Abstract
Background
Tuberculosis (TB) continues to result in high morbidity and mortality in children from resource-limited settings. Diagnostic challenges, including resource-intense sputum collection methods and insensitive diagnostic tests, contribute to diagnostic delay and poor outcomes in children. We evaluated the diagnostic utility of stool Xpert MTB/RIF (Xpert) compared with bacteriologic confirmation (combination of Xpert and culture of respiratory samples).
Methods
In a hospital-based study in Cape Town, South Africa, we enrolled children younger than 13 years of age with suspected pulmonary TB from April 2012- August 2015. Standard clinical investigations included tuberculin skin test, chest radiograph and HIV testing. Respiratory samples for smear microscopy, Xpert and liquid culture included gastric aspirates, induced sputum, nasopharyngeal aspirates and expectorated sputum. One stool sample per child was collected and tested using Xpert.
Results
Of 379 children enrolled (median age, 15.9 months, 13.7% HIV-infected), 73 (19.3%) had bacteriologically confirmed TB. The sensitivity and specificity of stool Xpert vs. overall bacteriologic confirmation were 31.9% (95% CI 21.84-44.50%) and 99.7% (95% CI 98.2-100%) respectively. 23/51 (45.1%) children with bacteriologically confirmed TB with severe disease were stool Xpert positive. Cavities on chest radiograph were associated with Xpert stool positivity regardless of age and other relevant factors (OR 7.05; 95% CI 2.16-22.98; p=0.001).
Conclusions
Stool Xpert can rapidly confirm TB in children who present with radiologic findings suggestive of severe TB. In resource-limited settings where children frequently present with advanced disease, Xpert on stool samples could improve access to rapid diagnostic confirmation and appropriate treatment.
Keywords: tuberculosis, diagnosis, children, stool
Introduction
Despite being preventable and treatable, tuberculosis (TB) contributed to at least 169,000 deaths among children in 2015 (1). TB-related mortality and morbidity in children are likely underestimated, due to challenges in diagnosing TB in children and incomplete reporting. TB frequently presents with non-specific symptoms, especially in young and HIV-infected children, and is a frequent co-morbid condition in children presenting with pneumonia and other respiratory childhood illnesses (2, 3). TB has been reported in up to 23% of children with severe pneumonia in high TB-burden settings (2), with autopsy studies from African urban hospitals identifying TB in 4-20% of paediatric deaths, the majority undiagnosed (3-5). Although early diagnosis and treatment of TB in children is associated with excellent treatment outcomes (6, 7), the majority of children with TB live in low-resource settings with poor access to timely TB diagnosis and intervention (8, 9).
TB in children is mostly intrathoracic (pulmonary) in > 75% of cases (10, 11), and is mainly paucibacillary (smear-negative). Current bacteriologic tests to confirm TB in children have limited sensitivity and resource-intensive methods are needed to collect adequate samples. Current available tests confirm a minority of children with TB: 5-10% of children with TB are smear microscopy positive and 10-40% culture positive depending on disease severity and spectrum (12, 13), resulting in a perceived unfavourable cost-benefit ratio for bacteriologic testing in most resource-limited settings. The Xpert MTB/RIF© assay (Xpert; Cepheid, Sunnydale, CA), endorsed by the World Health Organization for the diagnosis of TB and rifampicin resistance in adults and children including in resource-limited settings in 2010 (14), yields rapid results, but has <70% sensitivity compared to culture in children (15). Xpert also requires appropriate samples in young children, such as gastric aspirates (GA), induced sputum (IS), nasopharyngeal aspirates (NPA), fine needle aspiration (FNA) of peripheral lymph nodes or bronchoalveolar lavage (BAL)(13, 16), all resource-intense, invasive procedures.
Access to rapid diagnosis for childhood TB requires more than the availability of diagnostic tests. Bacteriologic diagnosis will remain largely confined to referral hospitals or well-resourced settings unless tests can be applied to samples that are easy to collect at the level of the healthcare system where children initially present, typically the primary healthcare level in resource-limited settings (17). The evaluation of any new diagnostic strategy for childhood TB should therefore consider not only diagnostic accuracy, but also the potential application of such strategies at relevant levels of healthcare (18-20).
Stool collection is potentially a more feasible and acceptable alternative to collecting respiratory samples, and can occur at home. Mycobacterium tuberculosis (M.tb) in swallowed sputum is recoverable from stool samples by culture or molecular methods (21-23). Stool does not require sophisticated collection equipment and poses low TB infection risk to healthcare workers. In particular, stool is easy to obtain from young children, a high-risk group for severe TB and where diagnosing TB is the most challenging. In critically ill children, stool collection may be a safer alternative to invasive sputum collection procedures.
In a prospective hospital-based study of children presenting with suspected intrathoracic TB in Cape Town, South Africa, we evaluated the diagnostic accuracy of Xpert on stool samples compared to bacteriologic confirmation from respiratory samples, and also to a clinical reference standard. We determined which clinical and bacteriologic factors were associated with positive stool Xpert.
Materials and Methods
Eligibility
Children <13 years of age who presented consecutively to two hospitals (Tygerberg Hospital and Karl Bremer Hospital, Cape Town, South Africa) with suspected intrathoracic TB, were screened for enrolment from April 2012 to August 2015. The enrolling facilities provide primary to tertiary level medical services to approximately 30% of the total Cape Town population of approximately 4 million. Children ≤13 years if age were eligible if they presented with ≥1 of: 1) cough ≥2 weeks, 2) unexplained fever ≥1 week, or 3) poor growth or weight loss over the preceding 3 months. With consideration of the fact that TB may present acutely, especially in young children, we also included children with any duration of cough, if ≥1 of the following were present: 1) exposure to an identified TB source case in the past 12 months, 2) positive tuberculin skin test (TST) if previously negative or unknown, or 3) a chest radiograph (CXR) suggestive of TB as assessed by the study clinician. Infants <3 months of age were also eligible if they had pneumonia unresponsive to appropriate antimicrobials, or unexplained and unresponsive sepsis syndrome. We excluded children who had received >1 dose of antituberculosis therapy (excluding isoniazid preventive therapy), had an established alternative diagnosis at screening, or who resided in areas too remote to ensure retention.
