Abstract
Incomplete understanding of the contributions of dispersants and engineered nanomaterial (ENM) agglomeration state to biological outcomes presents an obstacle for toxicological studies. Although reactive oxygen species (ROS) production is often regarded as the primary indicator of ENM bioactivity and toxicity, it remains unclear whether ENM produce ROS or whether ROS is an outcome of ENM-induced cell injury. Phagolyosomal disruption and cathepsin B release also promotes bioactivity through inflammasome activation. Therefore, specific particle parameters, i.e. pre-exposure dispersion status and particle surface area, of two ENM (NiO and CeO2) were used to evaluate the role of ROS generation and cathepsin B release during ENM-induced toxicity. Male C57BL/6J mice were exposed to 0, 20, 40, or 80 μg of poorly- or well-dispersed NiO-NP or CeO2-NP in four types of dispersion media. At 1 and 7 days post-exposure, lung lavage fluid was collected to assess inflammation, cytotoxicity, and inflammasome activation. Results showed that pre-exposure dispersion status correlated with post-exposure pulmonary bioactivity. The differences in bioactivity of NiO-NP and CeO2-NP are likely due to NiO-NP facilitating the release of cathepsin B and in turn inflammasome activation generating pro-inflammatory cytokines. Further, both metal oxides acted as free radical scavengers. Depending on the pH, CeO2-NP acted as a free radical scavenger in an acidic environment (an environment mimicking the lysosome) while the NiO-NP acted as a scavenger in a physiological pH (an environment that mimics the cytosol of the cell). Therefore, results from this study suggest that ENM-induced ROS is not likely a mechanism of inflammasome activation.
Keywords: Pulmonary toxicity, engineered nanoparticles, ROS, agglomeration, inflammasome, activation, inflammation, cytotoxicity
Background
Engineered nanoparticles/materials (ENM) are now being incorporated into a multitude of different products. The unique physiochemical properties of ENM support their wide variety of applications. However, the unique physiochemical properties of ENM introduce a wide range of concerns pertaining to human health (Shvedova et al. 2012; Manke et al. 2013). One of the challenges in evaluating potential adverse effects of ENM is due to their unique and extensive tendency to agglomerate and the lack of a uniform set of guidelines to prepare ENM suspensions for in vitro or in vivo studies. In order to develop procedures to evaluate potential ENM hazards, studies elucidating the roles of dispersion media and agglomerate size on bioactivity as well as the mechanisms facilitating bioactivity need to be undertaken to understand the roles of these factors to reduce experimental variability.
Numerous studies on size- and shape-dependent toxicity of nanoparticles have demon-strated the biological importance of these properties (Chithrani et al. 2006; Powers et al. 2007; Lewinski et al. 2008; Murphy et al. 2008; Wang et al. 2008). These parameters affect cellular uptake, protein adsorption, accumulation in organelles and distribution throughout the body. One reason for these effects is the correlation between particle size and surface area. In general, for a fixed mass of particles, surface area increases as particle size becomes smaller. Thus, particle surface area may explain the greater toxicity of nanoparticles compared with an equal mass of fine particles of the same material (Donaldson et al. 2002; Monteiller et al. 2007; Sager et al. 2009). Since it is the surface atoms and molecules that play a significant role in determining the bulk properties of the nanoparticle, most of the previous toxicity studies have demonstrated an inverse relationship between particle size and toxicity.
However, agglomeration of nanoparticles can dramatically change the original size and shape of the particles when delivered to biological models in vitro or in vivo (Sager et al. 2016). Nanoparticles tend to easily agglomerate due to their high surface energy, but can be partially dispersed by sonication when loosely bound. Agglomeration alters the size and shape of nano-particles within the suspension; this, in turn, greatly influences the cell-particle interactions. A lack of understanding of the contributions of dispersants and agglomeration state to biological effects hinders the study of the actual effects of particle size and shape on toxicity (Sager et al. 2007; Wang et al. 2008; Auffan et al. 2010; Keller et al. 2010).
Some of the paradigms for ENM-mediated toxicity include oxidative stress, inflamma-tion, genetic damage, and the inhibition of cell division and cell death (Stone et al. 2007; Ju-Nam and Lead 2008; Li et al. 2008; Johnston et al. 2010). Most work to date has suggested that ROS generation and consequent oxidative stress are frequently observed with ENM toxicity (Li et al. 2010 ; Shvedova et al., 2012). However, it may be inaccurate to assume that ROS generation is a prerequisite to NP-induced toxicity since a few studies have reported the direct toxicity of NP without causing ROS (Wang et al. 2010). Nevertheless, ROS generation may be a major event during NP-induced injury that needs to be thoroughly characterized in order to predict NP-induced toxicity.
Current evidence also suggests that toxic nanoparticles could potentially increase phago-lysosomal membrane permeability and release of cathepsin B which also promotes the activation of the NLRP3 inflammasome. Cathepsin B release, in turn, induces release of pro-inflammatory cytokines (IL-1β and IL-18) from alveolar macrophages (AM) (Beamer et al. 2012). It was previously shown that the NLRP3 inflammasome is activated in vivo, after pulmonary exposure to MWCNT (Sager et al. 2014). Studies have concluded that upon assembly of the intact NLRP3 inflammasome, caspase-1 becomes activated and produces the mature and secreted forms of the pro-inflammatory cytokines interleukin IL-1β and IL-18 (Jin and Flavell, 2010). The NLRP3 inflammasome activation pathway model uptake of nanoparticles causes the disruption of the phagolysosome acidic compartment and subsequent release of cathepsin B; inhibition of this process has been proven to block the inflammasome activation.
To date, an exploration into the cellular mechanisms driving inflammasome activation by nanoparticle induced pulmonary injury, whether it be ROS generation or cathepsin B release, is incomplete and remains to be fully elucidated. Therefore, the current study evaluated specific particle parameters, i.e. pre-exposure dispersion status and particle surface area, of two highly utilized metal oxides to evaluate the role of ROS generation and inflammasome activation during ENM-induced toxicity.
NiO was chosen as one particle for these experiments due to the fact that previous studies have reported that NiO nanoparticles stimulate pulmonary bioactivity (Gillespie et al. 2010; Horie et al. 2011; Sager et al. 2016). CeO2 was chosen as a comparison particle due to the evidence that it is a nanoparticle that can display either a beneficial (anti-oxidant) or toxic (oxidant) effect (Asati et al. 2010) in differing experimental situations. The purpose of this study was not to compare the bioactivity of CeO2 to NiO, as both particles have been shown to facilitate pulmonary inflammation and pathology in certain experimental situations, but instead to explore and assess how parameters, like pre-exposure dispersion and ROS production, and inflammasome activation may facilitate the differences between the pulmonary toxicity of these two metal oxides.
Materials and Methods
Particles and characterization
CeO2-nanoparticles (CeO2-NP) were obtained from Sigma (cerium (IV) oxide; < 25 nm; Cat. #544841; St. Louis, MO). Nickel oxide nanoparticles (NiO-NP) were also obtained from Sigma (nickel II oxide nanopowder; Cat. #637130). Both NiO- and CeO2-NP were separately suspended in one of four different, commonly utilized dispersion medias: phosphate buffered saline (PBS), dispersion media (DM; combination of dipalmitoyl-phosphatidyl choline (DPPC) and albumin in concentrations that mimic diluted alveolar lining fluid) (Porter et al. 2008) and two commercially available surfactants; Survanta® and pluronics (pluronics F-68). Both well-dispersed (20 min sonication) and poorly-dispersed (5 min sonication) suspensions were produced utilizing both CeO2-NP and NiO-NP in each media. Each particle suspension (1.6 mg/ml) was sonicated utilizing a Branson Sonifer 450 (Branson Ultrasonics Corp., Danbury, CT) at 25W continuous output for 20 min or for 5 min to generate well-dispersed or poorly-dispersed suspensions, respectively. During sonication, heat was dissipated by placing the samples on ice.
