ABSTRACT
Endogenous electric fields modulate many physiological processes by promoting directional migration, a process known as galvanotaxis. Despite the importance of galvanotaxis in development and disease, the mechanism by which cells sense and migrate directionally in an electric field remains unknown. Here, we show that electrophoresis of cell surface heparan sulfate (HS) critically regulates this process. HS was found to be localized at the anode-facing side in fetal neural progenitor cells (fNPCs), fNPC-derived astrocytes and brain tumor-initiating cells (BTICs), regardless of their direction of galvanotaxis. Enzymatic removal of HS and other sulfated glycosaminoglycans significantly abolished or reversed the cathodic response seen in fNPCs and BTICs. Furthermore, Slit2, a chemorepulsive ligand, was identified to be colocalized with HS in forming a ligand gradient across cellular membranes. Using both imaging and genetic modification, we propose a novel mechanism for galvanotaxis in which electrophoretic localization of HS establishes cell polarity by functioning as a co-receptor and provides repulsive guidance through Slit-Robo signaling.
KEY WORDS: Galvanotaxis, Brain tumor-initiating cells, Heparan sulfate, Electrophoresis
Highlighted Article: Cell surface heparan sulfate is a novel electric field sensor that regulates the galvanotaxis of glial cells through electrophoretic polarization and its function as a co-receptor for chemo-repulsive ligands such as Slit2.
INTRODUCTION
Endogenous electric fields (EFs) are known to drive many physiological processes including embryo development, wound healing and immune responses by promoting directional migration, a process known as galvanotaxis (Mycielska and Djamgoz, 2004; Lin et al., 2008). Disruption of endogenous EFs with pharmacological agents or externally applied EFs of opposite polarity disturbs these processes, whereas enhancement of endogenous EFs (same polarity, increased magnitude) increases the rate of regeneration by promoting the extent of nerve sprouting and the rate of wound healing in vivo (Song et al., 2004; Messerli and Graham, 2011). The brain exhibits one of the highest electrical activities amongst all organs in the body; electric fields in the brain are not an epiphenomenon but actively regulate cellular functions. For example, the endogenous electric field between the subventricular zone and olfactory bulb was found to direct the migration of neuroblasts and guide the migration of neural precursor cells along the rostral migratory stream (Cao et al., 2013). Furthermore, increased electrical activity stimulated by optogenetics accelerates glioma growth in vivo (Venkatesh et al., 2015). Taken together, these results suggest that endogenous electric fields modulate neural regeneration and glioma infiltration by regulating galvanotaxis; however, the mechanism by which brain cells sense and migrate directionally in an electric field remains unknown. Therefore, elucidating the mechanism of galvanotaxis can provide new insight into brain development and the progression of diseases such as glioma, and provide the foundations for new clinical interventions.
Proposed explanations for galvanotaxis include electrophoretic distribution of charged membrane components (Jaffe, 1977; Poo and Robinson, 1977; Allen et al., 2013), asymmetric activations of ion channels (Yang et al., 2013; Nakajima et al., 2015), and membrane-associated electro-osmotic forces (McLaughlin and Poo, 1981). Interestingly, while most cell types exhibit galvanotaxis, the response can be either cathodic or anodic, suggesting that there may be competing mechanisms (Mycielska and Djamgoz, 2004; Sato et al., 2009; Sun et al., 2013). Here, we investigate the galvanotaxis in three different types of glial cells including primary neural progenitor cells (fNPCs), fNPC-derived astrocytes, and malignant brain tumor-initiating cells (BTICs). We show that all three cell types exhibit a directional response to an external EF. More importantly, we identify the novel role of surface heparan sulfate (HS), a highly negatively charged sulfated glycosaminoglycan (GAG), in sensing and mediating galvanotaxis. HS was found to be highly localized towards the positive electrode (anode) of the cells in the presence of an EF in all cell types due to electrophoretic interactions. Enzymatic digestion of HS significantly abolished the cathodic response in cells. Furthermore, using non-viral siRNA knockdown, we showed that galvanotaxis is unlikely to be due to any single heparan sulfate proteoglycan, but is rather a collective outcome due to the localization of HS chains. HS was identified as a co-receptor, establishing a Slit2 gradient across cellular membranes as a consequence of electrophoretic localization. Slit2, a chemorepulsive ligand critical for central nervous system development (Shi and Borgens, 1994; Ba-Charvet et al., 1999; Kaneko et al., 2010), subsequently provides a repulsive guidance through Slit-Robo signaling as indicated by the attenuation of galvanotaxis in response to downregulation of Robo1. We propose that HS is a novel EF sensor that regulates galvanotaxis through electrophoretic interactions and its function as a co-receptor, to establish a ligand gradient. Our findings provide direct evidence in support of electrophoretic interactions in regulating galvanotaxis, and highlight the possibility of an EF in promoting autologous chemotaxis.
RESULTS
fNPCs, astrocytes and BTICs exhibit galvanotaxis with different characteristics
To understand the mechanisms regulating the galvanotaxis of brain cells, we first characterized the responses of fNPCs, astrocytes and BTICs using a custom galvanotaxis chip (Huang et al., 2013) (Fig. 1A). All experiments were conducted under the same culture conditions (see Materials and Methods) to avoid any bias. The trajectories of the cells in the presence of an EF were tracked and analyzed to characterize the cellular response. We showed that galvanotaxis is highly dependent on cell type: while 100% of fNPCs exhibited strong directional response towards the cathode (Movie 1 and Fig. 1B), astrocytes derived from fNPCs showed an anodic directional response opposite to fNPCs (Movie 2, Fig. 1C). Meanwhile, the majority of BTICs (73%) migrated towards the cathode in the presence of a 1 V cm−1 EF (Movie 3 and Fig. 1D). Further quantifying cell motility and directedness in the presence of an EF (Fig. 1E) showed that fNPCs exhibited the highest motility on a laminin-coated surface in the presence of an EF (0.87±0.08 μm min−1) followed by BTICs (0.75±0.15 μm min−1) and astrocytes (0.56±0.03 μm min−1). fNPCs also exhibited the highest directedness (d) towards the cathode (d=0.85±0.09) followed by BTICs (d=0.44±0.01), whereas astrocytes migrated strongly towards the anode (d=−0.85±0.06).
Fig. 1.
