Abstract
To date, there has been no convincing evidence for an association between Chlamydia pneumoniae or Helicobacter pylori and ectasia. In this case-control study, we have investigated the association of H. pylori and C. pneumoniae seropositivity with ectasia, severe coronary atherosclerosis, and normal vessels, which were so classified by coronary angiography. We have also evaluated the influence of these infections on inflammatory markers such as high-sensitive C-reactive protein (hsCRP) and interleukin 6 (IL-6).
Of the 796 patients undergoing coronary angiography for suspected ischemic heart disease, 244 patients were recruited. Of these, 91 had normal vessels, 88 had 3 or more obstructed vessels, and 65 had ectatic vessels without atherosclerosis. Eighty-seven atherosclerotic patients (98.9%) were positive for C. pneumoniae IgG, as were 64 ectatic patients (98.5%) and 76 controls (83.5%) (P < 0.001). Forty-two atherosclerotic patients (47.7%) were positive for C. pneumoniae IgM, as were 43 ectatic patients (66.2%) and 43 controls (47.3%) (P = 0.036). Seventy-two atherosclerotic patients (81.8%) were positive for H. pylori IgA, as were 26 ectatic patients (40.0%) and 44 controls (48.4%) (P < 0.001). High-sensitive CRP levels were significantly higher in ectatic patients (5.639 mg/L) than in controls (4.390 mg/L) (P = 0.032), and IL-6 levels were significantly higher in atherosclerotic patients (33.92 U/L) than in controls (14.01 U/L) (P < 0.001). Interleukin-6 levels were higher in H. pylori seropositive patients, and hsCRP levels were higher in C. pneumoniae seropositive patients, when compared with seronegatives.
We suggest that, as in atherosclerosis, C. pneumoniae infection is related to ectasia, with raised CRP levels.
Key words: Atherosclerosis; bacterial infections/complications; biological markers; C-reactive protein; chlamydia infections/complications; Chlamydia pneumoniae; Cardiovascular diseases/etiology; dilatation, pathologic/etiology; helicobacter infections/complications; Helicobacter pylori; inflammation; interleukin-6
The relationship between atherosclerosis and microorganisms has been a subject of research interest during the last 2 decades. However, the relationship between microorganisms and ectasia, and inflammatory markers in ectasia, has not been studied.
Among the suspected microorganisms, Chlamydia pneumoniae is the bacterium whose relationship with atherosclerosis has been suggested by epidemiologic and laboratory studies.1,2 Helicobacter pylori, which has proven associations with gastric ulcer, adenocarcinoma, and gastric mucosa-associated lymphoid tissue (MALT), has also been associated with atherosclerosis in a substantial number of studies, although other studies show an absence of this association.3 Gastroduodenal H. pylori infection, diagnosed by the identification of H. pylori stool antigen, has also been found to be related to atherosclerosis.4
Interleukin-6 (IL-6) is a multifunctional cytokine with both endocrine and paracrine effects, which mediates several functions in the host's defense and promotes atherogenesis, dyslipidemia, hypertension, and insulin resistance by mediating the effect of activated macrophages and lymphocytes.5 The role of IL-6 in cardiovascular epidemiology requires further evaluation; it has been proposed that IL-6 is a weaker risk marker for cardiovascular disease than is C-reactive protein (CRP). The pursuit of data that directly compare these 2 inflammatory markers is warranted.6 C-reactive protein is a relatively well established predictor for cardiovascular disease; however, interaction among infection, inflammatory response by host, and atherosclerosis remains unclear.
In this original study, we investigated the association of H. pylori and C. pneumoniae seropositivities with ectasia, severe coronary atherosclerosis, and the absence of either of these conditions, as revealed by coronary angiography. More specifically, we investigated whether these associations were influenced by serum levels of the inflammatory markers CRP and IL-6.
We also evaluated the relations between ectasia and age, sex, and the classical risk factors of atherosclerosis.
Patients and Methods
This study was designed as a case-control study.
Patients
The study group consisted of individuals who had been referred for coronary angiography because of chest pain or for noninvasive tests that were compatible with myocardial ischemia. The study was performed in compliance with human-studies guidelines, and informed consent was obtained from the eligible patients after the nature of the procedure was explained. Patients admitted to the Cardiology Department of the Suleyman Demirel University School of Medicine from January 2002 through February 2003 were recruited for the study. Patients were hospitalized overnight on the day of angiography and were discharged the next morning. Eligible participants had been informed about the study before angiography, and blood samples were drawn from the brachial vein during the angiography procedure. Statin and aspirin usage was stopped 7 days before the angiography procedure in all study groups. Patients who had a sustained myocardial infarction (MI) within the previous 3 months or who had significant valvular heart disease or nonatherosclerotic cardiomyopathy were excluded.
