Behavioral and neural responses to auditory stimuli are profoundly influenced by recent sounds, yet how this occurs is not known. Here, the authors show in the auditory cortex of awake mice that the quality of history-dependent effects is diverse and related to cell type, response latency, firing rates, and receptive field bandwidth. In a cortical model, differences in excitatory-inhibitory balance can produce this diversity, providing the cortex with multiple ways of representing temporally complex information.
Keywords: auditory cortex, forward suppression, stimulus history
Abstract
Responses to auditory stimuli are often strongly influenced by recent stimulus history. For example, in a paradigm called forward suppression, brief sounds can suppress the perception of, and the neural responses to, a subsequent sound, with the magnitude of this suppression depending on both the spectral and temporal distances between the sounds. As a step towards understanding the mechanisms that generate these adaptive representations in awake animals, we quantitatively characterize responses to two-tone sequences in the auditory cortex of waking mice. We find that cortical responses in a forward suppression paradigm are more diverse in waking mice than previously appreciated, that these responses vary between cells with different firing characteristics and waveform shapes, but that the variability in these responses is not substantially related to cortical depth or columnar location. Moreover, responses to the first tone in the sequence are often not linearly related to the suppression of the second tone response, suggesting that spike-frequency adaptation of cortical cells is not a large contributor to forward suppression or its variability. Instead, we use a simple multilayered model to show that cell-to-cell differences in the balance of intracortical inhibition and excitation will naturally produce such a diversity of forward interactions. We propose that diverse inhibitory connectivity allows the cortex to encode spectro-temporally fluctuating stimuli in multiple parallel ways.
NEW & NOTEWORTHY Behavioral and neural responses to auditory stimuli are profoundly influenced by recent sounds, yet how this occurs is not known. Here, the authors show in the auditory cortex of awake mice that the quality of history-dependent effects is diverse and related to cell type, response latency, firing rates, and receptive field bandwidth. In a cortical model, differences in excitatory-inhibitory balance can produce this diversity, providing the cortex with multiple ways of representing temporally complex information.
sensory systems do not create an exact neural representation of stimuli, but rather modify these representations based on the context of current and recent sensory experiences (Allman et al. 1985; Bregman 1990; Simons 1985). Such context-dependent representations are a critical aspect of auditory processing, in which the perception of successive auditory events as either isolated components or one integrated object depends on several factors, including the spectral composition, duration, and temporal separation of the events (Bregman 1990; Yost 1991). Numerous experimental paradigms reveal the sensitivity of the auditory system to context and have demonstrated that sounds with particular spectral and temporal characteristics can suppress, or enhance, neural responses to target sounds (Brosch and Schreiner 1997, 2000; Calford and Semple 1995; Nelken 2014; Schreiner et al. 2000).
A tractable and commonly used paradigm for investigating the context dependence of auditory responses is forward suppression, in which a preceding “masker” tone robustly suppresses responses to a later “probe” tone. The strength of this suppression depends both on the spectral content and on the relative timing of the tones, such that masker tones that are closer in time or more similar in frequency to the probe will produce greater suppression of the probe response. This effect of recent stimulus history occurs at multiple stages of the auditory system, but changes in quality from the periphery to the central nervous system.
These changes may be shaped through several mechanisms. Within the cochlea, the basilar membrane is thought to be composed of biophysical filters, called critical bands, which determine the frequency resolution of the cochlea (Greenwood 1961) and impose a limit on which frequencies can be simultaneously represented. These cochlear effects, however, last only milliseconds (Recio et al. 1998) and likely do not contribute significantly to longer-lasting suppression (Brosch and Schreiner 1997). In the auditory nerve, on the other hand, forward suppression can last for several tens of milliseconds (Harris and Dallos 1979), and the suppression of the response to the probe is proportional to the masker-driven firing rate (Smith 1977), suggesting that suppression arises through spike-frequency adaptation. Subcortically, in the cochlear nucleus (Boettcher et al. 1990; Ingham et al. 2016; Shore 1995; Watanabe and Simada 1971), the inferior colliculus (Malone and Semple 2001; Nelson et al. 2009), and the thalamus (Schreiner 1981), masker stimuli that do not evoke suprathreshold responses can still suppress probe responses, presumably through more complex circuit-generated mechanisms, such as synaptic depression or inhibition. Further transformations occur within the cortex. For example, cortical neurons cannot faithfully follow stimulus rates higher than 5–15 Hz, whereas thalamic neurons can follow rates above 100 Hz (Creutzfeldt et al. 1980; Miller et al. 2002; Wang et al. 2008; Yao et al. 2015), suggesting that the duration of forward suppression is longer in the cortex than in prior stages. Additionally, suppression in the cortex appears to be quite robust, as several studies have demonstrated that at short latencies, the majority of cortical neurons can have their firing suppressed by a broad range of masker tones (Brosch and Schreiner 1997; Calford and Semple 1995; Scholes et al. 2011; Scholl et al. 2008). Cortical processing of sequences, and forward suppression in particular, is thought to underlie the perceptual grouping or separation of objects within acoustic streams (Fishman et al. 2004; Fishman et al. 2001), a process that is at the core of speech perception and auditory scene analysis.
How such robust, history-dependent interactions arise within the cortex has been a longstanding topic of debate. Activity-dependent mechanisms, such as presynaptic short-term depression and spike-frequency adaptation, are pronounced in many cortical areas and have been hypothesized to dominate masking interactions in visual (Carandini et al. 2002; Sanchez-Vives et al. 2000) and somatosensory cortices (Chung et al. 2002; Díaz-Quesada and Maravall 2008), and to be the main contributors to forward suppression in auditory cortex (Abolafia et al. 2011; Bayazitov et al. 2013; Wehr and Zador 2005). However, the cortex also contains numerous, diverse populations of GABAergic interneurons. The synaptic inhibition that these neurons provide shapes the responses of all cortical cells and may also shape such interactions (Li et al. 2014; Zhang et al. 2003). Determining the relative contributions of these mechanisms is partly complicated by the widespread use of anesthesia, which alters the level of spontaneous activity (Kuwada et al. 1989; Syka et al. 2005; Zurita et al. 1994), modifies the pattern of cortical and thalamic firing (Gaese and Ostwald 2001; Massaux et al. 2004; Zurita et al. 1994), and distorts the effects of synaptic inhibition (Franks and Lieb 1994; Hara and Harris 2002). Thus, determining how cellular, synaptic, and inhibitory mechanisms interact to support temporal processing will require dissecting them in awake animals.
Here we establish a basic understanding of forward suppression in the auditory cortex of waking mice by providing a quantitative analysis of single unit responses to brief tone sequences. First, we find that there are multiple ways in which masker stimuli can alter responses to a probe stimulus, and that the strengths and shapes of forward interactions are strikingly heterogeneous. Moreover, these aspects of forward suppression vary between neurons with different response properties and waveform shapes, suggesting that encoding of temporally complex sound is distributed across functionally diverse neuronal populations. Finally, we use a layered network model to show that cell-to-cell variability in excitatory-inhibitory balance will naturally produce such a diversity of forward interactions. We propose that synaptic inhibition allows the cortex to encode spectro-temporally fluctuating stimuli and regulate the influence of stimulus history in multiple, parallel ways.
MATERIALS AND METHODS
Protocols.
All experiments were performed in accordance with the National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee at the University of California, San Francisco.
Animals.
For all experiments, we used 6- to 12-wk-old male and female mice (n = 82) that were housed in groups of 2–5 under a 12:12-h light-dark cycle, and all recordings were performed during the light cycle. All mice were genetic crosses of knock-in lines on C57BL/6 backgrounds.
Surgeries.
At least 2 days before recording, animals were induced under anesthesia with 3% isoflurane, and surgical anesthesia was maintained with 1.25–2% isoflurane. Pre- and postoperative multimodal analgesics were given. A custom metal headplate with a 5-mm diameter opening was affixed over the right temporal skull with dental cement, and a silicone elastomer was placed in the opening to prevent drying of the skull. Animals were allowed to recover for 2–5 days, after which a second surgery was performed (following the same anesthesia/analgesic protocol as before) to drill a hole in the skull (~2 mm) above the auditory cortex, and new silicone elastomer was placed in the opening to protect the brain. Animals recovered for 1–3 h before recordings.
Data acquisition and stimuli.
After recovery, animals were placed in a head holder on an air-floated, free-spinning spherical treadmill (modified from Niell and Stryker 2010); the sound pressure from the air-floated treadmill was maintained at or below 45 dB and had spectral power mainly at frequencies below 4 kHz). The silicone plug was removed from the opening in the skull, and recordings were made using a 16 site linear probe (50-μm spacing, Neuronexus), inserted approximately perpendicular to the cortical surface. Neural activity was amplified and digitized continuously with a 16-channel recording system (Tucker-Davis Technologies) at 24,414 Hz.
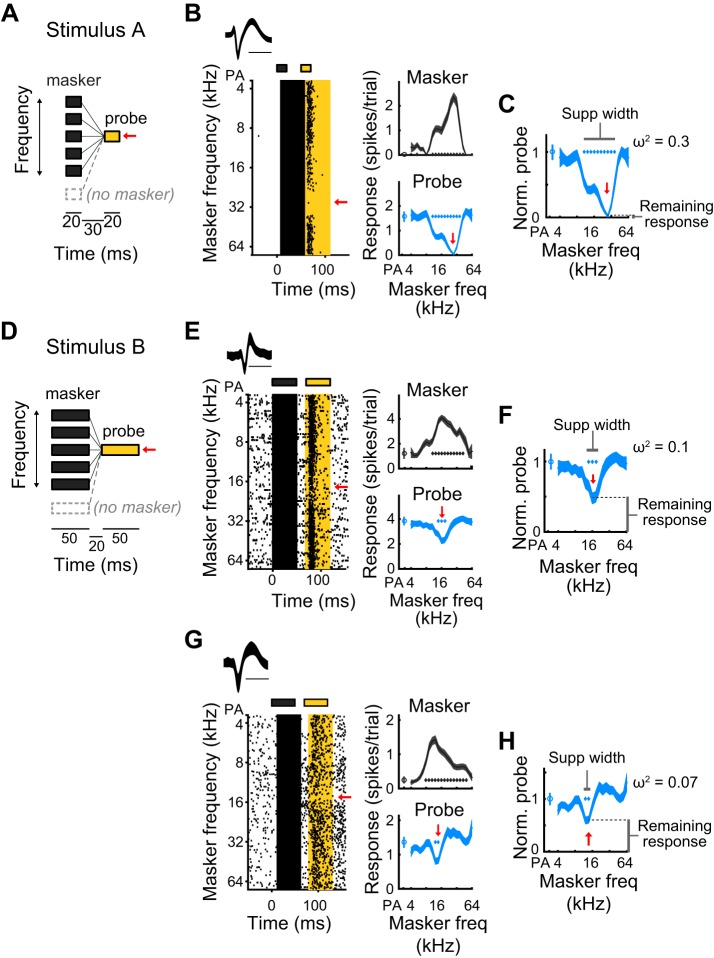
Auditory stimuli were presented through a free-field speaker (ES1, Tucker-Davis Technologies), directed toward the left ear of the animal. Fifty-millisecond tones of various frequencies (4 kHz to 64 kHz, 0.2-octave spacing) and intensities (0–60 dB, 5-dB increments) were played to determine the best frequency (BF) of the units in the recording site, after which a forward suppression experiment was conducted. Forward suppression stimuli consisted of a masker tone followed by a probe tone, separated by a gap (Fig. 1, A and D). The frequency of the probe tone was chosen as the best frequency of the site (10–15 dB above threshold) and was fixed across trials. The masker tone varied in frequency across trials (4 kHz to 64 kHz, 0.2-octave spacing) and was 5–10 dB louder than the probe tone. On randomly interleaved trials, the probe tone was presented without a preceding masker (probe alone, PA, trials) to determine the unsuppressed probe response. Because the timing of the two tones has been shown to influence the strength of suppression (Bartlett and Wang 2005; Brosch and Schreiner 1997 2000; Wehr and Zador 2005), we used two stimulus sets that differed in tone and gap duration. Stimulus A (stim A) consisted of two 20-ms tones separated by a 30-ms gap (Fig. 1A), while stimulus B (stim B) consisted of two 50-ms tones separated by a 20-ms gap (Fig. 1B). The onset-timing difference, or stimulus-onset asynchrony, was 50 ms and 70 ms, respectively.
Fig. 1.
