Abstract
Purpose
The aim was to investigate the effect of sleep improvement on desire for and intake of weight gain promoting foods in adolescents with late bedtimes.
Methods
A sample of 42 adolescents with late bedtimes was enrolled in an intervention designed to improve sleep. Their desire for and intake of food in the morning was assessed at pre-treatment and post-treatment.
Results
Adolescents with earlier bedtimes at post-treatment relative to pre-treatment increased their caloric intake of low Glycemic Index, fruit and dairy foods at post-treatment. This effect was not observed in adolescents who did not improve their bedtime at post-treatment.
Conclusions
These findings suggest that advancing bedtimes earlier can improve breakfast choices, an important meal for obesity prevention during adolescence.
Keywords: eveningness, bedtimes, sleep, adolescent, food
The average adolescent in the United States sleeps approximately 7 hours during the school year and 33% of adolescents go to bed later than 11:15pm (1). With the onset and progression through puberty there is a biologic shift toward both going to sleep and getting up later, often referred to as an eveningness circadian preference, which when combined with early school start times are thought to be major contributors to this sleep deficit (2, 3). The combined picture is of concern given that both late bedtimes and short total sleep time (TST) are independently associated with a range of adverse outcomes (1, 4), including higher risk of obesity (5, 6).
Rates of obesity are rising rapidly among children and adolescents (7). Eating behaviors tend to persist from adolescence into adulthood and thus adolescence is a particularly important period for obesity prevention (8). Increased intake of low glycemic index (GI) foods has been linked to obesity prevention (9, 10). GI is a measure of how quickly blood glucose levels (i.e., blood sugar) rise after eating a particular type of food. Low GI foods promote satiety by supporting fat instead of carbohydrate metabolism, and higher levels of the satiety promoting hormone leptin (11). Indeed, obese children who were given high GI breakfasts ate 53% more throughout the day than those with medium or low GI breakfasts (12). Moreover, a small experimental study in 9 healthy adult men indicated the timing and amount of leptin secreted were altered by consuming high-GI meals (13).
Accumulating evidence points to late bedtimes, shorter total sleep time (TST) and daytime sleepiness as independent risk factors for the selection and intake of more high GI foods and fewer low GI foods (5, 14). Late bedtime on non-school days has been associated with greater “fast food” consumption in adolescents (15), which may be influenced by less availability of healthier food options at night. One experimental study involving adolescent participants found that short TST (defined as 6.5 hours of less hours) over 5 nights resulted in increased intake of high glycemic index foods (16). Moreover, another group of researchers found that, in an experimental protocol, desire for weight-gain promoting foods increased after sleep deprivation in adults and was predicted by subjective daytime sleepiness (14).
To our knowledge, there are no published investigations as to whether sleep improvement results in healthier food choices in adolescents. There are several reasons why it is especially important to determine whether an intervention to improve sleep is effective in improving food choice in adolescents with an eveningness circadian preference (a risk factor for obesity) (5); first, food intake plays a key role in the development and maintenance of obesity (17) and second, there are few effective treatments for obesity (18, 19).
The present study was designed within the context of a NICHD-funded trial designed to improve sleep for 10–18 year olds with an eveningness circadian preference. The aim of the present study was to investigate the effect of sleep improvement (indexed by three sleep and circadian markers derived from sleep diary), following 6 sessions of either an active sleep or control treatment, on (a) desire for and (b) intake of weight gain promoting foods in the morning. The hypothesis tested is that sleep improvement from pre-treatment to post-treatment, across treatment groups, will be associated with both decreased desire for and intake of weight gain promoting foods.
Methods
Participants
The data for the present study were collected as part of a larger research project. Adolescents were eligible for the study if they scored within the lowest quartile, the cutoff for eveningness circadian preference, on the Children’s Morningness Eveninigness Preference Scale (20). Also, the adolescent must have had the current pattern of late bedtimes for the last 3 months based on self and parent-report. Adolescents were ineligible to participate, if (a) they could not communicate in English or Spanish, (b) they had an active, progressive physical illness or neurological degenerative disease directly related to the onset and course of the sleep disturbance, (c) there was evidence from clinical diagnosis or report of sleep apnea, restless legs or periodic limb movements during sleep, (d) they had an intellectual disability, autism spectrum disorder, or any other significantly impairing pervasive developmental disorder, (e) there was evidence from clinical diagnosis or report by youth or parent of Bipolar Disorder or Schizophrenia, or (e) they had a history of substance dependence in the past six months.
Participants were recruited for the current study via administrators and parent groups at local schools as well as advertisements on list serves, Craigslist and Facebook.
Design
All procedures were approved by the University of California, Berkeley, Committee for the Protection of Human Subjects (Protocol ID 2012-02-4007).
For adolescents who met eligibility criteria after an in-person assessment, seven to ten days of sleep diary was collected. Participants then spent the night prior to the food-desire and snack tasks in the laboratory. In the laboratory, each participant went to bed at their average weekday bedtime and woke at their average weekday rise time as defined by their sleep diary. In the morning, a standardized breakfast of one slice of toast and jam was provided. The food-desire task followed by the snack task was then administered. An identical protocol was repeated following completion of 6 weeks of intervention.
Using a computer-generated random numbers list, a research assistant conducted randomization of adolescents to either an active sleep or psychoeducation condition, stratified by age and sex. In the present study, adolescents were collapsed across conditions. 42 adolescents participated in the present study.