By means of diagnostic coronary angiography, we categorized our study groups as “normal” (subjects with angiographically normal coronary arteries); “severe coronary atherosclerosis” (subjects with atheromatous plaques or greater than 50% luminal stenosis in 3 or more coronary arteries); or “coronary ectasia” (subjects with segmental or diffuse luminal dilatation of one or more coronary arteries). A coronary artery segment with a diameter 1.5-fold greater than the diameter of the adjacent normal segment was defined as ectatic. If no adjacent normal segment could be identified, a coronary artery segment with a diameter 1.5-fold greater than the mean coronary diameter index of the corresponding coronary segment in the normal group was defined as ectatic, as previously described by Kruger and colleagues.7 Ectatic patients with atherosclerotic plaques were not included in this study.
Of the 796 patients undergoing coronary angiography for suspected ischemic heart diseases, 244 patients were recruited. Of these, 91 had normal vessels, 88 had 3 or more obstructed vessels, and 65 had ectatic vessels without atherosclerosis.
Determination of Risk Factors
Risk factor criteria for coronary artery disease (CAD) were derived from Ossei-Gerning and coworkers.8 Age, sex, cigarette-smoking, diabetes mellitus (DM), hypertension (HT), hypercholesterolemia (HCL), and obesity were recorded. A patient who had stopped smoking more than 20 years before and who was less than 30 years of age when he or she stopped smoking was considered not to have smoking as a risk factor. A patient was considered to have diabetes if he or she was taking insulin or oral hyperglycemic agents, or if he or she had previously received such treatment and was currently controlling the condition through dietary modification. A patient was considered to have HCL if he or she had a total serum cholesterol value higher than 240 mg/dL (6.2 mmol/L) or was receiving a cholesterol-lowering agent. A patient was considered to have HT if he or she had received that diagnosis on the basis of an arterial pressure >140/90 mmHg, or was being treated for HT with antihypertensive medications, dietary modifications, or both.
Laboratory Tests
Specimen Collection
Blood samples were drawn during angiography, and sera were stored at −80°C until analysis.
Sera Analysis
Seropositivities for all sera samples were tested for H. pylori IgG and IgA and for C. pneumoniae IgG and IgM by the ELISA method (Euroimmun GmbH; Am-Sonneberg, Germany). Levels above 20 “relative units/mL” were accepted as positive for qualitative assessment, in accordance with the manufacturer's instructions. Serum IL-6 concentrations were detected quantitatively by the ELISA method (BioSource International; Camarillo, Calif). High-sensitive C-reactive protein (hsCRP) levels (N High-sensitive CRP, Dade Behring; Schwalbach, Germany) were detected using particle-enhanced immunonephelometry and were analyzed by hsCRP calibration, which detects serum CRP levels up to 0.1 mg/L.
Statistical Analysis
Categorized data were analyzed by the χ2 test. Differences in means of continuous variables between groups were compared by means of the independent samples t-test and ANOVA if more than 2 groups were assessed. Logistic and linear regression models were used to assess the independent associations of various risk factors to CAD. All of the statistical analyses were performed using the SPSS statistics program, version 10.0 (SPSS Inc.; Chicago, Ill).
Results
C. pneumoniae IgG tests were positive in 87 of 88 atherosclerotic patients (98.9%), in 64 of 65 ectatic patients (98.5%), and in 76 of 91 control subjects (83.5%) (P < 0.001). C. pneumoniae IgM tests were positive in 42 atherosclerotic patients (47.7%), in 43 ectatic patients (66.2%), and in 43 control subjects (47.3%) (P = 0.036). H. pylori IgA tests were positive in 72 atherosclerotic patients (81.8%), in 26 ectatic patients (40.0%), and in 44 controls (48.4%) (P < 0.001). High-sensitive CRP levels were significantly higher in ectatic patients (5.639 mg/L) than in control subjects (4.390 mg/L) (P = 0.032, independent samples t-test). Interleukin-6 levels were significantly higher in atherosclerotic patients (33.92 U/L) than in control subjects (14.01 U/L) (P = 0.001) (independent samples t-test) (Table I).
