Abstract
Rapid iodine-131(131I) turnover in the thyroid gland is an important feature of Graves’ disease (GD) and also a strong predictor of radioiodine therapy failure. The aim of this study was to explore the predictors of rapid 131I turnover. The clinical data on 2543 patients were retrospectively reviewed. Patients were divided into 2 groups depending on present or absent with rapid 131I turnover defined as a 4-hour to 24-hour 131I uptake ratio of ≥1. Overall, 590 cases (23.2%) had a rapid 131I turnover. In the univariate analysis, gender, age, FT3/FT4 concentration, disease duration, with or without antithyroid drugs (ATD), time of ATD, thyroid weight and thyroid textures displayed significant differences. Cutoff values of age, FT3 and thyroid weight to predict rapid 131I turnover were 38 years, 35 pmol/l and 56 g by receiver operating characteristic curves. Binary logistic regression analysis further revealed higher probability of rapid 131I turnover in patients with thyroid weight ≥56 g (odds ratio [OR]:3.7, 95% confidence interval [CI]: 3.032–4.559), age <38 years (OR:2.3, 95%CI: 1.906–2.856), FT3 concentration ≥35 pmol/l (OR:7.6, 95%CI: 5.857–8.563) and females (OR:2.2, 95%CI: 1.757–2.791). In conclusion, larger goiters, younger age, higher FT3 concentration and females are independently associated with rapid 131I turnover in GD patients.
Introduction
Graves’ disease (GD) is the commonest cause of hyperthyroidism and accounted for nearly 90% cases. As an autoimmune disorder, women are more susceptible to hyperthyroidism than men1. Radioiodine-131 (131I), which has been used for approximately 70 years for the treatment of GD, was proven to be efficient and safe both as a primary therapy and secondary therapy when thyrotoxicosis can not be controlled by antithyroid drugs (ATD)2–4.
Radioactive iodine uptake (RAIU) is a useful tool which can be used to differentiate hyperthyroidism from thyroiditis, calculate the 131I dose for treatment of GD and predict therapeutic outcome5, 6. The dose of 131I to be administered could be fixed even if neither thyroid weight nor thyroid uptake is known and also adjusted using complex dosage formula based on gland size, the 131I concentration of per gram thyroid tissue as well as the residence time of 131I in the thyroid gland7–10.
At some institutions, traditionally, only 24-hour RAIU was measured for calculation of therapeutic doses of 131I11. However, it was impossible to calculate the residence time of thyroidal 131I by using a single measurement. It was reported that 4- to 24-hour uptake ratio could be used as an index of rapid iodine-131 turnover and a surrogate parameter for effective half life in hyperthyroidism12. Previous studies showed that about 12–32% GD patients had shortened residence time of 131I in the thyroid gland due to the rapid 131I turnover12–14. Additionally, several investigators reported that rapid 131I turnover was a strong predictor of radioiodine therapy (RIT) failure12, 14, 15, which increased the whole-body radiation dose secondary to the additional release of protein-bound 131I into the vascular system16. However, to our knowledge, there is lack of study to reveal the predictive factors of rapid 131I turnover in patients with GD.
In the present study, our aim was to explore the potential factors that could predict rapid thyroidal 131I turnover in patients with GD by retrospective review of their data.
Results
Patients and first-dose RIT failure
Baseline and pre-RIT patient clinical characteristics of the 2543 patients studied are listed in Table 1.
Table 1.
Baseline and pre-RIT patient clinical characteristics (n = 2543).
