The bAP-Ca2+ signal amplitudes in some apical tuft branches randomly vary from moment to moment. In repetitive measurements, successful AP invasions are followed by complete failures. Passive spread of voltage from the apical trunk into the tuft occasionally reaches the threshold for local Na+ spike, resulting in stronger Ca2+ influx. During a burst of three somatic APs, the peak of dendritic Ca2+ in the apical tuft occurs with a delay of 20-50 ms depending on AP frequency.
Keywords: prefrontal, somatosensory, layer 1, dendritic excitability, potassium current, AP flickering, voltage-sensitive dye
Abstract
In cortical pyramidal neurons, backpropagating action potentials (bAPs) supply Ca2+ to synaptic contacts on dendrites. To determine whether the efficacy of AP backpropagation into apical tuft dendrites is stable over time, we performed dendritic Ca2+ and voltage imaging in rat brain slices. We found that the amplitude of bAP-Ca2+ in apical tuft branches was unstable, given that it varied from trial to trial (termed “bAP-Ca2+ flickering”). Small perturbations in dendritic physiology, such as spontaneous synaptic inputs, channel inactivation, or temperature-induced changes in channel kinetics, can cause bAP flickering. In the tuft branches, the density of Na+ and K+ channels was sufficient to support local initiation of fast spikelets by glutamate iontophoresis. We quantified the time delay between the somatic AP burst and the peak of dendritic Ca2+ transient in the apical tuft, because this delay is important for induction of spike-timing dependent plasticity. Depending on the frequency of the somatic AP triplets, Ca2+ signals peaked in the apical tuft 20–50 ms after the 1st AP in the soma. Interestingly, at low frequency (<20 Hz), the Ca2+ peaked sooner than at high frequency, because only the 1st AP invaded tuft. Activation of dendritic voltage-gated Ca2+ channels is sensitive to the duration of the dendritic voltage transient. In apical tuft branches, small changes in the duration of bAP voltage waveforms cause disproportionately large increases in dendritic Ca2+ influx (bAP-Ca2+ flickering). The stochastic nature of bAP-Ca2+ adds a new perspective on the mechanisms by which pyramidal neurons combine inputs arriving at different cortical layers.
NEW & NOTEWORTHY The bAP-Ca2+ signal amplitudes in some apical tuft branches randomly vary from moment to moment. In repetitive measurements, successful AP invasions are followed by complete failures. Passive spread of voltage from the apical trunk into the tuft occasionally reaches the threshold for local Na+ spike, resulting in stronger Ca2+ influx. During a burst of three somatic APs, the peak of dendritic Ca2+ in the apical tuft occurs with a delay of 20-50 ms depending on AP frequency.
a backpropagating action potential (bAP) is thought to bring Ca2+ to synaptic contacts on distal dendrites, providing necessary conditions for synaptic plasticity and release of retrograde messengers as well as changes in local dendritic membrane excitability. bAP-induced Ca2+ transients must exhibit adequate amplitude and timing to have any impact on synaptic plasticity (e.g., precise timing between somatic AP and synaptic input; Feldman 2012). Layer 1 (L1) is the most superficial layer of the neocortex, where glutamatergic and neuromodulatory axons impinge on terminal apical tuft dendrites of pyramidal neurons (Cruikshank et al. 2012; Eccles 1984). Glutamatergic afferents in L1 convey information about cortical processing from the adjacent cortical areas. They also stream continuous feedback from the remote association cortices, nonspecific thalamic nuclei, and subcortical gray matter (Gilbert and Wiesel 1989). Neuromodulatory fibers, on the other hand, project from the brain stem and basal forebrain to inform cortical L1 about cognitive and emotional states (Arnsten et al. 2012). Given the importance of glutamatergic and neuromodulatory inputs, it is peculiar that a relatively large fraction of these inputs impinges onto apical tuft branches, because in pyramidal neurons, the apical tuft dendrites are the most electrically isolated dendritic region from the AP initiation site (Gulledge et al. 2005). In L5 pyramidal neurons of the rat prefrontal cortex (PFC), AP backpropagation is decremental over large distances along the apical trunk. Consequently, the AP-associated Ca2+ transients are seldom present in the most proximal segments of apical tuft dendrites and never present in dendritic tips (Barth et al. 2008; Gulledge and Stuart 2003).
A small change in a single voltage-gated dendritic conductance is sufficient for triggering a significant change (decrease or increase) in the bAP amplitude in distal apical segments (Barth et al. 2008; Hoffman et al. 1997; Stuart and Sakmann 1994). In both hippocampal and neocortical pyramidal neurons, the dendritic voltage-gated channels are prone to neuromodulation (Hoffman and Johnston 1999; Tsubokawa and Ross 1997; Zhou and Antic 2012). Furthermore, a patterned synaptic input from the Schaffer collaterals into proximal and mid-apical sites, commonly employed in long-term potentiation (LTP) induction protocols, has been shown to alter intrinsic membrane excitability of local dendritic segments, which is manifested by a detectable enhancement of AP amplitude in that segment (Frick et al. 2004; Rosenkranz et al. 2009). In this study we asked whether poor efficacy of AP backpropagation into the distal apical tuft is a stable feature of cortical L5 pyramidal neurons (Barth et al. 2008; Boudewijns et al. 2013; Gulledge and Stuart 2003). Toward this goal, we optically monitored AP-associated Ca2+ transients in apical tuft dendrites every 2–5 min, for ~100 min. In the absence of cortical afferent inputs (in brain slices), AP backpropagation in the apical tuft was often variable in subsequent trials. Backpropagating APs either failed to propagate into the distal apical tuft, resulting in a small or undetectable Ca2+ influx, or successfully invaded the same apical tuft branches to provide a greater influx of Ca2+. Potential benefits and functional implications of the stochastic nature of bAP-Ca2+, such as a paradoxical gain in the systems’ computational power, are discussed.
METHODS
Brain slice and electrophysiology.
Sprague-Dawley rats (RRID:RGD_737891; postnatal days 21–45) of either sex were anesthetized via inhalation of isoflurane before decapitation, according to the animal protocol approved by the Institutional Animal Care and Use Committee, University of Connecticut Health Center. Brains were quickly removed in ice-cold artificial cerebrospinal fluid (ACSF), which contained a mixture of (in mM) 125 NaCl, 26 NaHCO3, 10 glucose, 2.3 KCl, 1.26 KH2PO4, 2 CaCl2, and 2 MgSO4 (pH 7.4, osmolality 300–310 mosmol/kg) and was oxygenated continuously with a mixture of 95% O2 and 5% CO2. To block spontaneous synaptic inputs, the following drugs were purchased from Sigma or Tocris and bath applied: 6,7-dinitroquinoxaline-2,3(1H,4H)-dione (DNQX; 10 µM), dl-2-amino-5-phosphonopentanoic acid (APV; 25 µM), (+)-MK-801 hydrogen maleate (MK-801; 10 µM), (±)-α-methyl-(4-carboxyphenyl)glycine (MCPG; 10 µM), R(+)-SCH-23390 hydrochloride (SCH-23390, 5 µM), and (S)-(−)-sulpiride (sulpiride; 5 µM). Coronal brain slices (300 µm) were cut in drug-free ice-cold ACSF and then allowed to incubate for 30 min at 36°C. Slices were kept in ACSF at room temperature until they were transferred into the recording chamber. Somatic whole cell recordings were made on visually identified L5 cortical pyramidal neurons located in either the PFC (prelimbic and infralimbic areas) or the somatosensory cortex. All measurements were performed at 33 ± 1°C. Patch pipettes (7 MΩ) were filled with intracellular solution containing (in mM) 135 K-gluconate, 2 MgCl2, 3 Na2-ATP, 10 Na2-phosphocreatine, 0.3 Na2-GTP, and 10 HEPES (pH 7.3, adjusted with KOH). Neurons with APs exceeding 75 mV (measured from the baseline) and membrane potentials more hyperpolarized than −55 mV (not corrected for junction potential) were included in this study. Multiclamp 700B and Clampex9.2 software were used to attain and store the recordings. Three APs were evoked with brief somatic current injections [intensities 1–2 nA; duration 1.5–3 ms, 120-ms interstimulus interval (8.3 Hz) unless specified otherwise]. At 8.3 Hz, the AP-Ca2+ transients do not summate in the dendrite (Fig. 1), and the peak Ca2+ signal is identical to that obtained with a single AP. K+ channel antagonist 4-aminopyridine (4-AP) was applied locally via a micropipette used for patching (7 MΩ) attached to a Picospritzer. Intrapipette concentration of 4-AP was 4 mM dissolved in ACSF. The drug precipitates in the tip of the glass pipette, and only small amounts can be released on a single pressure pulse (1–3 psi). Each 4-AP treatment consisted of 3–6 brief pulses (pulse duration 200 ms, pulse interval 1 s) delivered in one location near the target dendritic branch, 20–30 μm away from the dendritic shaft.
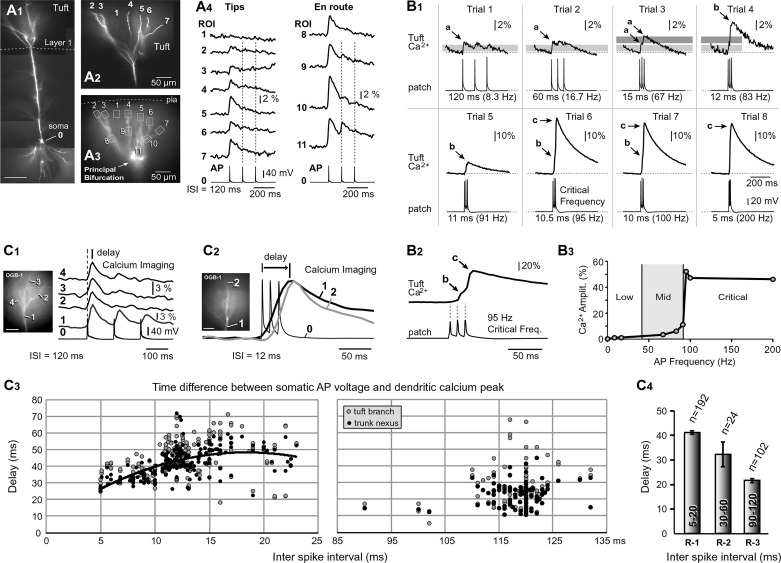
Fig. 1.
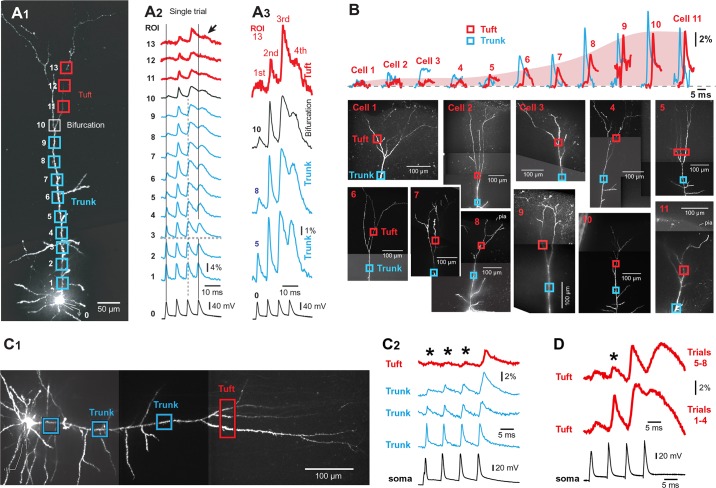
AP-mediated Ca2+ transients in the apical tuft. A1: composite image of a pyramidal cell. Scale, 100 μm. A2: apical tuft branches are numbered 2–7. A3: one video frame from the Ca2+ imaging data (125-Hz frame rate). Boxes mark regions of interest (ROIs). A4: APs recorded whole cell from the soma (ROI 0) and AP-Ca2+ optically from apical tuft dendrites (ROIs 2–11). ROI 1 records scattered light from the space between 2 tuft branches. B1: different cell from A. Triplets of APs at various interspike intervals (ISI range 120-5 ms) were triggered in the soma (patch) and Ca2+ influx recorded in the apical tuft branch, 100 μm distal to the principal bifurcation (tuft Ca2+). Gray bar marks amplitude of Ca2+ signal during simple AP backpropagation. The Ca2+ peaks and inflections marked by arrows a–c are mediated by different biophysical mechanisms. Arrow a is a simple AP backpropagation. Arrow b is an inflection on the rise phase of dendritic Ca2+ transient, due to nonlinear integration of dendritic voltage waveforms (Na+ electrogenesis). Arrow c is dendritic Ca2+ spike (Ca2+ electrogenesis; Larkum et al. 1999). B2: somatic (patch) and dendritic (Ca2+) signal displayed on a fast timescale to enhance the inflection point b in the dendritic Ca2+ waveform. B3: peak amplitude of dendritic Ca2+ signal across 3 frequency ranges: low (<20 Hz), mid (40–90 Hz), and critical (>90 Hz). C1 and C2: time delay between somatic AP (ROI 0) and dendritic Ca2+ signal (ROIs 1–4) at low AP rate (ISI = 120 ms) and high AP rate (ISI = 12 ms). Spatial scales, 50 μm. C3: time difference between peak of AP in the soma and peak of Ca2+ signal in dendrite plotted vs. ISI. Black circles are measurements in the apical trunk nexus, 50 μm below bifurcation (294 measurements from 102 cells). Gray circles are measurements in tuft branches of the same neurons. Twenty-four measurements in the ISI range (30–60 ms) are omitted from this display. C4: average time delay between peak of somatic AP and peak of dendritic Ca2+ signal for 3 ranges of ISI (R-1, 5–20 ms; R-2, 30–60 ms; and R-3, 90–120 ms; 318 measurements in 102 cells).
Dendritic Ca2+ imaging.
Calcium-sensitive dye (Oregon-Green BAPTA-1; OGB1) and neuronal tracer dye (AlexaFluor 594; AF594) were purchased from Invitrogen, dissolved in intracellular solution (150 µM and 40 µM, respectively), and injected via somatic patch pipette for at least 55 min before optical imaging. Navigation, focusing, and microphotography were performed in the AF594 channel. Ca2+ transients were captured at a camera frame rate of 125–200 Hz using Olympus BX51WI or Zeiss Axioskop 2FS microscopes equipped with the NeuroCCD-SMQ camera (80 × 80 pixels) and a ×0.3 coupler (Optem series RL091301-1). A Lumen 200 (Prior Scientific) or blue light-emitting diode (Luminus Devices) was used for epi-illumination. The following filter set was used for Ca2+ imaging: excitation, 500/25 nm; dichroic, 525 nm; and emitter, 530 nm long pass (LP). Neurons were loaded with OGB1 dye for ~55 min before the first optical recording was taken. Following the dye-loading phase, in the next ~100 min, optical recordings were taken every 2–5 min. In the first 20–50 min of the 100-min-long optical series, we observed a gradual increase in Ca2+ signal amplitude, consistent with Maravall et al. 2000 (their Fig. 4). However, this gradual increase in signal amplitude is very different from sudden changes occurring between two subsequent trials, i.e., “trial-to-trial variations.” If a subsequent trial had a more than 20% fractional fluorescence change (ΔF/F) difference from the preceding trial and this happened at least two times in the series (amplitude drop followed by amplitude recovery at least 2 times during the experiment), the AP-Ca2+ amplitude was deemed unstable, which we termed “amplitude flickering.”