TABLE I. Established Risk Factors and Laboratory Findings in Patient and Control Groups
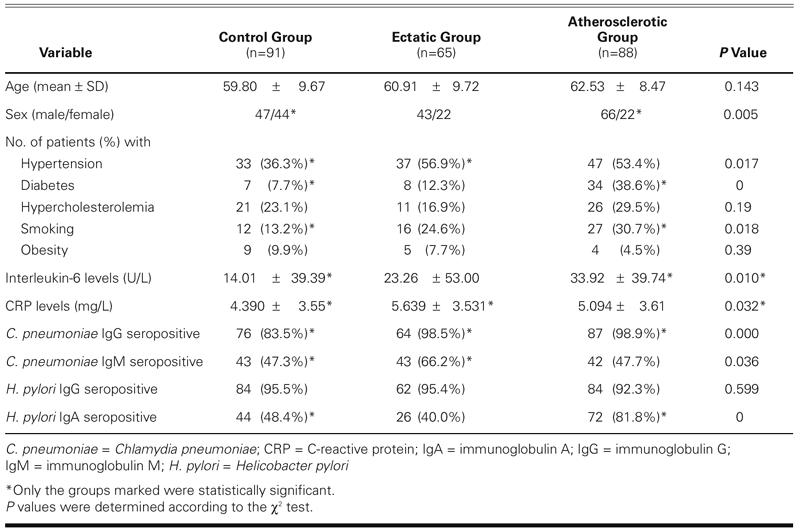
Comparisons of patient and control groups with established risk factors are summarized in Table I. Among the established risk factors, only hypertension (P = 0.011, χ2 = 6.542) was significantly higher in ectatic patients than in control subjects.
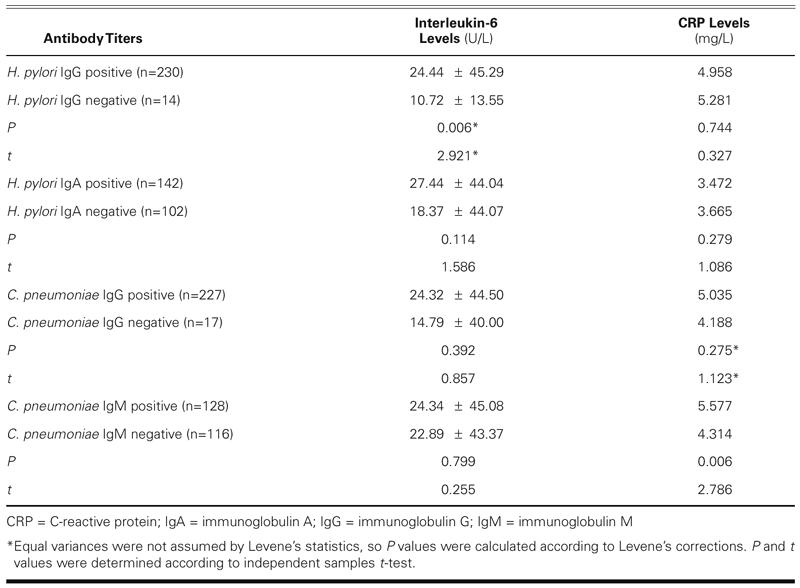
When comparisons were made between seropositive and seronegative cases with inflammatory markers (Table II), CRP levels were significantly higher in C. pneumoniae IgM-positive cases (5.577 mg/L) than in negatives (4.31 mg/L) (P = 0.006), and IL-6 levels were significantly higher in H. pylori IgG-positive cases than in negatives (P = 0.006).
TABLE II. Titers of Inflammatory Markers in Patients with Respect to Infection with Helicobacter pylori and Chlamydia pneumoniae
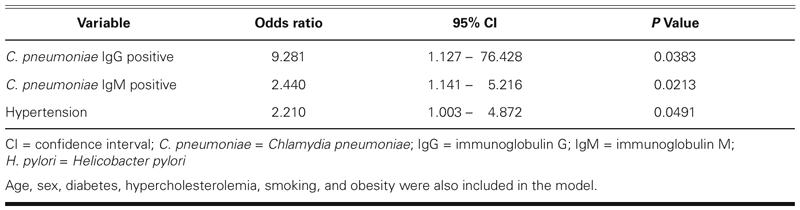
In odds ratio risk analysis, there was a significant relationship between ectasia and both C. pneumoniae IgG and IgM seropositivity (OR = 12.632, 95% CI = 1.624–98.256, χ2, P = 0.002; OR: 2.182, 95% CI = 1.13–4.214, χ2, P = 0.023, respectively). C. pneumoniae infection remained significantly associated with ectasia after adjusting for age, sex, hypertension, diabetes mellitus, and hypercholesterolemia in a logistic regression model8; however, there was a slight change in the odds ratio (Table III).
