Abstract
Antiviral treatment options for chronic Hepatitis E Virus (HEV) infections are limited and immunological determinants of viral persistence remain largely unexplored. We studied the antiviral potency of pegylated interferon-α (pegIFNα) against HEV infections in humanized mice and modelled intrahepatic interferon stimulated gene (ISG) responses. Human gene expression levels in humanized mouse livers were analyzed by qPCR and Nanostring. Human CXCL10 was measured in mouse serum. HEV genotype 3 (gt3) infections were cleared from liver and feces within 8 pegIFNα doses in all mice and relapsed after a single pegIFNα injection in only half of treated animals. Rapid viral clearance by pegIFNα was confirmed in HEV gt1, but not in Hepatitis B Virus infected animals. No ISG induction was observed in untreated HEV gt3 and gt1 infected humanized livers compared to control chimeric mice, irrespective of the human hepatocyte donor, viral isolate or HEV infection duration. Human specific ISG transcript levels in mouse liver increased significantly after pegIFNα treatment and induced high circulating human CXCL10 in mouse serum. In conclusion, HEV gt1 and gt3 infections do not elicit innate intrahepatic immune responses and remain highly sensitive to pegIFNα in immunocompromised humanized mice.
Introduction
Hepatitis E Virus (HEV) infections are emerging in western countries1. HEV is a non-enveloped positive-sense single-stranded RNA virus, belonging to the family Hepeviridae within the genus Orthohepevirus 2. Transmission mainly occurs through the fecal-oral route via contaminated water in developing countries or through the consumption of undercooked meat in industrialized countries3. Seven different genotypes have been described so far, of which genotype (gt) 1 and 3 are most prevalent in humans2. In healthy individuals, HEV mostly resolves spontaneously without severe symptoms, but pregnant women seem to be at risk of developing fulminant liver failure by HEV gt1 with mortality rates up to 25%4, 5. On the other hand, increasing rates of chronic gt3 infections have been described in immunocompromised patients in Europe, resulting in progressive liver fibrosis and cirrhosis6–8. These data indicate that host pathogen interactions differ between both genotypes.
Antiviral treatment options for chronic HEV infected immunocompromised patients are limited. Ribavirin (RBV) leads to sustained viral responses in roughly 75% of patients, but is hampered by RBV-induced anemia and the need for recombinant erythropoietin injections or transfusions in more than half of patients9, 10. As an alternative, pegylated interferon-alpha (pegIFNα) has been administered to a few patients in doses comparable to Hepatitis C virus (HCV) treatment regimens10, 11. However, factors associated with interferon (IFN)-susceptibility, the optimal pegIFNα dose or treatment duration have not been investigated in vivo.
The anti-HEV effects of IFNα in vitro differ according to the target cell and viral strain used. In vitro HEV models consist of human hepatoma and lung adenocarcinoma cell-lines, in which replication of subgenomic or full length replicons and seldom intact patient-derived viruses are studied12–16. Patient-derived HEV gt3 cultures show slow viral propagation, whereas HEV gt1 can only be cultured in vitro after induction of endoplasmic reticulum stress in the host cell line15–17. While HEV gt1 replication has been shown to be adequately suppressed by exogenous IFNα, HEV gt3 replication has not18–20. In addition, viral inhibition of the interferon stimulated gene (ISG) responses have been described as a determining factor for IFNα susceptibility in vitro 21. As the studied host cells are either no target cells in vivo (A549 cells) or are hampered by defects in their innate immune signaling (Huh7 and Huh7.5), the host response towards genuine patient-derived HEV in differentiated human hepatocytes remains to be established18, 22. In addition, several clinical observations are not matched by in vitro viral replication data. HEV containing an in vivo RBV acquired mutation (K1383N), showed conflicting results in vitro with decreased viral replication and increased RBV-sensitivity23. Furthermore, the antiviral efficacy of sofosbuvir against HEV showed discrepancies in different in vitro and in vivo studies24–27.
Recently, we and others have shown that human-liver chimeric mice can be used to study HEV infection in differentiated human hepatocytes in vivo 15, 28, 29. Here, we examined baseline ISG expression levels and susceptibility to pegIFNα in HEV gt1 and gt3 infected humanized mice. We demonstrate that HEV gt1 infections lead to higher virus loads in mouse feces, bile and liver compared to HEV gt3 infections, without the induction of intrahepatic human innate immune responses. Both HEV genotypes, but not Hepatitis B virus (HBV), are cleared after a few doses of pegIFNα in vivo, an effect accompanied by a clear increase of human ISG transcript levels in liver and of circulating human CXCL10 levels in mouse serum.
Results
Higher viral burden in HEV gt1 compared to gt3 infected human-liver chimeric mice
Humanized UPA+/+NOG mice were i.v. inoculated with a filtered feces suspension containing either HEV gt3 or HEV gt1 and were observed for 2, 6 or 14 weeks until euthanization. Infected mice were housed individually to prevent inter-mice contamination. During the infection course a higher percentage of HEV gt1 infected mice presented viremia, but the peak viral load in serum was similar to HEV gt3 infected mice (2.6 ± 0.4 and 1.4 ± 0.4 log HEV RNA IU/ml, respectively, Fig. 1a). The peak HEV RNA load in feces was significantly higher in HEV gt1, compared to HEV gt3 infected mice (5.9 ± 0.2 and 4.2 ± 0.5 log HEV RNA IU/gram, respectively, P = 0.029; Fig. 1b). HEV gt1 infected mice also had higher viral loads in bile (6.1 ± 0.2 vs. 5.2 ± 0.4 log HEV RNA IU/ml, respectively, P = 0.038; Fig. 1c) and liver (6.8 ± 0.2 vs 5.8 ± 0.3 log HEV RNA IU/gram, respectively, P = 0.015; Fig. 1d) at euthanasia, despite similar levels of serum human albumin compared to HEV gt3 infected mice, indicative for similar degrees of human chimerism (1.6 ± 0.4 and 1.9 ± 0.5 mg/ml, respectively, Fig. 1e). Despite lower absolute HEV gt1 inocula compared to HEV gt3, animals challenged with undiluted feces suspensions demonstrated similar results reaching higher HEV gt1 RNA levels in bile (P = 0.038), liver (P = 0.006), and feces (P = 0.06) compared to HEV gt3 RNA levels. These results point to a higher in vivo virulence of HEV genotype 1 compared genotype 3.