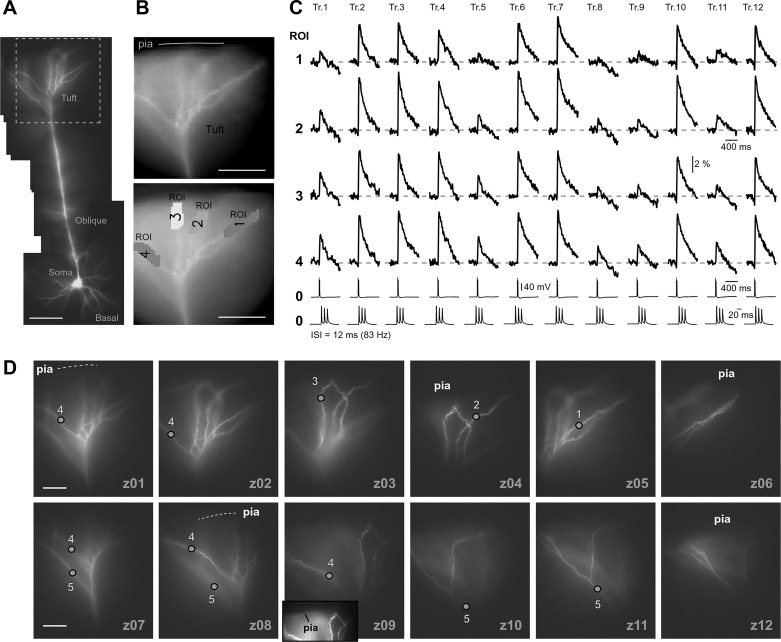
Fig. 4.
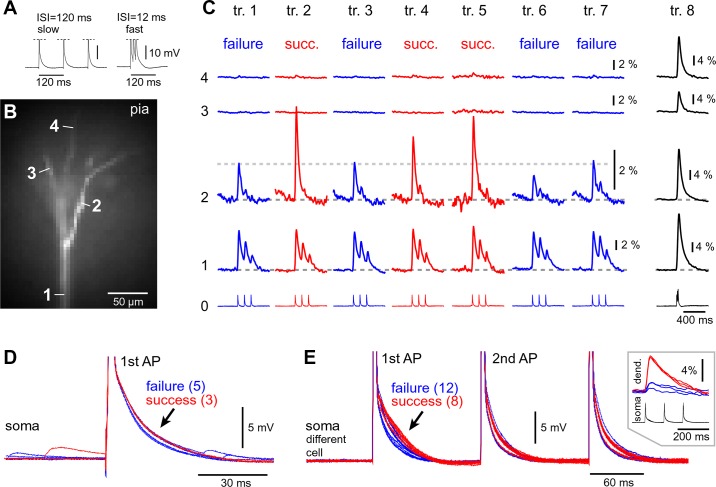
Flickering of the AP-induced dendritic Ca2+ spike. A: composite photograph of a pyramidal cell. Scale, 100 µm. B: one video frame (125 Hz) taken from the Ca2+ imaging sequence (top) and actual pixels used for spatial averaging (bottom). C: AP triplets (ISI = 12 ms, 83 Hz) recorded whole cell (ROI 0) and AP-Ca2+ optically (ROIs 1–4); repeated in 2- to 3-min intervals (trials 1–12). Somatic whole cell recording is displayed at 2 timescales. APs failed to invade the apical tuft in trials 1, 5, 8, 9, and 11. Successful AP invasions are interposed with AP failures (“AP Flickering”). D: apical tuft was photographed at 10 focal planes (z01–z10). Inset in z09 has enhanced contrast to show the positions of tuft branches in relation to the pia.
Dendritic voltage imaging.
Voltage-sensitive dye JPW-3028 (obtained from Leslie Loew, Univ. of Connecticut) dissolved in intracellular solution (400 µM) was applied by free diffusion from somatic patch pipette for at least 60 min, and the dye-loading patch pipette was removed. Brain slice was incubated for ~120 min to allow the dye to diffuse into distal dendrites, and the neuron was repatched with a dye-free electrode. For voltage imaging with laser-spot illumination (532-nm laser), the voltage transients were captured at as camera frame rate of 2,700 Hz. The filter set was as follows: excitation, 510/60 nm; dichroic, 570 nm; and emitter, 600 nm LP. Multisite voltage imaging was performed on an AxioExaminer D1 microscope (Carl Zeiss Microscopy) equipped with three camera ports for 1) infrared differential interference contrast video microscopy (CCD-300-RC; Dage-MTI, Michigan City, IN); 2) fast data acquisition (NeuroCCD-SM; RedShirt Imaging), and 3) spinning-disk confocal microscopy (Yokogawa CSU-10; Solamere Technology Group, Salt Lake City, UT). The NeuroCCD-SM camera frame rate was 5,000 Hz. Wide-field laser illumination (532-nm laser) was combined with the following filter set: excitation, no filter; dichroic, 560 nm; and emitter, 610 nm LP (Popovic et al. 2012).
Data analysis.
Optical signals were analyzed using NeuroPlex software (RedShirt Imaging). The voltage offset of the camera is measured in the dark and automatically subtracted by the imaging software during acquisition. Fluorescence (F) seen by a camera pixel consists of Fdye + Fbgnd, where Fbgnd is stray light and tissue autofluoresence. During the biological signal (e.g., Ca2+ influx), Fbgnd remains constant while Fdye changes with Ca2+ concentration. Changes in fluorescence can be represented as ΔF = Fdye-signal + Fbgnd − (Fdye-rest + Fbgnd) = Fdye-signal − Fdye-rest. To correct for differences in compartment volume and illumination intensity variation, it is important to represent the signal as fractional fluorescence change ΔF/F. ΔF/F without background subtraction is ΔF/F = (Fdye-signal − Fdye-rest)/(Fdye-rest + Fbgnd). Signals within one region of interest (ROI) can be compared between subsequent trials because the summand “Fdye-rest + Fbgnd” does not change during the intertrial interval, typically 2-5 min. The best case scenario is to perform background subtraction; however, if the contrast is low (Fbgnd ≈ Fdye), then small errors in determining the Fbgnd have a large impact on ΔF/F measurement, making it unreliable. Unfortunately, during imaging of distal structures, this is often the case. For this reason, background subtraction was not performed, and the reported amplitudes ΔF/F of Ca2+ transients in apical tuft dendrites are less than they would be if background subtraction were carried out. Nine to 16 pixels were selected from one dendritic location for spatial averaging of optical signals. Each pixel covered an area 5 µm × 5 µm in the object plane. Temporal averaging was not used in Ca2+ imaging experiments. In voltage imaging experiments, three to nine trials were averaged to improve the signal-to-noise ratio, unless specified differently. In the off-line analysis, optical recordings were conditioned by high-pass “tau” and low-pass “Butterworth” digital filters, available in NeuroPlex: Butterworth (35-Hz cutoff) filter for Ca2+ signals, 900-Hz cutoff filter for laser-spot voltage signals, and 2,000-Hz cutoff for multisite voltage signals. Color coding of Ca2+ signal amplitudes was based on a color scale in which red is high and blue is low amplitude. The amplitude of Ca2+ signal obtained in apical tuft branch at critical frequency (AP-induced Ca2+ spike) was set to red color, and the same color calibration was used for other frequencies. The baseline or “b-line” is an arbitrary amplitude calculated form trials used for display in figures. Four to five trials of similar amplitude were selected from an optical series and averaged to generate a b-line. Electrical recordings were analyzed using Clampfit 10.2. Only synaptic events >0.5 mV were taken into account (by setting the threshold at 0.5 mV). Peak amplitude, number of events, and instantaneous frequency were measured using the Event Detection function (Threshold Search) in Clampfit on 1-min epochs of current-clamp recordings. The duration of AP afterdepolarization (ADP; in ms) was measured 4–7 mV above baseline depending on a cell. All measurements in one cell were performed at the same level above baseline potential. The regression analysis was performed between ADP duration and peak amplitude of AP-Ca2+ signal in an apical tuft branch experiencing flickering.
Computational modeling of tuft dendrites.
The model neuron was built in NEURON (code RRID:SCR_005393; release 7.1) using morphology from a reconstructed PFC L5 pyramidal cell (Acker and Antic 2009) and by incorporating voltage-gated channels from previously published studies (Mainen and Sejnowski 1996; Migliore et al. 1999; Schaefer et al. 2003). Na+ channels were distributed on the soma, axon, and apical dendrites with maximal conductance densities of 900, 5,000, and 360 pS/μm2, respectively. The “na.mod” file used in the present study is a modification of the na.mod file published previously (Mainen and Sejnowski 1996). We eliminated the temperature adjustment factor “tadj = q10^((celsius - temp)/10))” (in NEURON code), which links the Na+ channel conductance density ḡNa to the nominal temperature of the model (Mainen and Sejnowski 1996). A ḡNa of 100 pS/μm2 in the present study (nominal temperature = 27°C) would be equal to a ḡNa of ~71.4 pS/μm2 in the Mainen and Sejnowski (1996) model at 27°C. Delayed rectifier K+ channel conductance density (“kv.mod”, again without the Mainen et al. temperature factor) was 40 pS/μm2, except in the model's axon, where it was 100 pS/μm2. In the soma, A-type K+ channels of the “proximal” type (“kaprox.mod”) (Migliore et al. 1999) were added with a conductance density of 150 pS/μm2. In the apical dendrites the total A-type K+ conductance density was uniformly 700 pS/μm2, but this total was a mixture of “proximal” and “distal” IA channels (Migliore et al. 1999) that included a linearly increasing portion of distal channels (“kadist.mod”), starting from zero at the soma and changing to purely distal channels beyond 300 μm. Because “kadist” is the only remaining A-type conductance in the apical tuft, the gKA values reported in figures and figure legends refer to “kadist.” The dendrites contained 2 pS/μm2 of high-voltage-activated (HVA) and low-threshold inactivating (iT) Ca2+ conductance combined. A ratio of 0.8 indicates 80% of HVA and 20% of iT Ca2+ conductance. For na.mod, kv.mod, kaprox.mod, kadist.mod and ca.mod files the Q10 was, 2.3, 2.3, 5, 5 and 2.3, respectively. Passive membrane properties were as follows: specific intracellular resistivity = 110 Ω·cm, specific membrane capacitance = 1 μF/cm2, and leak conductance = 0.04 mS/cm2. To account for dendritic spines, a “spine factor” of 2 was used to increase capacitance and leak conductance in the dendrites beyond a distance of 50 μm.
Statistical analysis.
Averages are means ± SE. Student’s t-test was performed when two groups were compared (paired for data from the same neuron and unpaired for data from different neurons). If the data did not pass the normality test, then the Mann-Whitney (Wilcoxon rank sum) test was performed (SigmaPlot 8.02, RRID:SCR_003210). Asterisks in figures denote P < 0.05.
RESULTS
Backpropagation at low AP frequency.
By implementing long dye-loading time periods (>55 min), the apical tuft of 196 neurons was successfully loaded with a mixture of OGB1 (150 µM) and AF594 (40 µM) (Fig. 1A1). Microscope (×40 objective) and camera magnifications (×0.3 coupler) were adjusted to allow simultaneous imaging of dendritic segments both proximal and distal to the principal apical bifurcation (Fig. 1, A2 and A3). Triplets of APs were elicited by somatic current injections at an interspike interval (ISI) of 120 ms, corresponding to an AP frequency of 8.3 Hz. In 69 of 196 pyramidal cells tested in this way, slow AP firing (120 ms, 8.3 Hz) failed to produce any detectable Ca2+ signal in the tuft. In 127 of 196 neurons, AP-associated Ca2+ transients were detected in mid sections of thin apical tuft branches (Fig. 1A4, en route). In 34 neurons, Ca2+ transients were also found in dendritic tips touching the pia (Fig. 1A4, tips). At a low firing frequency (120 ms, 8.3 Hz), the peak of the dendritic AP-Ca2+ signal belonged to the 1st AP in the train (Fig. 1A4; 127 of 127 neurons). The 2nd or 3rd AP-Ca2+ signal in the train (Fig. 1A4, dashed vertical lines) was either notably smaller than the 1st AP (52 of 127) or completely absent, undetectable (75 of 127 neurons). In accord with previous reports, these data show that APs occurring later (2nd or 3rd) in the train exhibited greater distance-dependent attenuation than the 1st AP (Colbert et al. 1997; Gulledge and Stuart 2003; Mickus et al. 1999; Spruston et al. 1995). At an ISI of 120 ms (8.3 Hz), voltage transients from 3 consecutive spikes do not summate in distal dendrites (Fig. 1B1, trial 1 and trial 2) and the Ca2+ signal has a peak amplitude identical to the signal obtained using a single AP.
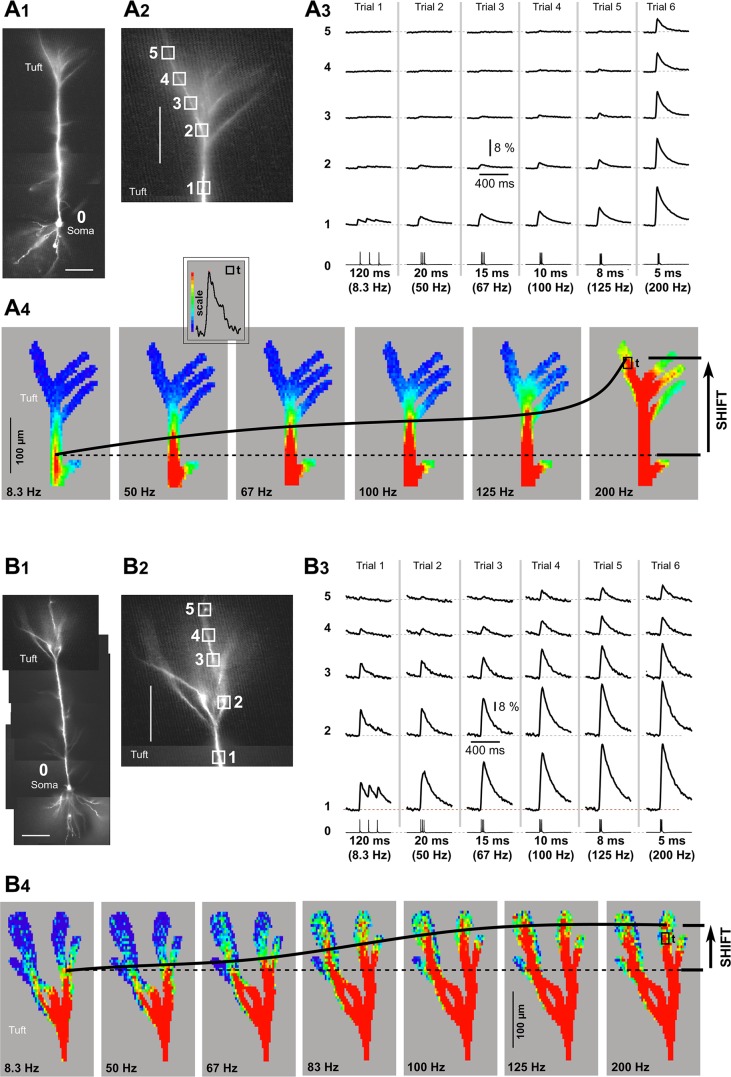
Fig. 2.
