Abstract
Pancreatitis represents nearly 3% of acute admissions to general surgery in United Kingdom hospitals and has a mortality of around 1%-7% which increases to around 10%-18% in patients with severe pancreatitis. Patients at greatest risk were those identified to have infected pancreatic necrosis and/or organ failure. This review seeks to highlight the potential vascular complications associated with pancreatitis that despite being relatively uncommon are associated with mortality in the region of 34%-52%. We examine the current evidence base to determine the most appropriate method by which to image and treat pseudo-aneurysms that arise as the result of acute and chronic inflammation of pancreas. We identify how early recognition of the presence of a pseudo-aneurysm can facilitate expedited care in an expert centre of a complex pathology that may require angiographic, percutaneous, endoscopic or surgical intervention to prevent catastrophic haemorrhage.
Keywords: Complication of pancreatitis, Splenicartery, Pancreatitis, Haemorrhage, Pseudoaneurysm
Core tip: Pancreatitis represents nearly 3% of acute admissions to general surgery in United Kingdom hospitals. The presence of a fluid collection, necrosis and infection can directly contribute to such vascular complications which are associated with a significant morbidity and mortality. Early recognition of the presence of a pseudo-aneurysm can facilitate expedited care in an expert centre of a complex pathology that may require angiographic, percutaneous, endoscopic or surgical intervention to prevent catastrophic haemorrhage.
INTRODUCTION
Pancreatitis represents nearly 3% of acute admissions to general surgery in United Kingdom hospitals[1]. Although this may seem like a relatively small proportion of patients the impact of the pancreatitic patient has a significant influence on hospital services due to the sizeable morbidity and mortality associated with pancreatitis. The annual incidence of acute pancreatitis ranges from 13 to 45 per 100000 persons worldwide and is around 13-45 per 100000 people in the United Kingdom which is increasing at a rate of 2.7% each year[2-6]. The number of admissions due to pancreatitis in the US has doubled since 1988. There are now nearly 275000 admissions each year due to pancreatitis[7,8]. It is estimated that between 20%-30% of patients will have a further episode of acute pancreatitis and 10% of patients will go on to develop chronic pancreatitis[9-12]. The current incidence rates of chronic pancreatitis are around 5-23 per 100000 people[5,13,14]. Pancreatitis has a mortality of around 1%-7% which increases to around 10%-18% in patients with severe pancreatitis[1-4,15]. Patients at greatest risk were those identified to have infected pancreatic necrosis and/or organ failure[16].
In order to appropriately manage patients with pancreatitis multiple scoring systems have been created to try to determine the severity of pancreatitis and the risk of morbidity and mortality including the Glasgow score, Ransom score, Balthazar score, Apache 2 score, and the modified Marshall score[17-19]. Many continue to be used routinely in clinical practice to improve the treatment of patients with pancreatitis. Understanding that certain patients with more severe pancreatitis have a higher risk of morbidity and mortality allows clinicians to maintain a high index of suspicion in order to identify early the complications of pancreatitis. The revised Atlanta guidelines identify two key phases to acute pancreatitis. In early phase it is associated with systemic disturbances as the result of the host response to local pancreatic injury[17] (Table 1). The cytokine cascade as the result of pancreatic inflammation can be clinically manifested as a systemic inflammatory response syndrome which in turn may precipitate organ failure. The presence and duration of organ failure is a key determinant in defining severity of acute pancreatitis (Table 2). Late phase pancreatic inflammation is defined by the presence of ongoing systemic symptoms of inflammation such as persistent organ failure or local complications.
Table 1.
Early vs late pancreatitis
| Early phase pancreatitis | Late phase pancreatitis |
| Systemic disturbances result from the host response to local pancreatic injury. | Persistence of systemic signs of inflammation. |
| Clinical manifestation with associated SIRS response. | Presence of local complications. |
| Usually lasts less than one week but may extend into the second week. | Compensatory inflammatory response syndrome. |
| Severity determined by presence of organ failure. Transient < 48 h. Persistent > 48 h. |
Table 2.
Defining pancreatic severity
| Mild acute pancreatitis | No organ failure |
| No local complications | |
| Moderately severe acute pancreatitis | Organ failure that resolves within 48 h (transient organ failure) and/or |
| Local or systemic complications without persistent organ failure | |
| Severe acute pancreatitis | Persistent organ failure (single/multiple) > 48 h |
Local complications of pancreatitis often cause patients to develop a late phase pancreatitis. Interstitial oedematous pancreatitis with or without necrosis may be complicated by the presence of pancreatic and peri-pancreatic collections. The presence of such collections can be broadly divided into five categories. Acute peri-pancreatic fluid collections usually arise in the acute phase of pancreatitis and are confined with normal anatomical planes. They commonly remain sterile however if they persist for greater than 4 wk they commonly form into a pseudocyst. A pancreatic pseudocyst is formed beyond 4 wk and demonstrates a walled off fluid collection in the absence of necrosis. The presence of necrosis can be categorised as either an acute necrotic collection (ANC) or a walled off necrosis (WON). Infected necrosis may occur in either ANC or WON and will present with the presence of gas within the collection on imaging or positive microbiological samples taken directly from the area of necrosis (Table 3). Acute and chronic inflammation as a result of pancreatitis can cause both thrombotic and haemorrhagic complications. The presence of a fluid collection, necrosis and infection can directly contribute to such vascular complications which are associated with a significant morbidity and mortality.
Table 3.
Defining pancreatic and peri-pancreatic collections
| Acute peri-pancreatic fluid collection | Don not have well defined walls |
| Homogenous, confined to normal fascial planes in retroperitoneum | |
| May be multiple | |
| Likely to develop into a pseudocyst if they persist > 4 wk | |
| Pancreatic pseudocyst | Fluid collection in peri-pancreatic tissues |
| Occasionally partly/totally intra-pancreatic | |
| Well defined wall with essentially no solid material | |
| Occur typically after 4 wk | |
| Acute necrotic collection | Fluid collection within the first 4 wk containing necrotic tissue and fluid. |
| Presence of necrosis differentiates it from APFC | |
| Walled off necrosis | Necrotic tissue contained within an enhancing wall of reactive tissue |
| Usually occurs > 4 wk after the onset of necrotising pancreatitis | |
| Infected necrosis | Presence of gas within collection |
| Positive cultures post FNA |
APFC: Acute peri-pancreatic fluid collection.