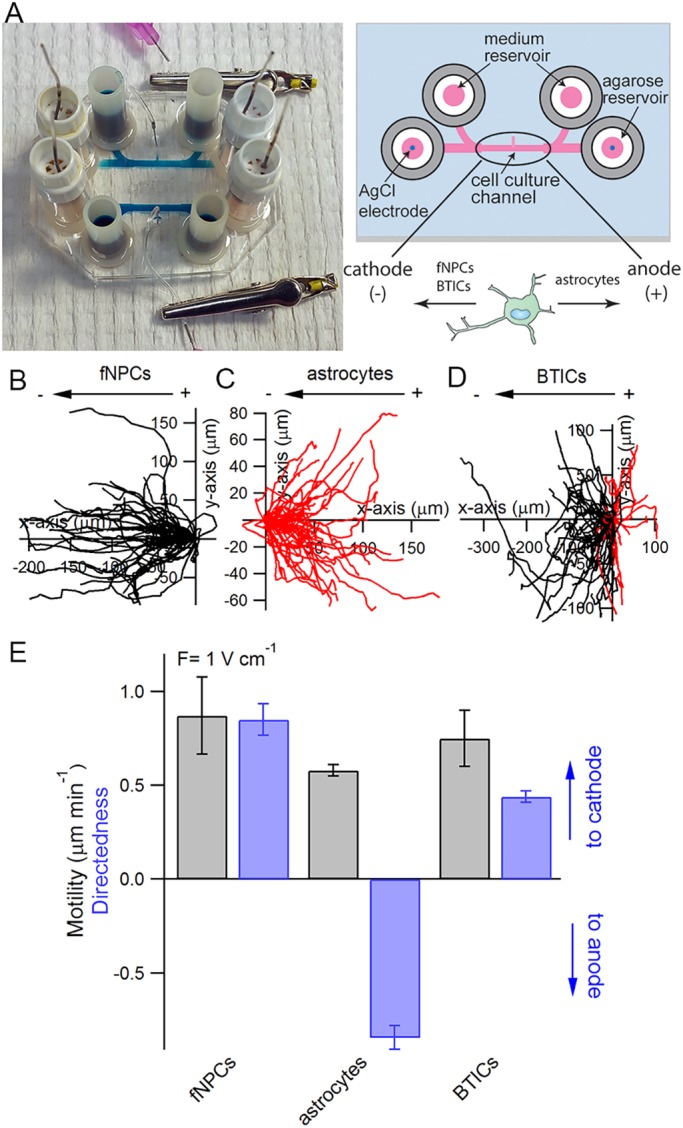
Galvanotaxis of fetal neural progenitor cells (fNPCs), astrocytes and brain tumor-initiating cells (BTICs). (A) Each galvanotaxis chip contains two symmetrical devices on a 35 mm×50 mm glass coverslip. Each device features two coiled Ag/AgCl electrodes embedded in agarose reservoirs located at each end of the cell culture channel, along with two media reservoirs also located at each end of the channels. The dimensions of the cell culture channel are 10 mm×1 mm×250 μm (length×width×height). A cell injection port located in the middle of the cell culture channel is used to introduce cells into the device and is clamped with an alligator clip afterwards to prevent evaporation. Trajectories of fNPCs (B), astrocytes (C) and BTICs (D) in the presence of an EF are analyzed and overlaid at the origin to characterize the galvanotaxis of each cell line. Each trajectory represents the actual path traveled by a cell in 3 h either to the cathode (left, black) or anode (right, red). (E) Quantitative analysis of cell motility and directedness in the presence of a 1 V cm−1 EF.
HS is localized towards the anode regardless of cell type and the direction of galvanotaxis
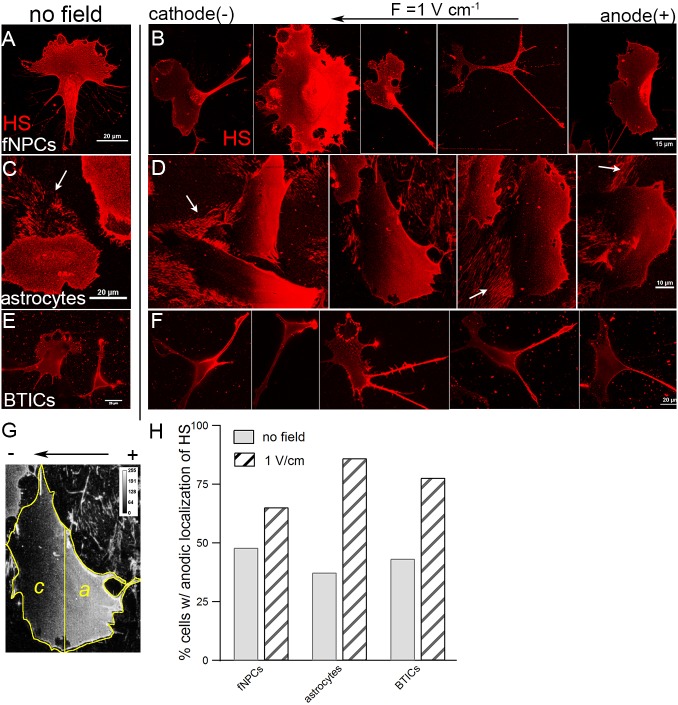
We next investigated the involvement of membrane components to understand how cells were able to sense and respond to an electric field. Heparan sulfate (HS) is a candidate as an EF sensor as it is not only ubiquitously expressed on all cellular membranes, but also highly negatively charged. To understand the involvement of HS, fNPCs, astrocytes and BTICs were stained with antibodies against HS both in the absence of an EF and after being stimulated with a 1 V cm−1 EF for 3 h. We showed that HS was indeed abundantly expressed in each cell type, as indicated by the punctate aggregates throughout the cellular membranes (Fig. 2A–F). In the absence of an EF, HS distributed uniformly across cellular membranes in each cell type (Fig. 2A,C,E). However, in the presence of an EF, where fNPCs and BTICs migrated towards the cathode and astrocytes migrated towards the anode, HS was found to be highly localized towards the anode (positive electrode) regardless of cell type and the direction of migration (Fig. 2B,D,F). The localization of HS is particularly obvious in astrocytes where the average size of the cells is much larger than fNPCs and BTICs. In both the absence and presence of an EF, astrocytes secreted and left behind trails of extracellular matrix (ECM) abundant with HS during migration (Fig. S1A,B). However, localization of HS was only observed in the presence of an EF (Fig. 2D), not in the absence of an external field (Fig. 2C). Further quantifying the localization of HS from analysis of processed images (Fig. 2G), we showed that an EF resulted in a substantial increase in the percentage of cells with anodic localization of HS (Fig. 2H). In the absence of an EF, the anodic localization of HS was close to 50%, indicating no polarization: 48% of fNPCs, 37.5% of astrocytes and 43.3% of BTICs. In contrast, in the presence of a 1 V cm−1 EF, the localization increased to 65.2, 86.1 and 77.8%, respectively. The distribution of HS in a smaller EF (0.5 V cm−1) was further examined in BTICs; however, there was no evidence for significant HS polarization (47.7%) (Fig. S1C,D).