Clinical investigations
Clinical investigations followed established local and international practice (24), including a detailed history of symptoms and TB exposure, a clinical examination, TST (Mantoux, 2 Tuberculin Units PPD RT-23, Statens Serum Institute, Copenhagen), CXR (antero-posterior and lateral films) and a minimum of two respiratory samples for TB bacteriology (see Laboratory Methods). CXR were read by two independent experts, using a standard reading tool. Consensus was determined on the certainty of TB diagnosis, the radiologic pattern, and extent of disease. Discrepant readings were resolved by a third reader or by a forum if consensus was not reached. Disease severity was determined using a pragmatic modification of a published classification (25). Radiologically severe TB was defined as any of: 1) evidence of complications resulting from typical radiologic manifestations of TB (e.g. cavities, expansile pneumonia, nodal airway obstruction), 2) bilateral parenchymal involvement, 3) overall parenchymal involvement more extensive than the total area of the right upper lobe, or 4) disseminated (miliary) TB. For CXR not typical of TB, criteria 2 and 3 were used to define severe disease.
Any additional imaging results (e.g. abdominal ultrasound, chest computed tomography) were incorporated into the final classification to describe the full spectrum of disease. Disseminated disease was defined as miliary TB or neurotuberculosis.
The final classification of the certainty of TB disease was determined retrospectively at the 2-month follow-up, when final culture results from enrolment were available and response to treatment was evaluated. Participants were classified using the revised clinical case definitions for diagnostic research of intrathoracic TB in children (20), into the categories “Confirmed TB”, “Unconfirmed TB” and “Unlikely TB”. The decision to treat for TB was made by the attending clinicians, based on clinical/ epidemiologic assessment and the results of all investigations (not based on research case definitions). Xpert results from study samples, including stool, were available to clinicians and may have influenced treatment decisions. However, this study did not evaluate the impact of Xpert results on decision to treat. All children were followed for 6 months by the study team regardless of final TB diagnosis, to assess clinical course and treatment response. Children with drug-resistant (DR)-TB were followed to treatment completion.
Sample collection
The complete sample schedule according to the study protocol consisted of one sample each of GA (in children <5 years of age) /SPT (older children who could expectorate), IS and NPA (Supplemental Digital Content 1) collected daily for 2 consecutive days, and a stool sample (maximum total 7 samples). Stool was collected within 7 days of enrolment. Some children also had FNA of peripheral lymph nodes and BAL, collected by the hospital staff if clinically indicated. If any of the samples required by the study schedule were collected by hospital personnel, these were not collected again by the research team; their bacteriologic results were documented.
Laboratory methods
Fluorescent smear microscopy, liquid culture and Xpert was completed on all respiratory samples collected by the research team. Due to cost constraints, samples collected by hospital personnel were generally only tested by smear and culture.
After collection, GA samples were titrated to neutral pH using 4% sodium bicarbonate solution (24). All respiratory specimens were kept refrigerated and transported to the laboratory in a cool box within 4 hours of collection. Stool samples collected at home from children discharged from hospital and those that could not be processed immediately were stored at 2-8°C for maximum 72 hours before processing.
Respiratory specimens were handled by the National Health Laboratory Service at Tygerberg Hospital. In brief, after decontamination with N-acetyl-L-cysteine-1.25% sodium hydroxide (NALC-NaOH), neutralization and concentration, the pellet was resuspended in 1.5 mL phosphate buffered saline (PBS). The sample was utilized for Auramine-O smear microscopy (26), liquid culture using the Mycobacteria Growth Indicator Tube (MGIT) system (Becton Dickinson, Sparks, MD, USA) (27) and Xpert. Positive cultures were confirmed for the presence of M. tb by Ziehl-Neelsen staining and microscopy, absence of any growth on blood agar plates and assessed for resistance against rifampicin and isoniazid using the GenoType® MTBDRplus line probe assay (Hain Life Science, Nehren, Germany). Rifampicin-resistant isolates underwent second-line drug-susceptibility testing (DST) for amikacin and ofloxacin using the agar proportion method.
Stool specimens were processed by the study laboratory technician. In the absence of Xpert manufacturer's specific instructions for stool, two methods were used [adapted: (28, 29)]. For method 1, up to 5g of stool was homogenized with 20mL PBS by vortexing: 5mL of the stool/PBS mixture was then processed as above with NALC-NaOH 1.25%. After concentration, 0.5mL of the resuspended pellet was used for MGIT culture and 1.0mL was mixed with 2.0mL of Xpert sample reagent (SR), and loaded into the GeneXpert instrument (Software Version 4.4a) following manufacturer's instructions.
In June 2014, stool culture was discontinued due to high contamination and low sensitivity and stool method 2 was implemented: 1-4g of specimen was homogenised with 10mL PBS. After centrifugation at 3000×g, 4°C for 20 minutes, the supernatant was discarded and the pellet resuspended in 10mL of PBS by vortexing for 20 seconds. Having settled large particulate matter by brief centrifugation (30 seconds; 2000×g), 1mL supernatant was mixed with 2mL Xpert SR and tested by Xpert.
This study was approved by the Stellenbosch University Health Research Ethics Committee (reference N11/09/282), by the participating hospitals and provincial department of health. Written informed consent for study participation was obtained from parents or legal caregivers, and written assent was obtained from older children.