| Characteristic | |
|---|---|
| Gender | |
| Male | 793(31.2%) |
| Female | 1750(68.8%) |
| Age(years) | |
| Mean ± SD | 40.8 ± 13.6 |
| Median (range) | 40.0(10–80) |
| FT3(pmol/l) | |
| Mean ± SD | 34.2 ± 11.2 |
| Median (range) | 33.6(6.6–50) |
| FT4(pmol/l) | |
| Mean ± SD | 79.3 ± 19.6 |
| Median (range) | 83.4(28.0–100) |
| Disease duration (mons) | |
| Mean ± SD | 31.8 ± 42.8 |
| Median (range) | 12.0(0.2–360) |
| ATD | |
| With | 1742(68.5%) |
| Without | 801(31.5%) |
| Time of ATD(mons) | |
| Mean ± SD | 18.3 ± 31.6 |
| Median (range) | 3.0(0–240) |
| 4 h thyroid 131I uptake(%) | |
| Mean ± SD | 41.3 ± 20.6 |
| Median (range) | 40(7–92) |
| 24 h thyroid 131I uptake(%) | |
| Mean ± SD | 75.6 ± 19.5 |
| Median (range) | 76(32–96) |
| Thyroid weight(g) | |
| Mean ± SD | 58.6 ± 27.0 |
| Median (range) | 52.0(15–212) |
| Complications | |
| With | 662(26.0%) |
| Without | 1881(74.0%) |
| Thyroid nodules | |
| With | 36414.3%) |
| Without | 2179(85.7%) |
| Thyroid texture | |
| Soft | 1275(50.1%) |
| Moderate | 943(37.1%) |
| Stiff | 325(12.8%) |
Data are presented as count (percentage) or mean ± SD and median (range). RAIU = radioiodine uptake, FT3 = free triiodothyronine, FT4 = free thyroxine, SD = standard deviation, ATD = antithyroid drugs.
Overall, 195 cases (7.7%) remained hyperthyroid after first-dose RIT (first-dose failure), and therefore, were given repeated RIT. Of them, 177 cases (7.0%) received second, 15 cases third and 3 subjects fourth 131I. Additionally, 590 patients (23.2%) had a rapid 131I turnover.
Comparison of patient characteristics between groups
Demographic and clinical characteristics of the 2 group patients studied are displayed in Table 2. We found subjects with rapid 131I turnover had a higher first-dose failure rate (12.0% vs. 6.3%, OR = 2.018, 95%CI: 1.484–2.744, P < 0.0001). When we compared the categorical variables between groups using the chi square test, we found no statistically significant association in the complications (P = 0.782) and thyroid nodules (P = 0.643). However, there was significant difference in the gender composition (P < 0.0001), thyroid textures (P = 0.004) and with or without ATD (P < 0.0001).
Table 2.
Comparison of patient characteristics between the 2 groups.
| Characteristic | Rapid 131I turnover | P | |
|---|---|---|---|
| Present (n = 590) | Absent (n = 1953) | ||
| Gender | |||
| Male | 126(21.4%) | 667(34.2%) | <0.0001* |
| Female | 464(78.6%) | 1286(65.8%) | |
| Age(years) | 33.0(11–78) | 42.0 (10–80) | <0.0001** |
| FT3(pmol/l) | 38.2(11.6–50) | 31.3(6.6–50) | <0.0001** |
| FT4(pmol/l) | 86.5(32.2–100) | 78.9(28.0–100) | 0.0010** |
| Disease duration (mons) | 12.5(0.3–264) | 12.0(0.2–360) | 0.1090 |
| ATD | |||
| With | 442(74.9%) | 1300(66.6%) | <0.0001* |
| Without | 148(25.1%) | 653(33.4%) | |
| Time of ATD | 6.0(0–240) | 2.0 (0–216) | 0.0010** |
| 4 h thyroid 131I uptake(%) | 42(7–89) | 40(9–92) | 0.2060 |
| 24 h thyroid 131I uptake(%) | 75(35–96) | 77(32–95) | 0.5340 |
| Thyroid weight(g) | 68.0(20–212) | 48.5(15–212) | <0.0001** |
| Complications | |||
| With | 151(25.60%) | 511(26.2%) | 0.7820 |
| Without | 439(74.4%) | 1442(73.8%) | |
| Thyroid nodules | |||
| With | 81(13.7%) | 283(14.5%) | 0.6434 |
| Without | 509(86.3%) | 1670(85.5%) | |
| Thyroid textures | |||
| Soft | 266(45.1%) | 1009 (51.7%) | 0.0040* |
| Moderate | 236(40.0%) | 707(36.2%) | |
| Stiff | 88(14.9%) | 237(12.1%) | |
| First-dose 131I failure | |||
| With | 71(12.0%) | 124(6.3%) | <0.0001* |
| Without | 519(88.0%) | 1829(93.7%) | |
Data are presented as count (percentage) or median (range). *P value < 0.01 using chi square test. **P value < 0.01 using Mann–Whitney U test. FT3 = free triiodothyronine, FT4 = free thyroxine, SD = standard deviation, ATD = antithyroid drugs.
