Abstract
Purpose
To detect and compare the vessel diameter effect of intravitreal vs subtenon injection of triamcinolone for diabetic macular edema (DME).
Methods
Sixty patients with DME who underwent triamcinolone injection either intravitreally (N=30) or under the tenon capsule (N=30) were included. Non-injected fellow eyes served as control. The main outcome measures were central retinal artery equivalent (CRAE), central retinal vein equivalent (CRVE), and artery–vein ratio (AVR).
Results
In the intravitreal group, pre-injection mean CRAE (147.07 μ) decreased to 141.03 μ at 1 week and to 139.43 μ at 1 month (P<0.001) while baseline CRVE (209.61 μ) decreased initially to 198.85 μ at 1 week then to 198.49 μ at 1 month (P<0.001). In the subtenon group, pre-injection CRAE (152.18 μ) decreased to 149.49 μ at 1 week and to 147.47 μ at 1 month (P=0.017), while baseline CRVE (215.60 μ) decreased initially to 208.69 μ at 1 week then to 207.25 μ at 1 month (P=0.003). Pre-injection AVR values did not change at 1 week and at 1 month in both injection groups (P=0.66 and P=0.196, respectively). In the control group, none of the 3 parameters changed throughout the study period compared to the baseline (P>0.28).
Conclusion
In eyes with DME, both intravitreal and subtenon triamcinolone injection led to a significant constriction of retinal arteries and veins.
Introduction
Diabetes mellitus (DM) is the most important systemic disease that gives rise to blindness, and it is the leading cause of visual function loss in people between 20 and 65 years of age.1 The most common cause of vision loss in patients with DM is diabetic maculopathy, and the most common cause of visual acuity loss in diabetic maculopathies is diabetic macular edema (DME).2
The exact pathogenesis of diffuse DME remains unclear. However, endogenous factors released by ischemic retinas are thought to break down the integrity of the blood–retina barrier. Prostaglandins, which cause an increase in vascular permeability, are the main endogenous factors. Cystoid changes characterized by diffuse intraretinal and subretinal macular fluid accumulation due to an increase in vascular permeability may also be observed in DME. Focal macular edema occurs as the result of leakage from microaneurysms and dilated capillaries. Although the efficacy of laser photocoagulation has been shown in focal edemas, its effects are poor in diffuse DME.1, 3, 4, 5
In addition to evidence showing that retinal vein thickness is affected by systemic inflammation, there are studies revealing that the retinal vein thickness of patients with diabetes and impaired fasting glucose is larger than that of individuals with normal fasting glucose.6, 7, 8, 9, 10 Inflammation and endothelial dysfunction are thought to be associated with enlargement of the retinal vein diameter in patients with diabetes and impaired fasting glucose.11 Diabetic retinopathy, hyperglycemia, and hypoxia induce retinal vasodilatation and lead to vascular hyperperfusion.12, 13
Corticosteroids are effective in treating DME due to their anti-inflammatory influence, by reducing vascular endothelial growth factor (VEGF) levels, and their stabilizing effect on the blood–retina barrier.14 VEGF, which is known as vascular permeability factor, shows this effect by increased phosphorylation of tight junction proteins, leading to increased retinal vascular permeability.15
The aim of this study is to compare the effect of a single dose of intravitreal triamcinolone (IVT) injection with subtenon triamcinolone (STT) injection on retinal vessel diameters for the treatment of DME.
Materials and methods
The study was conducted in accordance with the principles of the Declaration of Helsinki, and medical ethics committee approval was received. Written informed consent was obtained from each participant.
Patients
The data of this study were obtained from 120 eyes of 60 patients (mean age 59.8±8.3 years), consisting of 23 (38.3%) males and 37 (61.7%) females. The injected eyes were the study group while the fellow eyes served as the control group.
DME was detected with biomicroscopic fundus examinations and confirmed by fundus fluorescein angiography and optical coherence tomography (OCT). Patients who had not received retinal laser treatment within the last 6 months were included in the study. Eyes with a central macular thickness (CMT) of 300 μ or greater and with best-corrected visual acuity (BCVA) of 20/40 or less were selected as the injection group. The exclusion criteria were as follows: Patients with other ocular pathologies, such as uveitis, vitreomacular traction, stretched and attached posterior hyaloid, retinal vein occlusion, epiretinal membrane, and age-related macular degeneration that could lead to macular edema, and patients with uncontrolled glaucoma and hypertension (>160/90 mm Hg) despite medical therapy, smokers (current smoker or having smoked >100 cigarettes in the patient’s lifetime),16 high myopia (<−6.0 diopters), high hyperopia (>+4.0 diopters), a history of refractive surgery, and only-eye patients were excluded from this study.