Statistical Analysis
The primary objective of this study was to evaluate the sensitivity, specificity, and predictive values of stool Xpert for the diagnosis of intrathoracic TB in children, compared to a) overall bacteriologic confirmation and b) a clinical reference standard. The bacteriologic reference standard was defined as confirmation of M.tb by culture or Xpert of any samples collected by the research team or hospital personnel, including stool culture but excluding stool Xpert (index test and therefore not included in the reference standard). Not all children had all samples collected: children were included in analysis if they had a minimum of one stool and one respiratory sample collected (intention to diagnose analysis). All analyses of diagnostic accuracy were conducted per patient (not per sample). The reference was considered positive if any of the samples collected from any individual child were M tb positive on Xpert or culture. The reference was considered negative if none of the samples collected from any individual child were positive for M.tb on Xpert or culture. Non-evaluable results (invalid/error Xpert results) were reported, and were considered negative for the diagnostic accuracy analysis if the child had at least one other valid result.
We also compared the diagnostic accuracy of stool Xpert to the first GA and IS Xpert, with the first GA or IS culture as a reference respectively, in order to better assess the value of stool Xpert compared to a single respiratory sample.
The clinical reference standard was defined as the clinician's decision to treat for TB, where a “TB case” was defined as a child in whom antituberculosis treatment was initiated, and a “symptomatic control” was a child investigated for TB based on the same entry criteria, but who did not initiate treatment.
In addition, we report on the proportion of children with positive stool Xpert by international consensus case definitions of “confirmed”, “unconfirmed” and “unlikely” TB (20). According to this classification, “confirmed TB” is defined as bacteriologic confirmation by culture or Xpert of M. tb on any valid respiratory sample, including stool. We therefore included all children with any positive Xpert or culture result on any sample in this category (including stool Xpert).
Clinical and demographic characteristics were summarized by clinical case definitions using means and standard deviations if normally distributed and with medians and inter-quartile ranges if non-normally distributed (20). STARD guidelines were followed for reporting and analyses (30).
Univariable analysis was used to identify factors associated with Xpert-positive stool. Any factors associated at a p-value <0.10 were used to build a multivariable logistic regression model. For multivariable regression and comparative analyses, odds ratios (OR) and 95% confidence intervals (CI) were reported, with p-values; a p-value <0.05 was considered statistically significant. Analyses were generated using Stata 14.0 special edition software (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX, USA: StataCorp LP).
Results
Stool was collected and tested by Xpert in 379 children (Figure 1). According to research case definitions (20), 73 children had confirmed TB, 185 unconfirmed TB and 121 unlikely TB. The median age was 15.9 (IQR 9.2-29.3) months, the HIV prevalence was 13.7% and 27 (7.1%) children had a documented previous TB episode (Table 1).
Figure 1.
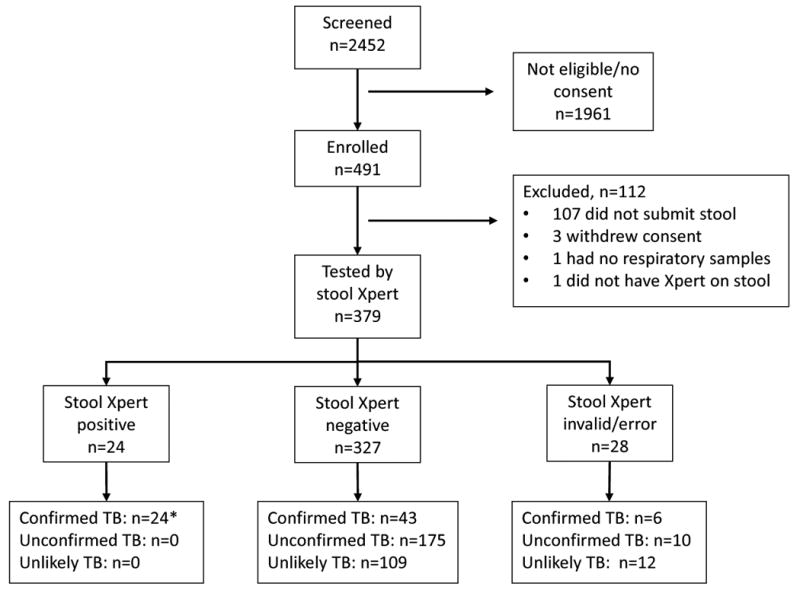
Study profile, including stool Xpert results compared to clinical consensus case definitions for pulmonary TB in children (20).
*One child was positive on stool Xpert, but all other samples were negative by Xpert and culture
Table 1.
Cohort characteristics overall and by international consensus diagnostic category (20), in children presenting with suspected tuberculosis (N= 379).