Figure 1.
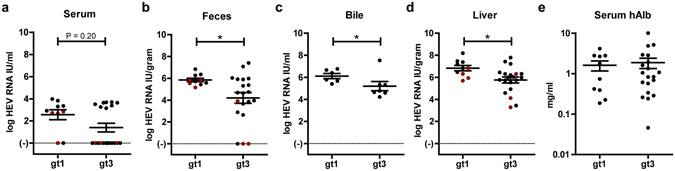
Higher viral loads in HEV gt1 compared to HEV gt3 infected mice despite similar degrees of human chimerism. Comparison of peak HEV RNA levels as measured by qRT-PCR in serum (a) and in feces (b). At sacrifice viral titers of HEV gt3 and gt1 infected mice were compared in bile (c, n = 7 and n = 6, respectively) and in liver (d). Human albumin levels were determined in mouse serum to quantify degree of chimerism at euthanasia (e). *P < 0.05, n = 20 for HEV gt3 and n = 10 for HEV gt1 (a,b,d,e). Data are pooled from 2, 6, and 14 weeks infection experiments. Red dots indicate mice who received a diluted HEV inoculum (a–d).
No induction of intrahepatic innate immune responses in HEV gt1 or gt3 infected human-liver chimeric mice
Because of the HEV gt1 and gt3 clinical differences4, 6–8 and different viral burdens in humanized mice, we examined the human host response in chimeric livers 2, 6, or 14 weeks after infection with either HEV gt1 or gt3. Using qRT-PCR we could not detect a significant increase in transcript levels of alpha or beta IFNs (data not shown), pathogen recognition receptors TLR3 and DDX58 (Fig. 2a), transcription factor STAT1 (Fig. 2b), or ISGs CXCL9, CXCL10, ISG15, RSAD2, OAS1, MX1, and IFIT1 (Fig. 2c). Furthermore, longer duration of HEV gt3, but not HEV gt1 infection led to significantly decreased STAT1, RSAD2 and MX1 expression levels in the liver (Fig. 2b+c). None of these human transcripts were detected in non-chimeric mouse livers.
Figure 2.
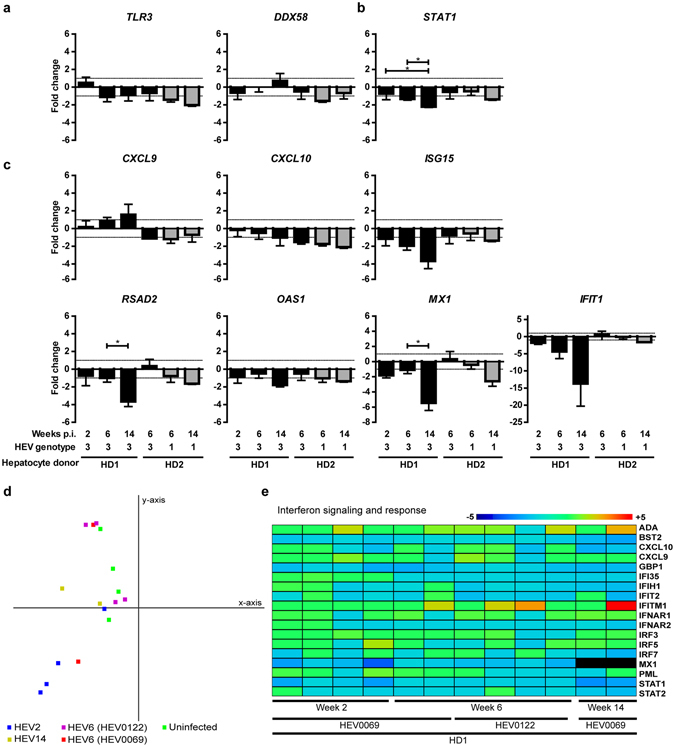
Minimal intrahepatic interferon-stimulated gene induction in HEV infected human-liver chimeric mice, between weeks 2 to 14 post infection. Whole chimeric-liver RNA was isolated from HEV gt3 and gt1 infected mice and analyzed for the human specific gene expression of sensing molecules TLR3 and DDX58 (a), transcription factor STAT1 (b), and interferon stimulated genes CXCL9, CXCL10, ISG15, RSAD2, OAS1, MX1 and IFIT1 (c) using qRT-PCR. Groups consist of n = 4, 6, 4, 3, 6 and 4 mice from left to right (a–c). Given values on y-axes are fold changes over HEV RNA negative chimeric-livers transplanted with the same hepatocyte donor. X-axes shows weeks post infection, HEV genotype, and hepatocyte donor. Significance was assessed within groups of the same hepatocyte donor using Krukskal-Wallis one-way Anova with Dunnett’s Multiple comparison test. *P < 0.05, Gray bars indicate HEV gt1, black bars HEV gt3 (a–c). In-depth human gene expression analysis was performed on RNA from chimeric mouse livers before infection, and after 2, 6 and 14 weeks of HEV gt3 infection using nCounter® Human Immunology V2 panel. Principal component 1 (x-axis) and 2 (y-axis) comprise 49% of the variance between samples using all non-cross reactive genes (d). Uninfected samples are indicated in green, infected samples are indicated in blue, red (HEV0069), purple (HEV0122) and yellow and by the number of weeks infected HEV2, HEV6, HEV6, HEV14, respectively (d). Heatmap shows fold change over average of 4 uninfected mice for interferon signaling and response genes (e). Gene legend is indicated on the right side and sample legend below the heatmap (e). Dark red indicates ≥ 5 fold change, and dark blue ≤ 5 fold change (e).