All patients underwent a complete ophthalmologic examination prior to injections. Visual acuity was determined based on the Early Treatment Diabetic Retinopathy Study (ETDRS) chart, and intraocular pressure (IOP) values were measured with Goldmann applanation tonometry. Slit lamp biomicroscopic examinations of the anterior segment were performed. Posterior segments were examined using indirect ophthalmoscopy and a +90 diopter lens, and the findings were recorded. Following the pharmacologic pupillary dilation, color fundus photographs (Digital Fundus Camera Canon CF-60DS, Canon Inc., New York, NY, USA) and OCT images (Cirrus HD-OCT 4000 model, software version 5.1.1.6 Carl Zeiss Meditec, Dublin, CA, USA) were obtained.
Triamcinolone acetonide injection
IVT injection was performed by an experienced physician in sterile conditions. Topical anesthetic was applied to the ocular surface, followed by preparation with 5% povidone iodine for at least two minutes. A total of 2 mg/0.05 ml of triamcinolone acetonide was injected into the mid vitreous with a 27-gauge needle 4 mm behind the limbus in patients who had not undergone cataract surgery, and 3.5 mm behind the limbus in patients who had undergone cataract surgery. Immediately after the needle was pulled back following injection, short-term pressure was gently applied with a cotton-tipped applicator to the injection point to prevent vitreous leakage and conjunctival hemorrhaging. A topical antibiotic was prescribed four times a day for 7 days after the injection.
For the posterior STT injection, following the same procedures as IVT in sterile conditions, a small incision (8 mm posterior to the limbus) was made through the conjunctiva and tenon capsule to bare sclera using a scissors. A total of 1 ml of a 40 mg/ml of triamcinolone acetonide was injected in the infero-temporal quadrant using a curved blunt cannula connected to a 2.5-ml syringe. After injection, a topical antibiotic drop was prescribed four times a day for 7 days.
All patients were invited to the clinic the next day, and biomicroscopic slit lamp examinations were performed and visual acuity and IOP values were measured. Earliest color fundus images for vessel diameter measurements were obtained 1 week after injections, as previously suggested.17
Patients were examined at 1 week and 1 month in the post-injection period. The same parameters were studied by performing detailed ophthalmological examinations in all visits, and OCT images and IOP measurements were repeated. Topical glaucoma treatment was started in patients with IOP measuring over 21 mm Hg. After assessment of IOP, pupils were dilated and color fundus photographs were obtained. Identified complications were recorded.
Measurement system of retinal vascular caliber
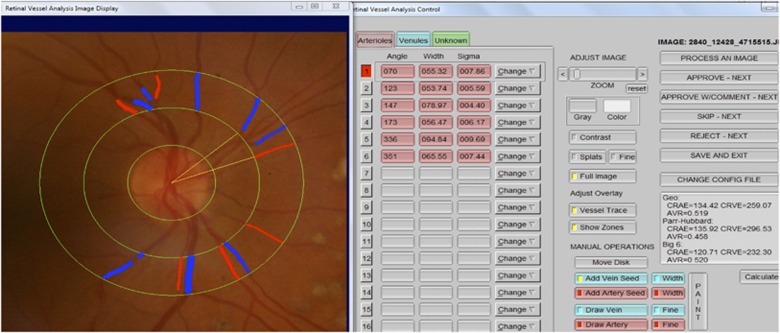
IVAN (with permission from Knudtson MD, University of Wisconsin, Madison, WI, USA) is a semi-automated system used to measure the width of retinal vessels using a digital retinal image (Nicole J. Ferrier, College of Engineering, Fundus Photography Reading Center, University of Wisconsin, Madison, WI, USA).18, 19 Three concentric rings centered on the optical disc were placed to determine the vascular measurement field, and thus two zones were formed. Zone A was defined as the area from the disc margin to half-disc diameter, and zone B was defined as the area from half-disc diameter to 1 disc diameter (from the disc margin). All retinal vessel diameters were measured by another masked researcher through zone B. Central retinal artery equivalent (CRAE) and central retinal vessel equivalent (CRVE) measurements were performed using the formula created by Hubbard19 and later revised by Knudtson20 (Figure 1).
Figure 1.
Digitized retinal photograph. Zone A is a half-disc diameter from the optic disc margin and Zone B is a half-disc to one and a half-disc diameter from the optic disc margin. Retinal vessel diameter measurements are performed by IVAN in Zone B.
