Abstract
We present the case of a 54-year-old man who had crescendo angina during nitrate therapy. Selective coronary angiograms showed no atherosclerotic lesions, but did show plexuses of intramural vessels that connected the distal thirds of the left and right coronary systems with the left ventricle. The cause of our patient's increased myocardial ischemia during nitrate therapy may have been the coronary “steal” phenomenon.
Key words: Angina pectoris, arteriovenous fistula/physiopathology, myocardial ischemia
Coronary artery fistula is the most common form of congenital anomaly of the coronary arteries. Drainage usually occurs into the right ventricle, the right atrium, the pulmonary artery, the coronary sinus, the left atrium, the left ventricle, or the superior vena cava.1 Approximately half of all patients with coronary artery fistulae remain asymptomatic; the other half develop congestive heart failure, infective endocarditis, myocardial ischemia induced by a coronary “steal” phenomenon, or rupture of an aneurysmal fistula.1
Multiple arterio–systemic fistulae, arising from all 3 major coronary arteries and draining into the left ventricle, are rare, and the clinical and hemodynamic sequelae are incompletely understood.
Case Report
In January 2004, a 54-year-old man presented with unstable angina. For more than 2 years before, he had experienced stable angina, which had worsened in recent weeks. Electrocardiography showed normal sinus rhythm and ST segment depression in V4–V6. Two-dimensional echocardiography showed no evidence of hypertrophy or other disorder. His medical history included hypertension but no known coronary artery disease, diabetes mellitus, hyperlipidemia, or smoking. Serum creatine kinase-MB and troponin T levels were normal. There was a mild diastolic murmur along the left sternal border, after the 2nd heart sound.
The patient was started on subcutaneous low-molecular-weight heparin, β-blockers, aspirin (300 mg), and parenteral nitrate. Despite intensive anti-ischemic therapy, his angina worsened. The patient therefore underwent cardiac catheterization.
Selective coronary angiography showed no atherosclerotic lesions; however, a heavy stream of contrast agent entered the left ventricle via an apparent plexus of intramural vessels, from the distal third of both the left and right coronary arterial systems. The left ventricle (LV) stained so thoroughly that all LV walls and borders were apparent throughout 3 cardiac cycles (Fig. 1).
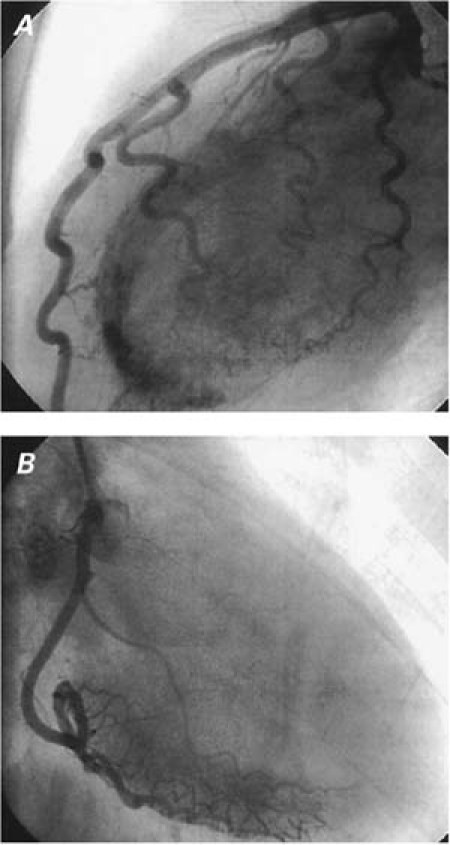
Fig. 1 A) Angiogram (left lateral projection) shows the multiple fistulae originating from the left coronary system. B) Angiogram (right anterior oblique projection) shows the multiple fistulae originating from the right coronary system.
Stress/rest Tc 99m sestamibi myocardial perfusion single-photon emission tomography and stress electrocardiography (ECG) were performed after coronary angiography. Both tests showed stress-induced myocardial ischemia. At stress ECG, ischemic alterations were recorded at the 1st minute of the 2nd stage of the Bruce protocol. The single-photon emission tomography for the evaluation of stress/rest perfusion detected a reversible perfusion defect of the posterolateral, lateral, and anterior walls, thus confirming the hemodynamic importance of the flow through multiple fistulae during stress cycloergometric (measurement of work cycles) testing (Fig. 2).
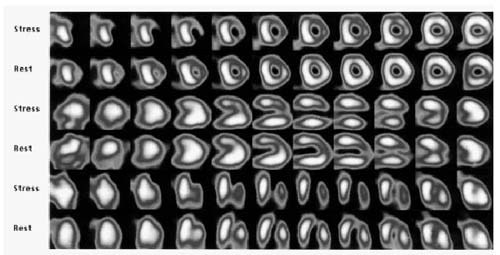
Fig. 2 Myocardial perfusion images with Tc 99m sestamibi shows stress-induced reversible ischemia in the anterior and lateral walls.
We inferred that the pathophysiologic mechanism of this patient's ischemia was myocardial steal, or reduction of blood flow at points distal to the fistulae. The mechanism is probably related to the diastolic pressure gradient caused by run-off from the coronary vasculature to a low-pressure receiving cavity. If the fistulae are large or multiple, as observed in our patient, we suggest that intracoronary diastolic perfusion pressure may diminish progressively. The myocardium beyond the origin of the multiple fistulae is at risk of ischemia, which is most frequently evident in association with exercise or other physical activity (increased myocardial oxygen demand). We found reversible perfusion defects in multiple myocardial areas by means of stress/rest Tc 99m sestamibi myocardial perfusion single-photon emission tomography. The correspondence between the sites of the coronary artery where the fistulae originated, identified by coronary angiography, and the sites of the reversible perfusion defects, identified by myocardial perfusion scintigraphy, indicated to us that anomalous flow through the fistulae was the likely cause of the myocardial ischemia.
