blood samples were obtained early in the morning after at least 12 hours of fasting. A blood biochemistry analysis was conducted by SRL Inc. (Tokyo, Japan). The following parameters were measured: Total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), creatinine, uric acid, fasting plasma glucose, insulin, and high-molecular weight adiponectin (HMW-ADP). Reactive oxygen metabolites (dROM) were analyzed as oxidative stress markers, and the biological antioxidant potential (BAP) was analyzed as the antioxidant capacity using reagent sets (Diacron Srl, Grosseto, Italy).
Depression levels were assessed using the Japanese version of the Beck Depression Inventory-Second Edition (BDI-II)9), which consists of 21 questions. Validation of the Japanese version has been confirmed10). Four response options (0-3) were provided for each question, and participants checked the option that best described them during the 2 weeks before the examination (including the actual examination date). BDI-II scores of 0 to 13, 14 to 19, 20 to 28, and 29 to 63 points indicated minimal, mild, moderate, and severe depression, respectively.
Psychological status was assessed using the Japanese version of the Profile of Mood States (POMS)11,12). The POMS consists of 65 items (including 7 dummy items) and assesses the following 6 identifiable moods: Tension-anxiety (T-A), depression (D), anger-hostility (A-H), vigor (V), fatigue (F), and confusion (C). Participants self-reported each item, with response options ranging from 0 (not at all) to 4 (extremely). A lower and higher score indicated a better mood state for the negative mood items (i.e., T-A, D, A-H, F, and C) and positive mood item (V), respectively. In this study, the total mood disturbance was calculated as the sum of negative mood scores minus the positive mood score.
Confidence in continuing exercise was also assessed by questionnaire using a 100-mm visual analog scale, more specifically with the question, "Are you confident to continue exercising?" For this measurement, 0 and 100 mm represented "not confident" and "fully confident," respectively.
The exercise questionnaire about exercise habit and awareness included the following questions: "How often do you exercise?," "Do you like exercise?," "Are you good at exercise?," and "Do you want to exercise more?" (Fig. 2). All participants were asked to complete a questionnaire using a 4-point Likert scale. This questionnaire was only used before the intervention.
Fig. 2.
Exercise questionnaire. The study participants self-reported their responses on a scale 1 to 4.
All participants were provided an exercise diary to record the exercises that they performed. The exercise diary was designed like a calendar and all participants were asked to check the date on which the exercise was performed. The exercise diary was collected after the 12-week exercise intervention.
Statistical analysis
Statistical analyses were performed with SPSS version 21.0 software (SPSS Inc., Chicago, IL, USA). Differences in baseline values between both groups were assessed using the Mann-Whitney U test. The Wilcoxon signed-rank test was used to compare data at baseline and after the intervention. The Fisher's exact test was used to compare the number of adverse events. To compare the effects of the intervention, we initially calculated the differences between data measured at baseline and after the intervention, and assessed the differences between both groups using the Mann-Whitney U test. A p-value of <0.05 was considered statistically significant. We calculated the Cohen's d-value using the G*Power 3 free software package for effect size analysis13). The d-value was defined as follows: 0.20 (small effect), 0.50 (medium effect), and 0.80 (large effect)14).
Results
A flow diagram of the study design is shown in Fig. 1. The final sample size of our study included 29 participants because 1 participant of the VG did not complete the study due to personal reasons. We found no significant difference in the clinical parameters and responses to the exercise questionnaire at baseline between both groups (Tables 1 and 2). The average exercise adherence in the SG and VG-was 45% (median = 54%, range 21-79%), 55% (median = 52%, range 38-88%), respectively, and no statistically significant difference was observed between both groups (p=0.235). There were some mild adverse events in the SG and VG, such as muscle pain (n=6 and n=2, respectively) and physical fatigue (n=6 and n=3, respectively) (p=0.215 and 0.427, respectively).
Table 1.