TABLE III. Risk Factors That Were Significant in a Logistic Regression Model with Ectasia as the Dependent Variable
Discussion
Atherosclerosis and Infection
Immunoglobulin A is the most important immunoglobulin for mucosal immunity. It is synthesized from gastric mucosa for initial protection of the host. In this study, H. pylori IgA seropositivity was significantly higher in severely atherosclerotic patients than in control subjects. H. pylori IgG seropositive results in our study were of no scientific value; because seroprevalence was higher than 95% in control subjects, it was impossible to confer any significance upon seroprevalence in atherosclerotic or ectatic patients. Some case control series or cross-sectional studies have shown a positive association between H. pylori seropositivity and CAD or cerebrovascular diseases8–12 or acute myocardial infarction,13 which tends to support our results. The detection of H. pylori DNA in atherosclerotic plaques14,15 further strengthens the possibility of a cause-and-effect relationship. However, there are also prospective studies that have failed to show any significant association.16–19
In this study, C. pneumoniae IgG-positive cases were significantly more frequent among severely atherosclerotic patients and ectatic patients than among the control subjects. In 1988, Saikku and associates20 provided the 1st serologic evidence of an association between C. pneumoniae and coronary heart disease. Patients with recent MI had significantly elevated IgG and IgA antibody titers against C. pneumoniae, when compared with controls. In 1996, Wimmer's group21 demonstrated that elevated serological markers of C. pneumoniae were also associated with cerebrovascular disease. Similar seroepidemiologic findings have now been confirmed by about 38 studies of varying designs (retrospective, cross-sectional, case-control, or prospective) worldwide.22 There are also some prospective studies that showed no correlation between C. pneumoniae IgG-seropositivity and future myocardial infarction or CRP.23,24 In conclusion, despite studies that have shown no correlation, our results are supported by large case-control studies that have found a relationship between C. pneumoniae infection and atherosclerosis.
Bacterial Infection and Inflammatory Markers
We determined seroprevalences of C. pneumoniae and H. pylori and assessed their relationships to CRP and IL-6, which have been proved to increase in the presence of atherosclerosis.25
In this study, hsCRP levels were significantly high-er in ectatic patients than in controls. In addition, hsCRP levels were higher in C. pneumoniae IgM-positive cases than in negatives. In a meta-analysis summarizing the results of 11 prospective studies with a total of 1,953 cases, patients in the top third of baseline measurements of CRP, when compared with patients in the bottom third, had an odds ratio for CAD of 2.0 (95% CI, 1.6–2.5), after adjustment of various confounding factors.26 Abdelmouttaleb and coworkers27 compared serum CRP levels in CAD patients (n = 142) and healthy controls (n = 37) who had been assessed by coronary angiography and found CRP levels to be higher in the CAD group. In 233 patients, Zhu and colleagues28 determined the serum CRP levels and the prevalence of serum IgG antibodies to 5 pathogens (C. pneumoniae, cytomegalovirus, hepatitis A virus, and herpes simplex virus types 1 and 2) and found an association between the total pathogen burden and CRP levels and CAD risk. C-reactive protein concentrations that are above the individuals' baselines but still within normal reference intervals have been observed in association with increased age, obesity, and smoking, and in individuals with chronic infections such as C. pneumoniae and H. pylori,29 which supports our results.
In this study, IL-6 was significantly higher in patients with C. pneumoniae infections and in those with atherosclerosis. Interleukin-6 is a central stimulus for acute phase response and is the primary determinant of the hepatic production of CRP.30 Elevated levels of circulating IL-6 have been found in CAD31 and MI5 patients, which supports our results. Acellular components of C. pneumoniae have been identified, which directly stimulate IL-6 production in human blood mononuclear cells in vitro.32 Because in vitro chlamydial infection also elicits IL-6 production from human smooth muscle cells,33 it is likely that chronic clinical chlamydial infection elicits such production. Anderson's group34 studied the effects of 3 months of azithromycin treatment or placebo in 302 patients with CAD who had been seropositive to C. pneumoniae for 3 to 6 months. Azithromycin reduced a global rank sum score of 4 inflammatory markers (CRP, IL-1, IL-6, and tumor necrosis factor-α), but C. pneumoniae IgG and IgA antibody titers were unchanged. In the study of MacIntyre and associates,35 C. pneumoniae led to endothelial damage of the human brain microvasculature, thereby increasing the expression of the zonula adherens proteins and decreasing the expression of the tight junctional protein occludin. Our study supports the concept that C. pneumoniae destroys the endothelium, laying the groundwork for atherosclerosis.