Before obtaining the study parameters, blood pressure, pulse rate, and respiration rate of the participants were measured after 5 min resting in a sitting position during every visit. Fundus imaging and IOP assessments were postponed unless the patients had blood pressure, pulse rate, and respiration rate values similar to those noted at the baseline.
Statistical analysis
To assess the results obtained in this study, SPSS (Statistical Package for Social Sciences) for Windows 18.0 software was used for statistical analysis. Continuous variables were expressed as ‘mean±SD.’ Eligibility of all quantitative variables to normal distribution was assessed by the Kolmogorov–Smirnov test for statistical analysis. Because data conformed to a normal distribution, a ‘repeated variance analysis’ test was used to compare the many repeated measurements within the groups. The Bonferroni test was used as a post hoc test. Intergroup analyses were performed using an ‘independent samples t-test.’ Pearson’s test was used to discover possible linear correlations between vessel size parameters and CMT as well as IOP. Fisher’s exact test was used to determine possible nonrandom associations between two categorical variables. P<0.05 was considered to represent statistical significance.
Results
IVT group
There were 30 patients, consisting of 15 (50%) males and 15 (50%) females in the IVT group. Eighteen (60%) eyes were phakic and 12 (40%) eyes were pseudophakic in the study group, and 19 (63.3%) eyes were phakic and 11 (36.3%) eyes were pseudophakic in the non-injected fellow eyes (control group).
Baseline BCVA (0.86±0.55, logMAR) improved immediately to 0.76±0.52 at 1 week and to 0.71±0.51 at 1 month (P=0.009), and pre-injection CMT (507.87±124.87 μ) decreased to 323.27±76.17 μ at 1 week and to 354.80±108.48 μ at 1 month after the injection (P<0.001).
Baseline CRAE, CRVE and AVR levels in the study group were similar to those of the control group (P=0.976, P=0.969 and P=0.929). IVT injection caused a significant decrease of baseline CRAE and CRVE and unaltered AVR values throughout the study period, whereas no alteration of these parameters was observed in the non-injected fellow eyes (Table 1).
Table 1. Alteration of study parameters within the intravitreal triamcinolone-injected eyes (IVT study group, n=30) and the non-injected fellow eyes (control group, n=30).
| Groups | Parameters | Pre-injection | 1 week | 1 month | P-value |
|---|---|---|---|---|---|
| CRAE | 147.07±22.33 | 141.03±21.61 | 139.43±19.17 | <0.001a | |
| IVT | CRVE | 209.61±26.84 | 198.85±28.32 | 198.49±27.20 | <0.001a |
| AVR | 0.70±0.08 | 0.72±0.08 | 0.71±0.08 | 0.66 | |
| CRAE | 146.91±18.84 | 148.61±20.12 | 147.54±21.79 | 0.72 | |
| Control | CRVE | 209.90±30.58 | 209.02±30.96 | 209.88±32.72 | 0.99 |
| AVR | 0.71±0.07 | 0.71±0.09 | 0.72±0.09 | 0.30 |
Abbreviations: AVR, artery to vein ratio; CRAE, central retinal artery equivalent (μ); CRVE, central retinal vein equivalent (μ); IVT, intravitreal triamcinolone.
Statistically significant with analysis of variance test, data were expressed as mean±SD.
After IVT injection, 21 mm Hg or higher IOP was observed in 3 eyes at 1 week and 4 eyes at 1 month. Baseline IOP (16.6±2.9 mm Hg) elevated to 18.5±3.4 mm Hg at 1 week and 18.9±3.6 mm Hg at 1 month (P<0.001) while no significant change of baseline IOP (16.8±2.8 mm Hg) was observed in the control group during the study period (16.8±3.2 mm Hg and 15.57±3.0 mm Hg, P=0.061).
In IVT-injected eyes, CRVE showed a positive correlation with CMT at 1 week (r=0.50, P=0.005) and at 1 month (r=0.37, P=0.045). At 1 week, CRVE was negatively correlated with IOP (r=−0.39, P=0.034).
STT group
STT group data were obtained from 8 (26.7%) males and 22 (73.3%) females. Eighteen (60%) eyes were phakic while 12 (40%) were pseudophakic in the study group, and 17 (56.7%) phakic eyes and 13 (43.3%) pseudophakic eyes were present in the non-injected fellow eyes (control group).
Baseline BCVA (0.66±0.55) improved to 0.59±0.41 at 1 week and 0.49±0.35 at 1 month (P=0.005), whereas pre-injection CMT (443.56±108.56 μ) decreased to 393.63±88.46 μ at 1 week and to 374.80±95.97 μ at 1 month after the injection (P<0.001).
