Abstract
There is conflicting evidence on the favorable effects of vitamin D supplementation on metabolic profile in Type 2 diabetes mellitus (T2DM) patients and this might be due to genetic variations in vitamin D receptors (VDRs). Thus, we studied the metabolic effects of a 12-month vitamin D supplementation in T2DM patients according to VDR polymorphisms. A total of 204 T2DM subjects received 2000 IU vitamin D3 daily for 12 months. Serum 25(OH)D and metabolic profiles were measured at baseline and after 12 months. VDR polymorphisms (Taq-I, Bsm-I, Apa-I and Fok-I) were identified using TaqMan genotyping assays. Vitamin D supplementation significantly increased HOMA β-cell function (p = 0.003) as well as significantly decreased triglycerides, total and LDL-cholesterol (p < 0.001). The lowest increment in 25(OH)D levels was detected in patients with Fok-I CC genotypes (p < 0.0001). With vitamin D supplementation, Taq-I GG genotype carriers showed significant improvements in triglycerides, LDL- and total cholesterol, insulin, HbA1c and HOMA-IR (p < 0.005, 0.01, < 0.001, < 0.005, 0.03 and 0.01, respectively). Similarly, Bsm-I TT genotype carriers showed significant improvements in triglycerides (p = 0.01), insulin and HOMA-IR (p-values < 0.05). In conclusion, improvements in metabolic profile due to vitamin D supplementation is influenced by VDR polymorphisms, specifically for carriers of Taq-I GG and Bsm-I TT genotypes.
Introduction
Vitamin D plays a crucial role in calcium homeostasis and bone metabolism. Over the past decade, extra-skeletal effects of vitamin D has attracted considerable interest, including type 2 diabetes mellitus (T2DM)1, 2. In vitro studies showed that the active form of vitamin D (1,25-dihydroxyvitamin D or 1,25(OH)2D3) stimulates the release of insulin by the pancreatic β-cells3, 4. Numerous observational and epidemiological studies also reported an inverse association between serum 25-hydroxy vitamin D (25(OH)D) levels and fasting glucose, insulin resistance and lipid profile excluding HDL-cholesterol5–10. Despite promising results in favor of vitamin D, interventional studies yielded divergent results. Recent systematic reviews of randomized control trials (RCTs) in T2DM patients suggest an insufficient evidence about the beneficial effects of vitamin D supplementation on glucose homeostasis or insulin resistance11, 12. Similarly, some RCTs failed to indicate an association between vitamin D supplementation and a favorable lipid profile13, 14. In contrast, other studies have reported that vitamin D supplementation improved lipid profile and insulin sensitivity15–18. Differences in responses to vitamin D supplementation may partially be explained by the genetic makeup and variations involved in vitamin D pathway19. Vitamin D receptor (VDR) gene is one such factor responsible for regulating responses to vitamin D. It is known that the active form of vitamin D (1,25 (OH)2D3) binds with the cytosolic/nuclear VDR which then heterodimerizes with retinoid X receptor and regulates expression of several target genes20. In fact, VDR is expressed in a variety of tissues including muscles and pancreatic cells21, 22.
Several single nucleotide polymorphisms (SNPs) have been identified in the VDR gene including Taq-I, Bsm-I, and Apa-I polymorphisms, which are located at the 3′ untranslated region (3′ UTR) of the gene and are suspected to alter VDR expression23, 24. While another polymorphism known with Fok-I restriction site is located within the 5′ end near the promoter region23. Fok-I is a functional polymorphism that results in different translation initiation sites on VDR that lead to three amino acids becoming longer and less effective protein25. Since these polymorphisms affect the stability and activity of VDR mRNA and protein, it is likely that the individuals who are not responding cardiometabolically to vitamin D treatment might be due to these VDR SNPs. Indeed, recent studies have reported that the VDR genotypes may potentially affect the individual’s response to treatment. For instance, women with the VDR Bsm-I CC genotype had greater improvement in bone mineral density after treatment than those with the TT genotype26. Likewise, in patients with benign prostate hyperplasia, response to standard drug therapy is significantly associated with the VDR Taq-I genotype27.
There is limited information regarding the cardiometabolic effects of vitamin D intake based on VDR gene polymorphisms in T2DM subjects. The only study by Neyestani et al.19 have shown that subjects with VDR Fok-I ff genotype responded the least to vitamin D-fortified yogurt intake in terms of improvement serum 25(OH)D levels and some inflammatory markers. Given the potential of other VDR SNPs (Taq-I, Bsm-I, Apa-I) to influence the stability of VDR mRNA herein, we aim to evaluate the effects of vitamin D supplementation according to four different VDR SNPs (Taq-I, Bsm-I, Apa-I and Fok-I) on glycemic and lipid responses in T2DM patients. For this study, 204 T2DM patients who were mostly vitamin D deficient were enrolled and given 2000 IU vitamin D daily for 12 months, and screened for four different VDR SNPs (Taq-I, Bsm-I, Apa-I and Fok-I).
Results
Improved glycemic and lipid profile after 12 months of vitamin D supplementation
Table 1 shows the differences between baseline and 12-months supplementation in the studied subjects. There was a significant increase in serum 25(OH)D levels from baseline to12 months after vitamin D supplementation (p < 0.001). Improved serum 25(OH)D levels had a parallel significant decrease in LDL- total cholesterol (p < 0.0001) as well as triglycerides (p < 0.001) (Table 1). There was also a significant increase in serum insulin (p < 0.005) and HOMA-β cell function (p < 0.003).
Table 1.
Anthropometrics and biochemical characteristics of all T2DM subjects at baseline and after 12 months of vitamin D supplementation.
| N = 204 | Baseline | Follow-up | p value |
|---|---|---|---|
| BMI (kg/m2) | 32.3 ± 5.9 | 32.5 ± 6.0 | 0.008 |
| Waist Circumference (cm) | 106.2 ± 12.9 | 106.2 ± 12.9 | 0.843 |
| Hips Circumference (cm) | 110.9 ± 12.9 | 111.6 ± 13.7 | 0.112 |
| Waist-Hip Ratio | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.581 |
| Systolic Blood Pressure (mmHg) | 127.0 ± 15.1 | 127.2 ± 14.5 | 0.897 |
| Diastolic Blood Pressure (mmHg) | 80.1 ± 9.2 | 79.2 ± 9.5 | 0.355 |
| Triglycerides (mmol/l) | 2.0 ± 0.8 | 1.9 ± 0.8 | 0.037 |
| Total Cholesterol (mmol/l) | 5.3 ± 1.1 | 4.9 ± 1.1 | 0.0001 |
| HDL-Cholesterol (mmol/l) | 1.0 ± 0.3 | 0.9 ± 0.3 | 0.0001 |
| LDL-Cholesterol | 3.3 ± 0.9 | 3.0 ± 1.0 | 0.0001 |
| Fasting Glucose (mmol/l) | 10.6 ± 4.6 | 10.7 ± 4.5 | 0.974 |
| Insulin (uU/ml) # | 15.8 ± 9.8 | 18.5 ± 12.0 | 0.005 |
| HbA1c | 7.5 ± 2.3 | 7.7 ± 2.5 | 0.274 |
| HOMA-IR # | 8.1 ± 7.9 | 8.7 ± 7.8 | 0.187 |
| HOMA β-cell function # | 60.3 ± 61.2 | 72.0 ± 67.4 | 0.003 |
| 25(OH)D (nmol/l) | 33.4 ± 12.2 | 54.2 ± 17.6 | 0.0001 |
Note: Data were presented as mean ± standard deviation. # log-transformed prior to analysis. BMI- Body mass index; HOMA-IR- Homeostasis model assessment for insulin resistance.