Participant characteristics at baseline
| Supervised exercise group(n=15) | Voluntary exercise group(n=14) | p-value | ||
|---|---|---|---|---|
| mean±SD | mean±SD | |||
| SD: standard deviation, VO2 max: maximum oxygen uptake, KET: knee extensor torque, TC: total cholesterol, LDL-C: low-density lipoprotein cholesterol, HDL-C: high-density lipoprotein cholesterol, TG: triglyceride, Cr: creatinine, UA: uric acid, FPG: fasting plasma glucose, HMW-ADP: high molecular weight adiponectin, dROM: reactive oxygen metabolites, BAP: biological anti-oxident potential, BDI-II: Beck depression inventory-II, POMS: profile of mood status, VAS: visual analogue scale. | ||||
| Anthropometry | ||||
| Age (yr) | 25.3±3.4 | 24.7±3.7 | 0.533 | |
| Height (cm) | 158.8±4.3 | 159.1±3.2 | 0.645 | |
| Weight (kg) | 48.6±5.6 | 50.6±7.6 | 0.455 | |
| Body mass index (kg/m2) | 19.3±1.9 | 19.9±2.5 | 0.359 | |
| Body fat (%) | 26.5±5.0 | 26.8±5.2 | 0.743 | |
| Muscle mass (%) | 31.7±7.9 | 34.9±3.0 | 0.089 | |
| Basal metabolic rate (kcal) | 1097±82 | 1130±115 | 0.295 | |
| Vital sign | ||||
| Systolic blood pressure (mmHg) | 105.4±5.9 | 106.2±11.3 | 0.710 | |
| Diastolic blood pressure (mmHg) | 66.7±5.6 | 67.8±6.8 | 0.760 | |
| Pulse rate (bpm) | 76.6±8.9 | 75.4±11.2 | 0.760 | |
| Physical fitness | ||||
| VO2max (ml/kg/min) | 38.4±4.8 | 37.3±4.7 | 0.527 | |
| 60 deg/sec KET (Nm/kg) | 198.1±35.1 | 202.8±37.3 | 0.541 | |
| 180 deg/sec KET (Nm/kg) | 120.4±23.8 | 129.0±22.6 | 0.407 | |
| Biochemistry | ||||
| TC (mg/dl) | 176.1±30.5 | 174.9±19.0 | 0.913 | |
| LDL-C (mg/dl) | 96.5±28.4 | 94.4±16.9 | 0.527 | |
| HDL-C (mg/dl) | 68.6±10.0 | 71.4±11.8 | 0.615 | |
| TG (mg/dl) | 49.6±15.4 | 48.3±12.4 | 0.793 | |
| Cr (mg/dl) | 0.6±0.1 | 0.7±0.1 | 0.101 | |
| UA (mg/dl) | 4.3±1.0 | 4.3±0.8 | 0.759 | |
| FPG (mg/dl) | 93.4±5.9 | 91.6±5.9 | 0.335 | |
| Insulin (μIU/ml) | 6.1±3.5 | 5.6±2.9 | 0.727 | |
| HMW-ADP (μg/ml) | 4.6±1.8 | 4.6±1.9 | 0.896 | |
| dROM (UCARR) | 239.6±70.0 | 272.6±51.4 | 0.138 | |
| BAP (μmol/l) | 1873±401 | 1971±364 | 0.600 | |
| Mental status | ||||
| BDI-II | 12.1±6.6 | 10.9±8.2 | 0.599 | |
| POMS | TMD: Total mood disturbance | 221.9±51.0 | 224.3±47.5 | 0.827 |
| T-A: tension-anxiety | 52.9±8.7 | 50.3±9.2 | 0.457 | |
| D: depression | 56.4±12.4 | 55.5±10.6 | 0.930 | |
| A-H: anger-hostility | 49.5±9.6 | 54.9±12.2 | 0.182 | |
| V: vigor | 46.6±7.5 | 45.6±9.2 | 0.497 | |
| F: fatigue | 54.3±9.0 | 56.1±9.3 | 0.497 | |
| C: confusion | 55.4±12.2 | 53.1±11.3 | 0.646 | |
| Self-efficacy | ||||
| Confidence to continue exercise | 40.1±23.0 | 40.4±15.4 | 0.662 | |
| (VAS: 0-100) | ||||
Table 2.