| Characteristic | All children | Confirmed TB | Unconfirmed TB | Unlikely TB |
|---|---|---|---|---|
| N=379 (100%) | N=73 | N=185 | N=121 | |
| Age, months: median (IQR) | 15.7 (9.2-29.4) | 19.1 (10.9-44.0) | 16.0 (9.6-28.3) | 13.3 (6.2-25.7) |
| Male (%) | 195 (51.5) | 27 (37.0) | 105 (56.8) | 63 (52.1) |
| Ethnicity: | ||||
| Mixed race (%) | 215 (56.7) | 42 (57.5) | 111 (60.0) | 62 (51.2) |
| Black African (%) | 163 (43.0) | 31 (42.5) | 73 (39.5) | 59 (48.8) |
| Caucasian (%) | 1 (0.3) | 0 | 1 (0.5) | 0 |
| Perinatal HIV exposure (%) | 105 (27.7) | 18 (24.7) | 54 (29.2) | 33 (27.3) |
| HIV-infected (%) | 51 (13.5) | 8 (11.0) | 30 (16.2) | 13 (10.7) |
| On ART at presentation (%) | 22 (43.1) | 4 (50.0) | 14 (46.7) | 4 (30.8) |
| Previous TB treatment (%) | 27 (7.1) | 4 (5.5) | 18 (9.7) | 5 (4.1) |
| Intrathoracic (%) | 19 (70.4) | 2 | 13 | 4 |
| Extrathoracic/disseminated (%) | 5 (18.5) | 2 | 2 | 1 |
| Unknown site of TB (%) | 3 (11.1) | 0 | 3 | 0 |
| Completed previous TB treatment* (%) | 24 (88.9) [n=27] | 4 (100) [n=4] | 15 (83.3) [n=18] | 5 (100) [n=5] |
| WAZ, median (IQR) | -1.8 (-2.9 to -0.7) | -1.7 (-2.9 to -0.7) | -2.2 (-3.2 to -0.9) | -1.4 (-2.1 to -0.7) |
| HAZ, median (IQR) | -1.7 (-2.9 to -0.7) | -1.7 (-2.8 to -0.6) | -2 (-3.1 to -0.9) | -1.4 (-2.5 to -0.5) |
| WAZ<-2 (%) | 168 (44.3) | 31 (42.5) | 103 (55.7) | 34 (28.1) |
| Evidence of BCG immunization** (%) | 360 (95.0) | 65 (89.0) | 178 (96.2) | 117 (96.7) |
| ≥1 well-defined TB symptom (20) (%) | 310 (81.8) | 66 (90.4) | 169 (91.4) | 75 (62.0) |
| TST positive# (%) | 82 (28.0) [n=293] | 39 (72.2) [n=54] | 38 (26.2) [n=145] | 5 (5.3) [n=94] |
| Exposure to identified TB source case (%) | 214 (56.5) | 51 (69.9) | 141 (76.1) | 22 (18.2) |
| CXR suggestive of TB§ (%) | 105 (28.3) [n=371] | 51 (71.8) [n=71] | 47 (25.7) [n=183] | 7 (6.0) [n=117] |
| Treated for TB (%) | 170 (44.9) | 73 (100) | 69 (37.3) | 28 (23.1) |
ART: antiretroviral therapy; WAZ: weight-for-age Z-score according to UK growth charts 1990; HAZ: height-for-age Z-score according to UK growth charts 1990; BCG: bacille Calmette-Guerin; TST: tuberculin skin test; CXR: chest radiograph.
3 children had missing/incomplete records of the reported previous TB episode, so site of TB and treatment outcome could not be determined;
Either documented BCG vaccination in the Road To Health Card or BCG scar present;
≥10mm if HIV-negative and BCG vaccinated, ≥5mm if HIV-positive or not BCG vaccinated;
As determined retrospectively by 2 independent expert readers.
Spectrum of disease among TB cases
Antituberculosis treatment was initiated in 170/379 (44.9%) children within 2 weeks of enrolment. In 164/170 (96.5%) children with CXR available for dual reading, 84/164 (51.2%) had radiologic patterns typical of TB, while 63/164 (38.4%) had non-typical patterns. Nine (5.5%) CXR were normal and in 8 (4.9%) the quality of the CXR was inadequate for evaluation. The most common radiologic pattern reported as typical of TB was intrathoracic lymph node disease in 64/164 (39.0%). Other typical patterns observed alone or in combination, were 17 with cavitating disease, 9 miliary, 8 expansile pneumonia, 4 Ghon complex and 2 pleural effusions. The most common non-typical changes were suggestive of lower respiratory tract infection (bronchopneumonia, lobar pneumonia, interstitial pattern) in 47 (28.7%) children. Overall, 84/164 (51.2%) TB cases had severe disease by CXR findings.
Thirty-five (20.6%) children also had extrathoracic TB: 15 disseminated (5/15 also had neurotuberculosis, 2/15 also abdominal TB and 1/15 also cervical TB adenitis), 11 abdominal TB and 9 cervical TB adenitis.
Twenty-four/170 (14.1%) children were treated for DR-TB: 16 were bacteriologically confirmed (6 multidrug-resistant [MDR], 4 isoniazid mono-resistant, 4 rifampicin mono-resistant and 2 extensively drug-resistant [XDR]); 8 were empirically treated for MDR-TB based on symptoms, suggestive CXR and close contact with a confirmed MDR-TB source case.
Bacteriology results and diagnostic utility of stool
In total, 1849 respiratory samples were collected (median samples/child = 4; range: 1-10).
Of the 73/170 (42.9%) children with bacteriologically confirmed TB, 71 were detected on culture or Xpert of non-stool samples, one only on stool culture, and one only on stool Xpert. Eleven/73 (15.1%) children had smear-positive TB; these were all also positive on Xpert and culture of respiratory samples. Nine of the 11 (81.8%) were stool Xpert positive, one (9.1%) was stool Xpert negative and another (9.1%) had an invalid stool Xpert result, which was not repeated.
Among the 170 TB cases (i.e. children treated for TB), children >5 years of age had a higher proportion of confirmed TB (13/18; 72.2%) compared to younger children (60/152; 39.5%, p=0.008). Eight/30 (26.7%) HIV-infected children were bacteriologically confirmed, compared to 65/140 (46.4% p=0.047) HIV-uninfected children, but a higher proportion of HIV-infected children overall initiated antituberculosis treatment (30/51; 58.8% vs 140/328; 42.7% respectively, p=0.031).
Stool Xpert detected 24 TB cases, of which 23 were also bacteriologically confirmed on non-stool samples. The sensitivity and specificity of stool Xpert vs. overall bacteriologic confirmation were 31.9% (95% CI 21.4-44.0%) and 99.7% (95% CI 98.2-100%), respectively (Table 2). Stool Xpert detected rifampicin resistance in 3/12 (25%) confirmed rifampicin-resistant cases. Invalid/error Xpert results occurred in 17/1611 (1.1%) respiratory samples vs. 28/379 (7.4%) stool samples: in 3/28 cases with invalid stool results, a second stool sample yielded negative results. Stool processed by method 1 vs method 2 yielded 21/191 (11.0%) vs. 7/188 (3.7%) invalid/error Xpert results on the first stool tested (p=0.007).
Table 2.