Effect of VDR genotypes on anthropometric and clinical parameters after vitamin D supplementation
Prevalence of vitamin D sufficiency (serum 25(OH)D > 50 nmol/L) increased after 12 months of vitamin D supplementation. However, around 42.2% of the subjects remained vitamin D deficient (Fig. 1). To explore whether VDR genotype has any influence on determining the vitamin D status (Deficiency/Sufficiency), subjects were compared according to VDR genotype. At baseline, there was no significant trend in distribution of vitamin D deficiency status among VDR Fok-I variants (P-trend = 0.626). However, after intervention, trend test was highly significant (P-trend < 0·0001), showing > 51% of subjects with Fok-I TC genotype and almost all subjects with CC genotype remained vitamin D deficient (Table 2). Further data analysis indicates that after 12 months of vitamin D supplementation, subjects in Fok-I TT group showed better improvement (increased) in serum 25(OH)D levels compared to CC group (TT 24.4 ± 15.8 vs CC 8.4 ± 9.6; p < 0.001) (Table 3 and Fig. 2). This association remained significant (p = 0.005) even after adjusting for confounders such as age, gender, BMI and baseline 25(OH)D levels. After Bonferroni correction, this association remained significant (P = 0.02). In addition, subjects with the rare Fok-I CC genotype also showed significant increase in BMI (1.0 ± 1.4 kg/m2, p = 0.02) compared to TT and TC genotypes, after 12 months of vitamin D supplementation (Table 3).
Figure 1.
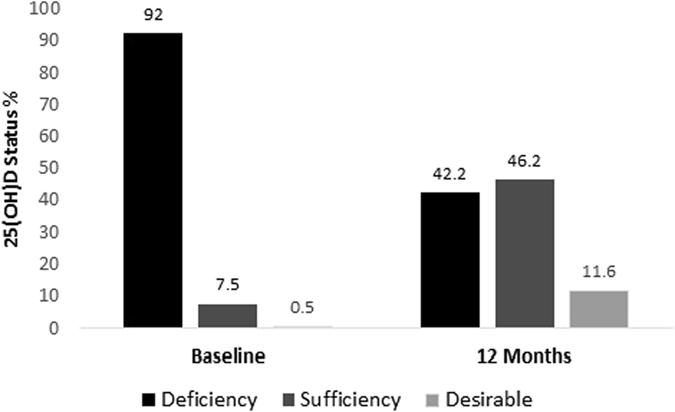
Comparison of the vitamin D status before and after 12 months of vitamin D supplementation. Deficiency: Serum 25(OH)D level < 50 nmol/L. Sufficiency: Serum 25(OH)D level ≥ 50 nmol/L. Desirable: Serum 25(OH)D level > 75 nmol/L.
Table 2.
Vitamin D status among the Fok-I genotypic groups before and after 12 months of vitamin D supplementation.
| Vitamin D status (N = 199) | TT (N = 113) | TC (70) | CC (16) | |||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | |
| Desirable | 0 (0.0) | 14 (12.4) | 1 (1.4) | 9 (12.9) | 0 (0.0) | 0 (0.0) |
| Sufficiency | 10 (8.8) | 66 (58.4) | 4 (5.7) | 25 (35.7) | 1 (6.3) | 1 (6.3) |
| Deficiency | 103 (91.2) | 33 (29.2) | 65 (92.9) | 36 (51.4) | 15 (93.8) | 15 (93.8) |
Note: Σ2 test showed a Ptrend of 0.626 at baseline; Ptrend at 12-month follow-up is < 0.0001. Data is presented as frequencies (%). Deficiency: Serum 25(OH)D level < 50 nmol/l. Sufficiency: Serum 25(OH)D level between 50 to 75 nmol/l. Desirable: Serum 25(OH)D level > 75 nmol/l.
Table 3.
Comparisons of the variables among the Fok-I genotypic before and after 12 months of vitamin D supplementation.
| Variables | TT (113) | TC (70) | CC (16) | P value | Pa | |
|---|---|---|---|---|---|---|
| BMI (kg/m2) | Baseline | 32.1 ± 5.9 | 32.4 ± 6.2 | 32.5 ± 5.4 | 0.02 | 0.01* |
| 12 Months | 32.4 ± 6.0 | 32.5 ± 6.2 | 33.6 ± 5.2 | |||
| Change | 0.2 ± 0.9 | 0.1 ± 1.1 | 1.0 ± 1.4aa | |||
| Systolic BP (mmHg) | Baseline | 126.7 ± 16.0 | 128.7 ± 13.9 | 116.0 ± 8.9 | 0.10 | 0.07 |
| 12 Months | 127.5 ± 14.6 | 126.4 ± 14.8 | 128.0 ± 11.0 | |||
| Change | 0.9 ± 14.9 | −2.2 ± 14.1 | 12.0 ± 11.0 | |||
| Diastolic BP (mmHg) | Baseline | 79.7 ± 8.9 | 81.8 ± 9.1 | 70.0 ± 7.1 | 0.16 | 0.26 |
| 12 Months | 79.5 ± 8.9 | 79.1 ± 10.1 | 76.0 ± 13.4 | |||
| Change | −0.3 ± 11.0 | −2.7 ± 9.2 | 6.0 ± 18.2 | |||
| Triglycerides (mmol/l) | Baseline | 2.0 ± 0.8 | 1.9 ± 0.8 | 2.0 ± 1.2 | 0.49 | 0.49 |
| 12 Months | 1.8 ± 0.8 | 1.9 ± 0.9 | 1.8 ± 0.9 | |||
| Change | −0.1 ± 0.7 | 0.0 ± 0.7 | −0.2 ± 0.7 | |||