Measuring contextual interactions using a sequential forward suppression paradigm. A: forward suppression stimulus in which a masker tone, whose frequency varies from trial to trial, which precedes a probe tone, whose frequency (indicated by the red arrow) is constant. On randomly interleaved trials, called probe alone (PA) trials, the masker tone is omitted. Stimulus A is composed of 20-ms masker and probe tones, separated by a 30-ms gap. B: an example single unit’s response to stimulus A. Left: spike waveform (means ± SD of unit’s action potentials; scale bar is 1 ms). Raster shows spike times relative to the onset of the masker, as a function of masker frequency. Gray and yellow rectangles above raster signify times of masker and probe tones, respectively. Gray and yellow highlighted regions show the 50-ms time periods used to calculate average masker and probe responses, respectively. Red arrow, frequency of probe tone. Right, top: tuning profile (means ± SE of responses to the masker, as a function of masker frequency). Gray diamonds, masker frequencies for which responses significantly exceeded spontaneous. Right, bottom: suppression profile (means ± SE of responses to the probe, as a function of masker frequency). Blue diamonds, masker frequencies for which responses to the probe are significantly suppressed below the PA response. C: the normalized suppression profile, as a function of masker frequency, is produced by dividing the suppression profile by the probe alone response. Remaining response at probe frequency (lower vertical bar) is defined as the normalized probe response at probe frequency. Suppression width (horizontal bar) is measured as the range of masker stimuli, in octaves, that significantly suppress the probe response. Frequency dependence (ω2) is a measure of how strongly the response to the probe depends on the frequency of the masker tone (see materials and methods). D: stimulus B is similar to stimulus A, but composed of 50-ms masker and probe tones with a 20-ms gap between the two. E and F: as B and C. An example single unit’s response to stimulus B shows a relatively large remaining response at probe frequency, modest frequency dependence, and a narrow suppression width. G and H: as B and C. Another example single unit’s response to stimulus B, demonstrating that the highest normalized probe response can be >1 (i.e., the probe alone response).
Since we could record from multiple units across the length of the recording electrode, occasionally units with different best frequencies were recorded simultaneously. Thus for some units, the probe frequency was far (>0.5 octaves) from the best frequency. Unless otherwise stated, only units for which the probe frequency was within 0.5 octaves of the best frequency were analyzed.
Constructing single unit tuning and suppression profiles.
To isolate single units, neural events that crossed a threshold of 4 standard deviations above background were collected and sorted using custom software written in MATLAB (MathWorks) by Matthew Fellows, based on Calabrese and Paninski (2011). A single unit was defined as auditory responsive if the activity in the 50 ms poststimulus onset was significantly (α = 0.01, rank-sum test) greater than the activity in the 50 ms before stimulus onset. For each auditory responsive unit (stim A: 133 of 153 units; stim B: 76 of 81 units), we constructed event rasters aligned to stimulus onset. We used these to calculate response onsets and to construct both frequency tuning profiles (masker responses as a function of masker frequency, at a fixed masker intensity) and forward suppression profiles (probe responses as a function of masker frequency, at a fixed probe intensity). To calculate masker response latencies, we pooled events across the trials in response to the best frequency (BF; stimulus frequency that elicited the largest response) and grouped these events into 1-ms bins. Onset and offset latencies were then defined as the times at which evoked firing at BF exceeded spontaneous firing by 3 standard deviations and then fell below this threshold, respectively. Response onset latency to the probe tone was taken to be the same as the response onset latency to the masker tone. Tuning profiles for each auditory responsive unit were then generated by taking the firing rate in the period 5 ms before masker onset latency to 45 ms after onset latency (total of 50 ms), as a function of masker frequency (Fig. 1, B, E, and G). Units were defined as tuned if the tuning profile was significantly (α = 0.05, Kruskal-Wallis) modulated by frequency.
For all tuned units (stim A: 123 of 133 units; stim B: 67 of 76 units), we then generated suppression profiles by measuring the average firing rate in the 5 ms before probe response onset to 45 ms after probe response onset (total of 50 ms), as a function of masker frequency (Fig. 1, B, E, and G). Normalized suppression profiles were generated by dividing suppression profiles by the probe alone response (Fig. 1, C, F, and H).
Forward suppression classification and characterization.
Units were considered “suppressed” if at least one of the masker stimuli significantly (α = 0.01, rank-sum) suppressed the probe response below the probe alone response. Units were considered “facilitated” if at least one masker stimulus significantly (α = 0.01, rank-sum) increased the probe response relative to the probe alone response. Thus, there were four possible categories of units: suppressed (Fig. 1, B, E, and G and Fig. 2, A and B), facilitated (Fig. 2C), mixed (facilitated and suppressed; Fig. 2D), or neither suppressed nor facilitated (Fig 2, E and F). In addition to this suppression profile classification, we measured three features of the normalized suppression profiles (Fig 1, C, F, and H). First, remaining response at probe frequency was measured as the normalized firing rate of the probe response when the masker frequency was at probe frequency (often the most suppressed probe response). Second, suppression width was quantified as the range of masker frequencies in octaves (0.2 octaves per masker frequency bin) that suppressed the probe response relative to the probe alone response. Last, the frequency dependence of suppression was measured as ω2, which is an unbiased measure of the proportion of variance in the response to the probe that is explained by the frequency of the masker tone, and was defined as:
where SSfreq is the sum of squares between masker frequencies (calculated from a one-way ANOVA), SStotal is the total sum of squares, MSerror is the mean square error, and df is the degrees of freedom.
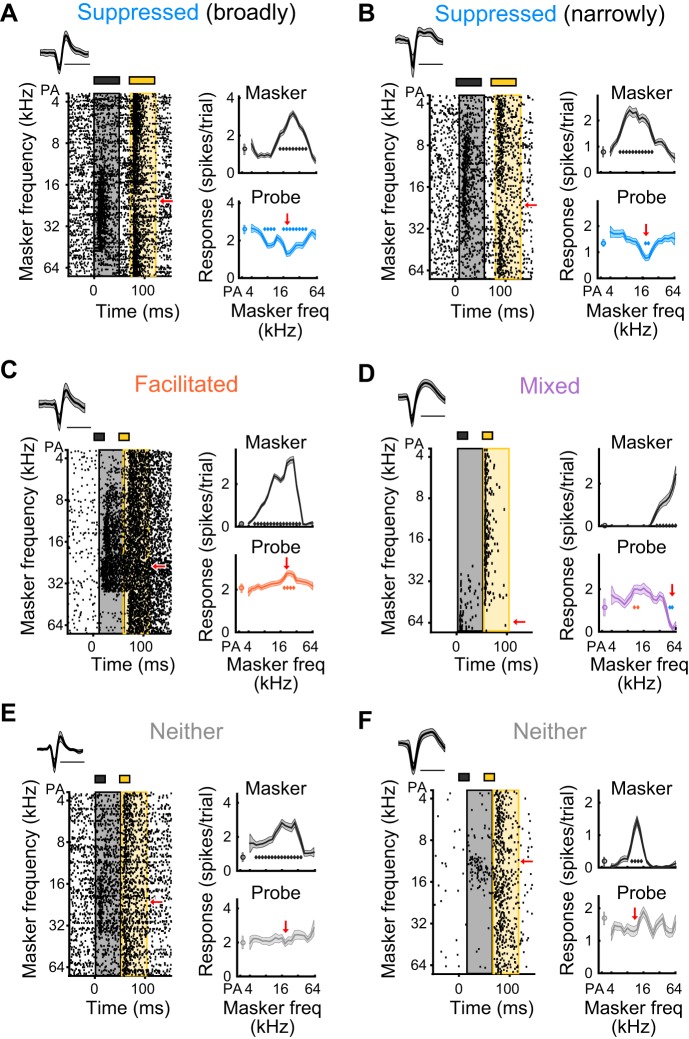
Fig. 2.
Heterogeneous responses in a forward suppression paradigm. A: a single unit, presented with stimulus B, whose probe response is suppressed by a wide range of masker frequencies (blue diamonds). Red arrow indicates frequency of probe tone. B: a single unit, presented with stimulus B, whose probe response is suppressed by a narrow range of masker frequencies (blue diamonds). C: a single unit, presented with stimulus A, whose probe response is facilitated by a narrow range of masker stimuli (orange diamonds). D: a single unit, presented with stimulus A, whose probe response is facilitated by some masker frequencies (orange diamonds), and suppressed by others (blue diamonds), showing a mixed effect. E and F: single units, presented with stimulus A, whose probe responses are not significantly affected by the prior presentation of a masker stimulus, and therefore are neither suppressed nor facilitated.
Correlating masker and probe responses.
Correlations between the response to the masker and the response to the probe for a given unit were calculated by plotting the average masker responses against the average probe responses. These points were then fit to a straight line using standardized major axis regression, which is necessary to account for the measurement variance in both the x- and y-axes (Sokal and Rohlf 2012).
Multilayered model.
The influences of short-term synaptic adaptation and synaptic inhibition on responses to the masker and probe stimuli were modeled in a two-tone forward suppression paradigm, using a linear threshold model containing three layers of neurons. Each neuron’s response ranges from 0 (no response) to 1 (maximum possible firing). The first layer contains a population of n “thalamic” neurons, whose responses as a function of tone frequency (f) are Gaussian-tuned, with standard deviations (δT) of ¼ octave and with evenly spaced center frequencies (5 per octave, spanning the range 2 octaves below the probe frequency to 2 octaves above the probe frequency):
[Throughout the model, tone frequency (f) is presented in terms of octaves from the probe frequency, and specific values for each term are presented at the end.] Each thalamic neuron synapses onto “pyramidal” cells in the cortical (second) layer, such that the synaptic output of thalamic cells onto the pyramidal cells is proportional to the thalamic response:
We modeled the T→Pyr synapses as dynamic. They depress instantaneously by an amount proportional to the synaptic output and recover over time t, according to an exponential function where τ is the recovery time constant of the synapse:
Thus, the availability of these T→Pyr synapses to respond to a stimulus is either 1 (if there was no prior stimulus) or it is the complement of depression (if there was a prior stimulus):
Each cortical excitatory cell k receives depressing synapses from multiple thalamic neurons; these are center-weighted and scaled by a Gaussian connectivity function:
Thus, the response of a cortical excitatory neuron, as a function of tone frequency, is the product of the synaptic outputs from the thalamus, their current availability, and their connection weights onto the cortical excitatory neuron:
Thalamic neurons also make excitatory, depressing synapses onto second-layer “interneurons” (T→Int) in the same way, the only difference being that the connectivity function that describes T→Int synapses is broader (parameters below). In addition, thalamic neurons make depressing connections onto the third-layer “cortical output” cells (T→CO), with similar bandwidth as T→Pyr connections, but with weaker synaptic gain.
The pyramidal neurons and interneurons in the second layer also make depressing synapses onto the cortical output cell in the third layer (Pyr→CO; Int→CO). The main differences between these two connections are that pyramidal cells add current to the output cell, while inhibitory cells subtract current from the output cell, and that the synaptic weights from inhibitory cells onto the output cell are weaker than the excitatory connections from pyramidal neurons.
The synaptic gains of all connections are:
The bandwidths of the Gaussian connectivity weight functions are:
The scaling factors for the Gaussian connectivity weights are:
The time constants for all synaptic connections are:
The time between masker and probe tones (i.e., the gap duration) is:
To model altered strength of inhibition onto the cortical output neuron, we additionally included a range of scaling factors for the Gaussian connectivity weights from Int→CO synapses:
RESULTS
Diversity of forward suppression.
We found that stimulus history (in a two-tone forward suppression paradigm; Fig. 1, A and D) influenced the responses of most units in the awake mouse auditory cortex, but in qualitatively and quantitatively diverse ways. In most units (63%), the response to the probe tone was significantly suppressed by a preceding masker tone (see materials and methods; Fig. 3A, blue bars). In addition to these suppressed units, a small portion of units (~9%) exhibited significant forward facilitation of the probe response (see materials and methods; Fig. 3A, orange bars), an even smaller fraction (~1%) showed mixed effects (both suppression and facilitation; Fig. 3A, purple bars), and the remainder (~25%) were neither significantly suppressed nor facilitated (Fig. 3A:, gray bars). Consistent with previous reports, the strength of suppression in suppressed units often depended on the spectral distance between the masker and the probe tones, such that the magnitude of suppression was greatest when the masker and probe were similar in frequency (Fig. 1, B, E, and G and Fig. 2, A and B); however, the strength and shape of suppression often appeared different between suppressed units. For instance, in some units, masker tones completely suppressed responses to the probe (Fig. 1B), whereas in other units, responses to the probe were only partially suppressed (Fig. 1, E and G and Fig. 2, A and B); in some units, a broad range of masker frequencies suppressed the probe response (Fig. 2A), whereas in other units, only a narrow range of masker frequencies produced suppression of the probe response (Fig. 2B).
Fig. 3.