After the particle suspensions were prepared as described above, 2-ml samples were immediately transferred to a cuvette and particle size range was immediately determined by dynamic light scattering (DLS). For each NP/suspension media combination, DLS analysis was conducted 10 separate times on 10 different samples. The 10 individual run results were then utilized to calculate the average hydrodynamic diameter for each sample analyzed. DLS analysis for each sample was conducted using a Nanotrac 252 (Microtrac; Montgomeryville, PA).
Electron microscopy (EM) analyses were also conducted on the same samples. For the EM analysis, each particle suspension was diluted in diH2O 1:1000 to prevent salt and protein components of the dispersion solutions from creating artifacts and preventing accurate interpretation. Next, a 0.5 ml sample of each suspension was then filtered through a 0.2-μm Nucleopore filter onto a Formvar-coated copper grid to dry. Each sample was then imaged using a Hitachi field emission scanning electron microscope (FESEM).
The surface charge of each nanoparticle/suspension media combination was also analyzed using a Malvern Zetasizer Nano ZS instrument (Malvern Instruments Inc., Malvern, UK). Each zeta potential measurement represents the arithmetic mean of 10 individual measure-ments made by the instrument for each NP/suspension media combination. The electrophoretic mobility of both NiO-NP and CeO2-NP in each media was converted into the zeta potential by utilizing the Helmholtz-Smoluchowski equation. The surface area of both the NiO-NP and the CeO2-NP were also measured by the Brunauer-Emmett-Teller (BET) method. BET measure-ments were taken on each particle sample in its dry state under N2 adsorption to measure specific surface area (Brunauer et al. 1938).
Electron Spin Resonance (ESR) Measurements
Xanthine, xanthine oxidase and 5-5-dimethyl-1-pyroline-oxide (DMPO) were purchased from Sigma. The spin trap, DMPO, was purified by charcoal de-colorization and vacuum distillation and was free of electron spin resonance (ESR) detectable impurities.
Free radical measurements
ESR and spin trapping were used to detect short-lived free radical intermediates. Radicals were measured using the addition-type reaction of a short-lived radical with a compound (spin trap) to form a relatively long-lived paramagnetic free radical product (spin adduct), which could then be studied using conventional ESR measurements. Concentrations given in the figure legends are final concentrations.
The intensity of the signal was used to measure the relative amount of short-lived radicals trapped, and the hyperfine couplings of the spin adduct are characteristic of the original trapped radicals. Spin trapping is the method of choice for detection and identification of free radical generation because of its specificity and sensitivity. All ESR measurements were conducted using a Bruker EMX spectrometer (Bruker Instruments, Billerica, MA) and a flat cell assembly. Hyperfine couplings were measured (to 0.1 G) directly from magnetic field separation using potassium tetra-peroxochromate (K3CrO8) and 1,1-diphenyl-2-picrylhydrazyl as reference standards. The relative radical concentration was estimated by multiplying half of the peak height by (ΔHpp)2, where ΔHpp represents peak-to-peak width. Acquisit (Bruker) was used for data acquisitions and analyses.
Reaction system
The free radical spin trap system was composed of DMPO (100 mM), particle (CeO2, NiO) xanthine (35 mM) and xanthine oxidase (4 μl/ml). Measurements were conducted in artificial lysosome fluid (ALF), a fluid which mimics the acidic environment inside a lysosome, and DM, which mimics the neutral pH in the cell cytosol. ALF was prepared as described in Marques et al (2011). The reaction was allowed to incubate for 3 min at room temperature. ESR settings are given in figure legends.
Animals
C57BL/6J mice (male, 7-wk-old) were purchased from the Jackson Laboratories (Bar Harbor, ME). Mice were housed in an AAALAC-accredited, specific-pathogen-free, environ-mentally-controlled facility and allowed to acclimate at least 7 days prior to use. The mice were monitored to be free of endogenous viral pathogens, parasites, mycoplasms, Helicobacter, and CAR Bacillus. Mice were kept in ventilated cages, that were provided HEPA-filtered air and both Diamond-Dry virgin cellulose and hardwood Sani-chips for bedding. The mice were maintained on a Harlan Teklad 7913, 6% fat, irradiated diet and tap water, both of where were available ad libitum. All experimental procedures were approved by the Animal Care and Use Committee of the University of Montana prior to beginning the study.
Mouse pharyngeal aspiration
Mice were exposed to 0, 20, 40, or 80 μg/mouse of a poorly- or well-dispersed NiO-NP or CeO2-NP/suspension media combination via pharyngeal aspiration. For pharyngeal aspiration, a stock solution of each CeO2-NP and NiO-NP/suspension media combination (1.6 mg/ml) (well-dispersed and poorly-dispersed) was prepared utilizing the previously described sonication parameters (5 min vs. 20 min, 25W, and continuous output). From the prepared stock solution of each NP/suspension media combination dilutions were made to produce final NP concentrations of 20, 40, or 80 μg/mouse. Immediately upon preparation of each CeO2-NP or NiO-NP/suspen-sion media combination, pharyngeal aspiration was conducted as described by Rao et al. (2003). In brief, the animals were anesthetized with isoflurane and placed on a board in a near vertical position. The tongue was extended with lined forceps; 50 μl respective particle suspension was placed on the back of the tongue which was held until the suspension was aspirated. Control mice were administered an equal volume of the dispersion media being utilized.
Collection of lung lavage fluid
At 1 and 7 day post-exposure, mice were euthanized by intraperitoneal (IP) injection of sodium pentobarbital (100–300 mg/kg body weight) followed by exsanguination. The trachea was cannulated with a blunt 22-G needle and whole lung lavage (WLL) was performed using cold sterile Ca+2, Mg+2-free PBS at a volume of 0.6 ml for first lavage (kept separate) and 1 ml for subsequent lavages. Approximately 4 ml WLL fluid/mouse was collected in sterile centrifuge tubes. Collected WLL cells were washed in PBS by centrifugation (600 × g, 5 min, 4°C) and re-suspended in PBS (Zeidler et al. 2004). Acellular first-fraction WLL aliquots were frozen or kept on ice for analysis.
Cell counts and differentials and WLL fluid analyses
Total WLL cell counts were obtained using a Coulter Multisizer 3 (Coulter Electronics, Hialeah, FL) and cytospin preparations of the cells were made using a cytocentrifuge (Shandon, London, UK). The preparations were stained with modified Wright-Giemsa stain and differen-tials determined by light microscopy. Lactate dehydrogenase (LDH) activity of the first WLL fluid was measured [to assess cytotoxicity] using a COBAS MIRA Plus chemical analyzer (Roche Diagnostics, Montclair, NJ) as previously described (Porter et al. 2002).
The presence of inflammatory mediators (cathepsin B, IL-1β and IL-18) in the first WLL fluid was measured 1 day post-exposure for the 80 μg/mouse dose level for both NiO-NP and CeO2-NP. Levels present were measured using commercially available ELISA kits (BioSource, Camarillo, CA) as previously described (Hamilton et al. 2009). To determine cathepsin B activity, in 96-well plates [using PBS as diluent], first WLL fluid (50 μl), 2 μg Z-LR-AMC (fluorogenic Peptide Substrate, R&D Systems, Minneapolis, MN) ± 66 μM inhibitor (Z-Phe-Phe-FMK, MBL International, Woburn, MA) were combined in a total volume of 150 μl. The samples were incubated at 37°C for 1 hr before fluorescence was measured using a plate reader with 380 nm excitation and 460 nm emission. Cathepsin B specific activity was calculated as: relative fluorescence units (RFU) from cathepsin B activity (no inhibitor) minus with inhibitor.
Statistical analysis
Statistical Analysis was conducted using SigmaStat® Statistical Analysis Software. Comparisons among doses was performed for each particle-suspension media combination at all post-exposure times using an analysis of variance (ANOVA) with significance set at p ≤ 0.05, with post-hoc tests for pair-wise comparison of dose groups. Analyses were conducted comparing pulmonary bioactivity of each CeO2-NP and NiO-NP/suspension media combination to control at each post-exposure timepoint. Comparisons of bioactivity responses between poorly- and well-dispersed CeO2-NP and NiO-NP/suspension media combination for each dose was also conducted for each post-exposure timepoint. Individual means were compared using a Tukey-pairwise multiple comparison procedure with an overall significance level of 0.05. Data are reported as mean ± standard error (SE).