Baseline CRAE, CRVE, and AVR in the study group was similar to that of the control group (P=0.296, P=0.732, and P=0.289, respectively). STT injection caused a significant decrease in baseline CRAE and CRVE while AVR values were unchanged throughout the study period. No alteration of these parameters was observed in the non-injected fellow eyes (Table 2).
Table 2. Alteration of study parameters within the subtenon triamcinolone-injected eyes (STT study group, n=30) and the non-injected fellow eyes (control group, n=30).
| Groups | Parameters | Pre-injection | 1 week | 1 month | P-value |
|---|---|---|---|---|---|
| CRAE | 152.18±18.10 | 149.49±20.56 | 147.47±20.53 | 0.017a | |
| STT | CRVE | 215.60±32.05 | 208.69±36.87 | 207.25±35.34 | 0.003a |
| AVR | 0.715±0.095 | 0.730±0.099 | 0.726±0.105 | 0.196 | |
| CRAE | 146.24±25.02 | 152.50±23.40 | 148.68±20.43 | 0.768 | |
| Control | CRVE | 212.87±29.42 | 217.10±33.11 | 214.11±31.36 | 0.283 |
| AVR | 0.689±0.090 | 0.692±0.076 | 0.699±0.084 | 0.394 |
Abbreviations: AVR, artery to vein ratio; CRAE, central retinal artery equivalent (μ); CRVE: central retinal vein equivalent (μ); STT, subtenon triamcinolone.
Statistical analysis was calculated by analysis of variance test, data were expressed as mean±SD.
After STT injection, 21 mm Hg or higher IOP was observed in 5 eyes at 1 week and 2 eyes at 1 month. Baseline IOP (15.2±3.2 mm Hg) elevated to 18.7±3.9 mm Hg at 1 week and 17.7±3.1 mm Hg at 1 month (P=0.02) while no significant change of baseline IOP (14.7±3.2 mm Hg) was observed in the control group during the study period (15.4±3.5 mm Hg and 15.6±3.9 mm Hg, P=0.765).
In STT-injected eyes, AVR was negatively correlated with CMT at 1 week (r=−0.37, P=0.044). At 1 month, CRAE was positively correlated with IOP (r=0.37, P=0.047).
IVT vs STT
There were no differences in the values of CRAE, CRVE, and AVR in the IVT and STT study groups at baseline, 1 week, and 1 month after injection (P>0.12; Table 3). After IVT and STT injections, there were no differences between topical glaucoma medication users at 1 week (3 eyes vs 5 eyes respectively, P=0.706) and at 1 month (4 eyes vs 2 eyes respectively, P=0.433).
Table 3. Alteration of study parameters comparing the subtenon triamcinolone-injected eyes (STT study group, n=30) and the intravitreal triamcinolone-injected eyes (IVT study group, n=30).
| Parameters | IVT | STT | P-value |
|---|---|---|---|
| CRAE | |||
| Pre-injection | 147.07±22.33 | 152.18±18.10 | 0.335 |
| 1 week | 141.03±21.61 | 149.49±20.56 | 0.126 |
| 1 month | 139.43±19.17 | 147.47±20.53 | 0.123 |
| CRVE | |||
| Pre-injection | 209.61±26.84 | 215.60±32.05 | 0.436 |
| 1 week | 198.85±28.32 | 208.69±36.87 | 0.251 |
| 1 month | 198.49±27.20 | 207.25±35.34 | 0.286 |
| AVR | |||
| Pre-injection | 0.703±0.083 | 0.715±0.095 | 0.631 |
| 1 week | 0.716±0.080 | 0.730±0.099 | 0.569 |
| 1 month | 0.707±0.082 | 0.726±0.105 | 0.450 |
Abbreviations: AVR, artery to vein ratio; CRAE, central retinal artery equivalent (μ); CRVE, central retinal vein equivalent (μ); IVT, intravitreal triamcinolone; STT, subtenon triamcinolone. Statistical analysis was calculated by analysis of independent samples t-test, data were expressed as mean±SD.