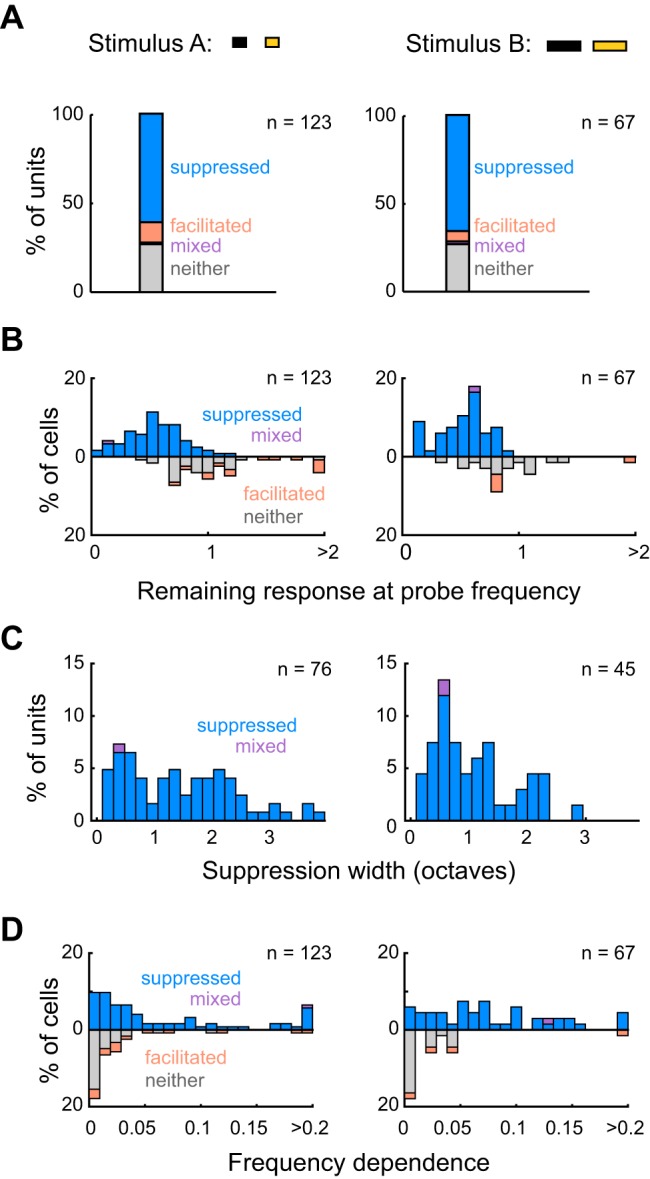
Forward suppression is similar between units presented with stimulus A (stim A) or stimulus B (stim B). A: percentages of all units presented with either stimulus A (left; n = 123) or stimulus B (right; n = 67) that were suppressed (stim A: 75 units; stim B: 44 units), facilitated (stim A: 14 units; stim B: 4 units), mixed (both suppressed and facilitated; stim A: 1 unit; stim B: 1 unit), or neither suppressed nor facilitated (stim A: 33 units; stim B: 14 units). These proportions are not different between stimulus A and stimulus B (Fisher’s exact test, P = 0.49). B: stacked distributions of remaining responses at probe frequency for units presented with stimulus A (left) or stimulus B (right). The remaining responses of all units are not significantly different between stimulus types (upward and downward bars; stim A: median remaining response = 0.7 ± 0.44; stim B: median remaining response = 0.61 ± 0.34; rank-sum, P = 0.11), nor are those among only suppressed/mixed units (upward bars; stim A: median remaining response = 0.54 ± 0.33; stim B: median remaining response = 0.55 ± 0.27; rank-sum, P = 0.91). C: stacked distributions of suppression widths for units with suppression widths >0 (i.e., suppressed/mixed units), presented with either stimulus A (left; n = 76) or stimulus B (right; n = 45). D: stacked distributions of frequency dependence for units presented with stimulus A (left) or stimulus B (right).
To provide a more quantitative characterization of forward suppression, we measured three main features of units’ normalized suppression profiles: the remaining response at probe frequency (which relates to the strength of suppression), the width of the suppression profile (corresponding to the frequency range of suppression), and the frequency dependence of suppression (ω2; a measure of how strongly responses depend on the frequency of the masker stimulus; see materials and methods; Fig. 1). Units exhibited broad distributions of these three measurements (Fig. 3); some units were nearly completely suppressed at probe frequency (small remaining response), while others were barely suppressed at probe frequency (large remaining response); some were suppressed by a narrow range of masker frequencies (narrower widths), while others were suppressed by broader ranges (broader widths); and some units had essentially flat probe responses (lower frequency dependence), while others had probe responses that were more substantially modulated by masker stimuli (higher frequency dependence). These results indicate that preceding tones have both qualitatively and quantitatively diverse effects on responses to later tones, suggesting a distributed representation of stimulus history across cortical cells.
Small variations in stimulus duration elicit similar suppression qualities.
The temporal properties of sequential stimuli, for instance the duration of the masker tone and the duration of the gap between the masker and probe tones, have been shown to influence suppression strength (Bartlett and Wang 2005; Brosch and Schreiner 1997, 2000; Wehr and Zador 2005). Here, we examined forward suppression using two sequential stimuli with relatively small variations in masker and gap duration (see materials and methods; Fig. 1, A and D). We found that the proportions of suppressed, facilitated, mixed, and unaffected units did not differ between units presented with either of the two stimulus types (Fisher’s exact test, P = 0.49; Fig. 3A). Moreover, among the units that exhibited significant suppression (suppressed and mixed units; stim A: n = 76; stim B: n = 45), the strength and shape of normalized forward suppression did not differ between the two stimuli (stim A: median remaining response = 0.54 ± 0.33, stim B: median remaining response = 0.55 ± 0.27, rank-sum, P = 0.91, Fig. 3B; stim A: median width = 1.4 ± 1.6 octaves, stim B: median width = 1 ± 0.86 octaves, rank-sum, P = 0.083, Fig. 3C; stim A: median frequency dependence = 0.035 ± 0.082, stim B: median frequency dependence = 0.068 ± 0.083, rank-sum, P = 0.052, Fig. 3D). These similarities indicate that small changes in masker and gap duration do not seem to have large impacts on the prevalence or the characteristics of forward suppression in awake mice. In the following main analyses, units presented with either of the two stimulus types are analyzed together.
Do neuronal firing properties relate to suppression strength?
Could the observed variability in the strength and shape of forward suppression be linked to the underlying properties of the units themselves? In other words, can we predict how suppressed a unit will be based on measurable factors that relate to its basic physiological attributes, such as response latency, maximum evoked firing rate, or spontaneous firing rate?
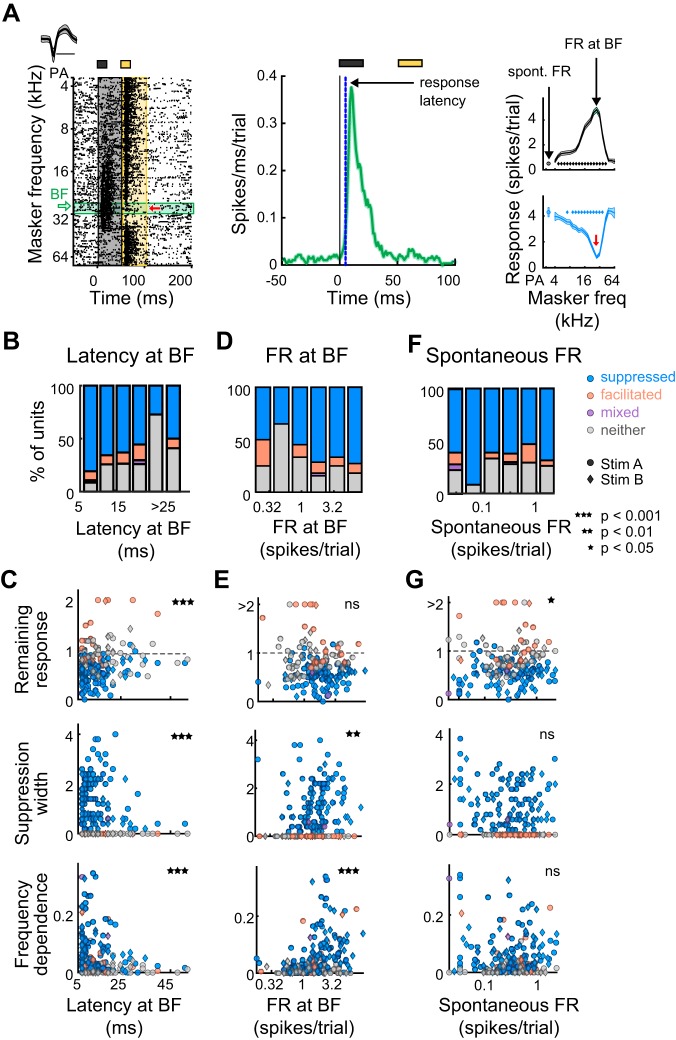
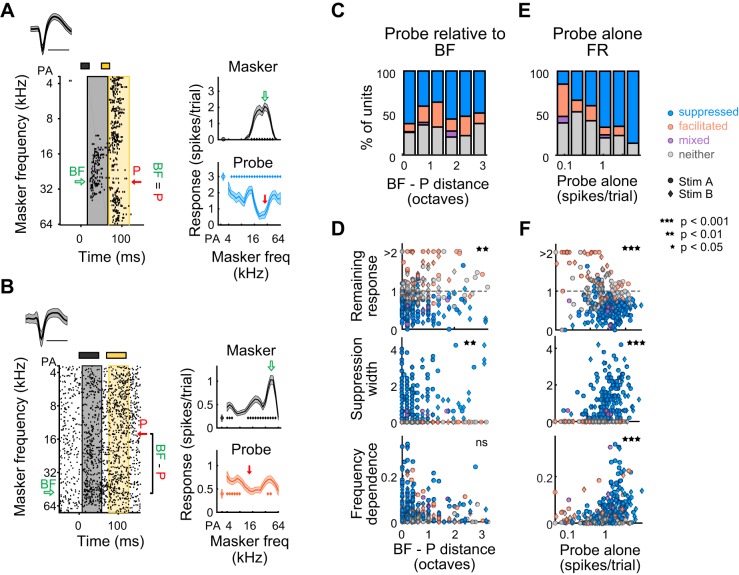
To address this, we measured the relationships between cellular response characteristics and suppression quality, the results of which are summarized in Table 1 (along with later results). We first measured the proportion of units that were suppressed, facilitated, mixed, or unaffected as a function of the latency of response onset at best frequency (BF) (Fig. 4B). Interestingly, the proportion of suppressed vs. nonsuppressed units tended to decrease with increased response onset latency (Cochran-Armitage, P = 0.0032). That is, short latency units were most likely to be forward suppressed. Moreover, response onset latency at BF was significantly correlated with the strength and shape of suppression profiles: specifically, short latency units tended to have lower remaining responses at probe frequency (Spearman correlation P = 0.0015, ρ = 0.26; Fig. 4C, top), broader suppression widths (Spearman correlation P = 0.0087, ρ = −0.28; Fig. 4C, middle), and higher frequency dependences (Spearman correlation P = 0019, ρ = −0.29; Fig. 4C, bottom), indicative of stronger suppression. The proportion of suppressed vs. nonsuppressed units was also related to the evoked firing at BF and tended to increase with increased evoked responses (Cochran-Armitage, P = 0.0079; Fig. 4D). Although evoked firing at BF did not correlate with remaining response at probe frequency (Spearman correlation P = 0.36, ρ = −0.068; Fig. 4E, top) it did positively correlate with suppression width (Spearman correlation P = 0.0014, ρ = 0.23; Fig. 4E, middle) and frequency dependence (Spearman correlation P = 8.3 × 10−9, ρ = 0.4; Fig. 4E, bottom). Spontaneous rate, however, was not related to the proportion of suppressed vs. nonsuppressed units (Cochran-Armitage, P = 0.35; Fig. 4F, top). Additionally, it was only marginally correlated with the strength of forward suppression, as there was a weak trend for units with higher spontaneous rates to have higher remaining responses at probe frequency (Spearman correlation P = 0.026, ρ = 0.16), but there was no relationship with suppression width (Spearman correlation P = 0.31, ρ = −0.075; Fig. 4F, middle) or frequency dependence (Spearman correlation P = 0.42, ρ = 0.059; Fig. 4F, bottom). Thus, although there is diversity in both the type and the shape of sequential interactions, units with shorter response latencies and higher evoked firing rates tend to be forward suppressed more often, and the precise shape of these forward interactions tends to vary based on response latency, evoked firing rates, and spontaneous firing rates. Thus, cortical neurons specialized for responding rapidly and robustly to acoustic events may be more important for detecting changes in ongoing sounds.
Table 1.
