Abstract
AIM
To assess the value of combined acoustic radiation force impulse (ARFI) imaging, serological indexes and contrast-enhanced ultrasound (CEUS) in distinguishing between benign and malignant liver lesions.
METHODS
Patients with liver lesions treated at our hospital were included in this study. The lesions were divided into either a malignant tumor group or a benign tumor group according to pathological or radiological findings. ARFI quantitative detection, serological testing and CEUS quantitative detection were performed and compared. A comparative analysis of the measured indexes was performed between these groups. Receiver operating characteristic (ROC) curves were constructed to compare the diagnostic accuracy of ARFI imaging, serological indexes and CEUS, alone or in different combinations, in identifying benign and malignant liver lesions.
RESULTS
A total of 112 liver lesions in 43 patients were included, of which 78 were malignant and 34 were benign. Shear wave velocity (SWV) value, serum alpha-fetoprotein (AFP) content and enhancement rate were significantly higher in the malignant tumor group than in the benign tumor group (2.39 ± 1.20 m/s vs 1.50 ± 0.49 m/s, 18.02 ± 5.01 ng/mL vs 15.96 ± 4.33 ng/mL, 2.14 ± 0.21 dB/s vs 2.01 ± 0.31 dB/s; P < 0.05). The ROC curve analysis revealed that the areas under the curves (AUCs) of SWV value alone, AFP content alone, enhancement rate alone, SWV value + AFP content, SWV value + enhancement rate, AFP content + enhancement rate and SWV value + AFP content + enhancement rate were 85.1%, 72.1%, 74.5%, 88.3%, 90.4%, 82.0% and 92.3%, respectively. The AUC of SWV value + AFP content + enhancement rate was higher than those of SWV value + AFP content and SWV value + enhancement rate, and significantly higher than those of any single parameter or the combination of any two of parameters.
CONCLUSION
The combination of SWV, AFP and enhancement rate had better diagnostic performance in distinguishing between benign and malignant liver lesions than the use of any single parameter or the combination of any two of parameters. It is expected that this would provide a tool for the differential diagnosis of benign and malignant liver lesions.
Keywords: Combined diagnosis, Liver lesions, Benign, Malignant, Differentiation
Core tip: This study investigated the diagnostic value of combined acoustic radiation force impulse (ARFI) imaging, serological indicators and contrast-enhanced ultrasound in differentiating between benign and malignant liver lesions. The results showed that the diagnostic performance of combined ARFI, alpha-fetoprotein (AFP) and contrast-enhanced ultrasound in distinguishing between benign and malignant liver lesions was higher than the use of any single parameter or the combination of any two of parameters. It is expected that this would provide a tool for the differential diagnosis of benign and malignant liver lesions.
INTRODUCTION
The gold standard for diagnosis of benign and malignant liver lesions is pathological examination. However, due to its invasive nature and the potential presence of infection, bleeding and other risks, pathological examination is only conducted in patients highly suspected of having malignant liver tumors. In addition, pathological diagnosis has many contraindications and is applicable in only a narrow range of patients. Thus, it is difficult to implement pathological diagnosis as a routine examination. For both benign and malignant liver lesions, detection of serological indicators and contrast-enhanced ultrasonography (CEUS) are routinely performed. Serological indicators alone often have a lower sensitivity and specificity than CEUS and acoustic radiation force impulse (ARFI) imaging. However, for small hepatocellular carcinoma, alpha-fetoprotein (AFP) is the most sensitive indicator[1,2]. Although CEUS is a mature examination method, its diagnostic accuracy may be affected by lesion depth, blood flow velocity and respiratory movement, which makes it not suitable for some lesions and patients[3-9]. ARFI imaging is simple and highly accepted by patients, and it can quantitatively assess the change in tissue hardness. However, it is vulnerable to biliary and intrahepatic vascular effects[10-17]. Although these three kinds of examinations have certain value in identifying benign and malignant lesions, each has its limitations. At present, the diagnostic accuracy of the combination of these three kinds of examinations in identifying benign and malignant liver lesions remains unknown, although the combined diagnosis has been widely used for other diseases. In the present study, by constructing receiver operating characteristic (ROC) curves, we compared the diagnostic accuracy of ARFI imaging, serological indexes and CEUS, alone or in different combinations, in identifying benign and malignant liver lesions, with an aim to provide a reliable tool for the differential diagnosis of benign and malignant liver lesions.
MATERIALS AND METHODS
Study subjects
A total of 43 patients with 112 liver lesions treated at our hospital from May 2016 to February 2017 were included in this study. Among these patients, 21 were male and 22 were female. The lesion size ranged from 8 mm × 7 mm to 123 mm × 100 mm. The exclusion criteria were: (1) patients in whom shear wave velocity (SWV) values could not be acquired during ARFI imaging; (2) patients who could not tolerate the ultrasound contrast agent or critically ill patients; (3) patients with completely liquefied cystic tumors; and (4) patients with contraindications to liver puncture biopsy. The study was approved by the Ethics Committee of Shanghai Yangpu District East Hospital, and informed consent was obtained from all patients.