ARTERIAL COMPLICATIONS IN ACUTE PANCREATITIS
Incidence, causes, and presentations
Vascular complications are relatively uncommon and occur in between 1%-23% of patients with pancreatitis. Venous complications are significantly more common than arterial complications. Arterial complications occur in around 1.3%-10% of patients[20-28]. Thrombosis of the portal and splenic vein has been shown to occur up to 23% and 22% of patients with pancreatitis respectively[23,29]. Portal vein thrombosis increases to 30% in those with peri-pancreatic collection and as high as 57% in patients with necrotising pancreatitis (Table 4)[29].
Table 4.
Different types and incidence of vascular complications in pancreatitis
| Vascular complications of pancreatitis | Incidence |
| Arterial complications | 1.3%-10% of patients with pancreatitis |
| Ruptured pseudo-aneurysm | 60% of all acute haemorrhage in pancreatitis |
| Haemorrhagic pseudocysts without pseudoaneurysms | 20% of all acute haemorrhage in pancreatitis |
| Capillary, venous or small vessel haemorrhage | 20% of all acute haemorrhage in pancreatitis |
| Venous complications | 1%-23% of patients with pancreatitis |
| Portal vein thrombosis | 23% of patients with pancreatitis |
| Splenic vein thrombosis | 22% of patients with pancreatitis |
| Superior mesenteric vein thrombosis | 19% of patients with pancreatitis |
It is important to identify arterial complications early and treat aggressively as they are associated with a mortality of between 34%-52%[26,28,30-35]. Sixty percent of all acute haemorrhage in the presence of pancreatitis occurs as the result of ruptured pseudo-aneurysms in the presence of necrotising pancreatitis. Haemorrhagic pseudocysts without pseudoaneurysms and capillary, venous or small vessel haemorrhage only account for approximately 20% of cases[22].
Arterial complications often arise as the result of arterial wall disruption in the presence of free lipolytic and proteolytic enzymes. Severe pancreatic inflammation and necrosis leads to the disruption of pancreatic tissue and subsequent pancreatic fluid release which has a high enzymatic content. These disruptive processes are further proliferated by the cytokine cascade that develops due to pancreatitis. Such processes in combination with pressure necrosis can lead to pseudo-aneurysm formation or spontaneous arterial rupture. Bleeding may also develop from the disruption of the wall of a pseudocyst or WON.
The presence of radiologically inserted drains can also cause direct trauma to vessels. Drains can perpetuate local inflammation which in turn can further diminish arterial wall integrity. Surgical intervention in the form of necrosectomy can disrupt arterial integrity and lead to the development of arterial complications. Multi organ failure, necrosis, peri-pancreatic infection, long-term anticoagulation and underlying vasculitis all increase the risk of vascular complications[35]. Pancreatic necrosis is specifically associated with an increased risk of major haemorrhage[28]. There is no evidence to confirm that fungal infections confer an increased risk of pseudoaneurysm formation in pancreatitis as compared to bacterial infection, however anecdotally in immunosuppressed patients fungal infections are closely associated with pseudoaneurysm formation. Due to the significant structural integrity of arteries the time taken for a pseudo-aneurysm to form can vary greatly from weeks to even years[22,29,36].
Although we are aware of certain risk factors for haemorrhage associated with pseudo-aneurysms it is challenging to quantify the impact that acute vs chronic inflammation has on vessel stability. Current evidence on acute haemorrhage is predominantly small volume case series of a mixed population of patients who have been treated for acute haemorrhage. However Zyromski et al[37] identified 24 patients with a pseudoaneurysm associated with pancreatitis of which 22 patients had acute on chronic pancreatitis and 2 patients presented during their first episode of acute pancreatitis. Twenty-one patients had a collection or pseudocyst and 2 had identifiable necrosis. Sethi et al[38] demonstrated that of those undergoing mesenteric angiography for bleeding associated with pancreatitis 8 had acute pancreatitis and 8 had chronic pancreatitis. Within the acute population three patients were diagnosed with necrotising pancreatitis and one required necrosectomy. Seven of the eight patients with chronic pancreatitis had confirmed pseudocyst formation and one developed acute necrosis on a background of chronic pancreatitis. Udd et al[21] looked at 33 patients with chronic pancreatitis and bleeding pancreatic pseudoaneurysms. They showed that 1.7% of admissions related to chronic pancreatitis were due to haemorrhage. They found that within the study population seven had pseudocysts two of which required percutaneous drainage and six patients had undergone a previous operation. There is no definitive evidence to show that patients with chronic pancreatitis and pseudocyst formation are at greater risk of developing arterial complications as compared to patients with severe acute pancreatitis with necrosis yet both are at high risk.
Symptomatic pseudo-aneurysms can present in varying ways however the most common symptom described is abdominal pain. Around 29.5% of patients will have abdominal pain which may reflect bleeding in the retroperitoneum. However there is a degree of ambiguity in the cause of such symptoms as pancreatitis in itself is painful. Bleeding into the gastrointestinal tract can occur in around 26.5% with haemosuccus pancreaticus (haemorrhage into pancreatic duct) being present in 20% of patients and also bleeding in pancreatic pseudocyst (Figure 1)[39]. Symptomatology is also dependent on the anatomical location of the pseudo-aneurysm.
Figure 1.
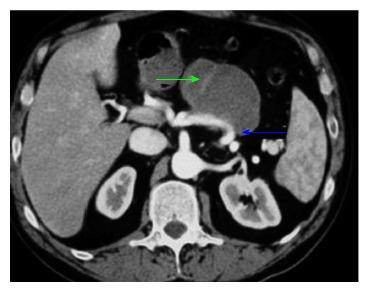
Computed tomography arterial and venous phase showing a pseudocyst (green arrows) eroding the splenic artery (blue arrows)[90].
Site of pseudoaneurysm
The most common site for pseudo-aneurysms is the splenic artery which occurs 35%-50% of occasions (Figures 2 and 3). The gastroduodenal and pancreaticoduodenal vessels each account for 20%-25% of pseudo-aneurysms. Mesenteric, colic and hepatic vessels commonly make up the remaining sites[20,31,40-50]. Aortic involvement can occur in up to 0.5% of cases[51,52]. Case reports have also identified individual cases of the left sub-capsular renal artery and the left phrenic artery[53,54]. Vessels in close proximity to the gastrointestinal tract have a greater risk of manifesting as luminal bleeding.
Figure 2.
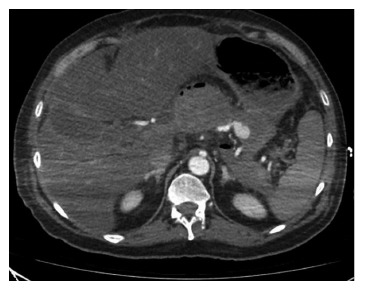
Computed tomography arterial and venous phases showing a pseudoaneurysm in a patient with necrotizing pancreatitis.