Exercise questionnaire data at baseline
| Supervised exercise group(n=15) | Voluntary exercise group(n=14) | p-value | |
|---|---|---|---|
| n | n | ||
| Exercise habits | |||
| Almost everyday | 0 | 0 | 0.715 |
| Sometimes | 1 | 1 | |
| Rarely | 2 | 1 | |
| Not at all | 12 | 12 | |
| Like or dislike exercise | |||
| Like | 0 | 0 | 0.643 |
| Somewhat like | 5 | 3 | |
| Somewhat dislike | 5 | 6 | |
| Dislike | 5 | 5 | |
| Good or poor at exercise | |||
| Good | 2 | 0 | 0.833 |
| Somewhat good | 4 | 6 | |
| Somewhat poor | 6 | 7 | |
| Poor | 3 | 1 | |
| Motivation for exercise | |||
| Agree | 5 | 2 | 0.175 |
| Somewhat agree | 8 | 8 | |
| Somewhat disagree | 2 | 4 | |
| Disagree | 0 | 0 |
Effects of exercise intervention by group and differences between groups (Table 3)
Table 3.
Effects of exercise intervention by group
| Supervised exercise group (n=15) | Voluntary exercise group (n=14) | ΔS vs. ΔV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ΔS (after intervention - baseline) | p-valuea) | Effect size | ΔV(after intervention-baseline) | p-valuea) | Effect size | p-valueb) | ||||
| mean±SD | mean±SD | |||||||||
| ΔS: value of the difference between after and before intervention in Supervised exercise group. ΔV: value of the difference between after and before intervention in Voluntary exercise group. SD: standard deviation, VO2max: maximum oxygen uptake, KET: knee extensor torque, TC: total cholesterol, LDL-C: low-density lipoprotein cholesterol, HDL-C: high-density lipoprotein cholesterol, TG: triglyceride, Cr: creatinine, UA: uric acid, FPG: fasting plasma glucose, HMW-ADP: high molecular weight adiponectin, dROM: reactive oxygen metabolites, BAP: biological anti-oxident potential, BDI-II: Beck depression inventory-II, POMS: profile of mood states,VAS: visual analogue scale. Effect size means Cohen’s d value. The thresholds for small, medium, and large effects were defined as d value s below 0.20, 0.50, and 0.80. a) Wilcoxon signed rank test, b) Mann-Whitney U test * p<0.05, ** p<0.01 | ||||||||||
| Anthropometry | ||||||||||
| Body mass index (kg/m2) | 0.2±0.7 | 0.334 | 0.32 | 0.2±0.3 | 0.064 | 0.56 | 0.983 | |||
| Body fat (%) | -0.5±4.5 | 0.513 | 0.12 | 1.4±2.8 | 0.041* | 0.50 | 0.143 | |||
| Muscle mass (%) | 2.2±9.0 | 0.509 | 0.30 | -0.6±0.8 | 0.017* | 0.75 | 0.149 | |||
| Basal metabolic rate (kcal) | -5.7±16.6 | 0.233 | 0.34 | -14.4±18.7 | 0.014* | 0.76 | 0.295 | |||
| Vital sign | ||||||||||
| Systolic blood pressure (mmHg) | -3.0±6.1 | 0.061 | 0.49 | -0.1±9.8 | 0.432 | 0.01 | 0.600 | |||
| Diastolic blood pressure (mmHg) | 1.1±4.4 | 0.280 | 0.47 | 1.2±6.6 | 0.753 | 0.19 | 0.678 | |||
| Pulse rate (bpm) | -2.9±8.4 | 0.244 | 0.35 | -1.7±9.2 | 0.463 | 0.19 | 0.879 | |||
| Physical fitness | ||||||||||
| VO2max (ml/kg/min) | 1.2±2.1 | 0.053 | 0.76 | -0.6±1.2 | 0.073 | 0.52 | 0.010* | |||
| 60 deg/sec KET (Nm/kg) | 6.0±16.1 | 0.211 | 0.37 | 1.9±23.4 | 0.778 | 0.08 | 0.694 | |||
| 180 deg/sec KET (Nm/kg) | 12.6±9.4 | 0.001** | 1.34 | 4.2±9.0 | 0.056 | 0.47 | 0.045* | |||
| Biochemistry | ||||||||||
| TC (mg/dl) | 5.3±10.3 | 0.059 | 0.51 | 9.3±13.3 | 0.030* | 0.76 | 0.304 | |||