Measures of Demographic Characteristics
Demographic characteristics assessed included parent reports of the adolescents’ age, biological sex, race/ethnicity and household income.
Investigators measured height and weight, from which BMI was calculated. BMI was transformed into z-scores for age and sex.
Sleep Measures
Sleep Diary (21, 22)
The daily sleep diary is a valid and sensitive measure in the detection of differences due to weekends, age, gender, sleep timing and sleep quality (23). In the present study, trained research assistants called the adolescents to collect their sleep diary each morning at an agreed-upon time.
While the full sleep diary was administered, based on a review of the literature (24, 25), bedtime and TST were determined to be the strongest risk factors for weight gain promoting food choice and thus were selected as the sleep parameters of interest for the current investigation. Bedtime and TST difference scores were calculated by subtracting average pre-treatment scores from average post-treatment scores. Earlier bedtimes at post-treatment compared to pre-treatment constituted the “Bedtime Improvement Group” (n=22); the same or later bedtimes at post-treatment compared to pre-treatment constituted the “No Bedtime Improvement Group” (n=20). Longer TST at post-treatment compared to pre-treatment constituted the “TST Improvement Group” (n=24); the same or shorter TST at post-treatment compared to pre-treatment constituted the “No TST Improvement Group” (n=18).
Sleepiness Scale (26)
The sleepiness scale is a well validated and widely used measure used to quantify subjective sleepiness throughout the day. The sleepiness scale showed a Pearson r test–retest reliability correlation of .65 (p<.01) and, based on the criterion of 0.70, the internal consistency coefficients of the subscale are near (0.65) or above (0.70), acceptable standards for the control and clinical samples, respectively (26). The sleepiness scale asks, “During the last two weeks, have you struggled to stay awake (fought sleep) or fallen asleep in the following situations?” It lists 10 situations (i.e. in a class at school) scored on a 4-point likert scale ranging from “no” to “both struggled to stay awake and fallen asleep”. The sleepiness scale was administered the night before the experimental protocol. Sleepiness difference scores were calculated by subtracting pre-treatment scores from post-treatment scores. Lower self-reported sleepiness at post-treatment compared to pre-treatment constituted the “Sleepiness Improvement Group” (n=24); the same or increased sleepiness at post-treatment compared to pre-treatment constituted the “No Sleepiness Improvement Group” (n=14).
Food Desire and Intake Measurements
Food-Desire Task (14)
A detailed description of the Food-desire task has been described elsewhere (14). The task was administered on a Dell Latitude E6430 laptop. Participants were seated at a computer and instructed to rate pictures of 80 food items on a 1–4 scale according to how much they wanted that food right now. Participants were informed that they would receive snacks following the task based on whichever items they rated wanting more.
The food items were evenly distributed across five categories (salty, sweet, starchy, fruit or dairy) and varied in GI. GI per serving was defined based on International Table of Glycemic Index (27). Foods were subsequently categorized as high versus low GI based on established cutoffs; low GI is defined as less than 55 and high GI is 55 or above. The mean desire for high and low GI foods and fruit, dairy, salt, starch, and sweet foods was then calculated.
Snack Task (28, 29)
The snack task has been used as a measure of food intake. In the present study, food choices were modified from previous protocols to match the foods displayed in the food-desire task. The 10 items available corresponded to items they had rated earlier in the food-desire task; donuts or cinnamon rolls (sweet), tortilla chips or cheese crackers (salty), bagels or croissants (starch), grapes or strawberries (fruit), and cheddar or mozzarella cubes (dairy). Participants were offered five bowls of food, one from each of five food categories: sweet, starch, salty, fruit, or dairy. Within each food category, two food items were available. The food offered in each category was based on which food the participant rated higher in the food-desire task. Items within categories were matched for calories per gram, GI, texture, and size. Based on the International Table of Glycemic Index (27), foods in the sweet, salty and starch categories were defined as high GI and foods in the dairy and fruit categories were defined as low GI.
All foods were unwrapped and each category of food was placed in a separate and identical plastic bowl on a tray in front of the participant. The arrangement of the bowls on the tray was counterbalanced to control for lateralization effects. All bowls appeared full and each participant was given the same amount of each food item. The research assistant said to each participant “these are the snacks you won. Feel free to eat whatever you like. Take a break and I’ll be back in 10 minutes.” After 10 minutes the research assistant returned to the room to retrieve the bowls.
The experimenter weighed each bowl before the participants arrived and then again after the task was completed. Measurements were computed in number of grams and were made using a 5-lb capacity, digital scale (with accuracy to the .10 gram). Calories consumed were calculated based on the calorie per gram of the food and the number of grams consumed. Food intake is defined as the number of calories of high GI, low GI, dairy, fruit, salt, starch and sweet foods consumed.
Treatment
Treatment began one week after the pre-treatment assessment and ended the week prior to the post-treatment assessment. Treatment involved 6 individual weekly 50–60 minute sessions delivered during the school year. The variable “Time” is defined as the pre-treatment and post-treatment time points.
Detailed descriptions of the active sleep (TranS-C-Youth) and Psychoeducation (PE) conditions have been described elsewhere (30). More details regarding treatment are available in the online supplement). All participants received at least 6 sessions of treatment.
