Abstract
Neurophysiology is the branch of physiology concerned with understanding the function of neural systems. Neural engineering (also known as neuroengineering) is a discipline within biomedical engineering that uses engineering techniques to understand, repair, replace, enhance, or otherwise exploit the properties and functions of neural systems. In most cases neural engineering involves the development of an interface between electronic devices and living neural tissue. This review describes the origins of neural engineering, the explosive development of methods and devices commencing in the late 1950s, and the present-day devices that have resulted. The barriers to interfacing electronic devices with living neural tissues are many and varied, and consequently there have been numerous stops and starts along the way. Representative examples are discussed. None of this could have happened without a basic understanding of the relevant neurophysiology. I also consider examples of how neural engineering is repaying the debt to basic neurophysiology with new knowledge and insight.
Keywords: neural engineering: neuroengineering, neural stimulators, neuroprostheses, cochlear implants, brain-computer interface, functional electrical stimulation
it could be argued that neural engineering commenced in the 1750s, when Jan Swammerdam enclosed a muscle in a glass tube containing water and activated it by tugging on its nerve, in order to determine whether its volume changed. In the same decade, Leopoldo Caldani electrically stimulated nerves with the use of an electrostatic machine (Brazier 1984). By the end of the eighteenth century, Giovanni Aldini and others had begun stimulating the nervous system in humans with the use of surface electrodes (Brazier 1988). First, Aldini performed what would now be called proof-of-principle experiments on the freshly severed heads of people who had just been guillotined, placing electrodes on the face, on the brain stem, or in the mouth. In the mid-nineteenth century Duchenne de Boulogne used electrical stimulation to study human facial expressions (Duchenne de Boulogne 1862). Duchenne’s photographs were used by Charles Darwin in his arguments regarding the evolutionary nature of the expression of emotions (Darwin 1872).
Electrical stimulation was soon adopted by clinicians to elicit muscle twitches, presumably to impress their patients rather than to treat their disorders (Licht 1971; McNeal 1977). By the mid-nineteenth century, induction coils enabled trains of electrical current pulses to be delivered, which elicited smoother muscle contractions. In the 1850s, the German inventor Isaac Pulvermacher marketed a belt comprised of a chain of batteries that delivered mechanically switched pulses of electrical current to the wearer’s abdomen. Pulvermacher’s chain was initially supported by the medical community (Bird 1851). Electrical belts and other garments became enormously popular, selling in their hundreds of thousands by the 1880s. However, vendors began making outlandish medical claims, with the result that by 1920 the field had become thoroughly discredited, as evidenced by articles in leading journals such as the Lancet and the British Medical Journal (British Medical Journal 1893).
By the mid-twentieth century, electrical stimulation of the nervous system had seen a resurgence and it had become an accepted clinical modality for pain mitigation, muscle strengthening, and rehabilitation. In 1956, with the development of the transistor, it became feasible to manufacture stimulators that could be implanted inside the body. This led to a remarkable burst of innovation and experimentation in the subsequent two decades. Devices that have their origin in this period include the cochlear implant (Chouard 2015; Djourno et al. 1957; Djourno and Eyries 1957), the cardiac pacemaker (Greatbatch 1962), the spinal cord epidural stimulator (Shealy et al. 1967), sacral nerve and root stimulators (Brindley and Lewin 1968), phrenic nerve and diaphragm pacers (Anagnostopoulos and Glenn 1966; Johnson and Eiseman 1971; Van Heeckeren and Glenn 1966), the foot-drop stimulator (Jeglic et al. 1970; Liberson et al. 1961; Waters et al. 1975), and the intraspinal stimulator (Friedman et al. 1972; Nashold et al. 1971b).
This article focuses mainly on neural engineering devices designed to treat nervous system dysfunction. To illustrate the relationship between such devices and the neurophysiology that underpins them, let us consider two examples: neurostimulators (NSs) and brain-computer interfaces (BCIs).
NSs, also known as neuroprostheses, are devices that activate neural tissue to help restore lost function. Figure 1 gives an idea of how rapidly this field is expected to grow over the next few years.
Fig. 1.
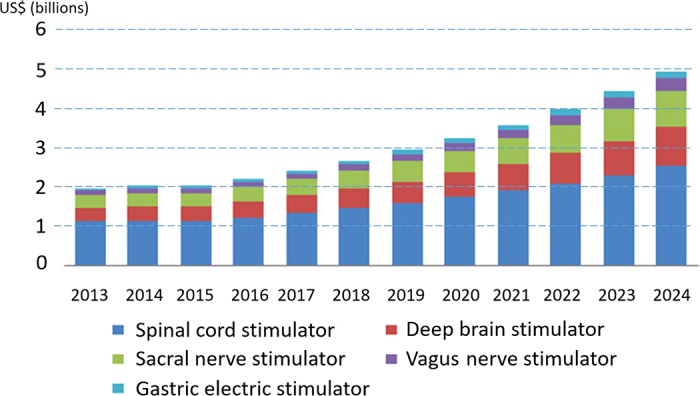
Global neurostimulation devices market in US, by device, 2013–2024. Reproduced from Grand View Research (2016) with permission.
The best-known and most successful implantable NS is the cochlear implant, arguably the most advanced neural engineering device in existence. As of 2012, cochlear implants had been implanted in over 320,000 people with severe sensorineural hearing loss worldwide (https://www.nidcd.nih.gov/health/statistics/quick-statistics-hearing). Sound vibrations are picked up by a microphone, typically located in an earpiece. The vibrations are transduced into voltage signals that are amplified, filtered, digitized, and resolved into components by a microprocessor. The microprocessor encodes this information in a radio-frequency (RF) signal and wirelessly transmits it to a pulse generator implanted under the scalp, which in turn delivers interleaved or simultaneous trains of stimuli to the sensory endings of the auditory nerve via a delicate multielectrode array inserted into the scala tympani of the cochlea. The evoked neural activity results in the hearing and discrimination of complex sounds such as speech and music. Parameters in the computer algorithms that convert the incoming signals into patterns of stimulation are fine-tuned through trial-and-error testing, whereby the recipients report the sounds they hear. The development of the cochlear implant relied on a detailed understanding of the neurophysiology of the auditory system, the physics of sound signals, the components of speech, electronics, computer processing, RF transmission, and electrode-tissue interactions. From an engineering point of view, a large variety of technical problems had to be overcome, not least those involving the multielectrode array (Chouard 2015; Clark 1978; House 1976). Unexpected societal barriers that soon arose are discussed below.
In a BCI, electrical activity is recorded from specific parts of the brain, via penetrating microelectrode arrays, electrode matrices resting on the brain surface, or electrodes attached to the scalp. The recorded signals are amplified, digitized, and resolved into components by a computer processor. For example, in a person with paralyzed arms neural activity signaling the intent to grasp and move external objects is recorded from motor areas of the cerebral cortex. The signals are decomposed analytically, and the resultant intention-related signals may be used to perform functional tasks with a robotic arm or to emulate a computer mouse, allowing the paralyzed person to use a computer. In the most recent research, the signals recorded from the brain have been used to generate trains of electrical stimuli delivered to muscle nerves via surface electrodes, eliciting movements of the user’s own arm. In this case the system is a combination of a BCI and a NS and has been called a “neural bypass” (Bouton et al. 2016; Sharma et al. 2016).
The above systems have two basic things in common: they sense one or more inputs and use this information to control one or more outputs. The same is true of most neurophysiological control systems. The inputs used in neural engineering devices either can come from artificial sensors such as accelerometers, strain gauges, and photoelectric devices or can come from the body itself, in the form of electrical activity of muscle and neural tissues. The outputs can range from computer displays and robotic arms to the recipient’s own limbs.
After the cochlear implant, the second most widely deployed implantable NS is the spinal cord stimulator. This is a fully implanted device that delivers trains of electrical pulses through an electrode array that is implanted on the surface of the dura mater, the membrane covering the spinal cord, or on the surface of the spinal cord itself (Iwahara et al. 1991, 1992). Epidural stimulation is used to reduce chronic pain, improve bladder control, and reduce spastic hyperreflexia. In a typical epidural stimulator a therapist sets the stimulation parameters with a wireless external programmer, and after that the device delivers steady trains of pulses for long periods of time. There is no continuous feedback control, and therefore there are no sensors other than wireless or magnetic on-off switches. The same is true of noninvasive transcutaneous electrical stimulators (TENS units) for pain mitigation, transcutaneous neuromuscular electrical stimulators (NMES) for exercising and strengthening muscles, implanted sacral nerve stimulators for bladder control, implanted phrenic nerve stimulators for respiration, implanted vagus nerve stimulators for epilepsy and depression, and deep brain stimulators for various motor and psychiatric disorders, intractable pain, epilepsy, and Alzheimer’s disease. The mechanism of action in some of these cases is assumed to be neuromodulation, an indirect effect mediated by intracellular second messengers. This is in contrast to the generally more rapid action of direct electrical excitation of neurons or classical chemical neurotransmission via ligand-gated postsynaptic receptors.
Sensing the Inputs
Artificial sensors.
The number of artificial sensors that can be deployed in a practical NS or BCI is insignificant when compared to the vast number of biological sensory receptors in the body. For example, foot-drop stimulators (NSs that activate the pretibial muscles to lift the foot at the onset of the swing phase of the locomotor step cycle) have at most two sensors, a force transducer or switch signaling under-heel pressure and an accelerometer signaling leg tilt. Another example is provided by grasp-release stimulators, in which signals from a shoulder or wrist sensor are used to control stimulation of muscles that open or close the hand (Peckham et al. 1980; Prochazka et al. 1996). This is in contrast to the body’s thousands of muscle, joint, connective tissue, and cutaneous receptors that provide highly detailed, multivariate information to the central nervous system (CNS) on the biomechanical state not only of the moving limb but also of the entire body (Prochazka 2015a). For example, in relation to proprioception (the sensing of the body’s own movements), there are 25,000–30,000 muscle spindles in the human body, including ~4,000 in each arm and 7,000 in each leg (Hulliger 1984; Voss 1971), nearly as many Golgi tendon organs, and many more deep pressure receptors and cutaneous receptors. In fact, it is interesting that NS devices such as foot-drop stimulators and grasp-release stimulators with only one or two sensors can significantly improve motor function after the nervous system has been damaged. This is because in some cases the sensing of simple events such as unloading of the leg during the locomotor step cycle (Liberson et al. 1961) or extension of the wrist during manual grasp-release actions (Prochazka et al. 1996) can suffice to trigger transitions from one phase of a movement to the next. It also shows that a single artificial sensor, when combined with an electronic analyzer, can provide information that is equivalent to the input from many biological sensory receptors. Again, the cochlear implant provides a good example. Sound vibrations impinging on a microphone are resolved electronically into many frequency components, just as the sound vibrations transmitted from the tympanic membrane (eardrum) to the cochlea are resolved into many frequency components by the 3,000 or so inner hair cells that line the basilar membrane of the cochlea. In this example a single artificial sensor and an electronic analyzer can replicate the function of thousands of biological sensors, albeit with a lower-frequency resolution and amplitude range.
What are some other limitations of artificial sensors compared with biological sensors? Artificial force and displacement sensors can match individual cutaneous and intramuscular receptors in their sensitivity and amplitude range, but it has proven difficult to attach them to body parts in ways that are convenient, cosmetically acceptable, and reliable over the long term. Under-heel switches and force sensors are generally held within shoes, which rules out their use when walking barefoot. In some functional electrical stimulation devices sensors are held in the same enclosures as the stimulators (e.g., wrist motion sensors, accelerometers, and leg tilt sensors; Prochazka 1997; Prochazka et al. 1997a; Stein et al. 2006). Tooth click sensors (Prochazka 2003) and head motion sensors (Prochazka 2016) built into earpieces are used to trigger hand grasp and release (e.g., Rehabtronics ReGrasp). Accelerometers and EMG amplifiers have been used experimentally in closed-loop tremor suppression systems (Gillard et al. 1999; Khobragade et al. 2015; Prochazka et al. 1992).
It took many years for the hardware of hearing aids and cochlear implants to be miniaturized to the point that they are unobtrusive and function for many years. In the cochlear implant, microphones and sound processors are generally external to the body. It was only recently that a fully implanted device that included a microphone and sound processor entered clinical trials (Briggs et al. 2008). Fully implantable hearing aids that monitor incoming sounds and apply corresponding mechanical vibrations to the ossicles have recently become available commercially (Barbara et al. 2011; Bruschini et al. 2016; Martin et al. 2009). In a few NSs, mechanical sensors have been implanted under the skin, for example, the microphones just discussed and wrist joint displacement sensors used with the Freehand upper limb device (Bhadra et al. 2002).
Visual systems in animals operate over an enormous range of light intensities, though some artificial optical sensors surpass them in terms of the range of detectable wavelengths (infrared to X-ray). Visual NSs have been developed recently that operate either by stimulating the visual cortex through electrode arrays implanted on the cortical surface or via electrode arrays attached to the back of the retina (see Table 2 for references). These devices receive inputs from miniature video cameras attached to goggles or glasses worn by the user. As in cochlear implants, the signals are processed electronically to derive trains of electrical pulses for delivery through multielectrode arrays. Learning algorithms are used to optimize this process through postimplant trial-and-error testing with the recipients.
Table 2.
Neurostimulators: studies
Commercially available,
discontinued commercially,
experimental.
Artificial sensors tend to be energy intensive, so regular battery charging or replacement is required. While this is not a major problem for sensors external to the body, it becomes a significant barrier for fully implanted systems. The approach currently taken is to implant rechargeable batteries that are charged through the skin by electromagnetic coupling. The external charger must regularly be held on, or close to, the body for significant periods of time, which can be inconvenient.
Sensing the body’s own neural signals.
Inputs to NSs and BCIs may be obtained from the body’s own neural input and output signals, for example, by monitoring the activity of neurons in the cerebral cortex (Schwartz 2016). Signals entering the spinal cord from muscle, joint, and skin receptors can be intercepted at the dorsal root ganglia with the use of implanted semimicroelectrode arrays (Loeb et al. 1977; Prochazka et al. 1976; Weber et al. 2006). This has been proposed as a means of providing intraspinal microstimulation devices with the kinematic feedback needed to activate paralyzed limbs (Holinski et al. 2013; Weber et al. 2007) and also as a means of restoring sensation by using the monitored signals to control stimulation of somatosensory regions of the brain (a “sensory neural bypass”) (Flesher et al. 2016). However, the technical barriers to long-term recording from peripheral nerves and dorsal root ganglia are formidable.
It is easier to record neural outputs indirectly, for example, by recording the electromyogram (EMG) of muscles that are still under voluntary control or even more simply by recording the movements generated by these muscles (Peckham et al. 1980; Prochazka et al. 1996). EMG control was first exploited in the AutoMove, a stimulator commercialized in the 1970s (van Overeem Hansen 1979). In this device, the EMG of a muscle under weak voluntary control is monitored through surface electrodes. When a preset threshold is reached, the device switches to stimulation mode, activating the same muscle through the same electrodes. This device, now known as the NeuroMove, is used to this day in some rehabilitation centers to strengthen paretic muscles after stroke and spinal cord injury (Francisco et al. 1998; Knutson et al. 2015).
Another approach is to use the EMG from a muscle under volitional control to continuously control functional electrical stimulation of a paretic or paralyzed muscle (Graupe et al. 1983, 1989; Hincapie and Kirsch 2007; Mercier et al. 2017; Williams and Kirsch 2015). In another variant, a pair of stimulating electrodes is attached to the skin over a paretic muscle. A pair of recording electrodes is attached at right angles about halfway between them (Hodgson 1986; Thorsen et al. 2001). This configuration minimizes artifacts in the EMG signal when the muscle is stimulated. A blocking amplifier eliminates any residual artifacts, making it possible to record EMG from the muscle between stimulating pulses, thus boosting voluntary contractions. When recording and stimulating electrodes are implanted, the stimulus currents and therefore the stimulus artifacts are smaller (Hincapie and Kirsch 2007; Liu et al. 2014).