Discussion
Vasodilatation occurs in response to inflammation and plays an especially important role in DME formation.6 There are studies indicating that the retinal vein diameters of patients with diabetes are larger than those of individuals with normal fasting glucose.8, 9 In addition, some studies have shown that both artery and vein diameters are larger in patients with diabetes.7, 8, 9, 10, 11, 12 The biological mechanism underlying the relationship of DM with the enlargement of retinal vein diameters is not yet clear, but it is thought that inflammation and endothelial dysfunction are associated with the enlargement of retinal vein diameters in diabetic retinopathy.10, 11
Corticosteroids are widely used for the treatment of DME. For this purpose, IVT, and STT injections are performed. Similar clinical results have been obtained with both administrations,21, 22, 23, 24 although there is some evidence from a systematic review and meta-analysis showing that IVT injection is more effective for short-term management of refractory diffuse DME than STT injection.25
The mechanism of action of intravitreal triamcinolone is proposed to be changes in retinal vessel diameters and perfusion regulations.26, 27, 28, 29, 30, 31 Corticosteroids are effective in DME due to their anti-inflammatory effects, by reducing VEGF levels, and their stabilization effects on the blood–retina barrier.14 It is thought that VEGF carries out its vascular effects via nitric oxide, which is a potent vasodilator.32 Nitric oxide plays an important role in retinal arteriolar tone and retinal blood flow autoregulation via its vasodilator effect.33
Vinten et al28 indicated that a single-dose 4 mg/0.1 ml IVT injection demonstrated a statistically significant reduction in both retinal arteriole and venule diameters in patients with DME 1 week after the injection. Our study showed significant vasoconstriction of retinal vessels even with half (2 mg) of the drug dose used in their study. Similar to our study’s design, Vinten et al28 applied IVT injections to 1 eye of 7 patients and considered the other (non-injected) eye as the control group. In addition, the authors also evaluated blood–retina barrier permeability: there was a significant decrease in the permeability of the blood–retina barrier consistent with the decrease in the diameters of the retinal arteries and veins. Non-injected fellow eyes showed no alteration.28 Sander et al29 reported that the increase in the permeability of the blood–retina barrier is coherent with retinal thickness and retinal vessel diameter changes in patients with DME.
Wickremasinghe et al30 demonstrated a reduction in the diameters of both arterioles and venules 3 months after a single-dose 4 mg/0.1 ml IVT injection in patients with refractory DME. There were 13 eyes in the injection group and 15 eyes in the placebo group, and the significant changes obtained in the injection group were not observed in the placebo group.
Some studies have indicated that injection of IVT has a significant effect on ocular hemodynamics. Çekiç et al26 observed a significant reduction in end-diastolic arterial blood velocity of posterior ciliary arteries in patients with DME 1 month after the injection of IVT. However, they reported that these changes returned to baseline values within 3 months. Anayol et al34 indicated that peak systolic velocity increased in ophthalmic arteries, whereas it decreased in central retinal arteries in patients with DME 1 month after IVT injection. Shahin et al35 demonstrated that IVT resulted in a decrease of end-diastolic velocity in ophthalmic arteries and central retinal arteries, and a reduction of peak systolic velocity in posterior ciliary arteries at 1 day and 1 week after injection.
Although some studies have examined the effects of IVT on retinal vessel calibers, to our knowledge, no study has yet documented the effect of STT on retinal vessel size or the comparative effects of IVT vs STT injections on retinal vessel diameters in diabetics.
In our study, statistically significant reductions in CRAE and CRVE values were obtained following both IVT and STT injections. We think that correlation of these values with reductions in CMT after IVT and STT injections will be useful in understanding DME and the action mechanism of triamcinolone. IVT and STT injections were proposed to lead to a decline in vascular permeability and leakage as a result of a decrease in retinal vessel diameters.36 Moreover, it is known that triamcinolone effects vascular permeability via VEGF inhibition, in addition to its anti-inflammatory effect.37 In our study, some correlations were detected between vessel size and CMT after triamcinolone injection. We believe that the significant constriction of retinal vessels with IVT and STT probably contributed to certain clinical outcomes, such as the decrease in CMT, which also yielded an improvement in BCVA. However, it should be kept in mind that vessel size alteration is not the only cause for CMT: variable metabolic and local factors act in a concerted manner in the etiopathogenesis of DME.
It has been reported that an IOP increase was observed in 20–80% of patients injected with IVT.37, 38, 39, 40 An IOP increase has also been indicated after STT injection.41 In the study conducted by Byun and Park42 to evaluate the complications and safety profile of STT, 18 of 159 eyes required glaucoma medication due to increased IOP after injection, but the IOP in all eyes was well controlled with the use of glaucoma medication. High IOP (26 mm Hg) was observed in only 1of 22 eyes in the study conducted by Özkurt et al,43 and it was controllable by medication. Kawamura et al44 demonstrated that repeated injections of STT increase the incidence of IOP elevation. Moreover, the elevation of IOP is more pronounced after IVT injections compared to STT injections.25 Similar IOP elevation was observed following both forms of triamcinolone injection in our study. However, there was no difference in the number of topical glaucoma medication users after IVT and STT injections during the study period. Normotensive values were achieved without the need for glaucoma surgery.