Advancing Ca2+ front in the apical tuft. A1: composite photograph of a pyramidal cell. Scale, 100 µm. A2: apical tuft region with marked ROIs. Scale, 100 µm. A3: triplets of APs were recorded whole cell from the soma (ROI 0) and AP-Ca2+ optically from apical tuft nexus (ROI 1) and apical tuft dendrite (ROIs 2–5). The ISI was varied in consecutive sweeps, from 120 ms (8.3 Hz, trial 1) to 5 ms (200 Hz, trial 6). A4: multisite display of Ca2+ imaging data. Signal amplitude is coded by color as shown in inset (“scale”). The peak amplitude at critical frequency (200 Hz) in the apical tuft branch is used to set the red scale value. The actual pixel used for calibration is marked by box (“t”). Dashed horizontal line indicates position of the leading edge of the Ca2+ front at 8.3 Hz. Thick black line marks displacement of the Ca2+ front with increasing frequencies. B1–B4: same as in A1–A4 except for a different pyramidal neuron and with different dynamics of Ca2+ front advancement. Note that the distance traveled by the leading edge of the Ca2+ front (shift) is notably shorter than in A.
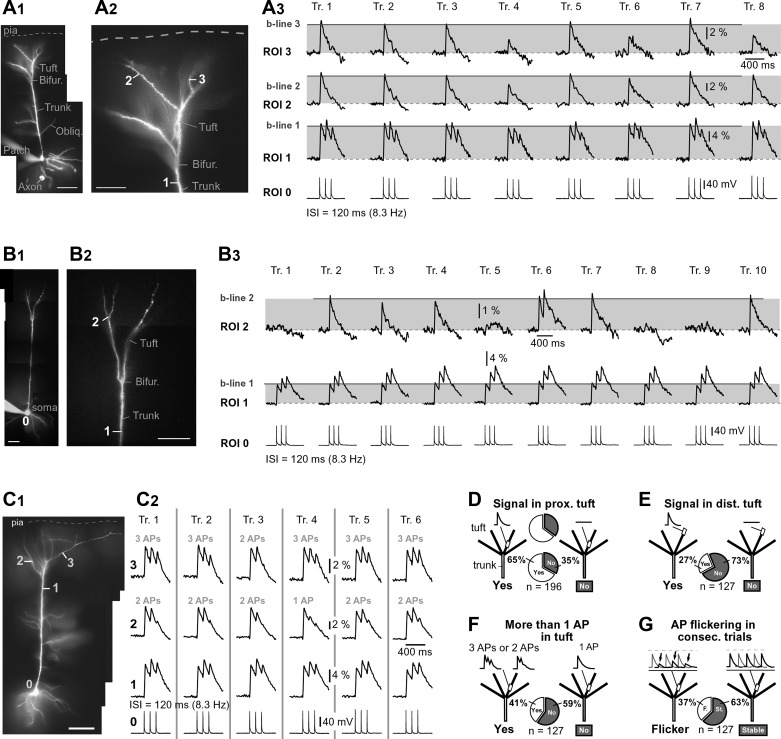
Fig. 3.
Dendritic Ca2+ signal from trial to trial. A1: composite photograph of a pyramidal cell. Scale, 100 µm. A2: apical tuft with ROIs marked. Bifur., bifurcation; Obliq., oblique dendrite. A3: 3 APs were evoked by somatic current injection (120-ms interval, 8.3 Hz) and recorded whole-cell from soma. The resulting AP-Ca2+ signals were recorded optically from apical trunk nexus (ROI 1) and tuft dendrites (ROIs 2 and 3). Recordings were repeated in 2- to 4-min intervals (trials 1–8). Tr., trial; b-line, baseline amplitude of the AP-Ca2+ signal. Moderate AP-Ca2+ signal amplitude variations were found in trials 4, 6, and 8. B1–B3: same as in A1–A3 except AP-Ca2+ signal amplitude varies notably from trial to trial (e.g., trials 1, 5, 8, and 9). C1: pyramidal cell with ROIs. Scale, 100 µm. C2: simultaneous electrical and optical recordings of AP triplets were repeated in 2- to 3-min intervals (trials 1–6). In all trials, the apical trunk (ROI 1) experienced 3 APs. The apical tuft branches (ROIs 2 and 3), on the other hand, experienced either 1, 2, or 3 APs. D–G: summary graphs in which all cells were stimulated to fire 3 APs at 8.3 Hz, pie charts show percentages within experimental groups, and n indicates no. of cells in each experimental group. D: in 112 of 167 cells tested in this way, AP-Ca2+ signals were detected in the proximal regions of apical tuft dendrites (67%). E: of 112 cells with AP-Ca2+ signal detected in apical tuft, 58 cells (52%) had signals in the most distal segment (tip) of at least one apical tuft branch. F: of 112 cells with AP-Ca2+ signal in apical tuft, 51 cells (46%) had more than one Ca2+ peak in apical tuft (3 APs or 2 APs). G: of 112 cells with signal in apical tuft, 67 cells (60%) exhibited trial-to-trial amplitude variations >20% above or below baseline (flicker). In the remaining 45 cells (40%), the peak amplitude of the AP-Ca2+ signal was stable.
Backpropagation at high AP frequency.
Fast sampling of dendritic Ca2+ transients (125–200 Hz) across a wide range of somatic firing frequencies (8.3–200 Hz; Fig. 1B1) allowed us to distinguish three characteristic features on the Ca2+ signal waveform: 1) the Ca2+ peaks marked by arrow a (Fig. 1B1) are due to a simple AP backpropagation (1 gray bar) or simple (linear) summation of two successive Ca2+ transients (Jaffe et al. 1992; Markram et al. 1995; Yuste et al. 1994) (Fig. 1B1, trial 3); 2) the inflection on the rise phase of dendritic Ca2+ transient marked by arrow b (Fig. 1B1) is due to nonlinear temporal summation of dendritic AP voltage waveforms (described by (Larkum et al. 1999 in their Fig. 3); and 3) the peaks marked by arrow c are due to successful initiation of dendritic Ca2+ spikes (Larkum et al. 1999) by regenerative activation of the local dendritic Ca2+ channels, i.e., active Ca2+ electrogenesis (Fig. 1B1, trial 6, critical frequency).
The Ca2+ signal amplitudes in distal apical tuft are not always all-or-none (Gulledge and Stuart 2003 in their Fig. 10). Instead, there is an intermediary range of dendritic signal amplitudes (Fig. 1B3, mid) interposed in between low-frequency APs on one side (Fig. 1B3, low) and Ca2+ spikes on the other side (Fig. 1B3, critical). The intermediary amplitudes are due to nonlinear temporal summation of dendritic AP voltage waveforms, unrelated to the Ca2+ electrogenesis firing (Fig. 1B1, trial 4 and trial 5). This is further explained below by dendritic voltage imaging.
Fig. 10.
Flickering of backpropagating APs in the somatosensory cortex. A1: somatosensory cortex L5 pyramidal neuron filled with JPW-3028. A2: optical recordings from 13 regions (ROI) are aligned with the somatic whole cell recording of 4 APs evoked by somatic current injection (ISI = 7 ms). Two groups of traces (ROIs 1–6 and 7–13) were recorded sequentially in 2 visual fields; 4 trials were averaged in each field. Arrow marks voltage transient associated with the 4th AP. A3: same data as in A2, displayed on a larger vertical scale. B: optical signals from 11 pyramidal neuron apical tufts (red traces) arranged by amplitude (ΔF/F). The peaks of tuft APs comprise a continuum (pink contour). Each tuft signal (red) is accompanied by one optical record from an apical trunk segment proximal to the principal bifurcation (blue). C1: somatosensory cortex L5 pyramidal neuron loaded with JPW-3028. C2: an optical trace from the apical tuft (red) is aligned with 3 traces from apical trunk and somatic whole cell. Asterisks denote failed APs. D: optical measurements were performed repeatedly at the same location. In the first period of time (trials 1–4), the 2nd AP invades the tuft, but not in the second period (trials 5–8). Asterisk denotes a failed AP.
Timing of dendritic AP-associated Ca2+ transients.
In this experiment the ISI was varied in the range of 120 to 5 ms, and fast Ca2+ imaging with a 125- or 200-Hz frame rate was used to measure the delay between somatic AP (Fig. 1C1, ROI 0) and peak Ca2+ signals in distal apical dendrites (ROI 2–4). Based on the 318 measurements performed in 102 cells, the peak-to-peak time delay between the somatic AP voltage and dendritic Ca2+ signal (Fig. 1, C1 and C2, delay) was a complex function of the somatic ISI (Fig. 1C3). The Ca2+ peak latency in the apical trunk nexus or principal trunk bifurcation (Fig. 1C1, ROI 1) increased from ~20 to ~50 ms as the ISI increased from 5 to 20 ms (Fig. 1C3, black circles and their trend line). In the ISI range from 50 to 120 ms, the peak latency of the Ca2+ signal in the apical trunk bifurcation is centered around 20 ms (Fig. 2C3). When data were binned into 3 ISI ranges: 5–20 ms (R-1), 30–60 ms (R-2), and 90–120 ms (R-3), the average time delays were 41 ± 0.7 (n = 192 measurements), 32 ± 4.9 (n = 24 measurements), and 21.6 ± 0.7 ms (n = 102 measurements), respectively (Fig. 1C4). On the basis of the fast voltage imaging (2,700-Hz frame rate), the amount of time between somatic AP (Fig. 1C1, ROI 0) and peak voltage signals in distal apical dendrites was invariably less than 2 ms, and it increased with the temporal order of spikes in a train. The time delay for the 1st, 2nd, and 3rd APs in the train was, on average, 1.09 ± 0.08 (n = 15), 1.34 ± 0.14 (n = 10), and 1.72 ± 0.15 ms (n = 10), respectively.
We also measured Ca2+ signal time delays in apical tuft branches of the same 102 neurons (Fig. 1C3, gray circles). Ca2+ signals in different dendritic segments belonging to the same neuron (i.e., apical nexus and apical tuft) did not always reach their peaks in synchrony. In 20 neurons, at least 1 tuft branch was more delayed than the trunk nexus, on average, 8.1 ± 0.8 ms (n = 59 measurements in 20 cells), meaning that Ca2+ signal first peaked in the apical trunk nexus (Fig. 1C2, ROI 1), and 8 ms later it peaked in the tuft (Fig. 1C2, ROI 2). Overall, the somatic slow-frequency AP trains (ISI = 120 ms, 8.3 Hz) produce Ca2+ peaks in apical tuft branches sooner than high-frequency AP trains (ISI = 5–20 ms, frequency = 200–50 Hz).
Advancing Ca2+ front along the apical axis.
“Critical AP firing frequency” (Larkum et al. 1999) was tested in 11 neurons by varying the somatic ISI and recording dendritic Ca2+. In 8 cells, at critical AP firing frequency, dendritic Ca2+ influx was, on average, severalfold greater than that below the critical frequency (Fig. 1B1, compare 95 vs. 91 Hz), a percent change of 550 ± 74% (mean ± SE, n = 8). The magnitude of a signal boost (signal amplitude at critical frequency/signal amplitude below critical frequency) varied considerably among pyramidal L5 neurons, from 207% to 1,486% (n = 8). In 3 of 11 cells, we could not determine critical frequency, i.e., evoke sharp transitions in dendritic Ca2+ signal amplitude, similar to what is reported in hippocampal CA1 pyramidal neurons (Maravall et al. 2000). The most obvious difference between cells with and cells without discernible critical frequency is the strength of AP backpropagation at low AP frequency (i.e., weak or strong). The cells with discernible critical frequency (8 of 11; Fig. 2B3) typically exhibited weak propagation into the apical tuft (Fig. 2A3, trials 1–5), strong boosting of signal amplitude by the critical frequency (Fig. 2A3, trial 6), and a large increase in dendritic length engulfed by strong Ca2+ influx [Fig. 2A4; observe the radial (vertical) shift of the red contour with increasing AP frequency]. Other cells (3 of 11) exhibited strong backpropagation already at low AP frequency (Fig. 2B3, trials 1–5), and for that reason, these cells had small amplitude boosting with increasing AP frequency with no discernible critical frequency. Gradual increase of the AP firing frequency from 8.3 to 200 Hz in these cells caused very small effective changes in dendritic membrane area (dendritic length) receiving strong Ca2+ influx (Fig. 2B4, shift). Regardless of the cell’s backpropagation strength, increasing the AP firing rate invariably caused the Ca2+ front to advance along the apical axis of the cortical pyramidal neuron, immersing more and more synapses in L1 (Fig. 2, A4 and B4, shift). Our spatially resolved data revealed that the somatic AP frequency regulates the depth of the cerebral cortex in which layered synaptic afferents encounter large internal Ca2+ concentrations in postsynaptic pyramidal neurons.
Inconsistent invasion of the apical tuft.