Similarly, we found no statistically significant difference in the disease duration (P = 0.109), 4 h/24 h thyroid 131I uptake (P = 0.206 and 0.534, respectively) when comparing the continuous variables in the 2 groups using the Mann-Whitney U test. However, we found younger patients, cases with higher FT3/FT4 concentration and heavier thyroid weight, and those with longer time of ATD use more likely had rapid 131I turnover (all P < 0.01).
Diagnostic values of age, FT3 and thyroid weight for rapid 131I turnover
Receiver operating characteristic (ROC) curves were drawn to evaluate the accuracy of age, FT3 concentration and thyroid weight in predicting rapid thyroidal 131I turnover (Fig. 1).
Figure 1.
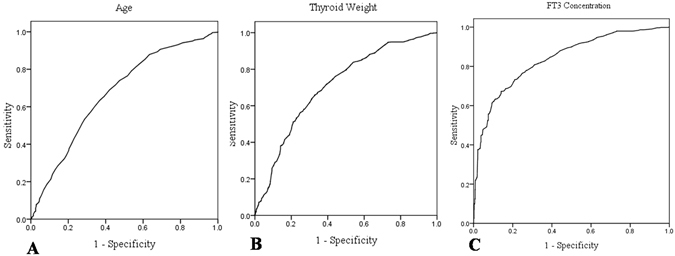
ROC curves for age (A), thyroid weight (B) and FT3 concentration (C) in predicting rapid thyroidal 131I turnover in GD patients.
The optimal cutoffs were the values yielding maximum sums of sensitivity and specificity from the ROC curves17, 18. The results demonstrated that the optimal cutoff values for age and FT3 concentration were 38 years old and 35 pmol/l, at which the sensitivity and specificity were 63.3%, 63.2% (for age) and 75.3%, 73.1% (for FT3), respectively (area under curve [AUC]: 0.672; 95% CI: 0.646–0.697 and AUC: 0.815; 95% CI: 0.796–0.834, P < 0.001, respectively). Similarly, we found a thyroid weight threshold of 56 g, with a sensitivity of 67.3% and specificity of 65.6% for rapid 131I turnover (AUC: 0.710; 95% CI: 0.687–0.733, P < 0.001).
Logistic regression analysis
Table 3 shows a multivariate logistic regression analysis of the potential risk factors of rapid 131I turnover. Variables that were significant in the univariate analysis were entered into the stepwise method. The multivariate logistic regression analysis revealed that patients with thyroid weight ≥56 g and FT3 concentration ≥35 pmol/l demonstrated a 3.7-fold and 7.6-fold higher probability of rapid 131I turnover, respectively, and cases with age < 38 years old showed a 2.3-fold higher probability. Additionally, female patients had a 2.2-fold higher probability of rapid 131I turnover.
Table 3.
Comparison of predictors for rapid 131I turnover by multivariate logistic regression analysis.
| Characteristics | OR | 95% CI | P value |
|---|---|---|---|
| Gender (female vs. male) | 2.214 | 1.757–2.791 | <0.0001 |
| Age (<38yrs vs. ≥38yrs) | 2.333 | 1.906–2.856 | <0.0001 |
| thyroid weight (≥56 g vs. <56 g) | 3.718 | 3.032–4.559 | <0.0001 |
| FT3 (≥35 pmol/l vs. <35 pmol/l) | 7.625 | 5.857–8.563 | <0.0001 |
FT3 = free triiodothyronine, FT4 = free thyroxine, OR = odds ratio, CI = confidence interval.