In order to evaluate a broader number of genes, Nanostring analysis of 594 human specific immunology-related genes was performed on chimeric (serum hAlb 2.5 ± 0.8 mg/ml) gt3 HEV-infected livers (6.1 ± 0.25 log HEV RNA IU/gr) at different time points post infection. Human transcript specificity was confirmed by including RNA from 3 non-chimeric livers and led to the removal of 50 cross-reactive genes from further analyses. Based on set criteria (<100 relative RNA counts and below four times the standard deviation in all samples), 255 genes were defined as non-expressed. Principal component analyses did not reveal clustering of samples (Fig. 2d). Of 18 genes related to interferon signaling and response, none showed consistent upregulation compared to non-infected chimeric mice (Fig. 2e). Down regulation of STAT1 and MX1 as observed by qRT-PCR, was confirmed in the Nanostring gene expression data (Fig. 2b+c,e). Taken together, these data show that ongoing HEV gt1 or gt3 replication for up to 14 weeks does not elicit an innate immune response in human hepatocytes in vivo.
HEV but not HBV is sensitive to pegIFNα-2a treatment in human-liver chimeric mice
Baseline ISG expression in hepatocytes has been shown to predict the response to IFNα treatment in chronic HCV infected patients30–32. As HEV did not induce an ISG response in vivo, we examined the HEV-sensitivity to pegIFNα treatment. As a negative antiviral control, we applied the same treatment to HBV gtA infected mice, which has been shown to only slightly reduce serum HBV DNA levels in a similar humanized mouse model33. After 1 to 2 pegIFNα injections, HEV gt3 RNA became undetectable in feces of all treated animals (Fig. 3a–c). Complete viral clearance in liver and bile was observed in all mice at euthanasia 24 hours after 4 or 8 pegIFNα injections (Fig. 3d). To examine whether a single dose of 30 µg/kg pegIFNα would suffice to clear HEV gt3 in vivo, 4 animals received one injection after 6 weeks of ongoing HEV gt3 replication and were observed for an additional 4 weeks. This led to a complete viral clearance in 2 out of 4 mice and relapse in feces in the remainder 2 (Fig. 3c+d). Four weeks after the initial single pegIFNα dose, the latter 2 animals received repetitive 10-fold lower pegIFNα doses for 2 weeks. Again a steep decline in fecal HEV RNA loads was noted, but HEV RNA reemerged in feces and was detectable in bile and liver at euthanasia one day after the second pegIFNα treatment course (Fig. 3c+d). The high in vivo HEV IFNα sensitivity was corroborated in HEV gt1 infected animals. Again rapid suppression of HEV replication was noted in feces (Fig. 3e), liver and bile (data not shown) after a 2 week treatment course with 30 µg/kg pegIFNα. In contrast, a similar treatment regimen of HBV gtA infected mice induced a maximum decline of 0.7 ± 0.2 log HBV DNA copies/ml in serum with high intrahepatic viral loads at necropsy (6.9 ± 0.6 log HBV DNA copies/gr liver) (Fig. 3f). Non-treated HEV gt1, HEV gt3 and HBV infected mice never showed spontaneous viral clearance (Fig. 1b–d and Suppl. Fig. 2a–c), nor was loss of human chimerism in pegIFNα-treated animals observed, based on persistent detection of human albumin levels in mouse serum (data not shown). These data indicate that HEV, but not HBV is highly sensitive to pegIFNα in humanized mice.
Figure 3.
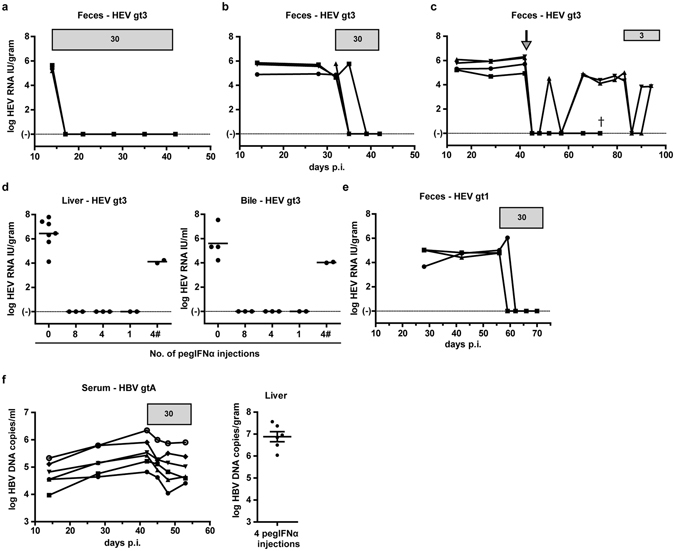
HEV is more sensitive than HBV to pegIFNα treatment in human-liver chimeric mice. HEV RNA was measured by qRT-PCR in feces of human-liver chimeric mice infected with HEV gt3 or HEV gt1 before and during 4 weeks (a, n = 3), 2 weeks (b, n = 4; e, n = 3). and single (c, n = 4) pegIFNα treatment. Horizontal gray bars indicate pegIFNα treatment duration and dosage in µg/kg (a,b). Arrow indicates time point of the single 30 µg/kg pegIFNα injection (c). One day after last dosage mice were sacrificed and viral load was determined in liver and bile (d). Non-treated infected mice were added as control (d). X-axes indicates number of pegIFNα injections (d). HBV DNA was measured in serum of HBV gtA infected human-liver chimeric mice before and during pegIFNα treatment, and one day after last treatment mice were euthanized and intrahepatic HBV DNA was measured (f, n = 6). #indicates 3 µg/kg pegIFNα dosages. All mice were transplanted with the same hepatocyte donor (HD2, a–f). Y-axes indicate log HEV RNA IU/gram (a–c,d left panel, e), log HEV RNA IU/ml (d right panel), and log HBV DNA copies/ml (f). X-axes indicate days post infection (a–c,e–f).