Figure 3.
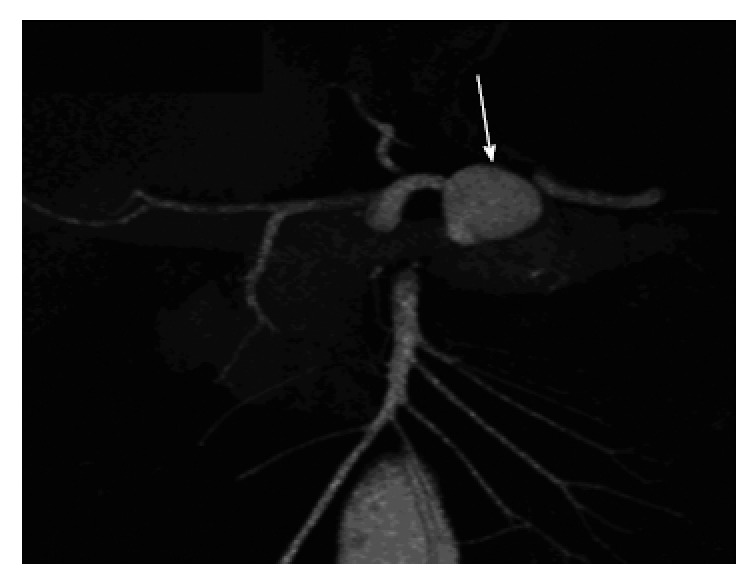
Three-D coronal maximum-intensity-projection computed tomography image in a 45-year-old man who had remote history of pancreatitis presented with back pain showing 2.0-cm pseudoaneurysm (arrow) arising from splenic artery[91].
Imaging diagnosis
Identification of a pseudo-aneurysm is typically by CT imaging however the indication for imaging can vary[22,30,55]. Ultrasound has been shown to be less sensitive than CT in identifying such complications however, still has an important role in identifying venous complications such as portal vein thrombosis[29,56]. It may also have a limited role in those allergic to iodine and certain cases of renal insufficiency[57]. Current guidance for patients with complicated pancreatitis with evidence of significant inflammation, infection and/or organ dysfunction is to undergo planned cross-sectional imaging in order to determine whether there is a complication of pancreatitis. Cross-sectional imaging for pancreatitis should occur a minimum of 72 h after the onset of symptoms unless there is diagnostic ambiguity[58]. At present there is no strong evidence to differentiate between the use of CT and MRI however MRI may show greater detail in differentiating the different types of peri- and intra-pancreatic collections, such as necrosis with pus, necrosis without pus and fluid collection without necrosis; these findings may be better defined by MRI[17,59].
Present evidence supports contrast enhanced triple phase CT as the best imaging modality for vascular complications[34,60,61]. When a pseudo-aneurysm is identified subsequent management can be planned accordingly with the aid of both CT and angiographic guidance[62,63]. Due to the greater accessibility and improved vascular imaging triple phase contrast CT has become the imaging modality of choice in severe pancreatitis. Cross-sectional imaging may identify local pancreatic complications that may benefit from antibiotics, radiological intervention or very rarely surgery which in turn will aim to potentially speed the recovery of the patient. Less frequently pseudo-aneurysms will rupture causing acute haemodynamic compromise and emergency imaging is performed to determine the underlying cause. There is currently little evidence to specify the timing of follow up imaging and this should be guided by clinical assessment.
MANAGEMENT OF ACUTE HAEMORRHAGE
Historically the management of pseudo-aneurysm was primarily surgical however over the past two decades the first line intervention is now interventional radiology. Since there have been marked improvements in the field of interventional radiology the spectrum of pathologies that can be treated successfully has expanded greatly. In the United Kingdom the provision of interventional radiology services varies significantly and is concentrated in tertiary centres. This is particularly evident out of hours where few hospitals have a 24-h on call interventional radiology service. A study of the provision of services for acute upper GI haemorrhage has shown that of hospitals in the United Kingdom 46% were able to offer out of hours interventional radiology services whether that be at the hospital in question or via a network[64]. Acute haemorrhage in district general hospitals is therefore unlikely to be treated with interventional radiology and certainly the mortality of acute haemorrhage from pseudo-aneurysm secondary to pancreatitis may reflect this. Some patients in the acute setting may be suitable for transfer depending on haemodynamic stability. In a semi-elective setting appropriate patients can be transferred to regional centres with greater expertise in managing pseudo-aneurysms where they would have greater facilities to manage such complicated patients.
Trans-arterial embolisation of pseudo-aneurysms is commonly achieved by the placement of stainless steel or complex helical coils in the proximal and distal feeding vessel in order to isolate inflow and prevent back filling via collaterals (Figure 4). Haemostasis can be augmented by N-butylcyanoacrylate glue, ethiodised oil, gelfoam, thrombin, polyvinyl alcohol and other particles[21,37,38,41,43,65-69]. Increasingly in visceral aneurysm treatment covered stents have been used to exclude inflow to the aneurysm however they are less commonly used in the treatment of pseudo-aneurysms. Complications can arise both in failed and successful procedures. Access related complications might occur in the form of haematoma, dissection, pseudo-aneurysm and emboli. Complications may arise local to the pseudo-aneurysm including rupture, end vessel infarction, dissection and coil migration.
Figure 4.
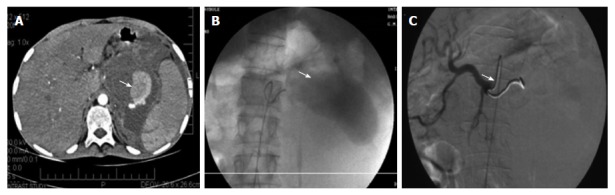
Splenic artery pseudoaneurysm before and after embolization. A: Post contrast computed tomography scan showing the pseudoaneurysm rising from the splenic artery; B: Pre embolization selective splenic arterial DSA angiography image showing pseudoaneurysm; C: Post embolization DSA image showing the coils inside the splenic artery with its resultant embolization[92].
Mortality of pseudo-aneurysms secondary to pancreatitis has been shown to be between 34%-52%[26,28,30-35], however the literature shows significant improvement in case series of those who have undergone radiological intervention. It is important to note that a proportion of patients will have been too unstable for intervention and therefore this mortality may not be reported as widely in the studies of radiological or surgical intervention. Kim et al[65] in 2015 demonstrated that of 37 patients undergoing angiographic embolisation it was unsuccessful in only three patients. Two of whom re-bled from the primary pseudo-aneurysm and one developed a new pseudo-aneurysm. Of the 34 patients who underwent successful angiographic embolisation there were no episodes of re-bleeding with a mean follow up of 38 wk. Two patients died as a result of splenic abscesses and subsequent sepsis due to procedure related splenic infarction giving a mortality of 5%.
