Abstract
Aims and Objectives:
The aims of the present study were to determine and compare the effects of different concentrations of Althaea officinalis extract, 0.2% chlorhexidine (CHX), and penicillin on Streptococcus mutans and Lactobacillus acidophilus in vitro.
Materials and Methods:
The laboratory study was done, for a period of 8 weeks. Minimum inhibitory concentration (MIC) in the test tube, minimum bactericidal concentration (MBC) in a plate culture medium, and growth inhibition zone diameter methods were used to compare the antibacterial effects of 0.2% CHX, penicillin, and different concentrations of A. officinalis root extract. The data were analyzed by SPSS version 24 using ANOVA and t-test analysis.
Results:
The results showed A. officinalis root extract had antibacterial effect, but significant differences were in MIC and MBC against L. acidophilus and S. mutans with penicillin and 0.2% CHX mouthwash. In addition, the mean growth inhibition zones of all the concentrations of the plant extract were less than that of the positive control group (P = 0.001). However, the difference in the maximum growth inhibition zone from that with the negative control group was significant. In addition, the antibacterial effect of the extract increased with an increase in its concentration. The extract exerted a greater antibacterial effect on S. mutans than on L. acidophilus. The plant polyphenols content is 3.7% which is equivalent to 29.93 g/ml.
Conclusion:
The root extract of A. officinalis exhibited antibacterial effects on S. mutans and L. acidophilus, but this effect was less than those of CHX mouthwash and penicillin. The antibacterial effect increased with an increase in the concentration of the extract.
KEYWORDS: Althaea officinalis, chlorhexidine mouthwash, Lactobacillus acidophilus, Streptococcus mutans
INTRODUCTION
Dental caries is the most prevalent chronic condition of childhood,[1] which is the result of interaction of various factors. Streptococcus mutans and Lactobacillus acidophilus are two main microorganisms responsible for dental caries.[2] The cariogenic properties of S. mutans and L. acidophilus include their capacity to adhere to tooth surfaces, to produce acid, and to survive in an acidic environment.[3] Plaque-related diseases tend to remain localized rather than exhibit invasion. The use of local antibacterial agents is more effective than the administration of systemic agents.[4]
Polyphenols are one of the main components of plants that are found in all the vegetables, flowers, and fruits.[5] The biologic properties of polyphenols include their antioxidative[6] and anti-inflammatory[7] effects. Advances in science and research have emphasized their potential activity in preventing orodental diseases including prevention of dental caries. It has been reported that polyphenols prevent adhesion of oral streptococci to tooth surfaces. However, further studies are necessary to evaluate the effect of polyphenols on dental caries.[8] Considering recent approaches in relation to the use of herbal medicines due to their compatibility with the human body, research has shown that polyphenols with a plant origin can be useful for patients contaminated with S. mutans and L. acidophilus.[9]
Althaea officinalis is one of the most important mucilaginous medicinal plants from Malvaceae family. This plant has been used in traditional medicine for the treatment of inflammatory reactions of the respiratory system and irritational coughing and to resolve inflammation including oral tissue inflammations and gingival abscesses.[10] In addition, researchers have evaluated the anti-inflammatory effects of the root extract of A. officinalis on inhibition of the activity of resistant periodontopathogens including Prevotella species and Porphyromonas gingivalis and Streptococcus pyogenes reporting that it clearly decreases the activity of these bacterial species.[11] Due to the fact that polyphenols exert inhibitory effects on the growth of bacteria. The antibacterial properties of medicinal marshmallow on various species, especially Gram-positive species can be attributed to its polyphenols compounds.[12] Investigation about cytotoxic activity and safety of extract showed that the liquid extracts had no significant cytotoxicity on human cells.[13]
Chlorhexidine (CHX) mouthwash is an antibacterial agent. However, it has some limitation for use in children under 7 years of age due to the possibility of swallowing and its unfavorable complications such as poisoning and tooth discoloration;[14] therefore, new dental caries prevention techniques have shifted toward the use of safer natural materials including probiotics[2] and polyphenols for young children with inhibitory effects on S. mutans and L. acidophilus.
The aim of the present in vitro study was to compare the antibacterial effects of different concentrations of ethanolic extract of A. officinalis root, 0.2% CHX, and penicillin on S. mutans and L. acidophilus.
MATERIALS AND METHODS
The laboratory study was performed in Microbiology laboratory in Kerman University of Iran, for a period of 8 weeks. The Institutional Ethical Committee of Shahed University (Tehran, Iran) approved the study (IR. Shahed.REC.1395.36).
