Abstract
Purpose of Review
Recognition of subclinical myocardial dysfunction offers clinicians and patients an opportunity for early intervention and prevention of symptomatic cardiovascular disease. We review the data on novel biomarkers in subclinical heart disease in the general population with a focus on pathophysiology, recent observational or trial data, and potential applicability and pitfalls for clinical use.
Recent Findings
High-sensitivity cardiac troponin and natriuretic peptide assays are powerful markers of subclinical cardiac disease. Elevated levels of these biomarkers signify subclinical cardiac injury and hemodynamic stress and portend an adverse prognosis. Novel biomarkers of myocardial inflammation, fibrosis, and abnormal contraction are gaining momentum as predictors for incident heart failure, providing new insight into pathophysiologic mechanisms of cardiac disease.
Summary
There has been exciting growth in both traditional and novel biomarkers of subclinical cardiac injury in recent years. Many biomarkers have demonstrated associations with relevant cardiovascular outcomes and may enhance the diagnostic and prognostic power of more conventional biomarkers. However, their use in “prime time” to identify patients with or at risk for subclinical cardiac dysfunction in the general population remains an open question. Strategic investigation into their clinical applicability in the context of clinical trials remains an area of ongoing investigation.
Keywords: Biomarkers, Heart failure, Subclinical, Cardiac, Population, Troponin
Introduction
Subclinical cardiac dysfunction precedes the development of heart failure and other cardiovascular diseases but often goes undiagnosed due to lack of screening paradigms and available testing strategies. Patients with subclinical cardiac dysfunction benefit from early diagnosis/recognition of the pathophysiological changes that lead to clinical heart disease by providing an opportunity for timely intervention and prevention. Biomarkers that are simple to measure in a clinical setting in an at-risk patient may help clinicians identify and treat pre-clinical heart disease more effectively.
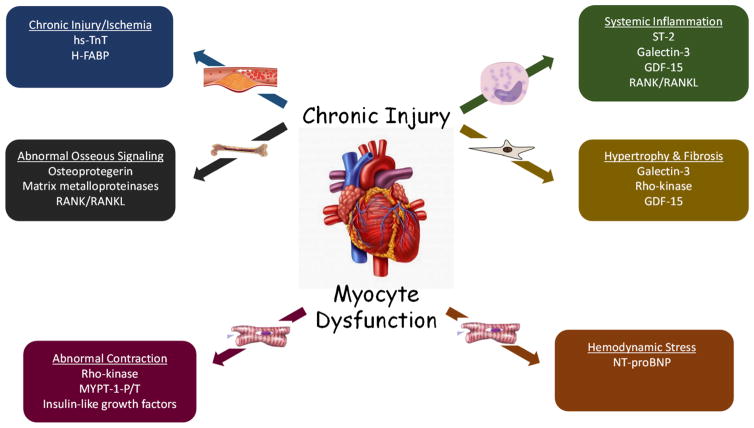
A central pathophysiological insult leading to clinical heart failure (HF) is cardiac remodeling. Remodeling is characterized as inappropriate dilation of the ventricular chambers and thinning of the myocardium as a consequence of activation of the sympathetic nervous and renin-angiotensin-aldosterone system (RAAS). Multiple other stressors likely contribute to adverse cardiac remodeling as well, including hemodynamic perturbations, myocardial injury, dysregulation of myocyte excitation and contraction, abnormal myocardial metabolism, and inflammation (Fig. 1). The study of these unique pathophysiological mechanisms has led to the identification of many novel blood-based biomarkers that can potentially be clinically applied to assist in the diagnosis, prognosis, and therapeutic applications in patients (Table 1). We will therefore discuss these novel biomarkers in greater detail, including the pathophysiology they represent, current observational or trial data focused on the general population, and potential applicability and pitfalls for clinical use.
Fig. 1.
Novel biomarkers and pathophysiological pathways of subclinical cardiac dysfunction
Table 1.
Overview of novel biomarkers of subclinical cardiac dysfunction in the general population
| Biomarker | Accepted values | Proposed mechanism | Clinical utility | At-risk populations |
|---|---|---|---|---|
| hs-cTnT | Abnormal range: 0.014 ng/mL–unknown upper limit of detection | Troponin T present in cardiac muscles bound to myofilaments is continuously released during chronic myocardial injury | Chronically elevated cardiac troponins in the general population is associated with all-cause mortality and HF Temporal increases were strongly associated with incident HF |
High-risk phenotypes, including patients with subclinical HF and left ventricular hypertrophy |
| NT-proBNP | Abnormal range: 300 pg/mL–unknown upper limit of detection | Family of proteins involved in maintenance of cardio-renal homeostasis through natriuretic, vasodilatory, and diuretic effects | Independent prognostic value for death and heart failure Predictive value for all-cause mortality was even stronger when combined with detectable troponin T |
Patients with hypertension and/or diabetes have twice odds of diastolic or systolic dysfunction on echo Chronic renal insufficiency showed higher rates of HF |
| ST-2 | Radix Biosolutions Range of detection: 0.40 to 200 μg/L Median: 1.3 μg/L Critical Diagnostics Prestige Normal range: 1.75–34.3 U/mL Median: 25.7 U/mL Values above the median approach abnormal |
Interleukin-33 binding, dummy receptor is released and sequesters cytokines to limit cytokine toxicity to myocardium | Good sensitivity and specificity (73.5 and 79.6%) for diagnosis of HF. Predicts adverse outcomes in HF including death | African Americans have twice the prevalence of elevated ST2 compared with other race/ethnic groups in the population |
| Heart type fatty acid binding protein | Range of detection: 0.5–24 ng/mL Median: 5.0 ng/mL Values above the median approach abnormal |
Myocardial cytosolic protein involved in transport of long-chain fatty acids across the cell and mitochondrial membrane is released during periods of chronic myocardial injury | Initially used as an indicator of myocardial ischemia alongside troponin I. Incremental risk shown when elevated with BNP. Raises the specificity of BNP in the diagnosis of HF | Acute coronary syndrome patients. Predicts outcomes in HF with reduced and preserved ejection fraction |
| Rho-kinase and myosin light chain phosphatase 1 activity MYPT1-P/T | Range of detection: unknown however activity levels reported 4.3–44 (no units) range in diseased individuals Normal mean value: 1.2 ± 0.2 (no units) |
The two enzymes are involved in activity of myosin molecules during contraction and relaxation. Mechanism is poorly understood | Elevated leukocyte Rho-kinase activity correlated with clinical and echocardiographic indices of HF risk | Potentially identifies hypertensive patients at risk for HF |
| GDF-15 | Range of detection: 90–13,790 ng/L Median value: 1109 ng/L Values above the median approach abnormal |
Considered a marker of inflammatory stress that is a mediator of myocardial fibrosis | Elevated during disease exacerbation. Inflammatory marker that supplements NT-proBNP disease prognosis | Risk stratification after myocardial infarction, atrial fibrillation, prognostication of heart failure, and prediction of bleeding events when on anticoagulation |
| Galectin 3 | Range of detection: 2.4–950 ng/mL Median value: 17.8 ng/mL Values above the median approach abnormal |
Paracrine molecule related to aldosterone signaling. Member of galactoside lectin binding family involved in the development of myocardial fibrosis | Elevated concentrations associated with adverse clinical outcomes of HF associated hospitalization and death | Galectin-3 concentrations are prognostic in HF and associated with a 2-fold to 3-fold increase in death and hospitalization in patients presenting with HF |