Udd et al[21] in 2007 identified 33 patients who were identified to have pancreatitis related pseudo-aneurysms at angiography however only 23 were suitable for embolization. Four of the 23 re-bled and only three of those patients received successful repeat radiological intervention. Radiological intervention was successful in 22/33 patients (67%). The remaining 10 patients went on to require surgical cessation of bleeding. One patient who underwent interventional radiology and one patient who underwent surgery died giving a mortality of 6%. Bergert et al[20] 2005 identified 35 patients with bleeding secondary to pancreatitis related pseudo-aneurysms. Twenty six patients were identified to have pseudo-aneurysms at angiography however angiographic embolisation was the primary treatment in only 16. Two patients re-bled post-angiographic embolization. Nineteen patients required surgery to potentially stop bleeding. Three patients undergoing radiological intervention and four patients undergoing surgery died giving a mortality of 20%. The discrepancies in the number of patients undergoing radiological intervention and the mortality between Bergert et al[20] and Kim et al[65] may be reflected in the improvement of clinical practices from the related study dates of 1993-2004 and 2000-2012 respectively. Over the past two decades radiological provision, equipment and technical skills have improved significantly.
Tulsyan et al[68] in 2007 identified a mixed demographic of elective and non-elective patients (48 patients) with both visceral artery aneurysms and pseudo-aneurysms (28 patients) which showed marked success of radiological intervention. Forty seven of the 48 patients were embolised radiologically however three patients re-bled. Four patients died in the perioperative period all of whom were among the 22 patients who required urgent or emergent intervention because of hemodynamic instability giving a mortality of 18% in the non-elective group. Zyromski et al[37] in 2007 identified 37 patients with visceral pseudo-aneurysms 24 as the result of pancreatitis and 13 as the result of pancreatic surgery. Within the pancreatitis group 23 of the 24 patients underwent embolization and in each case it was successful at arresting haemorrhage. Initially in one patient a pseudo-aneurysm could not be identified at angiography but was confirmed after a repeat procedure. Twelve patients underwent surgery in an attempt to resolve the on-going pancreatic inflammatory process but not for treatment of a pseudo-aneurysm. On patient died as a result of a stroke unrelated to pseudo-aneurysm treatment.
Smaller studies assessing the management of pseudo-aneurysms secondary to pancreatitis have shown similar outcomes. Hsu et al[41] in 2006 (9 patients), Beattie et al[66] in 2003 (19 patients), Gambiez et al[67] in 1997 (14 patients) and Sethi et al[38] in 2010 (16 patients) identified that of a total of 58 patients radiological intervention was successful in only 35 patients (60%). Twenty patients subsequently underwent surgery (34%) and seven patients died (12%).
Angiographic embolisation of pancreatitis related pseudo-aneurysm has been shown to be very successful however there are occasions where the pseudo-aneurysm is inaccessible and therefore embolisation is not feasible. Increasingly in these circumstance image guided thrombin injection has been shown to have a role. The current literature identifies a number of case reports detailing successful embolization of a pseudo-aneurysm with percutaneous thrombin[62,70-84]. Of the 23 patients identified who underwent percutaneous thrombin injections four patients re-bled and required further intervention. Two patients had repeated thrombin injections, one underwent angiographic coiling and one is unknown. Other than re-bleeding no significant complications were documented. Evidence is increasing to show that percutaneous therapy is a viable alternative to angiographic therapy. The delivery of thrombin has been successfully used angiographically and percutaneously however in certain scenarios neither can provide appropriate access to allow for successful embolisation. Endoscopic ultrasound has been shown in case reports to provide appropriate access for thrombin injection of pancreatitis related pseudo-aneurysms. Both aneurysms of the gastro-duodenal and splenic artery have been embolised endoscopically without the need for re-intervention[85-89].
Surgery in patients with pancreatitis is known to be associated with significant morbidity and mortality which only increases in the presence of acute haemorrhage. With the improvement of minimally invasive techniques the role of surgery has diminished as angiographic, percutaneous and endoscopic techniques can appropriately exclude pseudo-aneurysms. However in spite of the technological advances in radiology and endoscopy, surgery will continue to play an important role in haemorrhage control where other techniques are not technically possible or available. There is currently no consensus on the surveillance of pseudo-aneurysms and the need for follow up. Patients with pancreatitis of a severity that leads to the formation of a pseudo-aneurysm will often undergo follow up imaging to ensure the resolution of local pancreatitis complications and in turn individually designed imaging follow up can be determined in coordination with the clinician and radiologist to ensure that the future re-bleed risks are mediated.
CONCLUSION
Pancreatitis has a number of aetiologies and many more potential complications which leads to it being a common pathology seen in every surgical department. Arterial complications of pancreatitis although rare are associated with a high morbidity and mortality. It is important to maintain a high level of suspicion for pseudo-aneurysm as they can be easily missed despite cross-sectional imaging. Early recognition of the presence of a pseudo-aneurysm can facilitate expedited care of this complex pathology that may require angiographic, percutaneous, endoscopic or surgical intervention to prevent catastrophic haemorrhage.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: None of the authors has any potential conflicting financial interests relevant to this article.