For sample preparation, about 500 g roots of A. officinalis plant extract[15] gathered from Tehran suburb gardens in August 2016.
The percolation technique was used for the extraction process. The roots of A. officinalis were immersed in 70% ethanol and then extraction was carried out in a percolator. Extra solvent was evaporated with the use of a rotator and finally an extract with a concentration of 850 mL/mg was achieved.
To preparation of a standard culture medium of bacteria, the bacteria were procured from the Iranian Industrial Research Organization, Asre Enghelab Institute (Tehran, Iran), with the codes PTCC 1643 (L. acidophilus) and PTCC 1683 (S. mutans).
Liquid serial dilution technique was used to determine minimum inhibitory concentration (MIC). MIC is defined as the minimum concentration of the extract that completely inhibits visible bacterial growth compared to the negative control group. The liquid medium was Mueller-Hinton Broth) MERCK comp., Germany). A. officinalis root extracts were prepared at concentrations of 850 (100%), 637.5 (75%), 425 (50%), 212.5 (25%), 102 (12%), 51 (6%), and 25.5 (3%) mg/mL. A total of 50 μL of the microbial suspension, at 0.5 McFarland concentration, was inoculated into each test tube and then the test tubes were incubated at 37°C for 24 h. The lowest concentration at which no turbidity was observed was considered the MIC. A test tube with no microorganisms was considered as the negative control and a test tube with no extract was considered as the positive control.
To determine minimum bactericidal concentration (MBC), solid culture medium Mueller-Hinton broth was used. Fifty microliters were retrieved from the test tubes with consecutive dilutions with no turbidity and cultured on blood agar plates, followed by incubation for 24 h. The minimum concentration of the extract, in which no colony was detected and was considered as the MBC.
The MIC and MBC of CHX (Chlorhexidine, Iran Najo Comp, Tehran, Iran) and penicillin (Penicillin V, 500 mg, Cosar Pharmaceutical co., Tehran, Iran) too were determined in the same manner.
For disc diffusion, bacterial suspensions at 0.5 McFarland concentration were cultured on brain–heart agar plates. Then, discs impregnated with different concentrations of the extract were placed on the culture media at proper distances. In addition, discs containing 0.2 CHX and penicillin were used as positive controls. Dimethyl sulfoxide (DMSO (plate was used as a negative control. The plates were incubated at 37°C, and the growth inhibitory zone diameters were measured every 24 h up to 3 days. All test performed 3 times.
Amount of total phenolic contents (TPCs) was assessed using Folin-Ciocalteu reagent. Briefly, 50 mg of dry mass of A. officinalis extract was mixed with 0.5 mL of Folin-Ciocalteu reagent and 7.5 mL deionized water. The mixture was kept at room temperature for 10 min and then 1.5 mL of 20% NaCO (w/v) added. The mixture was heated on a water bath at 40°C for 20 min and then cooled in an ice bath. The absorbance was measured at 755 nm using a spectrophotometer (U-2001, Hitachi Instruments Inc., Tokyo, Japan). Amounts of TPC were calculated using gallic acid calibration curve within the range of 10–50 ppm. The results were expressed as gallic acid equivalents mg/100 g of dry plant matter. The results are reported on dry weight basis.
STATISTICAL ANALYSES
The data are presented as mean and analyzed by ANOVA. The t-test was used for two-by-two comparisons. P < 0.05 was considered statistically significant.
RESULTS
Table 1 presents the MIC and MBC values of A. officinalis extract for S. mutans and L. acidophilus. A. officinalis extract with MIC = 102 mg/mL had the bacteriostatic effect and at the concentration of MBC = 212.5 mg/mL showed the bactericidal effect on S. mutans. The MIC for L. acidophilus was observed at 212.5 mg/mL concentration, and MBC was 212.5 mg/mL and showed bacteriostatic and bactericidal effect, respectively.
Table 1.
The minimum inhibitory concentration and minimum bactericidal concentration values of Althaea officinalis extract, penicillin, and chlorhexidine for Streptococcus mutans and Lactobacillus acidophilus

As shown in Table 1, the MIC and MBC of the plant extract for these two bacterial species were different and higher from those of penicillin and 0.2% CHX.
By disc diffusion test, the results of compare three-time repeat on maximum inhibitory growth inhibition zone diameters for S. mutans and L. acidophilus showed no significant effect of repeat on the growth inhibition zones (S. mutans: F(2,60) = 0.119, P < 0.888 and L. acidophilus: F(2,60) = 0.484, P < 0.619).