Analysis Plan
All analyses were conducted in SPSS 22 (IBM Corporation, Armonk, NY, 2013). Statistical significance was evaluated using a 2-sided design with alpha set at 0.05.
To assess differences in bedtime, TST and sleepiness from pre-treatment to post-treatment independent sample t-tests were conducted within Sleep Improvement Groups. Repeated-measures ANOVAs were used to assess the impact of sleep improvement (separately for bedtime improvement, TST improvement, and sleepiness improvement) on desire for and intake of foods at pre-treatment versus post-treatment. T-tests were conducted to further explore significant interaction effects.
Results
Participant characteristics are presented in Table 1.
Table 1.
Demographic characteristics at pre-treatment
| Full Sample N=42 | |
|---|---|
| Age (years): M (SD) | 15.15 (1.68) |
| Sex: % male | 42 |
| Race: | |
| % Caucasian | 52.1 |
| % African American/Black | 8.3 |
| % Hispanic or Latino/Latina | 14.3 |
| % Asian | 10.4 |
| % Native Hawaiian or Pacific Islander | 8.3 |
| % Refused | 4.2 |
| % Mixed Race | 16.7 |
| Annual Income ($): % <$30,000 | 6.5 |
| Z-score: M (SD) | .37 (1.0) |
| Bedtime (hh:mm): M (SD) | 1:02 AM (1.33) |
| TST (hours): M (SD) | 6.64 (1.25) |
| 1 Sleepiness: M (SD) | 5.97 (4.71) |
Note. M=mean; SD=standard deviation; Z-score=BMI adjusted for age and sex; hh:mm=hours:minutes.
Sleepiness scores ranged from 0 to 19 in the present sample. In the measure validation study conducted in children, 9.64 and 11.99 were the mean scores in a control and clinical sleep disordered sample respectively (Owens, Spirito & McGuinn, 2000).
The mean bedtime, TST and sleepiness difference values for the three sets of Sleep Improvement Groups are displayed in Table 2. The Bedtime Improvement and No Bedtime Improvement Groups and TST Improvement and No TST Improvement Groups significantly differed in bedtime and TST from pre-treatment to post-treatment, but not in sleepiness. The Sleepiness Improvement and No Sleepiness Improvement Groups significantly differed in sleepiness from pre-treatment to post-treatment, but not in bedtime and TST.
Table 2.
Means, standard deviations, and t-tests of difference in bedtimes, TST, and sleepiness from pre-treatment to post-treatment among Sleep Improvement Groups.a
| Sleep Improvement Groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| BT Imprv N=22 |
No BT Imprv N=20 |
t | TST Imprv N=24 |
No TST Imprv N=18 |
t | Sleepiness Imprv N=13 |
No Sleepiness Imprv N=23 |
t | |
| BT Diff (hours) | −.96 (.71) | .56 (.48) | 8.05** | −.79 (.86) | .50 (.58) | 5.50** | −.49 (1.10) | −.07 (.98) | −1.19 |
| TST Diff (hours) | −1.62 (1.64) | .35 (.68) | 4.98** | −1.61 (1.53) | .55 (.46) | 5.77** | −.68 (1.28) | −.64 (1.82) | −.07 |
| Sleepiness Diff (score) | −.63 (2.91) | 1.12 (7.23) | .97 | 1.10 (6.64) | 1.07 (2.60) | −1.19 | −3.43 (1.99) | 2.17 (5.51) | −3.64** |
Note. Data are expressed as the mean (and standard deviation) or as t-values. Imprv=Improvement; Diff=Difference; t=t-values from independent samples t-tests; BT Diff=bedtime difference from pre-treatment to post-treatment; TST Diff=total sleep time difference from pre-treatment to post-treatment; Sleepiness Diff =sleepiness difference from pre-treatment to post-treatment.
This table does not reflect the full sample because 7 participants dropped out during treatment. 4 participants were missing sleepiness data. Values displayed are means (and standard deviations).
p <.05;
p <.001.
Two-way repeated-measures ANOVAs were conducted, one for each of the food desire and intake outcome variables1. Mean food desire and intake outcomes in the Sleep Improvement Groups at pre-treatment and post-treatment are listed in Table 3.
Table 3.
Means and standard deviations of food desire and intake in Sleep Improvement Groups at pre-treatment and post-treatment.