Peer-review started: March 18, 2017
First decision: April 5, 2017
Article in press: July 12, 2017
P- Reviewer: Du YQ, Fujino Y, Gonzalez-Ojeda A S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
References
- 1.McLean R, Jones M, Kanakala V, Dixon S, McCallum I. PWE-204 Acute pancreatitis: incidence, management and outcome trends over 15 years. Gut. 2015;64:A301–A302. [Google Scholar]
- 2.Roberts SE, Akbari A, Thorne K, Atkinson M, Evans PA. The incidence of acute pancreatitis: impact of social deprivation, alcohol consumption, seasonal and demographic factors. Aliment Pharmacol Ther. 2013;38:539–548. doi: 10.1111/apt.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamada S, Masamune A, Kikuta K, Hirota M, Tsuji I, Shimosegawa T; Research Committee of Intractable Diseases of the Pancreas. Nationwide epidemiological survey of acute pancreatitis in Japan. Pancreas. 2014;43:1244–1248. doi: 10.1097/MPA.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 4.Shen HN, Lu CL, Li CY. Epidemiology of first-attack acute pancreatitis in Taiwan from 2000 through 2009: a nationwide population-based study. Pancreas. 2012;41:696–702. doi: 10.1097/MPA.0b013e31823db941. [DOI] [PubMed] [Google Scholar]
- 5.Yadav D, Whitcomb DC. The role of alcohol and smoking in pancreatitis. Nat Rev Gastroenterol Hepatol. 2010;7:131–145. doi: 10.1038/nrgastro.2010.6. [DOI] [PubMed] [Google Scholar]
- 6.Vidarsdottir H, Möller PH, Vidarsdottir H, Thorarinsdottir H, Björnsson ES. Acute pancreatitis: a prospective study on incidence, etiology, and outcome. Eur J Gastroenterol Hepatol. 2013;25:1068–1075. doi: 10.1097/MEG.0b013e3283640fc8. [DOI] [PubMed] [Google Scholar]
- 7.Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, Gangarosa LM, Thiny MT, Stizenberg K, Morgan DR, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–1187.e1-e3. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang AL, Vadhavkar S, Singh G, Omary MB. Epidemiology of alcohol-related liver and pancreatic disease in the United States. Arch Intern Med. 2008;168:649–656. doi: 10.1001/archinte.168.6.649. [DOI] [PubMed] [Google Scholar]
- 9.Takeyama Y. Long-term prognosis of acute pancreatitis in Japan. Clin Gastroenterol Hepatol. 2009;7:S15–S17. doi: 10.1016/j.cgh.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 10.Lankisch PG, Breuer N, Bruns A, Weber-Dany B, Lowenfels AB, Maisonneuve P. Natural history of acute pancreatitis: a long-term population-based study. Am J Gastroenterol. 2009;104:2797–2805; quiz 2806. doi: 10.1038/ajg.2009.405. [DOI] [PubMed] [Google Scholar]
- 11.Nøjgaard C, Becker U, Matzen P, Andersen JR, Holst C, Bendtsen F. Progression from acute to chronic pancreatitis: prognostic factors, mortality, and natural course. Pancreas. 2011;40:1195–1200. doi: 10.1097/MPA.0b013e318221f569. [DOI] [PubMed] [Google Scholar]
- 12.Yadav D, O’Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. Am J Gastroenterol. 2012;107:1096–1103. doi: 10.1038/ajg.2012.126. [DOI] [PubMed] [Google Scholar]
- 13.Mayerle J, Hoffmeister A, Werner J, Witt H, Lerch MM, Mössner J. Chronic pancreatitis--definition, etiology, investigation and treatment. Dtsch Arztebl Int. 2013;110:387–393. doi: 10.3238/arztebl.2013.0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirota M, Shimosegawa T, Masamune A, Kikuta K, Kume K, Hamada S, Kihara Y, Satoh A, Kimura K, Tsuji I, et al. The sixth nationwide epidemiological survey of chronic pancreatitis in Japan. Pancreatology. 2012;12:79–84. doi: 10.1016/j.pan.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Gullo L, Migliori M, Oláh A, Farkas G, Levy P, Arvanitakis C, Lankisch P, Beger H. Acute pancreatitis in five European countries: etiology and mortality. Pancreas. 2002;24:223–227. doi: 10.1097/00006676-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139:813–820. doi: 10.1053/j.gastro.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 18.Bollen TL, Singh VK, Maurer R, Repas K, van Es HW, Banks PA, Mortele KJ. A comparative evaluation of radiologic and clinical scoring systems in the early prediction of severity in acute pancreatitis. Am J Gastroenterol. 2012;107:612–619. doi: 10.1038/ajg.2011.438. [DOI] [PubMed] [Google Scholar]
- 19.Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology. 2012;262:751–764. doi: 10.1148/radiol.11110947. [DOI] [PubMed] [Google Scholar]
- 20.Bergert H, Hinterseher I, Kersting S, Leonhardt J, Bloomenthal A, Saeger HD. Management and outcome of hemorrhage due to arterial pseudoaneurysms in pancreatitis. Surgery. 2005;137:323–328. doi: 10.1016/j.surg.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Udd M, Leppäniemi AK, Bidel S, Keto P, Roth WD, Haapiainen RK. Treatment of bleeding pseudoaneurysms in patients with chronic pancreatitis. World J Surg. 2007;31:504–510. doi: 10.1007/s00268-006-0209-z. [DOI] [PubMed] [Google Scholar]
- 22.Balthazar EJ. Complications of acute pancreatitis: clinical and CT evaluation. Radiol Clin North Am. 2002;40:1211–1227. doi: 10.1016/s0033-8389(02)00043-x. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal AK, Raj Kumar K, Agarwal S, Singh S. Significance of splenic vein thrombosis in chronic pancreatitis. Am J Surg. 2008;196:149–154. doi: 10.1016/j.amjsurg.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 24.Eckhauser FE, Stanley JC, Zelenock GB, Borlaza GS, Freier DT, Lindenauer SM. Gastroduodenal and pancreaticoduodenal artery aneurysms: a complication of pancreatitis causing spontaneous gastrointestinal hemorrhage. Surgery. 1980;88:335–344. [PubMed] [Google Scholar]
- 25.Kiviluoto T, Kivisaari L, Kivilaakso E, Lempinen M. Pseudocysts in chronic pancreatitis. Surgical results in 102 consecutive patients. Arch Surg. 1989;124:240–243. doi: 10.1001/archsurg.1989.01410020114019. [DOI] [PubMed] [Google Scholar]