Comparison between the effect of time (24, 48, 72 h) on maximum inhibitory growth inhibition zone diameters for S. mutans and L. acidophilus showed no significant effect (S. mutans: F(2,18) = 0.424, P < 0.661 and L. acidophilus: F(2,18) = 0.577, P < 0.571). One-way ANOVA was used for statistical test.
As shown in Table 2, comparison between inhibition zones of different concentration shows significant difference between the extract concentration with one-way ANOVA for both bacteria (S. mutans: F(6,14) = 99.795, P < 0.001 and L. acidophilus: F(6,14) = 4.866, P < 0.001). Since the result of ANOVA is significant, the LSD post hoc test must be reported.
Table 2.
Comparison of inhibitory growth zone diameter of Streptococcus mutans and Lactobacillus acidophilus with same concentrations of Althaea officinalis extract by independent t-test analysis
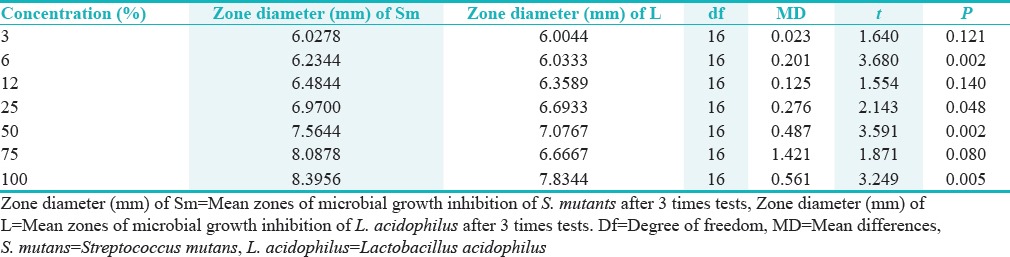
Comparisons of the mean growth inhibition zones for S. mutans at different concentrations of the extract showed no significant differences between the 3% and 6% (P < 0.121) and between 6% and 12% concentrations (P < 0.062). However, the differences between the other concentrations of the extract were significant (P < 0.001). In relation to L. acidophilus, there were significant differences between all the concentrations on one hand and the 100% concentration on the other hand (P < 0.001). Therefore, there was an increase in the inhibitory effect on both bacterial species with an increase in the concentration of the extract.
Comparison between the same extract concentration on S. mutans and L. acidophilus inhibition zone shows a significant difference between two tested bacteria with independent t-test (t = 2.43, df = 124, P < 0.017).
There were significant differences in the mean growth inhibition zones at similar concentrations of 6%, 25%, 50%, and 100% of S. mutans and L. acidophilus. Considering the means, the extract was more effective on S. mutans than on Lactobacillus.
The mean growth inhibition zone diameters for S. mutans and L. acidophilus with 500 mg of penicillin were 16.85 and 17.35 mm, respectively; these diameters for CHX were 13.85 and 14.23 mm, respectively, with 6 mm for the negative control group (DMSO). The results of statistical tests showed that means of growth inhibition zone diameters for all the concentrations of the extract were less than those in the positive control group (one sample t-test, df = 8, P < 0.001). However, the difference of the maximum growth inhibition zone of extract was significant from the negative control group (one-sample t-test, df = 8, P < 0.001).
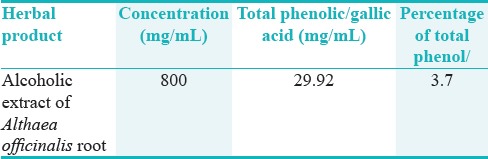
Based on the analysis of Folin-Ciocalteu of the A. officinalis, the total phenolic extract was shown in Table 3.
Table 3.