| BT Improvement Groups
|
TST Improvement Groups
|
Sleepiness Improvement Groups
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BT Imprv n=22 | No BT Imprv n=20 | TST Imprv n=24 | No TST Imprv n=18 | Sleepiness Impv n= 24 | No Sleepiness Imprv n=14 | |||||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Desirea | ||||||||||||
| High GI | 2.29 (.48) | 2.09 (.36) | 2.42 (.44) | 2.47 (.50) | 2.32 (.46) | 2.19 (.35) | 2.40 (.47) | 2.34 (.60) | 2.31 (.38) | 2.17 (.33) | 2.40 (.45) | 2.35 (.46) |
| Low GI | 2.42 (.49) | 2.39 (.45) | 2.40 (.54) | 2.36 (.52) | 2.36 (.51) | 2.38 (.43) | 2.47 (.51) | 2.38 (.53) | 2.49 (.40) | 2.39 (.21) | 2.42 (.51) | 2.45 (.55) |
| Fruit | 2.63 (.59) | 2.62 (.60) | 2.54 (.61) | 2.56 (.62) | 2.54 (.61) | 2.58 (.57) | 2.54 (.61) | 2.63 (.66) | 2.62 (.52) | 2.67 (.42) | 2.64 (.59) | 2.68 (.64) |
| Dairy | 2.13 (.64) | 2.11 (.53) | 2.06 (.61) | 1.95 (.67) | 2.10 (.60) | 2.08 (.56) | 2.09 (.66) | 2.00 (.66) | 2.31 (.59) | 2.03 (.41) | 2.08 (.62) | 2.11 (.67) |
| Salt | 1.96 (.58) | 1.89 (.59) | 1.96 (.58) | 2.07 (.57) | 1.99 (.52) | 1.98 (.51) | 2.07 (.69) | 1.93 (.70) | 2.03 (.66) | 1.75 (.55) | 1.99 (.54) | 2.12 (.55) |
| Starch | 2.35 (.50) | 2.08 (.42) | 2.46 (.47) | 2.44 (.57) | 2.31 (.49) | 2.13 (.41) | 2.52 (.46) | 2.40 (.62) | 2.43 (.42) | 2.15 (.38) | 2.43 (.47) | 2.33 (.53) |
| Sweet | 2.50 (.64) | 2.32 (.46) | 2.73 (.64) | 2.91 (.64) | 2.59 (.611) | 2.49 (.52) | 2.63 (.69) | 2.68 (.74) | 2.46 (.47) | 2.56 (.57) | 2.71 (.66) | 2.60 (.64) |
| Intakeb | ||||||||||||
| Calories | 374.28 (253.35) | 501.17 (191.68) | 513.05 (197.94) | 537.81 (204.46) | 435.07 (260.36) | 521.44 (207.97) | 447.41 (207.69) | 511.62 (182.03) | 386.24 (218.48) | 539.75 (193.32) | 471.36 (245.49) | 518.42 (204.83) |
| High GI | 282.95 (194.82) | 367.28 (146.00) | 376.48 (175.01) | 434.60 (189.33) | 333.06 (212.97) | 398.90 (175.25) | 320.06 (158.24) | 395.09 (161.82) | 283.72 (155.95) | 400.93 (140.28) | 36.56 (204.49) | 413.97 (180.40) |
| Low GI | 91.33 (72.86) | 133.64 (97.35) | 136.57 (81.19) | 103.03 (78.06) | 102.02 (77.11) | 122.31 (94.64) | 127.35 (82.19) | 116.33 (83.86) | 102.52 (88.23) | 138.57 (90.94) | 105.80 (68.75) | 104.27 (87.16) |
| Fruit | 26.27 (18.08) | 33.00 (17.05) | 32.75 (16.24) | 27.77 (13.93) | 26.48 (18.75) | 30.85 (15.91) | 33.19 (14.88) | 30.37 (16.06) | 28.12 (18.84) | 31.17 (15.02) | 30.77 (16.14) | 29.89 (15.28) |
| Dairy | 65.06 (66.93) | 100.64 (89.89) | 103.83 (77.38) | 75.26 (69.83) | 75.54 (72.77) | 91.46 (87.19) | 94.16 (76.02) | 85.96 (74.72) | 74.40 (76.84) | 107.41 (84.14) | 75.04 (65.15) | 74.38 (77.76) |
| Salt | 69.05 (81.94) | 54.36 (68.39) | 137.19 (126.16) | 120.54 (99.31) | 95.33 (115.46) | 78.12 (87.90) | 109.72 (103.84) | 92.94 (92.78) | 76.31 (87.32) | 74.86 (86.67) | 122.04 (119.29) | 105.66 (89.07) |
| Starch | 110.57 (104.91) | 114.99 (98.07) | 99.55 (71.01) | 116.70 (85.22) | 121.83 (99.49) | 120.77 (95.87) | 83.31 (70.83) | 108.07 (86.56) | 86.83 (88.99) | 124.90 (80.24) | 101.98 (87.64) | 109.50 (96.98) |
| Sweet | 103.33 (103.34) | 197.93 (105.14) | 139.74 (108.23) | 197.36 (103.19) | 127.03 (111.90) | 200.02 (113.05) | 127.03 (111.90) | 194.08 (88.65) | 120.58 (115.77) | 201.17 (90.71) | 141.01 (101.96) | 198.82 (99.85) |
Note. Data are expressed as the mean (and standard deviation). Pre=Pre-treatment; Post=Imprv=Improvement; BT=bedtime; TST=total sleep time.
Desire is measured on scale from 1 to 4.
Intake is measured in calories consumed.
Food Desire
There were significant main effects of Bedtime Improvement Group on high GI foods desired F(1,30)=8.07, MSE=1.58, p=.008, η2=.141, 90% CI [.006–.323] and sweets desired, F(1,30)=4.59, MSE=4.20, p=.04, η2=.212, 90% CI [.034–.395]. No other main effects of Sleep Improvement Group were observed.
Significant main effects of Time indicated that, regardless of Sleepiness Improvement Group, adolescents desired more starch calories, F(1,28)=7.08, MSE=.34, p=.01, η2=.202, 90% CI [.026–.390]. No other main effects of Time were observed.
No significant Sleep Improvement Group x Time interaction effects were observed on food desire (all p’s>.05).
Food Intake
Results for Repeated-measures ANOVA’s assessing the role of sleep improvement on food intake in the snack task are displayed in Table 4.