- 26.White AF, Baum S, Buranasiri S. Aneurysms secondary to pancreatitis. AJR Am J Roentgenol. 1976;127:393–396. doi: 10.2214/ajr.127.3.393. [DOI] [PubMed] [Google Scholar]
- 27.de Castro SM, Kuhlmann KF, Busch OR, van Delden OM, Laméris JS, van Gulik TM, Obertop H, Gouma DJ. Delayed massive hemorrhage after pancreatic and biliary surgery: embolization or surgery? Ann Surg. 2005;241:85–91. doi: 10.1097/01.sla.0000150169.22834.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flati G, Andrén-Sandberg A, La Pinta M, Porowska B, Carboni M. Potentially fatal bleeding in acute pancreatitis: pathophysiology, prevention, and treatment. Pancreas. 2003;26:8–14. doi: 10.1097/00006676-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Dörffel T, Wruck T, Rückert RI, Romaniuk P, Dörffel Q, Wermke W. Vascular complications in acute pancreatitis assessed by color duplex ultrasonography. Pancreas. 2000;21:126–133. doi: 10.1097/00006676-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Balthazar EJ, Fisher LA. Hemorrhagic complications of pancreatitis: radiologic evaluation with emphasis on CT imaging. Pancreatology. 2001;1:306–313. doi: 10.1159/000055829. [DOI] [PubMed] [Google Scholar]
- 31.Mortelé KJ, Mergo PJ, Taylor HM, Wiesner W, Cantisani V, Ernst MD, Kalantari BN, Ros PR. Peripancreatic vascular abnormalities complicating acute pancreatitis: contrast-enhanced helical CT findings. Eur J Radiol. 2004;52:67–72. doi: 10.1016/j.ejrad.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Bergert H, Dobrowolski F, Caffier S, Bloomenthal A, Hinterseher I, Saeger HD. Prevalence and treatment of bleeding complications in chronic pancreatitis. Langenbecks Arch Surg. 2004;389:504–510. doi: 10.1007/s00423-004-0478-7. [DOI] [PubMed] [Google Scholar]
- 33.Xiao B, Zhang XM, Tang W, Zeng NL, Zhai ZH. Magnetic resonance imaging for local complications of acute pancreatitis: a pictorial review. World J Gastroenterol. 2010;16:2735–2742. doi: 10.3748/wjg.v16.i22.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirby JM, Vora P, Midia M, Rawlinson J. Vascular complications of pancreatitis: imaging and intervention. Cardiovasc Intervent Radiol. 2008;31:957–970. doi: 10.1007/s00270-007-9138-y. [DOI] [PubMed] [Google Scholar]
- 35.Sharma PK, Madan K, Garg PK. Hemorrhage in acute pancreatitis: should gastrointestinal bleeding be considered an organ failure? Pancreas. 2008;36:141–145. doi: 10.1097/MPA.0b013e318158466e. [DOI] [PubMed] [Google Scholar]
- 36.Bretagne JF, Heresbach D, Darnault P, Raoul JL, Gosselin M, Carsin M, Gastard J. Pseudoaneurysms and bleeding pseudocysts in chronic pancreatitis: radiological findings and contribution to diagnosis in 8 cases. Gastrointest Radiol. 1990;15:9–16. doi: 10.1007/BF01888725. [DOI] [PubMed] [Google Scholar]
- 37.Zyromski NJ, Vieira C, Stecker M, Nakeeb A, Pitt HA, Lillemoe KD, Howard TJ. Improved outcomes in postoperative and pancreatitis-related visceral pseudoaneurysms. J Gastrointest Surg. 2007;11:50–55. doi: 10.1007/s11605-006-0038-2. [DOI] [PubMed] [Google Scholar]
- 38.Sethi H, Peddu P, Prachalias A, Kane P, Karani J, Rela M, Heaton N. Selective embolization for bleeding visceral artery pseudoaneurysms in patients with pancreatitis. Hepatobiliary Pancreat Dis Int. 2010;9:634–638. [PubMed] [Google Scholar]
- 39.Tessier DJ, Stone WM, Fowl RJ, Abbas MA, Andrews JC, Bower TC, Gloviczki P. Clinical features and management of splenic artery pseudoaneurysm: case series and cumulative review of literature. J Vasc Surg. 2003;38:969–974. doi: 10.1016/s0741-5214(03)00710-9. [DOI] [PubMed] [Google Scholar]
- 40.de Perrot M, Berney T, Bühler L, Delgadillo X, Mentha G, Morel P. Management of bleeding pseudoaneurysms in patients with pancreatitis. Br J Surg. 1999;86:29–32. doi: 10.1046/j.1365-2168.1999.00983.x. [DOI] [PubMed] [Google Scholar]
- 41.Hsu JT, Yeh CN, Hung CF, Chen HM, Hwang TL, Jan YY, Chen MF. Management and outcome of bleeding pseudoaneurysm associated with chronic pancreatitis. BMC Gastroenterol. 2006;6:3. doi: 10.1186/1471-230X-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vander Mijnsbrugge W, Laleman W, Van Steenbergen W, Heye S, Verslype C, Maleux G. Long-term clinical and radiological outcome of endovascular embolization of pancreatitis-related pseudoaneurysms. Acta Radiol. 2017;58:316–322. doi: 10.1177/0284185116648502. [DOI] [PubMed] [Google Scholar]
- 43.Mendelson RM, Anderson J, Marshall M, Ramsay D. Vascular complications of pancreatitis. ANZ J Surg. 2005;75:1073–1079. doi: 10.1111/j.1445-2197.2005.03607.x. [DOI] [PubMed] [Google Scholar]
- 44.Boudghène F, L’Herminé C, Bigot JM. Arterial complications of pancreatitis: diagnostic and therapeutic aspects in 104 cases. J Vasc Interv Radiol. 1993;4:551–558. doi: 10.1016/s1051-0443(93)71920-x. [DOI] [PubMed] [Google Scholar]
- 45.Walter JF, Chuang VP, Bookstein JJ, Reuter SR, Cho KJ, Pulmano CM. Angiography of massive hemorrhage secondary to pancreatic diseases. Radiology. 1977;124:337–342. doi: 10.1148/124.2.337. [DOI] [PubMed] [Google Scholar]
- 46.Nosher JL, Chung J, Brevetti LS, Graham AM, Siegel RL. Visceral and renal artery aneurysms: a pictorial essay on endovascular therapy. Radiographics. 2006;26:1687–1704; quiz 1687. doi: 10.1148/rg.266055732. [DOI] [PubMed] [Google Scholar]
- 47.Kim JH, Kim JS, Kim CD, Lee HS, Cho YJ, Lee JS, Choi DW, Han WS, Kim YH, Yeon JE, et al. [Clinical features of pseudoaneurysms complicating pancreatitis: single center experience and review of Korean literature] Korean J Gastroenterol. 2007;50:108–115. [PubMed] [Google Scholar]
- 48.Mallick IH, Winslet MC. Vascular complications of pancreatitis. JOP. 2004;5:328–337. [PubMed] [Google Scholar]
- 49.Mauro MA, Jaques P. Transcatheter management of pseudoaneurysms complicating pancreatitis. J Vasc Interv Radiol. 1991;2:527–532. doi: 10.1016/s1051-0443(91)72236-7. [DOI] [PubMed] [Google Scholar]