Diagnostic accuracy of stool Xpert compared to Xpert and culture of respiratory and non-respiratory samples (per patient analysis), and compared to a clinical decision to treat.
| a. Diagnostic accuracy of stool Xpert compared to Xpert and culture of respiratory and non-stool non-respiratory samples | ||||
|---|---|---|---|---|
| Sensitivity (%; 95% CI) | Specificity (%; 95% CI) | PPV (%; 95% CI) | NPV (%; 95% CI) | |
| Stool Xpert | 23/72 (31.9; 21.4-44.0) | 306/307 (99.7; 98.2-100) | 23/24 (95.8; 78.9-99.9) | 306/355 (86.2; 82.2-89.6) |
| b. Diagnostic accuracy of stool Xpert compared to clinical decision to treat | ||||
| Sensitivity (%; 95% CI) | Specificity (%; 95% CI) | PPV (%; 95% CI) | NPV (%; 95% CI) | |
| Stool Xpert | 24/170 (14.1; 9.3-20.3) | 209/209 (100; 98.3-100) | 24/24 (100; 85.8-100) | 209/355 (58.9; 53.6-64.0) |
PPV: Positive predictive value; NPV: negative predictive value.
Compared to the first GA culture, the sensitivity and specificity of stool Xpert were 45.5% (28.1-63.6) and 97.4% (98.3-100), while GA Xpert sensitivity and specificity were 54.5% (36.4-71.9) and 98.7% (96.2-99.7), respectively (Table 3). GA Xpert had an incremental detection of 3/33 (9.1%) over GA culture, while stool Xpert detected an additional 6/33 (18.2%).
Table 3.
Diagnostic accuracy of stool Xpert vs the first gastric aspirate or induced sputum sample
| Sensitivity (%; 95% CI) | Specificity (%; 95% CI) | PPV (%; 95% CI) | NPV (%; 95% CI) | |
|---|---|---|---|---|
| a. Diagnostic accuracy of the first GA Xpert compared to the first GA culture | ||||
| GA Xpert | 18/33 (54.5; 36.4-71.9) | 226/229 (98.7; 96.2-99.7) | 18/21 (85.7; 63.7-97.0) | 226/241 (93.4; 89.9-96.5) |
|
| ||||
| b. Diagnostic accuracy of stool Xpert compared to the first GA culture | ||||
| Stool Xpert | 15/33 (45.5; 28.1-63.6) | 223/229 (97.4; 98.3-100) | 15/21 (71.4; 47.8-88.7) | 223/241 (92.5; 88.5-95.5) |
|
| ||||
| c. Diagnostic accuracy of the first IS Xpert compared to the first IS culture | ||||
| IS Xpert | 16/24 (66.7; 44.7-84.4) | 240/246 (97.6; 94.8-99.1) | 16/22 (72.7; 49.8-89.3) | 240/248 (96.8; 93.7-98.6) |
|
| ||||
| d. Diagnostic accuracy of stool Xpert compared to the first IS culture | ||||
| Stool Xpert | 12/24 (50.0; 29.1-70.9) | 240/246 (97.6; 94.8-99.1) | 12/18 (66.7; 41.0-86.7) | 240/252 (95.2; 91.8-97.5) |
GA: gastric aspirate; PPV: Positive predictive value; NPV: negative predictive value; IS: induced sputum.
Compared to the first IS culture, the sensitivity and specificity of stool Xpert were 50.0% (29.1-70.9) and 97.6% (94.8-99.1), while IS Xpert sensitivity and specificity were 66.7% (44.7-84.4) and 97.6% (94.8-99.1), respectively (Table 3). IS Xpert had an incremental detection of 6/24 (25.0%) over IS culture, while stool Xpert detected an additional 8/24 (33.3%).
Spectrum of disease in stool-Xpert positive cases
Radiologically severe disease was observed in all 24 stool-Xpert positive cases: 12 (52.2%) had complicated lymph node disease, 9 (39.1%) had cavitating disease (2 also with miliary disease and 2 with expansile pneumonia) and 1 more each had miliary TB and expansile pneumonia, respectively. One had bilateral alveolar opacification not typical of TB. Of the 51 bacteriologically confirmed cases with severe intrathoracic disease, 23 (45.1%) were stool Xpert positive.
Only one of 13 cases with abdominal TB accompanying intrathoracic TB had positive stool Xpert.
Factors associated with having Xpert-positive stool
Univariable analysis of clinical features and their association with Xpert-positive stool is shown in Table 3. Cavities on CXR and expansile pneumonia were associated with having positive stool Xpert. HIV status, malnutrition, antibiotics before sample collection and stool processing method were not significantly associated with stool Xpert status. In multivariable logistic regression, adjusting for age and expansile pneumonia, cavities remained significantly associated with positive stool Xpert (OR 7.05; 95% CI 2.16-22.98; p=0.001).
Discussion
To our knowledge, this is the largest study to investigate the value of Xpert on stool samples for the diagnosis of TB in HIV-uninfected and -infected children. We explored not only the diagnostic accuracy but also the clinical applicability of stool Xpert. Our study population is representative of children with suspected TB from many high-burden settings, who are frequently referred to hospital for TB investigation. A large proportion of children presenting in this manner already have advanced TB disease, often with co-morbidity and co-infections (2, 3, 31), further compounding diagnostic challenges. In high-burden TB settings, resource-intensive methods for the collection of respiratory samples from young children and the relatively low yield of diagnostic tests in this population often pose a barrier to attempting diagnostic confirmation both at the community-based clinic and at referral level .