Fig. 2.
Anodic localization of HS in the presence of an EF. The distribution of HS was measured by performing a maximum intensity projection of confocal z-stacks of individual cells to minimize any error associated with out-of-focus pixels. In the absence of an EF, HS is distributed uniformly across the membranes of fNPCs (A), astrocytes (C) and BTICs (E). However, strong anodic localization of HS was observed across all three cell lines (B,D,F) in the presence of an EF despite fNPCs and BTICs migrating towards the cathode and astrocytes migrating towards the anode. (G) Distribution of HS was analyzed by performing a signed rank test among all the pixels in the cathode face (c) versus pixels in the anode face (a). (H) Percentage of cells with anodic localization of HS is higher in the presence of an EF than no EF among all three cell types. Each condition represents more than 50 cells. White arrows in panels C and D indicate the ECM left behind by cells during migration.
HS regulates the cathodic response in fNPCs and BTICs
To establish the relevance of anodic localization of HS in cellular galvanotaxis, we next used heparinase (HPase) to digest surface HS. The effectiveness of HPase treatments was verified by immunostaining of HS, where cells treated with HPase were devoid of any HS signal except at the periphery near focal adhesions (Fig. 3A, right, B, right). fNPCs, astrocytes and BTICs were each treated with HPase and stimulated with an EF to compare their responses to the corresponding wild-type cells (Fig. 3C-E). We showed that while HPase treatment had no significant effect on cell motility in either cell type (Fig. 3F), it significantly influenced the cathodic responses of fNPCs and BTICs (Fig. 3G). The directedness of fNPCs significantly decreased from 0.85 to 0.38 (P=0.038) after treatment with HPase, whereas the directedness of BTICs significantly decreased from 0.48 to −0.12 (P<0.01). The anodic directional response of astrocytes, however, remained unaffected even after treatment with HPase (Fig. 3G).
Fig. 3.
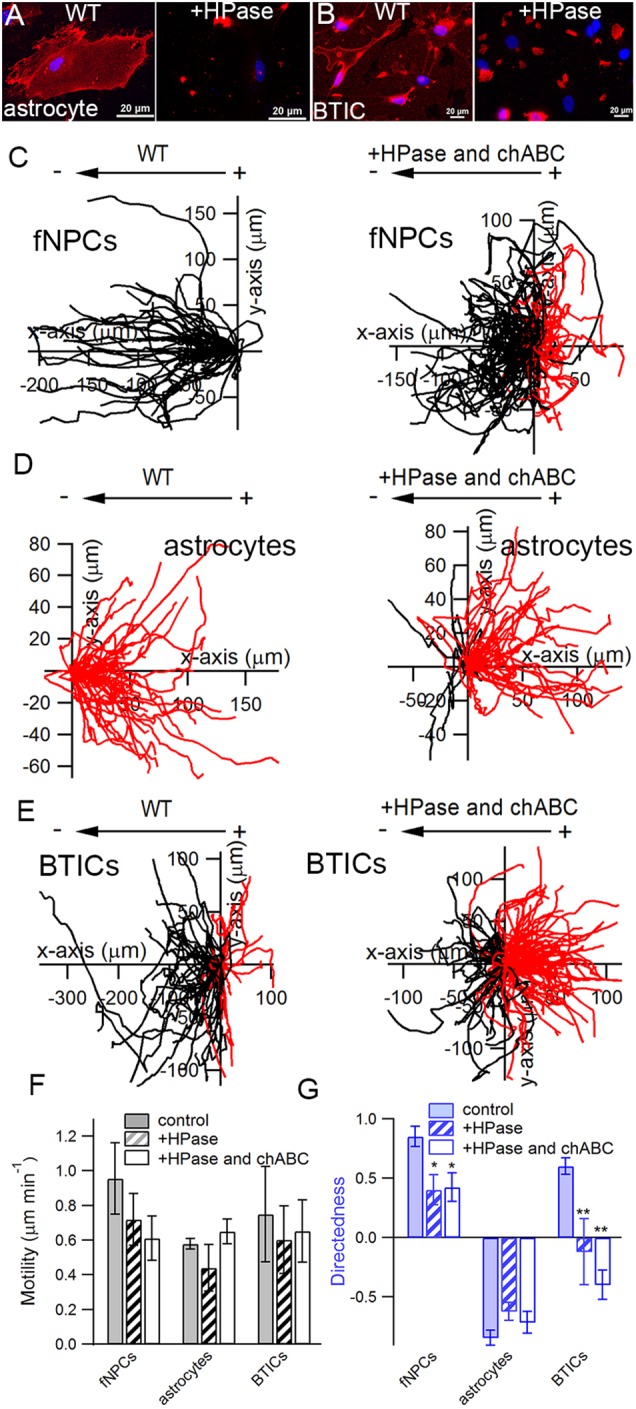
Enzymatic digestion of HS significantly attenuates cathodic galvanotaxis in fNPCs and BTICs. Heparinase (HPase) significantly reduced the amount of surface HS GAGs in astrocytes (A) and BTICs (B), as shown by the absence of fluorescence except near cell peripheries close to focal adhesions after enzymatic treatment. (C–E) Trajectories of wild-type cells (WT) and cells treated with both HPase and chondroitinase ABC (chABC) are shown side by side to highlight the effect of sulfated GAGs on cellular galvanotaxis. Each trajectory represents the actual path traveled by a cell in 3 h either to the cathode (left, black) or anode (right, red). (F) Enzymatic treatment with either HPase alone or a combination of HPase and chABC had no significant effect on cell motility when compared with the corresponding WT. (G) HPase significantly attenuated the cathodic response of fNPCs and completely abolished the galvanotaxis of BTICs, but had no effect on astrocytes. The combination of HPase and chABC did not further reduce the directedness of fNPCs but completely reversed the directional response in BTICs. *P<0.05; **P<0.01; Student's t-test. Statistics were obtained from at least three independent experiments with at least 60 cells in each experiment. Error bars represent standard deviation.