Total phenolic/galicacid of alcoholic extract of A. officinalis of root
DISCUSSION
Dental caries is still one of the health problems all over the world. The main bacteria associated with this disease are S. mutans and L. acidophilus. Since the attachment and proliferation are key steps in successful colonization in the dental plaque biofilm and pathogenesis of these microorganisms, different strategies such as inhibition of bacterial growth and prevention of microbial colonization were developed to protect dental caries. An ideal antimicrobial agent would inhibit both of these activities. Antimicrobial susceptibility of oral has been tested to a number of plant extracts and natural substances. Alcea species have a wide range of uses in herbal medicine in the treatment of some diseases such as sore throats, laryngitis and tonsillitis, coughs, dryness of the lungs, and digestive upsets.[16]
A. officinalis extract is one of the most important herbal medicines traditionally used in the treatment of inflammatory conditions of the respiratory system.[17] It is difficult to directly compare the results of different studies due to differences in analyses, the microbial techniques used, the presence of sucrose in the culture media available, the type of the material used, the manufacturers, and the extraction technique used. In addition, during the preparation processes of the extracts, some or all the active components of the extracts might be inactivated or the concentrations of the active ingredients might be different in terms of the geographical location, seasons, and cultivation processes[18] and all these factors affect the efficacy of A. officinalis extract
In reviewing the results of MBC and MIC, A. officinalis root extract against S. mutans and L. acidophilus is clear that A. officinalis has an antibacterial effect on both bacteria but less than antibacterial effect of penicillin antibiotics and CHX mouthwash. However, potency of the extract was lower than control agents, but this point is less important than safety of the herb.
In reviewing the diameter of inhibition zone due to significant differences in mean of inhibition zone of 100 percent extract with other concentrations to conclude that with increasing concentration, the effect of inhibition on bacteria increases. Furthermore, by comparing inhibition zone extract by positive and negative control groups showed that bacterial growth in the presence of penicillin and CHX was lower than herbal extract, but the growth of bacteria to extract significantly less than the negative control group. The extract had more potency on S. mutants than Lactobacillus that can be a base for further investigations.
Furthermore, the effect of time showed that the passage of time from 24 to 72 h had a significant effect on inhibition zone. In other words, over time, reduce anti-bacterial effect of extract did not find and this is the strength for this extract.
Ferrazzano et al. evaluated the polyphenolic and anticariogenic properties of plant extracts and reported that polyphenols, flavonoids, and anthocyanins exert their anticariogenic effects by inhibiting bacterial growth and interfering with bacteria adhesion to tooth surfaces and affecting the enzymatic activity of cariogenic bacteria, especially S. mutans.[9] Given the analysis of the components of A. officinalis extract and a high percentage of phenolic and flavonoid components in the extract (at a concentration of 850 mg containing 29.92 mg ml phenolic compounds(, the antibacterial properties of A. officinalis might be attributed to its polyphenolic components. Our TPC of A. officinalis extract was lower than Sadighara et al.[15] This diversity was related to different cultivation area of the plant.
In another study, Esmaeelian evaluated the effects of malvidin-3,5-diglucoside components extracted from the planet species Alcea longipedicellata on oral bacterial species and reported that it inhibited the growth and production of acids by S. mutans and other cariogenic bacteria in the oral cavity that are found in dental plaque. Malvin exhibited bactericidal activity, whereas the ethanolic extract and chloroform had bacteriostatic properties.[16] Considering the familial relationship between the plant mentioned above and the plant evaluated in the present study (Malvaceae family) and also the similarity of the chemical structure of their extracts, it is possible to attribute the antibacterial properties of A. officinalis against S. mutans to the properties and compositions of mucilaginous Malvaceae plant family.
Dehghan evaluated the antibacterial effect of the ethanolic extract of A. officinalis on S. pyogenes in comparison to commonly used antibiotics. The results showed that this extract had antibacterial properties and the bacterial growth inhibition exhibited a logarithmic relationship with an increase in concentration.[11] The results showed a significant antibacterial effect of the root extract of A. officinalis on the Gram-positive bacterial species mentioned above and Streptococcus mutans is also one of the Gram-positive bacteria.
Gautam (2015) evaluated the antibacterial effect of the extract of A. officinalis seed and essential oils on five pathogenic bacterial species and one pathogenic fungus of the respiratory tract.[10] In 2011 in the another study, antimicrobial effect of the hexane extract of A. officinalis root collected from the North-west of Iran was evaluated on a number of Gram-positive and Gram-negative bacteria.[19] Furthermore, in 2011, Walter carried out a study on the roots and leaves of A. officinalis in Pakistan. The antibacterial effects of this herbal medicine on E. coli, and P. aeruginosa Gram-negative bacterial species and S. aureus Gram-positive bacterial species were evaluated. The results of all these studies have shown a higher susceptibility of Gram-positive bacteria to this herbal extract compared to the Gram-negative bacteria,[20] which might be attributed to differences in the composition of the cell walls of these bacterial species.[21] It is possible that the antibacterial effect of the ethanolic extract of A. officinalis extract on Gram-positive bacteria is due to its adhesion to N-acetyl glucosamine found in the bacterial cell wall.[22] In addition, it might be attributed to the antioxidative effects of the plant extract components. The antibacterial effect of the extract can be explained by the fact that the plant polyphenols, plays a major role, exert their inhibitory effects on bacterial growth by producing hydrogen peroxide.[23]
Rezaei et al. evaluated the antibacterial effect and wound healing potential of A. officinalis extract in comparison to ciprofloxacin, gentamicin, and penicillin on P. aeruginosa, Listeria monocytogenes, S. aureus, and E. coli. The results showed that although A. officinalis extract was not effective on Gram-negative bacteria, it was effective on Gram-positive bacteria[24] similar to my research and clearly improved wound healing process. Therefore, it was concluded that the A. officinalis extract is a good choice for treating Gram-positive bacterial infections.
