Introduction
Key Teaching Points.
-
•
For patients continuing to experience palpitations, near syncope, and syncope after pacemaker or implantable cardioverter-defibrillator (ICD) implant, it is useful to correlate symptoms with arrhythmias.
-
•
External event monitoring can be a helpful adjunct in this evaluation but is inconvenient and not tolerated well by patients.
-
•
A valuable option in patients who already have a specific Medtronic pacemaker or ICD is the use of a Medtronic Patient activator in patients to mark symptomatic events for direct, convenient correlation of symptoms and rhythms.
Some patients continue to experience palpitations, near syncope, and syncope after pacemaker or implantable cardioverter-defibrillator (ICD) implantation. In these patients it is very useful to correlate symptoms with arrhythmias and to confirm that their implanted device is working appropriately and is programmed optimally. External event monitoring can be a helpful adjunct in this evaluation but is inconvenient and not tolerated well by many patients. A valuable alternative for patients who already have a specific Medtronic pacemaker or ICD may be the use of a Medtronic patient activator to mark symptomatic events for direct, convenient correlation of symptoms and rhythms.
Case report
A 73-year-old man presented to the pacemaker clinic for evaluation of palpitations. His history was significant for rheumatic mitral regurgitation and atrial fibrillation, for which he underwent bioprosthetic mitral valve replacement, left atrial maze procedure, and left atrial appendage ligation 3 years prior. He had recurrence of atrial fibrillation and atrial flutter postoperatively and ultimately underwent cryoballoon ablation for atrial fibrillation approximately 2 years prior.
He previously had a Medtronic Reveal LINQ implantable loop recorder (ILR) placed about 1 year prior for evaluation of recurrent syncopal episodes. ILR monitoring revealed several episodes of bradycardia, which were symptomatic. Additionally he was found to have chronotropic incompetence and therefore underwent implantation of a Medtronic Advisa MRI compatible dual-chamber pacemaker for irreversible symptomatic bradycardia 2 months prior to current presentation. For no particular reason, the ILR was not explanted at the time of pacemaker implantation.
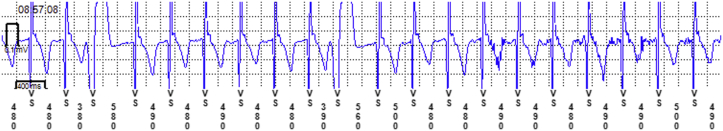
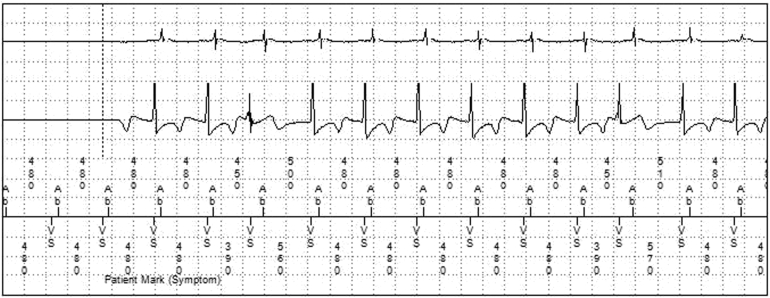
The patient experienced palpitations along with lightheadedness about 2 weeks prior to his visit with the pacemaker clinic. He used his only symptom marker (Model 96000 Patient Assistant) to activate his ILR during the episodes of palpitations. ILR review revealed the data shown in Figure 1. Interrogation of his Medtronic Advisa pacemaker at the time of his office visit showed a symptom-marked event and electrogram (EGM) at the time of the episodes, shown in Figure 2.
Figure 1.
Implantable loop recorder data at the time of a “symptom-marked event.”
Figure 2.
Device electrogram from the ADVISA pacemaker at the time of the same “symptom marked event” using the Patient Assistant Model PA96000.
Review of the symptom-marked EGMs from the pacemaker showed a short R-P interval tachycardia that correlated with the patient’s palpitations. A premature ventricular contraction produces an increase in the next Vs-Vs interval, which is reflected in a subsequent increase in Ab-Ab interval, making atrial tachycardia less likely. The V-A interval of 60 msec reduces the likelihood of atrioventricular reentrant tachycardia. Atrioventricular nodal reentrant tachycardia is the most likely diagnosis. In contrast, although the ILR recordings revealed a tachycardia, they were unable to point to a specific etiology owing to the lack of discernible P waves. However, the pacemaker EGMs recorded at the same time clearly showed a short R-P tachycardia as the culprit arrhythmia, resulting in treatment with beta-blockers and resolution of the patient’s symptoms.