Instruments and methods
ARFI imaging: A Siemens Acuson S3000 color Doppler ultrasound system with a 6C-1HD convex array probe (center frequency, 4.0 MHz) was used. First, a whole liver scan was performed using conventional ultrasound to observe the nature of the lesion. Then, this was switched to ARFI mode to avoid biliary ducts within the liver, blood vessels and liquid necrosis. Patients were instructed to completely hold their breath during the examination. Then, SWV measurement was initiated while keeping the probe stationary. This was repeated for 3 to 6 times at the lesion center, and repeated for 3 to 5 times at the periphery of the lesion, with average SWV value calculated.
CEUS: CEUS was performed with the same system for ARFI imaging in the contrast mode, with the mechanical index adjusted to the appropriate state. SonoVue suspension (2.0 mL) was administered by bolus injection via the elbow vain, followed by rapid injection of 5 mL of normal saline. The patient was breathing steadily throughout the procedure. After imaging, the records were exported. Then, analysis software was use to plot the time intensity curve of the lesion and obtain the initial time, peak time, initial strength, peak intensity and 180-s echo intensity of the lesion. Subsequently, peak acceleration time, intensity increment, enhancement rate, and 180-s dissipation rate were calculated.
Serological examination: An automatic biochemical analyzer was used for the quantitative analysis of the following serological indicators: AFP, serum fucosidase (AFU), alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyltranspeptidase (GGT), and alkaline phosphatase (ALP).
Statistical analysis
SPSS 17.0 statistical software was used for statistical analyses. Measurement data are presented as mean ± SD. Comparison of data between groups was performed using two independent samples t-test. Indicators with statistically significant differences were used to construct the ROC curves to calculate the area under the curve (AUC), in order to investigate the diagnostic accuracy. Diagnostic accuracy was compared between different combinations to determine the value of combined diagnosis in identifying benign and malignant liver lesions.
RESULTS
Pathological results
Pathological or radiological diagnosis confirmed 78 cases of malignant tumors and 34 cases of benign tumors. Among these cases, 18 were primary liver cancer, 60 were metastatic cancer, 16 were hemangiomas, 11 were liver cysts, 5 were adenomas, and 2 were focal nodular hyperplasia.
Comparison of SWV values between malignant and benign tumor groups
SWV values were significantly higher in the malignant tumor group (2.39 ± 1.20 m/s, a typical image is shown in Figure 1A) than in the benign tumor group (1.50 ± 0.49 m/s, a typical image is shown in Figure 1B) (P < 0.05; Figure 2).
Figure 1.
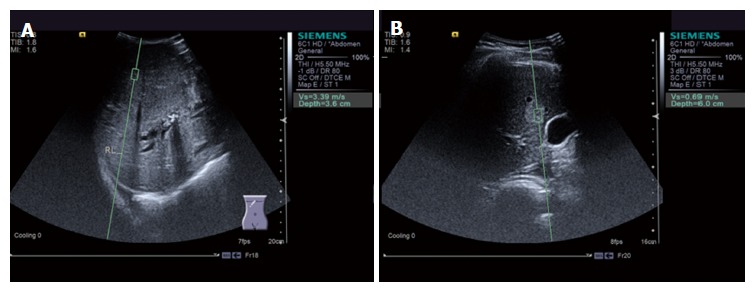
Acoustic radiation force impulse imaging of a liver tumor. A: Acoustic radiation force impulse (ARFI) detection of a malignant liver tumor [shear wave velocity (SWV) = 3.39 m/s]; B: ARFI detection of a benign liver tumor (SWV = 0.69 m/s).
Figure 2.
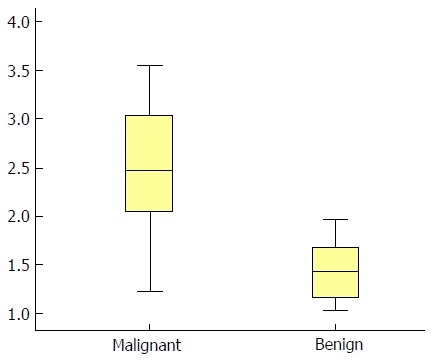
Shear wave velocity values of the malignant and benign tumor groups. The shear wave velocity of malignant tumors was 2.39 ± 1.20 m/s, while that of benign tumors was 1.50 ± 0.49 m/s.
Comparison of serological indicators between malignant and benign tumor groups
AFP level was significantly higher in the malignant tumor group than in the benign tumor group (P < 0.05), while the differences in AFU, ALT, AST, GGT and ALP levels were not statistically significant (P > 0.05) (Table 1).
Table 1.
Comparison of serological indicators between the malignant and benign tumor groups
| AFP (ng/mL) | AFU (U/L) | ALT (U/L) | AST (U/L) | GGT (U/L) | ALP (U/L) | |
| Malignant tumor group | 18.02 ± 5.01 | 29.74 ± 14.22 | 39.01 ± 3.60 | 46.92 ± 12.11 | 62.40 ± 27.30 | 139.40 ± 85.90 |
| Benign tumor group | 15.96 ± 4.33 | 25.61 ± 13.11 | 37.90 ± 4.33 | 44.40 ± 11.60 | 54.90 ± 22.30 | 117.99 ± 57.30 |
| t-value | 2.081 | 1.446 | 1.705 | 1.409 | 1.409 | 1.328 |
| P value | 0.04 | 0.151 | 0.09 | 0.162 | 0.162 | 0.187 |
AFP: Alpha-fetoprotein; AFU: Serum fucosidase; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ALP: Alkaline phosphatase; GGT: Glutamyltranspeptidase.