Table 4.
Results of Repeated-Measures ANOVA analyses of Sleep Improvement on Intake of Calories in the Snack Task
| Group | Time | Group x Time | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F | η2 | 90% CI | F | η2 | 90% CI | F | η2 | 90% CI | |
| Bedtime Improvement Groupsa | |||||||||
| Calories | 3.193 | .082 | 0–.239 | 3.346 | .085 | 0–.244 | 1.855 | .049 | 0–.193 |
| High GI | 3.696 | .093 | 0–.254 | 4.010* | .100 | 0.263 | .395 | .011 | 0–.118 |
| Low GI | .114 | .003 | 0–.083 | .515 | .014 | 0–.127 | 7.236* | .167 | .023–.337 |
| Fruit | .272 | .008 | 0–.106 | .005 | .0001 | 0–.006 | 4.253* | .106 | .009–.269 |
| Dairy | .070 | .002 | 0–.071 | .584 | .016 | 0–.132 | 5.621* | .135 | .010–.303 |
| Salt | 6.439* | .152 | .017–.321 | .424 | .012 | 0–.120 | .005 | .0001 | 0–.006 |
| Starch | .043 | .001 | 0–.046 | .847 | .023 | 0–.148 | .154 | .004 | 0–.091 |
| Sweet | .936 | .025 | 0–.153 | 11.732* | .246 | .065–.414 | 2.169 | .057 | 0–.205 |
| TST Improvement Groupsb | |||||||||
| Calories | .002 | .0001 | 0–.002 | 3.373 | .086 | 0–.245 | .071 | .002 | 0–.072 |
| High GI | .013 | .0004 | 0–.015 | 4.136* | .103 | .001–.266 | .003 | .0001 | 0–.003 |
| Low GI | .127 | .004 | 0–.086 | .514 | .014 | 0–.127 | .825 | .022 | 0–.146 |
| Fruit | 1.371 | .037 | 0–.173 | .001 | .000 | 0–.001 | 1.965 | .052 | 0–.198 |
| Dairy | .027 | .0007 | 0–.030 | .637 | .017 | 0–.135 | .404 | .011 | 0–.119 |
| Salt | .136 | .004 | 0–.088 | .334 | .009 | 0–.113 | .068 | .002 | 0–.071 |
| Starch | 1.370 | .037 | 0–.173 | 1.130 | .030 | 0–.162 | .821 | .022 | 0–.146 |
| Sweet | .169 | .005 | 0–.093 | 10.663* | .229 | .055–.397 | .830 | .023 | 0–.147 |
| Sleepiness Improvement Groupsc | |||||||||
| Calories | .335 | .010 | 0–.118 | 4.553* | .118 | .003–.288 | 1.361 | .039 | 0–.181 |
| High GI | .861 | .025 | 0–.155 | 4.817* | .124 | .005–.295 | .840 | .024 | 0–.154 |
| Low GI | .262 | .008 | 0–.111 | 1.552 | .044 | 0–.189 | 1.793 | .050 | 0–.200 |
| Fruit | .035 | .001 | 0–.040 | .110 | .003 | 0–.086 | .324 | .009 | 0–.117 |
| Dairy | .368 | .011 | 0–.121 | 1.618 | .045 | 0–.192 | 1.731 | .048 | 0–.197 |
| Salt | 2.369 | .065 | 0–.222 | .005 | .0001 | 0–.006 | .911 | .026 | 0–.158 |
| Starch | .012 | .0004 | 0–.014 | 1.228 | .035 | 0–.175 | .478 | .014 | 0–.131 |
| Sweet | .019 | .0006 | 0–.022 | 11.402* | .251 | .065–.422 | .117 | .003 | 0–.088 |
There were significant main effects of Bedtime Improvement Group, such that, regardless of Time, adolescents in the Bedtime Improvement Group ate fewer sweet and salty foods than adolescents in the No Bedtime Improvement Group.2 No other main effects of Sleep Improvement Group were observed.
Significant main effects of Time indicated that, regardless of Sleepiness Improvement group adolescents ate more calories total; regardless of Bedtime, TST or Sleepiness Improvement Group, adolescents ate more high GI, and sweet calories, post-treatment compared to pre-treatment.