Brain activity can be recorded with surface electroencephalogram (EEG) electrodes (McFarland and Wolpaw 2003; Wolpaw et al. 1991), implanted electrocorticogram (ECoG) electrodes (Fig. 2) (Branco et al. 2017; Flint et al. 2017; Hotson et al. 2016; Leuthardt et al. 2006; Vansteensel et al. 2016; Vinjamuri et al. 2009; Wang et al. 2009), or implanted penetrating microelectrode arrays (Bouton et al. 2016; Sharma et al. 2016). Surface EEG signals tend to be small, variable, susceptible to artifacts (especially eyeblinks), and poorly localized because the sources (ensembles of cortical neurons) and the recording sites are separated not only by a layer of cerebrospinal fluid and the meninges but also by the skull and the scalp. Nonetheless, EEG-based BCIs were highly represented at the 2016 Society for Neuroscience annual meeting. Furthermore, in a recent study feedback-controlled functional electrical stimulation driven by EEG signals enabled able-bodied subjects to perform a tracking task with their forearms (Vidaurre et al. 2016). In theory, ECoG electrode arrays implanted subdurally on the cortical surface, or epidurally on the dural surface, should provide more localized multichannel signals than surface EEGs. Research is underway to compare these modalities (Flint et al. 2017).
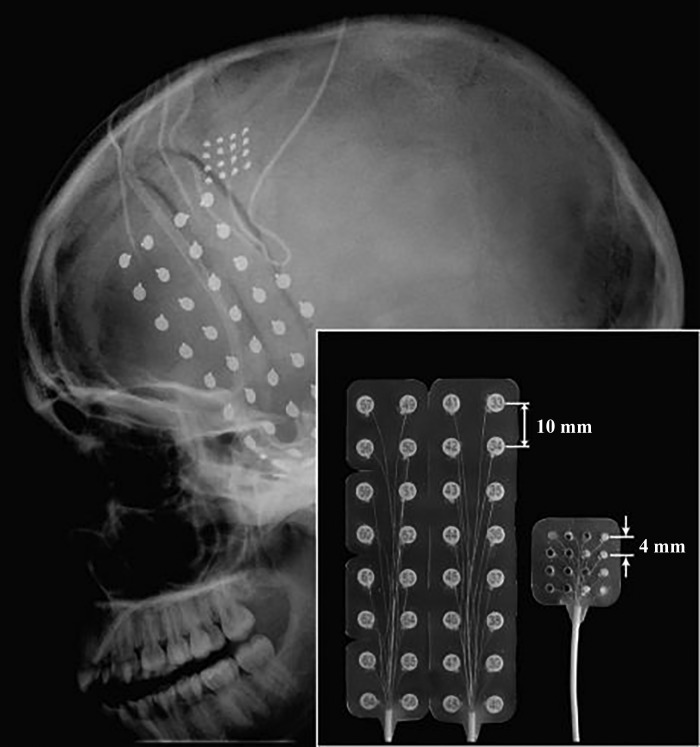
Fig. 2.
Head X-ray (lateral view) showing the locations and sizes of implanted ECoG electrodes: one micro-ECoG grid (16 contacts), one regular ECoG grid (32 contacts), and two 6-contact regular ECoG strips. Inset: side-by-side comparison of a regular ECoG grid and a micro-ECoG grid showing the center-to-center electrode spacing (reproduced from Wang et al. 2009 with permission).
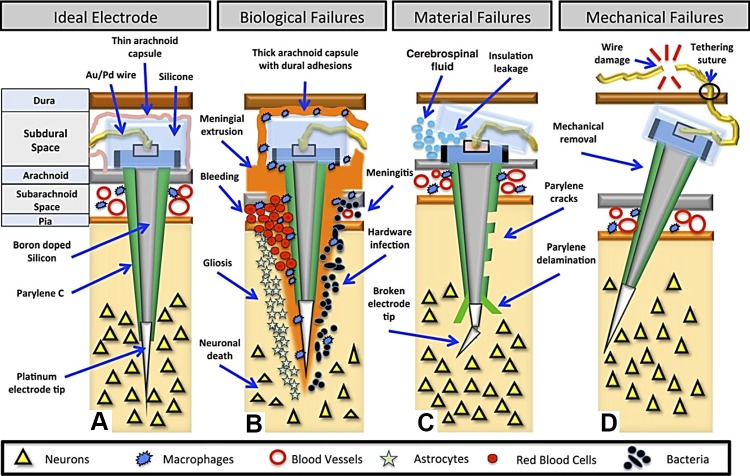
Multiple single-cell recordings with penetrating intracortical silicon microelectrode arrays provide the most localized and information-rich signals, but this approach is also the most invasive. With current devices the recordings degrade within a year or so (Wodlinger et al. 2015), though it is possible to extract useful information even after this has occurred (Perge et al. 2013, 2014; Simeral et al. 2011). In 2013 an important failure analysis of silicon microelectrode arrays was published (Barrese et al. 2013). Of 78 arrays implanted in 27 monkeys, the recording duration ranged from 0 to 5.75 yr, with a mean of 387 days and a median of 182 days. Fifty-six percent of failures occurred within a year, mechanical failures involving leads and connectors being the most common (48%) (Fig. 3). Meningeal reactions that separated the array from the parenchyma were the most common cause of biological failure (14.5%). Recordings showed a slow, progressive decline in spike amplitude, noise amplitude, and number of viable channels, which predicted complete signal loss by ~8 yr. The authors concluded that these arrays could potentially record for many years with the use of implantable wireless systems, better control of meningeal reactions, and improved insulation materials.
Fig. 3.
Major failure modes of microelectrode arrays. A: ideal placement, 1–1.5 mm into cortex. The top of the electrode becomes encased in a thin layer of arachnoid, which helps to stabilize the array. B: biological failures: bleeding, cell death, hardware infection, meningitis, gliosis, and meningeal encapsulation and extrusion. Macrophages originating in the subarachnoid space may mediate the encapsulation response. C: material failures: broken electrode tips, insulation leakage, and parylene cracks and delamination. D: mechanical failures: wire breakage and connector damage (reproduced from Barrese et al. 2013 with permission).
The slow deterioration of signal quality has been attributed to tissue encapsulation, inflammatory responses, rupture of the blood-brain barrier, and the death of neurons resulting from the insertion, micromotion, or physiological motion of microelectrodes (Kozai et al. 2010, 2012; McCreery et al. 2016). Smaller microelectrodes with coatings of dielectric and bioactive materials have been shown to result in significant improvements in this regard (Fattahi et al. 2014; Kozai et al. 2016). Syringe-injectable microelectronics are also on the horizon (Liu et al. 2015). Given the breadth of research underway in this field, it seems likely that the quality and reliability of signals recorded from the CNS will see major improvements over the next few years.
Activating the Outputs
Nerves innervating limb muscles are relatively accessible, allowing pulses of electrical current that activate them to be applied through the skin via self-adhesive, conductive gel electrodes, conductive rubber electrodes coated with gel, or metal plate electrodes with an intervening layer of spongy material that is moistened with water. The gel and water conform to skin contours and distribute the flow of current evenly, thus avoiding hot spots of high current density that would cause skin discomfort, inflammation, and burns. Transcutaneous stimulation is commonly used in surface stimulators such as TENS and NMES units and functional electrical stimulators. When the target stimulation sites are deep or close to the CNS (e.g., cochlear nerves, sacral nerves, and the vagus nerve), or when external components cause inconvenience, the stimulator and leads are implanted. The most common implantable stimulators, cardiac pacemakers, only deliver a single pulse every second or so and can therefore be powered by an implanted nonrechargeable battery that is surgically replaced every few years. In contrast, the implantable NSs in Tables 1 and 2 have to deliver stimuli at rates as high as 18,000 pulses/s. As mentioned above, such devices contain batteries or capacitors that are recharged either intermittently or continuously by electromagnetic coupling from an outside coil. The body-worn sound processors and transmitters in cochlear stimulators have high energy needs and incorporate batteries that require recharging every few hours. In an alternative approach where only a single channel is needed, an external stimulator delivers current pulses through the skin between two surface electrodes and some of the current flows through a fully implanted lead to a target nerve (the StimRouter) (Deer et al. 2010, 2016; Gan et al. 2011; Prochazka 2005). In this system, as the stimulator is external to the body, it can be recharged and serviced easily and pulse parameters and modes of control can be modified and updated as the technology improves.
Table 1.
Neurostimulators in approximate descending order of numbers deployed worldwide
| Device | Sensor | Sensed Variable | Signal Processing | Pulse Generator, Electrodes, Target |
|---|---|---|---|---|
| TENS and NMES stimulators, external | N/A | N/A | N/A | External PG and electrodes |
| Cochlear implant* | Microphone | Air pressure variations | Digitizing, filtering, compressing, encoding | Implanted RF-controlled PG, multichannel linear electrode array, auditory nerve endings |
| Spinal cord epidural stimulator implant* | N/A | N/A | Parameter adjustment via external wireless programmer | Implanted RF-controlled PG, multichannel epidural array, dorsal roots, columns |
| Sacral nerve stimulator implant* | N/A | N/A | Parameter adjustment via external programmer | Implanted RF-controlled PG, sacral spinal nerve root |
| Foot-drop stimulator (external)* | Under-heel switch or force sensor or tilt sensor | Under-heel pressure or leg tilt | On-off detection or digitizing, filtering, threshold detection | External PG and electrodes in garter, common peroneal nerve |
| Vagus nerve stimulator implant* | N/A | N/A | Parameter adjustment via external programmer | Implanted or external PG, vagus nerve |
| Deep brain stimulator implant* | N/A (EMG and accelerometers: experimental) | N/A (tremor: experimental) | Parameter adjustment via external programmer | External PG, midbrain nuclei |
| Phrenic nerve and diaphragm pacers (implanted)* | N/A | N/A | Parameter adjustment via external programmer | Implanted or external PG, phrenic nerve cuff or intramuscular electrodes in diaphragm |
| Sacral anterior root stimulator implant* | N/A | N/A | Parameter adjustment via external programmer | Implanted RF-controlled PG, sacral anterior nerve root |
| Grasp-release stimulators (external)* | Push-button or earpiece | Taps, tooth clicks, head nods | Digitizing, filtering, threshold detection | External PG and electrodes in brace or garment, nerves innervating hand muscles |
| Grasp-release stimulators (implant)† | Goniometers or implanted Hall effect sensors | Shoulder or wrist movements | Digitizing, filtering, proportional control of outputs | Implanted RF-controlled PG, epimysial electrodes, nerves innervating hand muscles |
| StimRouter implant* | N/A or earpiece | N/A or tooth clicks | Parameter adjustment via external programmer or digitizing, filtering, threshold detection | Implanted lead, external stimulator, skin electrodes, nerves suppressing pain, overactive bladder, or restoring hand function |
| Foot-drop stimulator (implant)§ | Under-heel force sensor or nerve cuff | Under-heel force, afferent activity | Digitizing, filtering, threshold detection | Implanted RF-controlled PG, nerve cuff electrodes, common peroneal nerve |
| Microstimulator implant§ | N/A | N/A | Parameter adjustment via external programmer | Implanted BIONs, various nerves |
| Visual cortex implant§ | Video | Visual field | Image analysis, stimulus encoding | Implanted RF-controlled PG, multichannel electrode array, visual cortex |
| Retinal implant§ | Video, eye position sensor | Visual field, eye position | Image analysis, stimulus encoding, eye position compensation | Implanted RF-controlled PG, multichannel electrode array, retinal ganglion cells |
| Somatosensory cortex implant§ | Force or strain sensors | Pressure or movement | Digitizing, filtering, threshold detection | External PG, intracortical electrode arrays or ECoGs, cortical cells |
| Spinal cord epidural stimulator implant (spatiotemporal)§ | Video motion capture, cortical electrode arrays | Limb position or cortical commands | Image analysis, digitizing, filtering, compressing, encoding | Implanted RF-controlled PG, multichannel epidural array, dorsal roots, dorsal columns |
| Intraspinal microstimulation (limb movement)§ | External goniometers | Limb position | Digitizing, filtering, threshold detection | Implanted penetrating semimicroelectrode arrays |
| Intraspinal microstimulation (bladder control)§ | Bladder and sphincter pressures | Urethral pressure sensor | Parameter adjustment to effect | Implanted penetrating semimicroelectrode arrays |
| Intraspinal microstimulation (respiration)§ | EMG | Genioglossus EMG | Parameter adjustment to effect | Implanted penetrating semimicroelectrode arrays |
PG, pulse generator; N/A, not applicable.
Commercially available,
discontinued commercially,
experimental.
Electrodes in implanted NSs generally consist of insulated leads with conductive terminals implanted on or adjacent to neural tissues. The terminals are made of a biologically compatible and corrosion-resistant material such as stainless steel, platinum, or silicon. They may be individually inserted, they may be attached to a shaft to form a linear array [e.g., deep brain stimulation (DBS) leads], or they may be mounted on a substrate to form a matrix (e.g., spinal epidural leads, ECoGs). In the case of penetrating microelectrode arrays, the impedance of the conductive tips of the electrodes is reduced with a variety of conductive and dielectric coatings (Campbell et al. 1991; Maynard et al. 1997).
From a theoretical point of view, the excitation of whole nerves or individual nerve axons and cell bodies with electrical stimulation is well understood (Stein and Prochazka 2009). In practice, the main problem is to activate just those whole nerves or groups of axons within nerves that elicit the desired action and not others. For example, when muscle nerves are stimulated via surface electrodes, afferents in cutaneous nerves are nearly always activated too, and this can cause discomfort. If the targeted motor nerves are deep lying, other motor nerves may be activated, producing unwanted components of movement. Implanted electrodes such as epimysial, intramuscular, and nerve cuff electrodes with terminals adjacent to, or attached to, the targeted nerves are much more selective, but they are subject to mechanical failure in the long term. When targeted and untargeted nerve terminals are in close proximity, as in the cochlea, selectivity is a major problem. This is currently the subject of intensive research and development (Roche and Hansen 2015).
Selectivity is also a crucial issue in implants targeting structures within the CNS, where ensembles of neurons with opposite functions may be located less than a millimeter apart. A good example is provided by attempts to develop intraspinal microstimulation systems to restore limb movements after spinal cord injury. Although it has occasionally been possible to activate single limb muscles or groups of synergists by stimulating through individual electrodes implanted in or near spinal motoneuron pools (Mushahwar et al. 2000, 2002), the more common result, particularly in the weeks and months after implantation, is a coactivation of multiple muscles (Moritz et al. 2007; Tai et al. 2003). As a result, intraspinal microstimulation may elicit whole limb synergies or stiffen the limb rather than moving it in a controlled manner (personal observations).
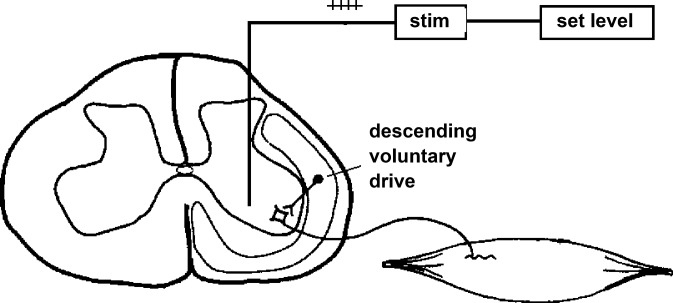
Voluntary control of paretic muscles can be boosted with nonspecific epidural or transcutaneous stimulation of the spinal cord (Angeli et al. 2014; Carhart et al. 2004; Dimitrijevic et al. 1998; Gerasimenko et al. 2002, 2015; Harkema et al. 2011; Herman et al. 2002; Lu et al. 2016; Sayenko et al. 2015), presumably by activating sensory axons in dorsal roots and the dorsal columns (Gaunt et al. 2006; Musienko et al. 2012; Rattay et al. 2000, 2003). Voluntary contractions of different muscles may also be boosted by intraspinal microstimulation at levels insufficient to activate any of the muscles in the absence of volitional drive (Prochazka et al. 2002b) (see Fig. 4). Given that intraspinal microstimulation recruits branches of sensory afferents before alpha motoneurons (Gaunt et al. 2006), the mechanism of boosting in this case may be the same as that for transcutaneous stimulation of the spinal cord.
Fig. 4.
Schematic showing how steady intraspinal microstimulation at a level close to threshold for activating motoneurons that elicit muscle contractions could boost descending commands to those motoneurons and muscles in a person with spinal cord injury (reproduced from Prochazka et al. 2002b with permission).