Comparison of CEUS indicators between malignant and benign tumor groups
CEUS enhancement rate was significantly higher in the malignant tumor group than in the benign tumor group (a typical image is shown in Figure 3A and B) (P < 0.05), while the differences in start time, initial strength, peak time, peak intensity, 180-second echo intensity, peak acceleration time, strength increment, and rate of regression were not statistically significant (P > 0.05) (Table 2).
Figure 3.
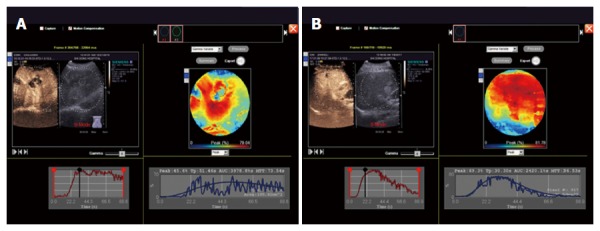
Contrast-enhanced ultrasound TIC curves used to detect benign and malignant liver tumors. A: TIC curve for the detection of malignant liver tumors; B: TIC curve for the detection of benign liver tumors.
Table 2.
Comparison of contrast-enhanced ultrasound parameters in the malignant and benign tumor groups
| Start time (s) | Initial strength (dB) | Peak time (s) | Peak intensity (dB) | 120s Echo intensity (dB) | Peak acceleration time (s) | Intensity increment (dB) | Enhancement rate (dB/s) | Extinction rate (dB/s) | |
| Malignant tumor group | 11.98 ± 2.95 | 8.35 ± 6.03 | 30.22 ± 9.65 | 39.27 ± 6.32 | 27.33 ± 17.86 | 17.94 ± 4.64 | 30.99 ± 6.67 | 2.24 ± 0.21 | 0.08 ± 0.07 |
| Benign tumor group | 13.01 ± 3.92 | 6.57 ± 5.66 | 33.33 ± 11.96 | 37.77 ± 7.30 | 32.01 ± 11.96 | 20.10 ± 10.32 | 31.22 ± 7.12 | 2.01 ± 0.31 | 0.06 ± 0.06 |
| t-value | -1.532 | 1.463 | -1.456 | 1.101 | -1.396 | -1.533 | -0.164 | 2.589 | 1.449 |
| P value | 0.128 | 0.146 | 0.148 | 0.273 | 0.166 | 0.128 | 0.870 | 0.011 | 0.150 |
Diagnostic accuracy of different combinations of SWV value, AFP content and enhancement rate
SWV value, AFP content and enhancement rate were combined in the following different ways: SWV value + AFP content, SWV value + enhancement rate, AFP content + enhancement rate, and SWV value + AFP content + enhancement rate. Then, the ROC curves were constructed to calculate the AUCs. The AUCs of SWV value alone, AFP content alone, enhancement rate alone, SWV value + AFP content, SWV value + enhancement rate, AFP content + enhancement rate and SWV value + AFP content + enhancement rate were 85.1%, 72.1%, 74.5%, 88.3%, 90.4%, 82.0% and 92.3%, respectively. Taking the maximum value of the Youden index, SWV = 1.60 m/s, AFP = 18.68 ng/mL, and enhancement rate = 2.21 dB/s were determined as the best cut-off values for the diagnosis of benign and malignant liver lesions (Figures 4 and 5). When the cut-off value for the regression coefficient of the SWV value, AFP content and enhancement rate was 0.8711451, it was found that 23 cases were false-negative lesions, which included 11 cases of liver metastases derived from the digestive tract and 12 cases of hepatocellular carcinoma; and there were no false-positive lesions.
Figure 4.
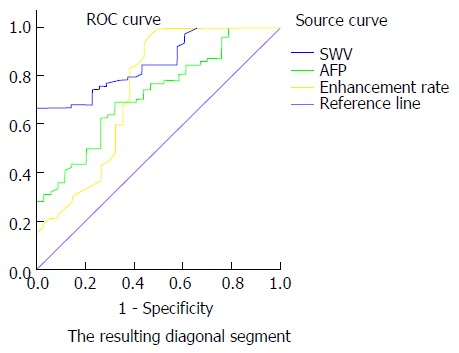
Comparison of the receiver operating characteristic curves of shear wave velocity, alpha-fetoprotein and enhancement rate for the identification of benign and malignant liver tumors.
Figure 5.
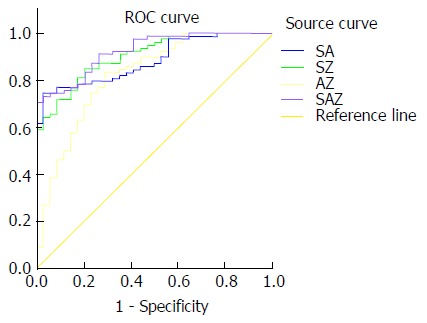
Comparison of the receiver operating characteristic curves of the combination of shear wave velocity value, alpha-fetoprotein content and enhancement rate for identifying benign and malignant liver tumors. SA = SWV value + AFP content; SZ = SWV value + enhancement rate; AZ = AFP content + enhancement rate; SAZ = SWV value + AFP content + enhancement rate.