Summary of the relationships between quality of forward suppression and unit properties
| Latency at BF | Firing Rate at BF | Spontaneous Firing Rate | Cortical Depth | BF – P Distance | Probe Alone Response | Tuning Width | |
|---|---|---|---|---|---|---|---|
| Proportion trend (Cochran-Armitage test) | †P = 0.0032 | †P = 0.0079 | §P = 0.35 | §P = 0.26 | ‡P = 0.047 | *P = 1.1 × 10−8 | *P = 2.7 × 10−5 |
| Remaining response at probe frequency (Spearman correlation) | †P = 0.0015, ρ = 0.26 | §P = 0.36, ρ = −0.068 | ‡P = 0.026, ρ = 0.16 | §P = 0.82 (Kruskal-Wallis) | †P = 0.0013, ρ = 0.17 | *P = 7.1 × 10−7, ρ = −0.28 | §P = 0.23, ρ = −0.09 |
| Suppression width (Spearman correlation) | †P = 0.0087, ρ = −0.28 | †P = 0.0014, ρ = 0.23 | §P = 0.31, ρ = −0.075 | §P = 0.89 (Kruskal-Wallis) | †P = 0.0019, ρ = −0.17 | *P = 4.7 × 10−12, ρ = 0.38 | *P = 2.7 × 10−6, ρ = 0.33 |
| Frequency dependence (Spearman correlation) | †P = 0.0019, ρ = −0.29 | *P = 8.3 × 10−9, ρ = 0.4 | §P = 0.42, ρ = 0.059 | §P = 0.24 (Kruskal-Wallis) | §P = 0.11, ρ = −0.089 | *P = 1.2 × 10−14, ρ = 0.42 | *P = 0.00024, ρ = 0.26 |
BF, best frequency; BF – P distance, distance of probe from BF. P values in the top row were calculated from Cochran-Armitage tests (a test for trends in proportions) on the proportion of suppressed units, as a function of the variable in each column. Values in the bottom three rows were calculated from Spearman correlations of the suppression metric in each row vs. the variable in each column, except for cortical depth, which was calculated using a Kruskal-Wallis test.
P < 0.001;
P < 0.01;
P < 0.05;
P > 0.05.
Fig. 4.
Latencies, evoked firing rates (FR), and spontaneous firing rates are related to the prevalence and quality of forward suppression. A, left: example single unit’s waveform (means ± SD) and spike raster. Green horizontal bar denotes trials when the masker tone was at the best frequency (BF). Red arrow, probe frequency. Middle: spikes are pooled across responses to the BF (from green trials in left diagram) and binned by 1 ms to create a peri-stimulus time histogram. Response latency is the time when evoked responses reach 3 standard deviations above spontaneous activity. Right: response to the masker and probe as a function of masker frequency. Spontaneous firing rate is defined as the average firing rate during the 50-ms period before the probe alone stimulus, while the firing rate at BF is defined as the average firing rate in the 50-ms period during which the BF is played. B: proportion of suppressed (blue), facilitated (orange), mixed (purple), and neither suppressed nor facilitated units (gray), as a function of response onset latency at BF. C: correlation of response latency with remaining response at probe frequency (top), suppression width (middle), and frequency dependence (bottom) (n = 190). Circles, units presented with stimulus A; diamonds, units presented with stimulus B; blue, suppressed units; orange, facilitated units; purple, mixed units; gray, units with neither effect. D: as B, as a function of evoked firing rate at BF. E: as C. Correlation of firing rate at BF with remaining response at probe frequency (top), frequency dependence (middle), and suppression width (bottom). F: as B, as a function of spontaneous firing rate. G: as C. Correlation of spontaneous firing rate with remaining response at probe frequency (top), suppression width (middle), and frequency dependence (bottom).
Some neurons showed relatively sustained responses to the masker, lasting up until the probe presentation (e.g., Fig. 2C). These prolonged masker responses may relate to differences in how they process rapid sequential stimuli. Indeed, we did find that units with prolonged responses that had not ended by the time of the probe (n = 25) had different suppression characteristics from units whose masker responses had ended by the time of the probe (n = 165). First, they were forward-facilitated more frequently (long-responders: n = 7/25; shorter-responders: n = 10/155; Fisher’s exact test, P = 0.0028); second, they had larger remaining responses at probe frequency [long-responders: median ± interquartile range (IQR) = 0.79 ± 0.35; shorter-responders: median ± IQR = 0.63 ± 0.37; rank-sum, P = 0.01] and larger frequency dependences (long-responders: median ± IQR = 0.061 ± 0.09; shorter-responders: median ± IQR = 0.026 ± 0.064; rank-sum, P = 0.011). These units with long-lasting responses may mediate a complementary form of temporal processing in which the representation of acoustic stimuli is summed over time.
To ensure that the significant correlations between the characteristics of suppression and cellular response properties were not driven by contamination of single units by multiunit “noise,” we identified the highest-quality units, or those with the least likelihood of contamination, by using a simple metric of variance in unit waveform shape: specifically, we measured the average R2 value from correlations across all waveform pairs in a unit cluster. We found that, between high-quality units with average R2 > 0.85 (n = 40) and units with average R2 < 0.85 (n = 150), the distributions of remaining responses at probe frequency (rank-sum P = 0.27), suppression widths (rank-sum, P = 0.66), and frequency dependences (rank-sum, P = 0.94) did not significantly differ, indicating that noise contamination from multiunit activity is not a likely contributor to these results.
Does spike waveform shape predict suppression strength?
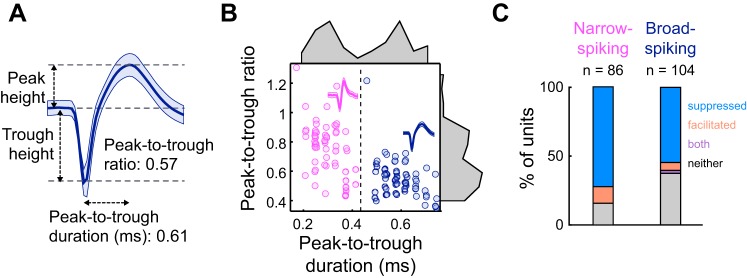
The relationship between suppression strength and certain firing characteristics suggests that certain subtypes of cortical neurons may be more likely to exhibit forward suppression. In particular, neurons with narrow-spiking (NS) and broad-spiking (BS) waveforms have different intrinsic and circuit properties that may enable them to respond to auditory sequences in distinct ways. We addressed whether waveform shape relates to suppression strength by separating units into NS or BS categories based on the bimodal distribution of waveform peak-to-trough durations (NS, <450 μs; BS, ≥450 μs; Fig. 5, A and B), consistent with other groups’ classifications (Cardin et al. 2007; Li et al. 2015; Moore and Wehr 2013; Niell and Stryker 2008). We found that the proportions of suppressed, facilitated, mixed, and unaffected units were significantly different between NS and BS units (Fisher’s exact test, P = 0.013) and, importantly, that the percentage of suppressed NS units (62%, n = 60 of 97 units) was greater than the percentage of suppressed BS units (43%, n = 59 of 137; Fig. 5C). However, of the NS and BS units that showed significant suppression, remaining responses at probe frequency, suppression widths, and frequency dependences were not significantly different (remaining responses: rank-sum, P = 0.71; widths: rank-sum, P = 0.34; frequency dependences: rank-sum, P = 0.67). Thus, although suppressed NS and BS units did not exhibit major differences in suppression shape, NS units more often exhibited suppressive sequential interactions. Given that NS units are generally able to fire rapidly over time without adapting, spike-frequency adaptation mechanisms within NS cells most likely do not contribute to forward suppression in these units; other circuit mechanisms, such as short-term synaptic depression at cortical synapses, spike-frequency adaptation of input neurons, or synaptic inhibition within the cortex, may make larger contributions.
Fig. 5.
Narrow-spiking units exhibit forward suppression more often than broad-spiking units. A: example single unit waveform (means ± SD). Peak-to-trough ratio is the peak height divided by the trough height, while the peak-to-trough duration is the time from the trough height to the peak height. B: peak-to-trough ratio as a function of peak-to-trough duration for all tuned units (n = 190). Narrow-spiking units (magenta) are defined by a peak-to-trough duration of ≤ 0.45 ms. Broad-spiking units (dark blue) are defined by a peak-to-trough duration of >0.45 ms. C: percentage of narrow-spiking (left) and broad-spiking (right) units that are suppressed, facilitated, mixed, or neither suppressed nor facilitated. Percentages are significantly different between narrow-spiking and broad-spiking units (Fischer’s exact test, P = 0.013).
Does the quality of suppression vary across depth or between neighboring units?
Recent work suggests that forward suppression and other history-dependent responses, such as stimulus-specific adaptation, may vary with cortical lamina or depth (Christianson et al. 2011; Li et al. 2014; Szymanski et al. 2009). We recorded the cortical depths of units (n = 115) by subtracting the relative position of the unit on the electrode from the depth that the electrode tip was lowered. We then segmented these units into eight nonoverlapping groups, each spanning 100 μm of the cortical depth. We found that the proportion of suppressed units did not change significantly as a function of depth (Cochran-Armitage, P = 0.26; Fig. 6A). Moreover, neither remaining response at probe frequency, suppression width, nor the frequency dependence of suppression varied as a function of cortical depth (remaining response: Kruskal-Wallis, P = 0.82; Fig. 6B, left; width: Kruskal-Wallis, P = 0.89; Fig. 6C, left; frequency dependence: Kruskal-Wallis, P = 0.24; Fig. 6D, left). Since these metrics of suppression are related to firing rates (Fig. 4), changes in firing rate across depth could influence how suppression strength and shape vary across depth; however, we found that firing rate did not systematically vary across depth (evoked: Kruskal-Wallis, P = 0.73; spontaneous: Kruskal-Wallis, P = 0.86) and therefore likely does not explain these results.
Fig. 6.
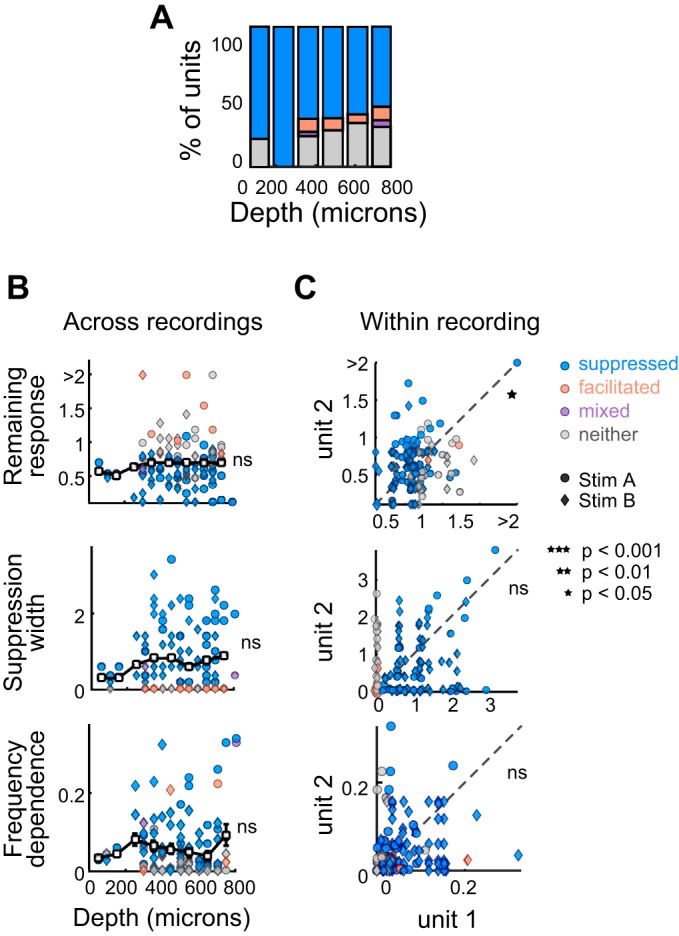
Suppression quality is variable across cortical depth and between neighboring units. A: proportion of suppressed (blue), facilitated (orange), mixed (purple), and neither suppressed nor facilitated units (gray), as a function of cortical depth. B: correlation of cortical depth with remaining response at probe frequency (top), suppression width (middle), and frequency dependence (bottom) for all units whose approximate depth from the cortical surface was recorded (n = 115). Units were divided into 8 nonoverlapping groups, each spanning 100 μm of the cortical depth. The white rectangles and black bars represent means ± SE for these groups. Circles, units presented with stimulus A; diamonds, units presented with stimulus B; blue, suppressed units; orange, facilitated units; purple, mixed units; gray, units with neither effect. C: comparison of remaining responses at probe frequency (top), suppression widths (middle), and frequency dependences (bottom) between neighboring pairs of simultaneously recorded units (n = 137 pairs). The deeper units within pairs are plotted on the x-axis. A unit may be represented more than once if it was recorded at the same time as more than one other unit. Circles, units presented with stimulus A; diamonds, units presented with stimulus B; blue, deeper unit is suppressed; orange, deeper unit is facilitated; purple, deeper unit is mixed; gray, deeper unit shows neither effect. ns, Not significant.