Another example of the importance of selective activation of neuronal populations is provided by systems designed to treat bladder-sphincter dyssynergia resulting from spinal cord injury. Here the descending drive from the pontine micturition center is absent, and a form of spasticity develops in which instead of relaxing when the bladder contracts to expel urine the external urethral sphincter (EUS) also contracts, thus preventing voiding. Experiments in animals with spinal cord transections showed that sacral ventral root stimulation could elicit voiding in such cases provided that reflex contractions of the EUS were abolished by transecting the dorsal roots (Brindley 1977; Heine et al. 1977). The problem of selectivity in this case is that ventral root stimulation activates not only the parasympathetic preganglionic axons that elicit bladder contractions but also the larger axons that innervate the EUS. Fortunately, upon cessation of stimulation, the EUS relaxes more quickly than the bladder, allowing a few seconds of poststimulus voiding. In clinical practice, brief trains of stimuli are repeated until sufficient voiding has occurred (Brindley et al. 1982; Tanagho et al. 1989). This is clearly not the normal physiological mechanism of voiding (McGee et al. 2015), yet the Finetech-Brindley System (Finetech Medical, Welwyn Garden City, UK) has been implanted in >2,500 people (Rijkhoff 2004), the majority of whom were able to void using their stimulator with residual volumes <30 ml and consequently were freed from catheter usage, which reduced the incidence of urinary tract infections (Martens et al. 2011; Van Kerrebroeck et al. 1993). Some recipients reported stimulator-driven erections and defecation (Egon et al. 1998).
The problem of activating neuronal populations additional to those intended was also encountered in numerous studies evaluating intraspinal microstimulation to treat bladder-sphincter dyssynergia (Gaunt and Prochazka 2006; Grill et al. 1999; Jonas et al. 1975a, 1976; Jonas and Tanagho 1975; Nashold et al. 1971a; Tai et al. 2004; Tanagho 1988). In the initial studies in lightly anesthetized dogs and cats, partial voiding could be elicited by stimulation within the sacral spinal cord, but bladder contractions were usually accompanied by EUS contractions (Friedman et al. 1972; Jonas et al. 1975a, 1975b). It was recognized that intraspinal microstimulation at separate sites would be needed to activate the bladder and at the same time inhibit the EUS. Poststimulus voiding (see above) was proposed as an intermediate solution (Jonas and Tanagho 1975). Between 1970 and 1980, 27 people with spinal cord injury were implanted with pairs of sacral intraspinal electrodes (Nashold et al. 1982). They remain the only recipients of intraspinal electrodes in the world. In most cases, stimulation produced some voiding, but the bladder-sphincter coactivation seen in the animal experiments remained a problem. Partial sphincterotomies improved voiding in some of the implanted people. The detailed outcomes of these human trials have been reviewed elsewhere (Gaunt and Prochazka 2006; Nashold et al. 1981).
Studies of intraspinal microstimulation for bladder control resumed in a number of centers in the late 1990s. In lightly anesthetized cats, interleaved pulse trains were delivered via implanted microelectrodes to the sacral parasympathetic nucleus to produce bladder contractions (de Groat 2006; Grill et al. 1999) and to the dorsal gray commissure to relax the EUS (Blok and Holstege 1998; Grill et al. 1999). The present author and his colleagues were involved in a related study in awake animals (Gaunt and Prochazka 2008). Although the combined, interleaved stimulation of the above sites sometimes elicited the desired combination of increased bladder pressure and decreased EUS pressure, it rarely resulted in complete voiding. More commonly, coactivation of the bladder and EUS occurred, and so the method was deemed insufficiently reliable by the present author and colleagues to justify clinical trials (Gaunt and Prochazka 2008). In retrospect, it was known from neurophysiological experiments that some interneurons in the dorsal gray commissure increase their firing during voiding and inhibit EUS motoneurons, while others decrease their firing during voiding and excite EUS motoneurons (Buss and Shefchyk 2003). Presumably, when intraspinal microstimulation in the dorsal gray commissure activated more of the latter than the former interneurons the EUS contracted, exacerbating bladder-sphincter dyssynergia rather than reducing it.
In a separate study, coordinated bladder contraction and EUS relaxation was elicited in cats lightly anesthetized with propofol by intraspinal microstimulation in, or just dorsal or lateral to, the dorsal gray commissure (Pikov et al. 2007). Voiding was elicited in two-thirds of the implanted animals, though in nearly all cases the voiding was incomplete. Stimulation at closely adjacent sites could elicit EUS contraction rather than relaxation, as found in the previous studies. The effects on the bladder and the EUS could reverse when the bladder filled. Three of the animals were tested after a low-thoracic spinal cord transection. The voiding responses to intraspinal microstimulation were maintained. Unlike humans, however, spinalized cats do not develop sustained bladder-sphincter dyssynergia, and therefore it is unclear whether intraspinal microstimulation would have the same effects in humans with spinal cord injury. Setting aside the difficulties and uncertainties, it may be possible in the future to use arrays of multicontact microelectrodes to select a small number of specific stimulation sites that produce reliable voiding in spinal cord-injured people.
Other, less invasive approaches to this problem that target the pudendal nerve have been explored. The pudendal nerve contains motor axons that innervate the EUS and axons that transmit sensory input from the pelvic floor, urethra, and external genitalia to the sacral spinal cord. The sensory input can either inhibit or facilitate bladder contractions, depending on its source (McGee et al. 2015). Selective stimulation of the sensory and motor branches of the pudendal nerve with specific amplitudes, rates, and patterns has been used either to inhibit the EUS and facilitate bladder contraction for voiding or to inhibit the bladder and activate the EUS to maintain continence (Boggs et al. 2006a, 2006b; Lee and Creasey 2002; McGee et al. 2015; Yoo et al. 2007; Yoo and Grill 2007). High-frequency blockade of the pudendal nerve to relax the EUS is another promising line of investigation (Bhadra et al. 2006; Boger et al. 2008; Kilgore and Bhadra 2004; Tai et al. 2007a, 2007b). It was shown recently in chronically spinalized animals that activation and blockade of the pudendal nerve can be achieved with the StimRouter system (Gaunt and Prochazka 2009). This may provide a low-cost type of neuroprosthesis for either maintaining continence or eliciting voiding, as only one or two leads would need to be implanted.
Failures and Successes
Most therapeutic interventions in biology are not always completely successful, and neural engineering interventions are no different. Some of the obstacles have been discussed above. To summarize, the most common problems are 1) to connect the recording or stimulating device to the target neurons and only those neurons; 2) to maintain this connection over long periods of time; 3) to achieve these outcomes without damaging the target or surrounding tissues; 4) to process the recorded signals and the stimulus trains so that they lead to useful outcomes; 5) to overcome or compensate for connective tissue encapsulation; and 6) to avoid infection. Failure reports on commercial NSs are available in the Manufacturer and User Facility Device Experience (MAUDE) database of the US Food and Drug Administration (Food and Drug Administration 2017).
What has been the impact on people’s lives of neural engineering devices? Cardiac pacemakers are not strictly neural prostheses, but they activate highly specialized cardiac muscles that have nervelike properties and many aspects of their design are common to those in neural prostheses. The benefits of cardiac pacemakers are well known: they have extended the lives and quality of life of hundreds of thousands of people.
The cochlear implant has provided functional hearing in over 300,000 deaf people. The history of its development over the last 60 years, its clinical adoption in many countries, and the controversies that have swirled around it are excellently reviewed in a book written by a sociologist faced with deciding whether his two deaf children should receive implants (Blume 2010). In the 1950s and 1960s there were concerns about the safety of implanting electrodes inside the cochlea, in relation to infection and the possible destruction of residual hearing, but as the devices and implant surgery improved the rate of adverse events has declined to below 5% (Farinetti et al. 2014). Nonetheless, lifetime follow-up is needed to detect and treat complications (Terry et al. 2015). In the early 1970s, neurophysiologists, finding that auditory nerve axons responded to sound differently than to electrical stimulation, were skeptical that electrical stimulation through one or more electrodes would ever produce useful hearing, and they recommended against proceeding in the absence of further basic research (Kiang and Moxon 1972). However, the clinicians, engineers, and manufacturers involved pressed ahead vigorously. Various electrode designs, speech encoding algorithms, and stimulation patterns were developed and tested in successive generations of devices (Roche and Hansen 2015). The current state of play, after a half century of technical development and clinical experience, is that a sizable proportion of implant recipients show remarkable hearing performance, including open-set speech recognition, acquisition of language skills, and even music appreciation (Roche and Hansen 2015).
To the surprise of those not directly involved, segments of the deaf community opposed the cochlear implant, particularly in relation to its application in children. Their greatest concern was that the cochlear implant undermined Deaf Culture. To quote Blume: “membership in the Deaf Community is psychologically and socially beneficial in itself. . . . parents of children newly diagnosed as deaf may not take the trouble to learn to sign language. However much or however little the child might ultimately profit from the implant, it would be deprived of access to language for a vital period of its early life.” The contrary point of view is that a deaf child not in receipt of a cochlear implant would be deprived of a level of hearing that in many cases has led to the acquisition of language through auditory means. The normal procedure is to implant a device on one side first, which reduces the risk of abolishing residual hearing. Furthermore, sign language and lip reading may still be taught, as recommended for example by the French National Consultative Ethics Committee on Health and Life Sciences (CCNE).
The lesson here is that it is important for neural engineers to understand not only the neurophysiology but also the relevant societal and psychological factors when developing novel interventions. At one of the first conference presentations on brain-machine interfaces, an audience member with quadriplegia told the present author “I need brain electrodes like I need a hole in the head.” But actually it was neither the trephination nor the implants that he was averse to, it was the large connector attached to the recipient’s head. If the device were fully implanted and invisible, he felt it might be acceptable and useful. Most users of assistive devices do not want to attract attention to their disability. Recognition of this factor has led to a focus on the design of less obtrusive and more cosmetically appealing neural prostheses (Kilgore et al. 2001).
Needless to say, the cost-benefit ratio is crucial and this goes well beyond purely monetary costs. For example, stimulator garments can improve upper limb function to some extent in people living with spinal cord injury, but if they take longer than a minute or two to don, they fall into disuse (personal observations). People with hemiplegia learn to perform many tasks one-handed, so any improvement in function of their affected hand has to be very substantial to result in a favorable cost-benefit ratio (Buick et al. 2016). Questionnaires that measure user satisfaction with assistive devices can provide useful information in this regard (Day et al. 2002; Demers et al. 2002; Jutai and Day 2002).
Neurophysiological Insights Arising from Neural Engineering Research and Development
Given the close relationship between neurophysiology and neural engineering, it was only to be expected that experience gained from the practical use of NSs and BCIs would lead to fundamental neurophysiological insights. Let us consider a few interesting examples.
Information conveyed by the relative timing of sensory inputs.
Interaural time differences of stimulation of ∼100 μs with bilateral cochlear implants improve speech and music perception, showing that the detection and decoding of minute differences in timing, presumably by neurons in the cochlear nuclei, lateral geniculate body, and auditory cortex, is an important mechanism, not only for directional discrimination of sound sources but also in higher-level processing of complex sounds (Laback et al. 2015).
Information content, modulation, and feedback effects of proprioceptive signals.
It is still not entirely clear how proprioceptive input from mammalian muscle spindles is modulated and used by the CNS to control normal voluntary movement. Neurographic recordings in awake humans have resulted in a number of different ideas about the way the CNS uses gamma fusimotor action to modulate spindle afferent stretch sensitivity. There is evidence for alpha-gamma coactivation, which serves to maintain spindle afferent firing during active muscle shortening (Hagbarth and Vallbo 1969; Matthews 1970; Proske and Gandevia 2012). There is also evidence for predictive gamma biasing action to compensate for future muscle length changes (Dimitriou and Edin 2010; Ellaway et al. 2015; Taylor et al. 2006). Neurography needles are easily dislodged, which has limited the freedom of movement in the human data (Prochazka and Hulliger 1998). Recordings from dorsal root afferents during a wide range of rapid, unrestricted movements in animals have tended to support a simpler, kinesthetic role for muscle spindles (Cody et al. 1975; Cody and Taylor 1973; Goodwin and Luschei 1975; Prochazka et al. 1976), whereby in addition to there being an alpha-linked component of gamma activity there is an additional component that is related to the difficulty, novelty, and context of the motor task (“fusimotor set”) (Prochazka 2015b; Prochazka et al. 1985; Prochazka and Ellaway 2012).
The latest recordings from sensory afferents in freely moving animals were obtained in the context of using dorsal root recordings as feedback signals to control motor NSs. It was found that signals from as few as 10 afferents enabled a fairly accurate reconstruction of the kinematics of a whole limb during free locomotion (Rigosa et al. 2011; Weber et al. 2006, 2007). Most of the relevant information was derived from the signals from muscle spindle afferents, which supports the idea that muscle spindles provide kinematic information. In addition, it became apparent from modeling studies that a crucial role for proprioceptive feedback is to switch the locomotor pattern generator from generating stance to generating swing and back at just the right time in the step cycle (Cruse 1990; Cruse and Warnecke 1992; Prochazka 1993; Prochazka and Yakovenko 2007a, 2007b). Brain control of balance and postural reactions has been shown to depend in part on proprioceptive input, and recent experiments in humans subjected to postural threats indicate that under these circumstances fusimotor input sensitizes muscle spindles, which increases the sensitivity of postural reflexes (Davis et al. 2011; Horslen et al. 2013).
Positive force feedback.
Another role for proprioception is to assist in load compensation, for example in supporting the weight of the body during the stance phase of locomotion. There is some debate as to the relative contributions of stretch reflexes and centrally generated drive in this case (Pearson 2004; Prochazka et al. 2002a). The Pearson laboratory has provided evidence that during locomotion reflexes mediated by extensor Golgi tendon organ afferents may generate significant levels of load-bearing extensor force (Donelan and Pearson 2004). This implies positive force feedback control, a mechanism supported by earlier data showing that whereas in static postures extensor motoneurons are inhibited by input from homonymous tendon organ afferents, during locomotion there is a reflex reversal such that tendon organ feedback becomes excitatory (Donelan and Pearson 2004; Pearson 2004). In technology, positive feedback is usually avoided, because it can lead to instability. On the other hand, when muscles shorten they produce progressively less force in response to a given increase in neural drive, and this has the effect of reducing the loop gain of positive force feedback to below the level that results in instability. From a theoretical point of view, positive force feedback to limb extensor muscles is in fact an effective load-compensating mechanism unless muscle length is constrained, as in isometric contractions (Prochazka et al. 1997b, 1997c, 2002a).
Spatiotemporal activation of spinal motoneuron pools during locomotion.
In attempts to elicit locomotion with arrays of intraspinal electrodes (Mushahwar et al. 2000), the need arose for an accurate three-dimensional map of the location of motoneuron pools in the lumbosacral spinal cord. Previously published histological data (Vanderhorst and Holstege 1997) were digitized, and a spatiotemporal animation of the activation of motoneuron pools was developed by combining this model with the known EMG activity of the main hindlimb muscles in normal locomotion (Yakovenko et al. 2002). This approach has since been used to model motoneuronal activation in human and rodent spinal cords (Ivanenko et al. 2006; Wenger et al. 2016). The animation revealed a rostrocaudal oscillation of motoneuronal activity within the spinal cord during the locomotor step cycle. The first impression was of a wave of activity propagating smoothly up and down the spinal cord (Yakovenko et al. 2000). Waves of activity traveling along the neuraxis had previously been suggested as controlling the orderly sequencing of muscles in locomotion in different species, particularly in limbless animals that move by undulating their bodies (Orlovsky et al. 1999). It was further suggested that the neural network producing these traveling waves had been conserved in the evolutionary transition from aquatic to terrestrial locomotion (Bem et al. 2003; Ijspeert et al. 2007). On closer analysis of the spatiotemporal model, it became evident that the rostrocaudal shifts of activity at the transitions between the swing and stance phases of locomotion were quite abrupt (Yakovenko et al. 2002). This suggests that the evolutionary modification of the spinal locomotor network from limbless to limbed animals must have involved a speeding up of the migrations of activity at the swing-stance and stance-swing transitions. A further insight came from neuromechanical modeling, which indicated that the stance phase of the locomotor step cycle lasts longer than the swing phase for biomechanical reasons and that the central pattern generator may be tuned to produce this relationship by virtue of persistent inward currents in timing interneurons (Prochazka et al. 2017; Prochazka and Yakovenko 2007a, 2007b; Spardy et al. 2011a, 2011b).
Hidden layer neurons and causality in corticospinal signals.