Chondroitin sulfate (CS) and dermatan sulfate are two other main types of GAG that bear a negative charge due to sulfation modification. To investigate its involvement in galvanotaxis, we treated cells with both HPase and chondroitinase ABC (chABC), enzymes that catalyze the removal of chondroitin sulfate and dermatan sulfate GAG chains. Addition of chABC did not affect the motility of either cell type (Fig. 3F), nor did it further affect the directedness of fNPCs (Fig. 3G). The directedness of astrocytes remained comparable to the wild-type cells despite the removal of HS, CS and dermatan sulfate (Fig. 3G). However, treatments with both HPase and chABC completely reversed the direction of galvanotaxis in BTICs from cathodic to anodic (Fig. 3E, right; Movie 4). BTICs treated with HPase and chABC migrated towards the anode with a mean directedness of −0.40±0.12, significantly different from wild-type BTICs (d=0.48±0.07, P<0.01).
HS-mediated directional response is unlikely to be due to any specific HSPG
HS exists as a heparan sulfate proteoglycan (HSPG), where a core protein is covalently attached to several sulfated HS chains. To understand how the anodic localization of HS mediates the cathodic directional migration of fNPCs and BTICs, we considered two possible explanations involving either HSPG core proteins or HS GAGs, and investigated them accordingly.
We first hypothesized that cathodic galvanotaxis is due to the asymmetric distribution of one of the HSPG core proteins that interacts with its downstream effectors and establishes cell polarity. To examine this hypothesis, the expression levels of selected individual HSPGs were systematically downregulated using siRNA. Two main types of HSPG are expressed on the membranes of mammalian cells: syndecan (SDC1–SDC4) and glypican (GPC1–GPC6) (Sarrazin et al., 2011). Using polymeric nanoparticles containing optimized siRNA sequences, we downregulated SDC1, SDC2, SDC3, SDC4 and GPC1; downregulation of the corresponding protein expression levels was confirmed by western blot and further validated by qRT-PCR (Fig. S2A–F). While the expression levels of selected HSPGs were downregulated by 40–60% based on western blots and the mRNA levels were downregulated by >80% (Fig. S2), the motility of BTICs remained unaffected by the transfection (Fig. S2G). Furthermore, downregulation of SDC1, SDC2, SDC3, SDC4 or GPC1 alone had no significant effect on the directedness of BTICs in the presence of an EF when compared with either wild-type cells or cells treated with a scrambled sequence (Fig. S2H).
Disrupting the sulfation of HS abolishes the galvanotaxis of fNPCs and BTICs
As cell polarity during galvanotaxis is unlikely to be due to any single HSPG, we considered the possibility that galvanotaxis is a collective outcome of the localization of sulfated GAG chains. We hypothesized that as a known co-receptor for many ligands (Sarrazin et al., 2011), HS is capable of binding and forming a ligand gradient across cellular membranes in the presence of an EF, and promotes directional migration in a way similar to chemotaxis. To test this hypothesis, each cell type was treated with NaClO3 to disrupt the sulfation of HS, as the ability of HS to bind to different ligands relies heavily on the degree of sulfation (Shipp and Hsieh-Wilson, 2007). Chlorate competitively inhibits the formation of high-energy sulfate donors required for sulfation reactions and thus substantially undermines the capability of HS to bind ligands such as fibroblast growth factor (FGF) and Slit (Safaiyan et al., 1999; Shipp and Hsieh-Wilson, 2007). fNPCs, astrocytes and BTICs were treated with 50 mM NaClO3 for 48 h before being subjected to an applied EF. While the motility of fNPCs and BTICs remained unaffected by the treatment (Fig. 4C), chlorate treatment completely abolished the cathodic galvanotaxis in both fNPCs and BTICs (Fig. 4A and B); the directedness of fNPCs decreased from 0.85 to −0.03 (P<0.01), whereas the directedness of BTICs decreased from 0.44 to 0.00 (P<0.01) (Fig. 4D). Astrocytes, however, remained unaffected by the chlorate treatment both in terms of motility and directedness (Fig. S3A).
Fig. 4.
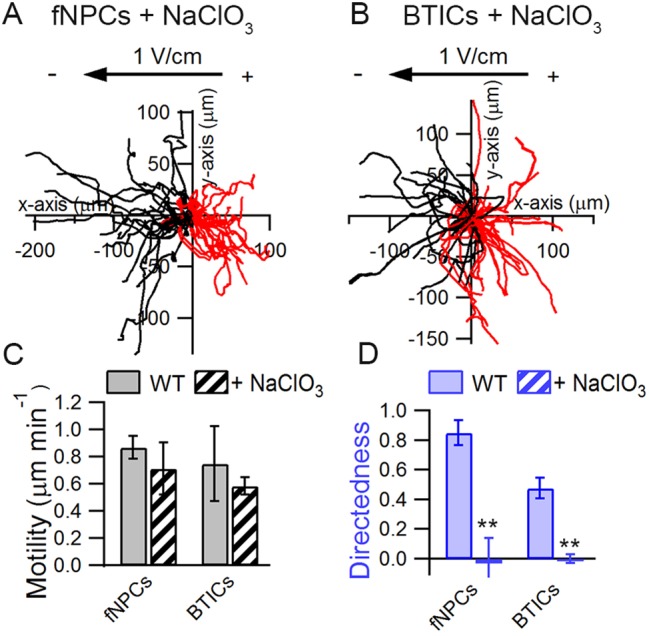
NaClO3 abolishes cathodic response in fNPCs and BTICs. fNPCs (A) and BTICs (B) were treated with 50 mM sodium chlorate for 48 h to investigate how the sulfation of HS influences cathodic galvanotaxis. Treatment with sodium chlorate had no effect on cell motility (C) but abolished the directional response of fNPCs and BTICs in the presence of an electric field (D). **P<0.01 (Student's t-test). Statistics were obtained from at least two independent experiments with at least 60 cells in each experiment. Error bars represent standard deviation.