| Total Cholesterol (mmol/l) (mmol/l) | Baseline | 5.3 ± 1.0 | 5.4 ± 1.1 | 5.0 ± 1.1 | 0.94 | 0.63 |
| 12 Months | 4.9 ± 1.0 | 5.0 ± 1.2 | 4.7 ± 1.0 | |||
| Change | −0.4 ± 0.9 | −0.4 ± 1.0 | −0.3 ± 1.2 | |||
| HDL-Cholesterol (mmol/l) | Baseline | 1.0 ± 0.3 | 1.1 ± 0.3 | 0.9 ± 0.4 | 0.47 | 0.14 |
| 12 Months | 0.9 ± 0.3 | 0.9 ± 0.3 | 0.9 ± 0.4 | |||
| Change | −0.1 ± 0.3 | −0.2 ± 0.3 | 0.0 ± 0.5 | |||
| LDL-Cholesterol (nmol/l) | Baseline | 3.3 ± 0.8 | 3.4 ± 1.1 | 3.0 ± 0.7 | 0.38 | 0.59 |
| 12 Months | 3.0 ± 0.9 | 3.0 ± 1.2 | 2.9 ± 0.9 | |||
| Change | −0.3 ± 0.9 | −0.4 ± 1.0 | −0.1 ± 0.8 | |||
| Glucose (mmol/l) | Baseline | 10.1 ± 3.9 | 11.9 ± 5.1 | 8.7 ± 5.0 | 0.33 | 0.12 |
| 12 Months | 10.5 ± 4.1 | 11.4 ± 5.1 | 8.9 ± 3.6 | |||
| Change | 0.4 ± 3.9 | −0.6 ± 4.9 | 0.2 ± 4.4 | |||
| Insulin (uU/ml) | Baseline | 16.1 ± 9.6 | 15.7 ± 10.2 | 12.6 ± 9.8 | 0.15 | 0.19 |
| 12 Months | 17.9 ± 11.7 | 18.9 ± 13.0 | 22.3 ± 5.6 | |||
| Change | 1.8 ± 12.1 | 3.2 ± 10.6 | 9.7 ± 11.3 | |||
| HbA1c | Baseline | 7.1 ± 2.1 | 8.1 ± 2.5 | 6.0 ± 1.7 | 0.28 | 0.25 |
| 12 Months | 7.5 ± 2.4 | 8.0 ± 2.6 | 6.8 ± 1.9 | |||
| Change | 0.4 ± 2.2 | −0.2 ± 2.7 | 0.8 ± 2.5 | |||
| HOMA-IR | Baseline | 8.1 ± 8.7 | 8.3 ± 6.9 | 4.9 ± 3.0 | 0.14 | 0.14 |
| 12 Months | 8.2 ± 8.1 | 9.4 ± 7.4 | 10.0 ± 7.4 | |||
| Change | 0.1 ± 5.9 | 1.0 ± 6.6 | 5.2 ± 6.3 | |||
| 25(OH)D (nmol/l) | Baseline | 33.0 ± 12.4 | 33.6 ± 12.3 | 36.0 ± 10.0 | 0.001 | 0.005** |
| 12 Months | 57.4 ± 17.3 | 51.3 ± 18.4 | 44.5 ± 9.2 | |||
| Change | 24.4 ± 15.8 | 17.7 ± 19.1 | 8.4 ± 9.6a |
Note: Change = Follow up − Baseline; p values presented are for the changes in different variables after vitamin D supplementation according to Fok-I genotypes; Pa indicates p values after adjusting age, gender, BMI and baseline 25(OH)D levels; Superscript “a” indicates significantly different from TT group after post hoc analysis; “*” indicates p < 0.05 after Bonferroni correction.
Figure 2.
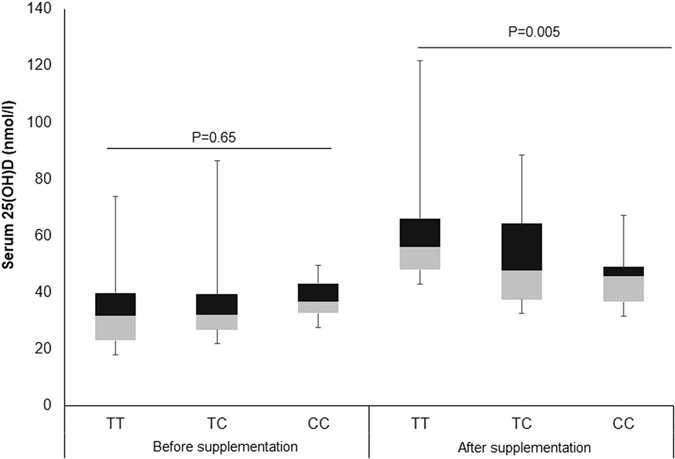
Box plot showing distribution of serum 25(OH)D levels (nmol/L) before and after vitamin D supplementation according to VDR fok-I genotypes.
Next, we compared the changes in glycemic and lipid profile according to VDR genotypes after intervention. Subjects with Taq-I GG genotype group showed significantly better improvements in glucose and lipid profile than other genotypes. A significant decrease in total cholesterol (−0.9 ± 0.9 mmol/l, p < 0.001), LDL-cholesterol (−0.7 ± 1.1, p = 0.01), triglycerides (−0.4 ± 0.5, p < 0.005), insulin (−2.3 ± 12.0 uU/ml, p < 0.005), HbA1c (p = 0.03) and HOMA-IR (p = 0.01) were observed in subjects with GG genotype than subjects with AA and AG genotypes (Table 4). However, significance for LDL-cholesterol (p = 0.09) and HOMA-IR (0.08) disappeared after adjustments for age, gender, BMI and baseline vitamin D levels. Associations of Taq-I genotypes with triglycerides, total-cholesterol, insulin and HbA1C remained significant after Bonferroni correction. Similarly, subjects carrying Bsm-I CC genotypes had better improvements in triglycerides (−0.4 ± 0.5, p = 0.01), insulin (−2.0 ± 11.7 uU/ml, p = 0.01) and HOMA-IR (p = 0.05) than other genotypes (Table 5). However, significance for HOMA-IR disappeared after adjustment with confounding factors. Likewise, subjects carrying Apa-I heterozygous AC genotype showed significantly better improvement in systolic blood pressure (−4.3 ± 14.5 mmHg, p = 0.009) than with AA and CC genotypes (Table 6).
Table 4.
Comparisons of the variables among the Taq-I genotypic before and after 12 months of vitamin D supplementation.