| LDL-C (mg/dl) | -0.8±9.3 | 0.776 | 0.09 | 7.9±12.2 | 0.030* | 0.64 | 0.034* | |||
| HDL-C (mg/dl) | 4.6±7.0 | 0.030* | 0.66 | -1.1±10.4 | 0.972 | 0.10 | 0.143 | |||
| TG (mg/dl) | 11.5±27.4 | 0.222 | 0.42 | 21.0±38.8 | 0.084 | 0.54 | 0.541 | |||
| Cr (mg/dl) | 0.0±0.0 | 0.975 | 0.07 | 0.0±0.0 | 0.160 | 0.38 | 0.263 | |||
| UA (mg/dl) | -0.1±0.6 | 0.183 | 0.13 | 0.1±0.5 | 0.503 | 0.19 | 0.212 | |||
| FPG (mg/dl) | 1.1±7.9 | 0.861 | 0.14 | 3.3±7.1 | 0.083 | 0.46 | 0.246 | |||
| Insulin (μU/ml) | 2.2±9.5 | 0.609 | 0.24 | 2.2±3.2 | 0.041* | 0.68 | 0.337 | |||
| HMW-ADP (μg/dl) | 0.5±0.6 | 0.007** | 0.83 | 0.6±3.1 | 0.272 | 0.27 | 0.793 | |||
| dROM (UCARR) | 12.6±32.2 | 0.281 | 0.39 | 35.5±27.0 | 0.002** | 1.31 | 0.045* | |||
| BAP (μmol/l) | 14.9±322.4 | 0.955 | 0.05 | 60.5±430.5 | 0.109 | 0.14 | 0.295 | |||
| Mental status | ||||||||||
| BDI-II | -3.6±4.9 | 0.012* | 0.73 | -0.9±3.6 | 0.419 | 0.24 | 0.079 | |||
| POMS | TMD: Total mood disturbance | -2.5±32.7 | 0.910 | 0.08 | -11.8±20.8 | 0.060 | 0.57 | 0.247 | ||
| T-A: tension-anxiety | 0.5±8.1 | 0.637 | 0.06 | -0.6±6.9 | 0.682 | 0.08 | 0.539 | |||
| D: depression | 0.4±8.2 | 0.622 | 0.05 | -1.1±5.2 | 0.445 | 0.21 | 0.270 | |||
| A-H: anger-hostility | 1.8±7.3 | 0.363 | 0.25 | -3.0±5.2 | 0.050 | 0.57 | 0.051 | |||
| V: vigor | 2.0±7.0 | 0.334 | 0.29 | 2.9±10.3 | 0.463 | 0.28 | 0.965 | |||
| F: fatigue | -0.7±7.3 | 0.925 | 0.09 | -2.4±6.9 | 0.232 | 0.36 | 0.339 | |||
| C: confusion | -2.5±7.4 | 0.212 | 0.34 | -1.6±5.4 | 0.254 | 0.30 | 0.758 | |||
| Self-efficacy | ||||||||||
| Confidence to continue exercise | -7.3±21.4 | 0.233 | 0.34 | -3.1±16.5 | 0.363 | 0.19 | 0.694 | |||
| (VAS: 0-100) | ||||||||||
Primary outcome
VO2 max increased in the SG whereas it decreased in the VG, but these changes were not statistically significant (p=0.053 and 0.073, respectively). However, the between-group difference was significant in the intervention effect (p=0.010).
Secondary outcome
In the SG, 180 deg/s knee extensor torque, HDL-C, and HMW-ADP significantly increased (p=0.001, 0.030, and 0.007, respectively), whereas BDI-II scores significantly decreased after a 12-week intervention (p=0.012). Other clinical assessments were not significantly different in the SG.
In the VG, body fat, TC, LDL-C, insulin, and dROM significantly increased (p=0.041, 0.030, 0.030, 0.041, and 0.002, respectively), whereas muscle mass and basal metabolic rate significantly decreased after a 12-week intervention (p=0.017 and 0.014, respectively). Other clinical assessments were not significantly different in the VG.
We also observed significant differences in the intervention effects (180 deg/s knee extensor torque, p=0.045; LDL-C, p=0.034; and dROM, p=0.045). Other clinical assessments were not significantly different between both groups.
Discussion
We demonstrated that exercise supervised by a PT was more effective than voluntary exercise for improving aerobic capacity, muscle strength, and some biochemical parameters in nurses conducting shift work with poor exercise habits (almost all participants' exercise habit was determined as "Rarely" or "Not at all." ) in this study.