Comparison of rapid 131I turnover in patients with thyroid weight <56 g or ≥56 g, age <38years or ≥38years and FT3 concentration <35 pmol/L or ≥35 pmol/L
A comparative analysis of the percent of rapid 131I turnover, using the chi square test, between the patients with thyroid weight <56 g or ≥56 g, age <38 years or ≥38years and FT3 concentration <35 pmol/l or ≥35 pmol/l was performed (Fig. 2).
Figure 2.
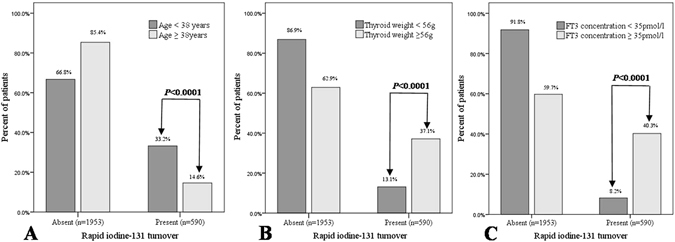
A comparative analysis of the percent of rapid 131I turnover between the patients with age <38 years or ≥38years (A), thyroid weight <56 g or ≥56 g (B) and FT3 concentration <35 pmol/l or ≥35 pmol/l (C).
We found a rapid 131I turnover rate of 37.1% among patients with thyroid weight ≥56 g and 13.1% with thyroid weight <56 g (P < 0.0001). Additionally, the rapid 131I turnover rates in patients with age <38 years and ≥38years were 33.2% and 14.6%, respectively (P < 0.0001). Similarly, we also found a rapid 131I turnover rate of 40.3% among patients with FT3 concentration ≥35 pmol/l and 8.2% with FT3 concentration <35 pmol/l (P < 0.0001).
Discussion
Radioactive iodine (131I) therapy is the most common modality for treatment of hyperthyroidism in the United States2. About 80–95% of GD patients could be controlled after first dose of 131I therapy, which is a relatively safe, simple and effective form of therapy4, 7, 9. In our institution, only 195 patients (7.7%) remained hyperthyroid after first-dose RIT.
Rapid 131I turnover in the thyroid gland is an important feature of GD and can be observed in 12–32% of patients with GD12–14. In the present study, we reviewed a large-sample GD patients and found the prevalence of rapid 131I turnover is 23.2% in our patient population. Some investigators reported that rapid 131I turnover was a strong predictor of RIT failure12, 14, 15, and Aktay et al.12 found up to 55% of the GD patients with rapid 131I turnover failed to respond to the initial 131I therapy. Similarly, our study showed patients with 131I uptake ratio of ≥1 have a higher first-dose RIT failure rate when comparing against those with 131I uptake ratio of <1 (12.0% vs. 6.3%, P < 0.0001), although we delivered a higher concentration of 131I per gram of thyroid tissue to patients with rapid 131I turnover in our routine work. The higher failure rate of 131I therapy among patients with rapid 131I turnover might be explained by the rapid clearance or turnover of iodine-131 from the thyroid gland, which results in a shorter effective half-life of 131I with less radiation subsequently delivered to the gland12. Therefore, patients with rapid 131I turnover should receive a larger dose of 131I in order to obtain higher RIT success rates.
The relatively high rapid 131I turnover and first-dose RIT failure rate in patients with GD highlight the importance of identifying predictors of rapid 131I turnover in this patient population. In our study, no differences were found in the disease duration, 4 h or 24 h thyroid 131I uptake, and complications. Meanwhile, gender, age, FT3/FT4 concentration, antithyroid medication, time of ATD, thyroid textures and thyroid weight could be used as the potential variables to predict rapid 131I turnover in the univariate analysis. Female patients had a higher rapid 131I turnover rate than males (26.5% vs. 15.9%). The FT3 and FT4 concentrations were higher in cases with rapid 131I turnover (38.2 pmol/l vs. 31.3 pmol/l, and 86.5 pmol/l vs.78.9 pmol/l, respectively). The values of thyroid weight in patients with 131I uptake ratio of ≥1 were heavier than those with 131I uptake ratio of <1 (68.0 g vs. 48.5 g), and patients with rapid 131I turnover were younger (33yrs vs. 42yrs). Furthermore, using multivariate logistic analysis, we found that gender, FT3 concentration, thyroid weight and age were the independent factors related to rapid 131I turnover. In our patient population, we verified that female patients had a 2.2-fold higher probability of rapid 131I turnover. Moreover, patients with thyroid weight ≥56 g and FT3 concentration ≥35pmol/l had a 3.7-fold and 7.6-fold higher probability of rapid 131I turnover, with an accuracy of 71.0% and 81.5%, respectively. Additionally, we verified that patients with age <38 years old showed 2.3 times more risk of rapid 131I turnover (accuracy 67.2%).