DISCUSSION
Pathological examination is the gold standard for diagnosing liver lesions. However, patient acceptance is low due to the invasiveness of the examination. Furthermore, pathological examination cannot be routinely used for screening of benign and malignant liver lesions. Serological indicators and CEUS are commonly used noninvasive examinations in clinical practice. However, serological indicators alone have a low sensitivity and specificity, and the CEUS examination process is complex and requires some skills[18-21]. ARFI imaging is simple and can quantitatively reflect changes in tissue hardness. Studies have shown that ARFI can be used to diagnose benign and malignant liver tumors, but it is easily affected by bile ducts and large blood vessels[22-29]. Thus, each of these three types of detection methods has its own advantages and disadvantages. Since the combined diagnosis has been widely used for other diseases at present, we hypothesized that the combination of these three kinds of examinations might have better accuracy in identifying benign and malignant liver lesions. Therefore, we constructed ROC curves to compare the diagnostic efficacy of ARFI imaging, serological indexes and CEUS, alone or in different combinations, in the present study.
Comparison of ARFI, serological parameters, CEUS indicators between malignant and benign tumor groups
Our results revealed that SWV value, AFP content and CEUS enhancement rate were significantly higher in the malignant tumor group than in the benign tumor group, suggesting that the use of these parameters is feasible for the differential diagnosis of benign and malignant liver lesions. Increased SWV value may indicate increased tumor hardness. This may be due to the richness of the liver cancer substance in tumor cells that sustains growth and promotes the invasion of capillaries, and the chaotic arrangement of tissues that show invasive growth. Hence, activity in the surrounding tissue is limited, because the hardness of malignant liver tumors is greater than that of benign liver tumors. Elevated AFP content may be due to malignant liver cells, in which the AFP gene is re-expressed and AFP is released to blood. CEUS enhancement rate represents the rich blood supply of the tumor, because the number of microvessels per unit volume of malignant tumors is more than that of benign tumors, and the unit time into the malignant tumor contrast agent also increases. Hence, the enhancement rate is higher[3,4,30-42]. The difference in AFP and CEUS enhancement rate between benign and malignant liver tumors can be expected, and the difference in SWV value between the two groups suggests that ARFI can also be used for the analysis of benign and malignant liver lesions.
Diagnostic accuracy of SWV value, AFP content and enhancement rate, alone or in different combinations, in distinguishing between malignant and benign tumors
The results of this study showed that the AUC of SWV value was slightly higher than that of CEUS enhancement rate and significantly higher than that of AFP content. This finding suggests that the diagnostic value of ARFI is higher than that of CEUS enhancement rate and AFP content and the diagnostic value of ARFI is higher than that of AFP. This might be because the change in the texture of liver lesions is more sensitive than that of AFP content. The diagnostic value of CEUS is less than that of ARFI, and the reason may be that CEUS reflects the hemodynamics of liver lesions, while ARFI reflects changes in tissue hardness. Tissue hardness reflects not only the vascular composition of tissue, but also the interstitial fiber composition of tissue, cell arrangement and other factors. Hence, compared to CEUS, ARFI is more comprehensive and intuitive.
The results of this study showed that the diagnostic accuracy of combined SWV value, AFP content and enhancement rate was slightly higher than that of SWV value plus AFP content and SWV value plus enhancement rate, and significantly higher than that of AFP content plus enhancement rate and any one of the three parameters. When the cut-off value for the regression coefficient of the SWV value, AFP content and enhancement rate was 0.8711451, the best diagnostic performance was achieved. With this cut-off value, the results of this study revealed that nine cases of hepatocellular carcinoma were false-negative lesions, which may have been transformed through liver cirrhosis to stage I liver cancer, in which the tumor composition was less obvious. Furthermore, SWV value and AFP content were low, which may be related to the partial necrosis in the tumor component. These led to the low SWV value, AFP content and enhancement rate regression coefficient, and then the false negative results were obtained. Meanwhile, studies have shown that the stiffness of hepatocellular carcinoma and cholangiocarcinoma is greater than that of liver metastases. Furthermore, metastatic carcinoma derived from the digestive tract has lower hardness, and AFP content is lower than the content of liver parenchyma. This led to low SWV value, AFP content and enhancement rate of regression coefficient, and then false negative results were obtained[43-51].
The results of this study were based on data from a small sample and did not cover all types of malignant and benign liver tumors. Therefore, the results of this study should be explained with caution, especially in clinical settings. However, the clinical pathological types of liver lesions studied in this study are common and have certain representative significance. Multi-center studies with a larger sample should be performed to verify our findings.
In summary, the diagnostic performance of combined ARFI, AFP and contrast-enhanced ultrasound in distinguishing between benign and malignant liver lesions was higher than the use of any single parameter or the combination of any two of parameters. It is expected that this would provide a tool for the differential diagnosis of benign and malignant liver lesions.
