Abstract
This meta-analysis was performed to determine the optimal use of anti-EGFR mAb in the treatment of metastasized colorectal cancer (mCRC). Seventeen randomized clinical trials were included, all evaluating the added value of anti-EGFR mAb to standard treatment line in patients with KRAS wild-type mCRC. Hazard and odds ratios were pooled using a random effect model, weighted according to cohort size. Pooled data of six first- and two second-line studies demonstrated a significantly improved ORR (OR 1.62, CI 1.27–2.04; OR 4.78, CI 3.39–6.75, respectively) and PFS (HR 0.79, CI 0.67–0.94; HR 0.80, CI 0.71–0.91, respectively) with the addition of anti-EGFR mAb to chemotherapy, while OS remained similar. Two third-line anti-EGFR mAb monotherapy studies revealed an improved PFS and OS (HR 0.44, CI 0.35–0.52; HR 0.55, CI 0.41–0.74). Addition of anti-EGFR versus anti-VEGF mAb to first-line chemotherapy was evaluated in three studies; ORR and PFS were comparable, while OS was improved (HR 0.8, CI 0.65–0.97). The influence of the chemotherapy backbone on anti-EGFR mAb efficacy, evaluated with meta-regression, indicated a higher ORR with irinotecan-based versus oxaliplatin-based regimens, but comparable PFS and OS. Reported toxicity (≥3 grade) increased ~20% in all treatment lines with the addition of anti-EGFR mAb. Anti-EGFR treatment significantly improves response and survival outcome of patients with (K)RAS wild-type mCRC, regardless of treatment line or chemotherapeutic backbone. Saving anti-EGFR mAb as third-line monotherapy is a valid and effective option to prevent high treatment burden caused by combination therapy. Combination treatment with anti-EGFR mAb to achieve radical resection of metastases needs further investigation.
Electronic supplementary material
The online version of this article (doi:10.1007/s10555-017-9668-y) contains supplementary material, which is available to authorized users.
Keywords: Colorectal cancer, Anti-EGFR monoclonal antibodies, Meta-analysis, Treatment response, Progression-free survival, Overall survival
Introduction
Worldwide colorectal cancer is the second most common cancer in women and the third in men [1]. Irresectable, non-curable colorectal cancer can be treated with palliative chemotherapy to reduce cancer symptoms, improve quality of life, and overall survival. In 1963, Heidelberger et al. discovered 5-fluorouracil (5-FU) as the first systemic chemotherapy for colorectal cancer. To date, this is the most effective and widely used systemic treatment for colorectal adenocarcinoma. In the course of time, combinations of fluoropyrimidines, with oxaliplatin and irinotecan, as well as the use of anti-EGFR and anti-VEGF targeted agents have improved survival of patients with metastasized colorectal cancer (mCRC) to about 2.5 years [2].
Cetuximab, a chimeric human and mouse monoclonal antibody (mAb), and panitumumab, a fully human mAb, both bind the epidermal growth factor receptor (EGFR). This prevents activation of the intracellular EGFR tyrosine kinase, resulting in an inhibition of the associated downstream signaling pathways, such as the RAS-RAF-MAPK and the PI3K-PTEN-AKT axis [3]. Additionally, antibody-dependent cell-mediated cytotoxicity (ADCC) may play a role in the efficacy of anti-EGFR mAb.
In 2008, a retrospective analysis revealed that the presence of mutations in Kirsten rat sarcoma viral oncogene homolog (KRAS) exon 2 has a negative predictive value for benefit from anti-EGFR therapy [4, 5]. Recently, the same was demonstrated for mutations in KRAS exons 3 and 4, and for the rare mutations in neuroblastoma rat sarcoma (NRAS) viral oncogene homolog exon 2–4 [6]. Despite patient selection based on wild-type (WT) RAS status, approximately 30% will not have clinical benefit from anti-EGFR mAb treatment [7]. Therefore, additional predictive biomarkers are needed.
In multiple clinical trials, the efficacy of anti-EGFR mAb has been evaluated as monotherapy or combined with different types of chemotherapy in patients with mCRC. Yet, the optimal sequence and combination for the use of anti-EGFR therapy remains unclear. With this meta-analysis, we aim to get more insight in the optimal clinical strategy for the use of anti-EGFR therapy. All included randomized controlled clinical trials in a KRAS WT mCRC population compared the additional benefit of anti-EGFR mAb therapy to first- or second-line chemotherapy treatment or to best supportive care in third-line treatment. We pooled efficacy data to objectify and compare overall response rate (ORR), progression-free survival (PFS), and overall survival (OS) for each treatment line. With meta-regression, the influence of the chemotherapeutic backbone and type of anti-EGFR mAb were analyzed. Furthermore, we evaluated whether the addition of anti-EGFR mAb is superior to anti-VEGF mAb in first-line treatment.
Methods
Search
A review protocol was developed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (www.prisma-statement.org). PubMed, Embase.com, and Wiley/Cochrane Library were searched from inception (by EvH and JCFK) up to 17 February 2016. Hereafter, the search was repeated weekly to evaluate new potential records. The following terms were used (including synonyms and closely related words) as index terms or free-text words: “colorectal neoplasms” and “cetuximab” and “RCT” and “survival.” Studies were selected using predefined inclusion criteria: randomized controlled trial, evaluation of efficacy (OS, PFS, and ORR) of anti-EGFR monoclonal antibodies, and KRAS WT (at least exon 2) population. Studies were screened and selected by two independent reviewers (EvH and RvO) using Reference Manager (version 12.0.3 Thomson Reuters). Risk for potential bias was assessed using the Cochrane collaboration’s tool (EvH and RvO). The full search strategies for all the databases and all used inclusion and exclusion criteria to screen for relevant articles can be found in the Supplementary Information 1. All languages were accepted. Duplicate articles were excluded.
Statistics
Hazard ratios (HRs) with standard errors (SEs) or confidence intervals (CIs) for PFS and OS were extracted from included studies. For ORR, odds ratios (ORs) with SE and CI were extracted. If the ORs were not stated in the publication, it was calculated from the percentage ORR and sample size if possible. SPSS (version 22, IBM Corp., Armonk, NY) was used for data entry; statistical analysis of the data was done in STATA version 12. A meta-analysis with a random effect model was used to generate a pooled summary effect size. All studies were weighted according to the number of included patients.
Heterogeneity between studies was visually evaluated using forest plots (non-overlapping confidence intervals indicate potential heterogeneity). To clarify potential heterogeneity between studies, meta-regression was used to test for different variables, such as chemotherapeutic backbone (5-FU, capecitabine, oxaliplatin, or irinotecan), type of anti-EGFR mAb (cetuximab or panitumumab), and summed points of the Cochrane collaboration’s tool. Differences with a p value <0.05 were considered relevant.