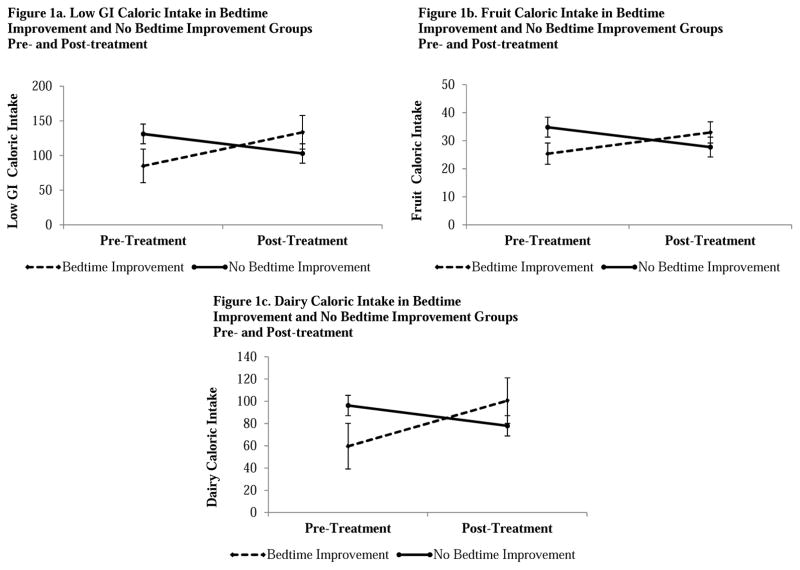
Significant results for the Bedtime Improvement Group x Time interaction effects, following 6 sessions of either treatment condition, on food intake are displayed in Figure 1. This indicates that the intake of low GI (Figure 1a), fruit (Figure 1b), and dairy (Figure 1c) calories at pre-treatment and post-treatment differed by Bedtime Improvement Group. Paired samples t-tests were performed comparing pre-treatment to post-treatment intake of low GI, fruit and dairy calories in the Bedtime Improvement Groups. Paired samples t-tests revealed that adolescents in the Bedtime Improvement Group ate significantly more low GI t(20)=−2.63, p=.02, d=.75, 95% CI [.12–1.37], and dairy calories t(20)= −2.46, p=.02, d=.75, 95% CI [.12–1.37], but not fruit calories t(20)= −1.48, p>.05 at pre-treatment compared to post-treatment. No significant differences were observed between pre-treatment and post-treatment food intake in the No Bedtime Improvement Group, (all p’s>.05). Independent sample t-tests revealed no significant differences between the Bedtime Improvement Group and the No Bedtime Improvement Group in food intake at pre-treatment or post-treatment (all p’s>.05). No other significant Sleep Improvement Group x Time interaction effects were observed.
Figure 1.
Bedtime Improvement Group x Time Interactions for (1a) Low GI, (1b) Fruit, and (1c) Dairy Caloric Intake: Means and Standard Error Bars. GI=Glycemic Index
Discussion
This study focused on food choices in adolescents with an eveningness circadian preference, a group at increased risk for obesity (4, 5). To our knowledge, this is the first study to a) investigate the relationship between sleep and breakfast choices - an important, independent risk factor for obesity in adolescents (12) and b) investigate sleep improvement as a potential mechanism for changes in food choice in adolescents.
The aim of the present study was to evaluate the effect of sleep improvement from pre-treatment to post-treatment on desire for and intake of weight gain promoting foods in the morning in a sample of adolescents. Moreover, the results provide some support for our hypothesis that sleep improvement from pre-treatment to post-treatment would be associated with decreased desire for and intake of weight gain promoting foods. Consistently, we found a medium to large effect (all η2>.10) such that, regardless of Time, adolescents in the Bedtime Improvement Group desired less high GI and sweet food and ate less salty food than those in the No Bedtime Improvement group. In other words, adolescents who adopted an earlier bedtime at the end of the treatment were more likely to desire and eat less unhealthy food. While differences in salty food eaten was observed between Bedtime Improvement Groups, no pre-treatment differences was observed in desire for high GI or sweet food. Recall that adolescents were not randomized to Bedtime Improvement Groups, rather they were categorized into the Bedtime Improvement and No Bedtime Improvement Groups based on bedtime difference between pre- and post-treatment. Importantly, a robust effect was observed (all η2>.106; d=.75) such that bedtime improvement was also associated with increased intake of low GI and dairy food at post-treatment compared to pre-treatment; meanwhile no bedtime improvement was associated with no significant change in intake of low GI food. These findings are consistent with research by Baron and colleagues (24) who reported that earlier bedtime was associated with intake of more health promoting and less weight gain promoting foods. While the underlying biological mechanisms of this shift in bedtime remains unknown, one possibility is a functional change within the brain. Specifically, the sleep-dependent restoration of activity in higher-order cortical regions, helping to better regulate sub-cortical limbic responsivity, which has previously been shown to promote the selection of weight gain promoting foods in adults (14).
Interestingly, while we found that a shift towards an earlier bedtime was associated with healthier food choices, associations between food choices and our other sleep measures (i.e. self-reported sleepiness; self-reported TST) were not statistically significant. Taken together, these findings raise the possibility that bedtime is a potential target for interventions to improve food intake in adolescents. This is consistent with literature, which identified bedtime as an important risk factor for weight gain, regardless of TST (5). Moreover, appetite regulating hormone concentrations such as leptin and ghrelin follow a normal diurnal variation strongly linked to circadian patterns (31, 32). Taken together, the literature and the present findings point to the potential importance of circadian rhythm/timing when studying the link between food choice and sleep.
There were several unexpected but noteworthy findings. Regardless of Sleepiness Improvement Group, adolescents desired more starch and consumed more total calories; regardless of Bedtime, TST or Sleepiness Improvement Group, adolescents ate more high GI and sweet calories at post-treatment compared to pre-treatment. Taken together, these findings suggest that following either a sleep focused or health-focused psychoeducation intervention, adolescents were eating more breakfast overall. On the one hand, eating regular breakfast is considered protective against weight gain (33, 34) so this finding is positive. On the other hand, we cannot rule out the possibility that there was a practice effect (i.e. the adolescents knew to expect a selection of breakfast items and therefore ate more during the task). However, in the present study within an experimental setting, the adolescents whose sleep improvement consisted of an earlier bedtime made healthier breakfast choices, a behavior associated with healthier weight and decreased obesity risk (11, 12).