Xpert testing of stool is likely to have the greatest impact in settings where TB in children is typically diagnosed on clinical grounds, and where diagnosis is therefore frequently delayed or missed (32). This is more common in very young children and in those with HIV co-infection, who may present acutely, with non-specific signs and symptoms (32, 33), and with severe disease, and who are at high risk of dying with undiagnosed TB (3, 5). The majority of children in our study belonged to these high-risk groups. Applied to this patient population which is at exquisitely high risk of rapid disease progression and poor clinical outcomes, stool Xpert may therefore potentially be a life-saving strategy, particularly where capacity for respiratory sample collection, culture and DST is limited. Furthermore, for children exposed to drug-resistant TB, rapid confirmation of TB and drug resistance is likely to improve access to timely and appropriate treatment. Our study indicates that targeting Xpert testing of stool in severely ill children referred to hospital with suspected severe intrathoracic TB has a high yield. If stool processing could be simplified and the diagnostic performance optimised, stool Xpert could be promoted for use in clinic settings where children have the first contact with the healthcare system.
Published data concur with our findings which suggest that stool Xpert is more useful in children with severe TB. Two pilot studies from South Africa (29, 34) and a multicentre study of Asian and African HIV-infected children with suspected intrathoracic TB (35) reported sensitivities of 41-75%, with specificities >97.5% for stool Xpert vs. culture of respiratory samples. The higher sensitivity reported compared to our results can be explained by the severe spectrum of TB in children with Xpert-positive stool included in these studies. In the study by Nicol, 100% (n=8) of children with positive stool Xpert had alveolar consolidation; 86% had nodal airway compression (34). In the study by Marcy, 48% of children (14/29) with bacteriologically confirmed TB were smear positive, higher than typically observed in pediatric paucibacillary TB (35). In our initial pilot study (n=14), all children with Xpert-positive stool had severe intrathoracic TB (29). In our current study, stool Xpert was positive only in children with radiologically severe TB, with a sensitivity of 82% for smear-positive TB and 45% for bacteriologically confirmed cases with severe disease. This is consistent with a laboratory study where the level of detection for Xpert on Macaque stools was determined to be approximately 1000 colony forming units (CFU)/mL (36), whereas in respiratory samples it is approximately 100 CFU/mL(37).
Given this higher detection threshold of Xpert on stool compared to respiratory samples, stool Xpert will only detect a proportion of children with bacteriologically confirmed TB. However, when direct comparison was made with the diagnostic potential of a single GA or IS sample, stool was not substantially inferior. Furthermore, stool Xpert had a substantial incremental yield over culture of the first respiratory sample, indicating that although stool Xpert will miss some cases, the total number detected is comparable to a single respiratory sample, which is more invasive than stool testing. Testing multiple stool samples could also improve sensitivity, although cost and feasibility would need to be considered. With more sensitive molecular tools soon to become available for evaluation, hopefully also in children (38), the clinical utility of stool for TB diagnosis in children may be further improved.
An important factor to consider when applying new diagnostic tests to stool samples is the sample processing method. The different protocols used in published studies could partly explain the variable results observed. For molecular analysis, stool samples require careful processing to remove PCR inhibitors and particulate matter that can interfere with assay performance (36, 39-41). Stool processing currently remains relatively labor intensive, but work is ongoing to develop centrifugation-free protocols applicable to primary/ district level healthcare facilities (36). As optimised methods for processing stool for TB testing have not yet been established, parallel comparisons of the most promising sample processing procedures for stool should be evaluated in future work.
Table 4.
Univariate and multivariate analysis using logistic regression to determine characteristics associated with positive stool Xpert (N=170).
| Factor | Univariable OR (95% CI) | p-value | Multivariable OR (95% CI) | p-value |
|---|---|---|---|---|
| Age > 5 years | 2.69 (0.86-8.40) | 0.088 | 2.04 (0.56-7.47) | 0.280 |
| Male sex | 0.75 (0.31-1.81) | 0.528 | ||
| Chest radiograph: cavities | 10.35 (3.48-30.82) | <0.001 | 7.05 (2.16-22.98) | 0.001 |
| Chest radiograph: miliary | 3.33 (0.77-14.35) | 0.106 | ||
| Chest radiograph: expansile pneumonia | 7.10 (1.64-30.66) | 0.009 | 3.97 (0.73-21.71) | 0.112 |
| Black ethnicity | 0.61 (0.24-1.50) | 0.280 | ||
| HIV exposed [n=166] | 0.77 (0.28-2.08) | 0.604 | ||
| HIV-infected | 0.63 (0.18-2.26) | 0.479 | ||
| WAZ < -2 | 1.56 (0.65-3.71) | 0.317 | ||
| Any antibiotic treatment before TB sample collection | 0.72 (0.30-1.71) | 0.453 | ||
| Stool processing method 2 vs method 1* | 1.47 (0.62 - 3.51) | 0.381 |
OR, odds ratio; CI, confidence interval; WAZ: weight-for-age Z-score according to UK growth charts 1990
See laboratory methods
Acknowledgments
Funding: This work received financial support from the Faculty of Medicine and Health Sciences at Stellenbosch University; the Harry Crossley Foundation; the South African Medical Research Council; the South African National Research Foundation; the Foundation for Innovative New Diagnostics; the Tuberculosis Trials Consortium (TBTC) at the Centers for Disease Control and Prevention (CDC).
This work forms part of the body of work towards a PhD degree for EW: the PhD work from which this study emanated was funded by the Medical Research Council of South Africa under MRC Clinician Researcher Programme and by the South African National Research Foundation (Thuthuka programme funding for doctoral students).
The funding sources did not influence the design, analysis or reporting of the study. The views and opinions expressed are not those of the funders but of the authors of the manuscript.