Upregulation of intrahepatic ISG and serum CXCL10 upon pegIFNα treatment
To examine whether pegIFNα induced HEV clearance was associated with an induction of human hepatocyte ISG responses, human specific transcript levels of several innate immune response genes were studied in chimeric livers of HEV gt3, HEV gt1 and HBV gtA infected and pegIFNα treated animals. PegIFNα treatment led to an 20-fold increase of CXCL10 transcription, conjointly with induction of TLR3, DDX58, STAT1, CXCL9, ISG15, RSAD2, OAS1, MX1, and IFIT1 genes in the livers of HEV infected humanized mice (Fig. 4a and Suppl. Fig. 3). In addition, treatment was associated with an increase in serum human CXCL10 levels of HEV gt1 and gt3 infected mice (59 ± 10 and 108 ± 14 pg/ml, respectively, Fig. 4b). Interestingly, intrahepatic CXCL10 expression levels remained elevated (3.4-fold compared to HEV-infected non-treated mice) 4 weeks after a single pegIFNα dose in the two mice that cleared HEV. Similar to previous reports, HBV persistence in vivo was not due to absence of an ISG response, as IFIT1, ISG15, MX1, STAT1, and CXCL10 all were strongly induced (Fig. 4a and data not shown)33. Overall, HEV but not HBV was found to be sensitive to pegIFNα induced hepatocyte-specific innate responses in vivo.
Figure 4.
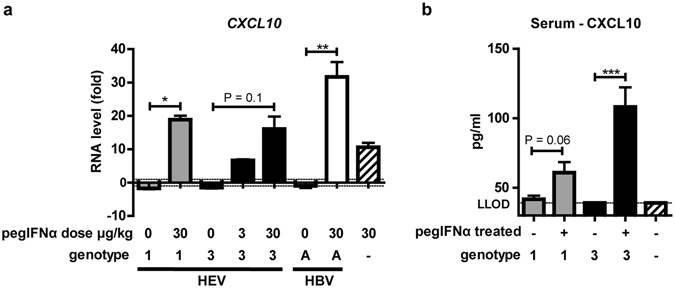
CXCL10 transcripts and protein are induced after pegIFNα treatment in HEV infected mice. RNA was isolated from non-treated and 2 weeks pegIFNα treated HEV gt1, gt3 and HBV infected mouse livers and was analyzed for the expression of human CXCL10 (a). X-axes indicate treatment dosage and virus genotype (a). Given values on y-axes are RNA levels in fold changes over uninfected non-treated mice (a). Human CXCL10 levels were measured using ELISA in mouse serum of uninfected, HEV-infected and HEV-infected pegIFNα treated mice (b). Dotted line indicates lower limit of detection (LLOD) (b). Gray bars indicate HEV gt1, black bars HEV gt3 and striped bar uninfected (a + b).
Discussion
Despite increasing reports on acute and chronic Hepatitis E Virus infections in Europe, antiviral treatment options are limited and immunological determinants of viral persistence remain largely unexplored5. Here we aimed to study baseline and therapeutically induced innate immune responses in a recently established humanized mouse model for chronic HEV infection. We demonstrate that (1) HEV is highly sensitive to pegIFNα treatment in vivo; (2) HEV infection in human hepatocytes doesn’t elicit an innate immune response; (3) HEV gt1 presents higher viral loads compared to HEV gt3.
HEV gt1 and gt3, but not HBV, showed to be highly sensitive to pegIFNα treatment in immune deficient human liver chimeric uPA+/+NOG mice. Viral clearance in feces, liver and bile was achieved after 4 and 2 weeks treatment and even after a single pegIFNα injection in 2/4 mice. PegIFNα associated viral clearance was accompanied by an increase of intrahepatic human ISGs and serum CXCL10 levels. In line with our data (Fig. 3f), the antiviral potency of pegIFNα against other hepatotropic viruses was less pronounced in similar humanized mouse models. PegIFNα reduced HBV viremia by 2.5 log IU/ml and HCV loads with 2.3 log IU/ml after 12 and 4 weeks of treatment respectively, without clearing the infection33–35. Successful IFNα treatment in immunocompetent woodchucks chronically infected with woodchuck hepatitis virus (a model for chronic HBV infection), is associated with an intrahepatic IFN-γ and NK/T cell gene signature, but not an ISG signature36. All together this suggests that pegIFNα has a strong direct anti-viral effect against HEV, whereas HBV and HCV require the immune system to achieve viral clearance or complete suppression.
Ongoing HEV gt1 or gt3 replication did not elicit human-innate immune responses in humanized livers of 30 uPA+/+NOG mice, irrespective of the infection duration, the human hepatocyte donor or viral isolate used. We specifically addressed the genomic response of human hepatocytes to HEV without that of infiltrating immune cells in our profound immune deficient uPA+/+NOG mice. In addition, we carefully eliminated cross hybridizing probes by including non-chimeric mice. After prolonged HEV gt3 infection for more than 3 months, significantly lower expression levels of STAT1, RSAD2 and MX1 compared to uninfected controls were observed, suggesting possible viral interference with the host’s cell innate immune signaling. Hepatotropic pathogens have developed different methods to evade innate immune defenses37. In our model, expression of TLR3 and DDX58 was detected in all HEV-infected chimeric livers indicating that these host sensing molecules were not counteracted at the transcription level (Fig. 2a). Several studies in HEK293T, A549 and Huh7 cells have suggested that HEV can directly interfere with phosphorylation of STAT1 and the induction of IFNα20, 21, 38. However, most of these studies use non-physiological HEV-infection models or are influenced by defects in the innate signaling of target cells22. Our findings indicate possible HEV mediated innate immune inhibitory effects in primary human hepatocytes. Further studies in differentiated human hepatocytes are required to determine how HEV is able to prevent immune sensing or disrupt innate signaling and how this influences viral fitness.