In our study, C. pneumoniae infection, not atherosclerosis, caused the major increase in hsCRP; we could not detect a statistically significant increase in hsCRP in atherosclerotic patients when they were compared with controls. In contrast, IL-6 increase was associated with atherosclerosis and H. pylori seropositivity. From these results, it is not easy to conclude that there is a causal relationship between C. pneumoniae seropositivity and inflammation in atherosclerosis or ectasia. If infection by C. pneumoniae does not initiate an inflammatory response that causes atherosclerosis or ectasia, the converse is possible: an inflammatory response to atherosclerosis might stimulate an anamnestic response that enables cross-reaction between pathogen-directed antigens and vascular “self” antigens. Infection might then modify the clinical course of disease without a direct role in causation.
Ectasia and Infection
Coronary artery ectasia (dilated coronopathy) is a relatively rare abnormality of the coronary arterial tree that is considered to be congenital (in 20%–30% of the cases) or acquired. Acquired coronary ectasias have been attributed most commonly to atherosclerosis (80%) and less commonly to inflammatory and connective tissue diseases. Pathologic studies reveal ectatic arterial enlargement, degeneration of the elastic lamina, medial atherosclerotic plaque formation or calcification, and thinning of the arterial wall.36–38 The cause of ectasia is still a subject of discussion.
In this study, C. pneumoniae IgM and IgG seropositive findings were significantly higher in ectatic patients than in control subjects. High-sensitive CRP levels were significantly higher in ectatic patients than in controls, and hsCRP levels were higher in C. pneumoniae IgM-positive patients (both findings indicate acute infection). But it is too early to conclude that there is a causal relationship between infection, inflammation, and ectasia.
C. pneumoniae IgM and IgG seropositive findings remained significant in our linear logistic regression model after adjusting for established risk factors for atherosclerosis. We conclude that C. pneumoniae infection is an independent risk factor for ectasia, which is not affected by known risk factors for atherosclerosis. To our knowledge, this is the 1st study designed to detect bacteria and inflammatory markers in ectatic vessels and to make comparisons with nonatherosclerotic controls; however, there are a few studies39,40 that concern aneurysms, in which C. pneumoniae IgA and IgG seroprevalences positively correlated with the expansion and progression of abdominal aortic aneurysms. These markers were thought to predict the need for elective surgical treatment of small abdominal aortic aneurysms.39,40 In our study, C. pneumoniae seropositivity and hsCRP levels were significantly higher in ectatic patients than in controls, which supports the hypothesis that inflammation plays a role in ectasia formation.
The relationship between ectasia and C. pneumoniae has been studied in an animal model. In the study by Tambiah and co-authors,41 periaortic application of live or formalin-inactivated C. pneumoniae to the abdominal aortas of New Zealand white rabbits was performed at laparotomy. Macrophage counting and aortic diameter measurements were performed 3 weeks after laparotomy, and C. pneumoniae antigens appeared to stimulate aortic dilatation—probably by specific activation of macrophages—which supports our study results.41 To our knowledge, there is no study on the relationship between ectasia and H. pylori.
We found ectasia to carry a risk of future atherosclerosis. In this study, among the established risk factors, only the frequency of hypertension was significantly higher in ectatic patients in comparison with nonatherosclerotic controls. This finding was in agreement with recent studies showing that the frequency of hypertension, an abnormal electrocardiogram, and a history of myocardial infarction were greater in patients with ectasia than in a control group whose members had obstructive coronary artery disease.42 However, there are conflicting results. Swaye and coworkers43 found no differences—when comparing aneurysmal and nonaneurysmal CAD patients—in hypertension, diabetes, lipid abnormalities, family history, or cigarette-smoking.