Regarding the cortical control of movement, in BCI research the firing of ensembles of motor cortical cells has been used to decode a variety of behavioral states: intention to perform a mouse click, attention, change in target direction, forward/backward walking direction, hold/release periods, grasp type, and movement onset (Velliste et al. 2014). When rest and active hold states in monkeys performing center-out arm movement tasks were compared, it was possible to distinguish these states from the firing of ensembles of neurons but not from those of single neurons. Furthermore, when neuronal population vectors were used to move displayed cursors to targets, on occasion the monkey rested its arm while still controlling cursor movement (Schwartz AB, personal communication). This is in line with a previous study that showed that the firing of neurons in motor areas of the cerebrum could be dissociated from actual movement performance, indicating that some, perhaps most, neurons in the cortex do not directly control spinal motoneurons (Alexander and Crutcher 1990). Rather, they might be the equivalent of “hidden layer” neurons in artificial neural nets, which are indirectly involved in controlling input-output behavior but whose activity is not obviously related to either inputs or outputs.
What is activated by electrical stimulation of CNS structures?
For many years it was assumed that epidural spinal cord stimulators activated axons in the dorsal columns and interneurons within the gray matter of the spinal cord. In fact, these devices were originally called dorsal column stimulators. It was then shown that epidural spinal stimulation activates axons in dorsal root filaments at lower amplitudes than axons in the dorsal columns (Ladenbauer et al. 2010; Rattay et al. 2000, 2003). Similarly, the identity of the neurons stimulated by DBS electrodes is unclear, which has impeded progress in understanding the mechanism of the therapeutic effects of DBS (McIntyre et al. 2004; Murrow 2014; Zhang and Grill 2010). Intraspinal microstimulation, originally assumed to activate alpha motoneurons and interneurons within a short distance of the electrode tips, was found to antidromically activate nearby branches of sensory axons at lower stimulus intensities and thereby to synaptically excite motoneurons in motoneuron pools spanning several spinal segments (Gaunt et al. 2006). This may explain how focal intraspinal stimulation can spread to activate many of the motoneurons of a synergistic muscle group.
What variable(s) are feedback controlled during movement?
BCI recordings in human tetraplegic participants have provided insight into cortical feedback control (Willett et al. 2017). In center-out cursor-control tasks, neural population activity gradually declined as the cursor approached the target from afar, then decreased more sharply as the cursor came into contact with the target. Predictive corrections to the cursor’s velocity were made during movement, and feedback corrections continued after the cursor reached the target. These observations are relevant to the basic question “what variable(s) does the nervous system control in limb movements?” (Stein 1982). Increasing the amplitude of electrical stimulation in the midbrain of the decerebrate cat increases the velocity of locomotion (Shik et al. 1966). Computer simulations have supported the idea that locomotor velocity is the basic command sent from supraspinal areas to the spinal locomotor pattern generator (Prochazka and Ellaway 2012). The BCI results suggest that velocity is also the controlled variable during arm movements, and that there may be a switch to positional or even force control once a target is reached.
Intermingling of neurons with opposite functions in the CNS.
The conclusion from the experiments involving intraspinal microstimulation to elicit bladder voiding, namely that neurons within the same small region of the spinal cord can have very different and even opposite functions (Gaunt and Prochazka 2006), has a broader implication. Most imaging methods that show the “lighting up” of regions of the CNS do not have the resolution to differentiate between functionally different subpopulations, and therefore should be interpreted with caution.
Therapeutic carryover effects.
Therapeutic electrical stimulation and functional electrical stimulation in people living with stroke and spinal cord injury have been shown to have carryover therapeutic effects (Andrews and Wheeler 1995; Shealy 1975; Vodovnik 1981) especially when performed in association with voluntary exercise training (Cauraugh et al. 2005; Cauraugh and Kim 2002, 2003; de Kroon et al. 2002; Kowalczewski et al. 2011; Musienko et al. 2012; Page et al. 2012; Popovic et al. 2006). Carryover effects lasting a few hours may result from short-term changes in the energetics of neuromuscular activation, whereas carryover effects lasting weeks or months have been attributed to muscle strengthening, neural plasticity, or both (Field-Fote 2004; Stein et al. 1992; Thomas and Gorassini 2005). Long-lasting carryover effects are also seen after TENS and sacral nerve stimulation for pain control, posterior tibial nerve stimulation to treat overactive bladder (Peters et al. 2010), and vagus nerve stimulation for epilepsy and depression (Yuan and Silberstein 2016a, 2016b, 2016c). The neurophysiological mechanisms involved are unclear and not necessarily the same in each case. In recent years they have been equated with neuromodulation, a process in which the activation of intracellular second messengers changes the responses of populations of neurons to subsequent inputs. Neuromodulation has a slower onset than direct ionotropic synaptic activation of neurons and is therefore associated with metabotropic synaptic transmission (Follesa et al. 2007; Yuan et al. 2016). It is interesting that despite TENS having become a standard therapeutic modality worldwide, the evidence for its efficacy in randomized controlled trials is still deemed inadequate and its presumed neurophysiological mechanisms of action remain uncertain (Catley et al. 2015; Johnson et al. 2015).
Future
In recent years several ambitious, multicenter neural engineering projects have been launched, with much publicity and large amounts of funding, These include the multiagency BRAIN Initiative (https://www.braininitiative.nih.gov), the Targeted Neuroplasticity Training Program (http://www.darpa.mil/program/targeted-neuroplasticity-training), and the European Human Brain Project (https://www.humanbrainproject.eu/en). These initiatives have been criticized by some as having unrealistic goals, ignoring basic neurophysiology, and depriving mainstream neuroscience researchers of funding. On the other hand, the goals are inspirational and will probably attract many young, innovative researchers to get involved in projects, some of which will fail and others will succeed, not always in the manner anticipated. Time will tell whether this shift in the organization and funding of neural engineering and neuroscience, which is taking place worldwide, will have been beneficial or not.
This also raises the interesting question, At what point of neurophysiological knowledge (or ignorance) is human implantation of devices justifiable? Recall that the cochlear stimulator was being implanted in deaf people in the early 1970s, when some expert neurophysiologists had concluded that “the present state of basic knowledge and technical competence argues strongly for additional preliminary work on animals” (Kiang and Moxon 1972). And yet within a few years, recipients were able to comprehend speech without visual input (Scott 2006). Recall too that deep brain stimulators have been implanted in tens of thousands of people without a clear understanding of the mechanism of action, yet they are often highly effective in suppressing tremor and unlocking rigidity (Birdno et al. 2014; Deeb et al. 2016). Perhaps the answer is that when the need is sufficiently pressing, when there is a reasonable chance of success based on the existing knowledge, and when the risks are judged to be acceptable, human trials are justified.
Conclusions
Neural engineering is a fast-growing, multidisciplinary field that has its foundations in neurophysiology and electrical engineering. The dawn of the transistor age in the late 1950s triggered an explosion of innovation, particularly with respect to implanted devices. In the intervening half century, there has been much progress in the development of materials, miniaturization, computerization, wireless communication, surgery, and, last but not least, the understanding of the underlying neurophysiological mechanisms. NSs are used clinically in their hundreds of thousands and in some cases have become the standard of care for specific neural disorders. None of this would have been possible without a detailed knowledge of basic neurophysiology. Neural engineering is repaying the debt with new basic knowledge and insight. Regarding the future, some developments are predictable. For example, there will no doubt be further miniaturization of electronics and improvements in the biocompatibility of implanted materials, in signal acquisition and processing, in surgical implantation, in selectivity of stimulation, and in the reliability and longevity of devices. Many problems await solutions. Past history indicates that some of these solutions will come from unexpected directions. The growth predictions for NSs indicate a bright and fascinating future for this domain of applied neurophysiology.
GRANTS
This work was supported by a grant from the Canadian Institutes of Health Research.
DISCLOSURES
I have a financial interest in Rehabtronics Inc., which markets two of the devices mentioned in this article (StimRouter and Regrasp).
AUTHOR CONTRIBUTIONS
A.P. drafted manuscript; A.P. edited and revised manuscript; A.P. approved final version of manuscript.
ACKNOWLEDGMENTS
I thank Dr. Stephen Scott for his insights regarding cochlear implants.
REFERENCES
- Al Asari S, Meurette G, Mantoo S, Kubis C, Wyart V, Lehur PA. Percutaneous tibial nerve stimulation vs. sacral nerve stimulation for faecal incontinence: a comparative case-matched study. Colorectal Dis 16: O393–O399, 2014. doi: 10.1111/codi.12680. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Neural representations of the target (goal) of visually guided arm movements in three motor areas of the monkey. J Neurophysiol 64: 164–178, 1990. [DOI] [PubMed] [Google Scholar]
- Alon G, Levitt AF, McCarthy PA. Functional electrical stimulation enhancement of upper extremity functional recovery during stroke rehabilitation: a pilot study. Neurorehabil Neural Repair 21: 207–215, 2007. doi: 10.1177/1545968306297871. [DOI] [PubMed] [Google Scholar]
- Anagnostopoulos CE, Glenn WW. Electronic pacemakers of the heart, gastrointestinal tract, phrenic nerve, bladder, and carotid sinus: current status. Surgery 60: 480–494, 1966. [PubMed] [Google Scholar]
- Andrews BJ, Wheeler GD. Functional and therapeutic benefits of electrical stimulation after spinal injury. Curr Opin Neurol 8: 461–466, 1995. doi: 10.1097/00019052-199512000-00012. [DOI] [PubMed] [Google Scholar]
- Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 137: 1394–1409, 2014. doi: 10.1093/brain/awu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbara M, Biagini M, Monini S. The totally implantable middle ear device “Esteem” for rehabilitation of severe sensorineural hearing loss. Acta Otolaryngol 131: 399–404, 2011. doi: 10.3109/00016489.2010.536994. [DOI] [PubMed] [Google Scholar]
- Barrese JC, Rao N, Paroo K, Triebwasser C, Vargas-Irwin C, Franquemont L, Donoghue JP. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J Neural Eng 10: 066014, 2013. doi: 10.1088/1741-2560/10/6/066014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry MP, Dagnelie G; Argus II Study Group . Use of the Argus II retinal prosthesis to improve visual guidance of fine hand movements. Invest Ophthalmol Vis Sci 53: 5095–5101, 2012. doi: 10.1167/iovs.12-9536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bem T, Cabelguen JM, Ekeberg O, Grillner S. From swimming to walking: a single basic network for two different behaviors. Biol Cybern 88: 79–90, 2003. doi: 10.1007/s00422-002-0340-3. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Deuschl G, Lang AE, Lyons KE, Rezai AR. Deep brain stimulation for Parkinson’s disease. Mov Disord 21, Suppl 14: S168–S170, 2006. doi: 10.1002/mds.20954. [DOI] [PubMed] [Google Scholar]
- Bethoux F, Rogers HL, Nolan KJ, Abrams GM, Annaswamy TM, Brandstater M, Browne B, Burnfield JM, Feng W, Freed MJ, Geis C, Greenberg J, Gudesblatt M, Ikramuddin F, Jayaraman A, Kautz SA, Lutsep HL, Madhavan S, Meilahn J, Pease WS, Rao N, Seetharama S, Sethi P, Turk MA, Wallis RA, Kufta C. The effects of peroneal nerve functional electrical stimulation versus ankle-foot orthosis in patients with chronic stroke: a randomized controlled trial. Neurorehabil Neural Repair 28: 688–697, 2014. doi: 10.1177/1545968314521007. [DOI] [PubMed] [Google Scholar]
- Bhadra N, Bhadra N, Kilgore K, Gustafson KJ. High frequency electrical conduction block of the pudendal nerve. J Neural Eng 3: 180–187, 2006. doi: 10.1088/1741-2560/3/2/012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadra N, Peckham PH, Keith MW, Kilgore KL, Montague F, Gazdik M, Stage T. Implementation of an implantable joint-angle transducer. J Rehabil Res Dev 39: 411–422, 2002. [PubMed] [Google Scholar]
- Bird G. Remarks on the hydro-electric chain of Dr. Pulvermacher. Lancet 58: 388–389, 1851. doi: 10.1016/S0140-6736(02)79703-1. [DOI] [Google Scholar]
- Birdno MJ, Tang W, Dostrovsky JO, Hutchison WD, Grill WM. Response of human thalamic neurons to high-frequency stimulation. PLoS One 9: e96026, 2014. doi: 10.1371/journal.pone.0096026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blok BF, Holstege G. The central nervous system control of micturition in cats and humans. Behav Brain Res 92: 119–125, 1998. doi: 10.1016/S0166-4328(97)00184-8. [DOI] [PubMed] [Google Scholar]
- Blume S. The Artificial Ear: Cochlear Implants and the Culture of Deafness. Piscataway, NJ: Rutgers Univ. Press, 2010, p. 226. [Google Scholar]
- Boger A, Bhadra N, Gustafson KJ. Bladder voiding by combined high frequency electrical pudendal nerve block and sacral root stimulation. Neurourol Urodyn 27: 435–439, 2008 10.1002/nau.20538. [DOI] [PubMed] [Google Scholar]
- Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Bladder emptying by intermittent electrical stimulation of the pudendal nerve. J Neural Eng 3: 43–51, 2006a. doi: 10.1088/1741-2560/3/1/005. [DOI] [PubMed] [Google Scholar]
- Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Frequency-dependent selection of reflexes by pudendal afferents in the cat. J Physiol 577: 115–126, 2006b. doi: 10.1113/jphysiol.2006.111815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton CE, Shaikhouni A, Annetta NV, Bockbrader MA, Friedenberg DA, Nielson DM, Sharma G, Sederberg PB, Glenn BC, Mysiw WJ, Morgan AG, Deogaonkar M, Rezai AR. Restoring cortical control of functional movement in a human with quadriplegia. Nature 533: 247–250, 2016. doi: 10.1038/nature17435. [DOI] [PubMed] [Google Scholar]
- Branco MP, Freudenburg ZV, Aarnoutse EJ, Bleichner MG, Vansteensel MJ, Ramsey NF. Decoding hand gestures from primary somatosensory cortex using high-density ECoG. Neuroimage 147: 130–142, 2017. doi: 10.1016/j.neuroimage.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazier MA. A History of Neurophysiology in the 17th and 18th Centuries. New York: Raven, 1984, p. 230. [Google Scholar]
- Brazier MA. A History of Neurophysiology in the 19th Century. New York: Raven, 1988, p. 265. [Google Scholar]
- Briggs RJ, Eder HC, Seligman PM, Cowan RS, Plant KL, Dalton J, Money DK, Patrick JF. Initial clinical experience with a totally implantable cochlear implant research device. Otol Neurotol 29: 114–119, 2008. doi: 10.1097/MAO.0b013e31814b242f. [DOI] [PubMed] [Google Scholar]
- Brindley GS. Emptying the bladder by stimulating sacral ventral roots. J Physiol 237: 15P–16P, 1974. [PubMed] [Google Scholar]