- 50.Tsiotos GG, Munoz Juarez MM, Sarr MG. Intraabdominal hemorrhage complicating surgical management of necrotizing pancreatitis. Pancreas. 1996;12:126–130. doi: 10.1097/00006676-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Giles RA, Pevec WC. Aortic pseudoaneurysm secondary to pancreatitis. J Vasc Surg. 2000;31:1056–1059. doi: 10.1067/mva.2000.102850. [DOI] [PubMed] [Google Scholar]
- 52.Balachandra S, Siriwardena AK. Systematic appraisal of the management of the major vascular complications of pancreatitis. Am J Surg. 2005;190:489–495. doi: 10.1016/j.amjsurg.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 53.Nitin J, Reddy DN, Singh JR. A Rare Location of Pseudoaneurysm in Chronic Pancreatitis: Left Sub-Capsular Renal Artery. J Gastrointest Dig Syst. 2016;6:474. [Google Scholar]
- 54.Arora A, Tyagi P, Gupta A, Arora V, Sharma P, Kumar M, Goyal M, Kumar A. Pseudoaneurysm of the inferior phrenic artery presenting as an upper gastrointestinal bleed by directly rupturing into the stomach in a patient with chronic pancreatitis. Ann Vasc Surg. 2012;26:860.e9–860.11. doi: 10.1016/j.avsg.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 55.Marshall GT, Howell DA, Hansen BL, Amberson SM, Abourjaily GS, Bredenberg CE. Multidisciplinary approach to pseudoaneurysms complicating pancreatic pseudocysts. Impact of pretreatment diagnosis. Arch Surg. 1996;131:278–283. doi: 10.1001/archsurg.1996.01430150056012. [DOI] [PubMed] [Google Scholar]
- 56.Fukatsu K, Ueda K, Maeda H, Yamashita Y, Itonaga M, Mori Y, Moribata K, Shingaki N, Deguchi H, Enomoto S, et al. A case of chronic pancreatitis in which endoscopic ultrasonography was effective in the diagnosis of a pseudoaneurysm. World J Gastrointest Endosc. 2012;4:335–338. doi: 10.4253/wjge.v4.i7.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai DM, Parajuly SS, Ling WW, Li YZ, Luo Y. Diagnostic value of contrast enhanced ultrasound for splenic artery complications following acute pancreatitis. World J Gastroenterol. 2014;20:1088–1094. doi: 10.3748/wjg.v20.i4.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balthazar EJ, Freeny PC, vanSonnenberg E. Imaging and intervention in acute pancreatitis. Radiology. 1994;193:297–306. doi: 10.1148/radiology.193.2.7972730. [DOI] [PubMed] [Google Scholar]
- 59.Xiao B, Zhang XM. Magnetic resonance imaging for acute pancreatitis. World J Radiol. 2010;2:298–308. doi: 10.4329/wjr.v2.i8.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koo BC, Chinogureyi A, Shaw AS. Imaging acute pancreatitis. Br J Radiol. 2010;83:104–112. doi: 10.1259/bjr/13359269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hyare H, Desigan S, Nicholl H, Guiney MJ, Brookes JA, Lees WR. Multi-section CT angiography compared with digital subtraction angiography in diagnosing major arterial hemorrhage in inflammatory pancreatic disease. Eur J Radiol. 2006;59:295–300. doi: 10.1016/j.ejrad.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 62.Nicholson AA, Patel J, McPherson S, Shaw DR, Kessel D. Endovascular treatment of visceral aneurysms associated with pancreatitis and a suggested classification with therapeutic implications. J Vasc Interv Radiol. 2006;17:1279–1285. doi: 10.1097/01.RVI.0000231948.08617.04. [DOI] [PubMed] [Google Scholar]
- 63.Yoon W, Jeong YY, Shin SS, Lim HS, Song SG, Jang NG, Kim JK, Kang HK. Acute massive gastrointestinal bleeding: detection and localization with arterial phase multi-detector row helical CT. Radiology. 2006;239:160–167. doi: 10.1148/radiol.2383050175. [DOI] [PubMed] [Google Scholar]
- 64.Nedjat-Shokouhi B, Glynn M, Denton ERE, Greenfield SM. Provision of out-of-hours services for acute upper gastrointestinal bleeding in England: results of the 2014–2015 BSG/NHS England national survey. Fron Gastroenterol. 2016:flgastro–2016-100706. doi: 10.1136/flgastro-2016-100706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim J, Shin JH, Yoon HK, Ko GY, Gwon DI, Kim EY, Sung KB. Endovascular intervention for management of pancreatitis-related bleeding: a retrospective analysis of thirty-seven patients at a single institution. Diagn Interv Radiol. 2015;21:140–147. doi: 10.5152/dir.2014.14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beattie GC, Hardman JG, Redhead D, Siriwardena AK. Evidence for a central role for selective mesenteric angiography in the management of the major vascular complications of pancreatitis. Am J Surg. 2003;185:96–102. doi: 10.1016/s0002-9610(02)01199-6. [DOI] [PubMed] [Google Scholar]
- 67.Gambiez LP, Ernst OJ, Merlier OA, Porte HL, Chambon JP, Quandalle PA. Arterial embolization for bleeding pseudocysts complicating chronic pancreatitis. Arch Surg. 1997;132:1016–1021. doi: 10.1001/archsurg.1997.01430330082014. [DOI] [PubMed] [Google Scholar]
- 68.Tulsyan N, Kashyap VS, Greenberg RK, Sarac TP, Clair DG, Pierce G, Ouriel K. The endovascular management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg. 2007;45:276–283; discussion 283. doi: 10.1016/j.jvs.2006.10.049. [DOI] [PubMed] [Google Scholar]
- 69.Bhasin DK, Rana SS, Sharma V, Rao C, Gupta V, Gupta R, Kang M, Singh K. Non-surgical management of pancreatic pseudocysts associated with arterial pseudoaneurysm. Pancreatology. 2013;13:250–253. doi: 10.1016/j.pan.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 70.Chauhan U, Puri SK, Jain N, Garg L, Kapoor A, Gupta N, Goel V. Percutaneous thrombin injection under sonographic guidance for exclusion of non-catheterizable post-pancreatitis pseudoaneurysm of the superior mesenteric artery: a minimally invasive and expeditious treatment option. J Med Ultrason (2001) 2016;43:295–299. doi: 10.1007/s10396-015-0687-4. [DOI] [PubMed] [Google Scholar]