COMMENTS
Background
The gold standard for identifying benign and malignant liver lesions is pathological diagnosis. However, due to its invasiveness, the presence of infection, bleeding and other risks, pathological examination may cause body damage. Hence, pathological examination is only suitable for patients with suspected liver malignancy. In addition, pathological diagnosis has many contraindications and is applicable in only a narrow range of patients. Thus, it is difficult to implement pathological diagnosis as a routine examination.
Research frontiers
Serological indicators and contrast-enhanced ultrasound (CEUS) are noninvasive routine examinations for both benign and malignant liver lesions. Serological indicators alone have a low specificity and sensitivity. CEUS is a well-established examination method; however, it is relatively complex to operate and has high technical requirements, and some patients cannot tolerate the contrast agent. Acoustic radiation force impulse (ARFI) imaging is simple to operate, has high patient acceptance, and can quantitatively reflect the change in tissue hardness; however, it is easily affected by bile ducts and large blood vessels. The diagnostic value of the combined of ARFI imaging, serological indicators, and CEUS remains unclear.
Innovations and breakthroughs
Although ARFI imaging, serological indexes and CEUS have certain value in identifying benign and malignant lesions, each has its limitations. At present, the diagnostic accuracy of the combination of these three kinds of examinations in identifying benign and malignant liver lesions remains unknown, although the combined diagnosis has been widely used for other diseases. The results of this study showed that the diagnostic performance of combined ARFI, serum alpha-fetoprotein (AFP) and contrast-enhanced ultrasound in distinguishing between benign and malignant liver lesions was higher than the use of any single parameter or the combination of any two of parameters.
Applications
It is expected that the combination of ARFI imaging, serological indicators and CEUS would provide a tool for the differential diagnosis of benign and malignant liver lesions.
Peer-review
The combination of acoustic radiation force impulse imaging, serological indicators, and contrast-enhanced ultrasound has high accuracy in identifying benign and malignant liver lesions. This result should be confirmed by large multi-center studies, in order to better promote its clinical use.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study was approved by Shanghai Yangpu District East Hospital Institutional Review Board.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: No potential conflicts of interest relevant to this article are reported.
Data sharing statement: No additional data are available.
Peer-review started: March 29, 2017
First decision: April 11, 2017
Article in press: May 9, 2017
P- Reviewer: Hoetker MS, Meropol NJ S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Li D
References
- 1.Park SJ, Jang JY, Jeong SW, Cho YK, Lee SH, Kim SG, Cha SW, Kim YS, Cho YD, Kim HS, et al. Usefulness of AFP, AFP-L3, and PIVKA-II, and their combinations in diagnosing hepatocellular carcinoma. Medicine (Baltimore) 2017;96:e5811. doi: 10.1097/MD.0000000000005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Best J, Bilgi H, Heider D, Schotten C, Manka P, Bedreli S, Gorray M, Ertle J, van Grunsven LA, Dechêne A. The GALAD scoring algorithm based on AFP, AFP-L3, and DCP significantly improves detection of BCLC early stage hepatocellular carcinoma. Z Gastroenterol. 2016;54:1296–1305. doi: 10.1055/s-0042-119529. [DOI] [PubMed] [Google Scholar]
- 3.D’Onofrio M, Romanini L, Serra C, Magnolfi F, Bertolotto M, Quaia E, Puntel G, Colleoni A, Fiorini E, Cenci C, et al. Contrast enhancement ultrasound application in focal liver lesions characterization: a retrospective study about guidelines application (SOCEUS-CEUS survey) J Ultrasound. 2015;19:99–106. doi: 10.1007/s40477-015-0185-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwamoto T, Imai Y, Kogita S, Igura T, Sawai Y, Fukuda K, Yamaguchi Y, Matsumoto Y, Nakahara M, Morimoto O, et al. Comparison of Contrast-Enhanced Ultrasound and Gadolinium-Ethoxybenzyl-Diethylenetriamine Pentaacetic Acid-Enhanced MRI for the Diagnosis of Macroscopic Type of Hepatocellular Carcinoma. Dig Dis. 2016;34:679–686. doi: 10.1159/000448855. [DOI] [PubMed] [Google Scholar]