We tested the stability of APs invasion of the apical tuft in repeated trials. The time interval between consecutive trials was in the range of 2–5 min; 10–33 experimental trials were acquired per neuron (n = 127 neurons) in the period of time ~100 min following the dye-loading phase (~55 min). In 63% of neurons examined in this way (80 of 127), dendritic Ca2+ signals were stable across all recording trials. That is, trial-to-trial dendritic signal amplitude variations, measured in subsequent trials (methods) were <20% ΔF/F. In 37% of neurons, dendritic signal amplitude in some trials deviated from the neighboring trials by >20% (Fig. 3A3, trial 4). Neurons were included in the analysis if the signal amplitude returned to the baseline in at least one of the later trials (Fig. 3A3, trial 5 or trial 7), thus eliminating the possibility that losses of signal were due to phototoxicity or some form of dendritic injury encountered during experimental measurement (e.g., rundown of the Ca2+ current or decreasing transient amplitudes reflecting the buffering of Ca2+ by increasing concentrations of Ca2+ concentration indicator; Helmchen et al. 1996; Maravall et al. 2000). Furthermore, simultaneous Ca2+ imaging of the apical trunk and apical tuft (Fig. 3A3, ROI 1) showed successful invasion of the apical trunk, but the backpropagating APs failed to maintain a stable amplitude of Ca2+ signal in the apical tuft branch of the same cell (Fig. 3A3, ROI 3, trials 1–8). It was not uncommon to observe experimental trials with large amplitude fluctuations in only one branch (Fig. 3A3, trial 6) or in more than one apical tuft branch at a time (Fig. 3A3, trial 4). In some cells, the large difference between two consecutive trials suggested a failure of the backpropagating AP to invade one or more apical tuft branches (Fig. 3B, trials 2, 3, 4, 6, 7, and 10 represent successful, whereas trials 1, 5, 8, and 9 represent unsuccessful passes of AP into the apical tuft). These data indicate that the efficacy of the AP invasion into the apical tuft branches varies from trial to trial so that successful invasions alternate with complete failures. This phenomenon is termed “AP flickering.”
Temporal pattern of AP flickering.
Multiple experimental trials were obtained from each neuron in the data set (10–33 trials), allowing us to examine if flickering occurred with the same pattern between individual neurons. The temporal pattern of AP-Ca2+ flickering was fairly random. The fraction of trials with successful invasions varied in a wide range from 0.18 to 0.67, on average, 0.42 ± 0.02 (n = 16 cells. Two neighboring successful invasions (large signal) can be separated by 0, 1, 2, or up to 7 trials showing “AP failures.” On the basis of these data, it does not seem that there are phases in which AP invasion is more likely than in other phases.
Flickering of the 2nd and 3rd spike.
A typical dendritic response to a slow triplet of somatic APs (<20 Hz) was a single Ca2+ peak belonging to the 1st AP, whereas the 2nd and 3rd AP did not produce any measurable signals in the tuft (Figs. 1 and 2). However, we acquired a number of cells (52 of 127 cells) with multiple peaks in apical tuft dendrites, suggesting that more than one AP waveform passed through the principal bifurcation and opened Ca2+ channels in the apical tuft branch (Fig. 3C, ROI 3, trial 1). For example, one apical tuft branch (Fig. 3C, ROI 2) experienced two APs on all trials except trial 4. The other apical tuft branch (ROI 1) experienced three APs on all trials except trial 3. This result indicates that AP flickering in one branch can occur independently from that in other sister branches (Fig. 3C, trial 4). Flickering of the 2nd or 3rd spike, as depicted in Fig. 3C2, was detected in 18 of 52 PFC pyramidal neurons (group of cells with more than one spike invading apical tuft branches).
Distribution of dendritic behaviors in the data set.
We analyzed a total of 196 neurons. At a firing rate of 8.3 Hz, 35% of neurons did not show any detectable AP-Ca2+ signal in proximal segments of apical tuft branches (Fig. 3D). In 127 pyramidal neurons, however, unambiguous AP-Ca2+ transients were detected in proximal segments of apical tuft branches when the “slow” AP firing paradigm was used (ISI = 120 ms, 8.3 Hz; Fig. 3D, 65%). Of 127 cells with AP-Ca2+ signals in proximal tuft, 34 cells also showed detectable Ca2+ transients in the most distal segment of at least one apical tuft branch (Fig. 3E, 27%). Of 127 cells with AP-Ca2+ signal in the proximal tuft, 52 cells showed multiple Ca2+ peaks, indicating invasion of more than one AP into the tuft region (Fig. 3F, 41%). AP flickering was detected in 47 of 127 neurons with AP-Ca2+ signals in the apical tuft (Fig. 3G, 37%).
Is “AP flickering” an experimental artifact?
We tested the reliability of high-frequency bursts of APs (ISI = 12 ms or 83 Hz) to repeatedly trigger Ca2+ influx in apical tuft branches (Fig. 4, A and B, tuft); data are shown for 10–21 trials per neuron, with an intertrial interval of 2–5 min (Fig. 4C). In each neuron examined in this way (n = 10), we found large differences between consecutive trials due to the successful and unsuccessful triggering of the dendritic Ca2+ spike (Fig. 4C, compare trial 4 vs. trial 5). Over the course of time (Fig. 4C, trials 1–12), seemingly identical somatic electrical signaling (ROI 0) was accompanied by stochastic internal Ca2+ concentration responses in the apical tuft (ROI 1–4). AP flickering in tuft dendrites (Figs. 3 and 4C) may be a pathological response of dendritic membranes injured during brain slicing. Harnett et al. (2013) reported that the behavior of tuft branches that are close to the slice surface, or are prematurely cut, exhibit different electrical properties than those that are deep in slices and terminate naturally. To address this possibility, at the end of the Ca2+ imaging session we photographed the apical tufts in multiple (5–18) focal planes, “z” (Fig. 4D, z01–z12). Dendritic branches showing AP flickering were regularly positioned at different depths inside the brain slice (Fig. 4B, ROIs). Flickering of AP-induced dendritic spikes was detected in apical tuft branches irrespective of their distance from the brain slice surface (Fig. 4C, trials 1, 5, 9, and 11).
Multisite Ca2+ imaging in tuft branches and z-stack photography of the apical tuft were also used to study low AP frequency (ISI = 120 ms, 8.3 Hz; intertrial interval 2–5 min, 10–21 trials per neuron, n = 11 neurons). In completely intact dendritic trees, showing zero premature cuts, we detected trial-to-trial amplitude variations >20%. In summary, the phenomenon of AP flickering at low AP frequency was detected in apical tuft branches of 11 pyramidal neurons irrespective of their distance from the brain slice surface.
Correlation between somatic ADP and AP-Ca2+ in apical tuft.
Activation of dendritic voltage-gated Ca2+ channels (VGCCs) is manifested in the cell body in the form of AP afterdepolarization (ADP) (Larkum et al. 1999; Williams and Stuart 1999). In two examples shown in Fig. 5, C and D, the AP-Ca2+ signals exhibit significantly larger amplitudes in trials colored red (successful invasion) compared with trials colored blue (failure to invade). Overall, the experimental trials shown in red had a broader somatic ADP than the blue trials, with a few exceptions; i.e., a few blue trials (failures) also showed a broad ADP (Fig. 5, C and D, arrow). To obtain a numerical characterization of data, the somatic ADP duration (methods) was measured in 20 neurons exhibiting AP-Ca2+ flickering in the apical tuft. Regression analysis revealed that Ca2+ signal amplitude (ΔF/F) in the apical tuft branch and the ADP duration in the soma (ms) were positively correlated in 9 of 20 neurons (R value in the range 0.5027–0.8736; P value in the range 0.0001–0.05; degrees of freedom in the range 8–19; n = 9 neurons). In the remaining 11 neurons, the P value of the regression analysis was >0.05, indicating no positive correlation between somatic ADP and amplitude of AP-Ca2+ in the apical tuft branch experiencing AP flickering (R value in the range 0.0467–0.5863; P value in the range 0.0675–0.9299; degrees of freedom in the range 4–12; n = 11 neurons). Overall, these data indicate that somatic ADP is not a reliable reporter of successful AP invasion into the apical tuft branch.
Fig. 5.
Somatic AP afterdepolarization in the context of AP-Ca2+ flickering in the apical tuft. A: “slow” and “fast” AP firing paradigms. B: apical tuft filled with OGB1. C: ROI 0 shows somatic whole cell records during Ca2+ imaging. ROIs 1–4 are Ca2+ transients from 4 apical tuft branches. In trials colored red, the amplitude of the Ca2+ signal in ROI 2 greatly exceeds the baseline level (horizontal gray bar), suggesting AP flickering occurring in dendrite 2. Dendrites 3 and 4 show no detectable optical signal at slow firing (trials 1–7) but strong signal at fast AP firing (trial 8). D: somatic whole cell records from trials 1–7 are superimposed on a faster time scale and APs are truncated. The cell body experiences stronger ADP (arrow) during successful invasions of tuft branch 2 (red) than during failures (blue). E: same as in D, except for a different pyramidal cell. Inset: dendritic Ca2+ transients (dend.) and somatic whole cell recordings (soma) from subsequent experimental trials are superimposed (only 6 Ca2+ trials are shown for clarity).
Spontaneous synaptic inputs.
Patterns of spontaneous synaptic activity come and go in cortical brains slices, and may be repeated in a particular structure (songs, avalanches), occurring at a rate of 1–5 per minute (Beggs and Plenz 2004; Ikegaya et al. 2004). A barrage of synaptic inputs on a given dendritic branch could alter the level of depolarization and thus control AP invasion into this branch (Hsieh and Levine 2013; Tsubokawa and Ross 1996; Waters and Helmchen 2004). To characterize spontaneous synaptic activity in our preparation, we counted synaptic potentials greater than 0.5 mV. The average peak amplitude per minute of recording was 0.68 ± 0.01 mV (72 epochs in 24 neurons, each epoch = 1 min), and it was relatively stable for all 24 cells. The average number of synaptic events per minute of recording was 203 ± 9.5 (72 epochs in 24 neurons, each epoch = 1 min) and it varied on average by ±20.2% from minute to minute. The average instantaneous frequency of synaptic inputs per minute of recording was 36 ± 1.4 Hz, and it varied from minute to minute. Although the visual inspection of traces did not show signs of structured synaptic patterns, bouts of increased synaptic activity interposed with periods of calm, the numerical analysis of the event count, and instantaneous frequency per minute recording indicated that we could not rule out the possibility that minute-to-minute changes in the amount of afferent synaptic input influenced AP flickering. Therefore, we repeated dendritic Ca2+ imaging experiments shown in Fig. 3 under conditions where synaptic input is blocked by a mixture of glutamatergic and dopaminergic antagonists (DNQX, 10 µM; APV, 25 µM; MK-801, 10 µM; MCPG, 10 µM; SCH-23390, 5 µM; and sulpiride, 5 µM). When neurons were driven to fire APs at ISI of 120 ms, they showed no signs of AP flickering behavior (n = 17). The experimental outcome in synaptic blockers (0 of 17 cells, 0%) compared with the experimental outcome obtained in regular saline (67 of 112 neurons, 60%; Fig. 3G) was significantly different (χ2; P < 0.01). These data suggested that spontaneous synaptic inputs may have an effect on the stability of the AP amplitude in apical tuft dendrites during slow AP firing (ISI = 120 ms). Next, we evaluated the effect of synaptic blockers on dendritic Ca2+ spikes using ISI = 12 ms. In 9 neurons perfused with the mixture of synaptic blockers, dendritic Ca2+ signals were tested with short ISI = 12 ms, to trigger AP-induced dendritic spikes. In two of nine cells tested in this way, AP flickering was observed in multiple trials, despite perfusing with synaptic blockers. Synaptic blockers did not prevent the stochastic nature of AP-induced dendritic Ca2+ spike firing.
A-type K+ conductance in the PFC.
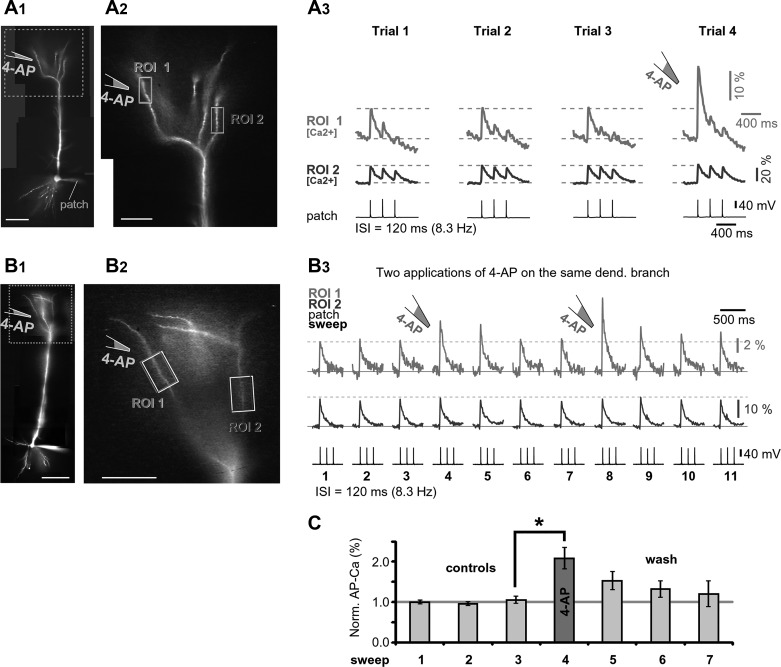
In hippocampal CA1 pyramidal neurons, the AP amplitude depends on the availability of the dendritic A-type K+ current (Hoffman et al. 1997). To test for the presence of A-type K+ conductance in the PFC L5 apical tuft, brief and focal applications of the K+ channel antagonist 4-aminopyridine (4-AP; intrapipette concentration 4 mM) were applied near a target dendritic branch (Fig. 6, A1 and A2). Note that 4-AP (5 mM in bath) selectively blocks A-type current in apical tuft dendrites of L5B pyramidal neurons in somatosensory cortex (Harnett et al. 2013). Brief focal applications of 4-AP (applied 2 s before somatic AP initiation) on an apical tuft branch in PFC caused a significant change in AP-Ca2+ amplitude (Fig. 6A3, ROI 1). The effect of the K+ channel antagonist was not detected in dendritic branches away from the drug application point (Fig. 6A3, ROI 2), indicating a highly localized drug action. In experiments in which 4-AP was applied more than once on the same dendritic branch (Fig. 6B, ROI 1), the amplitude of dendritic AP-Ca2+ increased markedly following each drug ejection (Fig. 6, B1–B3, trial 4 or trial 8), and it was possible to wash out the effect of the drug in 90 s (trials 6 and 7 and trials 10 and 11). The relative dendritic signal amplitude at the time of focal drug application was, on average, 208 ± 27% (n = 3 neurons, N = 6 applications) of the control value established just before the 4-AP application (Fig. 6C, compare trial 4 vs. trial 3). Consistent with Bekkers (2000) and Korngreen and Sakmann (2000), these data established two facts: 1) A-type K+ channels populate thin dendritic branches of L5 PFC pyramidal neurons; and 2) dendritic K+ channels have a strong impact on the efficacy of AP invasion into the PFC apical tuft.
Fig. 6.