Several studies confirmed safety of the extract.[25,26] Hence, it is compatible and safe for use in human.
It is suggested assessment of anticariogenic effect of the A. officinalis extract on other cariogenic bacteria's. Hence, extract of other cultural areas selects for extraction and compare with other mouth rinses. Finally, clinical researches should assess the in vivo effect of mouth rinses of this beneficial herb.
CONCLUSION
Considering the significant effect of A. officinalis extract on Gram-positive bacteria, further studies are recommended to evaluate the antibacterial effect of A. officinalis on other pathogenic microorganisms responsible for caries in vitro and in vivo.
FINANCIAL SUPPORT AND SPONSORSHIP
The study was funded through the National Institutes of Health, and, therefore, its publication must comply with the NIH Public Access Policy (http://grants.nih.gov/grants/guide/notice-files/NOT-OD-08-033.html).
CONFLICTS OF INTEREST
There are no conflicts of interest.
REFERENCES
- 1.Haghgoo R, Afshari E, Ghanaat T, Aghazadeh S. Comparing the efficacy of xylitol-containing and conventional chewing gums in reducing salivary counts of Streptococcus mutans: An in vivo study. J Int Soc Prev Community Dent. 2015;5(Suppl 2):S112–7. doi: 10.4103/2231-0762.172947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cagetti MG, Mastroberardino S, Milia E, Cocco F, Lingström P, Campus G. The use of probiotic strains in caries prevention: A systematic review. Nutrients. 2013;5:2530–50. doi: 10.3390/nu5072530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen L, Ren Z, Zhou X, Zeng J, Zou J, Li Y, et al. Inhibition of Streptococcus mutans biofilm formation, extracellular polysaccharide production, and virulence by an oxazole derivative. Appl Microbiol Biotechnol. 2016;100:857–67. doi: 10.1007/s00253-015-7092-1. [DOI] [PubMed] [Google Scholar]
- 4.Walsh T, Oliveira-Neto JM, Moore D. Chlorhexidine treatment for the prevention of dental caries in children and adolescents. Cochrane Database Syst Rev. 2015;13:CD008457. doi: 10.1002/14651858.CD008457.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishizu T, Tsutsumi H, Sato T. Mechanism of creaming down based on chemical characterization of a complex of caffeine and tea catechins. Chem Pharm Bull (Tokyo) 2016;64:676–86. doi: 10.1248/cpb.c16-00131. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharya A, Sood P, Citovsky V. The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol Plant Pathol. 2010;11:705–19. doi: 10.1111/j.1364-3703.2010.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duangjai A, Suphrom N, Wungrath J, Ontawong A, Nuengchamnong N, Yosboonruang A. Comparison of antioxidant, antimicrobial activities and chemical profiles of three coffee (Coffea arabica L.) pulp aqueous extracts. Integr Med Res. 2016;5:324–331. doi: 10.1016/j.imr.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrazzano GF, Cantile T, Roberto L, Ingenito A, Catania MR, Roscetto E, et al. Determination of the in vitro and in vivo antimicrobial activity on salivary Streptococci and Lactobacilli and chemical characterisation of the phenolic content of a Plantago lanceolata infusion. Biomed Res Int. 2015;2015:286817. doi: 10.1155/2015/286817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrazzano GF, Amato I, Ingenito A, Zarrelli A, Pinto G, Pollio A. Plant polyphenols and their anti-cariogenic properties: A review. Molecules. 2011;16:1486–507. doi: 10.3390/molecules16021486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gautan SS, Navneet, Kumar S, Chauhan R. Antimicrobial efficacy of Althaea officinalis Linn. Seed extract and essential oil against respiratory tract pathogens. J Appl Pharm Sci. 2015;5:115–9. [Google Scholar]