In contrast to our findings, one recent study infected a similar, but less profound immunodeficient uPA-SCID mouse model with the same HEV gt1 strain (Sar-55) and showed elevated ISG expression in 2 HEV-infected mice compared to one control animal28. While the impact of the hepatocyte donor type on expression levels cannot be disregarded as shown here (Suppl. Fig. 1), remnant mouse natural killer cell and Kupffer cell activity in the SCID compared to the NOG background might have contributed to the observed differences39, 40. The role of infiltrating innate immune cells in the liver during HEV-clearance was recently shown in the chimpanzee model41. In HEV-infected chimpanzees the intrahepatic expression levels of BST2 (present in monocytes, macrophages and dendritic cells) and not those of the adaptive immune system, corresponded with the expression kinetics of several ISG’s, including CXCL10, ISG15 and OAS1 41, 42.
An important finding of our study was that during both HEV gt1 and gt3 infections, no innate immune responses were induced despite higher HEV gt1 viral loads in mouse feces, bile and liver. These observations point to an intrinsic phenotypical difference of the distinct HEV genotypes, but cannot explain the different immune pathogenesis seen in patients, who have a strikingly different clinical presentation. Not only is disease severity higher in HEV gt1 infections, but also chronicity rates for HEV gt1 are found to be low or even zero. Possibly, different amounts of viral antigens or epitopes, could induce different magnitudes of natural killer cell or HEV-specific T cell responses resulting in respectively more clinical disease or less chronic infections for the different genotypes43, 44.
The clinical experience with pegIFN based therapies for chronic HEV is minimal. Eight cases have been published of which 5 showed a suppression of viremia at the first measured timepoint after initiation of pegIFNα treatment. PegIFNα treatment in HEV infected humanized mice modelled the viral decline seen in these 5 patients. It remains however unclear why some chronic HEV patients show slow viral declines upon IFN-treatment. We observed a viral relapse in feces, liver and bile of 2 humanized mice after a second pegIFNα treatment course (Fig. 3c+d). While animals received a 10-fold lower pegIFNα dose, the relapse might be partially ascribed to elevated intrahepatic ISG levels before retreatment. Increased CXCL10 levels were measured in the liver of 2 mice 4 weeks after a single pegIFNα injection, which corresponds to the timepoint at which retreatment was given to the remainder mice of that group. Since in chronic HCV patients the virologic response to pegIFNα is associated with low baseline ISG expression levels31, it would be interesting to examine whether this holds true for chronic HEV patients as well.
In conclusion, despite higher viral loads for HEV gt1 in human-liver chimeric mice, both HEV gt1 and gt3 do not induce an intrahepatic innate immune response. HEV, but not HBV, is highly sensitive to pegIFNα treatment in humanized mice.
Material and Methods
Ethics, consent and permissions
The use of patient material was approved by the medical ethical committees of Erasmus Medical Center and Antwerp University Hospital. Informed consent was obtained from all subjects. All animal work was conducted according to relevant Dutch national guidelines. The study protocol was approved by the animal ethics committee of the Erasmus Medical Center (DEC nr. 141-12-11).
Mouse origin and genotyping
Urokinase-type plasminogen activator (uPA)/NOD/Shi-scid/IL-2Rγnull (NOG) mice were kindly provided by the Central Institute for Experimental Animals (Kawasaki, Japan)45. Mice were bred at the Central Animal Facility of the Erasmus Medical Center. Zygosity of mice was determined as described previously15. Mice were co-housed with a maximum of 4 mice per individually ventilated cage and were fed normal chow ad libitum.
Human hepatocyte transplantation
Six to twelve week old male uPA-homozygous mice were transplanted as described previously46. In short, mice were anesthetized and transplanted via intrasplenic injection with 0.5 × 106 to 2 × 106 viable commercially available cryopreserved human hepatocytes from 1 of 3 donors (Corning, NY, USA; Lonza, Basel, Switzerland; Table 1). Graft take was determined by human albumin in mouse serum using an ELISA with human albumin cross-adsorbed antibody (Bethyl laboratories, Montgomery, TX, USA) as previously described15.
Table 1.
Hepatocyte donors.
| Donor ID | Gender | Age | Race |
|---|---|---|---|
| HD1 | Male | 2 years | Caucasian |
| HD2 | Female | 2 years | Caucasian |
| HD3 | Female | 7 months | Caucasian |
Viral strains, mouse infection and treatment
HEV gt3 was derived from feces of one of two chronic HEV patients (HEV0069 and HEV0122) as described previously15. HEV gt1 Sar-55 was derived from feces of a Rhesus macaque that had been originally inoculated with the human Sar-55 strain47. Eight weeks after transplantation human-liver chimeric mice were inoculated intravenously (i.v.) with 200 µl feces suspension containing HEV gt3 (8.8 log IU/ml or diluted to 6.8 log IU/ml), HEV gt1 (7.9 log IU/ml or diluted to 6.2 log IU/ml) or 200 µl patient serum containing HBV gtA (7.7 log IU/ml). After viral inoculation, mice were housed individually. Mice were treated with a single subcutaneous pegIFNα-2a (30 µg/kg unless stated otherwise, Pegasys, Roche, Basal, Switzerland) injection or every 3–4 days for 2 or 4 weeks. Overview of viral isolates are shown in Table 2. An overview of experimental groups is shown in Table 3 and Suppl. Figure 4.
Table 2.
Viral isolates.
| Virus | Genotype | Strain/Isolate* | Source | Inoculum |
|---|---|---|---|---|
| HEV | 1 | Sar-55 | Rhesus macaque feces | feces suspension |
| HEV | 3 | HEV0069* | Chronic HEV patient feces | feces suspension |
| HEV | 3 | HEV0122* | Chronic HEV patient feces | feces suspension |
| HBV | A | Chronic HBV patient serum | serum |
Table 3.