Drug-induced changes in apical tuft excitability. A1: L5 pyramidal neuron filled with OGB1. A2: apical tuft with 2 ROIs marked. A3: dendritic Ca2+ signals evoked by somatic triplets of APs (ISI = 120 ms). Local application of 4-AP (4 mM inside pipette) was delivered between trials 3 and 4. B: same as in A, except for a different cell and with 2 consecutive focal applications of 4-AP. Trials 1–3 are control trials, trial 4 is the 1st application of 4-AP, trials 5–7 are washout trials, trial 8 is the 2nd application of 4-AP, and trials 9–11 are washout trials. Interval between washout trials 5–7 and 9–11 is ~20 s. C: average change in dendritic signal amplitude in response to a focal application of 4-AP (n = 6 applications in 3 cells). *P < 0.05, paired t-test. All amplitudes are normalized (norm.) against the mean of 3 control recordings (trials 1–3).
Voltage-sensitive dye imaging in the apical tuft.
AP-Ca2+ signals do not provide sufficient mechanistic basis for the observed dendritic behaviors (Figs. 1–3), because changes in the internal Ca2+ are only indirectly linked to dendritic membrane potential. In the next series of experiments, voltage-sensitive dyes and the laser spot illumination technique (Zhou et al. 2008) were used to monitor voltage waveforms in distal dendrites. Apical tuft branches of PFC L5 pyramidal neurons (n = 15) were successfully filled with the voltage-sensitive dye JPW-3028 (Fig. 7A1) with the use of a dye-loading protocol that requires removal of the dye-loading pipette followed by 120 min of incubation (Antic et al. 1999). Neurons were repatched, and current pulses were injected into the cell body to evoke 10 APs (Fig. 7A2, soma). Voltage imaging of the apical trunk revealed frequency-dependent amplitude adaptation of backpropagating APs (Fig. 7A2, trunk), consistent with patch electrode recordings in the same cell type (Gulledge and Stuart 2003) as well as in the hippocampal CA1 pyramidal neurons (Colbert et al. 1997; Mickus et al. 1999; Spruston et al. 1995). These data indicate that voltage-sensitive dyes do not alter the basic physiology of the pyramidal cell apical dendrite in PFC.
Fig. 7.
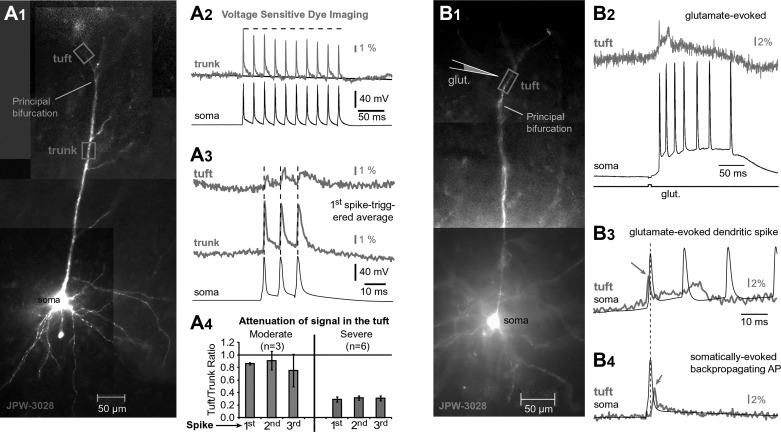
AP waveforms in apical tuft dendrites. A1: L5 pyramidal cell filled with voltage-sensitive dye JPW-3028. A2: dendritic voltage-sensitive dye signal (gray) recorded from a location marked by box in A1 (trunk). Somatic APs are black. A3: voltage imaging of AP triplets at 2 dendritic locations (tuft, trunk; gray) aligned with somatic whole cell (black). A4: signal amplitude ratio between tuft and trunk calculated for 2 groups of cells (moderate attenuation, n = 3; severe attenuation, n = 6). Local Na+ spike initiation. B1: same as in A1, except for a different cell. B2: glutamate-evoked dendritic potential in the apical tuft dendrite. B3: same pair of signals as in B2, shown on a faster timescale. B4: single backpropagating AP recorded in the apical tuft dendrite (gray). Dashed vertical line marks the peak of the somatic AP. Arrows mark negative latency of the dendritic AP (B3) and positive latency (B4) in the same tuft branch.
The average amplitude of the AP-associated optical signal in the apical trunk ~100 µm proximal to the principal bifurcation (6.3 ± 0.4% ΔF/F, n = 17) was notably larger than in the apical tuft ~50 µm distal to the principal bifurcation (2.4 ± 0.5% ΔF/F, n = 15). These data are consistent with a distance-dependent decline in AP amplitude as the AP propagates from trunk to tuft (Gulledge and Stuart 2003; Spruston et al. 1995). In nine neurons, we were able to obtain optical recordings from both trunk and tuft of the same cell (Fig. 7A3). In three of those nine neurons, we detected relatively strong voltage signals in the tuft. These cells presumably represent cells in which AP-Ca2+ signals were detected in the apical tuft (n = 112 of 167; Fig. 3D). In the remaining 6 neurons of this group, the amplitude of the AP optical signal in the apical tuft was very small (<1% ΔF/F) and the shape of the AP was highly distorted (Fig. 7A3, tuft). These cells may represent PFC neurons in which AP-Ca2+ signals were not detected in the apical tuft (n = 69 of 196; Fig. 3D). Overall, the voltage imaging experiments show that in some cells the backpropagating APs successfully invade, whereas in other cells APs fail to invade the apical tuft branches (Fig. 2A3, trial 1).
Na+ channels in the apical tuft.
We tested for the presence of Na+ channels in the apical tuft dendrites by using local application of glutamate (n = 5). Brief glutamate microiontophoresis (pulse duration = 5 ms) invariably triggered local depolarizations in the apical tuft dendrites (Fig. 7B1) that are best described as dendritic plateau potentials (Milojkovic et al. 2004; Oikonomou et al. 2014). The glutamate-evoked plateau potentials were sometimes preceded by fast Na+ spikes (Fig. 7B2). Fast Na+ spikelets can be distinguished from backpropagating APs on the basis of their timing with respect to somatic AP recording. The peak of the dendritic Na+ spikelet occurs before somatic AP (Fig. 7B3, arrow), whereas the peak of the backpropagating AP takes place after the somatic AP (Fig. 7B4, arrow).
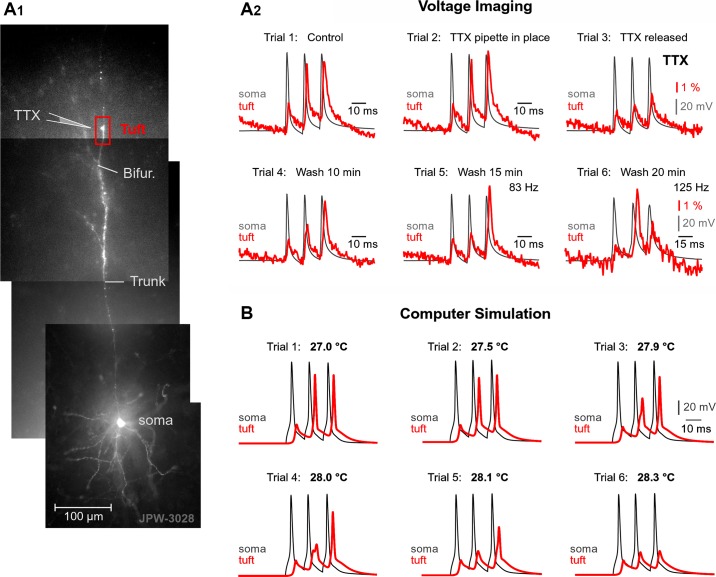
To determine whether dendritic Na+ channels participate in AP backpropagation in PFC L5 pyramidal neurons, we focally applied tetrodotoxin (TTX; 1 µM) to a dendritic segment 50–75 µm distal to the principal bifurcation (see Fig. 9A1). Local TTX application reduced the amplitudes of large voltage transients in tuft dendrites [see Fig. 9A2, trial 3; note the loss of the 2nd and 3rd dendritic (red) spikes] while having a negligible effect on the failed APs (1st dendritic spike) propagating passively. The activation of Na+ channels during AP backpropagation (see Fig. 9A2) and the local Na+ spike initiation (Fig. 7B3) indicate that voltage-gated Na+ channels are present in the apical tuft branches of PFC L5 pyramidal neurons, consistent with previously published work (Gulledge and Stuart 2003; Larkum et al. 2009; Seamans et al. 1997).
AP amplitude fluctuations in model neurons.
Experimental measurements in the current study provide four useful constraints for the biophysical model of apical tuft dendrites in PFC: 1) voltage-gated Na+ and 2) voltage-gated K+ channels are activated in apical tuft dendrites during AP backpropagation; and 3) the amplitude of backpropagating APs varies from trial to trial. 4) APs failed to invade the apical tuft branch in some trials, whereas in other trials the same apical tuft branch was successfully invaded by the AP voltage waveform (see Fig. 9A2, compare trials 2, 5, and 6).
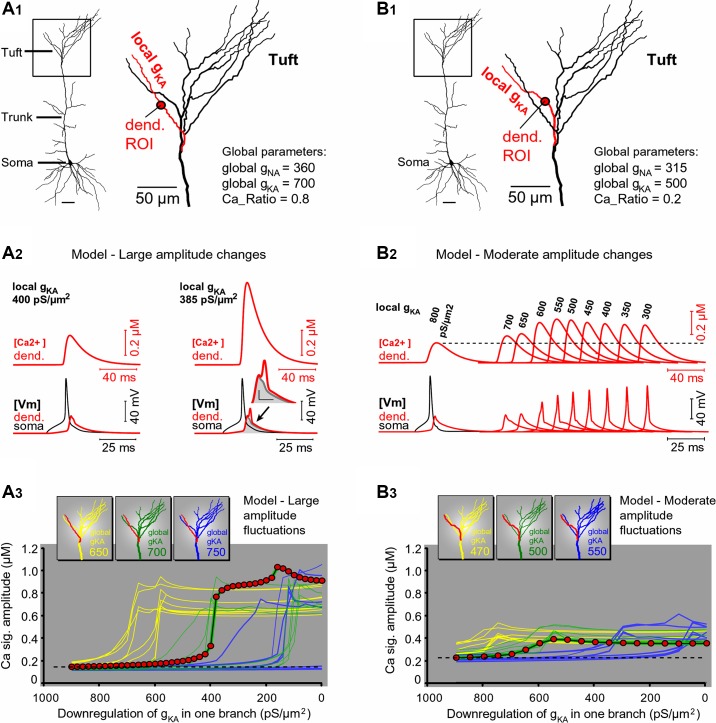
One possible explanation for the alteration in the backpropagating AP waveform is based on a variable availability of A-type K+ conductance in the dendritic membrane (Frick et al. 2004; Hoffman et al. 1997), due to phosphorylation states and/or inactivation states of the channel (Gasparini and Magee 2002; Labno et al. 2014; Lugo et al. 2008). We used computer simulations to test the effects of small changes in A-type K+ conductance availability in the apical tuft of PFC L5 pyramidal neurons. We asked if small and spatially restricted A-type K+ conductance alterations could reproduce experimentally observed changes in dendritic Ca2+ signal amplitude on subsequent recording trials (Fig. 3). A reconstructed PFC L5 neuron (Fig. 8A1) was endowed with passive and active membrane properties. The entire apical tuft was assigned global voltage-gated Na+ and global A-type K+ conductances (Fig. 8A1, global gNA = 360 pS/µm2; global gKA = 700 pS/µm2) except one apical tuft branch, in which local A-type K+ conductance was lower than the rest (Fig. 8A1, local gKA = 400 pS/µm2). Under these conditions, backpropagating AP failed to invade the selected apical tuft branch (Fig. 8A1, red branch), because the peak AP in the tuft dendrite dropped below the threshold for active propagation from the apical trunk into the apical tuft. The attenuated AP amplitude in the tuft branch caused a small activation of local VGCCs and a small increase in the internal Ca2+ concentration (Fig. 8A2, left, [Ca2+] dend.). This model mimics real neurons in a lower state of dendritic membrane excitability, when very small AP-induced Ca2+ transients occur in the apical tuft (Fig. 3A, trials 4, 6, and 8). To mimic occasional successful invasions into the same apical tuft dendrite (Fig. 5, A–C, trials 2, 4, and 5), we produced a spatially restricted IA current downregulation (Frick et al. 2004; Hoffman et al. 1997). The density of A-type conductance (local gKA) was reduced from 400 to 385 pS/µm2 in one dendritic branch only (Fig. 8A1, red branch). In response to this rather small downregulation of the A-type dendritic conductance (<4% decrease, restricted to only one branch), the backpropagating APs invaded the branch more strongly. Both the amplitude and duration of the dendritic voltage transients were increased in tandem (Fig. 8A2, right, [Vm] dend.), which resulted in a more efficient activation of local VGCCs, manifested by a large increase in the internal dendritic Ca2+ concentration ([Ca2+] dend.). Peak Ca2+ signal from the mid region of the affected tuft branch (red dot) plotted against the decreasing values of local gKA (Fig. 8A3, dark green line with red circle markers) showed a sharp transition around gKA = 400 pS/µm2. Every apical tuft dendritic branch was analyzed in the model by changing gKA in that branch only (Fig. 8A3, green family of lines). Each apical tuft branch experienced a sharp transition from failure to invasion (sigmoidal function), albeit at slightly different gKA threshold.
To test the robustness of the current computational model, the global apical gKA was shifted from default 700 pS/µm2 to either 650 or 750 pS/µm2 and the modeling analysis repeated for all branches in the apical tuft (Fig. 8A3, yellow and blue family of lines, respectively). Once more, the plot of the Ca2+ signal peak amplitude vs. local gKA showed a highly nonlinear relationship (sigmoidal function with hard threshold) for every apical tuft dendrite tested under three global apical gKA values (Fig. 8A3, sigmoidal lines). Regardless of the global apical gKA, each apical tuft branch exhibited a sudden jump in local AP-Ca2+ signal in response to a gradual decrease in local gKA. The only difference between branches was the exact gKA value (threshold) at which a jump occurred (Fig. 8A3). These modeling data gave three pointers. First, the transitions between AP failure and AP invasion can be induced by very small alterations in one dendritic conductance (e.g., A-type). Second, the transitions between AP failure and AP invasion are possible even if the conductance change was spatially restricted to one segment of the apical tuft (Fig. 8, A1 or B1). Third, individual branches experience unique gKA thresholds (Fig. 8, A3 or B3), which may explain independent flickering of individual apical tuft branches belonging to the same neuron (Fig. 3A3, trial 6). Neurons with large amplitude differences between consecutive trials (~100% change in dendritic Ca2+ signal) comprise a smaller fraction of cells in the current data set obtained in brain slices (9 of 47 cells). Neurons with moderate amplitude changes (20%– 50%) were more common (38 of 47 cells).
Fig. 8.