- Brindley GS. An implant to empty the bladder or close the urethra. J Neurol Neurosurg Psychiatry 40: 358–369, 1977. doi: 10.1136/jnnp.40.4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley GS, Lewin WS. The sensations produced by electrical stimulation of the visual cortex. J Physiol 196: 479–493, 1968. doi: 10.1113/jphysiol.1968.sp008519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley GS, Polkey CE, Rushton DN. Sacral anterior root stimulators for bladder control in paraplegia. Paraplegia 20: 365–381, 1982. doi: 10.1038/sc.1982.65. [DOI] [PubMed] [Google Scholar]
- British Medical Journal The Medical Battery Company, Limited, and the British Medical Association. Br Med J 1: 1193, 1893. [PMC free article] [PubMed] [Google Scholar]
- Bruschini L, Berrettini S, Forli F, Murri A, Cuda D. The Carina middle ear implant: surgical and functional outcomes. Eur Arch Otorhinolaryngol 273: 3631–3640, 2016. doi: 10.1007/s00405-016-3998-1. [DOI] [PubMed] [Google Scholar]
- Buick AR, Kowalczewski J, Carson RG, Prochazka A. Tele-supervised FES-assisted exercise for hemiplegic upper limb. IEEE Trans Neural Syst Rehabil Eng 24: 79–87, 2016. doi: 10.1109/TNSRE.2015.2408453. [DOI] [PubMed] [Google Scholar]
- Burridge J, Etherington R. A preliminary clinical study using RF BION microstimulators to facilitate upper limb function in hemiplegia. Adv Clin Neurosci Rehabil 4: 26–27, 2004. [Google Scholar]
- Buss RR, Shefchyk SJ. Sacral dorsal horn neurone activity during micturition in the cat. J Physiol 551: 387–396, 2003. doi: 10.1113/jphysiol.2003.041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell PK, Jones KE, Huber RJ, Horch KW, Normann RA. A silicon-based, three-dimensional neural interface: manufacturing processes for an intracortical electrode array. IEEE Trans Biomed Eng 38: 758–768, 1991. [DOI] [PubMed] [Google Scholar]
- Capogrosso M, Milekovic T, Borton D, Wagner F, Moraud EM, Mignardot JB, Buse N, Gandar J, Barraud Q, Xing D, Rey E, Duis S, Jianzhong Y, Ko WK, Li Q, Detemple P, Denison T, Micera S, Bezard E, Bloch J, Courtine G. A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature 539: 284–288, 2016. doi: 10.1038/nature20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart MR, He J, Herman R, D’Luzansky S, Willis WT. Epidural spinal-cord stimulation facilitates recovery of functional walking following incomplete spinal-cord injury. IEEE Trans Neural Syst Rehabil Eng 12: 32–42, 2004. doi: 10.1109/TNSRE.2003.822763. [DOI] [PubMed] [Google Scholar]
- Catley MJ, Gibson W, Wand BM, Meads C, O’Connell NE. Transcutaneous electrical nerve stimulation (TENS) for chronic pain—an overview of Cochrane reviews. Cochrane Database Syst Rev 2015: CD011890, 2015. doi: 10.1002/14651858.CD011890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauraugh JH, Kim S. Two coupled motor recovery protocols are better than one: electromyogram-triggered neuromuscular stimulation and bilateral movements. Stroke 33: 1589–1594, 2002. doi: 10.1161/01.STR.0000016926.77114.A6. [DOI] [PubMed] [Google Scholar]
- Cauraugh JH, Kim SB. Stroke motor recovery: active neuromuscular stimulation and repetitive practice schedules. J Neurol Neurosurg Psychiatry 74: 1562–1566, 2003. doi: 10.1136/jnnp.74.11.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauraugh JH, Kim SB, Duley A. Coupled bilateral movements and active neuromuscular stimulation: intralimb transfer evidence during bimanual aiming. Neurosci Lett 382: 39–44, 2005. doi: 10.1016/j.neulet.2005.02.060. [DOI] [PubMed] [Google Scholar]
- Chouard CH. Technical survey of the French role in multichannel cochlear implant development. Acta Otolaryngol 135: 523–531, 2015. doi: 10.3109/00016489.2014.968804. [DOI] [PubMed] [Google Scholar]
- Clark GM. Cochlear implant surgery for profound or total hearing loss. Med J Aust 2: 587–588, 1978. [DOI] [PubMed] [Google Scholar]
- Clark GM. Cochlear implants in the Third Millennium. Am J Otol 20: 4–8, 1999. [PubMed] [Google Scholar]
- Cody FW, Harrison LM, Taylor A. Analysis of activity of muscle spindles of the jaw-closing muscles during normal movements in the cat. J Physiol 253: 565–582, 1975. doi: 10.1113/jphysiol.1975.sp011207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody FW, Taylor A. The behaviour of spindles in the jaw-closing muscles during eating and drinking in the cat. J Physiol 231: 49P–50P, 1973. [PubMed] [Google Scholar]
- Cruccu G, Garcia-Larrea L, Hansson P, Keindl M, Lefaucheur JP, Paulus W, Taylor R, Tronnier V, Truini A, Attal N. EAN guidelines on central neurostimulation therapy in chronic pain conditions. Eur J Neurol 23: 1489–1499, 2016. doi: 10.1111/ene.13103. [DOI] [PubMed] [Google Scholar]
- Cruse H. What mechanisms coordinate leg movement in walking arthropods? Trends Neurosci 13: 15–21, 1990. doi: 10.1016/0166-2236(90)90057-H. [DOI] [PubMed] [Google Scholar]
- Cruse H, Warnecke H. Coordination of the legs of a slow-walking cat. Exp Brain Res 89: 147–156, 1992. doi: 10.1007/BF00229012. [DOI] [PubMed] [Google Scholar]
- da Cruz L, Dorn JD, Humayun MS, Dagnelie G, Handa J, Barale PO, Sahel JA, Stanga PE, Hafezi F, Safran AB, Salzmann J, Santos A, Birch D, Spencer R, Cideciyan AV, de Juan E, Duncan JL, Eliott D, Fawzi A, Olmos de Koo LC, Ho AC, Brown G, Haller J, Regillo C, Del Priore LV, Arditi A, Greenberg RJ; Argus II Study Group . Five-year safety and performance results from the Argus II Retinal Prosthesis System clinical trial. Ophthalmology 123: 2248–2254, 2016. doi: 10.1016/j.ophtha.2016.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. The Expression of the Emotions in Man and Animals. London: John Murray, 1872. [Chicago, IL: Univ. of Chicago Press, 1965, p. 368]. [Google Scholar]
- Davis JR, Horslen BC, Nishikawa K, Fukushima K, Chua R, Inglis JT, Carpenter MG. Human proprioceptive adaptations during states of height-induced fear and anxiety. J Neurophysiol 106: 3082–3090, 2011. doi: 10.1152/jn.01030.2010. [DOI] [PubMed] [Google Scholar]
- Day H, Jutai J, Campbell KA. Development of a scale to measure the psychosocial impact of assistive devices: lessons learned and the road ahead. Disabil Rehabil 24: 31–37, 2002. doi: 10.1080/09638280110066343. [DOI] [PubMed] [Google Scholar]
- de Groat WC. Integrative control of the lower urinary tract: preclinical perspective. Br J Pharmacol 147, Suppl 2: S25–S40, 2006. doi: 10.1038/sj.bjp.0706604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kroon JR, van der Lee JH, IJzerman MJ, Lankhorst GJ. Therapeutic electrical stimulation to improve motor control and functional abilities of the upper extremity after stroke: a systematic review. Clin Rehabil 16: 350–360, 2002. doi: 10.1191/0269215502cr504oa. [DOI] [PubMed] [Google Scholar]
- Deeb W, Giordano JJ, Rossi PJ, Mogilner AY, Gunduz A, Judy JW, Klassen BT, Butson CR, Van Horne C, Deny D, Dougherty DD, Rowell D, Gerhardt GA, Smith GS, Ponce FA, Walker HC, Bronte-Stewart HM, Mayberg HS, Chizeck HJ, Langevin JP, Volkmann J, Ostrem JL, Shute JB, Jimenez-Shahed J, Foote KD, Wagle Shukla A, Rossi MA, Oh M, Pourfar M, Rosenberg PB, Silburn PA, de Hemptine C, Starr PA, Denison T, Akbar U, Grill WM, Okun MS. Proceedings of the Fourth Annual Deep Brain Stimulation Think Tank: a review of emerging issues and technologies. Front Integr Neurosci 10: 38, 2016. doi: 10.3389/fnint.2016.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deer T, Pope J, Benyamin R, Vallejo R, Friedman A, Caraway D, Staats P, Grigsby E, Porter McRoberts W, McJunkin T, Shubin R, Vahedifar P, Tavanaiepour D, Levy R, Kapural L, Mekhail N. Prospective, multicenter, randomized, double-blinded, partial crossover study to assess the safety and efficacy of the novel neuromodulation system in the treatment of patients with chronic pain of peripheral nerve origin. Neuromodulation 19: 91–100, 2016. doi: 10.1111/ner.12381. [DOI] [PubMed] [Google Scholar]
- Deer TR, Levy RM, Rosenfeld EL. Prospective clinical study of a new implantable peripheral nerve stimulation device to treat chronic pain. Clin J Pain 26: 359–372, 2010. doi: 10.1097/AJP.0b013e3181d4d646. [DOI] [PubMed] [Google Scholar]
- Demers L, Weiss-Lambrou R, Ska B. The Quebec User Evaluation of Satisfaction with Assistive Technology (QUEST 2.0): an overview and recent progress. Technol Disabil 14: 101–105, 2002. [Google Scholar]
- Dimitrijevic MR, Gerasimenko Y, Pinter MM. Evidence for a spinal central pattern generator in humans. Ann NY Acad Sci 860: 360–376, 1998. doi: 10.1111/j.1749-6632.1998.tb09062.x. [DOI] [PubMed] [Google Scholar]
- Dimitriou M, Edin BB. Human muscle spindles act as forward sensory models. Curr Biol 20: 1763–1767, 2010. doi: 10.1016/j.cub.2010.08.049. [DOI] [PubMed] [Google Scholar]
- Djourno A, Eyries C [Auditory prosthesis by means of a distant electrical stimulation of the sensory nerve with the use of an indwelt coiling]. Presse Med 65: 1417, 1957. [PubMed] [Google Scholar]
- Djourno A, Eyries C, Vallancien P [Preliminary attempts of electrical excitation of the auditory nerve in man, by permanently inserted micro-apparatus]. Bull Acad Natl Med 141: 481–483, 1957. [PubMed] [Google Scholar]
- Dobelle WH, Mladejovsky MG, Evans JR, Roberts TS, Girvin JP. “Braille” reading by a blind volunteer by visual cortex stimulation. Nature 259: 111–112, 1976. doi: 10.1038/259111a0. [DOI] [PubMed] [Google Scholar]
- Donelan JM, Pearson KG. Contribution of force feedback to ankle extensor activity in decerebrate walking cats. J Neurophysiol 92: 2093–2104, 2004. doi: 10.1152/jn.00325.2004. [DOI] [PubMed] [Google Scholar]
- Duchenne de Boulogne GB. Mecanisme de la Physionomie Humaine [The Mechanism of Human Facial Expression]. Paris: Jules Renouard, 1862. [edited and translated by Cuthbertson RA. Cambridge, UK: Cambridge Univ. Press, 1990, p. 308]. doi: 10.1017/CBO9780511752841. [DOI] [Google Scholar]
- Egon G, Barat M, Colombel P, Visentin C, Isambert JL, Guerin J. Implantation of anterior sacral root stimulators combined with posterior sacral rhizotomy in spinal injury patients. World J Urol 16: 342–349, 1998. doi: 10.1007/s003450050078. [DOI] [PubMed] [Google Scholar]
- Ellaway PH, Taylor A, Durbaba R. Muscle spindle and fusimotor activity in locomotion. J Anat 227: 157–166, 2015. doi: 10.1111/joa.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinetti A, Ben Gharbia D, Mancini J, Roman S, Nicollas R, Triglia JM. Cochlear implant complications in 403 patients: comparative study of adults and children and review of the literature. Eur Ann Otorhinolaryngol Head Neck Dis 131: 177–182, 2014. doi: 10.1016/j.anorl.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Fattahi P, Yang G, Kim G, Abidian MR. A review of organic and inorganic biomaterials for neural interfaces. Adv Mater 26: 1846–1885, 2014. doi: 10.1002/adma.201304496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field-Fote EC. Electrical stimulation modifies spinal and cortical neural circuitry. Exerc Sport Sci Rev 32: 155–160, 2004. doi: 10.1097/00003677-200410000-00006. [DOI] [PubMed] [Google Scholar]
- Flesher SN, Collinger JL, Foldes ST, Weiss JM, Downey JE, Tyler-Kabara EC, Bensmaia SJ, Schwartz AB, Boninger ML, Gaunt RA. Intracortical microstimulation of human somatosensory cortex. Sci Transl Med 8: 361ra141, 2016. doi: 10.1126/scitranslmed.aaf8083. [DOI] [PubMed] [Google Scholar]
- Flint RD, Rosenow JM, Tate MC, Slutzky MW. Continuous decoding of human grasp kinematics using epidural and subdural signals. J Neural Eng 14: 016005, 2017. doi: 10.1088/1741-2560/14/1/016005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follesa P, Biggio F, Gorini G, Caria S, Talani G, Dazzi L, Puligheddu M, Marrosu F, Biggio G. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res 1179: 28–34, 2007. doi: 10.1016/j.brainres.2007.08.045. [DOI] [PubMed] [Google Scholar]
- Francisco G, Chae J, Chawla H, Kirshblum S, Zorowitz R, Lewis G, Pang S. Electromyogram-triggered neuromuscular stimulation for improving the arm function of acute stroke survivors: a randomized pilot study. Arch Phys Med Rehabil 79: 570–575, 1998. doi: 10.1016/S0003-9993(98)90074-0. [DOI] [PubMed] [Google Scholar]
- Friedman H, Nashold BS Jr, Senechal P. Spinal cord stimulation and bladder function in normal and paraplegic animals. J Neurosurg 36: 430–437, 1972. doi: 10.3171/jns.1972.36.4.0430. [DOI] [PubMed] [Google Scholar]
- Gan LS, Ravid EN, Kowalczewski J, Gauthier M, Olson J, Morhart M, Prochazka A. First permanent human implant of the Stimulus Router System, a novel neuroprosthesis: preliminary testing of a polarity reversing stimulation technique. Conf Proc IEEE Eng Med Biol Soc 2011: 3051–3054, 2011. [DOI] [PubMed] [Google Scholar]
- Gates GA, Daly K, Dichtel WJ, Dooling RJ, Gulya AJ, Hall JW 3rd, Jerger SW, Jones JE, Mayer MH, Pierschalla M, Ross LF, Schwartz RG, Weinstein BE, Young ED, Abbas PJ, Blarney P, Brackmann DE, Brimacombe JA, Chute PM, Cohen NL, Dorman MF, Eddington DK, Gantz BJ, Heller JW, Ketten DR, Knutson JF, Leake PA, McDermott HJ, Miyamoto RT, Moog JS, Osberger MJ, Shannon RV, Skinner MW, Summerfield Q, Tobey E, Waltzman SB, Wilson BS, Zwolan TA, Donahue AM, Allen MP, Beck LB, Bray EA, Cooper JA, Krawsnegor N, Monjan AA, Naunton RF, Snow JB Jr, Hodes RJ, Alexander DF, Hall ZW. NIH consensus conference. Cochlear implants in adults and children. JAMA 274: 1955–1961, 1995. doi: 10.1001/jama.1995.03530240065043. [DOI] [PubMed] [Google Scholar]
- Gaunt R, Prochazka A.Functional Microstimulation of the Lumbosacral Spinal Cord. Final Report, NIH-NINDS contract 1-NS-2-2342. Bethesda, MD: NIH-NINDS, 2008.
- Gaunt RA, Prochazka A. Control of urinary bladder function with devices: successes and failures. Prog Brain Res 152: 163–194, 2006. doi: 10.1016/S0079-6123(05)52011-9. [DOI] [PubMed] [Google Scholar]
- Gaunt RA, Prochazka A. Transcutaneously coupled, high-frequency electrical stimulation of the pudendal nerve blocks external urethral sphincter contractions. Neurorehabil Neural Repair 23: 615–626, 2009. [DOI] [PubMed] [Google Scholar]
- Gaunt RA, Prochazka A, Mushahwar VK, Guevremont L, Ellaway PH. Intraspinal microstimulation excites multisegmental sensory afferents at lower stimulus levels than local alpha-motoneuron responses. J Neurophysiol 96: 2995–3005, 2006. doi: 10.1152/jn.00061.2006. [DOI] [PubMed] [Google Scholar]
- Gerasimenko YP, Lu DC, Modaber M, Zdunowski S, Gad P, Sayenko DG, Morikawa E, Haakana P, Ferguson AR, Roy RR, Edgerton VR. Noninvasive reactivation of motor descending control after paralysis. J Neurotrauma 32: 1968–1980, 2015. doi: 10.1089/neu.2015.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko YP, Makarovskii AN, Nikitin OA. Control of locomotor activity in humans and animals in the absence of supraspinal influences. Neurosci Behav Physiol 32: 417–423, 2002. doi: 10.1023/A:1015836428932. [DOI] [PubMed] [Google Scholar]
- Gillard DM, Cameron T, Prochazka A, Gauthier MJ. Tremor suppression using functional electrical stimulation: a comparison between digital and analog controllers. IEEE Trans Rehabil Eng 7: 385–388, 1999. doi: 10.1109/86.788474. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, Luschei ES. Discharge of spindle afferents from jaw-closing muscles during chewing in alert monkeys. J Neurophysiol 38: 560–571, 1975. [DOI] [PubMed] [Google Scholar]
- Grand View Research Neurostimulation Devices Market Analysis by Product (Deep Brain Stimulators, Gastric Electric Stimulators, Spinal Cord Stimulators, Sacral Nerve Stimulators, Vagus Nerve Stimulators), by Application (Pain Management, Epilepsy, Essential Tremor, Urinary & Fecal Incontinence, Depression, Dystonia, Gastroparesis, Parkinson’s Disease, Other Diseases), and Segment Forecasts to 2024. San Francisco, CA: Grand View Research, 2016. [Google Scholar]
- Graupe D, Kohn KH, Basseas SP. Control of electrically-stimulated walking of paraplegics via above- and below-lesion EMG signature identification. IEEE Trans Automat Contr 34: 130–138, 1989. doi: 10.1109/9.21084. [DOI] [Google Scholar]
- Graupe D, Kohn KH, Kralj A, Basseas S. Patient controlled electrical stimulation via EMG signature discrimination for providing certain paraplegics with primitive walking functions. J Biomed Eng 5: 220–226, 1983. doi: 10.1016/0141-5425(83)90100-0. [DOI] [PubMed] [Google Scholar]
- Greatbatch W, inventor. Wilson Greatbatch Inc., assignee. Medical cardiac pacemaker. US patent 3,057,356, October 9, 1962.