The incidence of coronary artery ectasia in patients undergoing cardiac catheterization has ranged from 0.3% to 4.9% in different series, regardless of associated coronary artery stenosis.7,42–45 The incidence of coronary ectasia in the Turkish population has been reported to be 1.4% to 5.3%.46 The overall incidence of coronary artery ectasia in the Isparta region of Turkey was reported to be around 6.7% in a retrospective study of 1,521 consecutive patients who had undergone cardiac catheterization.47 The overall incidence of coronary artery ectasia in our patient group was even higher (8.0%) than that of the Isparta series.
Conclusions
Ectatic patients had more frequent C. pneumoniae infection and higher hsCRP levels than did nonath-erosclerotic controls. Atherosclerotic patients, on the other hand, had more frequent C. pneumoniae infection, more acute H. pylori infection, and higher levels of IL-6 than did nonatherosclerotic controls. These findings suggest that, like atherosclerosis, C. pneumoniae infection is related to ectasia, with raised CRP levels. Prospective clinical studies and laboratory research for molecular identification are needed to clarify the potential role of bacterial infections, including C. pneumoniae infection, in the pathogenesis of ectasia.
Footnotes
Address for reprints: Ali K. Adiloglu, MD, Posta Kutusu 61, 32100 Isparta, Turkey. E-mail: aadiloglu@yahoo.com
This research has been made possible by a government grant from the Prime Ministry State Planning Organization, Advanced Research Projects for Universities in Turkey. Project No: 002 K 120590.
References
- 1.Fong IW. Emerging relations between infectious diseases and coronary artery disease and atherosclerosis. CMAJ 2000;163:49–56. [PMC free article] [PubMed]
- 2.de Boer OJ, van der Wal AC, Becker AE. Atherosclerosis, inflammation, and infection. J Pathol 2000;190:237–43. [DOI] [PubMed]
- 3.Pakodi F, Abdel-Salam OM, Debreceni A, Mozsik G. Helicobacter pylori. One bacterium and a broad spectrum of human disease! J Physiol Paris 2000;94:139–52. [DOI] [PubMed]
- 4.Adiloglu AK, Nazli C, Cicioglu-Aridogan B, Kinay O, Can R, Ergene O. Gastroduodenal Helicobacter pylori infection diagnosed by Helicobacter pylori stool antigen is related to atherosclerosis. Acta Cardiol 2003;58:335–9. [DOI] [PubMed]
- 5.Bennet AM, Prince JA, Fei GZ, Lyrenas L, Huang Y, Wiman B, et al. Interleukin-6 serum levels and genotypes influence the risk for myocardial infarction. Atherosclerosis 2003;171:359–67. [DOI] [PubMed]
- 6.Blake GJ, Ridker PM. Inflammatory bio-markers and cardiovascular risk prediction. J Internal Med 2002;252:283–94. [DOI] [PubMed]
- 7.Kruger D, Stierle U, Herrmann G, Simon R, Sheikzadeh A. Exercise-induced myocardial ischemia in isolated coronary artery ectasias and aneurysms (“dilated coronopathy”). J Am Coll Cardiol 1999;34:1461–70. [DOI] [PubMed]
- 8.Ossei-Gerning N, Moayyedi P, Smith S, Braunholtz D, Wilson JI, Axon AT, Grant PJ. Helicobacter pylori infection is related to atheroma in patients undergoing coronary angiography. Cardiovasc Res 1997;35:120–4. [DOI] [PubMed]
- 9.de Luis DA, Lahera M, Canton R, Boixeda D, San Roman AL, Aller R, de la Calle H. Association of Helicobacter pylori infection with cardiovascular and cerebrovascular disease in diabetic patients. Diabetes Care 1998;21:1129–32. [DOI] [PubMed]
- 10.Markus HS, Mendall MA. Helicobacter pylori infection: a risk factor for ischaemic cerebrovascular disease and carotid atheroma. J Neurol Neurosurg Psychiatry 1998;64:104–7. [DOI] [PMC free article] [PubMed]
- 11.Smith D, Gupta S, Kaski JC. Chronic infections and coronary heart disease. Int J Clin Pract 1999;53:460–6. [PubMed]