| Variables | GG (46) | AG (99) | AA (54) | P Value | Pa | |
|---|---|---|---|---|---|---|
| BMI (kg/m2) | Baseline | 32.0 ± 6.3 | 31.7 ± 5.9 | 33.5 ± 5.7 | 0.21 | 0.18 |
| 12 Months | 32.1 ± 6.2 | 31.9 ± 6.0 | 33.9 ± 5.7 | |||
| Change | 0.1 ± 0.9 | 0.1 ± 1.1 | 0.4 ± 0.9 | |||
| Systolic BP (mmHg) | Baseline | 128.6 ± 17.3 | 126.3 ± 14.3 | 126.9 ± 15.1 | 0.58 | 0.35 |
| 12 Months | 130.9 ± 16.8 | 126.3 ± 13.0 | 125.2 ± 14.8 | |||
| Change | 2.3 ± 15.4 | 0.1 ± 15.8 | −1.7 ± 11.0 | |||
| Diastolic BP (mmHg) | Baseline | 80.7 ± 10.0 | 80.0 ± 8.4 | 79.7 ± 10.2 | 0.21 | 0.20 |
| 12 Months | 82.0 ± 9.8 | 79.3 ± 9.4 | 76.0 ± 8.8 | |||
| Change | 1.3 ± 11.3 | −0.7 ± 11.4 | −3.6 ± 8.3 | |||
| Triglycerides (mmol/l) | Baseline | 2.0 ± 0.9 | 2.0 ± 0.9 | 1.9 ± 0.8 | 0.005 | 0.01* |
| 12 Months | 1.6 ± 0.9 | 1.9 ± 0.8 | 1.9 ± 0.8 | |||
| Change | −0.4 ± 0.5 | −0.1 ± 0.7a | 0.0 ± 0.7 | |||
| Total Cholesterol (mmol/l) | Baseline | 5.3 ± 1.1 | 5.5 ± 1.0 | 5.0 ± 1.1 | 0.001 | 0.01* |
| 12 Months | 4.4 ± 0.9 | 5.2 ± 1.0 | 4.9 ± 1.1 | |||
| Change | −0.9 ± 0.9 | −0.3 ± 0.9 | −0.1 ± 0.9a | |||
| HDL-Cholesterol (mmol/l) | Baseline | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.0 ± 0.3 | 0.22 | 0.71 |
| 12 Months | 0.8 ± 0.3 | 0.9 ± 0.3 | 1.0 ± 0.3 | |||
| Change | −0.1 ± 0.4 | −0.2 ± 0.3 | −0.1 ± 0.3 | |||
| LDL-Cholesterol (nmol/l) | Baseline | 3.4 ± 1.0 | 3.4 ± 0.9 | 3.1 ± 1.0 | 0.01 | 0.09 |
| 12 Months | 2.7 ± 0.8 | 3.2 ± 1.1 | 2.9 ± 0.9 | |||
| Change | −0.7 ± 1.1 | −0.2 ± 0.9 | −0.2 ± 0.8 | |||
| Glucose (mmol/l) | Baseline | 11.0 ± 3.8 | 10.6 ± 4.6 | 10.5 ± 5.1 | 0.06 | 0.14 |
| 12 Months | 9.8 ± 4.1 | 10.8 ± 4.5 | 11.2 ± 4.6 | |||
| Change | −1.2 ± 3.3 | 0.2 ± 4.5 | 0.8 ± 4.6 | |||
| Insulin (uU/ml) | Baseline | 19.0 ± 10.2 | 15.7 ± 9.9 | 13.4 ± 8.8 | 0.005 | 0.01* |
| 12 Months | 16.7 ± 10.3 | 19.1 ± 11.8 | 18.8 ± 13.9 | |||
| Change | −2.3 ± 12.0 | 3.4 ± 11.4a | 5.4 ± 10.3a | |||
| HbA1c | Baseline | 7.9 ± 2.4 | 7.2 ± 2.1 | 7.6 ± 2.5 | 0.03 | 0.01* |
| 12 Months | 7.2 ± 2.8 | 7.7 ± 2.3 | 8.0 ± 2.5 | |||
| Change | −0.7 ± 2.2 | 0.5 ± 2.4a | 0.4 ± 2.5a | |||
| HOMA-IR | Baseline | 9.2 ± 5.5 | 8.4 ± 9.8 | 6.6 ± 5.0 | 0.01 | 0.04 |
| 12 Months | 7.6 ± 6.5 | 9.5 ± 8.9 | 8.4 ± 6.4 | |||
| Change | −1.6 ± 5.5 | 1.1 ± 6.9 | 1.9 ± 5.1 | |||
| 25(OH)D (nmol/l) | Baseline | 31.9 ± 12.7 | 34.4 ± 12.6 | 33.3 ± 10.7 | 0.56 | 0.62 |
| 12 Months | 51.2 ± 13.6 | 54.7 ± 20.2 | 56.1 ± 15.5 | |||
| Change | 19.2 ± 15.7 | 20.3 ± 18.9 | 22.8 ± 15.4 |
Note: Change = Follow up − Baseline; p values presented are for the changes in different variables after vitamin D supplementation according to Taq-I genotypes; Pa indicates p values after adjusting age, gender, BMI and baseline 25(OH)D levels; Superscript “a” indicates significantly different from GG group after post hoc analysis; “*” indicates p < 0.05 after Bonferroni correction.
Table 5.
Comparisons of the variables among the Bsm-I genotypic before and after 12 months of vitamin D supplementation.
| Variables | TT (41) | CT (103) | CC (55) | P value | Pa | |
|---|---|---|---|---|---|---|
| BMI (kg/m2) | Baseline | 32.2 ± 6.1 | 31.4 ± 5.5 | 34.1 ± 6.3 | 0.23 | 0.17 |
| 12 Months | 32.4 ± 6.0 | 31.5 ± 5.6 | 34.6 ± 6.2 | |||
| Change | 0.2 ± 0.9 | 0.1 ± 1.1 | 0.4 ± 0.9 | |||
| Systolic BP (mmHg) | Baseline | 128.6 ± 17.3 | 125.5 ± 14.0 | 128.6 ± 15.6 | 0.22 | 0.09 |
| 12 Months | 130.9 ± 16.8 | 126.6 ± 12.8 | 124.6 ± 15.3 | |||
| Change | 2.3 ± 15.4 | 1.0 ± 15.0 | −3.9 ± 12.9 | |||
| Diastolic BP (mmHg) | Baseline | 80.7 ± 10.0 | 79.5 ± 8.4 | 80.7 ± 10.2 | 0.07 | 0.10 |
| 12 Months | 82.0 ± 9.8 | 79.3 ± 9.3 | 75.9 ± 8.9 | |||
| Change | 1.3 ± 11.3 | −0.2 ± 11.1 | −4.8 ± 8.6 | |||
| Triglycerides (mmol/l) | Baseline | 3.2 ± 1.0 | 3.4 ± 0.9 | 3.2 ± 1.0 | 0.01 | 0.03* |
| 12 Months | 2.8 ± 0.9 | 3.2 ± 1.1 | 2.8 ± 0.9 | |||
| Change | −0.5 ± 1.2 | −0.2 ± 0.9a | −0.4 ± 0.9 | |||
| Total Cholesterol (mmol/l) | Baseline | 5.2 ± 1.1 | 5.5 ± 1.0 | 5.1 ± 1.0 | 0.27 | 0.17 |
| 12 Months | 4.6 ± 1.0 | 5.2 ± 1.0 | 4.7 ± 1.0 | |||
| Change | −0.6 ± 1.1 | −0.3 ± 0.9 | −0.4 ± 0.9 | |||
| HDL-Cholesterol (mmol/l) | Baseline | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.0 ± 0.3 | 0.32 | 0.44 |
| 12 Months | 0.9 ± 0.3 | 0.9 ± 0.3 | 0.9 ± 0.3 | |||
| Change | −0.1 ± 0.3 | −0.2 ± 0.3 | −0.1 ± 0.4 | |||
| LDL-Cholesterol (nmol/l) | Baseline | 2.1 ± 0.8 | 2.0 ± 0.8 | 1.9 ± 0.8 | 0.38 | 0.39 |
| 12 Months | 1.7 ± 0.9 | 1.9 ± 0.8 | 1.8 ± 0.8 | |||
| Change | −0.4 ± 0.5 | 0.0 ± 0.7 | −0.1 ± 0.7 | |||
| Glucose (mmol/l) | Baseline | 11.1 ± 3.7 | 10.5 ± 4.6 | 10.6 ± 5.0 | 0.30 | 0.41 |
| 12 Months | 10.2 ± 4.2 | 10.9 ± 4.6 | 10.7 ± 4.5 | |||
| Change | −0.9 ± 3.7 | 0.3 ± 4.4 | 0.1 ± 4.6 | |||
| Insulin (uU/ml) | Baseline | 18.6 ± 10.1 | 15.7 ± 9.9 | 13.6 ± 9.0 | 0.01 | 0.01* |
| 12 Months | 16.6 ± 10.0 | 19.3 ± 11.7 | 18.7 ± 14.2 | |||
| Change | −2.0 ± 11.7 | 3.6 ± 11.3a | 5.1 ± 10.8a | |||
| HbA1c | Baseline | 8.0 ± 2.3 | 7.1 ± 2.2 | 7.5 ± 2.5 | 0.06 | 0.02* |
| 12 Months | 7.5 ± 2.8 | 7.7 ± 2.4 | 7.7 ± 2.5 | |||
| Change | −0.5 ± 2.3 | 0.6 ± 2.3a | 0.2 ± 2.6 | |||
| HOMA-IR | Baseline | 9.0 ± 5.4 | 8.4 ± 9.8 | 6.6 ± 5.1 | 0.05 | 0.10 |
| 12 Months | 7.7 ± 6.4 | 9.6 ± 8.9 | 8.0 ± 6.5 | |||
| Change | −1.2 ± 5.5 | 1.2 ± 6.9 | 1.4 ± 5.2 | |||
| 25(OH)D (nmol/l) | Baseline | 31.1 ± 14.0 | 34.5 ± 12.4 | 33.6 ± 9.8 | 0.47 | 0.41 |
| 12 Months | 50.1 ± 14.7 | 54.6 ± 19.8 | 56.6 ± 14.9 | |||
| Change | 19.0 ± 15.9 | 20.2 ± 18.5 | 23.0 ± 15.9 |
Note: Change = Follow up − Baseline; p values presented are for the changes in different variables after vitamin D supplementation according to Bsm-I genotypes; Pa indicates p values after adjusting age, gender, BMI and baseline 25(OH)D levels; Superscript “a” indicates significantly different from TT group after post hoc analysis; “*” indicates p < 0.05 after Bonferroni correction.