- Grill WM, Bhadra N, Wang B. Bladder and urethral pressures evoked by microstimulation of the sacral spinal cord in cats. Brain Res 836: 19–30, 1999. doi: 10.1016/S0006-8993(99)01581-4. [DOI] [PubMed] [Google Scholar]
- Gupta P, Ehlert MJ, Sirls LT, Peters KM. Percutaneous tibial nerve stimulation and sacral neuromodulation: an update. Curr Urol Rep 16: 4, 2015. doi: 10.1007/s11934-014-0479-1. [DOI] [PubMed] [Google Scholar]
- Hagbarth KE, Vallbo AO. Activity in human muscle afferents during muscle stretch and contraction. Electroencephalogr Clin Neurophysiol 26: 341, 1969. [PubMed] [Google Scholar]
- Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377: 1938–1947, 2011. doi: 10.1016/S0140-6736(11)60547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugland MK, Hoffer JA, Sinkjaer T. Skin contact force information in sensory nerve signals recorded by implanted cuff electrodes. IEEE Trans Rehabil Eng 2: 18–28, 1994. doi: 10.1109/86.296346. [DOI] [Google Scholar]
- Health Quality Ontario Spinal cord stimulation for neuropathic pain: an evidence-based analysis. Ont Health Technol Assess Ser 5: 1–78, 2005. [PMC free article] [PubMed] [Google Scholar]
- Heine JP, Schmidt RA, Tanagho EA. Intraspinal sacral root stimulation for controlled micturition. Invest Urol 15: 78–82, 1977. [PubMed] [Google Scholar]
- Herman R, He J, D’Luzansky S, Willis W, Dilli S. Spinal cord stimulation facilitates functional walking in a chronic, incomplete spinal cord injured. Spinal Cord 40: 65–68, 2002. doi: 10.1038/sj.sc.3101263. [DOI] [PubMed] [Google Scholar]
- Hincapie JG, Kirsch RF. EMG-based control for a C5/C6 spinal cord injury upper extremity neuroprosthesis. Conf Proc IEEE Eng Med Biol Soc 2007: 2432–2435, 2007. doi: 10.1109/IEMBS.2007.4352819. [DOI] [PubMed] [Google Scholar]
- Hodgson JA, inventor. Electrode array for muscle stimulation and recording. US patent 4,619,266, October 28, 1986.
- Hoffer JA, Baru M, Bedard S, Calderon E, Desmoulin G. Initial results with fully implanted NeurostepTM FES system for foot drop. In: Proceedings of the 10th Annual Conference of the International FES Society, Montreal, Canada, 2005. [Google Scholar]
- Holinski BJ, Everaert DG, Mushahwar VK, Stein RB. Real-time control of walking using recordings from dorsal root ganglia. J Neural Eng 10: 056008, 2013. doi: 10.1088/1741-2560/10/5/056008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horslen BC, Murnaghan CD, Inglis JT, Chua R, Carpenter MG. Effects of postural threat on spinal stretch reflexes: evidence for increased muscle spindle sensitivity? J Neurophysiol 110: 899–906, 2013. doi: 10.1152/jn.00065.2013. [DOI] [PubMed] [Google Scholar]
- Hotson G, McMullen DP, Fifer MS, Johannes MS, Katyal KD, Para MP, Armiger R, Anderson WS, Thakor NV, Wester BA, Crone NE. Individual finger control of a modular prosthetic limb using high-density electrocorticography in a human subject. J Neural Eng 13: 026017, 2016. doi: 10.1088/1741-2560/13/2/026017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House WF. Cochlear implants. Ann Otol Rhinol Laryngol 85, Suppl 27: 1–93, 1976. doi: 10.1177/00034894760850S303. [DOI] [PubMed] [Google Scholar]
- Hulliger M. The mammalian muscle spindle and its central control. Rev Physiol Biochem Pharmacol 101: 1–110, 1984. doi: 10.1007/BFb0027694. [DOI] [PubMed] [Google Scholar]
- Ijspeert AJ, Crespi A, Ryczko D, Cabelguen JM. From swimming to walking with a salamander robot driven by a spinal cord model. Science 315: 1416–1420, 2007. doi: 10.1126/science.1138353. [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Poppele RE, Lacquaniti F. Spinal cord maps of spatiotemporal alpha-motoneuron activation in humans walking at different speeds. J Neurophysiol 95: 602–618, 2006. doi: 10.1152/jn.00767.2005. [DOI] [PubMed] [Google Scholar]
- Iwahara T, Atsuta Y, Garcia-Rill E, Skinner RD. Locomotion induced by spinal cord stimulation in the neonate rat in vitro. Somatosens Mot Res 8: 281–287, 1991. doi: 10.3109/08990229109144751. [DOI] [PubMed] [Google Scholar]
- Iwahara T, Atsuta Y, Garcia-Rill E, Skinner RD. Spinal cord stimulation-induced locomotion in the adult cat. Brain Res Bull 28: 99–105, 1992. doi: 10.1016/0361-9230(92)90235-P. [DOI] [PubMed] [Google Scholar]
- Jeglic A, Vanken E, Benedik M. Implantable muscle/nerve stimulator as part of an electronic brace. In: Proceedings of the 3rd International Symposium on External Control of Human Extremities. Belgrade, Yugoslavia: Nauka: Yugoslav Committee for Electronics and Automation, 1970, p. 593–603. [Google Scholar]
- Johnson MI, Paley CA, Howe TE, Sluka KA. Transcutaneous electrical nerve stimulation for acute pain. Cochrane Database Syst Rev 2015: CD006142, 2015. doi: 10.1002/14651858.CD006142.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson V, Eiseman B. Reinforcement of ventilation with electrophrenic pacing of the paralyzed diaphragm. J Thorac Cardiovasc Surg 62: 651–657, 1971. [PubMed] [Google Scholar]
- Jonas U, Heine JP, Tanagho EA. Studies on the feasibility of urinary bladder evacuation by direct spinal cord stimulation. I. Parameters of most effective stimulation. Invest Urol 13: 142–150, 1975a. [PubMed] [Google Scholar]
- Jonas U, Jones LW, Tanagho EA. Spinal cord stimulation versus detrusor stimulation. A comparative study in six “acute” dogs. Invest Urol 13: 171–174, 1975b. [PubMed] [Google Scholar]
- Jonas U, Jones LW, Tanagho EA. Controlled electrical bladder evacuation via stimulation of the sacral micturition center or direct detrusor stimulation. Urol Int 31: 108–110, 1976. doi: 10.1159/000280039. [DOI] [PubMed] [Google Scholar]
- Jonas U, Tanagho EA. Studies on the feasibility of urinary bladder evacuation by direct spinal cord stimulation. II. Poststimulus voiding: a way to overcome outflow resistance. Invest Urol 13: 151–153, 1975. [PubMed] [Google Scholar]
- Jutai J, Day H. Psychosocial Impact of Assistive Devices Scale (PIADS). Technol Disabil 14: 107–111, 2002. [Google Scholar]
- Kennedy PA, Schallert G. Practical issues and concepts in vagus nerve stimulation: a nursing review. J Neurosci Nurs 33: 105–112, 2001. doi: 10.1097/01376517-200104000-00007. [DOI] [PubMed] [Google Scholar]
- Khobragade N, Graupe D, Tuninetti D. Towards fully automated closed-loop deep brain stimulation in Parkinson’s disease patients: a LAMSTAR-based tremor predictor. Conf Proc IEEE Eng Med Biol Soc 2015: 2616–2619, 2015. doi: 10.1109/EMBC.2015.7318928. [DOI] [PubMed] [Google Scholar]
- Kiang NY, Moxon EC. Physiological considerations in artificial stimulation of the inner ear. Ann Otol Rhinol Laryngol 81: 714–730, 1972. doi: 10.1177/000348947208100513. [DOI] [PubMed] [Google Scholar]
- Kilgore KL, Bhadra N. Nerve conduction block utilising high-frequency alternating current. Med Biol Eng Comput 42: 394–406, 2004. doi: 10.1007/BF02344716. [DOI] [PubMed] [Google Scholar]
- Kilgore KL, Scherer M, Bobblitt R, Dettloff J, Dombrowski DM, Godbold N, Jatich JW, Morris R, Penko JS, Schremp ES, Cash LA. Neuroprosthesis consumers’ forum: consumer priorities for research directions. J Rehabil Res Dev 38: 655–660, 2001. [PubMed] [Google Scholar]
- Kim S, Callier T, Tabot GA, Gaunt RA, Tenore FV, Bensmaia SJ. Behavioral assessment of sensitivity to intracortical microstimulation of primate somatosensory cortex. Proc Natl Acad Sci USA 112: 15202–15207, 2015. doi: 10.1073/pnas.1509265112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson JS, Chae J, Hart RL, Keith MW, Hoyen HA, Harley MY, Hisel TZ, Bryden AM, Kilgore KL, Peckham H. Implanted neuroprosthesis for assisting arm and hand function after stroke: a case study. J Rehabil Res Dev 49: 1505–1516, 2012. doi: 10.1682/JRRD.2011.09.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson JS, Fu MJ, Sheffler LR, Chae J. Neuromuscular electrical stimulation for motor restoration in hemiplegia. Phys Med Rehabil Clin N Am 26: 729–745, 2015. doi: 10.1016/j.pmr.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczewski J, Chong SL, Galea M, Prochazka A. In-home tele-rehabilitation improves tetraplegic hand function. Neurorehabil Neural Repair 25: 412–422, 2011. doi: 10.1177/1545968310394869. [DOI] [PubMed] [Google Scholar]
- Kozai TD, Catt K, Du Z, Na K, Srivannavit O, Haque RU, Seymour J, Wise KD, Yoon E, Cui XT. Chronic in vivo evaluation of PEDOT/CNT for stable neural recordings. IEEE Trans Biomed Eng 63: 111–119, 2016. doi: 10.1109/TBME.2015.2445713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozai TD, Langhals NB, Patel PR, Deng X, Zhang H, Smith KL, Lahann J, Kotov NA, Kipke DR. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat Mater 11: 1065–1073, 2012. doi: 10.1038/nmat3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozai TD, Marzullo TC, Hooi F, Langhals NB, Majewska AK, Brown EB, Kipke DR. Reduction of neurovascular damage resulting from microelectrode insertion into the cerebral cortex using in vivo two-photon mapping. J Neural Eng 7: 046011, 2010. doi: 10.1088/1741-2560/7/4/046011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, Thomson S, O’Callaghan J, Eisenberg E, Milbouw G, Buchser E, Fortini G, Richardson J, North RB. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain 132: 179–188, 2007. doi: 10.1016/j.pain.2007.07.028. [DOI] [PubMed] [Google Scholar]
- Laback B, Egger K, Majdak P. Perception and coding of interaural time differences with bilateral cochlear implants. Hear Res 322: 138–150, 2015. doi: 10.1016/j.heares.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Ladenbauer J, Minassian K, Hofstoetter US, Dimitrijevic MR, Rattay F. Stimulation of the human lumbar spinal cord with implanted and surface electrodes: a computer simulation study. IEEE Trans Neural Syst Rehabil Eng 18: 637–645, 2010. doi: 10.1109/TNSRE.2010.2054112. [DOI] [PubMed] [Google Scholar]
- Lee YH, Creasey GH. Self-controlled dorsal penile nerve stimulation to inhibit bladder hyperreflexia in incomplete spinal cord injury: a case report. Arch Phys Med Rehabil 83: 273–277, 2002. doi: 10.1053/apmr.2002.28817. [DOI] [PubMed] [Google Scholar]
- Leuthardt EC, Miller KJ, Schalk G, Rao RP, Ojemann JG. Electrocorticography-based brain computer interface—the Seattle experience. IEEE Trans Neural Syst Rehabil Eng 14: 194–198, 2006. doi: 10.1109/TNSRE.2006.875536. [DOI] [PubMed] [Google Scholar]
- Lewis PM, Ackland HM, Lowery AJ, Rosenfeld JV. Restoration of vision in blind individuals using bionic devices: a review with a focus on cortical visual prostheses. Brain Res 1595: 51–73, 2015. doi: 10.1016/j.brainres.2014.11.020. [DOI] [PubMed] [Google Scholar]
- Li LF, Ka-Kit Leung G, Lui WM. Sacral nerve stimulation for neurogenic bladder. World Neurosurg 90: 236–243, 2016. doi: 10.1016/j.wneu.2016.02.108. [DOI] [PubMed] [Google Scholar]
- Liberson WT, Holmquest HJ, Scot D, Dow M. Functional electrotherapy: stimulation of the peroneal nerve synchronized with the swing phase of the gait of hemiplegic patients. Arch Phys Med Rehabil 42: 101–105, 1961. [PubMed] [Google Scholar]
- Licht SH. Electrodiagnosis and Electromyography. New Haven, CT: Licht, 1971, p. 533. [Google Scholar]
- Liu J, Fu TM, Cheng Z, Hong G, Zhou T, Jin L, Duvvuri M, Jiang Z, Kruskal P, Xie C, Suo Z, Fang Y, Lieber CM. Syringe-injectable electronics. Nat Nanotechnol 10: 629–636, 2015. doi: 10.1038/nnano.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Li X, Marciniak C, Rymer WZ, Zhou P. Extraction of neural control commands using myoelectric pattern recognition: a novel application in adults with cerebral palsy. Int J Neural Syst 24: 1450022, 2014. doi: 10.1142/S0129065714500221. [DOI] [PubMed] [Google Scholar]
- Loeb GE, Bak MJ, Duysens J. Long-term unit recording from somatosensory neurons in the spinal ganglia of the freely walking cat. Science 197: 1192–1194, 1977. doi: 10.1126/science.897663. [DOI] [PubMed] [Google Scholar]
- Loeb GE, Richmond FJ, Baker LL. The BION devices: injectable interfaces with peripheral nerves and muscles. Neurosurg Focus 20: E2, 2006. doi: 10.3171/foc.2006.20.5.3. [DOI] [PubMed] [Google Scholar]
- Loeb GE, Richmond FJ, Singh J, Peck RA, Tan W, Zou Q, Sachs N. RF-powered BIONs for stimulation and sensing. Conf Proc IEEE Eng Med Biol Soc 6: 4182–4185, 2004. [DOI] [PubMed] [Google Scholar]
- Lu DC, Edgerton VR, Modaber M, AuYong N, Morikawa E, Zdunowski S, Sarino ME, Sarrafzadeh M, Nuwer MR, Roy RR, Gerasimenko Y. Engaging cervical spinal cord networks to reenable volitional control of hand function in tetraplegic patients. Neurorehabil Neural Repair 30: 951–962, 2016. doi: 10.1177/1545968316644344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens FM, den Hollander PP, Snoek GJ, Koldewijn EL, van Kerrebroeck PE, Heesakkers JP. Quality of life in complete spinal cord injury patients with a Brindley bladder stimulator compared to a matched control group. Neurourol Urodyn 30: 551–555, 2011. doi: 10.1002/nau.21012. [DOI] [PubMed] [Google Scholar]
- Martin C, Deveze A, Richard C, Lefebvre PP, Decat M, Ibañez LG, Truy E, Mom T, Lavieille JP, Magnan J, Dubreuil C, Tringali S. European results with totally implantable carina placed on the round window: 2-year follow-up. Otol Neurotol 30: 1196–1203, 2009. doi: 10.1097/MAO.0b013e3181c34898. [DOI] [PubMed] [Google Scholar]
- Martin KD, Polanski WH, Schulz AK, Jöbges M, Hoff H, Schackert G, Pinzer T, Sobottka SB. Restoration of ankle movements with the ActiGait implantable drop foot stimulator: a safe and reliable treatment option for permanent central leg palsy. J Neurosurg 124: 70–76, 2016. doi: 10.3171/2014.12.JNS142110. [DOI] [PubMed] [Google Scholar]
- Martin KD, Polanski WH, Schulz AK, Jobges M, Ziemssen T, Schackert G, Pinzer T, Sobottka SB. ActiGait implantable drop foot stimulator in multiple sclerosis: a new indication. J Neurosurg 126: 1685–1690, 2017. doi: 10.3171/2016.4.JNS1660. [DOI] [PubMed] [Google Scholar]
- Matthews PB. The origin and functional significance of the stretch reflex. In: Excitatory Synaptic Mechanisms, edited by Andersen P, Jansen JK. Oslo: Universitetsforlaget, 1970, p. 301–315. [Google Scholar]
- Food and Drug Administration. MAUDE—Manufacturer and User Facility Device Experience. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm, 2017.