Rapid 131I turnover has been ascribed to the so-called “small iodine pool syndrome,” which can be seen in patients pretreated with ATD19, 20. Although ATD have short half-lives in blood, there is a high concentration and retention in the intra-thyroid environment, which may lead to a reduction in 131I uptake and effective half-life of 131I in the thyroid19. Thus, the 131I turnover is faster in comparison to patients who were not treated. Additionally, patients who maintained anti-thyroid drug use during RIT also had a 4.9-fold higher risk of treatment failure in comparison to those who discontinued the medication21. In our study, 74.9% patients with rapid 131I turnover had received antithyroid medications, comparing to 66.6% without rapid 131I turnover (P < 0.001). However, a significant different finding in the univariate analysis was not upheld in the multivariate model, indicating that antithyroid medication prior to RIT is not considered to be a significant factor predicting rapid 131I turnover.
As with most retrospective studies, this study has certain shortcomings. Firstly, the main weakness is the lack of data on thyroid autoantibodies titers, especially anti-thyrotrophin receptor antibody (TRAb) level which could be helpful in predicting disease severity and chance of RIT failure, whereas it was not available as a routine laboratory assessment during the time of data collection and we were unable to include it in our statistical analysis. Secondly, in this study, we only defined multiple 131I therapies as first-dose RIT failure, however, although few, some patients lost to follow-up or chose other forms of treatment such as antithyroid medication or surgery after the initial RIT. Therefore, first-dose failure rate in this study was slightly lower.
In conclusion, the 4- to 24-hour 131I uptake ratio appears to be a practical index for predicting early peaking of 131I uptake in GD patients. The incidence of rapid 131I turnover was high, which was expected in patients presenting larger goiters, younger age, higher FT3 concentration and females, particularly those with thyroid weight ≥56 g, age <38 years, FT3 concentration ≥35 pmol/l.
Materials and Methods
Subjects
Between June 2007 and March 2014, the medical records of hyperthyroid patients consecutively referred to the Thyroid Clinic for 131I therapy were reviewed. The 131I dose (MBq) = 131I dose for per gram of thyroid tissue (MBq/g) × thyroid weight (g)/24h-RAIU. Of all the 2940 patients, a total of 2543 patients (793 men and 1750 women; age, 10–80 years) with the clinical diagnosis of GD were selected and 350 cases with other etiologies for hyperthyroidism, including multinodular goiter, plummer’s disease and hashimoto’s thyroditis were excluded. Additionally, the remaining 47 patients who had received RIT before were also excluded. GD was diagnosed on the basis of diffuse goiter, elevated 4- or 24-hour RAIU of the thyroid gland, thyrotoxicosis, and/or positive TRAb. All medications that could interfere with thyroidal 131I uptake, such as seafood and some drugs (methimazole, propylthiouracil, compound iodine solution, probanthine, and so on), were stopped at least one week before RAIU measurements.
This study was approved by the medical ethics research committee of Tianjin Medical University General Hospital and written informed consent was obtained from each patient. We confrmed that all methods were carried out in accordance with the relevant guidelines and regulations.
Data collection and grouping
Data on gender, age, disease duration, thyroid function tests, with or without ATD, time of ATD, thyroid weight, 4 h/24 h thyroid 131I uptake, thyroid textures (soft, moderate or stiff), thyroid nodules or not, with complications or not prior to RIT were collected for all patients.
All the patients were divided into 2 groups depending on present or absent with rapid thyroidal 131I turnover (early peaking of 131I uptake), which was defined as an early (approximately 4 hour)/late (approximately 24 hour) 131I uptake ratio of ≥1.