Results
Particle Characterization
DLS and zeta potential measurements were made on each media/NP combination (Tables 1 and 2). After 5 min of sonication, with both CeO2-NP and NiO-NP suspensions, there were relatively large agglomerates in all four dispersion solutions. After 5 min of sonication, NiO-NP and CeO2-NP suspended in DM had the smallest hydrodynamic diameter (489.9 and 302.6 nm, respectively) while the NiO-NP and CeO2-NP suspended in Survanta® (5 min sonication) and pluronic (20 min sonication) had the largest agglomerates at 4460.1 and 694.2 nm, respectively (Table 1).
Table 1.
Effect of dispersion media and sonication time on average nanoparticle size (nm).
| Medium Type | 5 min sonication NiO |
5 min sonication CeO2 |
20 min sonication NiO |
20 min sonication CeO2 |
|---|---|---|---|---|
| Pluronics | 3060±13.5 | 2745±21.8 | 694±3.7 | 942±15.9 |
| PBS | 1312.5±8.4 | 1726±11.3 | 486±5.8 | 568±12.1 |
| Survanta | 4460.1±85.4 | 3530±32.7 | 221±6.6 | 261±10.9 |
| DM | 489.9±8.9 | 302±14.6 | 102±2.9 | 89±7.8 |
Dynamic light scattering (DLS) means of particle size (nm) ± standard errors (SE) are shown for each NiO/dispersion media combination and CeO2/dispersion media combination. DLS measurements for each particle/dispersion media combination (1.6 mg/ml) were immediately performed after the specified 5 or 20 minute sonication time periods. Values presented are means of hydrodynamic diameters of particle sizes (nm) from 10 sample recordings of each nano-sized particle/dispersion media combination analyzed.
Table 2.
Zeta potentials of each NiO-NP and CeO2-NP/dispersion media combination.
| Medium Type | NiO Zeta Potential (mV) | CeO2 Zeta Potential (mV) |
|---|---|---|
| Pluronics | −1.86 | −1.40 |
| PBS | −0.06 | −0.29 |
| Survanta | −0.04 | −0.51 |
| DM | −0.10 | −0.31 |
The surface charge of the NiO-NP and CeO2-NP in each dispersion media was analyzed using a Malvern Zetasizer Nano ZS instrument (Malvern Instruments Inc., UK). The electrophoretic mobility of the nanoparticles in each dispersion media was converted into the zeta potential by utilizing the Helmholtz-Smoluchowski equation.
When comparing the hydrodynamic diameters of the NiO-NP and CeO2-NP suspensions, there were no significant differences between the two metal oxide NP with regard to mean particle size in solution or zeta potential after either 5 or 20 min sonication (Tables 1 and 2). For example, after 5 min of sonication, the NiO-NP suspended in PBS had a mean diameter of 1312.5 nm while after 5 min of sonication the CeO2-NP suspended in PBS had a mean diameter of 1726.2 nm. However, increasing sonication time from 5 to 20 min did significantly reduce the mean diameter of both CeO2-NP and NiO-NP in all four dispersion solutions. The mean diameter of the CeO2-NP suspended in DM after 5 min of sonication was 302 nm, but after increasing the sonication time to 20 min, the mean diameter of the CeO2-NP significantly decreased to 89.3 nm (Table 1). Also, for both CeO2-NP and NiO-NP suspensions, the particles dispersed in DM produced the smallest hydrodynamic diameter at the end of either sonication period (Figures 1A and 1B, Table 1). In comparison, CeO2-NP and NiO-NP suspended in Survanta® produced particles with the largest hydrodynamic diameter after 5 min sonication, while after 20 min the particles suspended in pluronics had the largest diameter. BET surface area analysis of both NP in their dry state was measured. The BET specific area of NiO-NP was 113.0 m2/g while that of CeO2-NP was 46.8 m2/g.
Figure 1.

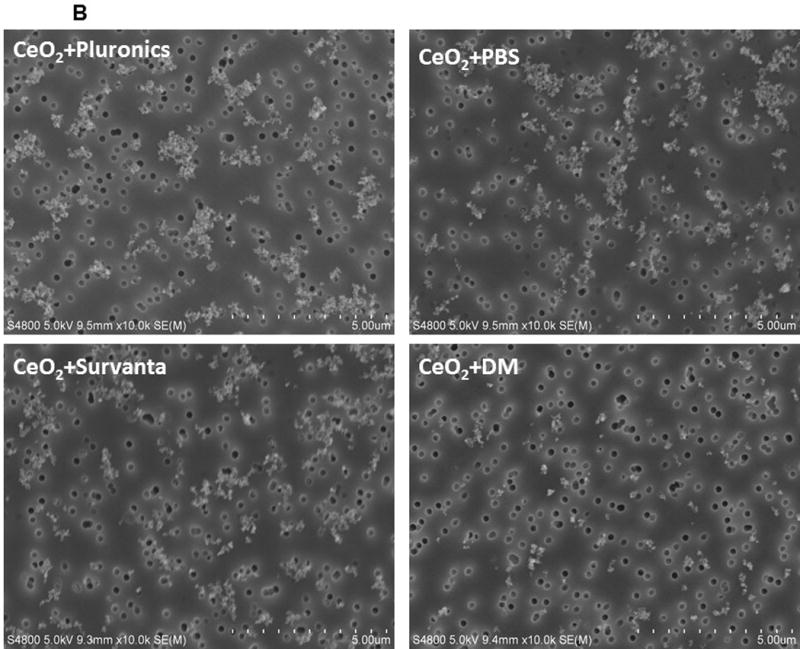
Panel A. Transmission electron microscopy (TEM) micrographs of CeO2-NP in pluronic (after 20 min sonication, well-dispersed, Panel A), PBS (after 20 min of sonication, well-dispersed, Panel B), Survanta® (after 20 min sonication, Well-dispersed, Panel C), and DM (after 20 min sonication, Well-dispersed, Panel D). Samples for TEM analyses were obtained from the same samples used for dynamic light scattering analyses (data presented in Table 1). Panel B. Field emission scanning electron microscopic (FESEM) micrographs of CeO2-NP in pluronic (after 20 min sonication, well-dispersed, Panel A), PBS (after 20 min sonication, well-dispersed, Panel B), Survanta® (after 20 min sonication, well-dispersed, Panel C), and DM (after 20 min of sonication, well-dispersed, Panel D). Samples for FESEM analyses were obtained from the same samples used for dynamic light scattering analyses.
Role of pre-exposure dispersion status on lung inflammation and injury from CeO2-NP
At 1 and 7 day post-exposure, there were no significant differences in PMN numbers (marker of inflammatory response) when comparing the various dispersed particle combinations for the CeO2-NP+pluronics irrespective of dose (Figure 2). For CeO2-NP dispersed in PBS, at 1 day post-exposure, a significant difference in PMN numbers between the poorly- and well-dispersed particle combinations was observed at both intermediate (40 μg/mouse) and high (80 μg/mouse) doses (Figure 2). At 7 day post-exposure, the well-dispersed CeO2-NP/PBS combina-tion caused a significant increase in PMN numbers at all doses compared to the poorly-dispersed CeO2-NP/PBS combinations. Similar to the CeO2-NP/pluronics combination, for CeO2-NP dispersed in Survanta® at 1 day post-exposure, there were no significant differences in PMN numbers compared with the various dispersed particle/media combinations. However, at 7 day post-exposure, the well-dispersed CeO2-NP+Survanta® caused a significant increase in PMN number when compared to the poorly-dispersed CeO2-NP+Survanta® combinations.
Figure 2.