| Osteoprotogerin (OPG) | Range of detection: 15–2211 ng/L Median value: 1164 ng/L Values above the median approach abnormal |
Dysregulation of OPG and RANK/RANKL signaling leads to progression of coronary plaque and stimulates MMP-related cardiomyocyte extracellular matrix degradation leading to myocardial injury | Elevated concentrations associated with adverse clinical outcomes including HF-associated hospitalization and death Incremental risk shown when elevated with NT-proBNP |
Post-myocardial infarction predictor of infarct size, HF hospitalization, and cardiovascular death |
| IGF B-7 | Range of detection: 0.10–79.80 ng/mL Median value: 50.30 ng/mL Values above the median approach abnormal |
IGF B-7 binds to IGF-1 blocking its pro-survival action on cardiac myocytes and promotes cell death | Elevated concentrations associated with adverse cardiovascular events Good correlation with echo-derived measurements for diastolic HF (E/A, E/E′, LA volume index, RVSP) |
Potential significance in the aging population given trend to lower IGF-1 in elderly |
HF heart failure, LA left atrial, RVSP right ventricular systolic pressure
High-Sensitivity Cardiac Troponin T
For over three decades, the presence of detectable serum cardiac troponins (cTnT) has been an important marker of acute myocardial injury [1]. Troponin molecules utilize calcium to modify downstream cardiac muscle contraction; loss of membrane integrity causes leakage of troponins into the blood [2]. In patients with irreversible, chronic myocardial injury, it is postulated that there may be a continuous release of troponin [2]. Recently, patients with chronic HF were found to have elevated troponin levels when compared to those without HF [3], likely due to chronic wall stress and subendocardial ischemia.
Prospective observational studies have also detected circulating troponin in a small percentage of the general population, raising the possibility of chronic, subclinical myocardial injury in patients without overt cardiovascular disease (CVD). In the Dallas Heart Study (DHS), a cohort of over 3500 ambulatory adults aged 30 to 65 years, a detectable cTnT was found in 0.7% patients and independently associated with high-risk phenotypes, including underlying HF and left ventricular hypertrophy (LVH) [4]. Furthermore, chronically elevated cardiac troponins in the general population may be a poor prognostic marker. In the Rancho Bernardo Study, an ambulatory cohort of over 950 elderly adults aged 60 to 97 years, a detectable cTnT was noted in 4.1% and independently increased the risk of all-cause and cardiovascular death (HR 2.06, p = 0.003 and HR 2.06, p = 0.04, respectively) [5].
One limitation of the current clinical assay for cardiac troponin T is a low prevalence of detectable cTnT in patients with or at risk for heart failure [6]. This reduces the clinical and practical value of the assay for subclinical disease detection in the general population. The advent of a high-sensitivity troponin T assay (hs-cTnT) allows for troponin detection at a 10-fold lower level than the standard assays [7]. The 99th percentile of the hs-cTnT assay, considered the upper reference limit for clinical use, is reported at 0.014 μg/L with a lower limit of detection <0.003 μg/L [8], compared with the lower limit of detection of <0.01 μg/L for standard assays. A significant proportion of individuals have a detectable hs-cTnT below the detection of the standard assay, which has allowed significant exploration of hs-cTnT as a biomarker of subclinical cardiovascular dysfunction.
In the DHS cohort (mean age 44 years), 25% had a detectable hs-cTnT at baseline, while 0.7% had a detectable cTnT using the standard assay [9]. By comparison, in the Atherosclerosis Risk in Communities (ARIC) Study, among a cohort of more than 9600 community-dwelling individuals without known coronary disease between 54 and 74 years of age, 66.5% had a detectable hs-cTnT, while <1% had a detectable cTnT using standard assays [10], and in the Cardiovascular Health Study (CHS), a cohort of more than 4200 community-dwelling elderly patients >65 years of age without prior HF, 66% had a detectable hs-cTnT [11].
The 99th percentile cutoff for clinical use of hs-cTnT merits further discussion, as it was derived from a small study of volunteers with minimal information about subject selection [8]. There are significant age and sex differences in the 99th percentile value of hs-cTnT, and universally using 0.014 μg/L may lead to improper diagnosis of acute CVD. For example, more than 10% of men between the ages of 65 and 74 without overt CVD were shown to have hs-cTnT levels >0.014 μg/L [12•]. A lack of current age- and sex-specific upper reference limit cutoff values may have not only a profound effect on disease diagnosis but also risk stratification, and is an important caveat in clinical application of the assay.
Multiple predictors of elevated hs-cTnT have been explored in the general population. In DHS, the prevalence of hypertension significantly increased from 27% in patients with undetectable troponins to 71% in those with levels above >0.014 μg/L [9]. Similarly, significant increases in diabetes prevalence (8 to 41%), metabolic syndrome (30 to 52%), and hypercholesterolemia (11 to 24%) were also noted. Elevated baseline hs-cTnT has also been associated with incident hypertension in the ARIC cohort (adjusted HR 1.31; 95% CI 1.07–1.61 for those with hs-cTnT ≥14 ng/L) [13]. Hs-cTnT can also help differentiate a malignant phenotype of LVH, an important precursor to HF. Elevated left ventricular mass and the presence of LVH are powerful predictors of depressed LV systolic function and cardiovascular death [13]. In a prospective analysis of 1072 Japanese males without overt CVD, hs-cTnT was detected in 81% participants, and independently associated with LVH on echocardiography [14]. In DHS, the prevalence of LVH on echocardiography increased from 8% in subjects with undetectable hs-cTnT to 48% in participants with levels >0.014 μg/L. LV mass increased and LV ejection fraction modestly decreased with increasing hs-cTnT levels [9]. Interestingly, our group found the cumulative incidence of HF and cardiovascular death to be significantly higher in DHS participants with the combination of LVH and detectable hs-cTnT, when compared to those with LVH alone (21 vs 6%, p < 0.0001) [15••]. This may suggest that certain patients with LVH have sustained cardiac injury, while others do not. Whether a more intensive blood pressure control regimen is needed for those with hypertension associated LVH and an elevated troponin to prevent cardiovascular events would require further clinical trials. McEvoy et al. stratified the ARIC cohort by diastolic blood pressure (DBP), and noted an inverse linear relationship between DBP and hs-cTnT for those with DBP <65 mmHg (adjusted OR 2.24; 95% CI 1.22–4.10 for those with DBP <60 mmHg) [16]. Also of interest for further studies would be to evaluate if intensive blood pressure control can prevent future troponin elevations in those with LVH and undetectable hs-cTnT at baseline.