Some previous studies demonstrated that VO2 max increases by 6.3 to 9.9% after exercise intervention15,16). On the other hand, the present study demonstrated that it increased by 3.1% in the SG and -1.6% in the VG. Moreover, VO2 max did not significantly improve after the exercise intervention in either group. These inconsistencies may be attributed to low exercise adherence and short duration of exercise intervention. However, we observed a significant difference in VO2 max between both groups, even though exercise adherence was lower in the SG. The workload of the ergometer used by participants in the SG was forcibly adjusted by the PT to reach the participants' target heart rates, and the majority of SG participants complained of the heavy workload. In contrast, participants of the VG self-adjusted the workload; therefore, it is possible that VG participants did not exercise using the proper workload. An increase in VO2 max (although non-significant) was only observed in the SG because participants in this group might have been appropriately supervised by the PT.
Inactivity not only causes adverse physical aspects17) but also mental aspects18). It is considered that implementing an effective exercise program that involves young and healthy workers as the primary prevention is considered important.
Nurses are prone to lack of exercise, particularly those who engage in shift work19). This study showed the significant effectiveness of exercise intervention supervised by a PT who is working at the same hospital.
Adherence of supervised exercise is generally considered to be better than adherence of voluntary exercise20). However, exercise adherence of the supervised group in this study was as low as that of the voluntary exercise group, showing a similar adherence of 45% among female healthcare workers in a previous study21). Low adherence may be attributed to the following reasons. In general, "a lack of time" is the main barrier for exercise among busy workers22). Nursing is a difficult, physical and mental work, which results in high prevalence of work-related fatigue23). Moreover, the majority of study participants did not have an exercise habit.
Nevertheless, the SG had lower exercise adherence than the VG; aerobic capacity and muscle strength in the SG showed more improvement than in the VG. Participants of the SG exercised with an appropriate workload under the supervision of a PT. Most participants felt that the workload was "somewhat heavy" or "rather heavy." Participants of the VG were asked to perform a self-adjustment exercise; thus, the workload may not be sufficient.
The special feature of this program is the role of the PT who functioned as a mental supporter as well. The presence of the PT in every exercise might have helped in maintaining the exercise intensity although it did not help in improving the adherence as expected. Participant encouragement by the PT in the same hospital may be significant. At last, this study showed the future possibility of occupational PTs in promoting the health of Japanese young nurses.
Limitations
This study has several limitations. First, the non-blinded design of this study was one of the major limitations because it might lead to inaccurate assessment. This study design might have also resulted in a difference in the attitude to exercise; that is, the SG might have taken more effort to exercise than the VG. Second, we did not calculate sample size. However, we have confirmed the primary outcome increases even if the number of subjects is about 1015). Third, this was not an intention-to-treat analysis of the results because we could not collect data of the participant who dropped out due to personal reasons. Fourth, our study sample was small and the statistical power was low. A future study with a large number of participants is necessary. Fifth, the majority of participants were young and healthy with a normal or small BMI; thus, showing an improvement of the biochemical data is difficult. This study could not generalize the findings on all Japanese nurses. At last, exercise adherence was about half of the target of twice a week in both groups, and some participants exhibited very low adherence. Participants were recruited from the hospital so that those with low motivation would be involved because of participation of fellow nurses. In this study, we showed the effectiveness of supervised exercise in nurses, including those with very low adherence.
Future study
A study with large participants or a different program with obligatory exercise during working hours is necessary to prove the effectiveness of PTs who engage in promoting health in the same healthcare facilities.
Conclusion
Supervised exercise at the workplace may be more effective for improving physical functions and several biochemical parameters than voluntarily exercise among young nurses with shift work and poor exercise habits. Thus, PTs may be valuable promoters of workers' health in healthcare facilities.
Acknowledgments: We are grateful to the staff (Hideo Shitama, Toru Akebi, Masanobu Kimura, Yuto Ogata, Ryosuke Ohya, Jun Hayakawa, Yudai Yano, Natsumi Hanada, and Chie Ikeda) of the Rehabilitation Center of the University Hospital, University of Occupational and Environmental Health, Japan for their support in this study.