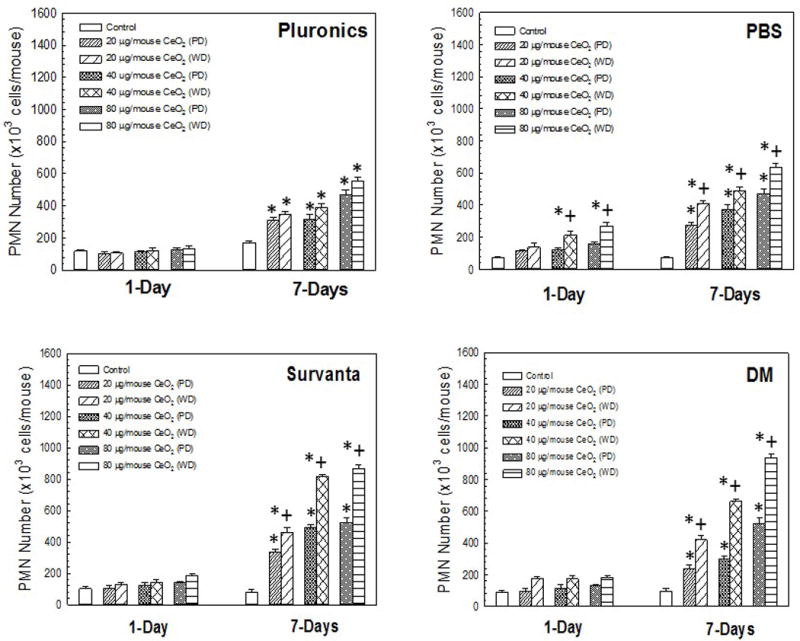
PMN number induced by pharyngeal aspiration exposure to 0, 20, 40, or 80 μg/mouse of either poorly-dispersed (PD) or well-dispersed (WD) CeO2-NP dispersed in differing dispersion medias (Panel A, pluronics, Panel B, PBS, Panel C, Survanta®, and Panel D, DM) at 1 and 7 days post-exposure. Values are given as means ± SE (n=8). *Significant difference between treatment and control groups at 1 and 7 day post-exposure (p < 0.05). +Significant difference between well-dispersed (20 min sonication) and poorly-dispersed (5 min sonication) CeO2-NP solutions at same post-exposure timepoint (p < 0.05). (NiO-NP PMN data shown in figure is from Sager et al. [2016]).
For the CeO2-NP dispersed in DM, no significant difference existed at 1 day post-exposure between the well-dispersed and poorly-dispersed particle/media combinations at any dose. However, at 7 day post-exposure, the well-dispersed CeO2-NP+DM produced a significant increase in PMN number at all doses when compared to the poorly-dispersed combinations. (NiO-NP PMN data shown in Figure 2 can be found in Sager et al. 2016).
LDH activity in lung lavage fluid was measured to assess cytotoxicity of CeO2-NP/media combinations at 1 and 7 day post-exposure. At both timepoints, significant differences in LDH activity were seen when comparing the poorly- to well-dispersed particle combinations for CeO2-NP+pluronics at the two highest doses (Figure 3, Panel A). In contrast, at 1 day post-exposure, no significant differences existed in LDH activity when comparing the poorly- and well-dispersed particle combinations for CeO2-NP+PBS at any dose. At 7 day post-exposure, the highest dose of well-dispersed CeO2-NP+PBS caused a significant increase in LDH activity when compared to poorly-dispersed CeO2-NP+PBS. Well-dispersed CeO2-NP+Survanta® caused a significant increase in LDH activity when compared to poorly-dispersed CeO2-NP+Survanta® at both 1 and 7 day post-exposure, at all doses. In a similar manner, well-dispersed CeO2-NP+DM produced a significant increase in LDH activity when compared to the poorly-dispersed particle/media combination at both 1 and 7 days post-exposure, at all doses. Taken together, the suspension studies showed that the well-dispersed particle suspensions were generally more cytotoxic when DM and Survanta® were dispersants, these provided the smallest agglomerates and highest bioactivity (NiO-NP LDH data shown in Figure 3 can be found in Sager et al. 2016).
Figure 3.
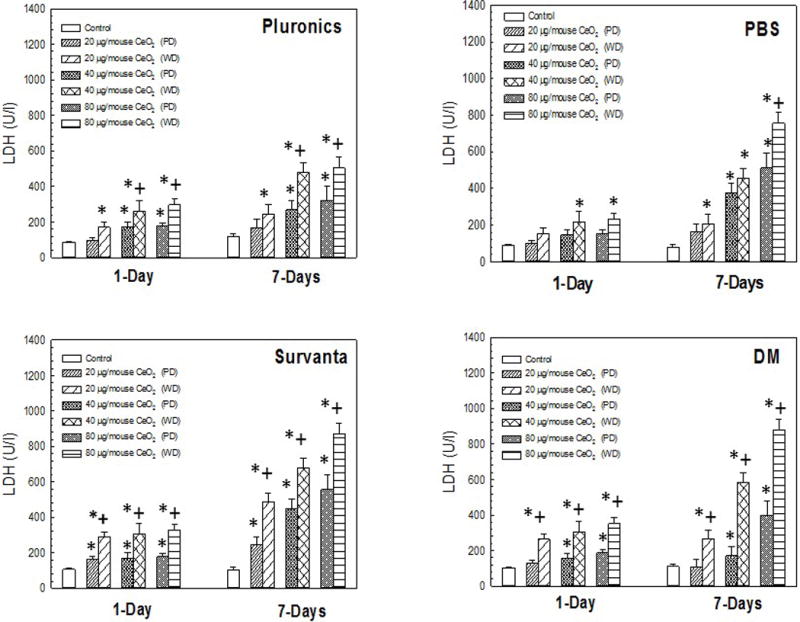
LDH activity induced by pharyngeal aspiration exposure to 0, 20, 40, or 80 μg/mouse of either poorly-dispersed (PD) or well-dispersed (WD) CeO2-NP dispersed in differing dispersion media (Panel A, Pluronics, Panel B, PBS, Panel C, Survanta®, and Panel D, DM) at 1 and 7 day post-exposure. Values are given as means ± SE (n=8). *Significant difference between treatment group and control group 1 and 7 day post-exposure (p < 0.05). +Significant difference between well-dispersed (20 min sonication) and poorly-dispersed (5 min sonication) CeO2-NP solutions at the same post-exposure time point (p < 0.05). (NiO-NP LDH data shown in figure from Sager et al. [2016]).
NLRP3 inflammasome activation
To determine if either NiO-NP and/or CeO2-NP/suspension combinations cause phago-lysosomal lysis and activation of the NLRP3 inflammasome, cathepsin B activities, as well as IL-18 and IL-1β levels were measured (respectively) in the first WLL fluid collected from mice 1 day after exposure for each CeO2-NP and NiO-NP/media combinations. Both CeO2-NP and NiO-NP (Figure 4) produced a dose-dependent increase in cathepsin B activity, regardless of dispersion media type. In regards to differences based on dispersion, for both CeO2-NP and NiO-NP, well-dispersed NP/suspension combinations produced significantly more cathepsin B release compared to poorly-dispersed NP/suspension combinations. When comparing the NiO-NP/combinations to the CeO2-NP/combinations, NiO-NP/suspension combinations resulted in an ≈ 20% greater increase in levels of cathepsin B activity than CeO2-NP/suspension combinations.
Figure 4.
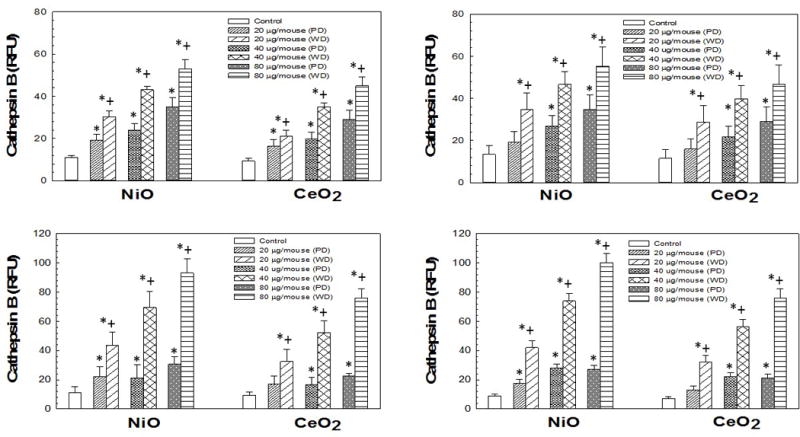
Cathepsin B activities induced by pharyngeal aspiration exposure to 0, 20, 40, or 80 μg/mouse of either poorly-dispersed (PD) or well-dispersed (WD) NiO- and CeO-NP dispersed in differing dispersion medias (Panel A: pluronics, Panel B: PBS, Panel C: Survanta®, and Panel D: DM) at 1 day post-exposure. Values are given as means ± SE (n=8). *Significant difference of treatment group from control at 1 day post-exposure (p < 0.05). +Significant difference between the poorly-dispersed (5 min sonication) and well-dispersed (20 min sonication) NiO- and CeO2-NP solutions at same post-exposure timepoint (p < 0.05). Cathepsin B activities determined as described in Methods.