Table 6.
Comparisons of the variables among the Apa-I genotypic before and after 12 months of vitamin D supplementation.
| Variables | AA (87) | AC (84) | CC (28) | P Value | Pa | |
|---|---|---|---|---|---|---|
| BMI (kg/m2) | Baseline | 33.7 ± 5.6 | 32.2 ± 6.2 | 31.9 ± 5.7 | 0.47 | 0.51 |
| 12 Months | 34.2 ± 5.4 | 32.4 ± 6.3 | 32.1 ± 5.8 | |||
| Change | 0.5 ± 1.0 | 0.1 ± 0.9 | 0.2 ± 1.1 | |||
| Systolic BP (mmHg) | Baseline | 126.9 ± 11.8 | 130.1 ± 16.3 | 124.1 ± 14.4 | 0.009 | 0.001* |
| 12 Months | 127.7 ± 10.9 | 125.8 ± 15.4 | 128.3 ± 14.4 | |||
| Change | 0.8 ± 10.4 | −4.3 ± 14.5a | 4.2 ± 14.7 | |||
| Diastolic BP (mmHg) | Baseline | 80.8 ± 9.5 | 81.2 ± 8.9 | 78.9 ± 9.3 | 0.05 | 0.07 |
| 12 Months | 76.2 ± 9.6 | 78.4 ± 9.1 | 80.6 ± 9.8 | |||
| Change | −4.6 ± 6.6 | −2.7 ± 10.6 | 1.6 ± 11.3 | |||
| Triglycerides (mmol/l) | Baseline | 1.9 ± 0.7 | 1.9 ± 0.8 | 2.0 ± 0.9 | 0.50 | 0.98 |
| 12 Months | 1.8 ± 0.8 | 1.9 ± 0.7 | 1.8 ± 0.9 | |||
| Change | −0.1 ± 0.7 | 0.0 ± 0.7 | −0.2 ± 0.7 | |||
| Total Cholesterol (mmol/l) | Baseline | 5.1 ± 1.1 | 5.4 ± 0.9 | 5.3 ± 1.1 | 0.99 | 0.93 |
| 12 Months | 4.7 ± 1.0 | 5.0 ± 1.1 | 4.9 ± 1.0 | |||
| Change | −0.4 ± 1.0 | −0.4 ± 1.0 | −0.4 ± 0.9 | |||
| HDL-Cholesterol (mmol/l) | Baseline | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.0 ± 0.3 | 0.90 | 0.98 |
| 12 Months | 0.9 ± 0.4 | 0.9 ± 0.3 | 0.8 ± 0.3 | |||
| Change | −0.1 ± 0.5 | −0.1 ± 0.3 | −0.2 ± 0.3 | |||
| LDL-Cholesterol (nmol/l) | Baseline | 3.0 ± 0.9 | 3.5 ± 0.8 | 3.2 ± 1.0 | 0.79 | 0.77 |
| 12 Months | 2.7 ± 0.9 | 3.1 ± 1.0 | 2.9 ± 1.0 | |||
| Change | −0.3 ± 0.7 | −0.4 ± 1.0 | −0.3 ± 1.0 | |||
| Glucose (mmol/l) | Baseline | 11.0 ± 5.9 | 10.7 ± 4.3 | 10.6 ± 4.3 | 0.69 | 0.62 |
| 12 Months | 10.4 ± 4.2 | 10.8 ± 4.6 | 10.6 ± 4.4 | |||
| Change | −0.6 ± 4.9 | 0.2 ± 4.7 | 0.1 ± 3.7 | |||
| Insulin (uU/ml) | Baseline | 14.4 ± 7.0 | 15.3 ± 10.1 | 16.8 ± 10.2 | 0.98 | 0.86 |
| 12 Months | 17.0 ± 11.6 | 18.3 ± 13.1 | 19.1 ± 11.1 | |||
| Change | 2.6 ± 7.9 | 3.0 ± 11.7 | 2.3 ± 12.4 | |||
| HbA1c | Baseline | 7.4 ± 2.7 | 7.4 ± 2.3 | 7.5 ± 2.2 | 0.90 | 0.83 |
| 12 Months | 7.8 ± 2.5 | 7.6 ± 2.4 | 7.7 ± 2.6 | |||
| Change | 0.4 ± 3.0 | 0.2 ± 2.3 | 0.1 ± 2.4 | |||
| HOMA-IR | Baseline | 6.7 ± 3.5 | 7.9 ± 6.6 | 8.7 ± 9.9 | 0.63 | 0.66 |
| 12 Months | 6.6 ± 3.4 | 8.5 ± 7.0 | 9.7 ± 9.3 | |||
| Change | −0.2 ± 2.9 | 0.6 ± 6.6 | 1.0 ± 6.6 | |||
| 25(OH)D (nmol/l) | Baseline | 35.1 ± 9.5 | 35.0 ± 13.6 | 31.7 ± 11.2 | 0.97 | 0.44 |
| 12 Months | 56.2 ± 13.3 | 55.9 ± 20.3 | 52.1 ± 16.1 | |||
| Change | 21.1 ± 14.3 | 20.9 ± 18.2 | 20.4 ± 17.4 |
Note: Change = Follow up − Baseline; p values presented are for the changes in different variables after vitamin D supplementation according to Apa-I genotypes; Pa indicates p values after adjusting age, gender, BMI and baseline 25(OH)D levels; Superscript “a” indicates significantly different from AA group after post hoc analysis; “*” indicates p < 0.05 after Bonferroni correction.