Discussion
The ILR has become the investigative tool of choice in recurrent unexplained syncope following negative initial examination. Several studies have established the utility of ILR in the diagnosis of syncope.1, 2, 3, 4 In 1 study the etiology of syncope as diagnosed with the use of ILR was arrhythmic in 22% of patients. Bradycardia was the most commonly detected arrhythmia (17% vs 6% tachycardia), usually leading to pacemaker implantation.3
The Medtronic Reveal Linq stores the recorded bipolar signal as a compressed signal. A compressed signal maximizes memory capability with only marginal loss of quality. The patient is instructed on the use of the symptom marker/activator at the time of implant. Once an episode is recorded (ie, a presyncopal or syncopal event occurs), the memory is “frozen” by the patient or a relative using a nonmagnetic hand-held activator (eg, Medtronic Patient Assistant Model PA96000). The episode is then uploaded for interrogation to a pacemaker programmer. Although heart rate is usually easily ascertained, P waves can often be challenging to discern or interpret. More recent versions of the ILR have programmable automatic detection of tachycardia-bradycardia arrhythmias and pauses and allows for comprehensive remote monitoring without an office visit. The Medtronic CareLink Home Monitor allows patients to transmit data from their Medtronic Reveal ILRs over a standard phone line for review by their physicians. The St Jude Medical Confirm ILR also has transtelephonic monitoring capability, enabling transmission of timely and accurate data.
Symptom markers used for activating ILRs are not known to communicate with pacemakers. That the Medtronic Advisa pacemaker can be “activated” by the Medtronic symptom marker is an incidental discovery that has not been reported previously. After discussion with the engineering team at Medtronic, we learned that certain Medtronic pacemakers and defibrillators are capable of being “activated” by the Medtronic symptom marker that is used for activating the Reveal loop recorders. The Medtronic PA96000 this patient was prescribed to mark symptoms in his implanted LINQ uses one of Medtronic’s proprietary telemetry protocols to communicate with the LINQ. Other Medtronic devices also use this proprietary telemetry protocol, and will have the capability to mark symptoms (for example: EnRhythm/EnTrust, Concerto/Virtuoso, Advisa, Secura/Consulta, Protecta, Viva/Evera/Brava/Amplia/Compai/Claria). All of these devices will respond to the PA96000 activator and mark symptomatic episodes in device memory.
Because certain models of Medtronic pacemakers are capable of “activation” by the symptom marker, patients with these pacemakers and with palpitations or other symptoms worrisome for an arrhythmia can potentially use a Medtronic ILR symptom marker to prompt the pacemaker to store an EGM of the symptom-marked event. The patient activator needs to be within 5 cm of the device to be detected. It could potentially continue to communicate at further ranges, but the 5 cm distance is a design requirement. Depending on a variety of other variables (eg, patient body habitus, implant position, competing electrical noise), the maximum distance for successful communication could vary.
The implication of being able to add an event marker to the Medtronic pacemaker/ICD marker channel using a Medtronic loop recorder patient activator is more convenient symptom-rhythm correlation for patients. Patients often continue to have symptoms after receiving a pacemaker or ICD, and it is helpful to get direct correlation (as opposed to approximate manual diary entries) between their symptoms and any arrhythmias. Because the programming and action of the pacemaker/ICD are not affected in any way, no adverse effect is expected with this novel use.
References
- 1.Krahn A.D., Klein G.J., Yee R., Takle-Newhouse T., Norris C. Use of an extended monitoring strategy in patients with problematic syncope. Reveal Investigators. Circulation. 1999;99:406–410. doi: 10.1161/01.cir.99.3.406. [DOI] [PubMed] [Google Scholar]
- 2.Krahn A.D., Klein G.J., Yee R., Skanes A.C. Randomized assessment of syncope trial: conventional diagnostic testing versus a prolonged monitoring strategy. Circulation. 2001;104:46–51. doi: 10.1161/01.cir.104.1.46. [DOI] [PubMed] [Google Scholar]
- 3.Krahn A.D., Klein G.J., Fitzpatrick A., Seidl K., Zaidi A., Skanes A., Yee R. Predicting the outcome of patients with unexplained syncope undergoing prolonged monitoring. Pacing Clin Electrophysiol. 2002;25:37–41. doi: 10.1046/j.1460-9592.2002.00037.x. [DOI] [PubMed] [Google Scholar]
- 4.Lombardi F., Calosso E., Mascioli G., Marangoni E., Donato A., Rossi S., Pala M., Foti F., Lunati M. Utility of implantable loop recorder (Reveal Plus) in the diagnosis of unexplained syncope. Europace. 2005;7:19–24. doi: 10.1016/j.eupc.2004.09.003. [DOI] [PubMed] [Google Scholar]