Retinal vessel diameters are also important in terms of glaucoma. There are some reports that have demonstrated a relationship between glaucoma and retinal vessel calibers.45, 46, 47 Retinal vessel diameter studies may also allow us to better understand the behavioral patterns of vessel calibers at various IOP levels after triamcinolone administrations. Our study revealed a negative correlation between IOP and CRVE in IVT-injected eyes at 1 week, and a positive correlation between IOP and CRAE after STT-injected eyes at 1 month.
The current study revealed relatively short-term effects of IVT or STT injection on retinal vessel calibers. Further studies to examine drug effects on vessel size for longer periods of time, with a much higher number of participants and also preferably accompanied by the detection of retinal hemodynamics, are warranted.
In summary, similar anatomic and functional improvements occurred after both IVT and STT administrations in diabetic eyes. Significant vasoconstriction in the retinal vessels was demonstrated following either method of triamcinolone injection. This study also revealed some correlations between retinal vessel size and central macular thickness as well as intraocular pressure values following triamcinolone injection.
Acknowledgments
Partly presented at the 16th Afro Asian Congress of Ophthalmology and the 5th Mediterranean Retina Meeting, Istanbul, Turkey, 13–16 June, 2012, and the 14th ESASO Retina Academy, Istanbul, Turkey, 13–15 November, 2014.
Footnotes
The authors declare no conflict of interest.
References
- Klein R, Klein BEK, Moss SE, Davis MD. The Wisconsin epidemiologic study of diabetic retinopathy, IV:diabetic macular adema. Ophthalmology 1984; 91: 1464–1474. [DOI] [PubMed] [Google Scholar]
- Moss SE, Klein R, Klein BEK. The incidence of vision loss in a diabetic population. Ophthalmology 1988; 95: 1340–1348. [DOI] [PubMed] [Google Scholar]
- Kang SW, Sa HS, Cho HY, Kim JI. Macular grid photocoagulation after intravitreal triamcinolone acetonide for diffuse diabetic macular edema. Arch Ophthalmol 2006; 124: 653–658. [DOI] [PubMed] [Google Scholar]
- Martidis A, Duker JS, Greenberg PB, Rogers AH, Puliafito CA, Reichel E et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology 2002; 109(5): 920–927. [DOI] [PubMed] [Google Scholar]
- Kang SW, Park CY, Ham DI. The correlation between fluorescein angiographic and optical coherence tomographic features in clinically significant diabetic macular edema. Am J Ophthalmol 2004; 137: 313–322. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Knudtson MD, Wong TY, Tsai MY. Are inflammatory factors related to retinal vessel caliber? The Beaver Dam Eye Study. Arch Ophthalmol 2006; 124(1): 87–94. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Wang JJ, Sharrett AR, Islam FM, Klein R, Klein BE et al. Relationship of retinal vascular caliber with diabetes and retinopathy: the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2008; 31(3): 544–549. [DOI] [PubMed] [Google Scholar]
- Kifley A, Wang JJ, Cugati S, Wong TY, Mitchell P. Retinal vascular caliber and the long-term risk of diabetes and impaired fasting glucose: the Blue Mountains Eye Study. Microcirculation 2008; 15(5): 373–377. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Moss SE, Wong TY, Sharrett AR. Retinal vascular caliber in persons with type 2 diabetes: the Wisconsin Epidemiological Study of Diabetic Retinopathy: XX. Ophthalmology 2006; 113(9): 1488–1498. [DOI] [PubMed] [Google Scholar]
- Patel V, Rassam S, Newsom R, Wiek J, Kohner E. Retinal blood flow in diabetic retinopathy. BMJ 1992; 305(6855): 678–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero AE. Metabolic and vascular abnormalities in subjects at risk for type 2 diabetes: the early start of a dangerous situation. Arch Med Res 2005; 36: 241–249. [DOI] [PubMed] [Google Scholar]