Because the coronary artery–cardiac chamber communications were multiple and diffuse, neither surgery nor transcatheter coil occlusion was considered in this case. In light of the worsening of angina under nitrate therapy, it is highly probable that nitrate therapy decreased coronary perfusion pressure and increased coronary steal in our patient. Inadequate angina control with β-blocker therapy showed us that such therapy is not enough to control ischemia in cases of multiple coronary artery fistulae. Because the patient did not comply, we were unable to perform follow-up myocardial perfusion scintigraphy.
Discussion
The pathophysiologic importance of a coronary arterial fistula is related to the amount of blood that flows through the communication and to the chamber or vessel into which the fistula drains; myocardial ischemia is believed to be the result of this fistulous bypass.2 If the fistulous pathway drains into the left ventricle, the hemodynamic results are similar to those of aortic regurgitation.3
The main presenting symptom of our patient was chest pain, with ECG changes indicative of ischemia. Symptoms and signs of coronary ischemia increased with nitrate therapy, and his angina developed a crescendo pattern. We think that coronary artery-to-left ventricle fistulae caused coronary steal and that nitrates increased the steal. Because angina developed in advanced age in our patient, we suspect that the fistulae to the left ventricle increased in number and size as the patient aged.
To the best of our knowledge, generalized arterio– systemic fistulae originating from all 3 major coronary vessels has been reported in only 19 cases in the literature. More than 14,000 selective coronary angiographic studies have been performed from 1997 to 2004 in our center, and this was the 1st case of multiple arterio–systemic fistulae that we have seen. The largest series was reported by Stierle and colleagues.4 They identified 8 cases of multiple arterio–systemic fistulae in 7,262 consecutive patients who underwent coronary angiography. This rate (8/7,202) is considerably higher than ours.
A necropsy in a case reported by Black and co-authors5 showed abnormal intramyocardial vessels communicating with the left ventricular lumen. So heavy was the stream of contrast solution into the left ventricle and so apparent was the plexus of contrast-filled arteries connecting with the left ventricle that we thought our patient might have a primary abnormality of the microcirculation.
There are 6 reported cases of multiple coronary artery–systematic fistulae with apical hypertrophic cardiomyopathy.6–9 Our patient did not have hypertrophic cardiomyopathy. We believe it is difficult to ascertain a causal relationship between these 2 anomalies. Considering the other reported cases without hypertrophy, such as ours, one can hardly conclude that local ischemia causes reactive myocardial hypertrophy.
In conclusion, multiple coronary–systematic fistulae are a rare cause of myocardial ischemia via the coronary steal phenomenon. The anatomical types of fistulous connections and the severity of leakage vary, and this may alter the myocardial ischemia level and clinical symptoms. Nitrate therapy probably increases ischemia by means of increased leakage to the left ventricle.
Footnotes
Address for reprints: Gulumser Heper, MD, Onur sokak 44/6, Maltepe 06570 Ankara, Turkey. E-mail: heperg@hotmail.com
References
- 1.Levin DC, Fellows KE, Abrams HL. Hemodynamically significant primary anomalies of the coronary arteries. Angiographic aspects. Circulation 1978;58:25–34. [DOI] [PubMed]
- 2.Kinard SA. Hypoplasia of the coronary sinus with coronary venous drainage into the left ventricle by way of the Thebesian system. Chest 1975;68:384–5. [DOI] [PubMed]
- 3.Reddy K, Gupta M, Hamby RI. Multiple coronary arteriosystemic fistulas. Am J Cardiol 1974;33:304–6. [DOI] [PubMed]
- 4.Stierle U, Giannitsis E, Sheikhzadeh A, Potratz J. Myocardial ischemia in generalized coronary artery-left ventricular microfistulae. Int J Cardiol 1998;63:47–52. [DOI] [PubMed]
- 5.Black IW, Loo CK, Allan RM. Multiple coronary artery-left ventricular fistulae: clinical, angiographic, and pathologic findings. Cathet Cardiovasc Diagn 1991;23:133–5. [DOI] [PubMed]
- 6.Lisanti P, Serino W, Petrone M. Multiple coronary artery-left ventricle fistulas in a patient with apical hypertrophic cardiomyopathy: an unusual cause of angina pectoris [in Italian]. G Ital Cardiol 1988;18:858–61. [PubMed]
- 7.Monmeneu JV, Bodi V, Sanchis J, Chorro FJ, Llopis R, Insa L, Lopez Merino V. Apical hypertrophic myocardiopathy and multiple fistulae between the coronary vessels and the left ventricle [in Spanish]. Rev Esp Cardiol 1995;48:768–70. [PubMed]
- 8.Delarche N, Colle JP. Multiple left coronary-ventricular microfistula and apical hypertrophy [in French]. Arch Mal Coeur Vaiss 1993;86:75–8. [PubMed]
- 9.Hong GR, Choi SH, Kang SM, Lee MH, Rim SJ, Jang YS, Chung NS. Multiple coronary artery-left ventricular microfistulae in a patient with apical hypertrophic cardiomyopathy: a demonstration by transthoracic color Doppler echocardiography. Yonsei Med J 2003;44:710–4. [DOI] [PubMed]