IL-18 levels for animals receiving NiO-NP dispersed in pluronics or PBS were not significantly different than control levels of IL-18 at any dose (Figure 5). However, the well-dispersed NiO-NP in Survanta® and DM did significantly increase IL-18 at all doses compared to control. Furthermore, the well-dispersed NiO-NP Survanta® and DM media combinations produced a significant increase in IL-18 at all doses compared to the corresponding poorly-dispersed NiO-NP Survanta® and DM media combinations. The two highest doses of all the CeO2-NP/media combinations produced a significant increase in IL-18 in the WLL compared to control, as well as compared to their corresponding poorly-dispersed counterparts.
Figure 5.
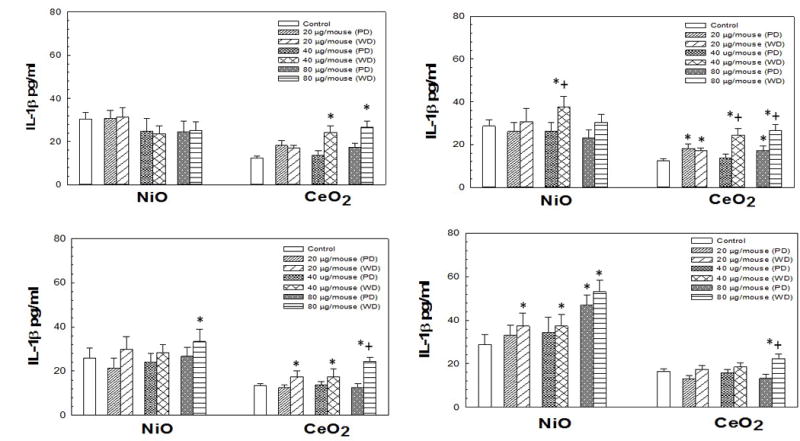
IL-18 levels induced by pharyngeal aspiration exposure to 0, 20, 40, or 80 μg/mouse of either poorly-dispersed (PD) or well-dispersed (WD) NiO- and CeO2-NP dispersed in differing dispersion medias (Panel A: pluronics, Panel B: PBS, Panel C: Survanta®, and Panel D: DM) at 1 day post-exposure. Values are given as means ± SE (n=8). *Significant difference of treatment group from control at 1 day post-exposure (p < 0.05). +Significant difference between the poorly-dispersed (5 min sonication) and well-dispersed (20 min sonication) NiO- and CeO2-NP solutions at same post-exposure timepoint (p < 0.05). IL-18 concentrations were determined as described in Methods.
IL-1β levels of NiO-NP dispersed in pluronics or PBS showed no trends in regard to dose or dispersion (Figure 6). However, the highest doses of the well-dispersed NiO-NP/Survanta® and DM combinations produced a significant increase in IL-1β compared to control (Figure 6). For the CeO2-NP pluronics combination, the two highest doses produced a significant increase in IL-1β compared to control. For the CeO2-NP PBS and Survanta® combinations, all doses of the well-dispersed particle/media combinations produced a significant increase in IL-1β compared to control, respectively. The highest dose of the CeO2-NP/DM combination produced a significant increase in IL-1β compared to control.
Figure 6.
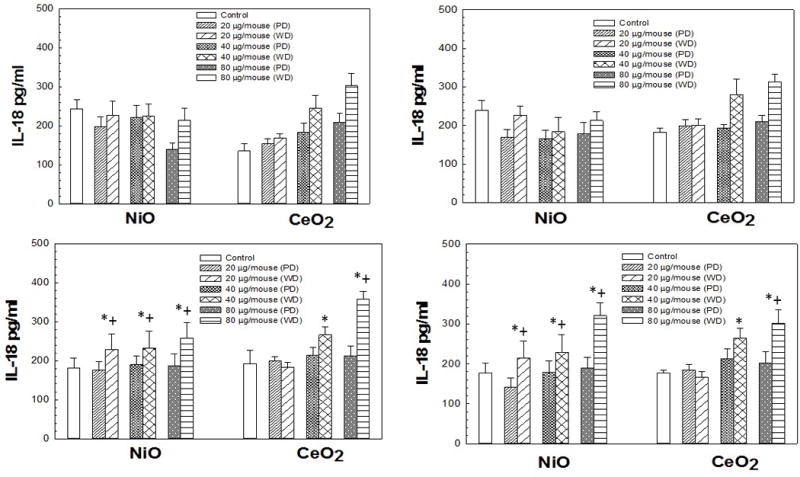
IL-1β levels induced by pharyngeal aspiration exposure to 0, 20, 40, or 80 μg/mouse of either poorly-dispersed (PD) or well-dispersed (WD) NiO- and CeO2-NP dispersed in differing dispersion medias (Panel A: pluronics, Panel B: PBS, Panel C: Survanta®, and Panel D: DM) at 1 day post-exposure. Values are given as means ± SE (n=8). *Significant difference of treatment group from control at 1 day post-exposure (p < 0.05). +Significant difference between poorly-dispersed (5 min sonication) and well-dispersed (20 min sonication) NiO- and CeO2-NP solutions at same post-exposure timepoint (p < 0.05). IL-1β concentrations were determined as described in Methods.
Taken together, the studies demonstrated that the well-dispersed particle suspensions facilitated activation of the NLRP3 inflammasome via pro-inflammatory cytokine production and cathepsin B release with DM and Survanta® as dispersants to a greater extent than the poorly dispersed particle suspensions in the same dispersing agents.
Electron Spin Resonance Studies
ESR studies were conducted to assess whether ROS production mediated the pulmonary bioactivity of CeO2-NP and NiO-NP. In order to better interpret where within the cells the particles were localizing and stimulating production of ROS, ESR was measured in a two-system set-up. First, radical generation facilitated by each nanoparticle was measured in artificial lysosome fluid (ALF), a fluid that mimics the lysosome acidic environment (ALF pH~4.5), or dispersion media (DM pH~7.4) that mimics the pH in a cell cytosol. Figure 7 shows results of measurements of radicals after the particles were exposed to a superoxide-generating system in ALF. CeO2-NP significantly decreased radical production, indicating a scavenging of superoxide radicals generated from xanthine/xanthine oxidase reaction. In contrast, NiO-NP had no signifi-cant effect in the ALF system on measured radicals compared to controls with no particles. When the reaction took place in DM (an experimental set-up used to mimic particle environment in a cell cytosol), NiO-NP reduced radical levels compared to the controls, while CeO2-NP had no apparent effect.
Figure 7.
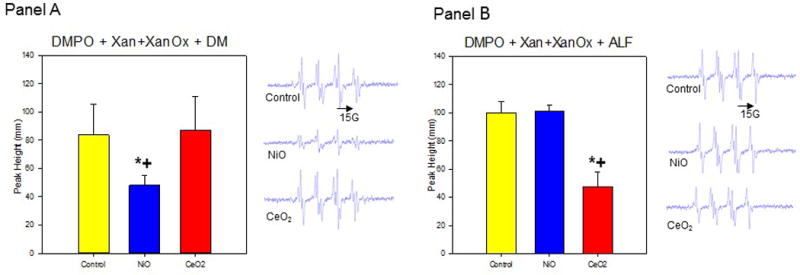
Induced superoxide radicals scavenged by particles in DM. DMPO (100 mM), Xan (2U), XanOx (35 mM), and particles in DM; Centerfield 3485G; sweep width 100 G; microwave frequency 9.79 GHz; power 63 mW; time constant 40.96 ms; Panel A (Left): Relative peak heights of ESR spectra (n=3); Panel A (Right): Representative ESR spectra. Induced super-oxide radicals scavenged by particles in ALF. DMPO (100 mM), Xan (2U), XanOx (35 mM), and particles in ALF; Centerfield 3485G; sweep width 100 G; microwave frequency 9.78 GHz; power 63 mW; time constant 40.96 ms; Panel B (Left): Relative peak heights of ESR spectra (n=3); Panel B (Right): Representative ESR spectra. *Significant difference between treatment group from control (p < 0.05). +Significant difference between CeO2-NP and NiO-NP solutions at same post-exposure timepoint (p < 0.05).