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
References
- 1). Addley K, Burke C, McQuillan P. Impact of a direct access occupational physiotherapy treatment service. Occup Med (Lond) 2010; 60: 651-653. [DOI] [PubMed] [Google Scholar]
- 2). Pizzari T, Davidson M. Health outcomes can be improved by implementing an occupational physiotherapy provider programme. Physiother Res Int 2013; 18: 47-54. [DOI] [PubMed] [Google Scholar]
- 3). Fujimura T, Takeda M, Asada F, et al. The investigation of low back pain among hospital nurses. JJOMT 2012; 60: 91-96 (in Japanese). [Google Scholar]
- 4). Cinar-Medeni O, Elbasan B, Duzgun I. Low back pain prevalence in healthcare professionals and identification of factors affecting low back pain. J Back Musculoskelet Rehabil [published online ahead of print Nov 11, 2016]. (doi: 10.3233/BMR. 160571). [DOI] [PubMed] [Google Scholar]
- 5). Moreira RF, Sato TO, Foltran FA, et al. Prevalence of musculoskeletal symptoms in hospital nurse technicians and licensed nurses: associations with demographic factors. Braz J Phys Ther 2014; 18: 323-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Kim MJ, Son KH, Park HY, et al. Association between shift work and obesity among female nurses: Korean Nurses' Survey. BMC Public Health 2013; 13: 1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Lee HY, Kim MS, Kim O, et al. Association between shift work and severity of depressive symptoms among female nurses: the Korea Nurses' Health Study. J Nurs Manag [published online ahead of print May 6, 2015]. (doi: 10.1111/jonm. 12298). [DOI] [PubMed] [Google Scholar]
- 8). Takano K. Effects of stretching for the prevention of shoulder stiffness and back pain in different jobs. JJOMT 2014; 62: 32-37 (in Japanese). [Google Scholar]
- 9). Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. San Antonio (TX): Psychological Corp; 1996. [Google Scholar]
- 10). Kojima M, Furukawa TA, Takahashi H, et al. Cross-cultural validation of the beck depression inventory-II in Japan. Psychiatry Res 2002; 31: 291-299. [DOI] [PubMed] [Google Scholar]
- 11). McNair D, Lorr M, Droppleman L. Manual for the Profile of Mood Status. San Diego (CA): Educational and Industrial Testing Services; 1971. [Google Scholar]
- 12). McNair D, Lorr M, Droppleman L. Revised Manual for the Profile of Mood Status. San Diego (CA): Educational and Industrial Testing Services; 1992. [Google Scholar]
- 13). Faul F, Erdfelder E, Lang AG, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39: 175-191. [DOI] [PubMed] [Google Scholar]
- 14). Cohen J. A power primer. Psychol Bull 1992; 112: 155-159. [DOI] [PubMed] [Google Scholar]
- 15). Eguchi M, Ohta M, Hiroshi Y. The effect of single long and accumulated short bouts of exercise on cardiovascular risks in male Japanese workers: a randomized controlled study. Ind Health 2013; 51: 563-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Jakicic JM, Winters C, Lang W, et al. Effects of intermittent exercise and use of home exercise equipment on adherence, weight loss, and fitness in overweight women: a randomized trial. JAMA 1999; 282: 1554-1560. [DOI] [PubMed] [Google Scholar]
- 17). Lee IM, Shiroma EJ, Lobelo F, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 2012; 380: 219-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Teychenne M, Ball K, Salmon J. Physical activity and likelihood of depression in adults: a review. Prev Med 2008; 46: 397-411. [DOI] [PubMed] [Google Scholar]
- 19). Chin DL, Nam S, Lee SJ. Occupational factors associated with obesity and leisure-time physical activity among nurses: a cross-sectional study. Int J Nurs Stud 2016; 57: 60-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Jordan JL, Holden MA, Mason EE, et al. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev 2010; 1: CD005956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Jakobsen MD, Sundstrup E, Brandt M, et al. Effect of workplace- versus home-based physical exercise on musculoskeletal pain among healthcare workers: a cluster randomized controlled trial. Sc and J Work Environ Health 2015; 41: 153-163. [DOI] [PubMed] [Google Scholar]
- 22). Ishii K, Inoue S, Ohya Y, et al. Sociodemographic variation in the perception of barriers to exercise among Japanese adults. J Epidemiol 2009; 19: 161-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Raftopoulos V, Charalambous A, Talias M. The factors associated with the burnout syndrome and fatigue in Cypriot nurses: a census report. BMC Public Health 2012; 12: 457. [DOI] [PMC free article] [PubMed] [Google Scholar]