RAIU, thyroid function tests and thyroid weight
The RAIU value was obtained at 4 and 24 hour after an oral tracer dose (about 74 kBq) of 131I through a nuclear multifunctional instrument/counter (MN-6300XT Apparatus, Technological University, China). The thyroidal 131I uptake was calculated according to the following equation: RAIU (%) = (neck counts − background counts) × 100/(standard counts − background counts). Thyroid function tests, including serum FT3 (normal range: 3.1–6.8 pmol/L) and FT4 (normal range: 12–22 pmol/L) concentrations etc, were measured by chemiluminescent immunoassays (Cobas 6000, Roche Diagnostics GmbH, Mannheim, Germany). Length, breadth, and depth of each lobe was measured respectively, the volume of each lobe was calculated using the formula for a prolate ellipsoid, and estimated thyroid weight(g) = length × breadth × depth × π/6.22
Statistical analysis
Statistical analysis was performed using SPSS (Statistical Package for Social Sciences) 12.0 for windows (SPSS, Chicago, IL, USA). Continuous and categorical variables were expressed as mean ± standard deviation (SD) (median [range]) and count (percentage), respectively. A chi square test was used to verify association or compare proportions. To compare continuous variables in the 2 groups, the Mann-Whitney U test was performed due to non-normal distributions. ROC curves were plotted to identify the best threshold for the potential predictors of rapid 131I turnover. AUC was used as an estimation of diagnostic accuracy. To identify associated factors of rapid 131I turnover, we performed multivariate logistic regression analysis with a variable entrance criterion of 0.05 or less. All P values presented were two-tailed, and values <0.05 were considered to be statistically significant.
Acknowledgements
This study was supported by China National Natural Science Foundation grant 81501510 (awarded to Renfei Wang).
Author Contributions
R.Z. and J.T. designed the study, R.Z. and R.W. wrote the main manuscript, R.Z., Q.J. and Y.Z. conducted the investigation and collected data, J.T., Z.M. and G.Z. performed the statistics and contributed to the critical revision of the manuscript. All authors reviewed the manuscript and gave the final approval for the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brent GA. Clinical practice. Graves’ disease. The New England journal of medicine. 2008;358:2594–2605. doi: 10.1056/NEJMcp0801880. [DOI] [PubMed] [Google Scholar]
- 2.Burch HB, Burman KD, Cooper DS. A 2011 survey of clinical practice patterns in the management of Graves’ disease. The Journal of clinical endocrinology and metabolism. 2012;97:4549–4558. doi: 10.1210/jc.2012-2802. [DOI] [PubMed] [Google Scholar]
- 3.Charkes ND. Retreatment of Graves’ disease with radioiodine 131I. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 1999;40:215–216. [PubMed] [Google Scholar]
- 4.Liu B, et al. Radiation Safety Precautions in (131)I Therapy of Graves’ Disease Based on Actual Biokinetic Measurements. The Journal of clinical endocrinology and metabolism. 2015;100:2934–2941. doi: 10.1210/jc.2015-1682. [DOI] [PubMed] [Google Scholar]
- 5.Hooper PL, Caplan RH. Thyroid uptake of radioactive iodine in hyperthyroidism. Jama. 1977;238:411–413. doi: 10.1001/jama.1977.03280050051020. [DOI] [PubMed] [Google Scholar]
- 6.de Jong JA, Verkooijen HM, Valk GD, Zelissen PM, de Keizer B. High failure rates after (131)I therapy in Graves hyperthyroidism patients with large thyroid volumes, high iodine uptake, and high iodine turnover. Clinical nuclear medicine. 2013;38:401–406. doi: 10.1097/RLU.0b013e3182817c78. [DOI] [PubMed] [Google Scholar]
- 7.Kita T, et al. Single dose planning for radioiodine-131 therapy of Graves’ disease. Annals of nuclear medicine. 2004;18:151–155. doi: 10.1007/BF02985106. [DOI] [PubMed] [Google Scholar]