These findings indicate NiO-NP would likely be a better scavenger of radicals than CeO2-NP in a cell cytosol, whereas the opposite is the case in an acidic environment (resembling internal environment of lysosome). Specifically, CeO2-NP were shown to scavenge radicals in the ALF (pH~ 4.5) experimental environment but had no scavenging behavior in a physiological pH (7.4) experimental environment. Therefore, from the ESR analysis, it can be concluded the difference in scavenging abilities of NiO-NP and CeO2-NP appears pH-related. However, in neither experimental environment did either metal oxide increase ROS production.
Discussion
The current study was performed to evaluate how specific particle parameters, i.e. pre-exposure dispersion status and particle surface area, of two highly utilized metal oxide NP (NiO and CeO2) influence inflammasome activation during ENM-induced toxicity. First, this study assessed whether pre-exposure dispersion differences (like mean hydrodynamic diameter in various medias) of each utilized NP could be facilitating differences in pulmonary bioactivity between the two particles. As studies from our laboratory have previously reported, dispersion of NP can be improved using various suspension strategies. For example, in vivo and in vitro studies have used a variety of dispersants, including media that mimic the alveolar fluid lining of the lung (Hamilton et al. 2009; Sager et al. 2014), 1% Tween 80 in PBS (Muller et al. 2005; Warheit et al. 2004) and cell culture media (Shvedova et al. 2003). For all biological applications of NP, it is important to understand the interactions a NP has with the surrounding biological environment to allow better prediction of their biological impact. When exposed to biological fluids, proteins and biomolecules in the medium tend to associate with the NP surface. In many cases the dispersion media used to make the particle suspension may alter the surface reactivity of the NP, in turn, modifying its toxicity. Therefore, either delivering agglomerated NP or using a dispersion media that coats the particle could lead to variability in assessment of toxicity of a specific NP.
In the current study, NiO was chosen since we and others reported that NiO NP stimulate pulmonary inflammation and injury (Asati et al. 2010; Horie et al. 2011; Sager et al. 2016). CeO2 was chosen for comparison due to the evidence that it can display either a beneficial (anti-oxidant) or toxic (oxidant) effect (Asati et al. 2010) under differing experimental situations. The current study demonstrated that pharyngeal aspiration of mice with CeO2-NP produced inflam-mation (an increase in PMN), as well as promoted cell injury (enhanced LDH activity in first WLL fluid) in vivo. Previously, our laboratory has shown that pharyngeal aspiration of mice with NiO-NP resulted in very similar trends in inflammation and cell injury over the course of 7 day post-exposure (Sager et al. 2016). General trends that were common between the CeO2-NP and NiO-NP exposures were: 1) a greater response 7 day post-exposure compared to 1 day post- aspiration; 2) dose dependence from 20–80 μg/mouse; and, 3) a relationship between degree of dispersion and pulmonary response.
The various physicochemical parameters of ENM, such as size, shape, structure, and elemental constituents, make the investigation of their toxic effects complex and challenging (Pojlak-Blazi et al. 2010). Some of the paradigms for ENM-mediated toxicity include oxidative stress, inflammation, genetic damage, and the inhibition of cell division and cell death (Stone et al. 2007; Ju-Nam and Lead, 2008; Li et al. 2008; Johnston, et al. 2010). Many studies suggest that ROS generation and in turn, consequent oxidative stress and inflammasome activation are frequently associated with ENM toxicity (Nel et al. 2006; Shevdova et al. 2012). The physico-chemical characterization of ENM including particle size, surface charge, and chemical composi-tion is a key indicator for the resulting ROS response and injury, since many intrinsic properties of ENM have been proposed to be associated with ROS production (Nel et al. 2006). However, it may be inaccurate to assume ROS generation is a prerequisite for all inflammasome activation and ENM-induced toxicity. In fact, some studies have reported the direct toxicity of ENM without ROS generation (Wang et al. 2010). Nevertheless, ROS generation facilitating inflam-masome activation may be a major event during ENM-induced injury that needs to be thoroughly characterized in order to predict ENM-induced toxicity.
In contrast to the general assumption of ENM-induced ROS generation, a unique NP, CeO2, has been suggested as having anti-oxidant properties (Tarnuzzer et al. 2006; Perez et al. 2008; Asati et al. 2010). In fact, the chemistry of engineered CeO2 NP supports its role of a biological free radical scavenger or anti-oxidant, since CeO2 nanoparticles cycle between Ce+3 and Ce+4 valence states. This means they possess oxygen vacancies that allow the NP to act as a regenerative catalyst (Heckert et al. 2008). CeO2-NP have been identified as one of the most economically valuable manufactured NP in current production (Wang et al. 2008). CeO2 demon-strates high oxygen storage capabilities, high oxygen ionic conductivity, as well as strong absorption of UV radiation (Lui et al. 2013). Thus, CeO2-NP are widely utilized in a variety of applications ranging from glass and ceramic polishing agents (Eom and Choi, 2009) to solar panels and fuel cells (Corma et al. 2004). Along with many industrial applications, a variety of biomedical applications utilizing CeO2-NP have also been explored. For example, CeO2- NP are currently being utilized to protect against ultra-violet radiation induced damage.
Studies from our laboratory show that both NiO-NP (Sager et al. 2016) and CeO2-NP promote divergent pulmonary inflammatory responses. Characterization of pre-exposure disper-sion status was explored as a possible variable contributing to the differences in bioactivity. The DLS experiments showed that increasing the sonication time from 5 min to 20 min significantly decreased the hydrodynamic diameter of each particle suspension. For example, after 5 min sonication, CeO2-NP suspended in DM had a hydrodynamic diameter of 302.6 nm. However, upon increasing the sonication time to 20 min, the hydrodynamic diameter of the CeO2-NP suspended in DM significantly decreased to 89.3 nm. There were no significant differences with regard to average hydrodynamic diameter size between NiO-NP and CeO2-NP suspended in the same media after 5 or 20 min sonication, representing poorly dispersed or well dispersed suspen-sions respectively. For example, after 20 min sonication, the average diameter of NiO-NP suspended in PBS was 486.8 nm while that of CeO2-NP in PBS was 568.6 nm. Therefore, pre-exposure dispersion status was subsequently ruled out as a probable variable to explain differ-ences in bioactivity between the two particles.
Another possible variable affecting ENM bioactivity is activation of the NLRP3 inflam-masome following phagolysosomal membrane permeability and cathepsin B release. Current evidence suggests that bioactive nanoparticles could potentially increase phagolysosomal membrane permeability, causing release of cathepsin B and activation of the NLRP3 inflamma-some with subsequent release of IL-1β and IL-18 from alveolar macrophages (Beamer et al. 2012). It has been shown previously that the NLRP3 inflammasome is activated in vivo after pulmonary exposure to MWCNT (Sager et al. 2014). In addition to the phagolysosomal release of cathepsin B, other possible NLRP3 inflammasome activation pathways have been explored. In addition to engaging the phagocytic pathway to activate the NLRP3 inflammasome, a second model of NLRP3 inflammasome activation suggests increased production of ROS activates the NLRP3 inflammasome (Halle et al. 2008; Jin and Flavell, 2010).