- 11.Dehghan E, Dashti H, Baghizadeh A. Antibacterial effect of ethanol extract (Althaea officinalis) on Streptococcus pyogenes compared with prevalent antibiotics in-vitro. J Rafsanjan Univ Med Scie. 2013;12960:461–74. [Google Scholar]
- 12.Tagashira M, Uchiyama K, Yoshimura T, Shirota M, Uemitsu N. Inhibition by hop bract polyphenols of cellular adherence and water-insoluble glucan synthesis of mutans streptococci. Biosci Biotechnol Biochem. 1997;61:332–5. doi: 10.1271/bbb.61.332. [DOI] [PubMed] [Google Scholar]
- 13.Benbassat N, Yoncheva K, Hadjimitova V, Hristova N, Konstantinov S, Lambov N. Influence of the extraction solvent on antioxidant activity of Althaea officinalis L. root extracts. Cent Eur J Biol. 2014;9:182–8. [Google Scholar]
- 14.Gupta D, Priya BM, Galgali SR. Comparison of amine fluoride and chlorhexidine mouth rinses in the control of plaque and gingivitis – A randomized controlled clinical trial. Indian J Dent Res. 2015;26:57–62. doi: 10.4103/0970-9290.156809. [DOI] [PubMed] [Google Scholar]
- 15.Sadighara P, Gharibi S, Moghadam Jafari A, Jahed Khaniki G, Salari S. The antioxidant and Flavonoids contents of Althaea officinalis L. flowers based on their color. Avicenna J Phytomed. 2012;2:113–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Esmaeelian B, Yari Kamrani Y, Amoozegar MA, Rahmani S, Rahimi M, Amanlou M. Anti-cariogenic properties of malvidin-3, 5-diglucoside isolated from Alcea longipedicellata against oral bacteria. Int J Pharmacol. 2007;3:468–74. [Google Scholar]
- 17.Kardosová A, Machová E. Antioxidant activity of medicinal plant polysaccharides. Fitoterapia. 2006;77:367–73. doi: 10.1016/j.fitote.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Smullen J, Koutsou GA, Foster HA, Zumbé A, Storey DM. The antibacterial activity of plant extracts containing polyphenols against Streptococcus mutans. Caries Res. 2007;41:342–9. doi: 10.1159/000104791. [DOI] [PubMed] [Google Scholar]
- 19.Valeie M, Shafaghat A, Salimi F. Chemical composition and antimicrobial activity of the flower and root hexane extracts of Althaea officinalis in Northwest Iran. J Med Plants Res. 2011;5:6972–6. [Google Scholar]
- 20.Walter C, Shinwari Z, Afzali I, Malik R. Antibacterial activity in herbal products used in Pakistan. Pak J Bot. 2011;43:155–62. [Google Scholar]
- 21.Ahmad I, Beg AZ. Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J Ethnopharmacol. 2001;74:113–23. doi: 10.1016/s0378-8741(00)00335-4. [DOI] [PubMed] [Google Scholar]
- 22.Sadeghi G. Vol. 11. University of Pharmacology Sciences of Islamic Azad University; 2003. Determination of Antibacterial Effects of Glycyrrhiza glabra on E. coli, Salmonella typhi, Shigella flexneri and Shigella sonnei. Thesis No. 1249; pp. 91–9. [Google Scholar]
- 23.Taleb H, Maddocks SE, Morris RK, Kanekanian AD. The Antibacterial activity of date syrup polyphenols against S. aureus and E. coli. Front Microbiol. 2016;7:198. doi: 10.3389/fmicb.2016.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rezaei M, Dadgar Z, Noori-Zadeh A, Mesbah-Namin SA, Pakzad I, Davodian E. Evaluation of the antibacterial activity of the Althaea officinalis L. leaf extract and its wound healing potency in the rat model of excision wound creation. Avicenna J Phtomed. 2015;5920:105–12. [PMC free article] [PubMed] [Google Scholar]
- 25.Ali Shah SM, Akhtar N, Akram M, Akhtar Shah P, Saeed T, Ahmed KH, et al. Pharmacological activity of Althaea officinalis L. J Med Plants Res. 2011;5:5662–6. [Google Scholar]
- 26.Benbassat N, Yoncheva K, Hadjimitova V, Hristova N, Kanstantinov S, Lambov N. In fluence of the extraction solvent on antioxidant activity of Althaea officinalis L. root extracts. Cent Eur J Biol. 2014;9:182–8. [Google Scholar]