- 5.Kondo S, Takagi K, Nishida M, Iwai T, Kudo Y, Ogawa K, Kamiyama T, Shibuya H, Kahata K, Shimizu C. Computer-Aided Diagnosis of Focal Liver Lesions Using Contrast-Enhanced Ultrasonography with Perflubutane Microbubbles. IEEE Trans Med Imaging. 2017;36:1427–1437. doi: 10.1109/TMI.2017.2659734. [DOI] [PubMed] [Google Scholar]
- 6.Loria F, Loria G, Basile S, Crea G, Frosina L, Di Carlo I. Contrast-enhanced ultrasound appearances of enhancement patterns of intrahepatic cholangiocarcinoma: correlation with pathological findings. Updates Surg. 2014;66:135–143. doi: 10.1007/s13304-014-0251-6. [DOI] [PubMed] [Google Scholar]
- 7.Roche V, Pigneur F, Tselikas L, Roux M, Baranes L, Djabbari M, Costentin C, Calderaro J, Laurent A, Rahmouni A, et al. Differentiation of focal nodular hyperplasia from hepatocellular adenomas with low-mechanical-index contrast-enhanced sonography (CEUS): effect of size on diagnostic confidence. Eur Radiol. 2015;25:186–195. doi: 10.1007/s00330-014-3363-y. [DOI] [PubMed] [Google Scholar]
- 8.Sparchez Z, Mocan T, Radu P, Anton O, Bolog N. Contrast enhanced ultrasonography in assessing the treatment response to transarterial chemoembolization in patients with hepatocellular carcinoma. Med Ultrason. 2016;18:96–102. doi: 10.11152/mu.2013.2066.181.scz. [DOI] [PubMed] [Google Scholar]
- 9.Tselikas L, Pigneur F, Roux M, Baranes L, Costentin C, Roche V, Calderaro J, Herin E, Laurent A, Zafrani E, et al. Impact of hepatobiliary phase liver MRI versus Contrast-Enhanced Ultrasound after an inconclusive extracellular gadolinium-based contrast-enhanced MRI for the diagnosis of benign hepatocellular tumors. Abdom Radiol (NY) 2017;42:825–832. doi: 10.1007/s00261-016-0921-6. [DOI] [PubMed] [Google Scholar]
- 10.Bao Z, Gu L, Liu J, Wei L, Ye Z. [Clinical value of acoustic radiation force impulse elastography in differential diagnosis of focal liver lesions] Zhonghua Ganzangbing Zazhi. 2016;24:123–126. doi: 10.3760/cma.j.issn.1007-3418.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Cho SH, Lee JY, Han JK, Choi BI. Acoustic radiation force impulse elastography for the evaluation of focal solid hepatic lesions: preliminary findings. Ultrasound Med Biol. 2010;36:202–208. doi: 10.1016/j.ultrasmedbio.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Davies G, Koenen M. Acoustic radiation force impulse elastography in distinguishing hepatic haemangiomata from metastases: preliminary observations. Br J Radiol. 2011;84:939–943. doi: 10.1259/bjr/97637841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fahey BJ, Nelson RC, Hsu SJ, Bradway DP, Dumont DM, Trahey GE. In vivo guidance and assessment of liver radio-frequency ablation with acoustic radiation force elastography. Ultrasound Med Biol. 2008;34:1590–1603. doi: 10.1016/j.ultrasmedbio.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frulio N, Laumonier H, Carteret T, Laurent C, Maire F, Balabaud C, Bioulac-Sage P, Trillaud H. Evaluation of liver tumors using acoustic radiation force impulse elastography and correlation with histologic data. J Ultrasound Med. 2013;32:121–130. doi: 10.7863/jum.2013.32.1.121. [DOI] [PubMed] [Google Scholar]
- 15.Gallotti A, D’Onofrio M, Romanini L, Cantisani V, Pozzi Mucelli R. Acoustic Radiation Force Impulse (ARFI) ultrasound imaging of solid focal liver lesions. Eur J Radiol. 2012;81:451–455. doi: 10.1016/j.ejrad.2010.12.071. [DOI] [PubMed] [Google Scholar]
- 16.Goya C, Hamidi C, Yavuz A, Hattapoglu S, Uslukaya O, Cetincakmak MG, Teke M, Urakci Z. The Role of Acoustic Radiation Force Impulse Elastography in the Differentiation of Infectious and Neoplastic Liver Lesions. Ultrason Imaging. 2015;37:312–322. doi: 10.1177/0161734614566697. [DOI] [PubMed] [Google Scholar]
- 17.Heide R, Strobel D, Bernatik T, Goertz RS. Characterization of focal liver lesions (FLL) with acoustic radiation force impulse (ARFI) elastometry. Ultraschall Med. 2010;31:405–409. doi: 10.1055/s-0029-1245565. [DOI] [PubMed] [Google Scholar]
- 18.Chen L, Chu F, Cao Y, Shao J, Wang F. Serum miR-182 and miR-331-3p as diagnostic and prognostic markers in patients with hepatocellular carcinoma. Tumour Biol. 2015;36:7439–7447. doi: 10.1007/s13277-015-3430-2. [DOI] [PubMed] [Google Scholar]