The hs-cTnT strongly associates with incident HF and cardiovascular mortality in the general population. In CHS, after a median follow-up of almost 12 years, those with the highest hs-cTnT had a >2-fold risk for incident HF and ~3-fold risk for cardiovascular death. The 1002 participants in CHS without coronary heart disease or major HF risk factors were separately analyzed, and the association between hs-cTnT, HF, and CV death remained [11]. In ARIC, there was a graded, concordant association between hs-cTnT and HF-related hospitalizations, most marked in subjects with levels >0.014 μg/L (HR 5.95). Notably, the hazard ratio for incident coronary heart disease was lower, suggesting that hs-cTnT may be a better predictor for HF. These associations were also significant in patients with minimally detectable hs-cTnT levels [10]. Hs-cTnT is also associated with increased all-cause mortality. After a median follow-up of 6.4 years in the DHS cohort, a detectable hs-cTnT was associated with a significant increase in adjusted all-cause mortality (1.9 vs 9.1%, p < 0.01) in those with detectable, increasing up to ~28% across higher hs-cTnT categories [9]. This graded association with all-cause mortality also persisted after adjustment for abnormal NT pro-BNP and C-reactive protein levels. Importantly, the adjusted association remained after exclusion of subjects with known CVD. A graded association with all-cause mortality was also found in ARIC, with a hazard ratio approaching 4-fold [10].
Recently, the prognostic implication of repeat hs-cTnT measurements has been explored. In an 11-year follow-up of the PREVEND cohort study, a community-based population in the Netherlands between the ages of 28 and 75 years, a doubling of hs-cTnT over time was associated with hazard ratio of 1.33 for incident HF after multivariable adjustment. In this study, participants with an LVEF 41–49% were excluded, and an elevated hs-cTnT was associated with increased risk for HF with reduced ejection fraction (HR 1.38, 95% CI 1.18–1.60), but not HF with preserved ejection fraction [17]. When troponin measurements were repeated between 2 and 3 years later in CHS, subjects with an increase in hs-cTnT >50% had a greater risk of HF and CV death, whereas a decrease of >50% was associated with lower risk of HF and CV death [11]. Temporal increases in hs-cTnT in ARIC also have strongly associated with incident HF independent of NT-pro BNP, suggesting that repeat measurements of this assay may further risk stratify patients [18••]. Of the subset of ARIC patients with undetectable hs-cTnT at baseline, those with abnormal assays at follow-up had a much higher risk of incident HF, with hazard ratios ranging up to 8-fold depending on the amount of troponin change from baseline. Thus, we now have multiple “healthy” cohorts which have serial measurements of hs-cTnT as a novel method of cardiovascular risk stratification in the general population. The appropriate time to repeat the test in an otherwise asymptomatic patient, however, remains unclear.
Natriuretic Peptides
Natriuretic peptides are a family of proteins that have a role in the maintenance of cardio-renal homeostasis through natriuretic, vasodilatory, and diuretic effects [19]. B-type natriuretic peptide (BNP) is manufactured in the ventricular myocytes and released into the blood stream. Atrial natriuretic peptide is also found mainly in the heart. These markers have been shown to be prognostic in patients with acute decompensated HF [20], and attempts have been made to use BNP to guide therapy in HF patients with varying degrees of success [21, 22]. Both BNP and NT-proBNP (the bio-inert circulating pro-hormone of BNP) are used regularly in clinical settings for evaluation and treatment of patients with both systolic and diastolic heart failure. Plasma levels of the two molecules are closely correlated (r = 0.90, <0.001) and have similar efficacy in predicting clinical severity of heart failure and prediction of event-free survival [23]. However, there do appear to be subtle differences in their prognostic ability for certain events. In the Val-HEFT cohort, the predictive ability for all-cause mortality was virtually identical, but NT-proBNP was superior to BNP in terms of predicting mortality and morbidity (AUC 0.688 [SE 0.009] vs 0.674 [0.009]; p = 0.0332) and heart failure hospitalizations (0.685 [0.011] vs 0.665 [0.012]; p = 0.0143) [24]. While their role in patients with known heart failure is well established, these biomarkers also have value in detecting subclinical cardiac disease in patients without clinical heart failure.
One possible use of natriuretic peptides is screening for asymptomatic left ventricular dysfunction (ALVD). In the PROBE-HF study, 1012 patients with hypertension and/or diabetes without any symptoms of heart failure were screened for ALVD with echocardiography [25]. In this study population, NT-proBNP was found to be an independent risk factor for the presence of significant ALVD, defined as either moderate to severe diastolic dysfunction or systolic dysfunction (OR 2.03, 95% CI 1.8–2.5; p < 0.0001). Similarly, the PREDICTOR study utilized a cohort of 1452 Italian patients without clinical evidence of heart failure and looked at the utility of NT-proBNP as a screening tool for ALVD [26]. In this cohort, NT-proBNP was a useful screening biomarker, with a negative predictive value of 99.4 (95% CI 98.4–99.5) for systolic dysfunction and 95.6 (95 CI 94.4–96.7) for moderate-to-severe diastolic dysfunction.
In addition to screening for the presence of LV dysfunction, there is evidence that these biomarkers have prognostic value in patients with risk factors. In the Olmstead County population, patients with either stage A or B heart failure, NT-proBNP levels greater than the age- and sex-adjusted 80th percentile added independent prognostic value for the death (HR 2.02, 95% CI 1.45–2.81; p < 0.001), heart failure (HR 1.56, 95% CI 1.18–2.06; p = 0.002), and myocardial infarction (HR 2.63, 95% CI 1.87–3.68; p < 0.001) even when adjusted for multiple traditional risk factors such as age, sex, BMI, diabetes, hypertension, creatinine, and cholesterol [27]. Similarly, a substudy of PROBE-HF found that men (3.6-fold increase) and women (2.9-fold increase) with increased NT-proBNP levels had an increased risk of cardiac death, heart failure hospitalization, and non-fatal myocardial infarction over approximately 50 months of follow-up [28]. In patients with evidence of LVH, NT-proBNP may play a role in identifying those at risk for progression to more serious disease. Evidence from the DHS noted that patients with LVH and elevated NT-proBNP levels had significantly more incident heart failure and mortality than those with LVH and normal NT-proBNP levels (20.2 vs 1.5%, p < 0.0001) [15••]. In patients with renal disease, this has also been demonstrated. The Chronic Renal Insufficiency Cohort of 3283 patients with a mean glomerular filtration rate of 45.7 mL/min per 1.73 m2 with no known cardiac disease at baseline showed that patients in the highest quintile of NT-proBNP had significantly higher rates of heart failure (HR 9.57, CI 4.40–20.83) [29].