Results
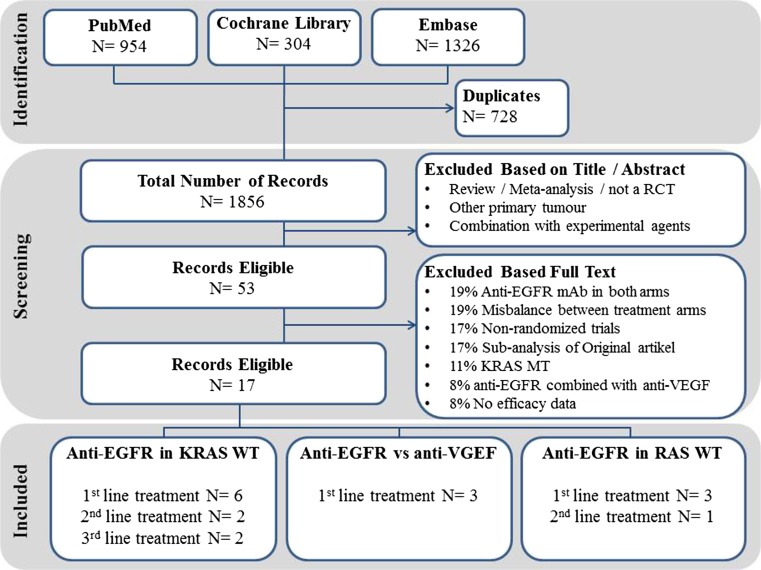
With our literature search, 1856 records were obtained; 1803 records did not meet the inclusion criteria based on title and abstract (Fig. 1). Of the remaining 53 records, 37 records were excluded based on full text review. Reasons for exclusion were anti-EGFR mAb in both arms (19%), a misbalance between treatment arms (19%), non-randomized trials (17%), sub-analysis of an original article (17%), KRAS-mutated population (11%), the combination of anti-EGFR mAb with anti-VEGF mAb (8%), and no reported efficacy data (8%) (Fig. 1). The 17 included publications are summarized in Tables 1 and 2. Pooled analyses were done for six first-line studies (n = 2580 patients), two second-line studies (n = 1057), and two third-line studies (n = 444). Of these trials, four studies published RAS WT data (n = 1464 patients), which were pooled. Additionally, three first-line studies that compared anti-EGFR mAb with anti-VEGF mAb were pooled (n = 2014 patients). Risk of bias was assessed using the Cochrane collaboration’s for randomized trials; all studies had a fairly low risk for bias (Supplementary Fig. 1).
Fig. 1.
ᅟIdentification, screening, and included records
Table 1.
ᅟSummary of included publications
| A. The addition of an anti-EGFR mAb to the first-line treatment of mCRC | |||||
| Study (author) | Combination (no. pt) | Control (no. pt) | Response rate % (OR, CI, p) | Median PFS (months) (HR, CI, p) | Median OS (months) (HR, CI, p) |
| OPUS (Bokemeyer) | Cetux + FOLFOX4 (82) | FOLFOX4 (97) | 57 versus 34 (2.6, 1.4–4.7, 0.003) | 8.3 versus 7.2 (0.57, 0.4–0.9, 0.006) | 22.8 versus 18.5 (0.894, 0.6–1.2, 0.56) |
| CRYSTAL (v Cutsem) | Cetux + FOLFIRI (316) | FOLFIRI (350) | 57 versus 40 (2.1, 1.5–2.8, <0.001) | 9.9 versus 8.4 (0.7, 0.6–0.9, 0.001) | 23.5 versus 20.0 (0.8, 0. 7–1.0, 0.009) |
| NORDIC-VII (Tveit) | Cetux + Nordic FLOX (97) | Nordic FLOX (97) | – | 7.9 versus 8.7 (1.07, 0.8–1.5, 0.66) | 20.1 versus 22.0 (1.14, 0.8–1.61, 0.48) |
| – (Ye) | Cetux + FOLFIRI or mFOLFOX6 (70) | FOLFIRI or mFOLFOX6 (86) | 57 versus 29 (2.1, 1.1–4.1, 0.02) | 10.2 versus 5.8 (0.6, 0.4–0.9, 0.004) | 30.9 versus 21.0 (0.54, 0.3–0.9, 0.013) |
| MRC COIN (Maughan) | Cetux + FOLFOX/CAPOX (362) | FOLFOX/CAPOX (367) | 64 versus 57 (1.4, 1.0–1.8, 0.049) | 8.6 versus 8.6 (0.96, 0.8–1.1, 0.6) | 17.0 versus 17.9 (0.96, 0.8–1.2, 0.67) |
| PRIME (Douillard) | Pani + FOLFOX4 (325) | FOLFOX4 (331) | 57 versus 48 (1.5, 1.1–2.0, 0.02) | 10.0 versus 8.6 (0.8, 0.6–1.0, 0.01) | 23.9 versus 19.7 (0.88, 0.7–1.1, 0.17) |
| B. The addition of an anti-EGFR mAb versus an anti-VEGF mAb to the first-line treatment of mCRC | |||||
| CALGB/SWOG 80405 (Vernook) | Cetux + FOLFOX or FOLFIRI (578) | Beva + (FOLFOX or FOLFIRI) (559) | – | 10.4 versus 10.8 (1.04, 0.9–1.2, 0.55) | 29.9 versus 29.0 (0.92, 0.78–1.09, 0.34) |
| FIRE-3 (Heinemann) | Cetux + FOLFIRI (297) | Beva + FOLFIRI (295) | 62 versus 58 (1.2, 0.9–1.6, 0.18) | 10.0 versus 10.3 (1.06, 0.9–1.3, 0.55) | 28.7 versus 25.0 (0.77, 0.62–0.96, 0.017) |
| PEAK (Schwartsberg) | Pani + mFOLFOX6 (142) | Beva + mFOLFOX6 (143) | 58 versus 54 (1.1, 0.7–1.8, 0.59) | 10.9 versus 10.1 (0.87, 0.7–1.2, 0.35) | 34.2 versus 24.3 (0.62, 0.44–0.89, 0.009) |
| C. The addition of an anti-EGFR mAb to the second-line treatment of mCRC | |||||
| 20,050,181 (Peeters) | Pani + FOLFIRI (303) | FOLFIRI (294) | 36 versus 10 (5.5, 3.3–8.9, <0.001) | 6.7 versus 4.9 (0.82, 0.7–1.0, 0.02) | 14.5 versus 12.5 (0.92, 0.8–1.1, 0.37) |
| PICCOLO (Seymour) | Pani + irinotecan (230) | Irinotecan (230) | 34 versus 12 (4.1, 2.5–6.8, <0.001) | 5.5 versus 4.7 (0.78, 0.6–1.0, 0.02) | 10.4 versus 10.9 (1.01, 0.83–1.23, 0.91) |
| D. The addition of an anti-EGFR mAb to the third-line treatment of mCRC | |||||
| 20,020,408 (Amado) | Pani + BSC (115) | BSC (114) | 17 versus 0 | 3.1 versus 1.8 (0.45, 0.3–0.6, <0.001) | 8.1 versus 7.6 (0.99, 0.8–1.3)a |
| CO.17 (Karapetis) | Cetux +BSC (110) | BSC (105) | 13 versus 0 | 3.7 versus 1.9 (0.4, 0.3–0.5, <0.001) | 9.5 versus 4.8 (0.55, 0.4–0.7, <0.001) |
mAb monoclonal antibodies, mCRC metastatic colorectal cancer, Cetux cetuximab, Pani panitumumab, Beva bevacizumab, BSC best supportive care, OR odds ratio, CI confidence interval, HR hazard ratio, OS overall survival, PFS progression-free survival
aCrossover design
Table 2.