- 19.Chen QW, Cheng CS, Chen H, Ning ZY, Tang SF, Zhang X, Zhu XY, Vargulick S, Shen YH, Hua YQ, et al. Effectiveness and complications of ultrasound guided fine needle aspiration for primary liver cancer in a Chinese population with serum α-fetoprotein levels ≤200 ng/ml--a study based on 4,312 patients. PLoS One. 2014;9:e101536. doi: 10.1371/journal.pone.0101536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui Z, Yu X, Guo L, Wei Y, Zheng S, Li W, Chen P, Zhu J, Peng J. Combined analysis of serum alpha-fetoprotein and MAGE-A3-specific cytotoxic T lymphocytes in peripheral blood for diagnosis of hepatocellular carcinoma. Dis Markers. 2013;35:915–923. doi: 10.1155/2013/907394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song P, Feng X, Inagaki Y, Song T, Zhang K, Wang Z, Zheng S, Ma K, Li Q, Kong D, et al. Clinical utility of simultaneous measurement of alpha-fetoprotein and des-γ-carboxy prothrombin for diagnosis of patients with hepatocellular carcinoma in China: A multi-center case-controlled study of 1,153 subjects. Biosci Trends. 2014;8:266–273. doi: 10.5582/bst.2014.01116. [DOI] [PubMed] [Google Scholar]
- 22.Kim JE, Lee JY, Bae KS, Han JK, Choi BI. Acoustic radiation force impulse elastography for focal hepatic tumors: usefulness for differentiating hemangiomas from malignant tumors. Korean J Radiol. 2013;14:743–753. doi: 10.3348/kjr.2013.14.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon HJ, Kang MJ, Cho JH, Oh JY, Nam KJ, Han SY, Lee SW. Acoustic radiation force impulse elastography for hepatocellular carcinoma-associated radiofrequency ablation. World J Gastroenterol. 2011;17:1874–1878. doi: 10.3748/wjg.v17.i14.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mărginean CO, Brănzaniuc K, Mărginean C, Azamfirei L, Pitea AM. Elastography, progression factor in liver ultrasound. Rev Med Chir Soc Med Nat Iasi. 2010;114:764–770. [PubMed] [Google Scholar]
- 25.Rizzo L, Nunnari G, Berretta M, Cacopardo B. Acoustic Radial Force Impulse as an effective tool for a prompt and reliable diagnosis of hepatocellular carcinoma - preliminary data. Eur Rev Med Pharmacol Sci. 2012;16:1596–1598. [PubMed] [Google Scholar]
- 26.Shuang-Ming T, Ping Z, Ying Q, Li-Rong C, Ping Z, Rui-Zhen L. Usefulness of acoustic radiation force impulse imaging in the differential diagnosis of benign and malignant liver lesions. Acad Radiol. 2011;18:810–815. doi: 10.1016/j.acra.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 27.Vermehren J, Polta A, Zimmermann O, Herrmann E, Poynard T, Hofmann WP, Bojunga J, Sarrazin C, Zeuzem S, Friedrich-Rust M. Comparison of acoustic radiation force impulse imaging with transient elastography for the detection of complications in patients with cirrhosis. Liver Int. 2012;32:852–858. doi: 10.1111/j.1478-3231.2011.02736.x. [DOI] [PubMed] [Google Scholar]
- 28.Ying L, Lin X, Xie ZL, Tang FY, Hu YP, Shi KQ. Clinical utility of acoustic radiation force impulse imaging for identification of malignant liver lesions: a meta-analysis. Eur Radiol. 2012;22:2798–2805. doi: 10.1007/s00330-012-2540-0. [DOI] [PubMed] [Google Scholar]
- 29.Yu H, Wilson SR. Differentiation of benign from malignant liver masses with Acoustic Radiation Force Impulse technique. Ultrasound Q. 2011;27:217–223. doi: 10.1097/RUQ.0b013e318239422e. [DOI] [PubMed] [Google Scholar]
- 30.Dong Y, Wang WP, Mao F, Ji ZB, Huang BJ. Application of imaging fusion combining contrast-enhanced ultrasound and magnetic resonance imaging in detection of hepatic cellular carcinomas undetectable by conventional ultrasound. J Gastroenterol Hepatol. 2016;31:822–828. doi: 10.1111/jgh.13202. [DOI] [PubMed] [Google Scholar]
- 31.Dong Y, Zhu Z, Wang WP, Mao F, Ji ZB. Ultrasound features of hepatocellular adenoma and the additional value of contrast-enhanced ultrasound. Hepatobiliary Pancreat Dis Int. 2016;15:48–54. doi: 10.1016/s1499-3872(15)60039-x. [DOI] [PubMed] [Google Scholar]
- 32.Kong WT, Ji ZB, Wang WP, Cai H, Huang BJ, Ding H. Evaluation of Liver Metastases Using Contrast-Enhanced Ultrasound: Enhancement Patterns and Influencing Factors. Gut Liver. 2016;10:283–287. doi: 10.5009/gnl14324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li R, Tang CL, Cai P, Ma KS, Zhang XH, Ding SY, Zhang Y, Guo DY, Yan XC. Comparison of CT and contrast-enhanced ultrasound findings in hepatic angiomyolipoma with pathological correlations. Abdom Radiol (NY) 2016;41:248–256. doi: 10.1007/s00261-015-0571-0. [DOI] [PubMed] [Google Scholar]
- 34.Liang X, Lin L, Cao Q, Huang R, Wang Y. Recognizing Focal Liver Lesions in CEUS With Dynamically Trained Latent Structured Models. IEEE Trans Med Imaging. 2016;35:713–727. doi: 10.1109/TMI.2015.2492618. [DOI] [PubMed] [Google Scholar]
- 35.Liu JJ, Li HX, Chen ZB, Yang WP, Zhao SF, Chen J, Bai T, Li H, Li LQ. Consistency analysis of contrast-enhanced ultrasound and contrast-enhanced CT in diagnosis of small hepatocellular carcinoma. Int J Clin Exp Med. 2015;8:21466–21471. [PMC free article] [PubMed] [Google Scholar]