The predictive value of BNP and NT-proBNP may even extend beyond groups with risk factors for cardiovascular disease. In community-based samples of healthy patients, there is evidence that these biomarkers are predictors of poor outcomes. A study of healthy individuals in Denmark found that NT-proBNP was predictive for a first major cardiovascular event (defined as non-fatal myocardial infarction, fatal coronary heart disease, unstable angina, heart failure, or stroke/transient ischemic attack) even after adjustment for a variety of traditional risk factors (HR 3.24, 95% CI 1.80–5.79) [30]. Larger cohorts have confirmed this result. Wang et al. prospectively studied 3346 asymptomatic patients with no evidence of heart failure [31]. After over 5 years of follow-up, participants in the 80th percentile or above had higher rates of all-cause mortality, stroke, and major cardiovascular event including heart failure and atrial fibrillation. Similarly, in the Rancho Bernardo Study, NT-proBNP elevations at baseline were predictive of both all-cause and cardiovascular mortalities (HR 1.85, p < 0.001 and HR 2.51, p < 0.001, respectively) [5]. The predictive value for all-cause mortality was even stronger when combined with detectable troponin T levels (HR 3.20, p < 0.001). More recent studies have confirmed these findings. In the Malmö Diet and Cancer study Cardiovascular Cohort of 5187 presumably healthy individuals, NT-proBNP was predictive of incident heart failure (HR 1.63, p < 0.001) [32]. Analysis of the Heinz Nixdorf Recall Study showed that in patients without evidence of cardiovascular disease, both BNP (HR 1.37, 95% CI 1.19–1.58; p < 0.0001) and NT-proBNP (HR 1.60, 95% CI 1.39–1.84; p < 0.0001) were predictive of first major cardiovascular event [33]. In this study, NT-proBNP was superior to BNP at predicting events in subjects <60 years old and among females. On the other hand, a study of over 2000 patients in Olmsted County, Minnesota, showed less promising results for the use of NT-proBNP in healthy populations [27]. The NT-proBNP levels above the age- and sex-adjusted 80th percentile in this normal, healthy subgroup—defined as subjects without traditional cardiac risk factors or structural and functional cardiac abnormalities—did not have increased risk of a combined endpoint of death, heart failure, stroke, or myocardial infarction.
Novel Markers of Inflammation, Fibrosis, and Contractility
A relatively underappreciated risk factor of HF disease progression is inflammation. The pathogenesis of HF is closely associated with abnormal cytokine release such as rapid increases in circulating levels of inflammatory molecules associated with fibrosis such as ST2 and GDF-15, abnormal fibroblast paracrine signaling with galectin-3, dysregulation of RANK/RANKL osseous cell signaling via increased osteoprotegerin (OPG), and, as consequence of OPG release, thrombotic mediators such as matrix metalloproteinases (MMPs).
ST2 was first identified as a molecular receptor of interleukin (IL)-33. It circulates in both protein bound and unbound forms [34]. Expression of soluble ST2 was found to increase during periods of cardiac loading with angiotensin II and phenylephrine [35]. When soluble ST2 binds to IL-33, the molecule functions as a dummy receptor and sequesters circulating IL-33 from binding to the myocardium, resulting in reduction of myocardial hypertrophy and fibrosis, effectively mitigating the detrimental effects of interleukin cytokine signaling [36]. Among diverse patient cohorts, the median soluble ST2 measured in the serum using the Radix Biosolutions ® (Georgetown, TX) or Critical Diagnostics Prestige ® (San Diego, CA) systems was significantly higher among those with heart failure as compared to those without heart failure. The mean sensitivity and specificity of ST2 for the diagnosis of HF in these cohorts were 73.5 and 79.6%, respectively [37, 38]. Further studies found that elevated ST2 levels were predictive of future death and heart failure events independent of BNP or NT-proBNP concentrations. The incident risk of heart failure with elevated ST2 (>34.2 U/mL) over 18 months was associated with a 2-fold increased risk (HR 1.9, 95% CI 1.2–3.2) [39, 40]. Using the Radix Biosolutions assay, ST2 was also independently associated with a similar risk of incident heart failure (HR 1.9, 95% CI 1.2–3.0), in a multiethnic population among those in the highest quartile (1.44–28.63 μg/L). ST2 however had lower predictive power than BNP or NT-proBNP [38]. African American patients were found to have twice the prevalence for heart failure when detectable ST2 (>0.40 μg/L) was found as compared to white patients [37]. Whether soluble ST2 provides detection of subclinical heart failure in the African American population is unknown, and research in this area would provide insight towards earlier detection as well as alternative mechanisms of treatment of HF. It is important to understand the differences in scale when using the two available ST2 assays. While the risk of incident heart failure risk is similar in estimation, the precise concentrations that are predictive for incident heart failure in each assay are unknown.
GDF-15 is a potent inflammatory molecule that is released during physiological stress from the peripheral vasculature and adipose tissue [39]. Clinical risk factors associated with elevated levels included advanced age, hypertension, coronary artery disease, atrial fibrillation, decreased creatinine clearance, and low ejection fraction [40]. GDF-15 has therefore found a role in the evaluation for a multitude of cardiovascular diseases including risk stratification after myocardial infarction, atrial fibrillation, prognostication of heart failure, and prediction of bleeding events when on anticoagulation [41–43]. Elevated levels of GDF-15, measured using Quantikine ELISA ® for research or Elecsys ELISA® (Roche), predict increased risk of developing cardiovascular disease independent of traditional risk factors. In the Framingham Heart Study, patients followed for 11 years who had elevated GDF-15 (median concentration 1066 ng/L ± 594) were found to have increased risk for incident heart failure (HR 1.52, 95% CI 1.29–1.78) [44]. While predictive for incident heart failure in isolation, the added value of GDF-15 to BNP or NT-proBNP is relatively modest [42].