The results of the present study should be interpreted within the confines of several limitations. First, objective measures for the assessment of sleep and circadian rhythms (35), (2) were not used. However, the daily sleep diary is a widely used and well-validated subjective measure of sleep (21, 22). Another limitation is the relatively small sample and the fact that not all participants completed treatment. Finally, multiple comparisons were used. According to Nakagawa and Cuthill (36), corrections for multiple comparisons such as the use of Bonferroni procedures further reduce power, increasing a Type II error to unacceptable levels, and they may also contribute to publication bias, hindering the advance of the field. Therefore we opted to include effect sizes and confidence intervals of effect sizes as suggested by Nakagawa and Cuthill (36), rather than correct for multiple comparisons. Indeed, effect sizes for significant findings observed throughout the present study are considered medium to large, and are considered to be significant (CI’s do not include zero). Moreover, it should be noted that this sample contained a majority healthy weight participants and findings may be different if examining participants with obesity.
In summary, earlier bedtime from pre-treatment to post-treatment was associated with increased intake of healthier foods (low GI foods, fruit and dairy) as compared to adolescents whose bedtimes did not improve from pre-treatment to post-treatment. These healthier eating choices, if maintained over time, could be protective against the development of obesity. Clinically, these findings suggest that bedtime improvement can improve breakfast choices, an important meal for obesity prevention (37) during adolescence, which is a central developmental period in terms of obesity prevention (5). Moreover, the current study demonstrates that both sleep and food choices are modifiable in a high-risk sample of adolescents. Given the high rate of sleep problems and obesity in this age group and potential impact of healthy sleep and diet on overall health and development these findings are particularly promising.
Implications & Contribution.
The unique contribution of the present study is to investigate the relationship between sleep and breakfast choices in adolescents, and sleep improvement as a potential mechanism for changes in food choice. The data suggest that earlier bedtimes are associated with an increased intake of healthier foods for breakfast.
Acknowledgments
A special thank you to Kerrie Hein and the amazing research assistants and research participants without whom this project would not have been possible. This project was supported by a National Science Foundation Graduate Research Fellowship DGE 1106400 awarded to LDA and grant 1R01HD071065-01A1 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development awarded to AGH.
Footnotes
Mauchly’s Test of Sphericity indicated that the assumption of sphericity had not been violated (all p’s >.05).
Follow up analyses were conducted to assess pre-treatment differences between groups. Independent samples t-tests were conducted between the Bedtime Improvement Group and the No Bedtime Improvement Group at pre-treatment in the food desire and food intake outcomes. Results indicate that at pre-treatment participants in the Bedtime Improvement Group ate less salty food than those in the No Bedtime Improvement Group t(40)=−2.10, p=.05. No other group differences were observed (all p’s >.05)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Asarnow LD, McGlinchey E, Harvey AG. The Effects of Bedtime and Sleep Duration on Academic and Emotional Outcomes in a Nationally Representative Sample of Adolescents. Journal of Adolescent Health. 2013 doi: 10.1016/j.jadohealth.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R. An approach to studying circadian rhythms of adolescent humans. Journal of Biological Rhythms. 1997;12(3):278–89. doi: 10.1177/074873049701200309. [DOI] [PubMed] [Google Scholar]
- 3.Carskadon M, Mindell J, Drake C, editors. Journal of sleep research. BLACKWELL PUBLISHING; 9600 GARSINGTON RD, OXFORD OX4 2DQ, OXON, ENGLAND: 2006. Contemporary sleep patterns of adolescents in the USA: results of the 2006 National Sleep Foundation Sleep in America Poll. [Google Scholar]
- 4.Goldstein D, Hahn CS, Hasher L, Wiprzycka UJ, Zelazo PD. Time of day, intellectual performance, and behavioral problems in Morning versus Evening type adolescents: Is there a synchrony effect? Personality and Individual Differences. 2007;42(3):431–40. doi: 10.1016/j.paid.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asarnow LD, McGlinchey E, Harvey AG. Evidence for a Possible Link between Bedtime and Change in Body Mass Index. Sleep. 2015;38(10):1523–7. doi: 10.5665/sleep.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olds TS, Maher CA, Matricciani L. Sleep duration or bedtime? Exploring the relationship between sleep habits and weight status and activity patterns. Sleep. 2011;34(10):1299. doi: 10.5665/SLEEP.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. International journal of pediatric obesity: IJPO: an official journal of the International Association for the Study of Obesity. 2006;1(1):11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- 8.Mikkilä V, Räsänen L, Raitakari OT, Pietinen P, Viikari J. Consistent dietary patterns identified from childhood to adulthood: The Cardiovascular Risk in Young Finns Study. British Journal of Nutrition. 2005;93(06):923–31. doi: 10.1079/bjn20051418. [DOI] [PubMed] [Google Scholar]