- Skovborg F, Nielsen AV, Lauritzen E, Hartkopp O. Diameters of the retinal vessels in diabetic and normal subject. Diabetes 1969; 18: 292–298. [DOI] [PubMed] [Google Scholar]
- Grunwald JE, Brucker AJ, Schwartz SS, Braunstein SN, Baker L, Petrig BL et al. Diabetic glycemic control and retinal blood flow. Diabetes 1990; 39(5): 602–607. [DOI] [PubMed] [Google Scholar]
- Shimura M, Nakazawa T, Yasuda K, Shiono T, Iida T, Sakamoto T et al. Comparative therapy evaluation of intravitreal bevacizumab and triamcinolone acetonide on persistent diffuse diabetic macular edema. Am J Ophthalmol 2008; 145(5): 854–861. [DOI] [PubMed] [Google Scholar]
- Joussen AM, Poulaki V, Qin W, Kirchhof B, Mitsiades N, Wiegand SJ et al. Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. Am J Pathol 2002; 160(2): 501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenborn CA, Adams PE. Health behaviors of adults: United States, 2005–2007. Vital Health Stat 10 2010; 245: 1–132. [PubMed] [Google Scholar]
- Çekiç O, Bardak Y. Oculometric alterations following intravitreal triamcinolone injection. Br J Ophthalmol 2010; 94: 1408–1409. [DOI] [PubMed] [Google Scholar]
- Wong TY, Knudtson MD, Klein R, Klein BE, Meuer SM, Hubbard LD. Computer-assisted measurement of retinal vessel diameters in the Beaver Dam Eye Study: methodology, correlation between eyes, and effect of refractive errors. Ophthalmology 2004; 111(6): 1183–1190. [DOI] [PubMed] [Google Scholar]
- Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology 1999; 106(12): 2269–2280. [DOI] [PubMed] [Google Scholar]
- Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res 2003; 27(3): 143–149. [DOI] [PubMed] [Google Scholar]
- Cardillo JA, Melo Jr LA, Costa RA, Skaf M, Belfort Jr R, Souza-Filho AA et al. Comparison of intravitreal versus posterior sub-Tenon’s capsule injection of triamcinolone acetonide for diffuse diabetic macular edema. Ophthalmology 2005; 112(9): 1557–1563. [DOI] [PubMed] [Google Scholar]
- Cellini M, Pazzaglia A, Zamparini E, Leonetti P, Campos EC. Intravitreal vs. subtenon triamcinolone acetonide for the treatment of diabetic cystoid macular edema. BMC Ophthalmol 2008; 8: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdek S, Bahçeci UA, Gürelik G, Hasanreisoğlu B. Posterior subtenon and intravitreal triamcinolone acetonide for diabetic macular edema. J Diabetes Complications 2006; 20: 246–251. [DOI] [PubMed] [Google Scholar]
- Bonini-Filho MA, Jorge R, Barbosa JC, Calucci D, Cardillo JA, Costa RA. Intravitreal injection versus sub-Tenon’s infusion of triamcinolone acetonide for refractory diabetic macular edema: a randomized clinical trial. Invest Ophthalmol Vis Sci 2005; 46(10): 3845–3849. [DOI] [PubMed] [Google Scholar]
- Qi HP, Bi S, Wei SQ, Cui H, Zhao JB. Intravitreal versus subtenon triamcinolone acetonide injection for diabetic macular edema: a systematic review and meta-analysis. Curr Eye Res 2012; 37(12): 1136–1147. [DOI] [PubMed] [Google Scholar]
- Cekiç O, Bardak Y, Tiğ SU, Demirkol A, Ekim MM, Altintaş O et al. Hemodynamic response to intravitreal triamcinolone in eyes with macular edema: intravitreal triamcinolone and ocular blood flow. Int Ophthalmol 2007; 27(5): 313–319. [DOI] [PubMed] [Google Scholar]
- Mawatari Y, Koga T, Inumaru J, Hirata A, Fukushima M, Tanihara H. The effect of subtenon triamcinolone acetonide injection for diabetic macular edema on retinal and choroidal circulation. Am J Ophthalmol 2005; 140(5): 948–949. [DOI] [PubMed] [Google Scholar]
- Vinten M, Larsen M, Lund-Andersen H, Sander B, La Cour M. Short-term effects of intravitreal triamcinolone on retinal vascular leakage and trunk vessel diameters in diabetic macular oedema. Acta Ophthalmol Scand 2007; 85(1): 21–26. [DOI] [PubMed] [Google Scholar]
- Sander B, Thornit DN, Colmorn L, Strøm C, Girach A, Hubbard LD et al. Progression of diabetic macular edema: correlation with blood retinal barrier permeability, retinal thickness, and retinal vessel diameter. Invest Ophthalmol Vis Sci 2007; 48(9): 3983–3987. [DOI] [PubMed] [Google Scholar]