The DTTC Is an International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT)-funded Clinical Research Site, and is part of the IMPAACT Clinical Trial Unit at Stellenbosch University. AMD is Principal Investigator for the IMPAACT International TB Specialty Laboratory. Although the published study was not an IMPAACT protocol, the network's support for this work is acknowledged. Overall support for the IMPAACT Network was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Global Tuberculosis Report. World Health Organization; Geneva, Switzerland: 2016. [Google Scholar]
- 2.Oliwa JN, Karumbi JM, Marais BJ, Madhi SA, Graham SM. Tuberculosis as a cause or comorbidity of childhood pneumonia in tuberculosis-endemic areas: a systematic review. Lancet Respir Med. 2015;3(3):235–43. doi: 10.1016/S2213-2600(15)00028-4. [DOI] [PubMed] [Google Scholar]
- 3.Bates M, Shibemba A, Mudenda V, Chimoga C, Tembo J, Kabwe M, et al. Burden of respiratory tract infections at post mortem in Zambian children. BMC Med. 2016;14:99. doi: 10.1186/s12916-016-0645-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates M, Mudenda V, Mwaba P, Zumla A. Deaths due to respiratory tract infections in Africa: a review of autopsy studies. Curr Opin Pulm Med. 2013;19(3):229–37. doi: 10.1097/MCP.0b013e32835f4fe4. [DOI] [PubMed] [Google Scholar]
- 5.Chintu C, Mudenda V, Lucas S, Nunn A, Lishimpi K, Maswahu D, et al. Lung diseases at necropsy in African children dying from respiratory illnesses: a descriptive necropsy study. Lancet. 2002;360(9338):985–90. doi: 10.1016/S0140-6736(02)11082-8. [DOI] [PubMed] [Google Scholar]
- 6.Abubakar I, Laundy MT, French CE, Shingadia D. Epidemiology and treatment outcome of childhood tuberculosis in England and Wales: 1999-2006. Arch Dis Child. 2008;93(12):1017–21. doi: 10.1136/adc.2008.139543. [DOI] [PubMed] [Google Scholar]
- 7.Seddon JA, Godfrey-Faussett P, Hesseling AC, Gie RP, Beyers N, Schaaf HS. Management of children exposed to multidrug-resistant Mycobacterium tuberculosis. Lancet Infect Dis. 2012;12(6):469–79. doi: 10.1016/S1473-3099(11)70366-8. [DOI] [PubMed] [Google Scholar]
- 8.Munoz-Sellart M, Yassin MA, Tumato M, Merid Y, Cuevas LE. Treatment outcome in children with tuberculosis in southern Ethiopia. Scand J Infect Dis. 2009;41(6-7):450–5. doi: 10.1080/00365540902865736. [DOI] [PubMed] [Google Scholar]
- 9.Cambanis A, Yassin MA, Ramsay A, Bertel Squire S, Arbide I, Cuevas LE. Rural poverty and delayed presentation to tuberculosis services in Ethiopia. Trop Med Int Health. 2005;10(4):330–5. doi: 10.1111/j.1365-3156.2005.01393.x. [DOI] [PubMed] [Google Scholar]
- 10.Marais BJ. Childhood tuberculosis: epidemiology and natural history of disease. Indian J Pediatr. 2011;78(3):321–7. doi: 10.1007/s12098-010-0353-1. [DOI] [PubMed] [Google Scholar]
- 11.Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Enarson DA, Beyers N. The spectrum of disease in children treated for tuberculosis in a highly endemic area. Int J Tuberc Lung Dis. 2006;10(7):732–8. [PubMed] [Google Scholar]
- 12.Marais BJ, Hesseling AC, Gie RP, Schaaf HS, Enarson DA, Beyers N. The bacteriologic yield in children with intrathoracic tuberculosis. Clin Infect Dis. 2006;42(8):e69–71. doi: 10.1086/502652. [DOI] [PubMed] [Google Scholar]
- 13.Zar HJ, Connell TG, Nicol M. Diagnosis of pulmonary tuberculosis in children: new advances. Expert Rev Anti Infect Ther. 2010;8(3):277–88. doi: 10.1586/eri.10.9. [DOI] [PubMed] [Google Scholar]
- 14.Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system for the diagnosis of pulmonary and extrapulmonary TB in adults and children: policy update. World Health Organization. 2013 [PubMed] [Google Scholar]
- 15.Detjen AK, DiNardo AR, Leyden J, Steingart KR, Menzies D, Schiller I, et al. Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in children: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(6):451–61. doi: 10.1016/S2213-2600(15)00095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicol MP, Zar HJ. New specimens and laboratory diagnostics for childhood pulmonary TB: progress and prospects. Paediatr Respir Rev. 2011;12(1):16–21. doi: 10.1016/j.prrv.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Detjen AK, Walters E. Editorial Commentary: Improving Children's Access to New Tuberculosis Diagnostic Tools Starts With the Collection of Appropriate Specimens. Clin Infect Dis. 2016;62(9):1169–71. doi: 10.1093/cid/ciw042. [DOI] [PubMed] [Google Scholar]
- 18.Cuevas LE, Browning R, Bossuyt P, Casenghi M, Cotton MF, Cruz AT, et al. Evaluation of tuberculosis diagnostics in children: 2. Methodological issues for conducting and reporting research evaluations of tuberculosis diagnostics for intrathoracic tuberculosis in children. Consensus from an expert panel. J Infect Dis. 2012;205(Suppl 2):S209–15. doi: 10.1093/infdis/jir879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roadmap for childhood tuberculosis: towards zero deaths. World Health Organization. 2013 [Google Scholar]
- 20.Graham SM, Cuevas LE, Jean-Philippe P, Browning R, Casenghi M, Detjen AK, et al. Clinical Case Definitions for Classification of Intrathoracic Tuberculosis in Children: An Update. Clin Infect Dis. 2015;61(Suppl 3):S179–87. doi: 10.1093/cid/civ581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen BW. Comparison of three methods for decontamination of faeces for isolation of Mycobacterium tuberculosis. Tubercle. 1991;72(3):214–7. doi: 10.1016/0041-3879(91)90011-g. [DOI] [PubMed] [Google Scholar]