Scar expansion attributable to myocardial fibrosis is a major contributor to adverse cardiac remodeling. Aldosterone release is implicated in the pathogenesis of cardiac fibrosis and is derived from activation of renin-angiotensin-aldosterone system resulting in oxidative stress, inflammation, activation of endothelin-1, Rho-kinase signaling, and leptin release [45, 46]. Inflammatory mediators such as galectin-3 are associated with aldosterone release and fibrosis [47]. Galectin-3 molecules are released by fibroblasts and macrophages during periods of heart failure. Galectin-3 concentrations are prognostic in HF and associated with a 2- to 3-fold increase in death and hospitalization in patients presenting with HF [48, 49]. The Framingham Offspring cohort followed participants for approximately 11 years where the median galectin-3 concentration was 14.3 ng/mL in women and 13.1 ng/mL in males. Galectin-3 was associated with risk for incident HF (HR 1.28, 95% CI 1.11–1.43) per standard deviation increment in the log of galectin-3 concentration [50]. Increased risk for incident heart failure was similarly observed in the PREVEND cohort which found that galectin-3 remained an independent predictor of new-onset HF (HR 1.85, 95% CI 1.10–3.13) over 4 years of follow-up [51]. When taken individually, galectin-3, like ST2 and GDF-15, lacks the predictive power of natriuretic peptides but may be an important biomarker used to explore mediators of myocardial fibrosis and inflammation.
Further understanding of the magnitude inflammation in the pathogenesis of heart failure has led to a better appreciation for alternative mechanisms of cytokine dysregulation. For example, osseous signaling via OPG, RANK/RANKL, and MMPs leads to formation of coronary atherosclerotic plaque, degradation of cardiac myocytes, and the development of heart failure in animals and humans [52]. The incremental risk of HF increases with each doubling of OPG and is associated with a 3-fold increase in incident HF over mean follow-up of 8.2 ± 1.6 years in men in one population-based study [53]. In patients with acute myocardial infarction, OPG levels predict myocardial infarct size as well post-infarction HF outcomes [54, 55]. Furthermore, adjusted 30-day outcomes for elevated OPG were associated with 2-fold greater hazard of hospitalization for new or worsening HF and cardiovascular death [56]. OPG therefore appears to be a versatile biomarker for multiple cardiovascular diseases. Its real-world clinical utility, however, remains to be seen.
Novel Markers of Ischemia
Myocardial ischemia plays a significant role in the pathogenesis of HF, and small polypeptides abundantly found from the ventricular cardiac myocyte can be detected in the serum during periods of injury. Fatty acid metabolism is essential for cardiac myocyte function, and heart-associated fatty acid binding peptides (H-FABPs) help transport long-chain fatty acids across the cell and mitochondrial membranes. After myocardial infarction, H-FABP is detectable in the blood within 3 h of chest pain onset [57]. Elevated H-FABP concentrations correspond with advanced age, HF symptom severity, elevated BNP, and echo findings of elevated left atrial pressure. In patients with cardiac events, elevations mildly correlated with CK-MB and troponin I or T concentrations, although H-FABP was more frequently (88 vs. 44%, p = 0.013) detected than troponin T and may improve the sensitivity of early identification of cardiac injury [58]. In the OPUS-TIMI 16 trial evaluating the efficacy of the oral GP IIb/IIIa inhibitor, orofiban, in patients with an acute coronary syndrome, patients with elevated H-FABP were older in age >65 years and were more likely to have a history of HF. Patients with elevated levels were also more likely to have sudden death, recurrent myocardial infarction, and HF over a 10-month follow-up period. The relative hazard for development of HF was reported to be 4.5 times higher compared to patients with lower H-FABP concentrations [58, 59]. Elevated concentrations of H-FABP were also associated with all-cause mortality and HF related re-hospitalization at 1 and 5 years [60, 61].
Novel Markers of Myocardial Contraction and Relaxation
As a consequence of myocardial dysfunction in HF, derangements in cardiac myosin regulatory pathways also occur. The central mechanism of normal myocardial contraction involves the interaction of Rho-kinases and myosin phosphatases on the myosin light chain of sarcomeres through phosphorylation reactions [62]. When Rho-kinase activation is increased relative to myosin phosphatase (MYPT1-P/T), regulation of the myosin contraction pathway is disrupted and leads to ventricular myocyte hypertrophy, necrosis, and fibrosis followed by HF [63]. Among patients with long-standing hypertension, nearly 2-fold higher levels of Rho-kinase activity in circulating leukocytes were found in patients with LVH as compared to patients with hypertension alone [64]. The risk of LVH may translate to greater HF risk allowing for added prognostication with MYPT1-P/T. Indeed, measured MYPT1-P/T activity in circulating leukocytes have been noted to be significantly elevated up to 100-fold among patients with clinical NYHA II or III HF symptoms. Elevations were similarly correlated with severity of ejection fraction and left ventricular end-diastolic diameter [65]. While clinical data appear to suggest that abnormal elevations of MYPT1-P/T will be associated with incident HF, prospectively derived cohort studies investigating this question are currently lacking.
Insulin-like growth factors (IGF-1 and IGF-II) are essential mediators of cardiac myocyte survival. IGF-1 helps maintain cardiac muscle by inducing protein synthesis and by blocking premature cell death. The overexpression of IGF-1 in experimental models demonstrated improvements in contractile performance of myocardium, and diminished levels were shown to contribute to premature senescence of the heart [66, 67]. Approximately seven binding proteins modulate IGF action via activation, prolonging IGF half-life, and aiding in both receptor binding and inhibition. IGFBP-7 binds to IGF-1 neutralizing its beneficial action on cardiac myocyte. As IGF-1 levels decline with age, concentrations of IGFB-7 remain unchanged, offsetting the ratio with IGF-1 and are clinically associated with the diagnosis of diastolic HF [68]. Elevated IGF B-7/IGF-1 ratio was shown to be associated with multiple surrogate endpoints including elevated NT-proBNP and ST2, and correlated well with HF echocardiographic indices of transmitral E/A velocities, E/E′ ratio, left atrial volume index, and right ventricular systolic pressure [69, 70]. Prospective observational cohort studies evaluating incident HF related to IGF B-7 are lacking.
Conclusions
The wide array of novel biomarkers and their various uses in the diagnosis and prognostication of patients with subclinical cardiac dysfunction leave many clinicians and researchers questioning which biomarkers are most reliable. Indeed, many of the biomarkers discussed have demonstrated associations with relevant cardiovascular outcomes and may enhance the diagnostic and prognostic power of more conventional biomarkers. However, their use in “prime time” to identify patients with or at risk for subclinical cardiac dysfunction in the general population remains an open question. Identifying key mediators of HF development such as inflammation, myocardial ischemia, abnormal myocardial contraction, and relaxation, imbalance of homeostatic signaling may offer valuable insight into the prediction of future HF. Certain biomarkers may have particular relevance identifying unique populations at risk for HF such as ST2 in African Americans, IGFB-7 in the aging population, H-FABP and OPG after myocardial infarction, and determining the burden of myocardial fibrosis with GDF-15 and galectin-3. Future applications may also include the development of biomarker risk scores for cumulative cardiovascular events in order to reliably predict survival independent of age, sex, or contractile function [71]. While many biomarkers are associated with outcomes in observational studies of the general population, strategic investigation into their clinical applicability in the context of clinical trials remains an open area of investigation.