Overview of experimental groups.
| Treatment | Chimeric liver | Virus | n= | Hepatocyte donor |
|---|---|---|---|---|
| None | no | None | 3 | n/a* |
| yes | None | 8 | HD1, HD2 | |
| yes | HEV gt1 | 10 | HD1 | |
| yes | HEV gt3 (HEV0069) | 16 | HD1, HD2, HD3 | |
| yes | HEV gt3 (HEV0122) | 4 | HD1 | |
| yes | HBV gtA | 5 | HD3 | |
| pegIFNα-2a | no | None | 2 | n/a* |
| yes | None | 2 | HD2 | |
| yes | HEV gt1 | 3 | HD2 | |
| yes | HEV gt3 (HEV0069) | 11 | HD2 | |
| yes | HBV gtA | 6 | HD2 |
*n/a, not applicable.
HEV RNA and HBV DNA detection
The presence of HEV RNA in mouse serum, feces, bile and liver was determined by an ISO15189:2012-validated, internally controlled quantitative real-time RT-PCR, described previously7, 15. Cycle threshold (Ct) values above 38 were considered background, which corresponds to a lower limit of detection of 2.16 log10 HEV RNA units/ml in undiluted human serum. HEV RNAs detected in samples with Ct values below 38 are indicated with their calculated values. HBV viral load was measured in mouse serum and liver using a dual target approach, using primers and probes targeting preS-gen, as described before48, 49, and the X gene (HBV XJfwd12 5′-ggtctgtgccaagtgtttgst-3′, HBV XJprobe 5′-FAM-acgcaacccccactggctggg-BHQ1–3′, HBV XJrev12, 5′-tycgcagtatggatcgsc-3′).
RNA isolation of whole liver, generation of cDNA and real-time qPCR
Whole liver RNA was isolated using RNeasy mini kit (Qiagen, Hilden, Germany) including DNAse treatment according to manufacturer’s protocol starting with homogenization of liver tissue in RLT buffer. cDNA was generated by using an iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s protocol. Human specific gene expression was measured using Taqman primer/probe quantitative PCR, in TaqMan® Gene Expression Master Mix (Thermo Fisher Scientific, Waltham, MA, USA). Primer/probe combinations were purchased from Thermo Fisher Scientific; CXCL10 (Hs01124251_g1), CXCL9 (Hs00171065_m1), DDX58 (Hs01061436_m1), GAPDH (Hs00266705_g1), IFIT1 (Hs01911452_s1), ISG15 (Hs01921425_s1), IFNA1 (Hs00855471_g1), IFNA4 (Hs01681284_sh), IFNB1 (Hs01077958_s1), MX1 (Hs00895608_m1), OAS1 (Hs00973637_m1), RSAD2 (Hs00369813_m1), STAT1 (Hs01013996_m1), TLR3 (Hs01551078_m1). Expression of target genes was normalized to the expression of GAPDH using the formula 2−ΔCt, ΔCt = Cttarget−CtGADPH. cDNA from non-chimeric mouse livers was used as control to test cross-reactivity of housekeeping and target genes. Due to the difference in hepatocyte donor baseline expression levels of examined genes (Suppl. Fig. 1), fold changes of transcripts were calculated to those of non-infected humanized livers from mice transplanted with the identical hepatocyte donor.
Cytokine measurement
Human CXCL10 was measured in 1:5 diluted mouse serum samples using the Human CXCL10/IP10 Quantikine ELISA Kit (R&D Systems, Minneapolis, MN, USA) according to manufacturer’s protocol.
Nanostring analyses
RNA was isolated from chimeric mouse livers as described above. The nCounter GX human Immunology V2 Kit (NanoString Technologies, Seattle, WA, USA) was used to measure the expression of 594 human genes in the RNA of these samples. Following hybridization, transcripts were quantitated using the nCounter Digital Analyzer. Samples were run at the Johns Hopkins Deep Sequencing & Microarray Core facility. To correct for background levels, the highest negative control value for each sample was subtracted from each count value of that sample, as described previously50, 51. Following background subtraction, any negative count values were considered as 0. The geometric mean of 5 housekeeping genes provided by the company panel was calculated and used to normalize expression values. RNA from non-chimeric mouse livers was used as control to test cross-reactivity of genes. Fifty cross-reactive genes were removed prior to analyses of the data set. Non-expressed genes were defined as < 100 relative RNA counts and below four times the standard deviation in all samples.
Statistics
Differences between groups were calculated using two tailed Mann-Whitney test or Kruskal-Wallis one-way ANOVA with Dunn’s all column comparison post-test (GraphPad Prism version 5.01; GraphPad Software). Differences were considered significant when P < 0.05. Results are presented as the mean ± SEM. Principal component analyses was performed on log 2 transformed data set and heatmap of IFN signaling/response genes was generated using Multi-experiment viewer (MeV) software version 4.9.
Electronic supplementary material
Acknowledgements
We would like to thank Vincent Vaes and Vincent Duiverman for their assistance in performing biotechnical manipulations, and Jolanda Voermans and Claudia Mulders for excellent technical assistance. This study was supported by the Virgo consortium, funded by the Dutch government project number FES0908 and the Netherlands Genomics Initiative (NGI) project number 050-060-452. TV is recipient of an Erasmus MC Fellowship 2011 and a 2014 research mandate of the Belgian Foundation against Cancer (2014–087).