- 9.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA: the journal of the American Medical Association. 2002;287(18):2414–23. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- 10.Pi-Sunyer FX. Glycemic index and disease. The American journal of clinical nutrition. 2002;76(1):290S–8S. doi: 10.1093/ajcn/76.1.264S. [DOI] [PubMed] [Google Scholar]
- 11.Brand-Miller JC, Holt SH, Pawlak DB, McMillan J. Glycemic index and obesity. The American journal of clinical nutrition. 2002;76(1):281S–5S. doi: 10.1093/ajcn/76/1.281S. [DOI] [PubMed] [Google Scholar]
- 12.Ludwig DS, Majzoub JA, Al-Zahrani A, Dallal GE, Blanco I, Roberts SB. High Glycemic Index Foods, Overeating, and Obesity. Pediatrics. 1999;103(3):e26-e. doi: 10.1542/peds.103.3.e26. [DOI] [PubMed] [Google Scholar]
- 13.Herrmann TS, Bean ML, Black TM, Wang P, Coleman RA. High glycemic index carbohydrate diet alters the diurnal rhythm of leptin but not insulin concentrations. Experimental Biology and Medicine. 2001;226(11):1037–44. doi: 10.1177/153537020122601111. [DOI] [PubMed] [Google Scholar]
- 14.Greer SM, Goldstein AN, Walker MP. The impact of sleep deprivation on food desire in the human brain. Nature Communications. 2013:4. doi: 10.1038/ncomms3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giannotti FCF, Sebastiani T, Ottaviano S. Circadian preference, sleep and daytime behaviour in adolescence. Journal of sleep research. 2002;11:191–9. doi: 10.1046/j.1365-2869.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- 16.Beebe DW, Simon S, Summer S, Hemmer S, Strotman D, Dolan LM. Dietary intake following experimentally restricted sleep in adolescents. Sleep. 2013;36(6):827–34. doi: 10.5665/sleep.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hensrud DD. Diet and obesity. Current Opinion in Gastroenterology. 2004;20(2):119–24. doi: 10.1097/00001574-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Thompson WG, Cook DA, Clark MM, Bardia A, Levine JA. Treatment of obesity. Mayo Clin Proc. 2007;82(1):93–101. doi: 10.4065/82.1.93. quiz -2. [DOI] [PubMed] [Google Scholar]
- 19.Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA: the journal of the American Medical Association. 2014;311(1):74–86. doi: 10.1001/jama.2013.281361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. SLEEP-NEW YORK- 1993;16:258. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- 21.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29(9):1155–73. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 22.Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287–302. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monk TH, Reynolds CF, Kupfer DJ, Buysse DJ, Coble PA, Hayes AJ, et al. The Pittsburgh Sleep Diary. Journal of sleep research. 1994;3(2):111–20. [PubMed] [Google Scholar]
- 24.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity. 2011;19(7):1374–81. doi: 10.1038/oby.2011.100. [DOI] [PubMed] [Google Scholar]
- 25.Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. The American journal of clinical nutrition. 2010;91(6):1550–9. doi: 10.3945/ajcn.2009.28523. [DOI] [PubMed] [Google Scholar]
- 26.Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69(4):875–87. [PubMed] [Google Scholar]
- 27.Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care. 2008;31(12):2281–3. doi: 10.2337/dc08-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Habhab S, Sheldon JP, Loeb RC. The relationship between stress, dietary restraint, and food preferences in women. Appetite. 2009;52(2):437–44. doi: 10.1016/j.appet.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Zellner DA, Loaiza S, Gonzalez Z, Pita J, Morales J, Pecora D, et al. Food selection changes under stress. Physiology & behavior. 2006;87(4):789–93. doi: 10.1016/j.physbeh.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Harvey AG. A transdiagnostic intervention for youth sleep and circadian problems. Cognitive and Behavioral Practice. 2015 doi: 10.1016/j.cbpra.2022.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bodosi B, Gardi J, Hajdu I, Szentirmai E, Obal F, Jr, Krueger JM. Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. American journal of physiology Regulatory, integrative and comparative physiology. 2004;287(5):R1071–9. doi: 10.1152/ajpregu.00294.2004. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez J, Oliver P, Pico C, Palou A. Diurnal rhythms of leptin and ghrelin in the systemic circulation and in the gastric mucosa are related to food intake in rats. Pflugers Archiv: European journal of physiology. 2004;448(5):500–6. doi: 10.1007/s00424-004-1283-4. [DOI] [PubMed] [Google Scholar]
- 33.Cho S, Dietrich M, Brown CJ, Clark CA, Block G. The effect of breakfast type on total daily energy intake and body mass index: results from the Third National Health and Nutrition Examination Survey (NHANES III) J Am Coll Nutr. 2003;22(4):296–302. doi: 10.1080/07315724.2003.10719307. [DOI] [PubMed] [Google Scholar]
- 34.Timlin MT, Pereira MA, Story M, Neumark-Sztainer D. Breakfast eating and weight change in a 5-year prospective analysis of adolescents: Project EAT (Eating Among Teens) Pediatrics. 2008;121(3):e638–45. doi: 10.1542/peds.2007-1035. [DOI] [PubMed] [Google Scholar]
- 35.Sadeh A, Alster J, Urbach D, Lavie P. Actigraphically based automatic bedtime sleep-wake scoring: validity and clinical applications. Journal of Ambulatory Monitoring. 1989;2(3):209–16. [Google Scholar]
- 36.Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biological Reviews. 2007;82(4):591–605. doi: 10.1111/j.1469-185X.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- 37.Buyken AE, Trauner K, Gunther AL, Kroke A, Remer T. Breakfast glycemic index affects subsequent daily energy intake in free-living healthy children. The American journal of clinical nutrition. 2007;86(4):980–7. doi: 10.1093/ajcn/86.4.980. [DOI] [PubMed] [Google Scholar]