Haplotype and linkage disequilibrium (LD) analysis
VDR gene polymorphisms at Taq-I were in LD with both Bsm-I and Apa-I (R2 = 0.87 and −0.44 respectively). In addition, Bsm-I and ApaI SNPs were also in LD with each other (R2 = −0.61) (Supplementary Table S1). As there was a strong positive LD between Taq-I and Bsm-I SNPs, indicating two alleles occurred on the same haplotype more than expected. Thus, we combined the different genotypes from Taq-I and Bsm-I to check the haplotype effect on various parameters. After 12 months of vitamin D supplementation, Taq-IGG + Bsm-ITT haplotype showed significantly higher decrease in total cholesterol (−0.8 ± 0.9; p = 0.015), glucose (−1.3 ± 3.6; p = 0.036), insulin (−2.3 ± 12.0; p = 0.010), triglycerides (−0.4 ± 0.5; p = 0.012), HbA1c (−0.7 ± 2.3; p = 0.03) and HOMA-IR (−1.6 ± 5.5; p = 0.024) compared to Taq-IAA + Bsm-ICC and Taq-IAG + Bsm-ICG (Supplementary Table S2).
Disscussion
The present study showed varying degrees of metabolic improvements from vitamin D supplementation, partly due to variability in the VDR gene. In patients with Fok-I CC genotypic group, improvement in serum 25(OH)D levels was the least compared to other Fok-I genotypic groups, implying that VDR gene polymorphisms might be crucial for vitamin D intervention. Intake of 2000 IU/D vitamin D has beneficial effects on glycemic and lipid profile, but these effects were more pronounced in patients with Taq-I GG and Bsm-I TT genotypes in VDR gene.
In the present study, 42.2% of the subjects were not able to achieve the desirable 25(OH)D levels (>50 nmol/L) despite a year-long supplementation. In a RCT on obese T2DM Emirati participants, receiving high doses vitamin D3 was not sufficient to achieve target serum 25(OH)D levels (≥75 nmol/L)28. In a pilot study on American subjects randomized to 800 IU/d of supplemental vitamin D, only 50% of the participants reached the desirable serum 25(OH)D levels29. Moreover, our previous data in Saudi T2DM patients treated with 2000 IU/D of vitamin D for 18 months, as much as 22% of subjects remained deficient30. Since genetic analyses was missing in these studies, variations in the genes that regulate 25(OH)D levels could be one explanation. To prove this, data from the present study indicated an unaltered vitamin deficiency status in subjects belonging to VDR Fok-I CC group. Individuals with homozygous major allele Fok-I (TT) had significantly better increase in serum 25(OH)D levels than CC genotype. It is likely that causal variant (Fok-I CC) could possibly alter their role in metabolic feedback loops or effect the speed at which 25(OH)D is metabolized31. Smolders et al. have reported that 1,25(OH)2D limits its own levels and the levels of its precursor 25(OH)D via the VDR and demonstrated that 25(OH)D and 1,25(OH)2D-hydroxylation is affected by the Fok-I VDR polymorphism32. However, these genotypic variants were not associated with baseline 25(OH)D levels in the present study. It can be speculated that Fok-I genotypic variants might require a higher threshold 25(OH)D levels to exhibit its feedback effects. Alternatively, this SNP may be in linkage disequilibrium with other neighboring functional polymorphisms which are known to affect baseline 25(OH)D levels. Perhaps, there could be different mechanisms that regulate 25(OH)D derived from supplementation vs cutaneous synthesis. In agreement with our results, Neyestani et al. reported T2DM patients with minor Fok-I ff (corresponds to Fok-I CC in current study) had significantly lower serum 25(OH)D levels after daily intake of vitamin D fortified yoghurt19. Similarly, in a randomized double blinded, placebo-controlled trial, Yao et al. demonstrated an association between Fok-I G allele and increase in 25(OH)D levels33. Data form previous studies and non-genetic factors including age, BMI and baseline 25(OH)D concentrations could influence vitamin D treatment outcome34, 35. The association between Fok-I genotypes and increase in serum 25(OH)D levels remained significant even after adjustments of the above mentioned confounding factors, indicating an independent association.
Longitudinal studies have indicated an association between elevated serum 25(OH)D levels with lower T2DM risk8, 36. However, results from various RCTs have yielded conflicting results. The current study suggests that vitamin D intake favorably affects insulin sensitivity and lipid profile of T2DM patients. Muñoz-Aguirre et al.15 have also reported that in T2DM patients, 4000 IU/D of vitamin D intake may improve serum triglycerides. Similarly, Pittas et al. reported that in the elderly people with impaired fasting glucose, vitamin D and calcium supplementation might attenuate insulin resistance37. On the contrary, a recent systematic review and meta-analysis by Sieda et al. involving 35 RCTs concluded no effect of vitamin D supplementation on glucose homeostasis or diabetes prevention11. One possible reason for varying results could be the heterogeneity of the studied cohorts and the genetic differences among various ethnic groups. Thus, studying the genetic polymorphisms involved in vitamin D pathway might elucidate the potential reasons for inconsistent responses to vitamin D supplementation. For instance, the frequency of VDR Taq-I GG was 19.2% in the current study, while it was 15.6% in Non-Hispanic whites, 7.2% in Non-Hispanic blacks and 4.9% in Mexican-Americans38. Vitamin D modulates the expression of target genes upon binding to VDR, thus studying SNPs in VDR gene might provide insightful inter-individual or inter-population variability in response to vitamin D supplementation.
VDR affects cholesterol and bile acid synthesis in hepatocytes and serum by controlling expression of genes involved in bile acid synthesis39. Results from humans, animals and in vitro studies indicated that vitamin D deficiency increases serum cholesterol levels by reducing VDR activity40. Disruption of the VDR signaling pathway is associated with a reduction in pancreatic insulin mRNA levels leading to impairment in oral glucose tolerance and reduced insulin secretory capacity in normally fed mice41. Several studies showed a correlation between VDR genotypes with risk of obesity, diabetes and metabolic syndrome. However, the information about the effects of vitamin D supplementation and VDR genotypes in improving cardiometabolic aspects in T2DM patients are limited. In the present study, though we found no significant variance in increment 25(OH)D levels among various Taq-I and Bsm-I genotypic groups after vitamin supplementation, patients with Taq-I GG showed significant decrease in LDL-, total cholesterol, triglycerides and HOMA-IR levels than their counterparts. Importantly, except for LDL-cholesterol and HOMA-IR, the association remained significant after Bonferroni adjustments. Similarly, patients carrying Bsm-I TT genotypes showed significant differences in LDL-cholesterol and HOMA-IR levels than their counterparts with Bsm-I CT and CC genotypes. Hence, despite a similar rise in serum 25(OH)D levels in all subjects after supplementation, patients carrying Taq-I GG and Bsm-I TT genotypes seem to have better improvement in glycemic and lipid parameters. In particular, Bsm-I, Apa-I, and Taq-I RFLPs are located near the 3′ UTR region, which is known to be involved in the regulation of mRNA stability. Hypothetically, the presence of Taq-I G allele and Bsm-I T allele might be providing better VDR mRNA stability and half-life resulting to a higher number of VDRs expressed for a longer time in target cells, thus leading in a better response to vitamin D supplementation.
The authors acknowledge several limitations. Findings cannot be generalized since only patients with T2DM were included. Other confounders such as lifestyle, non-genetic factors such BMI, type of anti-diabetic drugs and duration of diabetes were not accounted. Finally, the present study did not have a control/placebo group and therefore bias is likely in the reported changes post intervention.