Although the prevalence and quality of forward suppression was heterogeneous across cortical depth, neighboring units recorded simultaneously within a column may share local or external inputs and therefore may be similarly affected by acoustic stimulus history. To examine this, we compared the remaining responses at probe frequency, frequency dependences, and widths of pairs of units (n = 137) recorded simultaneously along the same electrode (inserted roughly orthogonally to the cortical surface). There was a significant, yet weak, relationship between the remaining responses at probe frequency of all unit pairs (Spearman correlation P = 0.016, ρ = 0.21), however there was no significant relationship between suppression widths (Spearman correlation P = 0.25, ρ = 0.1) or frequency dependences (Spearman correlation P = 0.43, ρ = 0.061) of all unit pairs. Overall, neither cortical depth, nor the correlations between units recorded simultaneously, could account for much of the variability in suppression quality across units.
Suppression quality depends on the probe frequency.
In the standard characterization of forward suppression, the frequency of the probe tone is chosen to match the unit’s best frequency (BF). Consistent with this standard paradigm, we have so far presented units for which the probe frequency was chosen to be near (<0.5 octaves from) BF. However, if forward suppression is caused by depression or inhibition of inputs driven by the probe (rather than spike-frequency adaptation in response to the masker) then changing the frequency of the probe, and its distance from the BF (BF – P distance), will alter the quality of forward suppression (Scholes et al. 2011). This does not have to be the case, as forward suppression in the auditory nerve is governed by the response to the masker and would not necessarily be altered by changing the probe frequency. To determine whether BF – P distance influences forward suppression, we measured the proportion of units that were suppressed vs. unsuppressed across BF – P distance for all units (including those for which BF – P distance was greater than 0.5 octaves, n = 323; Fig. 7C). We found that the proportion of suppressed vs. nonsuppressed units varied with BF – P distance (Cochran-Armitage, P = 0.047; Fig. 7C). Also, both the remaining response at probe frequency and suppression width were significantly related to BF – P distance (remaining response: Spearman correlation P = 0.0013, ρ = 0.17; width: Spearman correlation P = 0.0019, ρ = −0.17; frequency dependence: Spearman correlation P = 0.11, ρ = −0.089; Fig. 7D), indicating that, when the probe frequency is dissimilar to the BF, suppression tends to be relatively shallower and narrower.
Fig. 7.
Quality of forward suppression depends on the spectral distance between probe tone and best frequency. A, left: raster of an example unit in which the probe (P) frequency (red arrow) is the same as best frequency (BF, green arrow). Right: In this unit, the response to the probe alone (PA) is relatively strong, and the probe response is broadly and deeply suppressed. B, left: raster of an example unit in which the probe frequency (red arrow) is 1.8 octaves from BF (green arrow). Right: in this unit, the response to the PA is relatively weak, and the probe response is facilitated. C: proportion of suppressed (blue), facilitated (orange), mixed (purple), and neither suppressed nor facilitated units (gray) as a function of BF – P distance. D: correlation of BF – P distance with remaining response at probe frequency (top), suppression width (middle), and frequency dependence (bottom). E: as C, as a function of the PA response. F: as D. Correlation of the PA response with remaining response at probe frequency (top), suppression width (middle), and frequency dependence (bottom).
The strength of the probe alone (PA) response may also influence how well the probe response will be suppressed by a masker tone. We found that the proportion of suppressed units was strongly related to the PA response; units with high PA responses had the highest proportion of suppressed units (Cochran-Armitage, P = 1.1 × 10−8; Fig. 7E). Moreover, the PA response was significantly related to the remaining response at probe frequency, suppression width, and frequency dependence (remaining response: Spearman correlation P = 7.1 × 10−7, ρ = −0.28; width: Spearman correlation P = 4.7 × 10−12, ρ = 0.38; frequency dependence: Spearman correlation P = 1.2 × 10−14, ρ = 0.42; Fig. 7F). Interestingly, units with lower PA responses also exhibited facilitation of the probe response more frequently (Fig. 7E), which may be related to circuit processes such as disinhibition of inhibitory activity or synaptic facilitation.
Relating excitatory tuning profiles to forward suppression profiles.
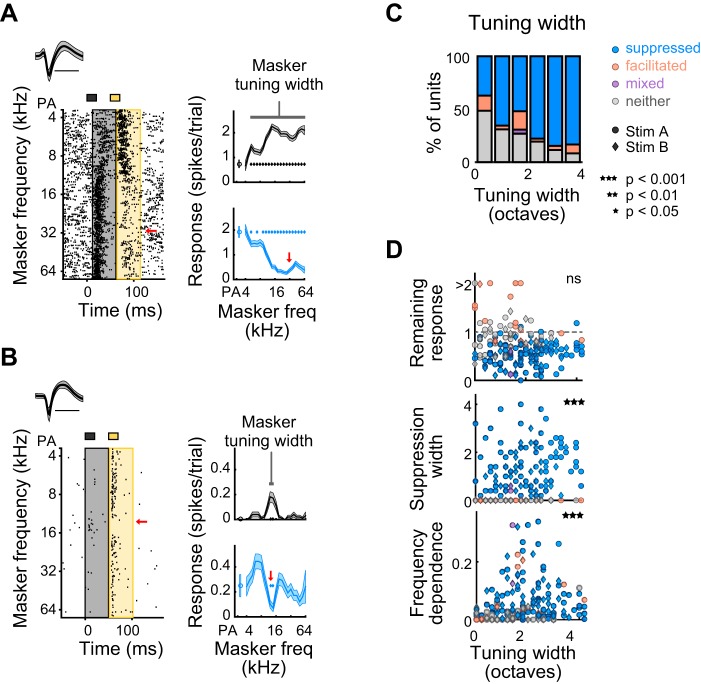
We next sought to determine to what extent the frequency tuning of the response to the masker relates to the strength of probe suppression for those units in which the probe frequency was chosen to be near (<0.5 octaves from) BF. Given that more broadly tuned neurons (Fig. 8A) likely receive broad tonotopic input, forward suppression of the probe response in these neurons may also be spectrally broad, potentially through several mechanisms: short-term depression of these broad inputs, adaptation of the neuron’s spiking response to these broad inputs, or through spectrally broad recruitment of synaptic inhibition by the masker tone. Indeed, tuning width (defined as the range of masker frequencies that elicited a significant response above spontaneous) significantly affected the proportion of suppressed units (Cochran-Armitage, P = 2.7 × 10−5; Fig. 8C); specifically, units with broader tuning bandwidths were more likely to be suppressed. Additionally, although tuning width was not significantly related to remaining response at probe frequency (Spearman correlation P = 0.23, ρ = −0.09; Fig. 8D, top), it was positively related to suppression width (Spearman correlation P = 2.7 × 10-6, ρ = 0.33; Fig. 8D, middle) and frequency dependence (Spearman correlation P = 0.00024, ρ = 0.26; Fig. 8D, bottom).
Fig. 8.
Units with broader frequency tuning show broader suppression. A: an example unit with broad tuning to the masker (measured as the range of masker frequencies, in octaves, that elicits a significant response) whose response to the probe is suppressed by a wide range of masker frequencies. B: an example unit with narrow tuning to the masker whose response to the probe is suppressed by a narrow range of masker frequencies. C: proportion of suppressed (blue), facilitated (orange), mixed (purple), and neither suppressed nor facilitated units (gray), as a function of masker tuning width. D: correlation of tuning width with remaining response at probe frequency (top), suppression width (middle), and frequency dependence (bottom) for all tuned units (n = 190). Circles, units presented with stimulus A; diamonds, units presented with stimulus B; blue, suppressed units; orange, facilitated units; purple, mixed units; gray, units with neither effect.
A strong relationship between tuning width and suppression width could result if a suprathreshold response to the masker tone directly prevents subsequent spiking activity in response to the probe tone, as in the auditory nerve where spike-frequency adaptation plays a key role in forward suppression (Harris and Dallos 1979). To more directly relate the strength of the masker response to the strength of the probe response, for each unit, we fit the probe response, as a function of masker response, to a straight line (Fig. 9B). Because measurement error is inherent to both masker and probe responses, we used reduced major-axis regression (which takes into account measurement error in both variables such that the regression is symmetrical) to fit a straight line to the data. R2 values from the regression indicate how well a linear fit captures the relationship between the masker and probe responses, whereas the slope indicates whether and to what extent the masker and probe responses are inversely or positively related. Counter to the idea that forward suppression in auditory cortex is largely generated by spike-frequency adaptation, most unit responses were not well fit by a straight line, indicated by relatively low R2 values (n = 190, median R2: 0.41 ± 0.48; Fig. 9G, upward and downward bars). Even when only including suppressed/mixed units, masker responses often did not linearly relate to probe responses (n = 121; median R2 = 0.5 ± 0.46; Fig. 9G, upward bars). Moreover, slopes were nearly always greater than −1 (all units: median slope = −0.51 ± 0.41; only suppressed/mixed units: median slope = −0.59 ± 0.36), indicating that the response to the probe was rarely the exact inverse of, and was often shallower than, the masker response (Fig. 9H). These results provide evidence that, in the awake mouse auditory cortex, the response to the masker most likely does not entirely account for the suppression of the probe response. Other potential mechanisms within the cortex, such as synaptic depression (Goudar and Buonomano 2015; May et al. 2015; Wehr and Zador 2005; Yao et al. 2015) or cortical inhibition (Calford and Semple 1995; Chen and Jen 2000; Li et al. 2014; Sutter and Loftus 2003; Zhang et al. 2003), are likely at play.
Fig. 9.
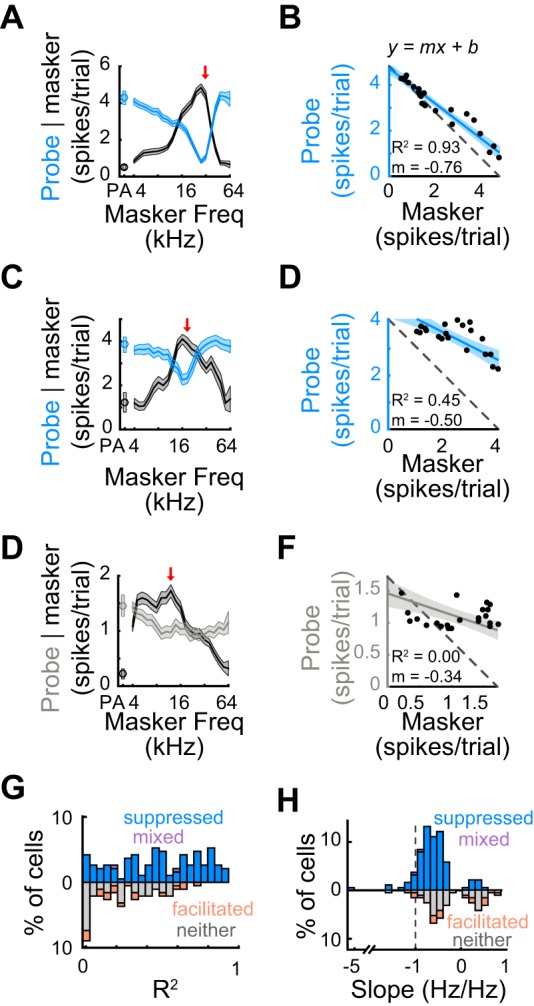
Probe responses often do not linearly relate to masker responses. A: means ± SE of response to the masker (black) and probe (blue) as a function of masker frequency for an example suppressed unit. Red arrow, probe frequency. B: linearly regressing the probe and masker responses reveals whether and to what extent the masker and probe responses are linearly related. In this example unit, an R2 of 0.93 indicates a good linear fit and a slope of −0.76 indicates that the range of probe responses was nearly that of the masker responses. C and D: as A and B. In this suppressed example unit, an R2 of 0.45 indicates a mediocre linear fit and a slope (m) of −0.50 indicates that the range of probe responses was half the range of masker responses. E and F: as A and B. In this example unit with no significant effect on the probe response, an R2 of 0 indicates a poor linear fit and a slope of −0.34 indicates that the range of probe responses was a third of the range of masker responses. G: distribution of R2 values for suppressed units (blue), facilitated units (orange), mixed units (purple), and units with no effect (gray). Most units do not exhibit high R2 values (all units, n = 190: median R2 = 0.41 ± 0.48; only suppressed/mixed units, n = 121: median R2 = 0.5 ± 0.46), indicating that the relationship between the probe responses and masker responses is more complex than a linear model would predict. H: as G, for the distribution of slope values. Median slope values >−1 (all units: median slope = −0.51 ± 0.41; only suppressed/mixed units: median slope = −0.59 ± 0.36) indicate that the probe responses of almost all units have a smaller range than masker responses.