A-type current-mediated changes in apical tuft excitability: model. A1: a reconstructed PFC L5 pyramidal cell. Inset: on each model run, only one apical tuft branch will be altered (e.g., red). A2: dendritic intracellular Ca2+ concentration ([Ca2+]; red) and dendritic voltage (Vm; red) recorded from a location marked by red circle in A1. Somatic AP is black. A change in dendritic gKA from 400 (left) to 385 pS/μm2 (right) was made in one apical tuft branch. Dendritic voltage waveform is copied from that at left, colored gray, and superimposed at right. Arrow indicates broadening of voltage waveform at the base. Inset: blowup of voltage waveforms (scales, 10 mV and 5 ms). A3: peak amplitude of Ca2+ signal from each tuft dendrite plotted against gKA in that dendrite. Each color (yellow, green, and blue) represents uniform (global) gKA in the apical trunk and tuft (650, 700, and 750 pS/μm2, respectively). Red circles indicate data from the red branch in A1. Dashed horizontal line represents a baseline level of AP-Ca2+ signal associated with high levels of local gKA. B1 and B2: same as in A1 and A2, except global apical gNa, gKA, and gCa were reduced to mimic moderate amplitude changes. Local gKA was gradually changed from 800 to 300 pS/μm2 in the red dendrite only (local gKA values indicated above Ca2+ signals). B3: same organization of curves as in A3. Note that in this model, a relative increase in Ca2+ signal above baseline level (dashed horizontal line) is less pronounced than in A3, hence “moderate.”
To simulate moderate changes in dendritic AP-Ca2+ signal, we used the same model shown in Fig. 8A, except the Ca2+ ratio (see methods) was set to 0.2, meaning a greater portion of low-threshold compared with high-threshold Ca2+ conductance, and the fast Na+ and A-type K+ conductances in apical dendrite were reduced by 12.5% and 28.5%, respectively. All parameters were kept the same between the runs except local gKA in one dendritic branch (Fig. 8B1, red). The amplitude of the dendritic Ca2+ signal increased moderately in response to a relatively large (on the order of 100 pS/µm2) decrease in local gKA (Fig. 8B2), unlike the previous model, in which a tiny (on the order of 10 pS/µm2) decrease in local gKA produced large boosting of dendritic Ca2+ (Fig. 8A2). Voltage waveforms underlying internal Ca2+ concentrations in apical tuft dendrites are shown in the bottom trace of Fig. 8B2 ([Vm] dend.). Interestingly, the computational model revealed a clear discrepancy between the trends of voltage and Ca2+ peaks in the apical tuft dendrite in response to gradually changing local gKA. For a range of gKA values between 550 and 300 pS/µm2, the peak of the dendritic Ca2+ signal was decreasing, whereas the peak of dendritic voltage was increasing in the same dendritic compartment (Fig. 8B2, compare the trend of [Ca2+] against [Vm]). The observed discrepancy between the trends of voltage peak amplitude and Ca2+ peak amplitude in the apical tuft dendrite is due to slow activation dynamics of dendritic VGCCs. Sharp spikes have large amplitude but very short duration. The duration of the sharp spike is insufficient for the activation of slow-activating VGCCs. Smaller spikes, on the other hand, were presented with longer durations at the base of the spike (Fig. 8B2, [Vm] dend.). Although smaller in amplitude, these prolonged voltage waveforms activate VGCCs more strongly. Basically, time spent at depolarized potential is more important for activation of VGCCs than the absolute amplitude of depolarization. This is a very interesting insight of the current study: smaller and longer waveforms produce stronger Ca2+ transients than large-amplitude sharp spikes.
At three different levels of global gKA (470, 500, and 550 pS/µm2), a gradual change in local gKA produced sigmoidal curves with moderate amplitudes and distinct thresholds for each apical tuft branch (Fig. 8B3, family of blue lines), as previously shown in the model with large amplitude changes (Fig. 8A3, family of blue lines). The magnitude of change in dendritic signal (20%–100%) matched the experimental data observed in real PFC L5 pyramidal neurons (Fig. 3, A and B).
Frequency-dependent boosting of AP amplitude in real and model neurons.
Repeated voltage-sensitive dye imaging of AP waveforms in the apical tuft (Fig. 9A1) revealed the failure of the 1st AP and successful invasion of later APs in the train (Fig. 9A2). This is consistent with the presence of rapidly inactivating A-type current in the apical tuft of PFC L5 neurons. The membrane depolarization induced by the 1st AP in the train inactivates local A-type conductance so that later APs are not opposed by the K+ current (Hoffman et al. 1997). Using the same multicompartmental model (Fig. 8), we next studied AP invasion of the apical tuft branch using a train of 3 APs at ISI = 12 ms (83 Hz; same as voltage imaging shown in Fig. 9A2). With membrane parameters identical to those in Fig. 8A1, the model produced a characteristic failure of the 1st and successful invasion of the 2nd and 3rd spikes (Fig. 9B, trial 1). In summary, voltage imaging (Fig. 9A) confirmed an interesting property of the apical tuft dendrites related to the boosting of later spikes in a high-frequency train, before initiation of dendritic Ca2+ spike (Larkum et al. 1999). This dendritic behavior is opposite to the activity-induced depression of later spikes in a train (Spruston et al. 1995). Both behaviors take place in the same pyramidal neuron, albeit at different frequencies of somatic AP firing. At lower frequencies, AP amplitudes decrease (Fig. 7A2), whereas at higher frequencies, AP amplitudes increase, with spike order (Fig. 9A2).
Fig. 9.
TTX-sensitive voltage waveforms in the apical tuft. A1: PFC L5 pyramidal cell filled with voltage-sensitive dye JPW-3028. A2: current pulses were used to evoke 3 APs at 83 Hz. Dendritic voltage-sensitive dye signal (red) recorded from a location marked by box in A1 (tuft) superimposed on whole cell somatic APs (gray). Six consecutive trials (1–6) are shown. In trial 2, a TTX-filled micropipette was just inserted near the ROI shown in A1. In trial 3, upon release of TTX (10 s), the AP waveforms have changed. In trials 4 and 5, a 10- and 15-min wash, respectively, improved the AP waveforms in the apical tuft. In trial 6, when AP frequency was set to 125 Hz, 2 of 3 APs failed to invade the apical tuft branch. Overall, the 2nd and 3rd AP “flicker” between consecutive recordings (trials 1–6). B: computational model: flickering of APs induced by miniature changes in channel kinetics. Same model as in Fig. 7A. Triplets of APs triggered in the cell body (gray trace) by 3 current pulses are superimposed with voltage waveforms obtained in the apical tuft branch (red trace). Six experimental trials are shown. Nominal temperature was varied from 27.0 to 28.3°C.
Effect of temperature in model neurons.
To mimic small perturbations of external or internal factors potentially affecting AP backpropagation in L5 pyramidal neurons, we gradually increased the model nominal temperature from 27.0°C to 28.3°C. Activation and inactivation kinetics of voltage gated currents in this model depend on temperature as described by the temperature coefficient Q10 (methods). We found that in a very narrow temperature range, less than 2°C, the apical tuft branch exhibited a variety of AP outcomes (Fig. 9B, trials 1–6). The 2nd spike successfully invaded the apical tuft branch at some but not all temperatures. The same held true for the 3rd spike in the train. Notably, in the same trial, one late spike can fail, whereas the other can be successful. For example, in trial 4 of Fig. 9B, the 2nd spike failed, whereas the 3rd spike was successful. Variations in the nominal temperature were made to show that small changes in the kinetics of the biophysical system under study (apical tuft) result in the AP-Ca2+ flickering behavior. For example, at 27.0°C, the 2nd AP invades apical tuft branch. At 28.0°C, the 2nd AP failed to invade the apical tuft branch. Reverting temperature back to 27.0°C resurrects the 2nd AP (AP-Ca2+ flickering behavior, Fig. 9B). Both experiments and computer simulations converge on the idea that small perturbations in dendritic excitability cause the 1st, 2nd, and 3rd APs to be successful in some trials but to fail in other trials (AP flickering). These perturbations could be based on many factors, including spontaneous synaptic inputs, downregulation-upregulation of voltage-gated channels (Figs. 6, 8, and 9A), or temperature-induced changes in channel activation-inactivation dynamics (Fig. 9B).
Somatosensory cortex.
We studied the reliability of AP backpropagation into distal tuft dendrites in the somatosensory cortex by voltage imaging (Fig. 10A1) and found backpropagation failure and trial-to-trial variability similar to that in the PFC. In the somatosensory cortex, high-frequency AP trains produced characteristic boosting of the 2nd or 3rd spike in the train, followed by the “Ca2+ spikes” in the apical trunk (Fig. 10A2), previously documented by dendritic patch electrodes (Larkum et al. 1999; their Fig. 1). Spatially resolved optical measurements (>10 recording sites simultaneously) showed that boosting of late spikes had occurred already in the apical trunk (Fig. 10A2, ROI 6) and was more prominent in the trunk (ROI 6) than in the tuft (ROI 13). The voltage waveform of the regenerative Ca2+ spike was present in a very long segment of the apical axis, from a proximal apical trunk near the cell body (Fig. 10A2, ROI 3) all the way up to the principal bifurcation (ROI 10). Passive propagation of the Ca2+ spike from the apical trunk through the principal bifurcation is manifested by significant depolarization in distal apical tuft branches (Fig. 10A2, arrow). Similar to the PFC (Fig. 9A2), in the somatosensory cortex too, the 1st AP in a train often failed to invade the apical tuft (Fig. 10, A2 and A3). The amplitude of the 1st AP varied considerably between L5 pyramidal cells, in the range of 0.5%–8% ΔF/F (n = 11). Voltage transients with small amplitudes typically had wide waveforms, whereas voltage transients of large amplitudes typically had narrow waveforms (Fig. 10B), consistent with the biophysics of AP propagation in weakly excitable cables (Rapp et al. 1996). Therefore, L5 pyramidal neurons of the rat somatosensory cortex comprise a highly heterogeneous group of neurons, in which multiple outcomes of AP invasion can be found ranging from a complete failure (Fig. 10B, cells 1–5) to a moderate invasion (cells 9–11). Early spikes in the AP train (e.g., 1st, 2nd, and 3rd AP) are prone to failures. For example, the 2nd AP is prominent in one neuron (Fig. 10A) but completely absent in another cortical L5 neuron (Fig. 10C). The stochastic nature of AP invasion into the apical tuft is mostly apparent when AP voltage waveforms are monitored repeatedly from one location; i.e., over many recording trials. For example, during one time period, the 2nd AP was successful (Fig. 10D, trials 1–4), but in the next time period, the AP invasion of the same apical tuft branch (same ROI) was considerably less successful (trials 5–8, asterisk).
In summary, the apical tuft branches of L5 pyramidal neurons of the somatosensory cortex experience a rich repertoire of voltage waveforms during a train of AP firing. Although the AP waveforms were similar near the AP initiation site at the cell body (Fig. 10A3, ROI 0), each AP in the train assumed a unique and highly unpredictable voltage waveform upon entering the apical tuft (ROI 13). The 1st AP in the train fails in almost every neuron, on almost every trial. Therefore, it can be said that the 1st AP failure in apical tuft is typical for cortical pyramidal cells. With respect to the 2nd AP of the train, the apical tufts of cortical L5 pyramidal neurons exhibit an astonishing variety of electrical responses, and the outcome of the AP invasion is variable even within the same cell (same apical tuft branch), on a moment-to-moment basis (Fig. 10D).
DISCUSSION
Multisite dendritic Ca2+ imaging, dendritic voltage imaging, focal application of drugs, and multicompartmental modeling were used to study backpropagation of AP into the apical tuft at different AP firing frequencies. Several novelties were found.
Ca2+ Signal in the Tuft
High-frequency AP bursts are not absolutely necessary for bringing small amounts of Ca2+ into the apical tuft branch. We found signals in the most distal segments of apical tuft branches during slow AP firing (<10 Hz). Our result differs from earlier studies (Barth et al. 2008; Boudewijns et al. 2013; Gulledge and Stuart 2003; Waters et al. 2005) due to simultaneous sampling of signals from several apical tuft branches, thus increasing chances for finding responsive branches. Additionally, the Ca2+-sensitive dye was allowed to diffuse for a very long time (methods), which improves the quality of optical signals in the most distal dendrites.
Boosting of bAP Amplitude
It was reported previously that the dendritic AP amplitude decreases abruptly after the first few APs in a low-frequency train (Spruston et al. 1995). The present study highlights an entirely opposite physiological response, previously observed by Larkum et al. (1999) (see their Fig. 3). That is, at AP frequencies >80 Hz, the dendritic AP amplitude increases for later spikes in train (Fig. 9B). This mechanism is based on dendritic regenerative Na+ spikes supported by K+ current inactivation, and it is distinctly different from the AP-induced initiation of dendritic Ca2+ spikes (Larkum et al. 1999). The simple boosting of bAP amplitude is characterized by sharp and narrow voltage waveforms of all three APs in the train separated by full repolarization between spikes (Fig. 9A), as opposed to broad voltage waveforms of the dendritic Ca2+ spikes exhibiting a Ca2+ shoulder and incomplete repolarization after the activation of the dendritic Ca2+ electrogenesis (Fig. 10A3; see also Larkum et al. 1999).
Rising Phase of the Ca2+ Waveform
We report an inflection on the rising phase of the Ca2+ waveform (Fig. 1, B1 and B2, arrow b) caused by dendritic Na+ spike preceding the dendritic Ca2+ spike (Larkum et al. 1999). Voltage-sensitive dye imaging reveals that local Na+ electrogenesis often precedes local Ca2+ electrogenesis (Fig. 10A3).
Variable AP Backpropagation
AP invasion into the apical tuft is highly variable, and this variability is presented at four levels. The AP invasion efficacy varies 1) between pyramidal cells of the same nominal class (e.g., medial PFC L5 pyramidal cells), 2) between individual apical tuft branches of the same cell (sister branches), 3) between consecutive spikes in a train (e.g., 1st vs. 2nd AP), and 4) with time (trial-to-trial variability, i.e., AP flickering). We observed a complete loss of Ca2+ signal in one trial and a return of this signal in the next trial (AP-Ca2+ flickering). AP flickering was not due to severing dendrites during brain slice preparation, because branches at any depth (in reference to the brain slice surface) experienced AP flickering. AP flickering was not due to phototoxic injury during optical imaging or rundown of Ca2+ current, which produces irreversible changes in signal amplitude. The AP flickering described in the present study comprises sudden increases and decreases in signal amplitude, with no change in the somatic AP shape (characteristic of phototoxic injury). Flickering of backpropagating APs in apical tuft branches could potentially be due to a failure of somatic current pulses to trigger all APs in all trials. However, in the present study, we provide evidence of AP flickering in apical tufts together with simultaneous recording of APs in the cell body and in the apical trunk of the same cell, so there is no ambiguity about successful initiation of the somatic AP in all recording trials.