Summery of included RAS WT publications
| Study (author, date) | Treatment line | Combination (no. pt) | Control (no. pt) | Response rate % (OR, CI, p) | Median PFS (months) (HR, CI, p) | Median OS (months) (HR, CI, p) |
|---|---|---|---|---|---|---|
| OPUS (Bokemeyer, May 2014) | First | Cetux + FOLFOX4 [8] | FOLFOX4 (49) | 58 versus 29 (3.3, 1.4–8.2, 0.008) | 12.0 versus 5.8 (0.53, 0.3–1.0, 0.06) | 19.8 versus 17.8 (0.94, 0.6–1.6, 0.8) |
| PRIME (Douillard, September 2013) | First | Pani + FOLFOX4 (259) | FOLFOX4 (253) | – | 10.1 versus 7.9 (0.72, 0.58–0.90, 0.004) | 25.8 versus 20.2 (0.77, 0.64–0.94, 0.009) |
| CRYSTAL (v Cutsem, January 2015) | First | Cetux + FOLFIRI (178) | FOLFIRI (189) | 61 versus 38 (2.64, 1.78–3.92, 0.001) | 11.3 versus 7.1 (0.58, 0.44–0.77, 0.001) | 26.1 versus 20.2 (0.75, 0.60–0.93, 0.008) |
| 20,050,181 (Peeters, December 2015) | Second | Pani + FOLFIRI (204) | FOLFIRI (294) | – | 6.4 versus 4.4 (0.70, 0.54–0.90, 0.006) | 16.2 versus 13.9 (0.80, 0.63–1.0, 0.077) |
mCRC metastatic colorectal cancer, Cetux cetuximab, Pani panitumumab, BSC best supportive care, OR odds ratio, CI confidence interval, HR hazard ratio, OS overall survival, PFS progression-free survival
First-line treatment
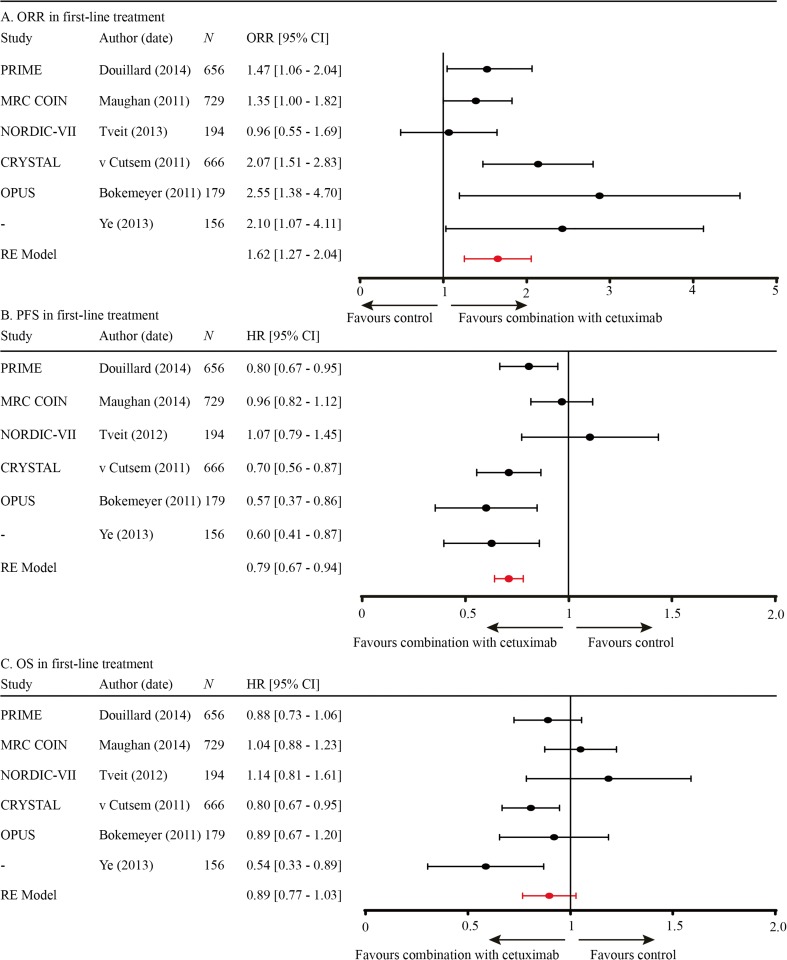
Of all publications included in this meta-analysis, six evaluated chemotherapy with and without anti-EGFR mAb in the first line [9–14]. Pooled data revealed that ORR and PFS significantly improved by the addition of anti-EGFR mAb treatment (OR 1.62, CI 1.27–2.07; HR PFS 0.79, CI 0.67–0.94; Fig. 2a, b, respectively), while OS did not improve (HR OS 0.89, CI 0.77–1.03; Fig. 2c).
Fig. 2.
a ORR in first-line treatment. b PFS in first-line treatment. c OS in first-line treatment
Using meta-regression, the effect of the chemotherapeutic backbone on efficacy data was evaluated in all six first-line studies. In two out of six studies, the chemotherapeutic backbone was irinotecan-based; in the remaining four studies, it was oxaliplatin-based. Although there was a beneficiary trend for the irinotecan-based combination, PFS and OS were not significantly different between these groups (p = 0.09 and p = 0.06, respectively). The ORR was significantly higher for the studies combining anti-EGFR mAb with irinotecan versus oxaliplatin (OR 2.07 versus 1.42; p = 0.04). Besides a subgroup in the MRC COIN study, none of the included studies used capecitabine as fluoropyrimidine backbone.
From three of the six first-line studies, retrospective analyses of RAS WT data were published [15–17]. Pooled analyses indicated that ORR, PFS, and OS were significantly improved with the addition of anti-EGFR mAb (OR 2.74, CI 1.91–3.94; HR 0.65, CI 0.55–0.77; HR 0.77, CI 0.67–0.89, respectively).
Second-line treatment
In two studies, second-line chemotherapy with or without anti-EGFR mAb was compared [18, 19]. Comparable to first-line studies, ORR and PFS were significantly improved in the arms that included anti-EGFR mAb (OR 4.78, CI 3.39–6.75; HR 0.80, CI 0.71–0.91). OS remained unaffected (HR 0.96, CI 0.84–1.10). In the 20,050,181 study, 45.5% of the patients in the FOLFIRI alone arm received anti-EGFR mAb therapy after progression; this could reduce the observed benefit in OS in the combination arm [19]. In the PICCOLO study, only 6% of the control group received subsequent anti-EGFR mAb therapy and data concerning other subsequent therapies were not collected [18].