- 71.Shrivastava A, Rampal JS, Reddy DN, Rao GV. Direct Needle Puncture and Embolization of Splenic Artery Pseudoaneurysm in Case of Chronic Atrophic Calcific Pancreatitis. Pol J Radiol. 2016;81:462–464. doi: 10.12659/PJR.898000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pacheco Jiménez M, Moreno Sánchez T, Moreno Rodríguez F, Guillén Rico M. [Pancreatic tail pseudoaneurysm: percutaneous treatment by thrombin injection] Radiologia. 2014;56:167–170. doi: 10.1016/j.rx.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 73.Barbiero G, Battistel M, Susac A, Miotto D. Percutaneous thrombin embolization of a pancreatico-duodenal artery pseudoaneurysm after failing of the endovascular treatment. World J Radiol. 2014;6:629–635. doi: 10.4329/wjr.v6.i8.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Rosa A, Gomez D, Pollock JG, Bungay P, De Nunzio M, Hall RI, Thurley P. The radiological management of pseudoaneurysms complicating pancreatitis. JOP. 2012;13:660–666. doi: 10.6092/1590-8577/1193. [DOI] [PubMed] [Google Scholar]
- 75.Fankhauser GT, Stone WM, Naidu SG, Oderich GS, Ricotta JJ, Bjarnason H, Money SR; Mayo Vascular Research Center Consortium. The minimally invasive management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg. 2011;53:966–970. doi: 10.1016/j.jvs.2010.10.071. [DOI] [PubMed] [Google Scholar]
- 76.McErlean A, Looby S, Lee MJ. Percutaneous ultrasound-guided thrombin injection as first-line treatment of pancreatic pseudoaneurysm. Cardiovasc Intervent Radiol. 2007;30:526–528. doi: 10.1007/s00270-006-0174-9. [DOI] [PubMed] [Google Scholar]
- 77.Laganà D, Carrafiello G, Mangini M, Dionigi G, Caronno R, Castelli P, Fugazzola C. Multimodal approach to endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Eur J Radiol. 2006;59:104–111. doi: 10.1016/j.ejrad.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 78.Williams M, Alderson D, Virjee J, Callaway M. CT-guided percutaneous thrombin injection for treatment of an inferior pancreaticoduodenal artery pseudoaneurysm. Cardiovasc Intervent Radiol. 2006;29:669–671. doi: 10.1007/s00270-004-0274-3. [DOI] [PubMed] [Google Scholar]
- 79.Ghassemi A, Javit D, Dillon EH. Thrombin injection of a pancreaticoduodenal artery pseudoaneurysm after failed attempts at transcatheter embolization. J Vasc Surg. 2006;43:618–622. doi: 10.1016/j.jvs.2005.11.051. [DOI] [PubMed] [Google Scholar]
- 80.Szopiński P, Ciostek P, Pleban E, Iwanowski J, Serafin-Król M, Marianowska A, Noszczyk W. Percutaneous thrombin injection to complete SMA pseudoaneurysm exclusion after failing of endograft placement. Cardiovasc Intervent Radiol. 2005;28:509–514. doi: 10.1007/s00270-004-0160-z. [DOI] [PubMed] [Google Scholar]
- 81.Geoghegan T, Tuite D, McAuley G, O’Keeffe S, Torreggiani WC. Percutaneous thrombin injection for the treatment of a post-pancreatitis pseudoaneurysm of the gastroduodenal artery. Eur Radiol. 2004;14:2144–2145. doi: 10.1007/s00330-004-2399-9. [DOI] [PubMed] [Google Scholar]
- 82.Sparrow P, Asquith J, Chalmers N. Ultrasonic-guided percutaneous injection of pancreatic pseudoaneurysm with thrombin. Cardiovasc Intervent Radiol. 2003;26:312–315. doi: 10.1007/s00270-003-0008-y. [DOI] [PubMed] [Google Scholar]
- 83.Manazer JR, Monzon JR, Dietz PA, Moglia R, Gold M. Treatment of pancreatic pseudoaneurysm with percutaneous transabdominal thrombin injection. J Vasc Surg. 2003;38:600–602. doi: 10.1016/s0741-5214(03)00454-3. [DOI] [PubMed] [Google Scholar]
- 84.Armstrong EM, Edwards A, Kingsnorth AN, Freeman S, Roobottom CA. Ultrasound guided thrombin injection to treat a pseudoaneurysm secondary to chronic pancreatitis. Eur J Vasc Endovasc Surg. 2003;26:448–449. doi: 10.1016/s1078-5884(03)00075-3. [DOI] [PubMed] [Google Scholar]
- 85.Rai P, Mohan S, Sharma M. Endoscopic ultrasound-guided thrombin injection in a large splenic artery aneurysm: first report in a patient with tropical chronic pancreatitis. Endoscopy. 2014;46 Suppl 1 UCTN:E355–E356. doi: 10.1055/s-0034-1377357. [DOI] [PubMed] [Google Scholar]
- 86.Gamanagatti S, Thingujam U, Garg P, Nongthombam S, Dash NR. Endoscopic ultrasound guided thrombin injection of angiographically occult pancreatitis associated visceral artery pseudoaneurysms: Case series. World J Gastrointest Endosc. 2015;7:1107–1113. doi: 10.4253/wjge.v7.i13.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roach H, Roberts SA, Salter R, Williams IM, Wood AM. Endoscopic ultrasound-guided thrombin injection for the treatment of pancreatic pseudoaneurysm. Endoscopy. 2005;37:876–878. doi: 10.1055/s-2005-870201. [DOI] [PubMed] [Google Scholar]
- 88.Robinson M, Richards D, Carr N. Treatment of a splenic artery pseudoaneurysm by endoscopic ultrasound-guided thrombin injection. Cardiovasc Intervent Radiol. 2007;30:515–517. doi: 10.1007/s00270-006-0081-0. [DOI] [PubMed] [Google Scholar]
- 89.Chaves DM, Costa FF, Matuguma S, Lera Dos Santos ME, de Moura EG, Maluf Filho F, Sakai P. Splenic artery pseudoaneurysm treated with thrombin injection guided by endoscopic ultrasound. Endoscopy. 2012;44 Suppl 2 UCTN:E99–100. doi: 10.1055/s-0030-1256740. [DOI] [PubMed] [Google Scholar]
- 90.Massani M, Bridda A, Caratozzolo E, Bonariol L, Antoniutti M, Bassi N. Hemosuccus pancreaticus due to primary splenic artery aneurysm: a diagnostic and therapeutic challenge. JOP. 2009;10:48–52. [PubMed] [Google Scholar]
- 91.Horton KM, Smith C, Fishman EK. MDCT and 3D CT angiography of splanchnic artery aneurysms. AJR Am J Roentgenol. 2007;189:641–647. doi: 10.2214/AJR.07.2210. [DOI] [PubMed] [Google Scholar]
- 92.Taori K, Rathod J, Disawal A, Mundhada R, Rewatkar A, Bakare V, Wavare P, Puria RP. Endovascular Embolization of Pseudoaneurysms Complicating Pancreatitis Using Microcoils: Case Series. Open J Radiol. 2013;3:33–40. [Google Scholar]