Differences in the ratio of excitation to inhibition contribute to the diversity of forward suppression in a multilayered model.
To gain intuition for how such diverse effects of stimulus history could arise in cortical neurons, we modeled the responses of a cortical output neuron in a multilayered network to masker and probe tone sequences (see materials and methods). Many models of temporal processing in the auditory cortex use short-term synaptic plasticity to explain changes in auditory responses based on acoustic context (David et al. 2009; May et al. 2015); for instance, it has been proposed that short-term plasticity at excitatory and inhibitory synapses might cause changes in the balance of excitation and inhibition (E/I ratio) that will be particularly important for explaining history-dependent processing (Goudar and Buonomano 2015). Thus, we hypothesized that cellular variability in E/I ratio, as has been observed in vivo and in vitro (Haider et al. 2006; Shu et al. 2003; Wehr and Zador 2003), may lead to the diversity of history-dependent responses we observed, and we explored this possibility in our model.
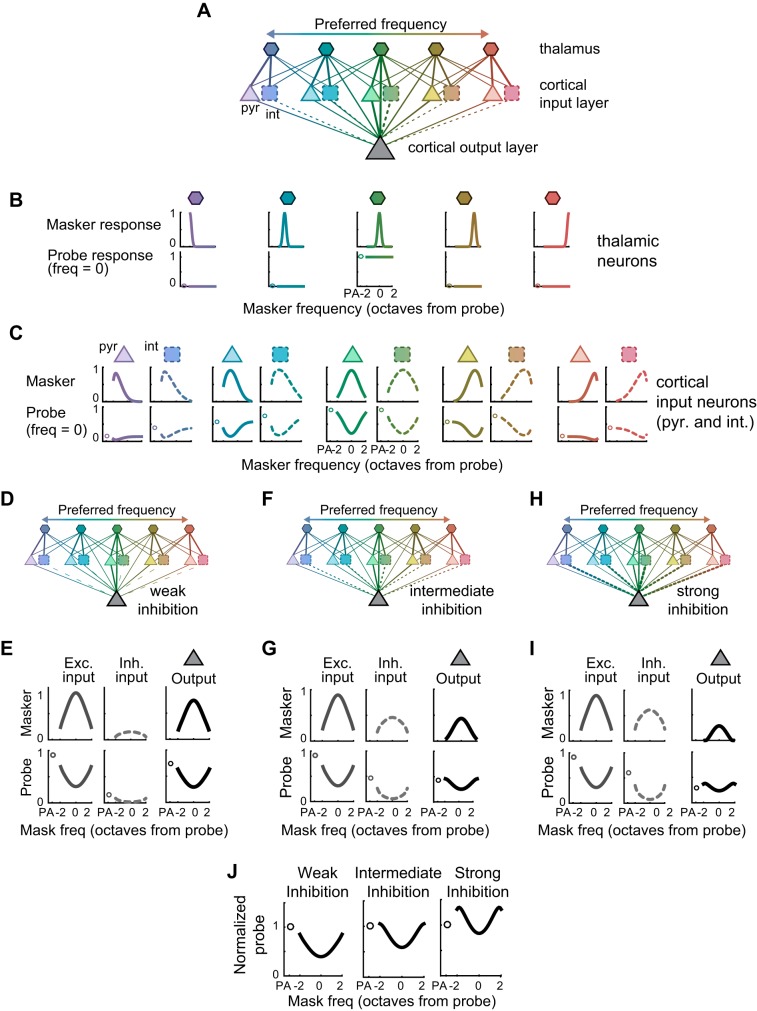
In the first layer of the model (Fig. 10A, top row) narrowly tuned thalamic neurons respond robustly, and without suppression, to the probe tone when the probe frequency is within their receptive fields (Fig. 10B, bottom row). This allowed us to isolate the contributions of potential cortical mechanisms from the effects of forward suppression inherited from subcortical structures [although it is known to be present within the thalamus (Schreiner 1981)]. Thalamic neurons then synapse onto cortical excitatory pyramidal neurons and cortical inhibitory interneurons in the second layer (Fig. 10A, middle row). In the model, as in the real auditory cortex, a wider range of thalamic neurons synapse onto interneurons [as observed in vitro (Cruikshank et al. 2007)], such that the interneurons’ responses to tones are broader than their pyramidal neighbors [as observed in vivo (Atencio and Schreiner 2008; Li et al. 2015); Fig. 10C, top row]. To model the effects of short-term synaptic plasticity, we modeled these thalamic synapses as dynamic, such that they instantaneously depress following a masker tone by an amount proportional to the strength of the masker response. Thus, the second-layer cortical neurons’ responses to the probe tone are suppressed following a masker tone (Fig. 10C, bottom row). Both the first-layer and second-layer neurons then synapse onto the cortical output neuron in the third layer, also with depressing connections, such that the net excitatory and net inhibitory inputs to the cortical output neuron exhibit forward suppression (Fig. 10E, left, middle). Overall, the result of synaptic depression in the network is robust forward suppression of the cortical output neuron (Fig. 10E, right).
Fig. 10.
Changing the ratio of excitation and inhibition alters forward suppression in a network with synaptic depression. A: schematic of the linear threshold model containing three layers of neurons: “thalamic” neurons (hexagons) in the first layer, excitatory “pyramidal” neurons (triangles) and inhibitory “interneurons” (squares) in the second layer, and a “cortical output” neuron (big triangle) in the third layer. The frequency preference of each neuron is indicated by its color. B, top: the responses of each thalamic neuron to masker tones, as a function of masker frequency (in units of octaves from the probe frequency). Bottom: the responses of each thalamic neuron to the probe alone (PA; circles), and to the probe tone as a function of masker frequency (lines). Thalamic neurons with the probe frequency in their receptive fields (e.g., middle, green) respond to the probe tone, but these responses are not forward suppressed. C: as B, for the pyramidal neurons and interneurons in the second layer, which receive depressing inputs from the thalamic neurons. Interneurons are more broadly tuned than pyramidal neurons and therefore often have higher responses to the PA. D: schematic of a network with weak inhibitory synaptic connections onto the cortical output neuron. E, left: the cortical output neuron receives tuned excitation in response to the masker, and depressed excitation in response to the probe. Middle: the cortical output neuron receives tuned, but weak, inhibition in response to the masker, and depressed and weak inhibition in response to the probe. Right: the net output of the cortical output neuron shows relatively strong forward suppression. F and G: as D and E, with intermediate inhibitory synaptic strength. H and I: as F and G, with strong inhibitory synaptic connections. J: normalized suppression curves of the cortical output neuron with either weak (left), intermediate (middle), or strong (right) inhibitory synaptic strength show strong forward suppression (left), weak forward suppression (middle), or a combination of forward suppression with forward facilitation (right).
To explore the contribution of synaptic inhibition, and E/I balance, to the diversity of forward effects observed in the data, we altered the synaptic weights of the inhibitory connections between the second-layer interneurons and the third-layer cortical output neuron (while keeping constant the weights of excitatory connections; see materials and methods). In a model with weak inhibitory connections (Fig. 10D), the inhibitory input to the output neuron is scaled down. Thus, in response to the probe, inhibition is weak and flat across tone frequency (Fig. 10E, middle), resulting in forward suppression that appears similar to that mediated purely by synaptic depression of excitatory synapses (Fig. 10E, left). In a model with intermediate inhibitory connections (Fig. 10F), the net inhibitory input to the cortical output neuron is scaled up, so that, in response to the probe, inhibition is stronger at the edges of the receptive field and weaker in the center (Fig. 10G, middle). This reduces the probe alone response relative to the rest of the probe responses, resulting in relatively weaker forward suppression (Fig. 10, G, right, and J, middle). Further strengthening the relative weight of synaptic inhibition increases the inhibitory input in response to the probe even more strongly at the edges of the receptive field (Fig. 10I, middle). This strong lateral inhibition suppresses the probe alone response relative to the other probe responses, resulting in relative facilitation at the edges of the suppression curve (Fig. 10, I, right, and J, right). Thus, cell-to-cell differences in the relative strengths of synaptic inhibition and excitation can produce some of the diverse effects observed in our data, from broad and deep suppression, to facilitation.
DISCUSSION
We characterized history-dependent responses in waking mice, using sequential, forward suppression stimuli with relatively short (<30 ms) gaps. We find that although 80–90% of neurons within the auditory cortex of waking mice exhibit tone responses, only ~60% of neurons exhibit robust forward suppression at these short intervals, and ~10% exhibit forward facilitation. We also find that how responses are modulated by a prior tone depends on multiple factors, including the basic physiological properties of the unit, in addition to stimulus parameters. We use a multilayered network model to demonstrate that some of the diversity in the quality of forward suppression can be explained by cellular differences in the relative strengths of excitation and inhibition. Here, we discuss how these results fit in with the existing literature on the nature of history-dependent effects and their underlying mechanisms in the auditory system.
Prevalence of forward suppression.
That only 70% of units’ probe responses significantly depend on the presence of the masker tone differs substantially from previous studies, which often report that nearly all recorded units’ responses exhibit forward suppression to some extent. For example, Brosch and Schreiner (1997), who recorded in anesthetized cats, reported a significant suppressive effect of masker tones on probe responses in 107 out of 111 units, and a facilitating effect in the other 4 units. Other studies conducted in anesthetized rats reported that all or most unit responses were suppressed by a masker stimulus when the interval between masker and probe was less than 128 ms (Scholl et al. 2008; Wehr and Zador 2005), and at longer intervals nearly half were facilitated (Wehr and Zador 2005). One factor that may explain much of this discrepancy is the anesthetic state of the animal: Pentobarbital anesthesia, used by Brosch and Schreiner (1997) and Calford and Semple (1995), enhances the sensitivity of GABAa receptors (MacDonald et al. 1989; Nicoll 1975) and decreases spontaneous activity (Antkowiak 1999; Gaese and Ostwald 2001; Zurita et al. 1994), potentially extending the time course and increasing the strength of stimulus-recruited synaptic inhibition (Tan et al. 2004; Wehr and Zador 2005). Ketamine anesthesia, used by Wehr and Zador (2005), blocks excitation through N-methyl-d-aspartate receptors, decreases spontaneous activity, and reduces frequency-tuning bandwidths (Anis et al. 1983; Antkowiak 1999; Zurita et al. 1994). On the other hand, during waking states, high background synaptic activity and spontaneous firing rates (Constantinople and Bruno 2011; Haider and McCormick 2009; Steriade et al. 2001) may place the cortex in a preadapted state in which sensory adaptation does not occur, or is attenuated (Castro-Alamancos 2004). In addition to higher background synaptic activity, waking states within auditory cortex are associated with sustained responses (Wang et al. 2005), which may also contribute to weaker forward suppression, or facilitation as we observed here.
Furthermore, dynamic fluctuations in animals’ arousal levels may influence how context or stimulus history affects auditory responses. For example, in somatosensory cortex, both history-dependent suppression and facilitation of whisker responses are diminished during active whisking compared with passive sensing (Castro-Alamancos and Connors 1996; Fanselow and Nicolelis 1999). In future studies, monitoring arousal level through pupil dilation or hippocampal sharp wave activity (McGinley et al. 2015; Reimer et al. 2014; Vinck et al. 2015) will inform how forward suppression and other context-dependent interactions are modulated by the internal state of the animal.
Technical differences between the current and previous studies may also contribute to the relatively low rate of forward suppressed units in our recordings. For our recordings we slowly lowered a 16-channel linear probe into the auditory cortex and allowed it to settle in one position for the duration of an experiment (~1 h). This technique allowed us to isolate units with both low and high firing rates from all cortical layers in a relatively unbiased fashion. This is in contrast to traditional single-electrode recordings, in which single units whose spikes can be easily isolated from multiunit activity (generally units with high firing rates) are targeted for recording. Because units with higher evoked firing rates were more likely to exhibit strong suppression, our unbiased sampling of both low and high firing units may have allowed us to uncover a higher proportion of units without significant forward suppression.
Diversity of forward suppression.