Localization of HS establishes a ligand gradient across cellular membranes
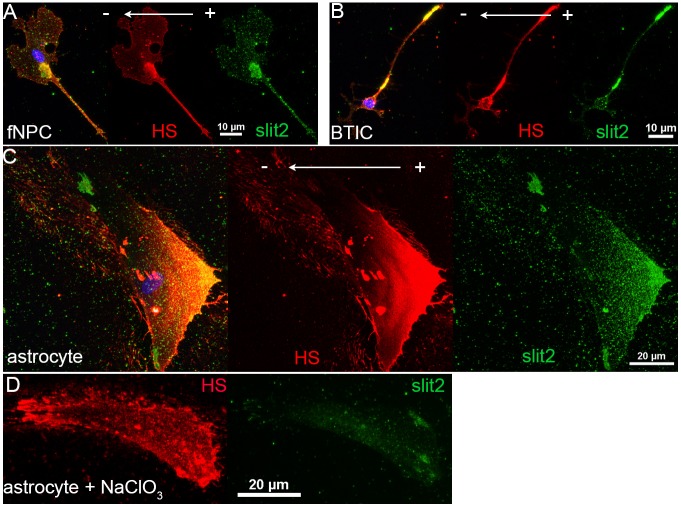
As NaClO3 completely abolished the cathodic response in fNPCs and BTICs, we continued to investigate the capability of HS in forming a gradient of ligands across a cellular membrane. Cells in the presence of an EF were stained with both HS and Slit2, a ligand of the Robo family of receptors essential for development in the central nervous system (Brose et al., 1999). Slit2 was chosen not only for its well-characterized affinity to HS (Hu, 2001), but also for its role in providing a repulsive migration cue in various types of brain cells (Shi and Borgens, 1994; Ba-Charvet et al., 1999; Kaneko et al., 2010). We have previously shown that Slit2-Robo1 signaling greatly enhanced the invasion of BTICs by providing a chemorepulsive signal in vitro (Guerrero-Cazares et al., 2015). The observation that HS localized towards the back of fNPCs and BTICs (anode) while cells migrated towards the cathode supports a mechanism involving Slit-mediated repulsive guidance. From immunofluorescence staining, we discovered that Slit2 was colocalized with HS towards the anode in the presence of an EF in all three cell types, demonstrating the capability of HS in forming a ligand gradient across a cellular membrane in an EF (Fig. 5A–C). Furthermore, we also showed that the ability of HS to bind Slit was significantly attenuated in cells treated with chlorate, as shown by a significant decrease in the intensity of Slit but not HS (Fig. 5D).
Fig. 5.
Slit2 colocalizes with HS and forms a gradient across cellular membranes in the presence of an EF. fNPCs (A), BTICs (B) and astrocytes (C) were stained for both HS (red) and Slit2 (green) to investigate whether localization of HS is capable of forming a ligand gradient across cellular membranes. Antibodies raised in different species were selected to avoid cross-reaction. Slit2 colocalized with HS as indicated in the overlays (yellow) and formed a gradient across cellular membranes in all three cell types. (D) Chlorate treatments significantly hindered the affinity of HS to Slit2, as indicated by the decrease in Slit2 fluorescence signal.
Downregulation of Robo1 attenuates galvanotaxis
To probe the involvement of Slit-Robo signaling in promoting galvanotaxis, BTICs were transduced with lentiviral particles containing a shRNA sequence against Robo1 receptor (Fig. 6A and B) and subjected to an EF. We showed that downregulation of Robo1 attenuated the galvanotaxis of cells as both cell motility and directedness decreased compared with the control group transduced with an empty virus (EV) (Fig. 6C,D). Downregulation of Robo1 decreased the motility of BTICs from 0.9 to 0.58 μm min−1 (P=0.039), whereas the directedness decreased from 0.72 to 0.36 (P=0.031) (Fig. 6E,F).
Fig. 6.
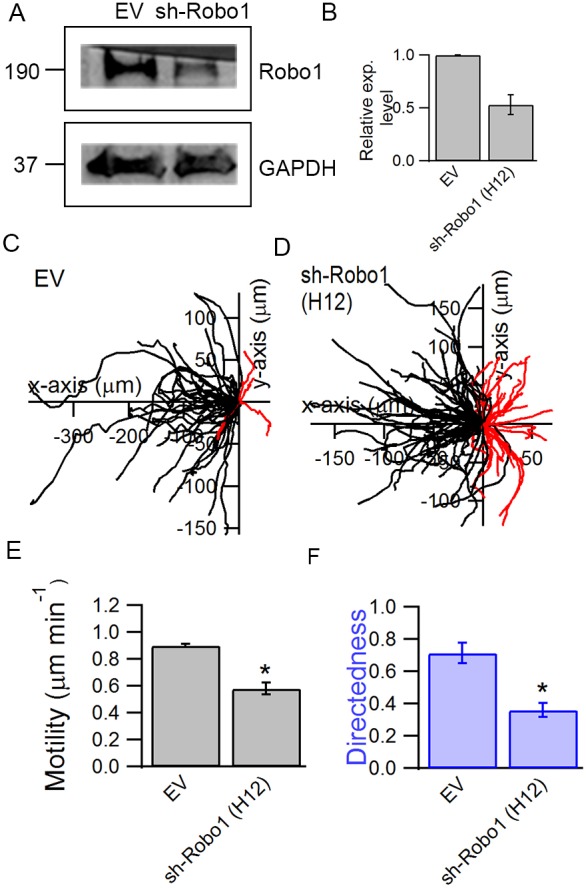
Downregulation of Robo1 attenuates the galvanotaxis of BTICs. BTICs were transduced with shRNA against Robo1 (sh-Robo1) to evaluate the contribution of Slit-Robo signaling in galvanotaxis. (A,B) The efficiency of the knockdown against cells transduced with an empty virus (EV) was evaluated using western blot, where the transduction resulted in a 47±9.7% downregulation of Robo1. Trajectories of EV cells (C) and sh-Robo1 cells (D) in the presence of an EF were analyzed to examine the involvement of Slit-Robo signaling in galvanotaxis. Robo1 knockdown resulted in a decrease in both cell motility (E) and directedness (F). *P<0.05; Student's t-test. Statistics were obtained from two independent experiments with at least 60 cells in each experiment. Error bars represent standard deviation.