With regard to the first model of inflammasome activation, the results showed that both NiO-NP and CeO2-NP facilitated a significant increase in cathepsin B release. However, for cathepsin B release, there was no significant difference between the effects of NiO-NP and CeO2-NP. Both NiO-NP and CeO2-NP (well-dispersed, 80 μg/mouse, 1 day post-exposure) produced a significant increase not only in cathepsin B, but also in levels of IL-1β and IL-18. Cathepsin B levels were not significantly different between NiO-NP- and CeO2-NP-exposed mice. However, mice exposed to 80 μg NiO-NP did have a greater increase in inflammatory IL-1β and IL-18. Thus, due to the similar trend in cathepsin B levels for the NiO-NP- and CeO2-NP-exposed mice, it was concluded that both ENM facilitated NLRP3 inflammasome activation via phagolysosome disruption. However, differences in responses of IL-1β and IL-18 between NiO-NP- and CeO2-NP-exposed mice may not be explained solely by cathepsin B release.
ESR studies were conducted to identify whether ROS production played a role in facilita-ting the difference in bioactivity between the NiO-NP and CeO2-NP. Previous studies identified CeO2 as a unique nanomaterial because it can, in certain experimental situations, exhibit anti-inflamm-tory properties. More specifically, CeO2 has been reported to scavenge ROS/possess superoxide-dismutase-like activity (Korsvik et al. 2007; Perez et al. 2008). If this was in fact, the case, CeO2 acting as an anti-oxidant could help explain the difference in the bioactivities of the two types of NP here.
Previous studies [Asati et al. 2010] reported that nanoceria displayed optimal anti-oxidant properties at physiological pH, but behaves as an oxidase at acidic pH. Asati et al. also reasoned that CeO2-NP cytotoxicity could depend on subcellular localization. Once in a cell, NP toxicity could depend on whether the NP are localized in particular cellular organelles (such as lysos-omes which are acidic) or distributed in the cytoplasm (which is at neutral pH). However, the outcome of the current ESR studies showed that in an acidic environment (ALF, pH~ 4.5) mimicking the lysosome internal environment, CeO2-NP acted as a scavenger, significantly reducing free radicals. At a physiological pH (DM, pH ~ 7.4) to mimic cell cytosol, CeO2-NP did not scavenge radicals. In contrast, NiO-NP at a physiological pH (in DM) did significantly scavenge free radicals; in an acidic environment (ALF) NiO-NP did not reduce radicals. This outcome supports the conclusions of previously-published studies that concluded there was a role for pH as a facilitator of the anti-oxidant behavior of CeO2. Furthermore, these findings also indicate that ENM, once co-localized inside a cell, do not require increased ROS production to activate the NLRP3 inflammasome.
Conclusions
Taken together, the current study showed that pre-exposure dispersion status was a factor that contributed to the post-exposure pulmonary bioactivity. This study showed that the smaller the hydrodynamic diameter of a particle suspension, the greater the pulmonary bioactivity of the particle suspension. However, pre-exposure dispersion differences between NiO-NP suspensions and CeO2-NP suspensions used in this study cannot contribute to helping explain the difference between pulmonary bioactivities of NiO-NP and CeO2-NP. These differences in bioactivity are likely due in part to NiO-NP facilitating production of IL-1β and IL-18. Further, in this study, both oxides acted as free radical scavengers, depending on the experimental environmental pH. CeO2-NP acted as free radical scavenger in an environment mimicking a lysosome while NiO-NP acted as scavenger in a physiological pH (environment mimicking cytosol). Another possible factor contributing to the difference in bioactivity of the CeO2-NP and NiO-NP may be the vast difference in their surface area; BET specific area of NiO-NP was 113.0 m2/g while that of CeO2-NP was 46.8 m2/g. Similar results have been noted in comparing pulmonary responses to ultrafine and fine titanium dioxide (Sager et al. 2008). Therefore, specific surface area may help explain the differences in bioactivity of ENM.
Acknowledgments
The authors would like to thank Diane Schwegler-Berry for her assistance on obtaining the TEM and SEM images presented in the MS. Mention of brand name does not constitute product endorsement. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Funding
This work was supported by NIH grant F32 ES021341.
Abbreviations
- ALF
Artifical lysosomal fluid
- AM
Alveolar macrophage
- ANOVA
Analysis of variance
- BAL
Bronchoalveolar lavage
- BET
Brunauer-Emmett-Teller
- CeO2
Cerium oxide
- CeO2-NP
Cerium oxide nanoparticles
- CAR
Cilia-associated-respiratory
- DLS
Dynamic light scattering
- DM
Dispersion media
- DMPO
5,5-dimethyl-1-pyroline-oxide
- ENM
Engineered nano-materials
- ESR
Electron spin resonance
- FESEM
Field emission scanning electron microscopy
- IL
Interleukin
- IP
Intraperitoneal
- K3CrO8
potassium tetra-peroxochromate
- LDH
Lactate dehydrogenase
- μm
Micrometer
- μg
Microgram
- mg
Milligram
- ml
Milliliter
- mm
Millimeter
- NiO
Nickel oxide
- NiO-NP
Nickel oxide nanoparticles
- NP
nanoparticle
- PBS
Phosphate-buffered saline
- PMN
Polymorphonuclear leukocyte
- PD
poorly-dispersed
- RFU
Relative fluorescence units
- TEM
Transmission electron microscopy
- WD
Well-dispersed
- WLL
Whole lung lavage
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
TMS and MW carried out all of the in vivo experiments involved in this study including the pharyngeal aspirations and animal sacrifices. TMS drafted the MS and performed statistical analysis. TMS, VC, and AH conceived of the study and participated in its design. SL and AM carried out the ESR particle analysis. TMS, VC, DWP, and AH participated in the study coordination, data analysis and interpretation, and helped draft the MS. All authors read and approved the final MS.
References
- Asati A, Santra S, Kaittanis C, Perez M. Surface-charge-dependent cell localization and cytotoxicity of cerium oxide nanoparticles. ACS Nano. 2010;4:5321–5331. doi: 10.1021/nn100816s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffan M, Bottero J, Chaneac C, Rose J. Inorganic manufactured nanoparticles: How their physico-chemical properties influence their biological effects in aqueous environments. Nanomedicine. 2010;5:999–1007. doi: 10.2217/nnm.10.61. [DOI] [PubMed] [Google Scholar]
- Beamer C, Girtsman T, Seaver B, Finsaas K, Migliaccio C, Perry V. IL-33 mediates mutli-walled carbon nanotube (MWCNT)-induced airway hyper-reactivity via the mobilization of innate helper cells in the lung. Nanotoxicology. 2012;7:1070–1081. doi: 10.3109/17435390.2012.702230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunauer S, Emmett PH, Teller E. Adsorption of gases in multi-molecular layers. JACS. 1938;60:309–319. [Google Scholar]
- Chithrani B, Ghazani A, Chan W. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- Corma A, Atienzar P, Garcia H, Chane-ching J. Hierarchially-meso-structured doped CeO2 with potential for solar-cell use. Nat Mater. 2004;3:394–397. doi: 10.1038/nmat1129. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Brown D, Clouter A, Duffin R, MacNee W, Renwick L, Tran L, Stone V. The pulmonary toxicology of ultrafine particles. J Aerosol Med. 2002;15:213–220. doi: 10.1089/089426802320282338. [DOI] [PubMed] [Google Scholar]
- Eom H, Choi J. Oxidative stress of CeO2 nanoparticles via p38-Nfr-2 signaling pathway in human bronchial epithelial cell, Beas-2B. Toxicol Lett. 2009;187:77–83. doi: 10.1016/j.toxlet.2009.01.028. [DOI] [PubMed] [Google Scholar]