In addition to the relatively lower prevalence of forward suppression, we also found that the quality of forward suppression is highly variable and depends on the innate response properties of the units. For example, units with low response latencies, high evoked firing rates, narrow spikes, and broad tuning profiles are more likely to be suppressed by a preceding tone; on the other hand, units with long response durations are more likely to be facilitated by a preceding tone. These results are in line with Brosch and Schreiner (1997), who found weak, but significant correlations between both response latency and tuning bandwidth and the duration and extent of forward interactions. That certain cellular traits are associated with both qualitative and quantitative aspects of forward suppression suggests that encoding of short temporal sequences may be distributed across a range of cell types or subgroups; some cells do not exhibit forward suppression, respond to multiple sounds in quick succession, and thus have high temporal resolution, whereas others exhibit robust forward suppression, integrating sounds over longer durations. Since neurons in cat and primate auditory cortex are topographically organized along the iso-frequency contours of the primary auditory cortex according to specific response features, such as latency and bandwidth, neurons that differentially specialize in temporal processing may also exhibit topographic organization (Atencio and Schreiner 2012). Whether such topography of temporal coding exists in smaller species, such as rats and mice, is a question for future experiments; however, the variable shape of forward suppression that we observed along a single electrode array (Fig. 6C) suggests otherwise. This variability in temporal coding between neurons may allow the cortex to simultaneously represent different features of natural sounds that fluctuate across multiple time scales.
Not only are suppression strength and shape affected by properties of the units themselves, but we also found that the extent of forward suppression depends on the frequency of the probe tone with respect to the best frequency of the unit: as the probe frequency deviates from the best frequency, the response to the probe alone decreases and suppression of the probe response weakens. These results conflict with recent studies that found that, as the probe alone response decreases, either when the probe frequency is off best frequency (Scholes et al. 2011) or when the probe level is low (Scholl et al. 2008), suppression strength increases. This discrepancy may be explained by the use of ketamine in those studies, which reduces tonic spontaneous firing rates. Since spontaneous firing rates under ketamine are usually very low (close to 0), the range over which probe responses can be suppressed is as large as the probe response itself; even relatively low responses can be significantly suppressed. However, in our awake preparation in which spontaneous activity was substantially higher, the spontaneous activity seemed to act as a floor below which probe responses were rarely suppressed. Thus, the anesthetic or behavioral state of the animal, and how it impacts spontaneous activity, may influence the readout of suppression.
Potential mechanisms of forward suppression.
A longstanding question in sensory neuroscience is how history-dependent effects arise within the cortex. Within the somatosensory field, one view is that history-dependent suppression at the level of the cortex is simply an amplification of suppression at subcortical levels imposed by the nonlinear transformation from synaptic input to spike output (Higley and Contreras 2007). While this may be one factor, studies suggest that multiple other mechanisms do contribute, including spike-frequency adaptation within cortical neurons (Abolafia et al. 2011), cortical synaptic inhibition (Brosch and Schreiner 1997; Calford and Semple 1995; Li et al. 2014; Zhang et al. 2003), and synaptic depression at thalamo- and cortico-cortical synapses (Bayazitov et al. 2013; David et al. 2009; Goudar and Buonomano 2015; May et al. 2015; Wehr and Zador 2005; Yao et al. 2015). The results from this study support the notion that spike-frequency adaptation is not a large contributor to forward suppression. Unlike spike-frequency adaptation in the auditory nerve, in which the strength of the masker response linearly corresponds to the strength of probe suppression (Harris and Dallos 1979), the relationship between the masker response and the suppression of the probe response in our data was often nonlinear, as has also been observed in the auditory thalamus (Schreiner 1981). Moreover, we found that narrow-spiking units [in which afterhyperpolarizations are quite brief (Rudy and McBain 2001) and relative refractory periods are modest (Hasenstaub et al. 2005)] are the most likely to exhibit forward suppression. Thus, suppressive mechanisms that do not necessarily directly track the firing rate of the output neuron—for instance, synaptic depression or inhibition of its inputs—may also be required to explain the diverse strength and variable shapes of forward suppression within the cortex.
In a network model similar to ours, with synaptic depression at both excitatory and inhibitory synapses (Goudar and Buonomano 2015), it has been shown that short-term synaptic dynamics account for forward interactions by differentially altering the ratio of inhibition and excitation. This is supported by work in vivo showing that sensory adaptation induced by repeated sensory stimulation alters E/I balance (Heiss et al. 2008), and that E/I balance changes from active network states, in which synapses are depressed, to quiet states, in which synapses are relieved from depression (Taub et al. 2013). As a complement to this, in our multilayered model, we find that cell-to-cell differences in the relative strength of inhibition vs. excitation produce both qualitative and quantitative variability in cortical responses to sequential stimuli: neurons in which inhibition strongly dominates excitation show more forward facilitation, while in neurons in which inhibition is weaker, forward interactions more closely track the forward suppression implemented by short-term synaptic depression at excitatory synapses. This is consistent with many experimental observations. While excitation and inhibition are often continuously synchronized such that fluctuations in one co-occur with proportional fluctuations in the other [both during spontaneous and sensory-evoked activity (Okun and Lampl 2008; Vogels et al. 2005)], the exact proportionality of excitation and inhibition varies from cell to cell; this is true during spontaneous activity, in both cortical slices (Shu et al. 2003) and in vivo (Haider et al. 2006), as well as in responses to sensory stimulation in the visual (Anderson et al. 2000) and auditory cortices (Wehr and Zador 2003). These differences in the exact excitatory-inhibitory balance between cortical cells may be one of many biologically plausible mechanisms by which the cortex can generate variable representations of temporally complex stimuli, which may be important for resolving numerous temporal features of ongoing sound such as speech. Multiple mechanisms, other than E/I balance, may also be important for generating long-lasting, facilitative responses observed in the data.
Importantly, the synaptic inhibition that balances excitation is not mediated by a homogenous population; rather multiple inhibitory cell types with remarkably different gene expression, morphology, and synaptic connectivity cooperate to shape the activity of auditory cortex in various ways, depending on the state of the network (Phillips and Hasenstaub 2016; Seybold et al. 2015). Which interneurons might mediate forward suppression is not yet clear, but the careful use of cell-type-specific tools, such as optogenetics, may allow us to probe their specific roles in generating diverse representations of temporally complex stimuli.
GRANTS
Research was supported by National Institutes of Health National Institute on Deafness and Other Communication Disorders Grants RO1 DC014101 (A. R. Hasenstaub) and NIH R01 DC02260 (C. E. Schreiner); Hearing Research Inc. (San Francisco); the Klingenstein Foundation; and the J. C. and E. Coleman Memorial Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.A.K.P., C.E.S., and A.R.H. conceived and designed research; E.A.K.P. performed experiments; E.A.K.P. and A.R.H. analyzed data; E.A.K.P., C.E.S., and A.R.H. interpreted results of experiments; E.A.K.P., C.E.S., and A.R.H. prepared figures; E.A.K.P. drafted manuscript; E.A.K.P., C.E.S., and A.R.H. edited and revised manuscript; E.A.K.P., C.E.S., and A.R.H. approved final version of manuscript.
REFERENCES
- Abolafia JM, Vergara R, Arnold MM, Reig R, Sanchez-Vives MV. Cortical auditory adaptation in the awake rat and the role of potassium currents. Cereb Cortex 21: 977–990, 2011. doi: 10.1093/cercor/bhq163. [DOI] [PubMed] [Google Scholar]
- Allman J, Miezin F, McGuinness E. Stimulus specific responses from beyond the classical receptive field: neurophysiological mechanisms for local-global comparisons in visual neurons. Annu Rev Neurosci 8: 407–430, 1985. doi: 10.1146/annurev.ne.08.030185.002203. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Carandini M, Ferster D. Orientation tuning of input conductance, excitation, and inhibition in cat primary visual cortex. J Neurophysiol 84: 909–926, 2000. [DOI] [PubMed] [Google Scholar]
- Anis NA, Berry SC, Burton NR, Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol 79: 565–575, 1983. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antkowiak B. Different actions of general anesthetics on the firing patterns of neocortical neurons mediated by the GABA(A) receptor. Anesthesiology 91: 500–511, 1999. doi: 10.1097/00000542-199908000-00025. [DOI] [PubMed] [Google Scholar]
- Atencio CA, Schreiner CE. Spectrotemporal processing differences between auditory cortical fast-spiking and regular-spiking neurons. J Neurosci 28: 3897–3910, 2008. doi: 10.1523/JNEUROSCI.5366-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atencio CA, Schreiner CE. Spectrotemporal processing in spectral tuning modules of cat primary auditory cortex. PLoS One 7: e31537, 2012. doi: 10.1371/journal.pone.0031537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett EL, Wang X. Long-lasting modulation by stimulus context in primate auditory cortex. J Neurophysiol 94: 83–104, 2005. doi: 10.1152/jn.01124.2004. [DOI] [PubMed] [Google Scholar]
- Bayazitov IT, Westmoreland JJ, Zakharenko SS. Forward suppression in the auditory cortex is caused by the Ca(v)3.1 calcium channel-mediated switch from bursting to tonic firing at thalamocortical projections. J Neurosci 33: 18940–18950, 2013. doi: 10.1523/JNEUROSCI.3335-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher FA, Salvi RJ, Saunders SS. Recovery from short-term adaptation in single neurons in the cochlear nucleus. Hear Res 48: 125–144, 1990. doi: 10.1016/0378-5955(90)90203-2. [DOI] [PubMed] [Google Scholar]
- Bregman AS. Auditory Scene Analysis. The Perceptual Organization of Sound. Cambridge, MA: MIT Press, 1990. [Google Scholar]
- Brosch M, Schreiner CE. Time course of forward masking tuning curves in cat primary auditory cortex. J Neurophysiol 77: 923–943, 1997. [DOI] [PubMed] [Google Scholar]
- Brosch M, Schreiner CE. Sequence sensitivity of neurons in cat primary auditory cortex. Cereb Cortex 10: 1155–1167, 2000. doi: 10.1093/cercor/10.12.1155. [DOI] [PubMed] [Google Scholar]
- Calabrese A, Paninski L. Kalman filter mixture model for spike sorting of non-stationary data. J Neurosci Methods 196: 159–169, 2011. doi: 10.1016/j.jneumeth.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Calford MB, Semple MN. Monaural inhibition in cat auditory cortex. J Neurophysiol 73: 1876–1891, 1995. [DOI] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ, Senn W. A synaptic explanation of suppression in visual cortex. J Neurosci 22: 10053–10065, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Palmer LA, Contreras D. Stimulus feature selectivity in excitatory and inhibitory neurons in primary visual cortex. J Neurosci 27: 10333–10344, 2007. doi: 10.1523/JNEUROSCI.1692-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Absence of rapid sensory adaptation in neocortex during information processing states. Neuron 41: 455–464, 2004. doi: 10.1016/S0896-6273(03)00853-5. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Connors BW. Short-term plasticity of a thalamocortical pathway dynamically modulated by behavioral state. Science 272: 274–277, 1996. doi: 10.1126/science.272.5259.274. [DOI] [PubMed] [Google Scholar]
- Chen QC, Jen PH. Bicuculline application affects discharge patterns, rate-intensity functions, and frequency tuning characteristics of bat auditory cortical neurons. Hear Res 150: 161–174, 2000. doi: 10.1016/S0378-5955(00)00197-0. [DOI] [PubMed] [Google Scholar]
- Christianson GB, Sahani M, Linden JF. Depth-dependent temporal response properties in core auditory cortex. J Neurosci 31: 12837–12848, 2011. doi: 10.1523/JNEUROSCI.2863-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Li X, Nelson SB. Short-term depression at thalamocortical synapses contributes to rapid adaptation of cortical sensory responses in vivo. Neuron 34: 437–446, 2002. doi: 10.1016/S0896-6273(02)00659-1. [DOI] [PubMed] [Google Scholar]