Anti-EGFR mAb monotherapy
Two monotherapy studies compared anti-EGFR mAb monotherapy versus best supportive care in chemotherapy-refractory patients with mCRC. ORR was not evaluable using ORs, as none of the patients in the best supportive care (BSC) arm had a response. The 20,020,408 [4] and the CO.17 study [5] reported an ORR of 17 and 13%, respectively, in patients treated with anti-EGFR monotherapy. Pooled data demonstrated a significantly longer PFS in the arm with anti-EGFR therapy (HR 0.44, CI 0.35–0.52). The 20,020,408 study had a crossover design; therefore, OS is not comparable. Karapetis et al. reported that overall survival doubled (9.5 versus 4.8 months) with the addition of anti-EGFR mAb to best supportive care (HR 0.55, CI 0.41–0.74) [5].
Wild-type RAS
Of all included studies in which the added benefit of anti-EGFR to chemotherapy was evaluated, one second-line and three first-line studies retrospectively assessed the effect in a RAS WT group (n = 1464 patients), excluding patients whose tumor harbored additional mutations in KRAS exon 3–4 and NRAS exon 2–4 [15–17, 20]. All efficacy data of the combination arm improved compared to the KRAS exon 2 WT group (ORR OR 2.74 and PFS HR 0.67). In the RAS WT group, overall survival was significantly improved with the addition of an anti-EGFR mAb, with a HR of 0.78 (CI 0.69–0.88) (Table 2).
Chemotherapeutic backbone
In six first-line and two second-line studies, chemotherapy with or without anti-EGFR mAb in patients with KRAS WT mCRC were evaluated. Pooled efficacy data of these eight studies demonstrated an improved ORR (OR 2.14, CI 1.47–3.12) and PFS (HR 0.8, CI 0.71–0.9). There was no benefit observed for OS (HR 0.92, CI 0.83–1.03).
Meta-regression of first- and second-line studies demonstrated that for the irinotecan-based group, the addition of anti-EGFR mAb rendered a significantly higher ORR with an OR of 3.41 versus 1.45 in the oxaliplatin-based group (p = 0.002). PFS and OS did not significantly differ between the irinotecan- or oxaliplatin-based groups with the addition of anti-EGFR mAb (p = 0.10 and p = 0.51, respectively).
Anti-EGFR versus anti-VEGF mAb in first-line treatment
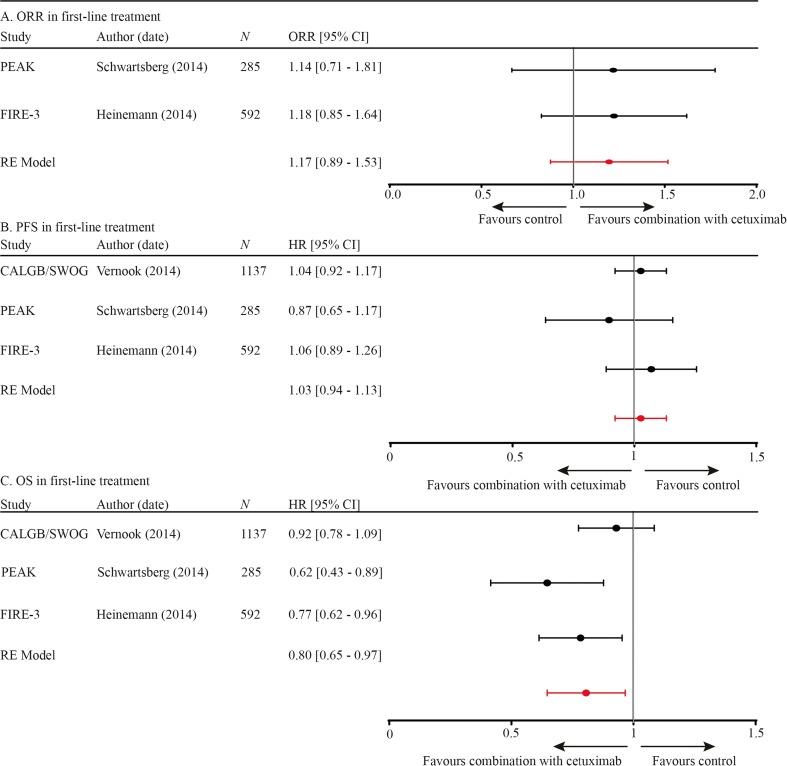
Three randomized controlled trials evaluated the addition of anti-EGFR mAb or anti-VEGF mAb to first-line palliative chemotherapy [2, 21, 22]. The PEAK and the FIRE-3 studies revealed similar overall response rates of about 50–60% in both arms. Pooled ORR data were also equal between the two arms (OR 1.17, CI 0.89–1.53). Furthermore, PFS was similar for both arms in all three studies; pooled data demonstrated the same results (HR 1.03, CI 0.94–1.13; Fig. 3). OS was significantly improved for the anti-EGFR mAb arm in the PEAK and FIRE-3 studies with a HR of 0.62 and 0.77, respectively. In the large CALGB/SWOG 80405 study, there was a beneficiary trend towards the anti-EGFR mAb arm, but the difference in OS was not significant (HR 0.92, CI 0.78–1.09). Pooled data revealed an overall survival benefit with a HR of 0.80 (CI 0.65–0.97; Fig. 3). Based on the forest plots, no obvious heterogeneity was observed between the three studies; therefore, meta-regression was not done.
Fig. 3.
a ORR in first-line treatment. b PFS in first-line treatment. c OS in first-line treatment
Toxicity
Another consideration for the addition of an anti-EGFR mAb to chemotherapy is its potential additive toxicity. In Table 3, the percentage of grade ≥3 adverse events are listed for all included studies. As expected, anti-EGFR mAb-specific adverse events, such as (acneiform) rash, diarrhea, hypomagnesaemia, and infusion-related reaction, occurred more often in the combination groups. Adverse events such as anemia, thrombocytopenia, leucopoenia, neutropenia, fatigue, and palmar-plantar erythrodysesthesia were comparable between the two arms, probably since these adverse events were more likely to be caused by the chemotherapeutic backbone. In first-line treatment, any reported grade ≥3 adverse events occurred in 82% of all included patients in the combination arm versus 62% in the control arm. For the second line, these percentages were 67 versus 46%, and in third line, it was 58 versus 40% for best supportive care. Thus, the addition of anti-EGFR mAb in all treatment lines resulted in an absolute increase of grade ≥3 adverse events of approximately 20%.
Table 3.