- Wickremasinghe SS, Rogers SL, Gillies MC, Zhu M, Wong TY. Retinal vascular caliber changes after intravitreal triamcinolone treatment for diabetic macular edema. Invest Ophthalmol Vis Sci 2008; 49(11): 4707–4711. [DOI] [PubMed] [Google Scholar]
- Cekiç O, Bardak Y, Tiğ US, Yildizoğlu U, Bardak H. Quantitative evaluation of reduction of plaque-like hard exudates in diabetic macular edema after intravitreal triamcinolone injection. Int Ophthalmol 2008; 28(2): 95–99. [DOI] [PubMed] [Google Scholar]
- Tilton RG, Chang KC, LeJeune WS, Stephan CC, Brock TA, Williamson JR. Role for nitric oxide in the hyperpermeability and hemodynamic changes induced by intravenous VEGF. Invest Ophthalmol Vis Sci 1999; 40(3): 689–696. [PubMed] [Google Scholar]
- Pournaras CJ, Rungger-Brändle E, Riva CE, Hardarson SH, Stefansson E. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res 2008; 27(3): 284–330. [DOI] [PubMed] [Google Scholar]
- Anayol MA, Toklu Y, Kamberoglu EA, Raza S, Arifoglu HB, Simavli H et al. Short-term effects of intravitreal triamcinolone acetonide injection on ocular blood flow evaluated with color Doppler ultrasonography. Int J Ophthalmol 2014; 7(5): 811–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahin M, Gad MA, Hamza W. Impact of intravitreal triamcinolone acetonide versus intravitreal bevacizumab on retrobulbar hemodynamic in patients with diabetic macular edema. Cutan Ocul Toxicol 2014; 33: 49–53. [DOI] [PubMed] [Google Scholar]
- Nagel E, Vilser W. Autoregulative behavior of retinal arteries and veins during changes of perfusion pressure: a clinical study. Graefes Arch Clin Exp Ophthalmol 2004; 242: 13–17. [DOI] [PubMed] [Google Scholar]
- Perretti M, Ahluwalia A. The microcirculation and inflammation: site of action for glucocorticoids. Microcirculation 2000; 7: 147–161. [PubMed] [Google Scholar]
- Wingate RJ, Beaumont PE. Intravitreal triamcinolone and elevated intraocular pressure. Aust N Z J Ophthalmol 1999; 27: 431–432. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Kreissig I, Degenring RF. Intraocular pressure after intravitreal triamcinolone acetonide. Br J Ophthalmol 2003; 87: 24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithen LM, Ober MD, Maranan L, Spaide RF. Intravitreal triamcinolone acetonide and intraocular pressure. Am J Ophthalmol 2004; 138: 740–743. [DOI] [PubMed] [Google Scholar]
- Mueller AJ, Jian G, Banker AS. The effect of deep posterior subtenon injection of corticosteroids on intraocular pressure. Am J Ophthalmol 1998; 125: 158–163. [DOI] [PubMed] [Google Scholar]
- Byun YS, Park YH. Complications and safety profile of posterior subtenon injection of triamcinolone acetonide. J Ocul Pharmacol Ther 2009; 25: 159–162. [DOI] [PubMed] [Google Scholar]
- Ozkurt YB, Akkaya S, Aksoy S, Evciman T, Haboğlu M. Posterior Subtenon’s capsule triamcinolone acetonide injection for the treatment of diabetic macular edema. J Ocul Pharmacol Ther 2015; 31(8): 455–460. [DOI] [PubMed] [Google Scholar]
- Kawamura R, Inoue M, Shinoda H, Shinoda K, Itoh Y, Ishida S et al. Incidence of increased intraocular pressure after subtenon injection of triamcinolone acetonide. J Ocul Pharmacol Ther 2011; 27(3): 299–304. [DOI] [PubMed] [Google Scholar]
- Lee JY, Yoo C, Park JH, Kim YY. Retinal vessel diameter in young patients with open-angle glaucoma: comparison between high-tension and normal-tension glaucoma. Acta Ophthalmol 2012; 90: e570–e571. [DOI] [PubMed] [Google Scholar]
- Gao J, Liang Y, Wang F, Shen R, Wong T, Peng Y et al. Retinal vessels change in primary angle-closure glaucoma: the Handan Eye Study. Sci Rep 2015; 5: 9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M, Yoo C, Kim SW, Kim YY. Retinal vessel diameter, retinal nerve fiber layer thickness, and intraocular pressure in Korean patients with normal-tension glaucoma. Am J Ophthalmol 2011; 151: 100–105. [DOI] [PubMed] [Google Scholar]