Abbreviations
- CVD
Cardiovascular disease
- DHS
Dallas Heart Study
- HF
Heart failure
- H-FABP
Heart-associated fatty acid binding peptides
- hs-cTnT
High-sensitivity cardiac troponin T
- IGF
Insulin-like growth factor
- LVH
Left ventricular hypertrophy
- MMPs
Matrix metalloproteinases
- MYPT1-P/T
Myosin light chain phosphatase 1 activity
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- OPG
Osteoprotegerin
Footnotes
Compliance with Ethical Standards
Conflict of Interest Kamal Shemisa, Anish Bhatt, and Daniel Cheeran declare no conflicts of interest.
Ian J. Neeland is supported by grant K23 DK106520 from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institute of Health and by the Dedman Family Scholarship in Clinical Care from UT Southwestern.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Jneid H, Alam M, Virani SS, et al. Redefining myocardial infarction: what is new in the ESC/ACCF/AHA/WHF third universal definition of myocardial infarction? Methodist Debakey Cardiovasc J. 2013;9:169–72. doi: 10.14797/mdcj-9-3-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sato Y, Fujiwara H, Takatsu Y. Cardiac troponin and heart failure in the era of high-sensitivity assays. J Cardiol. 2012;60:160–7. doi: 10.1016/j.jjcc.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Missov E, Mair J. A novel biochemical approach to congestive heart failure: cardiac troponin T. Am Heart J. 1999;138:95–9. doi: 10.1016/s0002-8703(99)70252-8. [DOI] [PubMed] [Google Scholar]
- 4.Wallace TW, Abdullah SM, Drazner MH, et al. Prevalence and determinants of troponin T elevation in the general population. Circulation. 2006;113:1958–65. doi: 10.1161/CIRCULATIONAHA.105.609974. [DOI] [PubMed] [Google Scholar]
- 5.Daniels LB, Laughlin GA, Clopton P, et al. Minimally elevated cardiac troponin T and elevated N-terminal pro-B-type natriuretic peptide predict mortality in older adults: results from the Rancho Bernardo Study. J Am Coll Cardiol. 2008;52:450–9. doi: 10.1016/j.jacc.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Latini R, Masson S, Anand IS, et al. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation. 2007;116:1242–9. doi: 10.1161/CIRCULATIONAHA.106.655076. [DOI] [PubMed] [Google Scholar]
- 7.Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858–67. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 8.Giannitsis E, Kurz K, Hallermayer K, et al. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56:254–61. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 9.de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–12. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the atherosclerosis risk in communities study. Circulation. 2011;123:1367–76. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.Gore MO, Seliger SL, Defilippi CR, et al. Age- and sex-dependent upper reference limits for the high-sensitivity cardiac troponin T assay. J Am Coll Cardiol. 2014;63:1441–8. doi: 10.1016/j.jacc.2013.12.032. This study highlights the significant age and sex variability in the distribution of values for hs-cTnT. It shows that implementing the current clinical abnormal cutoff value for hs-cTnT (99th percentile of the population) equivalent to 0.014 μg/L may lead to widespread improper diagnosis of acute myocardial infarction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drazner MH, Rame JE, Marino EK, et al. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J Am Coll Cardiol. 2004;43:2207–15. doi: 10.1016/j.jacc.2003.11.064. [DOI] [PubMed] [Google Scholar]
- 14.Otsuka T, Kawada T, Ibuki C, et al. Association between high-sensitivity cardiac troponin T levels and the predicted cardiovascular risk in middle-aged men without overt cardiovascular disease. Am Heart J. 2010;159:972–8. doi: 10.1016/j.ahj.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 15••.Neeland IJ, Drazner MH, Berry JD, et al. Biomarkers of chronic cardiac injury and hemodynamic stress identify a malignant phenotype of left ventricular hypertrophy in the general population. J Am Coll Cardiol. 2013;61:187–95. doi: 10.1016/j.jacc.2012.10.012. This study is the first to show a major interaction between left ventricular hypertrophy, hs-cTnT, and NT-proBNP on the outcome of heart failure and cardiovascular death in a community dwelling population. Asymptomatic individuals with LVH and either elevated hs-cTnT or NT-proBNP had a >4-fold higher risk for heart failure or cardiovascular death compared with persons without LVH or elevated biomarkers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McEvoy JW, Chen Y, Rawlings A, et al. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: implications for blood pressure control. J Am Coll Cardiol. 2016;68:1713–22. doi: 10.1016/j.jacc.2016.07.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brouwers FP, de Boer RA, van der Harst P, et al. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J. 2013;34:1424–31. doi: 10.1093/eurheartj/eht066. [DOI] [PubMed] [Google Scholar]
- 18••.McEvoy JW, Chen Y, Ndumele CE, et al. Six-year change in high-sensitivity cardiac troponin T and risk of subsequent coronary heart disease, heart failure, and death. JAMA Cardiol. 2016;1:519–28. doi: 10.1001/jamacardio.2016.0765. This study showed that temporal increases in hs-cTnT are strongly associated with incident heart failure independent of NT-pro BNP, suggesting that repeat measurements of this assay may further help to identify and risk stratify patients in the general population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volpe M, Rubattu S, Burnett J., Jr Natriuretic peptides in cardiovascular diseases: current use and perspectives. Eur Heart J. 2014;35:419–25. doi: 10.1093/eurheartj/eht466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masson S, Latini R, Anand IS, et al. Prognostic value of changes in N-terminal pro-brain natriuretic peptide in Val-HeFT (Valsartan Heart Failure Trial) J Am Coll Cardiol. 2008;52:997–1003. doi: 10.1016/j.jacc.2008.04.069. [DOI] [PubMed] [Google Scholar]
- 21.Sanders-van Wijk S, Maeder MT, Nietlispach F, et al. Long-term results of intensified, N-terminal-pro-B-type natriuretic peptide-guided versus symptom-guided treatment in elderly patients with heart failure. Circ Heart Fail. 2014;7:131. doi: 10.1161/CIRCHEARTFAILURE.113.000527. [DOI] [PubMed] [Google Scholar]