Percentage of grade ≥3 adverse events listed for all included studies
| (Acneiform) rash | Diarrhea | Anemia | Thrombocytopenia | Leucopoenia | Neutropenia | Hypomagnesaemia | Fatigue (lethargy) | (Peripheral) neuropathy | Palmar-plantar erythrodysesthesia | Infusion-related reactions | Any | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First-line treatment | OPUS | Combination | 18 | 9 | 4 | 3 | 7 | 35 | – | 1 | 14 | 4 | 2 | 82 |
| Control | 0 | 5 | 2 | 0 | 5 | 32 | – | 3 | 7 | 1 | 1 | 64 | ||
| CRYSTAL | Combination | 21 | 16 | – | – | 25 | 31 | – | 14 | – | – | 5 | 81 | |
| Control | <1 | 10 | – | – | 17 | 24 | – | 20 | – | – | 0 | 60 | ||
| NORDIC-VII | Combination | 22 | 17 | 1 | 4 | 14 | 46 | – | 16 | 23 | – | 7 | – | |
| Control | 1 | 10 | 1 | 3 | 21 | 47 | – | 10 | 32 | – | 3 | – | ||
| Ye et al. | Combination | 13 | 6 | – | – | 11 | – | – | 4 | – | 3 | – | ||
| Control | 3 | 4 | – | – | 9 | – | – | 6 | – | 2 | – | |||
| MRC COIN | Combination | 20 | 24 | 5 | 3 | 4 | 13 | 4 | 26 | 14 | 10 | – | – | |
| Control | 1 | 14 | 2 | 3 | 4 | 12 | 0 | 18 | 18 | 4 | – | – | ||
| PRIME | Combination | 37 | 18 | – | – | – | 43 | 7 | 10 | – | – | <1 | – | |
| Control | 2 | 9 | – | – | – | 42 | <1 | 3 | – | – | 0 | – | ||
| Mean toxicity first line | Combination | 22 | 15 | 3 | 3 | 12 | 34 | 6 | 13 | 14 | 7 | 4 | 82 | |
| Control | 1 | 9 | 2 | 2 | 11 | 31 | 0 | 11 | 16 | 3 | 1 | 62 | ||
| Second line | 20,050,181 | Combination | 37 | 14 | – | – | – | 20 | 3 | – | – | – | <1 | 73 |
| Control | 2 | 9 | – | – | – | 23 | 1 | – | – | – | 0 | 52 | ||
| PICCOLO | Combination | 19 | 30 | 4 | 2 | – | 22 | – | 21 | – | – | – | 61 | |
| Control | 0 | 19 | 1 | 0 | – | 12 | – | 11 | – | – | – | 39 | ||
| Mean toxicity second line | Combination | 28 | 22 | 4 | 2 | – | 21 | 3 | 21 | – | – | <1 | 67 | |
| Control | 1 | 14 | 1 | 0 | – | 18 | 1 | 11 | – | – | 0 | 46 | ||
| Third line | 20020408a | Combination | 8 | 1 | – | – | – | – | – | 4 | – | – | – | 37 |
| Control | 0 | 0 | – | – | – | – | – | 3 | – | – | – | 20 | ||
| CO.17b | Combination | 34 | – | – | – | – | – | 15 | 33 | – | – | 13 | 79 | |
| Control | 1 | – | – | – | – | – | 0 | 26 | – | – | 0 | 59 | ||
| Mean toxicity third line | Combination | 21 | 1 | – | – | – | – | 15 | 19 | – | – | 13 | 58 | |
| Control | 1 | 0 | – | – | – | – | 0 | 15 | – | – | 0 | 40 | ||
| Anti-VEGF versus anti-EGFR agent | C/S 80405 | Anti-EGFR | – | – | – | – | – | – | – | – | – | – | – | – |
| Anti-VEGF | – | – | – | – | – | – | – | – | – | – | – | – | ||
| FIRE-3 | Anti-EGFR | 26 | 11 | <1 | – | <1 | <1 | 4 | <1 | 0 | 3 | 4 | – | |
| Anti-VEGF | 2 | 14 | 0 | – | 0 | 0 | 1 | 1 | <1 | <1 | 0 | – | ||
| PEAK | Anti-EGFR | 32 | – | – | 1 | – | – | 7 | 11 | – | – | – | 63 | |
| Anti-VEGF | 1 | – | – | 0 | – | – | 0 | 9 | – | – | – | 56 | ||
aBased on original KRAS unselected group v Cutsem et al. JCO 2007
bBased on original KRAS unselected group Jonker et al. NEJM 15 2007
Proposed criteria to evaluate optimal use of anti-EGFR mAb
Differences in (progression-free) survival between treatment lines
The addition of anti-EGFR mAb in first- or second-line treatment renders the same beneficiary effect (first-line HR 0.79 versus second-line HR 0.80). The HR of PFS in the third line is not comparable to first or second line as it is compared to BSC.
OS in first and second line for the KRAS WT population was similar between the combination arm versus the control arm. Yet, in the RAS WT group, a significant improvement was seen in first-line treatment (HR of 0.77, CI 0.67–0.89). Only one second-line study, 20,050,181, reported survival in RAS WT data, with a non-significantly different survival between the two arms (median OS combination 16.2 versus 13.9 months, HR of 0.80, p = 0.08) [20]. OS in the third line was only evaluable in the CO.17, which revealed an improved OS with a HR of 0.55 (p < 0.001).
Differences in efficacy data due to the chemotherapeutic backbones
Between the included first- and second-line studies, ORR, PFS, and OS for combinations with irinotecan versus oxaliplatin were compared using meta-regression. ORR was significantly different, with an OR of 3.41 in the irinotecan combinations versus an OR of 1.45 in the oxaliplatin combinations (p = 0.0016). However, this benefit for irinotecan combinations was not reflected by PFS and OS gain (p = 0.10 and p = 0.51, respectively).
Differences in toxicity between treatment lines
In all treatment lines, there was an added absolute incidence of grade ≥3 adverse events of approximately 20% with the addition of anti-EGFR mAb. The total incidence of any grade ≥3 adverse events was 82% in the first-line combination therapy group, while this was 58% in third-line setting.
Discussion
The aim of this meta-analysis was to define the optimal use of anti-EGFR mAb in the non-curative treatment of mCRC. To determine its optimal use, we evaluated benefit (e.g., ORR, PFS, and OS) and toxicity that resulted from anti-EGFR mAb addition to standard chemotherapy or as monotherapy. Additionally, we assessed the influence of the treatment line as well as the chemotherapeutic backbone on efficacy of anti-EGFR mAb.
To determine optimal treatment for patients with non-curative mCRC, OS is the most important clinical outcome measure. However, benefit in OS is difficult to objectify due to the influence of subsequently applied systemic or, in some cases, local treatment strategies. Additionally, subsequent treatment data are often incompletely collected and reported. In the first- and second-line studies, there was no clear benefit in OS for the KRAS WT cohorts receiving anti-EGFR mAb therapy (HR 0.89, p = 0.13; HR 0.96, p = 0.54, respectively), but retrospective analyses in the RAS WT population indicated that the addition of anti-EGFR mAb to first-line chemotherapy significantly improved OS (HR 0.77, CI 0.67–0.89). In the third line, only the CO.17 trial provided a correct representation of benefit in OS with a HR of 0.55 (p < 0.001) compared to BSC. Benefit in PFS due to the addition of anti-EGFR mAb was comparable in first and second line (HR 0.79 and 0.80, respectively). In third line, the HR for PFS was 0.43. Yet, this greater effect of anti-EGFR mAb treatment is partly caused by the fact that it is compared to BSC, as the added median time to PFS with the addition of anti-EGFR mAb was comparable between all treatment lines. The last outcome measure, ORR, is often not paramount in a non-curative setting. An exception is the intent to convert unresectable to resectable disease, frequently a point of discussion for patients with unresectable liver-limited CRC metastases. In literature, the addition of anti-EGFR mAb to neo-adjuvant treatment has given contradictory results [23, 24]. Currently, a prospective multicenter RCT is including patients with liver-limited CRC metastases to further investigate the role of anti-EGFR mAb to convert irresectable to resectable disease [25]. In our study, pooled efficacy data revealed a significantly higher ORR in all treatment lines with the addition of an anti-EGFR mAb. However, based on the evaluated data, no obvious differences in added gain of OS, PFS, and ORR were demonstrated with the addition of anti-EGFR mAb in first-, second-, and third-line treatment to guide its optimal clinical use.