- 12.Pasceri V, Cammarota G, Patti G, Cuoco L, Gasbarrini A, Grillo RL, et al. Association of virulent Helicobacter pylori strains with ischemic heart disease. Circulation 1998;97:1675–9. [DOI] [PubMed]
- 13.Pellicano R, Mazzarello MG, Morelloni S, Allegri M, Arena V, Ferrari M, et al. Acute myocardial infarction and Helicobacter pylori seropositivity. Int J Clin Lab Res 1999;29:141–4. [DOI] [PubMed]
- 14.Farsak B, Yildirir A, Akyon Y, Pinar A, Oc M, Boke E, et al. Detection of Chlamydia pneumonia and Helicobacter pylori DNA in human atherosclerotic plaques by PCR. J Clin Microbiol 2000;38:4408–11. [DOI] [PMC free article] [PubMed]
- 15.Ameriso SF, Fridman EA, Leiguarda RC, Sevlever GE. Detection of Helicobacter pylori in human carotid atherosclerotic plaques. Stroke 2001;32:385–91. [DOI] [PubMed]
- 16.Pilotto A, Rumor F, Franceschi M, Leandro G, Novello R, Soffiati G, et al. Lack of association between Helicobacter pylori infection and extracardiac atherosclerosis in dyspeptic elderly subjects. Age Ageing 1999;28:367–71. [DOI] [PubMed]
- 17.McDonagh TA, Woodward M, Morrison CE, McMurray JJ, Tunstall-Pedoe H, Lowe GD, et al. Helicobacter pylori infection and coronary heart disease in the North Glasgow MONICA population. Eur Heart J 1997;18:1257–60. [DOI] [PubMed]
- 18.Folsom AR, Nieto FJ, Sorlie P, Chambless LE, Graham DY. Helicobacter pylori seropositivity and coronary heart disease incidence. Atherosclerosis Risk In Communities (ARIC) Study Investigators. Circulation 1998;98:845–50. [DOI] [PubMed]
- 19.Ossewaarde JM, Feskens EJ, De Vries A, Vallinga CE, Kromhout D. Chlamydia pneumoniae is a risk factor for coronary heart disease in symptom-free elderly men, but Helicobacter pylori and cytomegalovirus are not. Epidemiol Infect 1998;120:93–9. [DOI] [PMC free article] [PubMed]
- 20.Saikku P, Leinonen M, Mattila K, Ekman MR, Nieminen MS, Makela PH, et al. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet 1988; 2:983–6. [DOI] [PubMed]
- 21.Wimmer ML, Sandmann-Strupp R, Saikku P, Haberl RL. Association of chlamydial infection with cerebrovascular disease. Stroke 1996;27:2207–10. [DOI] [PubMed]
- 22.Grayston JT. Background and current knowledge of Chlamydia pneumoniae and atherosclerosis. J Infect Dis 2000; 181 Suppl 3:S402–10. [DOI] [PubMed]
- 23.Ridker PM, Kundsin RB, Stampfer MJ, Poulin S, Hennekens CH. Prospective study of Chlamydia pneumoniae IgG seropositivity and risks of future myocardial infarction. Circulation 1999;99:1161–4. [DOI] [PubMed]
- 24.Wald NJ, Law MR, Morris JK, Zhou X, Wong Y, Ward ME. Chlamydia pneumoniae infection and mortality from ischaemic heart disease: large prospective study. BMJ 2000; 321:204–7. [DOI] [PMC free article] [PubMed]
- 25.Erren M, Reinecke H, Junker R, Fobker M, Schulte H, Schurek JO, et al. Systemic inflammatory parameters in patients with atherosclerosis of the coronary and peripheral arteries. Arterioscler Thromb Vasc Biol 1999;19:2355–63. [DOI] [PubMed]
- 26.Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ 2000;321:199–204. [DOI] [PMC free article] [PubMed]
- 27.Abdelmouttaleb I, Danchin N, Ilardo C, Aimone-Gastin I, Angioi M, Lozniewski A, et al. C-Reactive protein and coronary artery disease: additional evidence of the implication of an inflammatory process in acute coronary syndromes. Am Heart J 1999;137:346–51. [DOI] [PubMed]
- 28.Zhu J, Quyyumi AA, Norman JE, Csako G, Waclawiw MA, Shearer GM, Epstein SE. Effects of total pathogen burden on coronary artery disease risk and C-reactive protein levels. Am J Cardiol 2000;85:140–6. [DOI] [PubMed]
- 29.Ledue TB, Rifai N. High sensitivity immunoassays for C-reactive protein: promises and pitfalls. Clin Chem Lab Med 2001;39:1171–6. [DOI] [PubMed]