Nevertheless, VDR polymorphisms influence metabolic response to vitamin D supplementation. VDR genotypic variations might help identify those who will benefit the most from vitamin D treatment. Least improvement in serum 25(OH)D levels was observed in patients carrying VDR Fok-I CC genotypes and might need higher doses of vitamin D than their counterparts in order to achieve sufficient levels. Cardiometabolic improvement was more evident in carriers of Taq-I GG and Bsm-I TT polymorphisms. These carriers may be the better responders to vitamin D therapy in terms of improving metabolic profile and a more tailored approach to vitamin D intervention is warranted. However, further analyses in other ethnic populations are needed to fully validate these findings.
Material and Methods
Subjects
This study is a part of our previously described vitamin D intervention study30. Briefly, a multi-center, interventional study was setup in the primary health care centers (PHCCs) in Riyadh, Saudi Arabia. 204 T2DM Saudi adults who were mostly vitamin D deficient [serum 25(OH)D < 50 nmol/l)] (men, N = 90: women, N = 114) were given 2000 IU vitamin D3 (Merck Pharma, Germany) daily for 12 months. All subjects answered a generalized questionnaire including present and past medical history. They underwent physical examination and submitted their written informed consents prior to inclusion. Patients taking multivitamins, calcium, cortisone or any other steroids, products with mineral oil, regular antacids, diuretics, phenytoin and phenobarbital medications, weight-loss drugs, gallbladder or gastrointestinal disorders and liver problems, were excluded as well as evidence of metabolic disease (Paget’s disease or osteomalacia), renal stone disease, hyperparathyroidism and abnormal levels of calcium, alkaline phosphatase and phosphorous. Ethical approval of the study including all methods and experimental protocols were obtained from the Institutional Review Board (IRB) of the College of Medicine, King Saud University in Riyadh, Saudi Arabia. All methods were performed in accordance with the relevant guidelines and regulations.
Anthropometry and blood withdrawal
After an overnight fast, subjects returned to their respective PHCCs for anthropometry and blood withdrawal. Anthropometry included weight, height, hip and waist circumference (cm), systolic and diastolic blood pressure. Body mass index (BMI) was calculated (kg/m2). Blood was transferred to a non-heparinized tube for centrifugation. Serum was then aliquoted into plain tubes and stored at −20 °C until further use.
Biochemical analysis
Methods for biochemical analysis was described in our previous paper30. In brief, fasting glucose and lipid profile were measured using chemical analyzer (Konelab, Finland). Serum 25(OH)D levels were measured using ELISA (IDS Ltd., Boldon Colliery, Tyne & Wear, UK).The inter and intra-assay variabilities were 5.3% and 4.6% respectively.. Serum insulin was measured using multiplex assay kits (LuminexW xMAPW Technology platform) (Luminexcorp, Texas). The inter-assay variation was <21% and intra-assay variation was 1.4–7.9%. Vitamin D deficiency was defined as serum 25(OH)D levels < 50 nmol/L. Homeostasis model assessment for insulin resistance (HOMA-IR) was calculated as fasting insulin (IU) x fasting glucose (mmol/L)/22.5.HOMA-β secretion (%) was calculated as 20 × fasting insulin (IU)/(fastingglucose-3.5).
VDR gene analysis
Genomic DNA was extracted from whole blood using DNeasy blood and tissue kit (Qiagen, Hilden, Germany). The DNA purity (260/280ratio) and concentration were measured using a Nano-drop spectrophotometer. The four VDR SNPs: Taq-I(rs731236), Bsm-I(rs1544410), Apa-I(rs7975232), and Fok-I(rs10735810) were evaluated by allelic discrimination real-time PCR using predesigned TaqMan genotyping assays (Applied Biosystems, Foster City, CA). The PCR steps comprisedof a hot start at 95 °C for 10 minutes followed by 45 cycles of 94 °C for 15 seconds and 60 °C for 1 minute; fluorescence detection occurs at of 60 °C. All the genotyping was performed in 10 µl reactions, using TaqMan genotyping mastermix in 96-well plates in an ABI 7000 instrument (Applied Biosystems).
Five samples were excluded from the analysis due to the failure of genotyping results.
Statistical Analysis
Data was analyzed using SPSS version 21.0, IBM (Armonk, NY, USA). Variables are expressed as mean ± standard deviation (SD). Normality assumption of data was tested using Kolmogrov-Smirnov test. Non-Gaussian variables were logarithmically transformed. Paired T-test was done to compare pre and post supplementation and analysis of variance (ANOVA) to compare genotype groups. Post hoc analysis was done using Tukey’s test. Mantel-Haenzsel test (χ 2 linear by linear association) for trend for checking p-trend. Bonferroni correction was applied to adjust for multiple comparisons among genotypes. Significance was set at p < 0.05.
Electronic supplementary material
Acknowledgements
The project was financially supported by Vice Deanship of Research Chairs, King Saud University, Riyadh, Saudi Arabia. Authors are grateful to Mr. Malak Nawaz Khan Khattak for the statistical analysis of data.
Author Contributions
N.M.A. designed the experiments and revised the manuscript. A.K.M. designed, executed the experiments and drafted the manuscript. O.S.A. analyzed the data. M.G.A.A. and K.W. participated in sample collection, data collection and experimental analysis. S.S. revised and edited the manuscript. G.T. helped drafting and revision of the manuscript. M.S.A. interpreted and analyzed the data. All authors have read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-08621-7
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bikle D. Nonclassic actions of vitamin D. The Journal of Clinical Endocrinology & Metabolism. 2009;94:26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. New England Journal of Medicine. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Bland R, et al. Expression of 25-hydroxyvitamin D 3-1α-hydroxylase in pancreatic islets. The Journal of steroid biochemistry and molecular biology. 2004;89:121–125. doi: 10.1016/j.jsbmb.2004.03.115. [DOI] [PubMed] [Google Scholar]
- 4.Zehnder D, et al. Extrarenal Expression of 25-Hydroxyvitamin D3-1α-Hydroxylase 1. The Journal of Clinical Endocrinology & Metabolism. 2001;86:888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among US adults. Diabetes care. 2005;28:1228–1230. doi: 10.2337/diacare.28.5.1228. [DOI] [PubMed] [Google Scholar]
- 6.Forouhi NG, Luan Ja, Cooper A, Boucher BJ, Wareham NJ. Baseline serum 25-hydroxy vitamin d is predictive of future glycemic status and insulin resistance the medical research council ely prospective study 1990–2000. Diabetes. 2008;57:2619–2625. doi: 10.2337/db08-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes care. 2004;27:2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 8.Knekt P, et al. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology. 2008;19:666–671. doi: 10.1097/EDE.0b013e318176b8ad. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhuri, J. R. et al. Deficiency of 25-hydroxyvitamin d and dyslipidemia in Indian subjects. Journal of lipids2013 (2013). [DOI] [PMC free article] [PubMed]
- 10.Karhapää P, et al. Diverse associations of 25‐hydroxyvitamin D and 1,25‐dihydroxy‐vitamin D with dyslipidaemias. Journal of internal medicine. 2010;268:604–610. doi: 10.1111/j.1365-2796.2010.02279.x. [DOI] [PubMed] [Google Scholar]