- Maynard EM, Nordhausen CT, Normann RA. The Utah Intracortical Electrode Array: a recording structure for potential brain-computer interfaces. Electroencephalogr Clin Neurophysiol 102: 228–239, 1997. doi: 10.1016/S0013-4694(96)95176-0. [DOI] [PubMed] [Google Scholar]
- Mazzone P, Vilela Filho O, Viselli F, Insola A, Sposato S, Vitale F, Scarnati E. Our first decade of experience in deep brain stimulation of the brainstem: elucidating the mechanism of action of stimulation of the ventrolateral pontine tegmentum. J Neural Transm (Vienna) 123: 751–767, 2016. doi: 10.1007/s00702-016-1518-5. [DOI] [PubMed] [Google Scholar]
- McCreery D, Cogan S, Kane S, Pikov V. Correlations between histology and neuronal activity recorded by microelectrodes implanted chronically in the cerebral cortex. J Neural Eng 13: 036012, 2016. doi: 10.1088/1741-2560/13/3/036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland DJ, Wolpaw JR. EEG-based communication and control: speed-accuracy relationships. Appl Psychophysiol Biofeedback 28: 217–231, 2003. doi: 10.1023/A:1024685214655. [DOI] [PubMed] [Google Scholar]
- McGee MJ, Amundsen CL, Grill WM. Electrical stimulation for the treatment of lower urinary tract dysfunction after spinal cord injury. J Spinal Cord Med 38: 135–146, 2015. doi: 10.1179/2045772314Y.0000000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol 115: 1239–1248, 2004. doi: 10.1016/j.clinph.2003.12.024. [DOI] [PubMed] [Google Scholar]
- McNeal D. 2000 Years of electrical stimulation. In: Functional Electrical Stimulation, edited by Hambrecht FT, Reswick JB. New York: Dekker, 1977, p. 3–35. [Google Scholar]
- Mercier LM, Gonzalez-Rothi EJ, Streeter KA, Posgai SS, Poirier AS, Fuller DD, Reier PJ, Baekey DM. Intraspinal microstimulation and diaphragm activation after cervical spinal cord injury. J Neurophysiol 117: 767–776, 2017. doi: 10.1152/jn.00721.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz CT, Lucas TH, Perlmutter SI, Fetz EE. Forelimb movements and muscle responses evoked by microstimulation of cervical spinal cord in sedated monkeys. J Neurophysiol 97: 110–120, 2007. doi: 10.1152/jn.00414.2006. [DOI] [PubMed] [Google Scholar]
- Moya P, Parra P, Arroyo A, Peña E, Benavides J, Calpena R. Sacral nerve stimulation versus percutaneous posterior tibial nerve stimulation in the treatment of severe fecal incontinence in men. Tech Coloproctol 20: 317–319, 2016. doi: 10.1007/s10151-016-1443-5. [DOI] [PubMed] [Google Scholar]
- Murrow RW. Penfield’s prediction: a mechanism for deep brain stimulation. Front Neurol 5: 213, 2014. doi: 10.3389/fneur.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushahwar VK, Collins DF, Prochazka A. Spinal cord microstimulation generates functional limb movements in chronically implanted cats. Exp Neurol 163: 422–429, 2000. doi: 10.1006/exnr.2000.7381. [DOI] [PubMed] [Google Scholar]
- Mushahwar VK, Gillard DM, Gauthier MJ, Prochazka A. Intraspinal micro stimulation generates locomotor-like and feedback-controlled movements. IEEE Trans Neural Syst Rehabil Eng 10: 68–81, 2002. doi: 10.1109/TNSRE.2002.1021588. [DOI] [PubMed] [Google Scholar]
- Musienko P, Heutschi J, Friedli L, van den Brand R, Courtine G. Multi-system neurorehabilitative strategies to restore motor functions following severe spinal cord injury. Exp Neurol 235: 100–109, 2012. doi: 10.1016/j.expneurol.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Nardone R, Höller Y, Leis S, Höller P, Thon N, Thomschewski A, Golaszewski S, Brigo F, Trinka E. Invasive and non-invasive brain stimulation for treatment of neuropathic pain in patients with spinal cord injury: a review. J Spinal Cord Med 37: 19–31, 2014. doi: 10.1179/2045772313Y.0000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashold BS Jr, Friedman H, Boyarsky S. Electrical activation of micturition by spinal cord stimulation. J Surg Res 11: 144–147, 1971b. doi: 10.1016/0022-4804(71)90039-4. [DOI] [PubMed] [Google Scholar]
- Nashold BS, Friedman H, Glenn JF, Grimes JH, Barry WF, Avery R. Electromicturition in paraplegia: implantation of a spinal neuroprosthesis. Proc Veterans Adm Spinal Cord Inj Conf 18: 161–165, 1971a. [PubMed] [Google Scholar]
- Nashold BS Jr, Friedman H, Grimes J. Electrical stimulation of the conus medullaris to control the bladder in the paraplegic patient. A 10-year review. Appl Neurophysiol 44: 225–232, 1981. [DOI] [PubMed] [Google Scholar]
- Nashold BS, Friedman H, Grimes J. Electrical stimulation of the conus medullaris to control bladder emptying in paraplegia: a ten-year review. Appl Neurophysiol 45: 40–43, 1982. [DOI] [PubMed] [Google Scholar]
- Nathan RH, inventor. Ben-Gurion University of the Negev Research & Development Authority, assignee. Device for generating hand function. US Patent 5,330,516, July 19, 1994.
- O’Dell MW, Dunning K, Kluding P, Wu SS, Feld J, Ginosian J, McBride K. Response and prediction of improvement in gait speed from functional electrical stimulation in persons with poststroke drop foot. PMR 6: 587–601, 2014. doi: 10.1016/j.pmrj.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Orlovsky GN, Deliagina TG, Grillner S. Neuronal Control of Locomotion. Oxford, UK: Oxford Univ. Press, 1999, p. 322. doi: 10.1093/acprof:oso/9780198524052.001.0001. [DOI] [Google Scholar]
- Page SJ, Levin L, Hermann V, Dunning K, Levine P. Longer versus shorter daily durations of electrical stimulation during task-specific practice in moderately impaired stroke. Arch Phys Med Rehabil 93: 200–206, 2012. doi: 10.1016/j.apmr.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Pearson KG. Generating the walking gait: role of sensory feedback. Prog Brain Res 143: 123–129, 2004. doi: 10.1016/S0079-6123(03)43012-4. [DOI] [PubMed] [Google Scholar]
- Peckham PH, Kilgore KL. Challenges and opportunities in restoring function after paralysis. IEEE Trans Biomed Eng 60: 602–609, 2013. doi: 10.1109/TBME.2013.2245128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham PH, Marsolais EB, Mortimer JT. Restoration of key grip and release in the C6 tetraplegic patient through functional electrical stimulation. J Hand Surg Am 5: 462–469, 1980. doi: 10.1016/S0363-5023(80)80076-1. [DOI] [PubMed] [Google Scholar]
- Peckham PH, Poon CW, Ko WH, Marsolais EB, Rosen JJ. Multichannel implantable stimulator for control of paralyzed muscle. IEEE Trans Biomed Eng 28: 530–536, 1981. doi: 10.1109/TBME.1981.324740. [DOI] [PubMed] [Google Scholar]
- Perge JA, Homer ML, Malik WQ, Cash S, Eskandar E, Friehs G, Donoghue JP, Hochberg LR. Intra-day signal instabilities affect decoding performance in an intracortical neural interface system. J Neural Eng 10: 036004, 2013. doi: 10.1088/1741-2560/10/3/036004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perge JA, Zhang S, Malik WQ, Homer ML, Cash S, Friehs G, Eskandar EN, Donoghue JP, Hochberg LR. Reliability of directional information in unsorted spikes and local field potentials recorded in human motor cortex. J Neural Eng 11: 046007, 2014. doi: 10.1088/1741-2560/11/4/046007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters KM, Carrico DJ, Perez-Marrero RA, Khan AU, Wooldridge LS, Davis GL, Macdiarmid SA. Randomized trial of percutaneous tibial nerve stimulation versus Sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trial. J Urol 183: 1438–1443, 2010. doi: 10.1016/j.juro.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Pikov V, Bullara L, McCreery DB. Intraspinal stimulation for bladder voiding in cats before and after chronic spinal cord injury. J Neural Eng 4: 356–368, 2007. doi: 10.1088/1741-2560/4/4/002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic MR, Thrasher TA, Adams ME, Takes V, Zivanovic V, Tonack MI. Functional electrical therapy: retraining grasping in spinal cord injury. Spinal Cord 44: 143–151, 2006. doi: 10.1038/sj.sc.3101822. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Comparison of natural and artificial control of movement. IEEE Trans Rehabil Eng 1: 7–17, 1993. doi: 10.1109/86.242403. [DOI] [Google Scholar]
- Prochazka A, inventor. Neuromotion Inc., owner. Garment having controller that is activated by mechanical impact. Canadian Patent 2217920, 1997.
- Prochazka A, inventor. Method and apparatus for controlling a device or process with vibrations generated by tooth clicks. US patent provisional application 60/421,633, October 25, 2002.
- Prochazka A, inventor. Rehabtronics Inc., assignee. Method and apparatus for controlling a device or process with vibrations generated by tooth clicks. US patent application 10/688,865, October 17, 2003.
- Prochazka A, inventor. Rehabtronics Inc., owner. Method of routing electrical current to bodily tissues via implanted passive conductors. Canadian patent PCT application, PCT/CA 2005/000074, 2005.
- Prochazka A. Proprioceptor models. In: Encyclopedia of Neurocomputation, edited by Jung R, Jaeger D, Tresch M. New York: Springer, 2015a, p. 2501–2518. [Google Scholar]
- Prochazka A. Sensory control of normal movement and of movement aided by neural prostheses. J Anat 227: 167–177, 2015b. doi: 10.1111/joa.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A. Neural prostheses for neurotrauma. In: Translational Neuroscience Fundamental Approaches for Neurological Disorders, edited by Tuszynski MH. New York: Springer, 2016, p. 457–478. [Google Scholar]
- Prochazka A, Elek J, Javidan M. Attenuation of pathological tremors by functional electrical stimulation. I: Method. Ann Biomed Eng 20: 205–224, 1992. doi: 10.1007/BF02368521. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Ellaway PH. Sensory systems in the control of movement. Compr Physiol 2: 2615–2627, 2012. doi: 10.1002/cphy.c100086. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Gauthier M, Wieler M, Kenwell Z. The bionic glove: an electrical stimulator garment that provides controlled grasp and hand opening in quadriplegia. Arch Phys Med Rehabil 78: 608–614, 1997a. doi: 10.1016/S0003-9993(97)90426-3. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Gillard D, Bennett DJ. Implications of positive feedback in the control of movement. J Neurophysiol 77: 3237–3251, 1997b. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Gillard D, Bennett DJ. Positive force feedback control of muscles. J Neurophysiol 77: 3226–3236, 1997c. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Gosgnach S, Capaday C, Geyer H. Neuromuscular models for locomotion. In: Bioinspired Legged Locomotion, edited by Sharbafi M, Seyfarth A. New York: Elsevier, 2017. [Google Scholar]
- Prochazka A, Gritsenko V, Yakovenko S. Sensory control of locomotion: reflexes versus higher-level control. Adv Exp Med Biol 508: 357–367, 2002a. doi: 10.1007/978-1-4615-0713-0_41. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Hulliger M. The continuing debate about CNS control of proprioception. J Physiol 513: 315, 1998. doi: 10.1111/j.1469-7793.1998.315bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A, Hulliger M, Zangger P, Appenteng K. “Fusimotor set”: new evidence for alpha-independent control of gamma-motoneurones during movement in the awake cat. Brain Res 339: 136–140, 1985. doi: 10.1016/0006-8993(85)90632-8. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Mushahwar VK, McCreery DB. Neural prostheses. J Physiol 533: 99–109, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A, Mushahwar V, Yakovenko S. Activation and coordination of spinal motoneuron pools after spinal cord injury. Prog Brain Res 137: 109–124, 2002b. doi: 10.1016/S0079-6123(02)37011-0. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Westerman RA, Ziccone SP. Discharges of single hindlimb afferents in the freely moving cat. J Neurophysiol 39: 1090–1104, 1976. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Wieler M, Kenwell Z, Gauthier MJ, inventors. Sim & McBurney, assignee. Garment for applying controlled electrical stimulation to restore motor function. US patent 5,562,707, October 8, 1996.