It has been suggested that the addition of an anti-EGFR mAb to irinotecan-based regimen has a synergetic effect, opposed to oxaliplatin-based regimens [26, 27]. With meta-regression, we have shown that anti-EGFR mAb rendered a significantly better ORR in the irinotecan-based regimen compared to the oxaliplatin-based regimen (p = 0.04). However, this superior effect was not confirmed for PFS and OS (p = 0.09 and p = 0.06, respectively). These results are in concordance with multiple randomized trials, which demonstrated no differences in treatment benefit for irinotecan and anti-EGFR mAb compared to oxaliplatin and anti-EGFR [8, 28–30].
The addition of either anti-EGFR mAb or anti-VEGF mAb to first-line non-curative chemotherapy is a much-debated current clinical issue. Pooled data of all available first-line studies, which compared the addition of both antibodies, demonstrated a comparable ORR and PFS, while OS was significantly longer in the anti-EGFR arm (HR of 0.8, CI 0.65–0.97). The benefit in OS was unexpected, as it is not in line with the other outcome measurements such as ORR and PFS. The significant benefit in OS was observed in two studies, the FIRE-3 [22] and the PEAK [21]. In the FIRE-3, ORR (the primary endpoint) as well as the PFS were similar in the cetuximab arm compared to the bevacizumab arm (62 versus 58% and 10.0 versus 10.3 months), while OS was 28.7 versus 25.0 months (p = 0.017, respectively) [22]. Although a recent post hoc analysis on centrally reviewed CT images reported a significant improvement with the addition of anti-EGFR mAb (72 versus 56%; p = 0.003), PFS remained similar between the anti-EGFR and the anti-VEGF mAb arms (8.4 versus 9.7 months; p = 0.53) [31]. The observed discrepancy between PFS and OS is most likely caused by small imbalances in subsequent treatments, such as more use of oxaliplatin and fluoropyrimidine in the anti-EGFR mAb arm [32]. Additionally, only 52% of the patients in the anti-VEGF mAb arm received anti-EGFR mAb treatment, compared to all patients in the anti-EGFR arm [32]. In fact, the PFS for the second-line therapy in the anti-EGFR mAb arm was significantly longer (HR 0.68, p < 0.001) [32], indicating that indeed the differences in OS were not caused by first-line treatment but by differences in later treatment. Perhaps, data of the patient distribution of left versus right sidedness in both arms might provide more insight in this discrepancy between ORR, PFS, and OS. For the smaller PEAK trial, no data concerning the subsequent treatment were published [21]. In the largest trial, which evaluated anti-EGFR mAb versus anti-VEGF mAb as addition to first-line treatment, the CALGB/SWOG 80405 trial, 1137 patients were treated with FOLFIRI and cetuximab or bevacizumab. No significant differences in PFS or OS were found between the two arms. Unfortunately, no full paper of this study has been published yet, precluding detailed analysis. In the second line, the SPIRITT trial is the only study that compared anti-EGFR with anti-VEGF mAb in addition to combination chemotherapy (FOLFIRI), after progression on first-line, oxaliplatin-based treatment with bevacizumab. As there are no other studies comparing these two agents in second line, we could not pool data. We did not pool this study with the three first-line studies, because of the difference in treatment setting. In the SPIRITT phase 2 trial, PFS and OS were similar for the two regimens [33]. It is remarkable that no difference was observed, because patients received bevacizumab in first line, probably not all patients were truly resistant to the first-line treatment regimen. Oxaliplatin toxicity may have influenced the switch to second-line treatment. Nevertheless, these data suggest that the addition of either mAb to this treatment setting improves clinical outcome.
It might be argued that first-line treatment is the most important treatment line, and responsible for the main gain in OS. This is the key rationale to add anti-EGFR mAb to first-line treatment. However, in the last decade, OS improved with 10 months, whereas PFS of the first-line treatment remained the same [34], indicating that the gain in OS most likely results from improved care for patients by using the total arsenal of available systemic treatments. In addition, multiple studies showed that survival benefit of combination therapy is comparable to sequential therapy, whereas combination therapy is significantly more toxic [35–40]. Indeed, in our meta-analysis, the occurrence of any ≥3 grade adverse events in first-line combination treatment was 82% compared to 64% with standard chemotherapy. Based on these data, using anti-EGFR mAb as third-line monotherapy is a sensible and tolerable treatment option after progression on standard (combination) chemotherapy.
In order to improve efficacy of anti-EGFR treatment, predictive biomarkers are urgently needed. An obvious biomarker, EGFR expression on tumor tissue, did not correlate with treatment benefit [8, 27–29]. A well-established biomarker that predicts primary resistance to anti-EGFR mAb are RAS mutations (KRAS and NRAS exon 2–4). This meta-analysis only included studies excluding patients with KRAS exon 2 mutated tumors, as these were the first and most common mutations known to induce resistance. Thus, our results are most likely an underestimation of the efficacy of anti-EGFR mAb treatment for the RAS wild-type cohort. Retrospective analyses of these additional RAS mutations were performed in four of the included studies [36]. Additional RAS mutations were confirmed in 14–26% of patients, resulting in improved efficacy data upon exclusion of these patients. A potential explanation for primary resistant patients with RAS wild-type mCRC is intralesional and interlesional heterogeneity in RAS mutation [41], making the RAS mutation determination on a single needle biopsy or old resection material prone for sampling errors. A promising novel approach is to evaluate these mutations in circulating cell-free DNA [42] or circulating tumor cells [43].
Recently, right-sided location of the primary tumor has been reported to negatively influence treatment benefit of anti-EGFR mAb [34, 44]. In the meta-analysis of Arnold et al., single patient data of six trials, which were also included in this meta-analysis, were pooled to evaluate the prognostic and predictive value of sidedness [45]. Indeed, they demonstrated that the addition of anti-EGFR mAb in patients with left-sided tumors significantly improved OS (HR = 0.75, CI 0.67–0.84) and PFS (HR = 0.78, CI 0.70–0.87), in contrast to patients with right-sided tumors (HR = 1.12, CI 0.87–1.45; HR = 1.12, CI 0.87–1.44, for OS and PFS, respectively). Further research is needed to evaluate the clinical utility of this biomarker and understand the underlying mechanisms of resistance.