- 36.Menozzi G, Maccabruni V, Marini G, Froio E, Garlassi E. Contrast-enhanced ultrasound (CEUS) appearance of hepatic myelolipoma. J Ultrasound. 2014;19:61–65. doi: 10.1007/s40477-014-0137-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seitz K, Strobel D. A Milestone: Approval of CEUS for Diagnostic Liver Imaging in Adults and Children in the USA. Ultraschall Med. 2016;37:229–232. doi: 10.1055/s-0042-107411. [DOI] [PubMed] [Google Scholar]
- 38.Shiozawa K, Watanabe M, Ikehara T, Matsukiyo Y, Kogame M, Shinohara M, Kikuchi Y, Shinohara M, Igarashi Y, Sumino Y. [Evaluation of Sorafenib for Hepatocellular Carcinoma with Low α-Fetoprotein by Arrival Time Parametric Imaging Using Contrast-Enhanced Ultrasonography with Sonazoid] Gan To Kagaku Ryoho. 2016;43:215–218. [PubMed] [Google Scholar]
- 39.Sirli R, Sporea I, Săndulescu DL, Popescu A, Dănilă M, Săftoiu A, Spârchez Z, Badea R. Contrast enhanced ultrasound for the diagnosis of liver hemangiomas - results of a Romanian multicentre study. Med Ultrason. 2015;17:444–450. doi: 10.11152/mu.2013.2066.174.csu. [DOI] [PubMed] [Google Scholar]
- 40.Spârchez Z, Radu P, Kacso G, Spârchez M, Zaharia T, Al Hajjar N. Prospective comparison between real time contrast enhanced and conventional ultrasound guidance in percutaneous biopsies of liver tumors. Med Ultrason. 2015;17:456–463. doi: 10.11152/mu.2013.2066.174.deu. [DOI] [PubMed] [Google Scholar]
- 41.Sugimori K, Numata K, Okada M, Nihonmatsu H, Takebayashi S, Maeda S, Nakano M, Tanaka K. Central vascular structures as a characteristic finding of regenerative nodules using hepatobiliary phase gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid-enhanced MRI and arterial dominant phase contrast-enhanced US. J Med Ultrason (2001) 2017;44:89–100. doi: 10.1007/s10396-016-0750-9. [DOI] [PubMed] [Google Scholar]
- 42.Yang W, Yan K, Wang S, Dai Y, Wu W, Yin SS, Chen MH. Differential Diagnosis of Arterial Phase Enhanced Hepatic Inflammatory Lesions and Hepatocellular Carcinomas with Contrast-enhanced Ultrasound. Ultrasound Med Biol. 2016;42:82–91. doi: 10.1016/j.ultrasmedbio.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 43.Beyer LP, Pregler B, Wiesinger I, Stroszczynski C, Wiggermann P, Jung EM. Continuous dynamic registration of microvascularization of liver tumors with contrast-enhanced ultrasound. Radiol Res Pract. 2014;2014:347416. doi: 10.1155/2014/347416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corvino A, Catalano O, Setola SV, Sandomenico F, Corvino F, Petrillo A. Contrast-enhanced ultrasound in the characterization of complex cystic focal liver lesions. Ultrasound Med Biol. 2015;41:1301–1310. doi: 10.1016/j.ultrasmedbio.2014.12.667. [DOI] [PubMed] [Google Scholar]
- 45.Gatos I, Tsantis S, Spiliopoulos S, Skouroliakou A, Theotokas I, Zoumpoulis P, Hazle JD, Kagadis GC. A new automated quantification algorithm for the detection and evaluation of focal liver lesions with contrast-enhanced ultrasound. Med Phys. 2015;42:3948–3959. doi: 10.1118/1.4921753. [DOI] [PubMed] [Google Scholar]
- 46.Liu J, Wang D, Li H, Li H, Zhou T, Zhao S, Ding Z. Clinical Value of Contrast-Enhanced Ultrasound in Diagnosis of Hyperechoic Liver Lesions. Med Sci Monit. 2015;21:2845–2850. doi: 10.12659/MSM.894115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lyu P, Li S, Xu H, Xu L, Lyu J, Shou J, Zhao B. [Application value of contrast-enhanced ultrasonography for assessing the high-risk population of hepatic malignant tumor] Zhonghua Zhongliu Zazhi. 2015;37:545–548. [PubMed] [Google Scholar]
- 48.Pschierer K, Grothues D, Rennert J, da Silva NP, Schreyer AG, Melter M, Stroszczysnski C, Jung EM. Evaluation of the diagnostic accuracy of CEUS in children with benign and malignant liver lesions and portal vein anomalies. Clin Hemorheol Microcirc. 2015;61:333–345. doi: 10.3233/CH-152003. [DOI] [PubMed] [Google Scholar]
- 49.Sporea I, Martie A, Bota S, Sirli R, Popescu A, Dănila M. Characterization of focal liver lesions using contrast enhanced ultrasound as a first line method: a large monocentric experience. J Gastrointestin Liver Dis. 2014;23:57–63. [PubMed] [Google Scholar]
- 50.Tarantino L, Ambrosino P, Di Minno MN. Contrast-enhanced ultrasound in differentiating malignant from benign portal vein thrombosis in hepatocellular carcinoma. World J Gastroenterol. 2015;21:9457–9460. doi: 10.3748/wjg.v21.i32.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim TK, Jang HJ. Contrast-enhanced ultrasound in the diagnosis of nodules in liver cirrhosis. World J Gastroenterol. 2014;20:3590–3596. doi: 10.3748/wjg.v20.i13.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]