Author Contributions
Designed research: M.D.B.v.d.G., A.B., T.V. Performed experiments: M.D.B.v.d.G., S.D.P., G.v.O., L.G., T.V. Analysis and interpretation of the data: M.D.B.v.d.G., S.D.P., A.B., T.V. Wrote manuscript: M.D.B.v.d.G., T.V. Provided essential research tools: S.D.P., L.G., Y.C., R.A.d.M., T.V. Critical revision of the manuscript for important intellectual content: S.D.P., R.A.d.M., A.B., T.V. Approved final version of manuscript: all.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-07434-y
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dalton HR, Seghatchian J. Hepatitis E virus: Emerging from the shadows in developed countries. Transfusion and apheresis science: official journal of the World Apheresis Association: official journal of the European Society for Haemapheresis. 2016 doi: 10.1016/j.transci.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Smith DB, et al. Consensus proposals for classification of the family Hepeviridae. The Journal of general virology. 2014;95:2223–2232. doi: 10.1099/vir.0.068429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aggarwal R, Jameel S. Hepatitis E. Hepatology. 2011;54:2218–2226. doi: 10.1002/hep.24674. [DOI] [PubMed] [Google Scholar]
- 4.Khuroo MS, Kamili S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. Journal of viral hepatitis. 2003;10:61–69. doi: 10.1046/j.1365-2893.2003.00398.x. [DOI] [PubMed] [Google Scholar]
- 5.Krain LJ, Nelson KE, Labrique AB. Host immune status and response to hepatitis E virus infection. Clinical microbiology reviews. 2014;27:139–165. doi: 10.1128/CMR.00062-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamar N, et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140:1481–1489. doi: 10.1053/j.gastro.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 7.Pas SD, et al. Hepatitis E virus infection among solid organ transplant recipients, the Netherlands. Emerging infectious diseases. 2012;18:869–872. doi: 10.3201/eid1805.111712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nijskens CM, et al. Hepatitis E virus genotype 3 infection in a tertiary referral center in the Netherlands: Clinical relevance and impact on patient morbidity. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2016;74:82–87. doi: 10.1016/j.jcv.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 9.Kamar N, et al. Ribavirin for chronic hepatitis E virus infection in transplant recipients. The New England journal of medicine. 2014;370:1111–1120. doi: 10.1056/NEJMoa1215246. [DOI] [PubMed] [Google Scholar]
- 10.Peters van Ton AM, Gevers TJ, Drenth JP. Antiviral therapy in chronic hepatitis E: a systematic review. Journal of viral hepatitis. 2015;22:965–973. doi: 10.1111/jvh.12403. [DOI] [PubMed] [Google Scholar]
- 11.Alric L, Bonnet D, Laurent G, Kamar N, Izopet J. Chronic hepatitis E virus infection: successful virologic response to pegylated interferon-alpha therapy. Annals of internal medicine. 2010;153:135–136. doi: 10.7326/0003-4819-153-2-201007200-00256. [DOI] [PubMed] [Google Scholar]
- 12.Berto A, et al. Replication of hepatitis E virus in three-dimensional cell culture. Journal of virological methods. 2013;187:327–332. doi: 10.1016/j.jviromet.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen HT, Shukla P, Torian U, Faulk K, Emerson SU. Hepatitis E virus genotype 1 infection of swine kidney cells in vitro is inhibited at multiple levels. Journal of virology. 2014;88:868–877. doi: 10.1128/JVI.02205-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen HT, et al. A naturally occurring human/hepatitis E recombinant virus predominates in serum but not in faeces of a chronic hepatitis E patient and has a growth advantage in cell culture. The Journal of general virology. 2012;93:526–530. doi: 10.1099/vir.0.037259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van de Garde MD, et al. Hepatitis E Virus (HEV) Genotype 3 Infection of Human Liver Chimeric Mice as a Model for Chronic HEV Infection. Journal of virology. 2016;90:4394–4401. doi: 10.1128/JVI.00114-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka T, Takahashi M, Kusano E, Okamoto H. Development and evaluation of an efficient cell-culture system for Hepatitis E virus. The Journal of general virology. 2007;88:903–911. doi: 10.1099/vir.0.82535-0. [DOI] [PubMed] [Google Scholar]
- 17.Nair VP, et al. Endoplasmic Reticulum Stress Induced Synthesis of a Novel Viral Factor Mediates Efficient Replication of Genotype-1 Hepatitis E Virus. PLoS pathogens. 2016;12:e1005521. doi: 10.1371/journal.ppat.1005521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devhare PB, Desai S, Lole KS. Innate immune responses in human hepatocyte-derived cell lines alter genotype 1 hepatitis E virus replication efficiencies. Scientific reports. 2016;6:26827. doi: 10.1038/srep26827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Todt D, et al. Antiviral Activities of Different Interferon Types and Subtypes against Hepatitis E Virus Replication. Antimicrobial agents and chemotherapy. 2016;60:2132–2139. doi: 10.1128/AAC.02427-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou X, et al. Disparity of basal and therapeutically activated interferon signalling in constraining hepatitis E virus infection. Journal of viral hepatitis. 2016;23:294–304. doi: 10.1111/jvh.12491. [DOI] [PubMed] [Google Scholar]
- 21.Dong C, et al. Suppression of interferon-alpha signaling by hepatitis E virus. Hepatology. 2012;55:1324–1332. doi: 10.1002/hep.25530. [DOI] [PubMed] [Google Scholar]
- 22.Keskinen P, et al. Impaired antiviral response in human hepatoma cells. Virology. 1999;263:364–375. doi: 10.1006/viro.1999.9983. [DOI] [PubMed] [Google Scholar]
- 23.Debing Y, et al. Hepatitis E virus mutations associated with ribavirin treatment failure result in altered viral fitness and ribavirin sensitivity. Journal of hepatology. 2016;65:499–508. doi: 10.1016/j.jhep.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 24.van der Valk M, Zaaijer HL, Kater AP, Schinkel J. Sofosbuvir shows antiviral activity in a patient with chronic hepatitis E virus infection. Journal of hepatology. 2016 doi: 10.1016/j.jhep.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Donnelly MC, et al. Sofosbuvir and Daclatasvir Anti-Viral Therapy Fails to Clear HEV Viremia and Restore Reactive T Cells in a HEV/HCV Co-Infected Liver Transplant Recipient. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.05.060. [DOI] [PubMed] [Google Scholar]