4. Conclusion
Based on our meta-analysis, we conclude that the anti-EGFR treatment significantly improves response and survival outcome of patients with (K)RAS wild-type mCRC, regardless of treatment line or chemotherapeutic backbone. It is a sensible treatment strategy to save anti-EGFR mAb as third-line monotherapy for patients with mCRC in a true non-curative setting, as combination therapy is more toxic and has no clinically significant benefit compared to sequential therapy. For patients with limited disease, first-line combination therapy with anti-EGFR mAb can be considered, if local radical treatment may still be an option upon downstaging. As sound data to support this last consideration are lacking, further research is necessary.
Electronic supplementary material
(DOCX 14 kb)
(DOCX 18 kb)
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Disclosures
HV is member of the advisory board of Erbitux (Merck). HV also received honoraria from Boehringer Ingelheim and Roche for his consultancy/advisory work. HV received research funding (outside this work) from Amgen, Vitromics Healthcare, Immunovo BV, Roche, Novartis. The other authors declare no disclosures.
Funding
This work was supported by KWF-Alpe d’Huez (2012-5565). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s10555-017-9668-y) contains supplementary material, which is available to authorized users.
References
- 1.Cosmai L, Gallieni M, Porta C. Renal toxicity of anticancer agents targeting HER2 and EGFR. Journal of Nephrology. 2015;28(6):647–657. doi: 10.1007/s40620-015-0226-9. [DOI] [PubMed] [Google Scholar]
- 2.Venook AP, Niedzwiecki D, Lenz H-J, Innocenti F, Mahoney MR, O'Neil BH, et al. (2014). CALGB/SWOG 80405: phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (MFOLFOX6) with bevacizumab (bv) or cetuximab (CET) for patients (PTS) with kras wild-type (WT) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). Journal of Clinical Oncology. 32(18).
- 3.Vincenzi B, Schiavon G, Silletta M, Santini D, Tonini G. The biological properties of cetuximab. Critical Reviews in Oncology/Hematology. 2008;68(2):93–106. doi: 10.1016/j.critrevonc.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Amado RG, Wolf M, Peeters M, Van CE, Siena S, Freeman DJ, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. Journal of Clinical Oncology. 2008;26(10):1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 5.Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. The New England Journal of Medicine. 2008;359(17):1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 6.Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. The New England Journal of Medicine. 2013;369(11):1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 7.Price TJ, Peeters M, Kim TW, Li J, Cascinu S, Ruff P, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. The Lancet Oncology. 2014;15(6):569–579. doi: 10.1016/S1470-2045(14)70118-4. [DOI] [PubMed] [Google Scholar]
- 8.Folprecht G, Gruenberger T, Bechstein W, Raab HR, Weitz J, Lordick F, et al. Survival of patients with initially non-resectable colorectal liver metastases following systemic treatment with FOLFOX/cetuximab or FOLFIRI/cetuximab in a multidisciplinary concept (CELIM-study) Onkologie. 2013;36:70. doi: 10.1159/000176390. [DOI] [Google Scholar]
- 9.Bokemeyer C, Bondarenko I, Hartmann JT, de BF, Schuch G, Zubel A, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Annals of Oncology. 2011;22(7):1535–1546. doi: 10.1093/annonc/mdq632. [DOI] [PubMed] [Google Scholar]
- 10.Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. Journal of Clinical Oncology. 2011;29(15):2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 11.Tveit KM, Guren T, Glimelius B, Pfeiffer P, Sorbye H, Pyrhonen S, et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. Journal of Clinical Oncology. 2012;30(15):1755–1762. doi: 10.1200/JCO.2011.38.0915. [DOI] [PubMed] [Google Scholar]
- 12.Ye LC, Liu TS, Ren L, Wei Y, Zhu DX, Zai SY, et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. Journal of Clinical Oncology. 2013;31(16):1931–1938. doi: 10.1200/JCO.2012.44.8308. [DOI] [PubMed] [Google Scholar]
- 13.Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377(9783):2103–2114. doi: 10.1016/S0140-6736(11)60613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Annals of Oncology. 2014;25(7):1346–1355. doi: 10.1093/annonc/mdu141. [DOI] [PubMed] [Google Scholar]
- 15.Bokemeyer, C., Kohne, C-H., Ciardiello, F., Lenz, H-J., Heinemann, V., Klinkhardt, U., et al. (2014). Treatment outcome according to tumor RAS mutation status in OPUS study patients with metastatic colorectal cancer (mCRC) randomized to FOLFOX4 with/without cetuximab. Journal of Clinical Oncology. 32(15).
- 16.Cutsem E, Lenz HJ, Köhne CH, Heinemann V, Tejpar S, Melezínek I, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2015;33:692–700. doi: 10.1200/JCO.2014.59.4812. [DOI] [PubMed] [Google Scholar]
- 17.Douillard, J-Y., Siena, S., Tabernero, J., Burkes, R. L., Barugel, M. E., Humblet, Y., et al. (2013). Overall survival (OS) analysis from PRIME: randomized phase III study of panitumumab (pmab) with FOLFOX4 for first-line metastatic colorectal cancer (mCRC). Journal of Clinical Oncology. 31(15).