- 11.Seida JC, et al. Effect of vitamin D3 supplementation on improving glucose homeostasis and preventing diabetes: a systematic review and meta-analysis. The Journal of Clinical Endocrinology & Metabolism. 2014;99:3551–3560. doi: 10.1210/jc.2014-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George P, Pearson E, Witham M. Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta‐analysis. Diabetic Medicine. 2012;29:e142–e150. doi: 10.1111/j.1464-5491.2012.03672.x. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Xia N, Yang Y, Peng D-Q. Influence of vitamin D supplementation on plasma lipid profiles: a meta-analysis of randomized controlled trials. Lipids in health and disease. 2012;11 doi: 10.1186/1476-511X-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ponda MP, Dowd K, Finkielstein D, Holt PR, Breslow JL. The Short-Term Effects of Vitamin D Repletion on Cholesterol A Randomized, Placebo-Controlled Trial. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:2510–2515. doi: 10.1161/ATVBAHA.112.254110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munoz-Aguirre P, et al. The effect of vitamin D supplementation on serum lipids in postmenopausal women with diabetes: a randomized controlled trial. Clinical Nutrition. 2015;34:799–804. doi: 10.1016/j.clnu.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Major GC, Alarie F, Doré J, Phouttama S, Tremblay A. Supplementation with calcium+ vitamin D enhances the beneficial effect of weight loss on plasma lipid and lipoprotein concentrations. The American journal of clinical nutrition. 2007;85:54–59. doi: 10.1093/ajcn/85.1.54. [DOI] [PubMed] [Google Scholar]
- 17.von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient–a randomised, placebo-controlled trial. British Journal of Nutrition. 2010;103:549–555. doi: 10.1017/S0007114509992017. [DOI] [PubMed] [Google Scholar]
- 18.Sun X, et al. Vitamin D supplementation reduces insulin resistance in Japanese adults: a secondary analysis of a double-blind, randomized, placebo-controlled trial. Nutrition Research. 2016;36:1121–1129. doi: 10.1016/j.nutres.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Neyestani TR, et al. Vitamin D Receptor Fok-I Polymorphism Modulates Diabetic Host Response to Vitamin D Intake Need for a nutrigenetic approach. Diabetes care. 2013;36:550–556. doi: 10.2337/dc12-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jurutka PW, et al. Molecular nature of the vitamin D receptor and its role in regulation of gene expression. Reviews in Endocrine and Metabolic Disorders. 2001;2:203–216. doi: 10.1023/A:1010062929140. [DOI] [PubMed] [Google Scholar]
- 21.Johnson JA, Grande JP, Roche PC, Kumar R. Immunohistochemical localization of the 1, 25 (OH) 2D3 receptor and calbindin D28k in human and rat pancreas. American Journal of Physiology-Endocrinology and Metabolism. 1994;267:E356–E360. doi: 10.1152/ajpendo.1994.267.3.E356. [DOI] [PubMed] [Google Scholar]
- 22.Bozic, M. et al. Hepatocyte vitamin D receptor regulates lipid metabolism and mediates experimental diet-induced steatosis. Journal of Hepatology (2016). [DOI] [PubMed]
- 23.Uitterlinden AG, Fang Y, van Meurs JB, Pols HA, van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338:143–156. doi: 10.1016/j.gene.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Ogunkolade B-W, et al. Vitamin D receptor (VDR) mRNA and VDR protein levels in relation to vitamin D status, insulin secretory capacity, and VDR genotype in Bangladeshi Asians. Diabetes. 2002;51:2294–2300. doi: 10.2337/diabetes.51.7.2294. [DOI] [PubMed] [Google Scholar]
- 25.Colin EM, et al. Consequences of vitamin D receptor gene polymorphisms for growth inhibition of cultured human peripheral blood mononuclear cells by 1, 25‐dihydroxyvitamin D3. Clinical endocrinology. 2000;52:211–216. doi: 10.1046/j.1365-2265.2000.00909.x. [DOI] [PubMed] [Google Scholar]
- 26.Palomba S, et al. BsmI vitamin D receptor genotypes influence the efficacy of antiresorptive treatments in postmenopausal osteoporotic women. A 1-year multicenter, randomized and controlled trial. Osteoporos Int. 2005;16:943–952. doi: 10.1007/s00198-004-1800-5. [DOI] [PubMed] [Google Scholar]
- 27.Manchanda PK, Kibler AJ, Zhang M, Ravi J, Bid HK. Vitamin D receptor as a therapeutic target for benign prostatic hyperplasia. Indian journal of urology: IJU: journal of the Urological Society of India. 2012;28 doi: 10.4103/0970-1591.105745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadiya A, et al. Vitamin D 3 supplementation and body composition in persons with obesity and type 2 diabetes in the UAE: a randomized controlled double-blinded clinical trial. Clinical Nutrition. 2016;35:77–82. doi: 10.1016/j.clnu.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 29.McCullough ML, et al. Vitamin D status and impact of vitamin D3 and/or calcium supplementation in a randomized pilot study in the Southeastern United States. Journal of the American College of Nutrition. 2009;28:678–686. doi: 10.1080/07315724.2009.10719801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Daghri NM, et al. Vitamin D supplementation as an adjuvant therapy for patients with T2DM: an 18-month prospective interventional study. Cardiovascular diabetology. 2012;11 doi: 10.1186/1475-2840-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orton S-M, et al. Evidence for genetic regulation of vitamin D status in twins with multiple sclerosis. The American journal of clinical nutrition. 2008;88:441–447. doi: 10.1093/ajcn/88.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smolders J, Damoiseaux J, Menheere P, Tervaert JWC, Hupperts R. Fok-I vitamin D receptor gene polymorphism (rs10735810) and vitamin D metabolism in multiple sclerosis. Journal of neuroimmunology. 2009;207:117–121. doi: 10.1016/j.jneuroim.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Yao, P. et al. Effects of Genetic and Non-genetic Factors on Total and Bioavailable 25 (OH) D Responses to Vitamin D Supplementation. The Journal of Clinical Endocrinology & Metabolism, jc. 2016–2930 (2016). [DOI] [PubMed]
- 34.Mazahery H, von Hurst PR. Factors affecting 25-hydroxyvitamin D concentration in response to vitamin D supplementation. Nutrients. 2015;7:5111–5142. doi: 10.3390/nu7075111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang, M. et al. SNP rs11185644 of RXRA gene is identified for dose-response variability to vitamin D3 supplementation: a randomized clinical trial. Scientific Reports7 (2017). [DOI] [PMC free article] [PubMed]
- 36.Mitri J, Muraru M, Pittas A. Vitamin D and type 2 diabetes: a systematic review. European journal of clinical nutrition. 2011;65:1005–1015. doi: 10.1038/ejcn.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes care. 2007;30:980–986. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 38.Genomics, P. H. VDR Allele and Genotype Frequencies. Centers for Disease Control and Preventionhttps://www.cdc.gov/genomics/population/genvar/frequencies/vdr.htm (2009).
- 39.Gonzalez FJ, Moschetta A. Potential role of the vitamin D receptor in control of cholesterol levels. Gastroenterology. 2014;146:899–902. doi: 10.1053/j.gastro.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li, S. et al. Increase of circulating cholesterol in vitamin D deficiency is linked to reduced vitamin D receptor activity via the Insig‐2/SREBP‐2 pathway. Molecular nutrition & food research (2016). [DOI] [PubMed]
- 41.Zeitz U, et al. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. The FASEB journal. 2003;17:509–511. doi: 10.1096/fj.02-0424fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