- 22.Wolf H, Mendez M, Gilman RH, Sheen P, Soto G, Velarde AK, et al. Diagnosis of pediatric pulmonary tuberculosis by stool PCR. Am J Trop Med Hyg. 2008;79(6):893–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Cordova J, Shiloh R, Gilman RH, Sheen P, Martin L, Arenas F, et al. Evaluation of molecular tools for detection and drug susceptibility testing of Mycobacterium tuberculosis in stool specimens from patients with pulmonary tuberculosis. J Clin Microbiol. 2010;48(5):1820–6. doi: 10.1128/JCM.01161-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guidance for national tuberculosis programmes on the management of tuberculosis in children. World Health Organization (second edition) 2014 [PubMed] [Google Scholar]
- 25.Wiseman CA, Gie RP, Starke JR, Schaaf HS, Donald PR, Cotton MF, et al. A proposed comprehensive classification of tuberculosis disease severity in children. Pediatr Infect Dis J. 2012;31(4):347–52. doi: 10.1097/INF.0b013e318243e27b. [DOI] [PubMed] [Google Scholar]
- 26.Steingart KR, Henry M, Ng V, Hopewell PC, Ramsay A, Cunningham J. Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis. 2006;6:570–81. doi: 10.1016/S1473-3099(06)70578-3. [DOI] [PubMed] [Google Scholar]
- 27.Siddiqi H, Rüsch-Gerdes S. MGIT procedure Manual. 2007 [Google Scholar]
- 28.Oberhelman RA, Soto-Castellares G, Gilman RH, Caviedes L, Castillo ME, Kolevic L, et al. Diagnostic approaches for paediatric tuberculosis by use of different specimen types, culture methods, and PCR: a prospective case-control study. Lancet Infect Dis. 2010;10(9):612–20. doi: 10.1016/S1473-3099(10)70141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walters E, Gie RP, Hesseling AC, Friedrich SO, Diacon AH, Gie RP. Rapid diagnosis of pediatric intrathoracic tuberculosis from stool samples using the Xpert MTB/RIF Assay: a pilot study. Pediatr Infect Dis J. 2012;31(12):1316. doi: 10.1097/INF.0b013e318266c21c. [DOI] [PubMed] [Google Scholar]
- 30.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Ann Clin Biochem. 2003;40(Pt 4):357–63. doi: 10.1258/000456303766476986. [DOI] [PubMed] [Google Scholar]
- 31.Wiseman CA, Schaaf HS, Cotton MF, Gie RP, Jennings T, Whitelaw A, et al. Bacteriologically confirmed tuberculosis in HIV-infected infants: disease spectrum and survival. Int J Tuberc Lung Dis. 2011;15(6):770–5. doi: 10.5588/ijtld.10.0501. [DOI] [PubMed] [Google Scholar]
- 32.Lolekha R, Anuwatnonthakate A, Nateniyom S, Sumnapun S, Yamada N, Wattanaamornkiat W, et al. Childhood TB epidemiology and treatment outcomes in Thailand: a TB active surveillance network, 2004 to 2006. BMC Infect Dis. 2008;8:94. doi: 10.1186/1471-2334-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiwanuka JP. Tuberculosis in children at Mbarara University Teaching Hospital, Uganda: diagnosis and outcome of treatment. Afr Health Sci. 2002;2(3):82–8. [PMC free article] [PubMed] [Google Scholar]
- 34.Nicol MP, Spiers K, Workman L, Isaacs W, Munro J, Black F, et al. Xpert MTB/RIF testing of stool samples for the diagnosis of pulmonary tuberculosis in children. Clin Infect Dis. 2013;57(3):e18–21. doi: 10.1093/cid/cit230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marcy O, Ung V, Goyet S, Borand L, Msellati P, Tejiokem M, et al. Performance of Xpert MTB/RIF and Alternative Specimen Collection Methods for the Diagnosis of Tuberculosis in HIV-Infected Children. Clin Infect Dis. 2016 doi: 10.1093/cid/ciw036. [DOI] [PubMed] [Google Scholar]
- 36.Banada PP, Naidoo U, Deshpande S, Karim F, Flynn JL, O’Malley M, et al. A Novel Sample Processing Method for Rapid Detection of Tuberculosis in the Stool of Pediatric Patients Using the Xpert MTB/RIF Assay. PLoS One. 2016;11(3):e0151980. doi: 10.1371/journal.pone.0151980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blakemore R, Story E, Helb D, Kop J, Banada P, Owens MR, et al. Evaluation of the analytical performance of the Xpert MTB/RIF assay. J Clin Microbiol. 2010;48(7):2495–501. doi: 10.1128/JCM.00128-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alland D, Rowneki M, Smith L, et al. Xpert MTB/RIF Ultra: A New Near-Patient TB Test With Sensitivity Equal to Culture. Conf Retroviruses Opportunistic Infect; 23-25 Feb; Seattle, Washington. p. US2015. Abstr 91. [Google Scholar]
- 39.Taylor N, Gaur RL, Baron EJ, Banaei N. Can a simple flotation method lower the limit of detection of Mycobacterium tuberculosis in extrapulmonary samples analyzed by the GeneXpert MTB/RIF assay? J Clin Microbiol. 2012;50(7):2272–6. doi: 10.1128/JCM.01012-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DiNardo AR, Hahn A, Leyden J, Stager C, Baron EJ, Graviss EA, et al. Use of string test and stool specimens to diagnose pulmonary tuberculosis. Int J Infect Dis. 2015;41:50–2. doi: 10.1016/j.ijid.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alvarez-Uria G, Azcona JM, Midde M, Naik PK, Reddy S, Reddy R. Rapid Diagnosis of Pulmonary and Extrapulmonary Tuberculosis in HIV-Infected Patients. Comparison of LED Fluorescent Microscopy and the GeneXpert MTB/RIF Assay in a District Hospital in India. Tuberc Res Treat. 2012;2012:932862. doi: 10.1155/2012/932862. [DOI] [PMC free article] [PubMed] [Google Scholar]