- 18.Seymour MT, Brown SR, Middleton G, Maughan T, Richman S, Gwyther S, et al. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): a prospectively stratified randomised trial. The Lancet Oncology. 2013;14(8):749–759. doi: 10.1016/S1470-2045(13)70163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, et al. Final results from a randomized phase 3 study of FOLFIRI {+/−} panitumumab for second-line treatment of metastatic colorectal cancer. Annals of Oncology. 2014;25(1):107–116. doi: 10.1093/annonc/mdt523. [DOI] [PubMed] [Google Scholar]
- 20.Peeters M, Oliner KS, Price TJ, Cervantes A, Sobrero AF, Ducreux M, et al. Analysis of KRAS/NRAS mutations in a phase III study of panitumumab with FOLFIRI compared with FOLFIRI alone as second-line treatment for metastatic colorectal cancer. Clinical Cancer Research. 2015;21(24):5469–5479. doi: 10.1158/1078-0432.CCR-15-0526. [DOI] [PubMed] [Google Scholar]
- 21.Schwartzberg LS, Rivera F, Karthaus M, Fasola G, Canon JL, Hecht JR, et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. Journal of Clinical Oncology. 2014;32(21):2240–2247. doi: 10.1200/JCO.2013.53.2473. [DOI] [PubMed] [Google Scholar]
- 22.Heinemann, V., von Weikersthal, L. F., Decker, T., Kiani, A., Vehling-Kaiser, U., Al-Batran, S. E., et al. (2014) FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncology. [DOI] [PubMed]
- 23.Primrose J, Falk S, Finch-Jones M, Valle J, O'Reilly D, Siriwardena A, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the new EPOC randomised controlled trial. The Lancet Oncology. 2014;15(6):601–611. doi: 10.1016/S1470-2045(14)70105-6. [DOI] [PubMed] [Google Scholar]
- 24.Basso M, Dadduzio V, Ardito F, Lombardi P, Strippoli A, Vellone M, et al. Conversion chemotherapy for technically unresectable colorectal liver metastases: a retrospective, STROBE-compliant, single-center study comparing chemotherapy alone and combination chemotherapy with cetuximab or bevacizumab. Medicine (Baltimore) 2016;95(20):e3722. doi: 10.1097/MD.0000000000003722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huiskens J, van Gulik TM, van Lienden KP, Engelbrecht MR, Meijer GA, van Grieken NC, et al. Treatment strategies in colorectal cancer patients with initially unresectable liver-only metastases, a study protocol of the randomised phase 3 CAIRO5 study of the Dutch Colorectal Cancer Group (DCCG) BMC Cancer. 2015;15:365. doi: 10.1186/s12885-015-1323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan DL, Pavlakis N, Shapiro J, Price TJ, Karapetis CS, Tebbutt NC, et al. Does the chemotherapy backbone impact on the efficacy of targeted agents in metastatic colorectal cancer? A systematic review and meta-analysis of the literature. PloS One. 2015;10(8):e0135599. doi: 10.1371/journal.pone.0135599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou SW, Huang YY, Wei Y, Jiang ZM, Zhang YD, Yang Q, et al. No survival benefit from adding cetuximab or panitumumab to oxaliplatin-based chemotherapy in the first-line treatment of metastatic colorectal cancer in KRAS wild type patients: a meta-analysis. PloS One. 2012;7(11):e50925. doi: 10.1371/journal.pone.0050925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ocvirk J, Brodowicz T, Wrba F, Ciuleanu TE, Kurteva G, Beslija S, et al. Cetuximab plus FOLFOX6 or FOLFIRI in metastatic colorectal cancer: CECOG trial. World Journal of Gastroenterology : WJG. 2010;16(25):3133–3143. doi: 10.3748/wjg.v16.i25.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng AL, Cornelio G, Shen L, Price T, Yang TS, Chung IJ, et al. First-line cetuximab with folfox or FOLFIRI every 2 weeks in KRAS wild-type metastatic colorectal cancer: phase II APEC study. Annals of Oncology. 2013;24:iv34–iiv5. doi: 10.1093/annonc/mdt202.27. [DOI] [PubMed] [Google Scholar]
- 30.Moosmann N, Weikersthal LF, Vehling-Kaiser U, Stauch M, Hass HG, Dietzfelbinger H, et al. Cetuximab plus capecitabine and irinotecan compared with cetuximab plus capecitabine and oxaliplatin as first-line treatment for patients with metastatic colorectal cancer: AIO KRK-0104—a randomized trial of the German AIO CRC study group. Journal of Clinical Oncology. 2011;29(8):1050–1058. doi: 10.1200/JCO.2010.31.1936. [DOI] [PubMed] [Google Scholar]
- 31.Stintzing, S., Modest, D. P., Rossius, L., Lerch, M. M., von Weikersthal, L. F., Decker, T., et al. (2016). FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. The Lancet Oncology, 17(10), 1426–1434. [DOI] [PubMed]
- 32.Modest, D.P., Stintzing, S., von Weikersthal, L. F., Decker, T., Kiani, A., Vehling-Kaiser, U., et al. Impact of subsequent therapies on outcome of the FIRE-3/AIO KRK0306 trial: first-line therapy with FOLFIRI plus cetuximab or bevacizumab in patients with KRAS wild-type tumors in metastatic colorectal cancer. (1527–7755 (Electronic)). [DOI] [PubMed]
- 33.Hecht JR, Cohn A, Dakhil S, Saleh M, Piperdi B, Cline BM, et al. SPIRITT: a randomized, multicenter, phase II study of panitumumab with FOLFIRI and bevacizumab with FOLFIRI as second-line treatment in patients with unresectable wild type KRAS metastatic colorectal cancer. Clinical Colorectal Cancer. 2015;14:72–80. doi: 10.1016/j.clcc.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Grothey, A. (2016). RAS wild-type patients should receive anti-VEGF therapy in first line. Presented at the ASCO Annual Meeting: June 2016; Chicago, IL.
- 35.Koopman M, Antonini NF, Douma J, Wals J, Honkoop AH, Erdkamp FL, et al. Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trial. Lancet. 2007;370(9582):135–142. doi: 10.1016/S0140-6736(07)61086-1. [DOI] [PubMed] [Google Scholar]
- 36.Seymour MT, Maughan TS, Ledermann JA, Topham C, James R, Gwyther SJ, et al. Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): a randomised controlled trial. Lancet. 2007;370(9582):143–152. doi: 10.1016/S0140-6736(07)61087-3. [DOI] [PubMed] [Google Scholar]
- 37.Cunningham D, Sirohi B, Pluzanska A, Utracka-Hutka B, Zaluski J, Glynne-Jones R, et al. Two different first-line 5-fluorouracil regimens with or without oxaliplatin in patients with metastatic colorectal cancer. Annals of Oncology. 2009;20(2):244–250. doi: 10.1093/annonc/mdn638. [DOI] [PubMed] [Google Scholar]
- 38.Ducreux M, Malka D, Mendiboure J, Etienne PL, Texereau P, Auby D, et al. Sequential versus combination chemotherapy for the treatment of advanced colorectal cancer (FFCD 2000–05): an open-label, randomised, phase 3 trial. The Lancet Oncology. 2011;12(11):1032–1044. doi: 10.1016/S1470-2045(11)70199-1. [DOI] [PubMed] [Google Scholar]
- 39.Seymour MT, Thompson LC, Wasan HS, Middleton G, Brewster AE, Shepherd SF, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet. 2011;377(9779):1749–1759. doi: 10.1016/S0140-6736(11)60399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asmis T, Berry S, Cosby R, Chan K, Coburn N, Rother M. Strategies of sequential therapies in unresectable metastatic colorectal cancer: a meta-analysis. Current Oncology. 2014;21(6):318–328. doi: 10.3747/co.21.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeantet, M., Tougeron, D., Tachon, G., Cortes, U., Archambaut, C., Fromont, G., et al. (2016). High intra- and inter-tumoral heterogeneity of RAS mutations in colorectal cancer. International Journal Molecular Science. 17(12). [DOI] [PMC free article] [PubMed]
- 42.Diaz LA, Jr, Williams RT, Wu J, Kinde I, Hecht JR, Berlin J, et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature. 2012;486(7404):537–540. doi: 10.1038/nature11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gasch C, Bauernhofer T, Pichler M, Langer-Freitag S, Reeh M, Seifert AM, et al. Heterogeneity of epidermal growth factor receptor status and mutations of KRAS/PIK3CA in circulating tumor cells of patients with colorectal cancer. Clinical Chemistry. 2013;59(1):252–260. doi: 10.1373/clinchem.2012.188557. [DOI] [PubMed] [Google Scholar]
- 44.Schrag, D.. (2016). The relationship between primary tumor sidedness and prognosis in colorectal cancer. Presented at the ASCO Annual Meeting, abstract no 3505, June 2016; Chicago, IL.
- 45.Arnold, D., Lueza, B., Douillard, J. Y., Peeters, M., Lenz, H. J., Venook, A., et al. (2017). Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomised trials. Annals Oncology. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 14 kb)
(DOCX 18 kb)