DISCUSSION
Gradients of molecular cues along the cell migration path is widely believed to be the driving mechanism for brain cell migration during development and pathogenesis. Our findings suggest that an endogenous or an applied EF can also establish a gradient of molecular cues at the cellular level through electrophoresis of HS, and resulted in directional migration. These findings have implications in understanding brain homeostasis and may be utilized for disease treatments.
Electrophoresis of cellular membrane components has long been hypothesized to be the driving mechanism for galvanotaxis (Jaffe, 1977). Results from galvanotaxis experiments in response to changes in media pH and viscosity also provide support for this mechanism (Allen et al., 2013). Membrane components such as ConA receptors, ricin receptors, sialic acids and EGF receptor (EGFR) have been shown to be polarized in an electric field (Poo et al., 1979; Zagyansky and Jard, 1979; Fang et al., 1999; Finkelstein et al., 2007; Nakajima et al., 2015). However, the identity of a macromolecule that exhibits both electrophoretic polarization and is also necessary for galvanotaxis has not been reported. For example, the ConA receptor has been shown to be polarized in an EF and its direction of polarization reversed when cells were treated with neuraminidase, but the effects of its polarization on galvanotaxis is unclear (McLaughlin and Poo, 1981). EGFR, however, has been shown to be important for galvanotaxis as pharmacological inhibition abolished galvanotaxis in keratinocytes, although it is unclear whether the polarization of EGFR towards the cathode is due to an electrophoretic force. Here, we present direct evidence in support of the electrophoresis in regulating galvanotaxis. As HS and other sulfated GAGs are amongst the most highly negative charged biopolymers in nature (Sarrazin et al., 2011) and HS is polarized towards the anode-facing side of glial cells regardless of their direction of galvanotaxis, localization of HS is probably a physical process involving an electrophoretic force. Furthermore, HS is also critical for the cathodic response observed in fNPCs and astrocytes. Taken together, we conclude that HS is a novel EF sensor that relays electrical signals to directional migration cues through electrophoretic polarization.
However, since the anodic directional response of astrocytes was not affected upon removal of HS, other mechanisms, perhaps in competition with HS, probably exist and collectively determine the direction of galvanotaxis. This could explain the surprising observation that the directional response of BTICs was reversed from cathodic to anodic upon removal of different types of sulfated GAGs (Fig. 3E); as we weakened the cathodic response mediated by sulfated GAGs, the mechanisms governing an anodic response dominated and cells migrated towards the anode. Interestingly, we have also previously observed that the galvanotaxis of BTICs can also be reversed by their surrounding microenvironment (Huang et al., 2016); galvanotaxis of BTICs switched from cathodic to anodic upon addition of poly-l-ornithine on top of a laminin-coated substrate, similar to the galvanotropism of neurons reported previously (Movie 5 and Fig. S4) (Rajnicek et al., 1998). These results highlight the existence of potential competing mechanisms. We hypothesize that one of the contributing factors in mediating the competing mechanisms may be the cellular level of cAMP, as poly- l-lysine was shown to elevate cellular cAMP levels more than twofold in oligodendrocytes (Vartanian et al., 1988). Cellular levels of cAMP have been shown to modulate the repulsive versus attractive response towards netrin-1 in Xenopus spinal neurons (Ming et al., 1997) and elevated cAMP levels abolished the galvanotaxis of keratinocytes and keratocyte fragments (Pullar and Isseroff, 2005; Zhu et al., 2015). Another candidate in regulating the anodic galvanotaxis is sialic acid, a negatively charged sugar molecule that contributes to the overall charge of the membrane. Removal of sialic acid has been shown to reverse the polarization of ConA receptors and impair the cathodic response in HeLa and 3T3 cells (McLaughlin and Poo, 1981; Finkelstein et al., 2007).
In an attempt to find out how the anodic localization of HS leads to a directional response towards the cathode, we tested two hypotheses; one involved the function of the core proteins of HSPGs and the other involved sulfated GAGs. We examined the first hypothesis by selectively knocking down the expression level of five individual syndecans and glypicans, as both types of surface HSPG have been shown to influence cell migration (Wight et al., 1992). For example, syndecan-1 has been shown to localize at the uropods (trailing edge) of polarized myeloma cells and promote cell adhesion (Børset et al., 2000). Similarly, syndecan-4 has also been observed to inhibit Rac at the back of neural crest cells in vivo during development (Matthews et al., 2008) and mediate the persistent migration of fibroblasts by locally regulating Rac activity (Bass et al., 2007). These results support our observations and hypothesis, where HSPGs were found to be localized at the anodal face (trailing end) while cells migrated towards the cathode. However, knocking down any individual HSPG alone did not have any significant effect on the cathodic response of BTICs, suggesting that galvanotaxis is unlikely to be dependent on the function of any individual HSPG core protein. Given that mammalian cells have four syndecan and six glypican genes, and our knock-downs yield 40–60% downregulation at the protein level, the possibility of redundancy/compensation between different family members remains.
We tested the possible role of sulfated HS GAG, as HS is known to serve as a co-receptor for many different ligands based on various sulfation modifications (Sarrazin et al., 2011). Treatment with sodium chlorate completely abolished the cathodic response in fNPCs and BTICs (Fig. 4D), suggesting the important role of sulfated HS in sensing and migrating during galvanotaxis. In addition, as Slit2 was found to be colocalized with HS in forming a positive surface gradient towards the anode (Fig. 5A–C) and downregulation of Robo1 attenuated galvanotaxis (Fig. 6), we propose a model where galvanotaxis is mediated by the electrophoresis of HS and its function to serve as a co-receptor for ligands such as Slit2 (Fig. 7). However, it is likely that other mechanisms and ligands, such as other family members of Slit and Robo as well as FGF, may have also contributed to the process as downregulating Robo1 only partially attenuated galvanotaxis. Nevertheless, our results highlight the novel role of HS as an EF sensor during galvanotaxis and provide direct evidence in support of the electrophoretic galvanotaxis model. These findings could have broad implications in many physiological events as cells ubiquitously express HS and small EFs have been associated with neural development (Burr, 1941; Cao et al., 2013), wound healing (Zhao et al., 2006) and metastatic disease (Mycielska and Djamgoz, 2004). Understanding how endogenous EFs could guide cell migration and how an applied EF could potentially be leveraged to modulate this process provide a rationale for new therapeutics. For example, transcranial direct current stimulation (tDCS) has shown clinical benefits for central nervous system diseases (Fregni et al., 2015) and was able to promote cathodal accumulation of endogenous neural stem cells (Rueger et al., 2012). As more is learned about the role of bioelectricity on cell function it is likely that new opportunities for interventions will emerge.