- 8.Leslie WD, et al. A randomized comparison of radioiodine doses in Graves’ hyperthyroidism. The Journal of clinical endocrinology and metabolism. 2003;88:978–983. doi: 10.1210/jc.2002-020805. [DOI] [PubMed] [Google Scholar]
- 9.Alexander EK, Larsen PR. High dose of (131)I therapy for the treatment of hyperthyroidism caused by Graves’ disease. The Journal of clinical endocrinology and metabolism. 2002;87:1073–1077. doi: 10.1210/jcem.87.3.8333. [DOI] [PubMed] [Google Scholar]
- 10.Catargi B, et al. Optimized radioiodine therapy of Graves’ disease: analysis of the delivered dose and of other possible factors affecting outcome. European journal of endocrinology / European Federation of Endocrine Societies. 1999;141:117–121. doi: 10.1530/eje.0.1410117. [DOI] [PubMed] [Google Scholar]
- 11.Osaki Y, Sakurai K, Arihara Z, Hata M, Fukazawa H. Prediction of late (24-hour) radioactive iodine uptake using early (3-hour) uptake values in Japanese patients with Graves’ disease. Endocrine journal. 2012;59:173–177. doi: 10.1507/endocrj.EJ11-0279. [DOI] [PubMed] [Google Scholar]
- 12.Aktay R, Rezai K, Seabold JE, Bar RS, Kirchner PT. Four- to twenty-four-hour uptake ratio: an index of rapid iodine-131 turnover in hyperthyroidism. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 1996;37:1815–1819. [PubMed] [Google Scholar]
- 13.Morris LF, Waxman AD, Braunstein GD. Accuracy considerations when using early (four- or six-hour) radioactive iodine uptake to predict twenty-four-hour values for radioactive iodine dosage in the treatment of Graves’ disease. Thyroid: official journal of the American Thyroid Association. 2000;10:779–787. doi: 10.1089/thy.2000.10.779. [DOI] [PubMed] [Google Scholar]
- 14.Marcocci C, et al. A reappraisal of the role of methimazole and other factors on the efficacy and outcome of radioiodine therapy of Graves’ hyperthyroidism. Journal of endocrinological investigation. 1990;13:513–520. doi: 10.1007/BF03348615. [DOI] [PubMed] [Google Scholar]
- 15.van Isselt JW. & Broekhuizen-de Gast, H. S. The radioiodine turnover rate as a determinant of radioiodine treatment outcome in Graves’ disease. Hellenic journal of nuclear medicine. 2010;13:2–5. [PubMed] [Google Scholar]
- 16.Berg GE, Michanek AM, Holmberg EC, Fink M. Iodine-131 treatment of hyperthyroidism: significance of effective half-life measurements. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 1996;37:228–232. [PubMed] [Google Scholar]
- 17.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clinical chemistry. 1993;39:561–577. [PubMed] [Google Scholar]
- 18.Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 19.Moka D, Voth E, Schicha H. Effect of antithyroid medication on the effective half-life and uptake of 131-iodine following radioiodine therapy] Nuklearmedizin. Nuclear medicine. 1997;36:87–92. [PubMed] [Google Scholar]
- 20.Burch HB, et al. The effect of antithyroid drug pretreatment on acute changes in thyroid hormone levels after (131)I ablation for Graves’ disease. The Journal of clinical endocrinology and metabolism. 2001;86:3016–3021. doi: 10.1210/jcem.86.7.7639. [DOI] [PubMed] [Google Scholar]
- 21.Zantut-Wittmann DE, et al. High pre-therapy [99mTc]pertechnetate thyroid uptake, thyroid size and thyrostatic drugs: predictive factors of failure in [131I]iodide therapy in Graves’ disease. Nuclear medicine communications. 2005;26:957–963. doi: 10.1097/01.mnm.0000183795.59097.42. [DOI] [PubMed] [Google Scholar]
- 22.Perry RJ, Hollman AS, Wood AM, Donaldson MD. Ultrasound of the thyroid gland in the newborn: normative data. Archives of disease in childhood. Fetal and neonatal edition. 2002;87:F209–211. doi: 10.1136/fn.87.3.F209. [DOI] [PMC free article] [PubMed] [Google Scholar]