- 26.Wang W, et al. Distinct Antiviral Potency of Sofosbuvir Against Hepatitis C and E Viruses. Gastroenterology. 2016;151:1251–1253. doi: 10.1053/j.gastro.2016.09.061. [DOI] [PubMed] [Google Scholar]
- 27.Dao Thi VL, et al. Sofosbuvir Inhibits Hepatitis E Virus Replication In Vitro and Results in an Additive Effect When Combined With Ribavirin. Gastroenterology. 2016;150:82–85 e84. doi: 10.1053/j.gastro.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Sayed IM, et al. Study of hepatitis E virus infection of genotype 1 and 3 in mice with humanised liver. Gut. 2016 doi: 10.1136/gutjnl-2015-311109. [DOI] [PubMed] [Google Scholar]
- 29.Allweiss L, et al. Human liver chimeric mice as a new model of chronic hepatitis E virus infection and preclinical drug evaluation. Journal of hepatology. 2016;64:1033–1040. doi: 10.1016/j.jhep.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Chen L, et al. Hepatic gene expression discriminates responders and nonresponders in treatment of chronic hepatitis C viral infection. Gastroenterology. 2005;128:1437–1444. doi: 10.1053/j.gastro.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 31.McGilvray I, et al. Hepatic cell-type specific gene expression better predicts HCV treatment outcome than IL28B genotype. Gastroenterology. 2012;142:1122–1131 e1121. doi: 10.1053/j.gastro.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 32.Feld JJ, et al. Hepatic gene expression during treatment with peginterferon and ribavirin: Identifying molecular pathways for treatment response. Hepatology. 2007;46:1548–1563. doi: 10.1002/hep.21853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allweiss L, et al. Immune cell responses are not required to induce substantial hepatitis B virus antigen decline during pegylated interferon-alpha administration. Journal of hepatology. 2014;60:500–507. doi: 10.1016/j.jhep.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 34.Kneteman NM, et al. Anti-HCV therapies in chimeric scid-Alb/uPA mice parallel outcomes in human clinical application. Hepatology. 2006;43:1346–1353. doi: 10.1002/hep.21209. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe T, et al. Hepatitis C virus kinetics by administration of pegylated interferon-alpha in human and chimeric mice carrying human hepatocytes with variants of the IL28B gene. Gut. 2013;62:1340–1346. doi: 10.1136/gutjnl-2012-302553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fletcher SP, et al. Intrahepatic Transcriptional Signature Associated with Response to Interferon-alpha Treatment in the Woodchuck Model of Chronic Hepatitis B. PLoS pathogens. 2015;11:e1005103. doi: 10.1371/journal.ppat.1005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Protzer U, Maini MK, Knolle PA. Living in the liver: hepatic infections. Nature reviews. Immunology. 2012;12:201–213. doi: 10.1038/nri3169. [DOI] [PubMed] [Google Scholar]
- 38.Nan Y, et al. Hepatitis E virus inhibits type I interferon induction by ORF1 products. Journal of virology. 2014;88:11924–11932. doi: 10.1128/JVI.01935-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tateno C, et al. Morphological and microarray analyses of human hepatocytes from xenogeneic host livers. Laboratory investigation; a journal of technical methods and pathology. 2013;93:54–71. doi: 10.1038/labinvest.2012.158. [DOI] [PubMed] [Google Scholar]
- 40.Tournoy KG, Depraetere S, Meuleman P, Leroux-Roels G, Pauwels RA. Murine IL-2 receptor beta chain blockade improves human leukocyte engraftment in SCID mice. European journal of immunology. 1998;28:3221–3230. doi: 10.1002/(SICI)1521-4141(199810)28:10<3221::AID-IMMU3221>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 41.Yu C, et al. Pathogenesis of hepatitis E virus and hepatitis C virus in chimpanzees: similarities and differences. Journal of virology. 2010;84:11264–11278. doi: 10.1128/JVI.01205-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans DT, Serra-Moreno R, Singh RK, Guatelli JC. BST-2/tetherin: a new component of the innate immune response to enveloped viruses. Trends in microbiology. 2010;18:388–396. doi: 10.1016/j.tim.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suneetha PV, et al. Hepatitis E virus (HEV)-specific T-cell responses are associated with control of HEV infection. Hepatology. 2012;55:695–708. doi: 10.1002/hep.24738. [DOI] [PubMed] [Google Scholar]
- 44.Brown A, et al. Characterization of the Specificity, Functionality, and Durability of Host T-Cell Responses Against the Full-Length Hepatitis E Virus. Hepatology. 2016;64:1934–1950. doi: 10.1002/hep.28819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suemizu H, et al. Establishment of a humanized model of liver using NOD/Shi-scid IL2Rgnull mice. Biochemical and biophysical research communications. 2008;377:248–252. doi: 10.1016/j.bbrc.2008.09.124. [DOI] [PubMed] [Google Scholar]
- 46.Vanwolleghem T, et al. Factors determining successful engraftment of hepatocytes and susceptibility to hepatitis B and C virus infection in uPA-SCID mice. Journal of hepatology. 2010;53:468–476. doi: 10.1016/j.jhep.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 47.Tsarev SA, et al. Characterization of a prototype strain of hepatitis E virus. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:559–563. doi: 10.1073/pnas.89.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pas SD, Fries E, De Man RA, Osterhaus AD, Niesters HG. Development of a quantitative real-time detection assay for hepatitis B virus DNA and comparison with two commercial assays. J Clin Microbiol. 2000;38:2897–2901. doi: 10.1128/jcm.38.8.2897-2901.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pas SD, Niesters HG. Detection of HBV DNA using real time analysis. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2002;25:93–94. doi: 10.1016/S1386-6532(02)00016-1. [DOI] [PubMed] [Google Scholar]
- 50.Movita D, et al. Inflammatory monocytes recruited to the liver within 24 hours after virus-induced inflammation resemble Kupffer cells but are functionally distinct. Journal of virology. 2015;89:4809–4817. doi: 10.1128/JVI.03733-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van de Garde MD, et al. Liver Monocytes and Kupffer Cells Remain Transcriptionally Distinct during Chronic Viral Infection. PloS one. 2016;11:e0166094. doi: 10.1371/journal.pone.0166094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