Fig. 7.
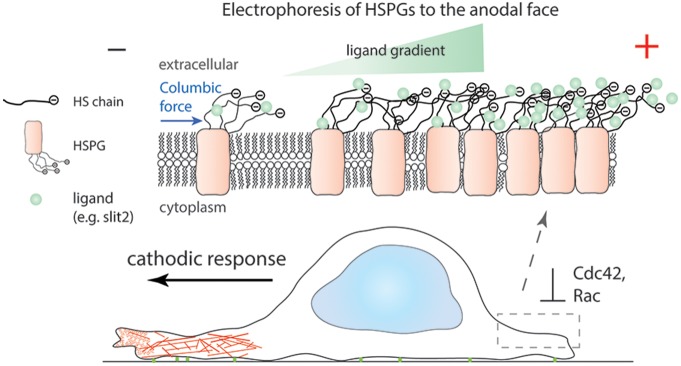
Proposed mechanism for cathodic galvanotaxis mediated by electrophoretic localization of HSPG. Electrophoresis of HS in the presence of an EF redistributes HSPGs towards the anode and consequently establishes a ligand gradient (for example, Slit2) across a cellular membrane, as HS is a co-receptor for many ligands. A ligand gradient across a cellular membrane asymmetrically activates downstream signaling, in this case Slit-Robo repulsive guidance, and promotes cell migration towards the cathode possibly by locally suppressing the activation of Cdc42 and Rac1 at the anode.
MATERIALS AND METHODS
Cell lines
All cell cultures were established with institutional approval by the Johns Hopkins University Internal Review Board.
fNPCs: F54 cells were derived after 17 weeks of gestation, obtained from elective abortion (Tzeng et al., 2011) and were maintained in 2:1 high-glucose Dulbecco's modified Eagle's medium (DMEM; Invitrogen)/Ham's F12 (Cellgro) mixture supplemented with 2% B-27, 1% antibiotic–antimyotic, 20 ng ml−1 bFGF, 20 ng ml−1 EGF, 20 ng ml−1 leukemia inhibitory factor (LIF; Millipore, Billerica, MA) and 5 μg ml−1 heparin (Sigma).
Astrocytes: Astrocytes were derived by plating fNPCs in a tissue culture flask in DMEM/F12 medium (Sigma) supplemented with 10% fetal bovine serum (Sigma) and 1% penicillin-streptomycin (Invitrogen) (Placone et al., 2015).
BTICs: GBM612 cells were used and previously validated by Johns Hopkins Genetic Resources Core Facility (Li et al., 2014). GBM612 cells isolated from intra-operative tissue are multipotent and are able to form diffuse tumors when implanted into animal models (Guerrero-Cázares et al., 2009; Li et al., 2014; Kondapalli et al., 2015). BTICs were grown in culture flasks coated with laminin and cultured in DMEM/F12 media supplemented with 2% B-27, 1% antibiotic–antimyotic, 20 ng ml−1 FGF and 20 ng ml−1 EGF.
Two-dimensional galvanotaxis and cell tracking
Galvanotaxis experiments were carried out using a customized galvanotaxis device reported previously (Huang et al., 2013), with standard microfabrication techniques (Fig. 1A). Briefly, a galvanotaxis chip is fabricated from polydimethylsiloxane (PDMS) and oxygen plasma bonded onto a glass coverslip. Ag/AgCl electrodes embedded in agarose are inserted into the reservoirs, and the exposed glass slide in the channel is coated with laminin. Before each experiment, different cell media were replaced with a standard medium for 6 h to ensure that the reported phenotypes are not medium dependent. The standard medium was DMEM/F12 supplemented with 2% B-27, 1% antibiotic–antimycotic, 20 ng ml−1 bFGF and 20 ng ml−1 EGF. The galvanotaxis device was mounted onto an inverted microscope (Nikon Ti-E 2000) equipped with a live-cell chamber at 37°C and 5% CO2. The cells were imaged through the glass slide. Cells were stimulated in a 1 V cm−1 DC EF for 3–9 h before being fixed for immunofluorescence studies.
The trajectories of cells from time-lapse images were automatically tracked using MetaMorph software (Molecular Devices, Sunnyvale, CA, USA) to minimize any tracking biases. Only isolated cells that remained in the field of view and did not undergo mitosis were selected for analysis. Cell trajectories were further analyzed using a customized MATLAB (MathWorks, Natick, MA, USA) script to characterize physical parameters including cell motility and directedness. Here, we define cell motility as the total path length traveled by a cell divided by the elapsed time. The directedness is defined as Σcosθi/n and ranges between −1 and +1, where n is the total number of cells and θi is the angle between the vector of cell displacement and electric field vector (Huang et al., 2013). A directedness close to zero indicates random motion, whereas positive and negative values indicate cathodic and anodic responses, respectively. To determine statistical significance, we use a two-tailed Student's t-test (*P≤0.05; **P≤0.01; ***P≤0.001).
Confocal immunofluorescence imaging
For immunofluorescence imaging, cells were fixed with 3.7% formaldehyde and stained with selected antibodies without a permeabilization step to minimize any fluorescence background arising from inside the cells. Cells were then blocked with tris-buffered saline with 0.1% Tween 20 (TBST) containing 5% bovine serum albumin (BSA) for 1 h and incubated with mouse anti-HS (1:100, US Biological, 10E4 epitope) or rabbit anti-Slit2 (1:100, Abcam, ab7665) overnight at 4°C. After incubation, cells were extensively washed with TBST and stained with a secondary antibody conjugated to a fluorophore (1:200, Invitrogen).
To quantify the localization of membrane proteins, confocal fluorescence images were collected to minimize any out-of-focus pixel due to any variation throughout the cell surface (TiE, Nikon). Confocal z-stacks at 1 μm per step were collected for each cell at the excitation wavelength corresponding to the fluorophore used.