- Prochazka A, Yakovenko S. The neuromechanical tuning hypothesis. Prog Brain Res 165: 255–265, 2007a. doi: 10.1016/S0079-6123(06)65016-4. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Yakovenko S. Predictive and reactive tuning of the locomotor CPG. Integr Comp Biol 47: 474–481, 2007b. doi: 10.1093/icb/icm065. [DOI] [PubMed] [Google Scholar]
- Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev 92: 1651–1697, 2012. doi: 10.1152/physrev.00048.2011. [DOI] [PubMed] [Google Scholar]
- Rachitskaya AV, Yuan A. Argus II retinal prosthesis system: an update. Ophthalmic Genet 37: 260–266, 2016. doi: 10.3109/13816810.2015.1130152. [DOI] [PubMed] [Google Scholar]
- Rattay F, Minassian K, Dimitrijevic MR. Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 2. Quantitative analysis by computer modeling. Spinal Cord 38: 473–489, 2000. doi: 10.1038/sj.sc.3101039. [DOI] [PubMed] [Google Scholar]
- Rattay F, Resatz S, Lutter P, Minassian K, Jilge B, Dimitrijevic MR. Mechanisms of electrical stimulation with neural prostheses. Neuromodulation 6: 42–56, 2003. doi: 10.1046/j.1525-1403.2003.03006.x. [DOI] [PubMed] [Google Scholar]
- Révész D, Rydenhag B, Ben-Menachem E. Complications and safety of vagus nerve stimulation: 25 years of experience at a single center. J Neurosurg Pediatr 18: 97–104, 2016. doi: 10.3171/2016.1.PEDS15534. [DOI] [PubMed] [Google Scholar]
- Richmond FJ, Bagg SD, Olney SJ, Dupont AC, Creasy J, Loeb GE. Clinical Trial of BIONsTM for Therapeutic Electrical Stimulation in Queen's University, Kingston, Ontario, Canada. In: Proceedings of the 5th Annual Conference of the International Functional Electrical Stimulation Society, edited by Sinkjaer T. Aalborg, Denmark: Univ. of Aalborg, 2000. [Google Scholar]
- Rigosa J, Weber DJ, Prochazka A, Stein RB, Micera S. Neuro-fuzzy decoding of sensory information from ensembles of simultaneously recorded dorsal root ganglion neurons for functional electrical stimulation applications. J Neural Eng 8: 046019, 2011. doi: 10.1088/1741-2560/8/4/046019. [DOI] [PubMed] [Google Scholar]
- Rijkhoff NJ. Neuroprostheses to treat neurogenic bladder dysfunction: current status and future perspectives. Childs Nerv Syst 20: 75–86, 2004. doi: 10.1007/s00381-003-0859-1. [DOI] [PubMed] [Google Scholar]
- Roche JP, Hansen MR. On the horizon: cochlear implant technology. Otolaryngol Clin North Am 48: 1097–1116, 2015. doi: 10.1016/j.otc.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero FJ, Gambarrutta C, Garcia-Forcada A, Marín MA, Diaz de la Lastra E, Paz F, Fernandez-Dorado MT, Mazaira J. Long-term evaluation of phrenic nerve pacing for respiratory failure due to high cervical spinal cord injury. Spinal Cord 50: 895–898, 2012. doi: 10.1038/sc.2012.74. [DOI] [PubMed] [Google Scholar]
- Santangeli P, Marchlinski FE. Left phrenic nerve pacing from the left subclavian vein: novel method to monitor for left phrenic nerve injury during catheter ablation. Circ Arrhythm Electrophysiol 8: 241–242, 2015. doi: 10.1161/CIRCEP.114.002302. [DOI] [PubMed] [Google Scholar]
- Sayenko DG, Atkinson DA, Floyd TC, Gorodnichev RM, Moshonkina TR, Harkema SJ, Edgerton VR, Gerasimenko YP. Effects of paired transcutaneous electrical stimulation delivered at single and dual sites over lumbosacral spinal cord. Neurosci Lett 609: 229–234, 2015. doi: 10.1016/j.neulet.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AB. Movement: how the brain communicates with the world. Cell 164: 1122–1135, 2016. doi: 10.1016/j.cell.2016.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SH. Neuroscience: converting thoughts into action. Nature 442: 141–142, 2006. doi: 10.1038/442141a. [DOI] [PubMed] [Google Scholar]
- Shaikhouni A, Donoghue JP, Hochberg LR. Somatosensory responses in a human motor cortex. J Neurophysiol 109: 2192–2204, 2013. doi: 10.1152/jn.00368.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Friedenberg DA, Annetta N, Glenn B, Bockbrader M, Majstorovic C, Domas S, Mysiw WJ, Rezai A, Bouton C. Using an artificial neural bypass to restore cortical control of rhythmic movements in a human with quadriplegia. Sci Rep 6: 33807, 2016. doi: 10.1038/srep33807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shealy CN. The viability of external electrical stimulation as a therapeutic modality. Med Instrum 9: 211–212, 1975. [PubMed] [Google Scholar]
- Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg 46: 489–491, 1967. doi: 10.1213/00000539-196707000-00025. [DOI] [PubMed] [Google Scholar]
- Sheffler LR, Chae J. Neuromuscular electrical stimulation in neurorehabilitation. Muscle Nerve 35: 562–590, 2007. doi: 10.1002/mus.20758. [DOI] [PubMed] [Google Scholar]
- Shik ML, Severin FV, Orlovsky GN. Control of walking and running by means of electrical stimulation of the mid-brain. Biophysics (Oxf) 11: 756–765, 1966. [Google Scholar]
- Simeral JD, Kim SP, Black MJ, Donoghue JP, Hochberg LR. Neural control of cursor trajectory and click by a human with tetraplegia 1000 days after implant of an intracortical microelectrode array. J Neural Eng 8: 025027, 2011. doi: 10.1088/1741-2560/8/2/025027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spardy LE, Markin SN, Shevtsova NA, Prilutsky BI, Rybak IA, Rubin JE. A dynamical systems analysis of afferent control in a neuromechanical model of locomotion: I. Rhythm generation. J Neural Eng 8: 065003, 2011a. doi: 10.1088/1741-2560/8/6/065003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spardy LE, Markin SN, Shevtsova NA, Prilutsky BI, Rybak IA, Rubin JE. A dynamical systems analysis of afferent control in a neuromechanical model of locomotion: II. Phase asymmetry. J Neural Eng 8: 065004, 2011b. doi: 10.1088/1741-2560/8/6/065004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein RB. What muscle variable(s) does the nervous system control in limb movements? Behav Brain Sci 5: 535–541, 1982. doi: 10.1017/S0140525X00013327. [DOI] [Google Scholar]
- Stein RB, inventor. Neuromotion Inc., assignee. Assembly for functional electrical stimulation during movement. US patent 5,814,093, September 29, 1998.
- Stein RB, Chong S, Everaert DG, Rolf R, Thompson AK, Whittaker M, Robertson J, Fung J, Preuss R, Momose K, Ihashi K. A multicenter trial of a footdrop stimulator controlled by a tilt sensor. Neurorehabil Neural Repair 20: 371–379, 2006. doi: 10.1177/1545968306289292. [DOI] [PubMed] [Google Scholar]
- Stein RB, Gordon T, Jefferson J, Sharfenberger A, Yang JF, de Zepetnek JT, Belanger M. Optimal stimulation of paralyzed muscle after human spinal cord injury. J Appl Physiol (1985) 72: 1393–1400, 1992. [DOI] [PubMed] [Google Scholar]
- Stein RB, Prochazka A. Impaired motor function: functional electrical stimulation. In: Textbook of Stereotactic and Functional Neurosurgery, edited by Lozano AM, Gildenberg PL, Tasker RR. Berlin: Springer, 2009, p. 3047–3060. [Google Scholar]
- Tai C, Booth AM, de Groat WC, Roppolo JR. Bladder and urethral sphincter responses evoked by microstimulation of S2 sacral spinal cord in spinal cord intact and chronic spinal cord injured cats. Exp Neurol 190: 171–183, 2004. doi: 10.1016/j.expneurol.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Tai C, Booth AM, Robinson CJ, de Groat WC, Roppolo JR. Multi-joint movement of the cat hindlimb evoked by microstimulation of the lumbosacral spinal cord. Exp Neurol 183: 620–627, 2003. doi: 10.1016/S0014-4886(03)00210-3. [DOI] [PubMed] [Google Scholar]
- Tai C, Wang J, Wang X, de Groat WC, Roppolo JR. Bladder inhibition or voiding induced by pudendal nerve stimulation in chronic spinal cord injured cats. Neurourol Urodyn 26: 570–577, 2007a. doi: 10.1002/nau.20374. [DOI] [PubMed] [Google Scholar]
- Tai C, Wang J, Wang X, Roppolo JR, de Groat WC. Voiding reflex in chronic spinal cord injured cats induced by stimulating and blocking pudendal nerves. Neurourol Urodyn 26: 879–886, 2007b. doi: 10.1002/nau.20430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanagho EA. Neural stimulation for bladder control. Semin Neurol 8: 170–173, 1988. doi: 10.1055/s-2008-1041373. [DOI] [PubMed] [Google Scholar]
- Tanagho EA, Schmidt RA, Orvis BR. Neural stimulation for control of voiding dysfunction: a preliminary report in 22 patients with serious neuropathic voiding disorders. J Urol 142: 340–345, 1989. doi: 10.1016/S0022-5347(17)38751-7. [DOI] [PubMed] [Google Scholar]
- Taylor A, Durbaba R, Ellaway PH, Rawlinson S. Static and dynamic gamma-motor output to ankle flexor muscles during locomotion in the decerebrate cat. J Physiol 571: 711–723, 2006. doi: 10.1113/jphysiol.2005.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PN, Wilkinson Hart IA, Khan MS, Slade-Sharman DE. Correction of footdrop due to multiple sclerosis using the STIMuSTEP implanted dropped foot stimulator. Int J MS Care 18: 239–247, 2016. doi: 10.7224/1537-2073.2015-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry B, Kelt RE, Jeyakumar A. Delayed complications after cochlear implantation. JAMA Otolaryngol Head Neck Surg 141: 1012–1017, 2015. doi: 10.1001/jamaoto.2015.2154. [DOI] [PubMed] [Google Scholar]
- Thomas SL, Gorassini MA. Increases in corticospinal tract function by treadmill training after incomplete spinal cord injury. J Neurophysiol 94: 2844–2855, 2005. doi: 10.1152/jn.00532.2005. [DOI] [PubMed] [Google Scholar]
- Thorsen R, Spadone R, Ferrarin M. A pilot study of myoelectrically controlled FES of upper extremity. IEEE Trans Neural Syst Rehabil Eng 9: 161–168, 2001. doi: 10.1109/7333.928576. [DOI] [PubMed] [Google Scholar]
- Van Heeckeren DW, Glenn WW. Electrophrenic respiration by radiofrequency induction. J Thorac Cardiovasc Surg 52: 655–665, 1966. [PubMed] [Google Scholar]
- Van Kerrebroeck PE, Koldewijn EL, Debruyne FM. Worldwide experience with the Finetech-Brindley sacral anterior root stimulator. Neurourol Urodyn 12: 497–503, 1993. doi: 10.1002/nau.1930120511. [DOI] [PubMed] [Google Scholar]
- van Overeem Hansen G. EMG-controlled functional electrical stimulation of the paretic hand. Scand J Rehabil Med 11: 189–193, 1979. [PubMed] [Google Scholar]
- Vanderhorst VG, Holstege G. Organization of lumbosacral motoneuronal cell groups innervating hindlimb, pelvic floor, and axial muscles in the cat. J Comp Neurol 382: 46–76, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- Vansteensel MJ, Pels EG, Bleichner MG, Branco MP, Denison T, Freudenburg ZV, Gosselaar P, Leinders S, Ottens TH, Van Den Boom MA, Van Rijen PC, Aarnoutse EJ, Ramsey NF. Fully implanted brain-computer interface in a locked-in patient with ALS. N Engl J Med 375: 2060–2066, 2016. doi: 10.1056/NEJMoa1608085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velliste M, Kennedy SD, Schwartz AB, Whitford AS, Sohn JW, McMorland AJ. Motor cortical correlates of arm resting in the context of a reaching task and implications for prosthetic control. J Neurosci 34: 6011–6022, 2014. doi: 10.1523/JNEUROSCI.3520-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaurre C, Klauer C, Schauer T, Ramos-Murguialday A, Müller KR. EEG-based BCI for the linear control of an upper-limb neuroprosthesis. Med Eng Phys 38: 1195–1204, 2016. doi: 10.1016/j.medengphy.2016.06.010. [DOI] [PubMed] [Google Scholar]
- Vinjamuri R, Weber DJ, Degenhart AD, Collinger JL, Sudre GP, Adelson PD, Holder DL, Boninger ML, Schwartz AB, Crammond DJ, Tyler-Kabara EC, Wang W. A fuzzy logic model for hand posture control using human cortical activity recorded by micro-ECog electrodes. Conf Proc IEEE Eng Med Biol Soc 2009: 4339–4342, 2009. doi: 10.1109/IEMBS.2009.5332746. [DOI] [PubMed] [Google Scholar]
- Vodovnik L. Therapeutic effects of functional electrical stimulation of extremities. Med Biol Eng Comput 19: 470–478, 1981. doi: 10.1007/BF02441314. [DOI] [PubMed] [Google Scholar]
- Voss H. Tabelle der absoluten und relativen Muskelspindelzahlen der menschlichen Skelettmuskulatur. Anat Anz 129: 5562–5572, 1971. [PubMed] [Google Scholar]
- Wang W, Degenhart AD, Collinger JL, Vinjamuri R, Sudre GP, Adelson PD, Holder DL, Leuthardt EC, Moran DW, Boninger ML, Schwartz AB, Crammond DJ, Tyler-Kabara EC, Weber DJ. Human motor cortical activity recorded with Micro-ECoG electrodes, during individual finger movements. Conf Proc IEEE Eng Med Biol Soc 2009: 586–589, 2009 10.1109/IEMBS.2009.5333704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters RL, McNeal D, Perry J. Experimental correction of footdrop by electrical stimulation of the peroneal nerve. J Bone Joint Surg Am 57: 1047–1054, 1975. doi: 10.2106/00004623-197557080-00002. [DOI] [PubMed] [Google Scholar]
- Weber DJ, Stein RB, Chan KM, Loeb G, Richmond F, Rolf R, James K, Chong SL. BIONic WalkAide for correcting foot drop. IEEE Trans Neural Syst Rehabil Eng 13: 242–246, 2005. doi: 10.1109/TNSRE.2005.847385. [DOI] [PubMed] [Google Scholar]
- Weber DJ, Stein RB, Everaert DG, Prochazka A. Decoding sensory feedback from firing rates of afferent ensembles recorded in cat dorsal root ganglia in normal locomotion. IEEE Trans Neural Syst Rehabil Eng 14: 240–243, 2006. doi: 10.1109/TNSRE.2006.875575. [DOI] [PubMed] [Google Scholar]
- Weber DJ, Stein RB, Everaert DG, Prochazka A. Limb-state feedback from ensembles of simultaneously recorded dorsal root ganglion neurons. J Neural Eng 4: S168–S180, 2007. doi: 10.1088/1741-2560/4/3/S04. [DOI] [PubMed] [Google Scholar]
- Wenger N, Moraud EM, Gandar J, Musienko P, Capogrosso M, Baud L, Le Goff CG, Barraud Q, Pavlova N, Dominici N, Minev IR, Asboth L, Hirsch A, Duis S, Kreider J, Mortera A, Haverbeck O, Kraus S, Schmitz F, DiGiovanna J, van den Brand R, Bloch J, Detemple P, Lacour SP, Bézard E, Micera S, Courtine G. Spatiotemporal neuromodulation therapies engaging muscle synergies improve motor control after spinal cord injury. Nat Med 22: 138–145, 2016. doi: 10.1038/nm.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett FR, Pandarinath C, Jarosiewicz B, Murphy BA, Memberg WD, Blabe CH, Saab J, Walter BL, Sweet JA, Miller JP, Henderson JM, Shenoy KV, Simeral JD, Hochberg LR, Kirsch RF, Ajiboye AB. Feedback control policies employed by people using intracortical brain-computer interfaces. J Neural Eng 14: 016001, 2017. doi: 10.1088/1741-2560/14/1/016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MR, Kirsch RF. Evaluation of head orientation and neck muscle EMG signals as three-dimensional command sources. J Neuroeng Rehabil 12: 25, 2015. doi: 10.1186/s12984-015-0016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodlinger B, Downey JE, Tyler-Kabara EC, Schwartz AB, Boninger ML, Collinger JL. Ten-dimensional anthropomorphic arm control in a human brain-machine interface: difficulties, solutions, and limitations. J Neural Eng 12: 016011, 2015. doi: 10.1088/1741-2560/12/1/016011. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, McFarland DJ, Neat GW, Forneris CA. An EEG-based brain-computer interface for cursor control. Electroencephalogr Clin Neurophysiol 78: 252–259, 1991. doi: 10.1016/0013-4694(91)90040-B. [DOI] [PubMed] [Google Scholar]
- Wolter T. Spinal cord stimulation for neuropathic pain: current perspectives. J Pain Res 7: 651–663, 2014. doi: 10.2147/JPR.S37589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovenko S, Mushahwar V, VanderHorst V, Holstege G, Prochazka A. Spatiotemporal activation of lumbosacral motoneurons in the locomotor step cycle. J Neurophysiol 87: 1542–1553, 2002. doi: 10.1152/jn.00479.2001. [DOI] [PubMed] [Google Scholar]
- Yakovenko S, Mushahwar VK, Vanderhorst VG, Holstege G, Prochazka A. Locus of activation of spinal motoneuron pools in the cat step cycle. J Physiol 525P: 42P, 2000. [Google Scholar]
- Yoo PB, Grill WM. Minimally-invasive electrical stimulation of the pudendal nerve: a pre-clinical study for neural control of the lower urinary tract. Neurourol Urodyn 26: 562–569, 2007. doi: 10.1002/nau.20376. [DOI] [PubMed] [Google Scholar]
- Yoo PB, Klein SM, Grafstein NH, Horvath EE, Amundsen CL, Webster GD, Grill WM. Pudendal nerve stimulation evokes reflex bladder contractions in persons with chronic spinal cord injury. Neurourol Urodyn 26: 1020–1023, 2007. doi: 10.1002/nau.20441. [DOI] [PubMed] [Google Scholar]
- Yuan H, Silberstein SD. Vagus nerve and vagus nerve stimulation, a comprehensive review: Part I. Headache 56: 71–78, 2016a. doi: 10.1111/head.12647. [DOI] [PubMed] [Google Scholar]
- Yuan H, Silberstein SD. Vagus nerve and vagus nerve stimulation, a comprehensive review: Part II. Headache 56: 259–266, 2016b. doi: 10.1111/head.12650. [DOI] [PubMed] [Google Scholar]
- Yuan H, Silberstein SD. Vagus nerve and vagus nerve stimulation, a comprehensive review: Part III. Headache 56: 479–490, 2016c. doi: 10.1111/head.12649. [DOI] [PubMed] [Google Scholar]
- Yuan TF, Li A, Sun X, Arias-Carrión O, Machado S. Vagus nerve stimulation in treating depression: a tale of two stories. Curr Mol Med 16: 33–39, 2016. doi: 10.2174/1566524016666151222143609. [DOI] [PubMed] [Google Scholar]
- Zhang TC, Grill WM. Modeling deep brain stimulation: point source approximation versus realistic representation of the electrode. J Neural Eng 7: 066009, 2010. doi: 10.1088/1741-2560/7/6/066009. [DOI] [PMC free article] [PubMed] [Google Scholar]