Time Delay of the Ca2+ Peak
Previous studies either lacked temporal resolution (slow sampling rate) or did not explore in detail the period of time between somatic AP and peak of Ca2+ signal in the apical tuft. How precise are our current measurements of the Ca2+ peak delay? Timing of dendritic Ca2+ peak in optical recordings is influenced by the affinity of the Ca2+-sensitive dye and the concentration reached in distal dendrite (Helmchen et al. 1996). In proximal dendrites of pyramidal cells loaded with OGB1 (100 µM), the rise time of single-AP fluorescence change is less than 2 ms (Maravall et al. 2000). The maximal sampling rate in the present study (5 ms per frame) and the OGB1 buffering (2 ms) do not prohibit our overall conclusion that dendritic Ca2+ signal in apical tuft occurs 30–60 ms after the 1st somatic AP in the AP triplet, if AP frequency is in the range from 80 to 200 Hz. Dendritic Ca2+ signal in apical tuft occurs 10–30 ms after the 1st somatic AP, if AP frequency is less than 20 Hz (Fig. 1C3). During slow AP firing (<20 Hz), the 2nd and 3rd spikes either do not show in Ca2+ records from the apical tuft or have significantly smaller amplitudes than the 1st AP (Figs. 1–6).
The spike timing-dependent plasticity (STDP) rule for potentiation, “EPSP followed by AP” (Bi and Poo 1998), may not work well in distal apical tufts when it comes to pairing excitatory postsynaptic potentials (EPSPs) with AP spike triplets (Letzkus et al. 2006). Although our measurements show that the voltage time delays for simple AP backpropagation are less than 2 ms (voltage peak in dendrite), the AP burst-induced Ca2+ signal is delayed 30 ms or more (Ca2+ peak in dendrite; Fig. 1). Brief and strong postsynaptic Ca2+ elevations are deemed necessary for LTP, whereas smaller, more prolonged Ca2+ elevations induce long-term depression (LTD; Lisman 1989). To ensure that an EPSP is received during the peak intracellular Ca2+ concentration in the tuft branch, a somatic spike triplet (e.g., 50 Hz), which is often used in LTP induction protocols, should occur 30–40 ms before the EPSP. Typically, the published LTP induction protocols use positive time intervals at +10 ms (Feldman 2012). However, our data suggested that this interval should be negative, at approximately −30 ms, for engaging the plasticity of the apical tuft EPSPs in neocortical L1. In accordance to this, EPSP-to-AP-triplet pairing at negative time intervals of −15 ms successfully induced robust LTP in L5 pyramidal neurons (Kampa et al. 2006). If L1 inputs require negative spike-timing delays, this would mean that their STDP depends on a different set of cortical inputs (e.g., inputs on proximal dendrites) to bring the axon above the firing threshold before the arrival of L1 inputs, which is a form of a cooperative LTP, and may have an impact on our understanding of functional interactions between cortical layers in learning and development.
Our spatially resolved data (Fig. 2) revealed that the somatic AP frequency regulates the depth of the cerebral cortex in which layered synaptic afferents encounter large internal Ca2+ concentrations in postsynaptic pyramidal neurons. These data explain why increasing the somatic AP frequency in LTP protocols improves the likelihood and amplitude of LTP (Feldman 2012).
Flickering Mechanisms
AP-Ca2+ flickering behavior can be caused by perturbations in several factors, including 1) background network activity (spontaneous synaptic inputs), 2) the availability of activatable dendritic channels (downregulation-upregulation), or 3) channel activation-inactivation dynamics (temperature). For example, small temperature variations that have been shown to occur naturally in the brain during feeding, mating, and injury (Aronov and Fee 2012; Grossman and Rechtschaffen 1967; Mrozek et al. 2012) may have an impact on the AP invasion process in the apical tuft.
Apical tuft branches in PFC contain 4-AP-sensitive K+ channels. The density of the putative A-type K+ channels in apical tuft branches is so high that blocking it with a drug (4-AP) doubles the local AP-Ca2+ signals. This quantitative estimate is a useful constrain for modeling apical tuft excitability.
The Na+ channel density in apical tuft branches is not only capable of boosting the amplitude of backpropagating APs (TTX experiment; Fig. 9A2), but also dendritic gNA is high enough to support local Na+ spike initiation (Fig. 7B).
Activation of dendritic VGCCs is very sensitive to the duration of dendritic voltage transient. Even without significant increases in peak millivolt amplitude of failing bAPs, small changes in the duration of these waveforms can cause a disproportionately large increase in dendritic Ca2+ influx (Fig. 8A).
AP Bursts Below Critical Frequency
Synapses in the neocortex exhibit low release probability. Activation of a single AP or low-frequency spike trains in presynaptic neurons may result in zero synaptic transmission. For that reason, burst firing is a particularly effective means for boosting the reliability of the intranetwork communications, for the rhythm to be reliably transmitted to the postsynaptic cell via unreliable synapses, and for recruiting feedback inhibition in cortical circuits (Kim and McCormick 1998; Wang 1999). Our dendritic imaging data showed that AP trains of certain frequencies (e.g., 50–90 Hz) are also necessary for inactivating the K+ A-type current in apical dendrite and boosting the voltage waveforms of the late (2nd or 3rd) bAPs (Fig. 9). This boosting of the late AP waveforms occurs at frequencies that are lower than the frequencies (>100 Hz) required for the generation of bAP-induced dendritic Ca2+ spikes (Larkum et al. 1999) and is likely to occur more often in vivo than are dendritic Ca2+ spikes, because pyramidal neurons in vivo rarely fire above 90 Hz. In summary, AP bursts serve important physiological roles at two neuronal terminals. In the axon terminal, they secure chemical transmission via unreliable cortical synapses. In the apical tuft terminal, they boost the voltage waveforms of the backpropagating spikes by inactivating dendritic A-type conductance.
AP backpropagation, spectrum of efficacies, and temporal fluctuations.
There is a large amount of variability in the strength of AP backpropagation among cortical L5 neurons. Experimental results show a complete failure of single AP in apical tufts of some neurons (Gulledge and Stuart 2003; Waters et al. 2005); present study) and relatively successful invasion of the apical tuft in other L5 pyramidal neurons (Holthoff et al. 2010); present study). Heterogeneous AP invasion efficacy has been previously detected between individual neurons of the same class (Barth et al. 2008; Boudewijns et al. 2013; Golding et al. 2001; Larkum et al. 2001). In the present study we show that variable strengths of AP backpropagation exist even within the same cell and same apical tuft branch, just at different instances of time.
Mechanism of AP flickering.
Flickering of backpropagating AP in apical tuft dendrites is the consequence of weak dendritic excitability (Rapp et al. 1996), unique morphology of the apical nexus, and the presence of Na+ and K+ channels in terminal apical tuft branches. A bAP is on the verge of failing as it enters the apical nexus, a complex branch point with large impedance change. Our data show that Na+ and K+ conductances can support local regenerative spikelets in the tuft (Fig. 7B). Minute changes in dendritic voltage waveform would result in AP success or AP failure to invade terminal tuft branch. Tiny fluctuations in any biophysical parameter will have a large impact on the outcome of invasion (success or failure). Our experiments did not directly determine which specific biophysical parameter was changing while we were observing trial-to-trial variations in AP amplitude (AP-Ca2+ flickering). In this study we were only able to show that when we artificially manipulate one biophysical parameter, the bAP-Ca2+ undergoes dramatic changes in apical tuft branches. We found four biological factors with significant impact on the outcome of bAP invasion and bAP-Ca2+ signal: 1) spontaneous synaptic inputs, 2) temperature, 3) availability of K+ channels and 4) availability of Na+ channels in distal dendritic branches.
Coupling of several tuft branches to the tip of the apical trunk provides the basis for a sudden drop in local membrane resistance complicated by a sudden increase in local membrane capacitance. This formidable obstacle (complex branch point) is located several hundred micrometers away from the cell body, at a distance at which AP amplitude has already lost much of its initial value achieved at the site AP initiation (Stuart et al. 1997). The grade of this loss (amplitude attenuation along the apical trunk), and the previous history (depolarization-induced inactivation) and phosphorylation status of Na+ and K+ conductances in the apical trunk and tuft (Barth et al. 2008; Gasparini and Magee 2002; Kim et al. 2007; Labno et al. 2014; Varga et al. 2000; Williams and Stuart 2000), as well as the spontaneous excitatory, inhibitory, and modulatory inputs into the tuft, or fluctuations in temperature, are just one group of factors that we show in our experiments to influence the outcome of the AP invasion into the apical tuft. It is possible that additional biological and biophysical factors also influence the success rate of AP invasion into the apical tuft.
Functional significance of AP flickering.
Apical tufts of L5 pyramidal neurons are endowed with 1) unique morphology, i.e., apical nexus followed by extensive branching, 2) ample spine density in terminal branches, and 3) voltage-gated conductances that support local nonlinear voltage responses (Berger et al. 2001; Larkum et al. 2009; Seamans et al. 1997). These intrinsic features combined with massive neuromodulatory input into L1 (dopaminergic, serotonergic, noradrenergic, cholinergic, and glutamatergic) render apical tuft as a highly malleable environment in terms of the local voltage and Ca2+ responses (Hoffman and Johnston 1999; Hsieh and Levine 2013; Tsubokawa and Ross 1997; Verhoog et al. 2016; Zhou and Antic 2012). Relatively small and spatially restricted changes in intrinsic or extrinsic conditions may drastically alter the efficacy of AP backpropagation, creating a dynamic switch between “ineffectual” to “significant” AP infiltration into the apical tuft branches. We found that neither low- nor high-frequency AP bursts are reliable suppliers of Ca2+ ions to the synaptic contacts in cortical L1. This finding has repercussions on our understanding of 1) spike-timing-dependent plasticity (Dan and Poo 2006; Feldman 2012; Letzkus et al. 2006) involving synapses inside the cortical L1, 2) the AP-mediated release of second messengers (Brenowitz et al. 2006; Fortin et al. 2004) in L1, and 3) Ca2+-dependent processes (Ghosh and Greenberg 1995; Qiu and Cheng 2010; West et al. 2001) occurring in the most superficial cortical zone.
Stochasticity is built into the cerebral cortex at virtually every level of the cortical structure and function, from membrane channel gating to synaptic transmission. Highly variable backpropagating APs in individual dendritic branches may serve to 1) increase the complexity of signal integrations at the level of an individual neuron, 2) expand the list and range of neuronal parameters eligible for modulatory/plastic changes, and 3) further reduce the deterministic aspect while increasing the stochastic nature of cortical processing (Guigon and Burnod 1995). Paradoxically, information processing is dependent not only on signal strength but also on a certain amount of basic noise and randomness (Bellay et al. 2015; Korn and Faure 2003; Rudolph and Destexhe 2001; Winterer et al. 1999). In a neuronal network composed of highly unpredictable individual neurons, the “decision” is formed when a large number of neurons manage to overcome stochastic forces and come to a consensus. In this way, the neuronal networks exploit individual uncertainties of their founding elements (neurons) to arrive at decisions with greater levels of confidence.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant MH109091 (to S. D. Antic), a National Association for Research on Schiozophrenia and Depression Young Investigator Award (to S. D. Antic), an Institutional Health Center Research Advisory Council grant (to S. D. Antic), NIH Grant NS068407 (to D. Zecevic), and National Science Foundation Grant IOS-0817969 (to D. Zecevic). Dendritic imaging experiments were in part performed in the Stem Cell Physiology and Chemistry Core, supported by Connecticut Innovations Grant 09-SCA-UCHC-13 (to S. D. Antic).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.M.S., K.D.O., W.-L.L.Z., C.D.A., and M.A.P. performed experiments; S.M.S. and S.D.A. analyzed data; D.Z. and S.D.A. interpreted results of experiments; C.D.A., M.A.P., and S.D.A. prepared figures; S.M.S. and S.D.A. drafted manuscript; S.M.S., K.D.O., W.-L.L.Z., M.A.P., D.Z., and S.D.A. edited and revised manuscript; S.M.S., K.D.O., W.-L.L.Z., C.D.A., M.A.P., D.Z., and S.D.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Leslie M. Loew (UConn Health) for providing dyes.