- 22.Gaggin HK, Mohammed AA, Bhardwaj A, et al. Heart failure outcomes and benefits of NT-proBNP-guided management in the elderly: results from the prospective, randomized ProBNP Outpatient Tailored Chronic Heart Failure Therapy (PROTECT) Study. J Card Fail. 2012;18:626–34. doi: 10.1016/j.cardfail.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Richards M, Nicholls MG, Espiner EA, et al. Comparison of B-type natriuretic peptides for assessment of cardiac function and prognosis in stable ischemic heart disease. J Am Coll Cardiol. 2006;47:52–60. doi: 10.1016/j.jacc.2005.06.085. [DOI] [PubMed] [Google Scholar]
- 24.Masson S, Latini R, Anand IS, et al. Direct comparison of B-type natriuretic peptide (BNP) and amino-terminal proBNP in a large population of patients with chronic and symptomatic heart failure: the valsartan heart failure (Val-HeFT) data. Clin Chem. 2006;52:1528–38. doi: 10.1373/clinchem.2006.069575. [DOI] [PubMed] [Google Scholar]
- 25.Betti I, Castelli G, Barchielli A, et al. The role of N-terminal PRO-brain natriuretic peptide and echocardiography for screening asymptomatic left ventricular dysfunction in a population at high risk for heart failure. The PROBE-HF Study. J Card Fail. 2009;15:377–84. doi: 10.1016/j.cardfail.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Mureddu GF, Tarantini L, Agabiti N, et al. Evaluation of different strategies for identifying asymptomatic left ventricular dysfunction and pre-clinical (stage B) heart failure in the elderly. Results from ‘PREDICTOR’, a population based-study in central Italy. Eur J Heart Fail. 2013;15:1102–12. doi: 10.1093/eurjhf/hft098. [DOI] [PubMed] [Google Scholar]
- 27.McKie PM, Cataliotti A, Lahr BD, et al. The prognostic value of N-terminal pro-B-type natriuretic peptide for death and cardiovascular events in healthy normal and stage A/B heart failure subjects. J Am Coll Cardiol. 2010;55:2140–7. doi: 10.1016/j.jacc.2010.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ballo P, Betti I, Barchielli A, et al. Prognostic role of N-terminal pro-brain natriuretic peptide in asymptomatic hypertensive and diabetic patients in primary care: impact of age and gender. Clin Res Cardiol. 2016;105:421–31. doi: 10.1007/s00392-015-0937-x. [DOI] [PubMed] [Google Scholar]
- 29.Bansal N, Hyre Anderson A, Yang W, et al. High-sensitivity troponin T and N-terminal pro-B-type natriuretic peptide (NT-proBNP) and risk of incident heart failure in patients with CKD: the Chronic Renal Insufficiency Cohort (CRIC) Study. J Am Soc Nephrol. 2015;26:946–56. doi: 10.1681/ASN.2014010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kistorp C, Raymond I, Pedersen F, et al. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA. 2005;293:1609–16. doi: 10.1001/jama.293.13.1609. [DOI] [PubMed] [Google Scholar]
- 31.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–63. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 32.Smith JG, Newton-Cheh C, Almgren P, et al. Assessment of conventional cardiovascular risk factors and multiple biomarkers for the prediction of incident heart failure and atrial fibrillation. J Am Coll Cardiol. 2010;56:1712–9. doi: 10.1016/j.jacc.2010.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kara K, Lehmann N, Neumann T, et al. NT-proBNP is superior to BNP for predicting first cardiovascular events in the general population: the Heinz Nixdorf Recall Study. Int J Cardiol. 2015;183:155–61. doi: 10.1016/j.ijcard.2015.01.082. [DOI] [PubMed] [Google Scholar]
- 34.Palmer G, Lipsky BP, Smithgall MD, et al. The IL-1 receptor accessory protein (AcP) is required for IL-33 signaling and soluble AcP enhances the ability of soluble ST2 to inhibit IL-33. Cytokine. 2008;42:358–64. doi: 10.1016/j.cyto.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Miyama N, Hasegawa Y, Suzuki M, et al. Investigation of major genetic polymorphisms in the renin-angiotensin-aldosterone system in subjects with young-onset hypertension selected by a targeted-screening system at university. Clin Exp Hypertens. 2007;29:61–7. doi: 10.1080/10641960601096968. [DOI] [PubMed] [Google Scholar]
- 36.Miller AM, Xu D, Asquith DL, et al. IL-33 reduces the development of atherosclerosis. J Exp Med. 2008;205:339–46. doi: 10.1084/jem.20071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen LQ, de Lemos JA, Das SR, et al. Soluble ST2 is associated with all-cause and cardiovascular mortality in a population-based cohort: the Dallas Heart Study. Clin Chem. 2013;59:536–46. doi: 10.1373/clinchem.2012.191106. [DOI] [PubMed] [Google Scholar]
- 38.Aldous SJ, Richards AM, Troughton R, et al. ST2 has diagnostic and prognostic utility for all-cause mortality and heart failure in patients presenting to the emergency department with chest pain. J Card Fail. 2012;18:304–10. doi: 10.1016/j.cardfail.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Schlittenhardt D, Schober A, Strelau J, et al. Involvement of growth differentiation factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in oxLDL-induced apoptosis of human macrophages in vitro and in arteriosclerotic lesions. Cell Tissue Res. 2004;318:325–33. doi: 10.1007/s00441-004-0986-3. [DOI] [PubMed] [Google Scholar]
- 40.Rohatgi A, Patel P, Das SR, et al. Association of growth differentiation factor-15 with coronary atherosclerosis and mortality in a young, multiethnic population: observations from the Dallas Heart Study. Clin Chem. 2012;58:172–82. doi: 10.1373/clinchem.2011.171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan MM, Santhanakrishnan R, Chong JP, et al. Growth differentiation factor 15 in heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail. 2016;18:81–8. doi: 10.1002/ejhf.431. [DOI] [PubMed] [Google Scholar]
- 42.Baggen VJ, van den Bosch AE, Eindhoven JA, et al. Prognostic value of N-terminal pro-B-type natriuretic peptide, troponin-T, and growth-differentiation factor 15 in adult congenital heart disease. Circulation. 2017;135:264–79. doi: 10.1161/CIRCULATIONAHA.116.023255. [DOI] [PubMed] [Google Scholar]
- 43.Wollert KC, Kempf T, Wallentin L. Growth differentiation factor 15 as a biomarker in cardiovascular disease. Clin Chem. 2017;63:140–51. doi: 10.1373/clinchem.2016.255174. [DOI] [PubMed] [Google Scholar]
- 44.Wang TJ, Wollert KC, Larson MG, et al. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. 2012;126:1596–604. doi: 10.1161/CIRCULATIONAHA.112.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huby AC, Antonova G, Groenendyk J, et al. Adipocyte-derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation. 2015;132:2134–45. doi: 10.1161/CIRCULATIONAHA.115.018226. [DOI] [PubMed] [Google Scholar]