- 30.Haddy N, Sass C, Droesch S, Zaiou M, Siest G, Ponthieux A, et al. IL-6, TNF-alpha and atherosclerosis risk indicators in a healthy family population: the STANISLAS cohort. Atherosclerosis 2003;170:277–83. [DOI] [PubMed]
- 31.Brevetti G, Piscione F, Silvestro A, Galasso G, Di Donato A, Oliva G, et al. Increased inflammatory status and higher prevalence of three-vessel coronary artery disease in patients with concomitant coronary and peripheral atherosclerosis. Thromb Haemost 2003;89:1058–63. [PubMed]
- 32.Netea MG, Selzman CH, Kullberg BJ, Galama JM, Weinberg A, Stalenhoef AF, et al. Acellular components of Chlamydia pneumoniae stimulate cytokine production in human blood mononuclear cells. Eur J Immunol 2000;30:541–9. [DOI] [PubMed]
- 33.Rodel J, Woytas M, Groh A, Schmidt KH, Hartmann M, Lehmann M, Straube E. Production of basic fibroblast growth factor and interleukin 6 by human smooth muscle cells following infection with Chlamydia pneumoniae. Infect Immun 2000;68:3635–41. [DOI] [PMC free article] [PubMed]
- 34.Anderson JL, Muhlestein JB. The ACADEMIC study in perspective (Azithromycin in coronary artery disease: elimination of myocardial infection with Chlamydia). J Infect Dis 2000;181 Suppl 3:S569–71. [DOI] [PubMed]
- 35.MacIntyre A, Hammond CJ, Little CS, Appelt DM, Balin BJ. Chlamydia pneumoniae infection alters the junctional complex proteins of human brain microvascular endothelial cells. FEMS Microbiol Lett 2002;217:167–72. [DOI] [PubMed]
- 36.Seabra-Gomes R, Somerville J, Ross DN, Emanuel R, Parker DJ, Wong M. Congenital coronary artery aneurysms. Br Heart J 1974;36:329–35. [DOI] [PMC free article] [PubMed]
- 37.Falsetti HL, Carroll RJ. Coronary artery aneurysm. A review of the literature with a report of 11 new cases. Chest 1976;69:630–6. [DOI] [PubMed]
- 38.Swanton RH, Thomas ML, Coltart DJ, Jenkins BS, Webb-Peploe MM, Williams BT. Coronary artery ectasia—a variant of occlusive coronary arteriosclerosis. Br Heart J 1978; 40:393–400. [DOI] [PMC free article] [PubMed]
- 39.Vammen S, Lindholt JS, Andersen PL, Henneberg EW, Ostergaard L. Antibodies against Chlamydia pneumoniae predict the need for elective surgical intervention on small abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2001; 22:165–8. [DOI] [PubMed]
- 40.Lindholt JS, Ashton HA, Scott RA. Indicators of infection with Chlamydia pneumoniae are associated with expansion of abdominal aortic aneurysms. J Vasc Surg 2001;34:212–5. [DOI] [PubMed]
- 41.Tambiah J, Franklin IJ, Trendell-Smith N, Peston D, Powell JT. Provocation of experimental aortic inflammation and dilatation by inflammatory mediators and Chlamydia pneumoniae. Br J Surg 2001;88:935–40. [DOI] [PubMed]
- 42.Markis JE, Joffe CD, Cohn PF, Feen DJ, Herman MV, Gorlin R. Clinical significance of coronary arterial ectasia. Am J Cardiol 1976;37:217–22. [DOI] [PubMed]
- 43.Swaye PS, Fisher LD, Litwin P, Vignola PA, Judkins MP, Kemp HG, et al. Aneurysmal coronary artery disease. Circulation 1983;67:134–8. [DOI] [PubMed]
- 44.Hartnell GG, Parnell BM, Pridie RB. Coronary artery ectasia. Its prevalence and clinical significance in 4993 patients. Br Heart J 1985;54:392–5. [DOI] [PMC free article] [PubMed]
- 45.Oliveros RA, Falsetti HL, Carroll RJ, Heinle RA, Ryan GF. Atherosclerotic coronary artery aneurysm. Report of five cases and review of literature. Arch Intern Med 1974;134:1072–6. [DOI] [PubMed]
- 46.Arslan N, Demirkan D. Coronary artery aneurysms [in Turkish]. Arch Turk Soc Cardiol 1993;21:123–6.
- 47.Altinbas A, Nazli C, Kinay O, Ergene O, Gedikli O, Ozaydin M, et al. Predictors of exercise-induced myocardial ischaemia in patients with isolated coronary artery ectasia. Int J Cardiovasc Imaging 2004;20:3–17. [DOI] [PubMed]