- Gillespie P, Kang G, Elder A, Gelein R, Chen L, Moreira A. Pulmonary response after exposure to inhaled nickel hydroxide nanoparticles: Short and long-term studies in mice. Nanotoxicology. 2010;4:106–109. doi: 10.3109/17435390903470101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle A, Hornung V, Petzold G, Stewart C, Monks B, Reinheckel T. The NALP3 inflam-masome is involved in the innate immune response to amyloid-β. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R, Wu N, Porter D, Buford M, Wolfarth M, Holian A. Particle length-dependent titanium dioxide nanomaterials’ toxicity and bioactivity. Particle Fiber Toxicol. 2009;6:35–41. doi: 10.1186/1743-8977-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckert E, Karakoti A, Seal S, Self W. The role of cerium redox state in the SOD mimetric activity of nanoceria. Biomaterials. 2008;29:2705–2709. doi: 10.1016/j.biomaterials.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M, Fukui H, Nishio K, Endoh S, Kato H, Fujita K. Evaluation of acute oxidative stress induced by NiO2 nanoparticles in vivo and in vitro. Metallomics. 2011;11:1244–1252. doi: 10.1539/joh.l10121. [DOI] [PubMed] [Google Scholar]
- Jin C, Flavell R. The missing link: How the inflammasome senses oxidative stress. Immunol Cell Biol. 2010;88:510–512. doi: 10.1038/icb.2010.56. [DOI] [PubMed] [Google Scholar]
- Johnston H, Hutchison G, Christensen F, Peters S, Hankin S, Stone V. A review of the in vivo and in vitro toxicity of silver and gold particulates: Particle attributes and biological mechanisms responsible for the observed toxicity. Crit Rev Toxicol. 2010;40:328–346. doi: 10.3109/10408440903453074. [DOI] [PubMed] [Google Scholar]
- Ju-Nam Y, Lead J. Manufactured nanoparticles: Overview of their chemistry, interactions and potential environmental implications. Sci Total Environ. 2008;400:396–414. doi: 10.1016/j.scitotenv.2008.06.042. [DOI] [PubMed] [Google Scholar]
- Keller A, Wang H, Zhou D. Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ Sci Technol. 2010;44:1962–1967. doi: 10.1021/es902987d. [DOI] [PubMed] [Google Scholar]
- Korsvik C, Patil S, Seal S, Self W. Vacancy engineered ceria oxide nanoparticles catalyze superoxide dismutase activity. Chem Commun. 2007;35:1056–1058. doi: 10.1039/b615134e. [DOI] [PubMed] [Google Scholar]
- Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4:26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- Li JJ, Muralikrishnan S, Ng CT, Yung LY, Bay HB. Nanoparticle-induced pulmonary toxicity. Experimental Biology and Medicine. 2010;235(9):1025–1033. doi: 10.1258/ebm.2010.010021. [DOI] [PubMed] [Google Scholar]
- Li N, Xia T, Nel A. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Rad Biol Med. 2008;44:1689–1699. doi: 10.1016/j.freeradbiomed.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui T, Hon M, Teoh L. Structure and optical properties of CeO2 nanoparticles synthesized by precipitation. J Elect Mater. 2013;42:2536–2543. [Google Scholar]
- Manke A, Wang L, Rojanasakul Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Bio-Med Res Intl. 2013;201:1–15. doi: 10.1155/2013/942916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques M, Loebenberg R, Almukainzi M. Simulated biological fluids with possible application in dissolution testing. Dissol Technol. 2011:15–28. [Google Scholar]
- Monteiller C, Tran L, MacNee W, Faux S, Jones A, Miller B, Donaldson K. The pro-inflammatory effects of low-toxicity low-solubility particles, nanoparticles and fine particles, on epithelial cells in vitro: Role of surface area. Occup Environ Med. 2007;64:609–615. doi: 10.1136/oem.2005.024802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C, Gole A, Stone J. Gold nanoparticles in biology: Beyond toxicity to cellular imaging. Acc Chem Res. 2008;41:1721–1730. doi: 10.1021/ar800035u. [DOI] [PubMed] [Google Scholar]
- Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- Perez JM, Asati A, Nath S, Kaittanis C. Synthesis of biocompatible dextran-coated nanoceria with pH-dependent anti-oxidant properties. Small. 2005;4:552–556. doi: 10.1002/smll.200700824. [DOI] [PubMed] [Google Scholar]
- Pojlak-Blazi M, Jaganjac M, Zarkovic N. Handbook of Nanophysics: Nanomedicine and Nanorobotics. New York: CRC Press; 2010. Cell oxidative stress: Risk of metal nanoparticles; pp. 17–22. [Google Scholar]
- Porter D, Barger M, Robinson V, Leonard S, Landsittel D, Castranova V. Comparison of low doses of aged and freshly fractures silica on pulmonary inflammation and damage in the rat. Toxicology. 2002;175:63–71. doi: 10.1016/s0300-483x(02)00061-6. [DOI] [PubMed] [Google Scholar]
- Powers K, Palazuelos M, Moudgil B, Roberts S. Characterization of the size, shape, and state of dispersion of nanoparticles for toxicological studies. Nanotoxicology. 2007;1:42–51. [Google Scholar]
- Rao G, Tinkle S, Weissman D, Antonini J, Kashon M, Salmen R. Efficacy of a technique for exposing the mouse lung to particles aspirated from the pharynx. J Toxicol Environ Health. 2003;66:1441–1452. doi: 10.1080/15287390306417. [DOI] [PubMed] [Google Scholar]
- Sager T, Porter D, Robinson V, Lindsley W, Schwegler-Berry D, Castranova V. Improved method to disperse nanoparticles for in vitro and in vivo investigation of toxicity. Nanotoxi-cology. 2007;1:118–129. [Google Scholar]
- Sager T, Kommineni C, Castranova V. Pulmonary response to intratracheal instillation of ultrafine vs. fine titanium dioxide: Role of surface area. Particle Fiber Toxicol. 2008;5:17. doi: 10.1186/1743-8977-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager T, Castranova V. Surface area of particle administered versus mass of particle administered in determining the pulmonary toxicity of ultrafine and fine carbon black: a sub-chronic in vivo exposure. Particle Fiber Toxicol. 2009;6:1. doi: 10.1186/1743-8977-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager T, Wolfarth M, Andrews M, Hubbs A, Porter D, Wu N. Analysis of effect of multi-walled carbon nanotube surface modification on bioactivity and inflammasome activa-tion. Nanotoxicology. 2014;8:317–327. doi: 10.3109/17435390.2013.779757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager T, Wolfarth M, Keane M, Porter D, Castranova V, Holian A. Effects of nickel-oxide nanoparticle pre-exposure dispersion status on bioactivity in the mouse lung. Nanotoxi-cology. 2016;10:151–161. doi: 10.3109/17435390.2015.1025883. [DOI] [PubMed] [Google Scholar]
- Shvedova A, Pietroiusti P, Fadeel B, Kagan V. Mechanisms of carbon nanotube-induced toxicity: Focus on oxidative stress. Toxicol Appl Pharmacol. 2012;261:121–133. doi: 10.1016/j.taap.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone V, Johnston H, Clift J. Air pollution, ultrafine and nanoparticle toxicology: Cellular and molecular interactions. Trans Nanobiosci. 2007;6:331–340. doi: 10.1109/tnb.2007.909005. [DOI] [PubMed] [Google Scholar]
- Tarnuzzer R, Colon J, Patil S, Seal S. Vacancy engineered ceria nanostructures for protection from radiation-induced cellular damage. Nano Lett. 2005;5:2573–2577. doi: 10.1021/nl052024f. [DOI] [PubMed] [Google Scholar]
- Wang L, Mercer R, Rojanasakul Y. Direct fibrogenic effects of dispersed single-wall carbon nanotubes on human lung fibroblasts. J Toxicol Environ Health. 2010;73:410–422. doi: 10.1080/15287390903486550. [DOI] [PubMed] [Google Scholar]
- Wang R, Crozier P, Sharma R, Adams J. Measuring the redox activity of individual catalytic nanoaprticles in cerium-based oxides. Nano Lett. 2008;8:962–967. doi: 10.1021/nl073135c. [DOI] [PubMed] [Google Scholar]
- Wang S, Lu W, Tovmachenko O, Rai U, Yu H, Ray P. Challenge in understanding size and shape dependent toxicity of gold nanomaterials in human skin keratinocytes. Chem Phys Lett. 2008;463:145–149. doi: 10.1016/j.cplett.2008.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler P, Hubbs A, Battelli L, Castranova V. Role of inducible nitric oxide in silica-induced pulmonary inflammation and fibrosis. J Toxicol Environ Health. 2004;67:1021–1026. doi: 10.1080/15287390490447296. [DOI] [PubMed] [Google Scholar]