- Constantinople CM, Bruno RM. Effects and mechanisms of wakefulness on local cortical networks. Neuron 69: 1061–1068, 2011. doi: 10.1016/j.neuron.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt O, Hellweg FC, Schreiner C. Thalamocortical transformation of responses to complex auditory stimuli. Exp Brain Res 39: 87–104, 1980. doi: 10.1007/BF00237072. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci 10: 462–468, 2007. doi: 10.1038/nn1861. [DOI] [PubMed] [Google Scholar]
- David SV, Mesgarani N, Fritz JB, Shamma SA. Rapid synaptic depression explains nonlinear modulation of spectro-temporal tuning in primary auditory cortex by natural stimuli. J Neurosci 29: 3374–3386, 2009. doi: 10.1523/JNEUROSCI.5249-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Quesada M, Maravall M. Intrinsic mechanisms for adaptive gain rescaling in barrel cortex. J Neurosci 28: 696–710, 2008. doi: 10.1523/JNEUROSCI.4931-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow EE, Nicolelis MA. Behavioral modulation of tactile responses in the rat somatosensory system. J Neurosci 19: 7603–7616, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman YI, Arezzo JC, Steinschneider M. Auditory stream segregation in monkey auditory cortex: effects of frequency separation, presentation rate, and tone duration. J Acoust Soc Am 116: 1656–1670, 2004. doi: 10.1121/1.1778903. [DOI] [PubMed] [Google Scholar]
- Fishman YI, Reser DH, Arezzo JC, Steinschneider M. Neural correlates of auditory stream segregation in primary auditory cortex of the awake monkey. Hear Res 151: 167–187, 2001. doi: 10.1016/S0378-5955(00)00224-0. [DOI] [PubMed] [Google Scholar]
- Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature 367: 607–614, 1994. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- Gaese BH, Ostwald J. Anesthesia changes frequency tuning of neurons in the rat primary auditory cortex. J Neurophysiol 86: 1062–1066, 2001. [DOI] [PubMed] [Google Scholar]
- Goudar V, Buonomano DV. A model of order-selectivity based on dynamic changes in the balance of excitation and inhibition produced by short-term synaptic plasticity. J Neurophysiol 113: 509–523, 2015. doi: 10.1152/jn.00568.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood DD. Critical bandwidth and the frequency coordinates of the basilar membrane. J Acoust Soc Am 33: 1344–1356, 1961. doi: 10.1121/1.1908437. [DOI] [Google Scholar]
- Haider B, Duque A, Hasenstaub AR, McCormick DA. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci 26: 4535–4545, 2006. doi: 10.1523/JNEUROSCI.5297-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider B, McCormick DA. Rapid neocortical dynamics: cellular and network mechanisms. Neuron 62: 171–189, 2009. doi: 10.1016/j.neuron.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Harris RA. The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analg 94: 313–318, 2002. doi: 10.1213/00000539-200202000-00015. [DOI] [PubMed] [Google Scholar]
- Harris DM, Dallos P. Forward masking of auditory nerve fiber responses. J Neurophysiol 42: 1083–1107, 1979. [DOI] [PubMed] [Google Scholar]
- Hasenstaub A, Shu Y, Haider B, Kraushaar U, Duque A, McCormick DA. Inhibitory postsynaptic potentials carry synchronized frequency information in active cortical networks. Neuron 47: 423–435, 2005. doi: 10.1016/j.neuron.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Heiss JE, Katz Y, Ganmor E, Lampl I. Shift in the balance between excitation and inhibition during sensory adaptation of S1 neurons. J Neurosci 28: 13320–13330, 2008. doi: 10.1523/JNEUROSCI.2646-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Contreras D. Cellular mechanisms of suppressive interactions between somatosensory responses in vivo. J Neurophysiol 97: 647–658, 2007. doi: 10.1152/jn.00777.2006. [DOI] [PubMed] [Google Scholar]
- Ingham NJ, Itatani N, Bleeck S, Winter IM. Enhancement of forward suppression begins in the ventral cochlear nucleus. Brain Res 1639: 13–27, 2016. doi: 10.1016/j.brainres.2016.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwada S, Batra R, Stanford TR. Monaural and binaural response properties of neurons in the inferior colliculus of the rabbit: effects of sodium pentobarbital. J Neurophysiol 61: 269–282, 1989. [DOI] [PubMed] [Google Scholar]
- Li LY, Ji XY, Liang F, Li YT, Xiao Z, Tao HW, Zhang LI. A feedforward inhibitory circuit mediates lateral refinement of sensory representation in upper layer 2/3 of mouse primary auditory cortex. J Neurosci 34: 13670–13683, 2014. doi: 10.1523/JNEUROSCI.1516-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LY, Xiong XR, Ibrahim LA, Yuan W, Tao HW, Zhang LI. Differential receptive field properties of parvalbumin and somatostatin inhibitory neurons in mouse auditory cortex. Cereb Cortex 25: 1782–1791, 2015. doi: 10.1093/cercor/bht417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald RL, Rogers CJ, Twyman RE. Barbiturate regulation of kinetic properties of the GABAA receptor channel of mouse spinal neurones in culture. J Physiol 417: 483–500, 1989. doi: 10.1113/jphysiol.1989.sp017814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone BJ, Semple MN. Effects of auditory stimulus context on the representation of frequency in the gerbil inferior colliculus. J Neurophysiol 86: 1113–1130, 2001. [DOI] [PubMed] [Google Scholar]
- Massaux A, Dutrieux G, Cotillon-Williams N, Manunta Y, Edeline JM. Auditory thalamus bursts in anesthetized and non-anesthetized states: contribution to functional properties. J Neurophysiol 91: 2117–2134, 2004. doi: 10.1152/jn.00970.2003. [DOI] [PubMed] [Google Scholar]
- May PJ, Westö J, Tiitinen H. Computational modelling suggests that temporal integration results from synaptic adaptation in auditory cortex. Eur J Neurosci 41: 615–630, 2015. doi: 10.1111/ejn.12820. [DOI] [PubMed] [Google Scholar]
- McGinley MJ, David SV, McCormick DA. Cortical membrane potential signature of optimal states for sensory signal detection. Neuron 87: 179–192, 2015. doi: 10.1016/j.neuron.2015.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LM, Escabí MA, Read HL, Schreiner CE. Spectrotemporal receptive fields in the lemniscal auditory thalamus and cortex. J Neurophysiol 87: 516–527, 2002. doi: 10.1152/jn.00395.2001. [DOI] [PubMed] [Google Scholar]
- Moore AK, Wehr M. Parvalbumin-expressing inhibitory interneurons in auditory cortex are well-tuned for frequency. J Neurosci 33: 13713–13723, 2013. doi: 10.1523/JNEUROSCI.0663-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelken I. Stimulus-specific adaptation and deviance detection in the auditory system: experiments and models. Biol Cybern 108: 655–663, 2014. doi: 10.1007/s00422-014-0585-7. [DOI] [PubMed] [Google Scholar]
- Nelson PC, Smith ZM, Young ED. Wide-dynamic-range forward suppression in marmoset inferior colliculus neurons is generated centrally and accounts for perceptual masking. J Neurosci 29: 2553–2562, 2009. doi: 10.1523/JNEUROSCI.5359-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA. Pentobarbital: action on frog motoneurons. Brain Res 96: 119–123, 1975. doi: 10.1016/0006-8993(75)90582-X. [DOI] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Highly selective receptive fields in mouse visual cortex. J Neurosci 28: 7520–7536, 2008. doi: 10.1523/JNEUROSCI.0623-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65: 472–479, 2010. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun M, Lampl I. Instantaneous correlation of excitation and inhibition during ongoing and sensory-evoked activities. Nat Neurosci 11: 535–537, 2008. doi: 10.1038/nn.2105. [DOI] [PubMed] [Google Scholar]
- Phillips EA, Hasenstaub AR. Asymmetric effects of activating and inactivating cortical interneurons. eLife 5: e18383, 2016. doi: 10.7554/eLife.18383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recio A, Rich NC, Narayan SS, Ruggero MA. Basilar-membrane responses to clicks at the base of the chinchilla cochlea. J Acoust Soc Am 103: 1972–1989, 1998. doi: 10.1121/1.421377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer J, Froudarakis E, Cadwell CR, Yatsenko D, Denfield GH, Tolias AS. Pupil fluctuations track fast switching of cortical states during quiet wakefulness. Neuron 84: 355–362, 2014. doi: 10.1016/j.neuron.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci 24: 517–526, 2001. doi: 10.1016/S0166-2236(00)01892-0. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, Nowak LG, McCormick DA. Membrane mechanisms underlying contrast adaptation in cat area 17 in vivo. J Neurosci 20: 4267–4285, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholes C, Palmer AR, Sumner CJ. Forward suppression in the auditory cortex is frequency-specific. Eur J Neurosci 33: 1240–1251, 2011. doi: 10.1111/j.1460-9568.2010.07568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl B, Gao X, Wehr M. Level dependence of contextual modulation in auditory cortex. J Neurophysiol 99: 1616–1627, 2008. doi: 10.1152/jn.01172.2007. [DOI] [PubMed] [Google Scholar]
- Schreiner C. Post-stimulatory effects in the medial geniculate body of guinea pigs. In: Neuronal Mechanisms of Hearing, edited by Syka J, Aitkin L. New York: Plenum Press, 1981, p. 191–196. doi: 10.1007/978-1-4684-3908-3_20. [DOI] [Google Scholar]
- Schreiner CE, Read HL, Sutter ML. Modular organization of frequency integration in primary auditory cortex. Annu Rev Neurosci 23: 501–529, 2000. doi: 10.1146/annurev.neuro.23.1.501. [DOI] [PubMed] [Google Scholar]
- Seybold BA, Phillips EA, Schreiner CE, Hasenstaub AR. Inhibitory actions unified by network integration. Neuron 87: 1181–1192, 2015. doi: 10.1016/j.neuron.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore SE. Recovery of forward-masked responses in ventral cochlear nucleus neurons. Hear Res 82: 31–43, 1995. doi: 10.1016/0378-5955(94)00160-R. [DOI] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature 423: 288–293, 2003. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- Simons DJ. Temporal and spatial integration in the rat SI vibrissa cortex. J Neurophysiol 54: 615–635, 1985. [DOI] [PubMed] [Google Scholar]
- Smith RL. Short-term adaptation in single auditory nerve fibers: some poststimulatory effects. J Neurophysiol 40: 1098–1111, 1977. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: The Principles and Practice of Statistics in Biological Research (4th ed.). New York: W. H. Freeman, 2012. [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol 85: 1969–1985, 2001. [DOI] [PubMed] [Google Scholar]
- Sutter ML, Loftus WC. Excitatory and inhibitory intensity tuning in auditory cortex: evidence for multiple inhibitory mechanisms. J Neurophysiol 90: 2629–2647, 2003. doi: 10.1152/jn.00722.2002. [DOI] [PubMed] [Google Scholar]
- Syka J, Suta D, Popelár J. Responses to species-specific vocalizations in the auditory cortex of awake and anesthetized guinea pigs. Hear Res 206: 177–184, 2005. doi: 10.1016/j.heares.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Szymanski FD, Garcia-Lazaro JA, Schnupp JW. Current source density profiles of stimulus-specific adaptation in rat auditory cortex. J Neurophysiol 102: 1483–1490, 2009. doi: 10.1152/jn.00240.2009. [DOI] [PubMed] [Google Scholar]
- Tan AY, Zhang LI, Merzenich MM, Schreiner CE. Tone-evoked excitatory and inhibitory synaptic conductances of primary auditory cortex neurons. J Neurophysiol 92: 630–643, 2004. doi: 10.1152/jn.01020.2003. [DOI] [PubMed] [Google Scholar]
- Taub AH, Katz Y, Lampl I. Cortical balance of excitation and inhibition is regulated by the rate of synaptic activity. J Neurosci 33: 14359–14368, 2013. doi: 10.1523/JNEUROSCI.1748-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinck M, Batista-Brito R, Knoblich U, Cardin JA. Arousal and locomotion make distinct contributions to cortical activity patterns and visual encoding. Neuron 86: 740–754, 2015. doi: 10.1016/j.neuron.2015.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels TP, Rajan K, Abbott LF. Neural network dynamics. Annu Rev Neurosci 28: 357–376, 2005. doi: 10.1146/annurev.neuro.28.061604.135637. [DOI] [PubMed] [Google Scholar]
- Wang X, Lu T, Bendor D, Bartlett E. Neural coding of temporal information in auditory thalamus and cortex. Neuroscience 154: 294–303, 2008. doi: 10.1016/j.neuroscience.2008.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lu T, Snider RK, Liang L. Sustained firing in auditory cortex evoked by preferred stimuli. Nature 435: 341–346, 2005. doi: 10.1038/nature03565. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Simada J. Auditory temporal masking: an electrophysiological study of single neurons in the cat’s cochlear nucleus and inferior colliculus. Jpn J Physiol 21: 537–549, 1971. doi: 10.2170/jjphysiol.21.537. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 426: 442–446, 2003. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Synaptic mechanisms of forward suppression in rat auditory cortex. Neuron 47: 437–445, 2005. doi: 10.1016/j.neuron.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Yao JD, Bremen P, Middlebrooks JC. Emergence of spatial stream segregation in the ascending auditory pathway. J Neurosci 35: 16199–16212, 2015. doi: 10.1523/JNEUROSCI.3116-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost WA. Auditory image perception and analysis: the basis for hearing. Hear Res 56: 8–18, 1991. doi: 10.1016/0378-5955(91)90148-3. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Tan AY, Schreiner CE, Merzenich MM. Topography and synaptic shaping of direction selectivity in primary auditory cortex. Nature 424: 201–205, 2003. doi: 10.1038/nature01796. [DOI] [PubMed] [Google Scholar]
- Zurita P, Villa AE, de Ribaupierre Y, de Ribaupierre F, Rouiller EM. Changes of single unit activity in the cat’s auditory thalamus and cortex associated to different anesthetic conditions. Neurosci Res 19: 303–316, 1994. doi: 10.1016/0168-0102(94)90043-4. [DOI] [PubMed] [Google Scholar]