REFERENCES
- Acker CD, Antic SD. Quantitative assessment of the distributions of membrane conductances involved in action potential backpropagation along basal dendrites. J Neurophysiol 101: 1524–1541, 2009. doi: 10.1152/jn.00651.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antic S, Major G, Zecevic D. Fast optical recordings of membrane potential changes from dendrites of pyramidal neurons. J Neurophysiol 82: 1615–1621, 1999. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron 76: 223–239, 2012. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronov D, Fee MS. Natural changes in brain temperature underlie variations in song tempo during a mating behavior. PLoS One 7: e47856, 2012. doi: 10.1371/journal.pone.0047856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth AM, Vizi ES, Zelles T, Lendvai B. α2-Adrenergic receptors modify dendritic spike generation via HCN channels in the prefrontal cortex. J Neurophysiol 99: 394–401, 2008. doi: 10.1152/jn.00943.2007. [DOI] [PubMed] [Google Scholar]
- Beggs JM, Plenz D. Neuronal avalanches are diverse and precise activity patterns that are stable for many hours in cortical slice cultures. J Neurosci 24: 5216–5229, 2004. doi: 10.1523/JNEUROSCI.0540-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers JM. Distribution and activation of voltage-gated potassium channels in cell-attached and outside-out patches from large layer 5 cortical pyramidal neurons of the rat. J Physiol 525: 611–620, 2000. doi: 10.1111/j.1469-7793.2000.t01-2-00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellay T, Klaus A, Seshadri S, Plenz D. Irregular spiking of pyramidal neurons organizes as scale-invariant neuronal avalanches in the awake state. eLife 4: e07224, 2015. doi: 10.7554/eLife.07224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger T, Larkum ME, Lüscher HR. High Ih channel density in the distal apical dendrite of layer V pyramidal cells increases bidirectional attenuation of EPSPs. J Neurophysiol 85: 855–868, 2001. [DOI] [PubMed] [Google Scholar]
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci 18: 10464–10472, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudewijns ZS, Groen MR, Lodder B, McMaster MT, Kalogreades L, de Haan R, Narayanan RT, Meredith RM, Mansvelder HD, de Kock CP. Layer-specific high-frequency action potential spiking in the prefrontal cortex of awake rats. Front Cell Neurosci 7: 99, 2013. doi: 10.3389/fncel.2013.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz SD, Best AR, Regehr WG. Sustained elevation of dendritic calcium evokes widespread endocannabinoid release and suppression of synapses onto cerebellar Purkinje cells. J Neurosci 26: 6841–6850, 2006. doi: 10.1523/JNEUROSCI.1280-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert CM, Magee JC, Hoffman DA, Johnston D. Slow recovery from inactivation of Na+ channels underlies the activity-dependent attenuation of dendritic action potentials in hippocampal CA1 pyramidal neurons. J Neurosci 17: 6512–6521, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank SJ, Ahmed OJ, Stevens TR, Patrick SL, Gonzalez AN, Elmaleh M, Connors BW. Thalamic control of layer 1 circuits in prefrontal cortex. J Neurosci 32: 17813–17823, 2012. doi: 10.1523/JNEUROSCI.3231-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity: from synapse to perception. Physiol Rev 86: 1033–1048, 2006. doi: 10.1152/physrev.00030.2005. [DOI] [PubMed] [Google Scholar]
- Eccles JC. The cerebral neocortex: A theory of its operation. In: Functional Properties of Cortical Cells, edited by Jones EG and Peters A. New York: Plenum, 1984, p. 1–36. doi: 10.1007/978-1-4615-6610-6_1. [DOI] [Google Scholar]
- Feldman DE. The spike-timing dependence of plasticity. Neuron 75: 556–571, 2012. doi: 10.1016/j.neuron.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin DA, Trettel J, Levine ES. Brief trains of action potentials enhance pyramidal neuron excitability via endocannabinoid-mediated suppression of inhibition. J Neurophysiol 92: 2105–2112, 2004. doi: 10.1152/jn.00351.2004. [DOI] [PubMed] [Google Scholar]
- Frick A, Magee J, Johnston D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat Neurosci 7: 126–135, 2004. doi: 10.1038/nn1178. [DOI] [PubMed] [Google Scholar]
- Gasparini S, Magee JC. Phosphorylation-dependent differences in the activation properties of distal and proximal dendritic Na+ channels in rat CA1 hippocampal neurons. J Physiol 541: 665–672, 2002. doi: 10.1113/jphysiol.2002.020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Greenberg ME. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science 268: 239–247, 1995. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. Columnar specificity of intrinsic horizontal and corticocortical connections in cat visual cortex. J Neurosci 9: 2432–2442, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding NL, Kath WL, Spruston N. Dichotomy of action-potential backpropagation in CA1 pyramidal neuron dendrites. J Neurophysiol 86: 2998–3010, 2001. [DOI] [PubMed] [Google Scholar]
- Grossman SP, Rechtschaffen A. Variations in brain temperature in relation to food intake. Physiol Behav 2: 379–383, 1967. doi: 10.1016/0031-9384(67)90055-8. [DOI] [Google Scholar]
- Guigon E, Burnod Y. Modelling the acquisition of goal-directed behaviors by populations of neurons. Int J Psychophysiol 19: 103–113, 1995. doi: 10.1016/0167-8760(94)00079-T. [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Kampa BM, Stuart GJ. Synaptic integration in dendritic trees. J Neurobiol 64: 75–90, 2005. doi: 10.1002/neu.20144. [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Stuart GJ. Action potential initiation and propagation in layer 5 pyramidal neurons of the rat prefrontal cortex: absence of dopamine modulation. J Neurosci 23: 11363–11372, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett MT, Xu NL, Magee JC, Williams SR. Potassium channels control the interaction between active dendritic integration compartments in layer 5 cortical pyramidal neurons. Neuron 79: 516–529, 2013. doi: 10.1016/j.neuron.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmchen F, Imoto K, Sakmann B. Ca2+ buffering and action potential-evoked Ca2+ signaling in dendrites of pyramidal neurons. Biophys J 70: 1069–1081, 1996. doi: 10.1016/S0006-3495(96)79653-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DA, Johnston D. Neuromodulation of dendritic action potentials. J Neurophysiol 81: 408–411, 1999. [DOI] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature 387: 869–875, 1997. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Holthoff K, Zecevic D, Konnerth A. Rapid time course of action potentials in spines and remote dendrites of mouse visual cortex neurons. J Physiol 588: 1085–1096, 2010. doi: 10.1113/jphysiol.2009.184960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh LS, Levine ES. Cannabinoid modulation of backpropagating action potential-induced calcium transients in layer 2/3 pyramidal neurons. Cereb Cortex 23: 1731–1741, 2013. doi: 10.1093/cercor/bhs168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegaya Y, Aaron G, Cossart R, Aronov D, Lampl I, Ferster D, Yuste R. Synfire chains and cortical songs: temporal modules of cortical activity. Science 304: 559–564, 2004. doi: 10.1126/science.1093173. [DOI] [PubMed] [Google Scholar]
- Jaffe DB, Johnston D, Lasser-Ross N, Lisman JE, Miyakawa H, Ross WN. The spread of Na+ spikes determines the pattern of dendritic Ca2+ entry into hippocampal neurons. Nature 357: 244–246, 1992. doi: 10.1038/357244a0. [DOI] [PubMed] [Google Scholar]
- Kampa BM, Letzkus JJ, Stuart GJ. Requirement of dendritic calcium spikes for induction of spike-timing-dependent synaptic plasticity. J Physiol 574: 283–290, 2006. doi: 10.1113/jphysiol.2006.111062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jung SC, Clemens AM, Petralia RS, Hoffman DA. Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons. Neuron 54: 933–947, 2007. doi: 10.1016/j.neuron.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U, McCormick DA. The functional influence of burst and tonic firing mode on synaptic interactions in the thalamus. J Neurosci 18: 9500–9516, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn H, Faure P. Is there chaos in the brain? II. Experimental evidence and related models. C R Biol 326: 787–840, 2003. doi: 10.1016/j.crvi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Korngreen A, Sakmann B. Voltage-gated K+ channels in layer 5 neocortical pyramidal neurones from young rats: subtypes and gradients. J Physiol 525: 621–639, 2000. doi: 10.1111/j.1469-7793.2000.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labno A, Warrier A, Wang S, Zhang X. Local plasticity of dendritic excitability can be autonomous of synaptic plasticity and regulated by activity-based phosphorylation of Kv4.2. PLoS One 9: e84086, 2014. doi: 10.1371/journal.pone.0084086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME, Kaiser KM, Sakmann B. Calcium electrogenesis in distal apical dendrites of layer 5 pyramidal cells at a critical frequency of back-propagating action potentials. Proc Natl Acad Sci USA 96: 14600–14604, 1999. doi: 10.1073/pnas.96.25.14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME, Nevian T, Sandler M, Polsky A, Schiller J. Synaptic integration in tuft dendrites of layer 5 pyramidal neurons: a new unifying principle. Science 325: 756–760, 2009. doi: 10.1126/science.1171958. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ, Sakmann B. Dendritic mechanisms underlying the coupling of the dendritic with the axonal action potential initiation zone of adult rat layer 5 pyramidal neurons. J Physiol 533: 447–466, 2001. doi: 10.1111/j.1469-7793.2001.0447a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus JJ, Kampa BM, Stuart GJ. Learning rules for spike timing-dependent plasticity depend on dendritic synapse location. J Neurosci 26: 10420–10429, 2006. doi: 10.1523/JNEUROSCI.2650-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc Natl Acad Sci USA 86: 9574–9578, 1989. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo JN, Barnwell LF, Ren Y, Lee WL, Johnston LD, Kim R, Hrachovy RA, Sweatt JD, Anderson AE. Altered phosphorylation and localization of the A-type channel, Kv4.2 in status epilepticus. J Neurochem 106: 1929–1940, 2008. doi: 10.1111/j.1471-4159.2008.05508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainen ZF, Sejnowski TJ. Influence of dendritic structure on firing pattern in model neocortical neurons. Nature 382: 363–366, 1996. doi: 10.1038/382363a0. [DOI] [PubMed] [Google Scholar]
- Maravall M, Mainen ZF, Sabatini BL, Svoboda K. Estimating intracellular calcium concentrations and buffering without wavelength ratioing. Biophys J 78: 2655–2667, 2000. doi: 10.1016/S0006-3495(00)76809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Helm PJ, Sakmann B. Dendritic calcium transients evoked by single back-propagating action potentials in rat neocortical pyramidal neurons. J Physiol 485: 1–20, 1995. doi: 10.1113/jphysiol.1995.sp020708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickus T, Jung H, Spruston N. Properties of slow, cumulative sodium channel inactivation in rat hippocampal CA1 pyramidal neurons. Biophys J 76: 846–860, 1999. doi: 10.1016/S0006-3495(99)77248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliore M, Hoffman DA, Magee JC, Johnston D. Role of an A-type K+ conductance in the back-propagation of action potentials in the dendrites of hippocampal pyramidal neurons. J Comput Neurosci 7: 5–15, 1999. doi: 10.1023/A:1008906225285. [DOI] [PubMed] [Google Scholar]
- Milojkovic BA, Radojicic MS, Goldman-Rakic PS, Antic SD. Burst generation in rat pyramidal neurones by regenerative potentials elicited in a restricted part of the basilar dendritic tree. J Physiol 558: 193–211, 2004. doi: 10.1113/jphysiol.2004.061416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrozek S, Vardon F, Geeraerts T. Brain temperature: physiology and pathophysiology after brain injury. Anesthesiol Res Pract 2012: 989487, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikonomou KD, Singh MB, Sterjanaj EV, Antic SD. Spiny neurons of amygdala, striatum, and cortex use dendritic plateau potentials to detect network UP states. Front Cell Neurosci 8: 292, 2014. doi: 10.3389/fncel.2014.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M, Gao X, Zecevic D. Voltage-sensitive dye recording from axons, dendrites and dendritic spines of individual neurons in brain slices. J Vis Exp: e4261, 2012. doi: 10.3791/4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z, Cheng J. The role of calcium-dependent gene expression in autism spectrum disorders: lessons from MeCP2, Ube3a and beyond. Neurosignals 18: 72–81, 2010. doi: 10.1159/000320970. [DOI] [PubMed] [Google Scholar]
- Rapp M, Yarom Y, Segev I. Modeling back propagating action potential in weakly excitable dendrites of neocortical pyramidal cells. Proc Natl Acad Sci USA 93: 11985–11990, 1996. doi: 10.1073/pnas.93.21.11985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Frick A, Johnston D. Kinase-dependent modification of dendritic excitability after long-term potentiation. J Physiol 587: 115–125, 2009. doi: 10.1113/jphysiol.2008.158816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph M, Destexhe A. Do neocortical pyramidal neurons display stochastic resonance? J Comput Neurosci 11: 19–42, 2001. doi: 10.1023/A:1011200713411. [DOI] [PubMed] [Google Scholar]
- Schaefer AT, Larkum ME, Sakmann B, Roth A. Coincidence detection in pyramidal neurons is tuned by their dendritic branching pattern. J Neurophysiol 89: 3143–3154, 2003. doi: 10.1152/jn.00046.2003. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Gorelova NA, Yang CR. Contributions of voltage-gated Ca2+ channels in the proximal versus distal dendrites to synaptic integration in prefrontal cortical neurons. J Neurosci 17: 5936–5948, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N, Schiller Y, Stuart G, Sakmann B. Activity-dependent action potential invasion and calcium influx into hippocampal CA1 dendrites. Science 268: 297–300, 1995. doi: 10.1126/science.7716524. [DOI] [PubMed] [Google Scholar]
- Stuart G, Spruston N, Sakmann B, Häusser M. Action potential initiation and backpropagation in neurons of the mammalian CNS. Trends Neurosci 20: 125–131, 1997. doi: 10.1016/S0166-2236(96)10075-8. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature 367: 69–72, 1994. doi: 10.1038/367069a0. [DOI] [PubMed] [Google Scholar]
- Tsubokawa H, Ross WN. IPSPs modulate spike backpropagation and associated [Ca2+]i changes in the dendrites of hippocampal CA1 pyramidal neurons. J Neurophysiol 76: 2896–2906, 1996. [DOI] [PubMed] [Google Scholar]
- Tsubokawa H, Ross WN. Muscarinic modulation of spike backpropagation in the apical dendrites of hippocampal CA1 pyramidal neurons. J Neurosci 17: 5782–5791, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga AW, Anderson AE, Adams JP, Vogel H, Sweatt JD. Input-specific immunolocalization of differentially phosphorylated Kv4.2 in the mouse brain. Learn Mem 7: 321–332, 2000. doi: 10.1101/lm.35300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoog MB, Obermayer J, Kortleven CA, Wilbers R, Wester J, Baayen JC, De Kock CP, Meredith RM, Mansvelder HD. Layer-specific cholinergic control of human and mouse cortical synaptic plasticity. Nat Commun 7: 12826, 2016. doi: 10.1038/ncomms12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ. Fast burst firing and short-term synaptic plasticity: a model of neocortical chattering neurons. Neuroscience 89: 347–362, 1999. doi: 10.1016/S0306-4522(98)00315-7. [DOI] [PubMed] [Google Scholar]
- Waters J, Helmchen F. Boosting of action potential backpropagation by neocortical network activity in vivo. J Neurosci 24: 11127–11136, 2004. doi: 10.1523/JNEUROSCI.2933-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J, Schaefer A, Sakmann B. Backpropagating action potentials in neurones: measurement, mechanisms and potential functions. Prog Biophys Mol Biol 87: 145–170, 2005. doi: 10.1016/j.pbiomolbio.2004.06.009. [DOI] [PubMed] [Google Scholar]
- West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci USA 98: 11024–11031, 2001. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ. Mechanisms and consequences of action potential burst firing in rat neocortical pyramidal neurons. J Physiol 521: 467–482, 1999. doi: 10.1111/j.1469-7793.1999.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ. Backpropagation of physiological spike trains in neocortical pyramidal neurons: implications for temporal coding in dendrites. J Neurosci 20: 8238–8246, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Ziller M, Dorn H, Frick K, Mulert C, Dahhan N, Herrmann WM, Coppola R. Cortical activation, signal-to-noise ratio and stochastic resonance during information processing in man. Clin Neurophysiol 110: 1193–1203, 1999. doi: 10.1016/S1388-2457(99)00059-0. [DOI] [PubMed] [Google Scholar]
- Yuste R, Gutnick MJ, Saar D, Delaney KR, Tank DW. Ca2+ accumulations in dendrites of neocortical pyramidal neurons: an apical band and evidence for two functional compartments. Neuron 13: 23–43, 1994. doi: 10.1016/0896-6273(94)90457-X. [DOI] [PubMed] [Google Scholar]
- Zhou WL, Antic SD. Rapid dopaminergic and GABAergic modulation of calcium and voltage transients in dendrites of prefrontal cortex pyramidal neurons. J Physiol 590: 3891–3911, 2012. doi: 10.1113/jphysiol.2011.227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou WL, Yan P, Wuskell JP, Loew LM, Antic SD. Dynamics of action potential backpropagation in basal dendrites of prefrontal cortical pyramidal neurons. Eur J Neurosci 27: 923–936, 2008. doi: 10.1111/j.1460-9568.2008.06075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]