- 46.Cavalera M, Wang J, Frangogiannis NG. Obesity, metabolic dysfunction, and cardiac fibrosis: pathophysiological pathways, molecular mechanisms, and therapeutic opportunities. Transl Res. 2014;164:323–35. doi: 10.1016/j.trsl.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma UC, Pokharel S, van Brakel TJ, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–8. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 48.van Kimmenade RR, Januzzi JL, Jr, Ellinor PT, et al. Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol. 2006;48:1217–24. doi: 10.1016/j.jacc.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 49.Meijers WC, Januzzi JL, deFilippi C, et al. Elevated plasma galectin-3 is associated with near-term rehospitalization in heart failure: a pooled analysis of 3 clinical trials. Am Heart J. 2014;167:853–60. e4. doi: 10.1016/j.ahj.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 50.Ho JE, Liu C, Lyass A, et al. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60:1249–56. doi: 10.1016/j.jacc.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Velde AR, Meijers WC, Ho JE, et al. Serial galectin-3 and future cardiovascular disease in the general population. Heart. 2016;102:1134–41. doi: 10.1136/heartjnl-2015-308975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ueland T, Yndestad A, Oie E, et al. Dysregulated osteoprotegerin/RANK ligand/RANK axis in clinical and experimental heart failure. Circulation. 2005;111:2461–8. doi: 10.1161/01.CIR.0000165119.62099.14. [DOI] [PubMed] [Google Scholar]
- 53.di Giuseppe R, Biemann R, Wirth J, et al. Plasma osteoprotegerin, its correlates, and risk of heart failure: a prospective cohort study. Eur J Epidemiol. 2016 doi: 10.1007/s10654-016-0172-4. [DOI] [PubMed] [Google Scholar]
- 54.Ueland T, Jemtland R, Godang K, et al. Prognostic value of osteoprotegerin in heart failure after acute myocardial infarction. J Am Coll Cardiol. 2004;44:1970–6. doi: 10.1016/j.jacc.2004.06.076. [DOI] [PubMed] [Google Scholar]
- 55.Andersen GO, Knudsen EC, Aukrust P, et al. Elevated serum osteoprotegerin levels measured early after acute ST-elevation myocardial infarction predict final infarct size. Heart. 2011;97:460–5. doi: 10.1136/hrt.2010.206714. [DOI] [PubMed] [Google Scholar]
- 56.Roysland R, Bonaca MP, Omland T, et al. Osteoprotegerin and cardiovascular mortality in patients with non-ST elevation acute coronary syndromes. Heart. 2012;98:786–91. doi: 10.1136/heartjnl-2011-301260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Viswanathan K, Kilcullen N, Morrell C, et al. Heart-type fatty acid-binding protein predicts long-term mortality and re-infarction in consecutive patients with suspected acute coronary syndrome who are troponin-negative. J Am Coll Cardiol. 2010;55:2590–8. doi: 10.1016/j.jacc.2009.12.062. [DOI] [PubMed] [Google Scholar]
- 58.Niizeki T, Takeishi Y, Arimoto T, et al. Heart-type fatty acid-binding protein is more sensitive than troponin T to detect the ongoing myocardial damage in chronic heart failure patients. J Card Fail. 2007;13:120–7. doi: 10.1016/j.cardfail.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 59.O’Donoghue M, de Lemos JA, Morrow DA, et al. Prognostic utility of heart-type fatty acid binding protein in patients with acute coronary syndromes. Circulation. 2006;114:550–7. doi: 10.1161/CIRCULATIONAHA.106.641936. [DOI] [PubMed] [Google Scholar]
- 60.Hoffmann U, Espeter F, Weiss C, et al. Ischemic biomarker heart-type fatty acid binding protein (hFABP) in acute heart failure—diagnostic and prognostic insights compared to NT-proBNP and troponin I. BMC Cardiovasc Disord. 2015;15:50. doi: 10.1186/s12872-015-0026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kutsuzawa D, Arimoto T, Watanabe T, et al. Ongoing myocardial damage in patients with heart failure and preserved ejection fraction. J Cardiol. 2012;60:454–61. doi: 10.1016/j.jjcc.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 62.Hartshorne DJ, Ito M, Erdodi F. Myosin light chain phosphatase: subunit composition, interactions and regulation. J Muscle Res Cell Motil. 1998;19:325–41. doi: 10.1023/a:1005385302064. [DOI] [PubMed] [Google Scholar]
- 63.Ding P, Huang J, Battiprolu PK, et al. Cardiac myosin light chain kinase is necessary for myosin regulatory light chain phosphorylation and cardiac performance in vivo. J Biol Chem. 2010;285:40819–29. doi: 10.1074/jbc.M110.160499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gabrielli L, Winter JL, Godoy I, et al. Increased Rho-kinase activity in hypertensive patients with left ventricular hypertrophy. Am J Hypertens. 2014;27:838–45. doi: 10.1093/ajh/hpt234. [DOI] [PubMed] [Google Scholar]
- 65.Ocaranza MP, Gabrielli L, Mora I, et al. Markedly increased Rho-kinase activity in circulating leukocytes in patients with chronic heart failure. Am Heart J. 2011;161:931–7. doi: 10.1016/j.ahj.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 66.Ungvari Z, Csiszar A. The emerging role of IGF-1 deficiency in cardiovascular aging: recent advances. J Gerontol A Biol Sci Med Sci. 2012;67:599–610. doi: 10.1093/gerona/gls072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Groban L, Pailes NA, Bennett CD, et al. Growth hormone replacement attenuates diastolic dysfunction and cardiac angiotensin II expression in senescent rats. J Gerontol A Biol Sci Med Sci. 2006;61:28–35. doi: 10.1093/gerona/61.1.28. [DOI] [PubMed] [Google Scholar]
- 68.Faxen UL, Hage C, Benson L, et al. HFpEF and HFrEF display different phenotypes as assessed by IGF-1 and IGFBP-1. J Card Fail. 2016 doi: 10.1016/j.cardfail.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 69.Barroso MC, Kramer F, Greene SJ, et al. Serum insulin-like growth factor-1 and its binding protein-7: potential novel biomarkers for heart failure with preserved ejection fraction. BMC Cardiovasc Disord. 2016;16:199. doi: 10.1186/s12872-016-0376-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gandhi PU, Gaggin HK, Sheftel AD, et al. Prognostic usefulness of insulin-like growth factor-binding protein 7 in heart failure with reduced ejection fraction: a novel biomarker of myocardial diastolic function? Am J Cardiol. 2014;114:1543–9. doi: 10.1016/j.amjcard.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 71.Berezin AE, Kremzer AA, Martovitskaya YV, et al. The utility of biomarker risk prediction score in patients with chronic heart failure. Clin Hypertens. 2015;22:3. doi: 10.1186/s40885-016-0041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]