Quantification of protein localization
For each confocal z-stack, a maximum intensity projection was created using NIS-Elements software to generate a 2D image and converted to an 8-bit grayscale to quantify the localization of heparan sulfate. The contour of a 2D cell image was identified and vertically divided along its geometric center into a cathode- and an anode-facing side using ImageJ (Fig. 2G). Subsequently, a Wilcoxon signed rank test was performed among all the pixels in the cathode face to pixels in the anode face using R software, where P<0.05 is defined as a cell with anodic localization of HS.
Enzymatic removal of surface glycosaminoglycans
Cells were treated with 12.5 U ml−1 of HPase I and III blend (Sigma) at 37°C for 6 h before being subjected to an EF for galvanotaxis experiments. To remove CS and dermatan sulfate, cells were incubated with chondroitinase ABC (chABC) (2.5 U ml−1; Sigma) at 37°C for 6 h.
siRNA transfection using polymeric nanoparticles
Polymers (R646) designed to condense siRNA into bioreducible nanoparticles were made of poly(β-amino ester) as described previously (Kozielski et al., 2014). The particles have been shown to be capable of efficient gene knock-down in primary human glioblastoma cells without significant cytotoxicity (Tzeng et al., 2011; Guerrero-Cázares et al., 2014). BTICs plated in a 12-well plate at a density of 200,000 cells/well were allowed to adhere overnight before transfection. Cells were then transfected with siRNAs (OriGene Technologies, Rockville, MD, USA) for SDC1, SDC2, SDC3, SDC4 and GPC1 genes or a scrambled siRNA (SC) at a final concentration of 120 nM using polymeric nanoparticles (R646) following procedures reported previously (Kozielski et al., 2014). Transfected cells were collected for western blot and qRT-PCR analysis after 72 h.
Western blot and qRT-PCR
Cells in culture were lysed with RIPA buffer (Santa Cruz Biotechnology, Dallas, TX, USA) on ice for 30 min before collection with a cell scraper. Lysates were centrifuged at 10,000 g at 4°C for 15 min, and the supernatants were removed from the pellets and collected. Protein collected from each lysate was measured with a Pierce BCA protein assay kit (ThermoFisher Scientific) to ensure equal loading. Denatured lysates were loaded onto 4–15% gradient SDS-polyacrylaminde gels (Bio-Rad) and transferred to a nitrocellulose membrane (Bio-Rad). Membranes were blocked with TBST containing 5% milk at room temperature for 1 h and incubated with primary antibodies at 4°C overnight. For each experiment, GAPDH-HRP (Santa Cruz Biotechnology, FL-335, 1:400) was used as a loading control. The following antibodies were used at the specified concentrations: rabbit anti-syndecan 1 (Santa Cruz Biotechnology, H-174, 1:200), rabbit anti-syndecan 2 (Abcam, ab79978, 1:200), rabbit anti-syndecan 3 (Proteintech, 10886-1-AP, 1:500), rabbit anti-syndecan 4 (Abcam, ab24511, 1:200), mouse anti-glypican 1 (Santa Cruz Biotechnology, A-10, 1:200) and rabbit anti-Robo1 (Abcam, ab85312, 1:800). Secondary antibodies conjugated to HRP (Bio-Rad) were used at 1:2000. Immunoactive bands were detected using enhanced chemiluminescence and quantified using Image Lab software (Bio-Rad).
Samples for qRT-PCR were prepared using a Taqman cells-to-CT kit (ThermoFisher Scientific) following the manufacturer's recommended protocol and measured using a StepOnePlus system (ThermoFisher Scientific). mRNA expression level was calculated using [Δ(ΔCt)] relative to scrambled control, with GAPDH being used as a housekeeping gene.
Stable transduction of cells with shRNA
To induce the knockdown of Robo1 expression, we used pLKO.1 lentiviral particles containing shRNA sequences specific for human Robo1 transcripts (Mission shRNA TRCN00000414 and TRCN00000417; Sigma Aldrich). An empty vector was used as control (SHC001; Sigma Aldrich). Human fetal neural progenitor cells were treated with one of the three viruses at 1 μl per 2 ml with polybrene (8 μl ml−1) overnight. Virus-containing media were replaced the next day with complete media with 0.25 μg ml−1 puromyocin as selection agent. After one week, the concentration of puromycin was reduced to 0.125 μg ml−1 for maintenance. Knockdown was confirmed using western blot.
Acknowledgements
We thank Dr Cheng Ran (Lisa) Huang for scientific discussions.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: Y.-J.H., P. Searson; Methodology: Y.-J.H.; Formal analysis: Y.-J.H., P. Searson; Investigation: Y.-J.H.; Resources: P. Schiapparelli, K.K., J.G., E.L.; Writing – original draft: Y.-J.H.; Writing – review and editing: Y.-J.H., H.G.-C., A.Q., P. Searson; Supervision: P. Searson.
Funding
The work was supported by the National Institutes of Health (grant numbers R01CA170629, R01NS070024). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.203752.supplemental
References
- Allen G. M., Mogilner A. and Theriot J. A. (2013). Electrophoresis of cellular membrane components creates the directional cue guiding keratocyte galvanotaxis. Curr. Biol. 23, 560-568. 10.1016/j.cub.2013.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ba-Charvet K. T. N., Brose K., Marillat V., Kidd T., Goodman C. S., Tessier-Lavigne M., Sotelo C. and Chédotal A. (1999). Slit2-mediated chemorepulsion and collapse of developing forebrain axons. Neuron 22, 463-473. 10.1016/S0896-6273(00)80702-3 [DOI] [PubMed] [Google Scholar]
- Bass M. D., Roach K. A., Morgan M. R., Mostafavi-Pour Z., Schoen T., Muramatsu T., Mayer U., Ballestrem C., Spatz J. P. and Humphries M. J. (2007). Syndecan-4-dependent Rac1 regulation determines directional migration in response to the extracellular matrix. J. Cell Biol. 177, 527-538. 10.1083/jcb.200610076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Børset M., Hjertner Ø., Yaccoby S., Epstein J. and Sanderson R. D. (2000). Syndecan-1 is targeted to the uropods of polarized myeloma cells where it promotes adhesion and sequesters heparin-binding proteins. Blood 96, 2528-2536. [PubMed] [Google Scholar]
- Brose K., Bland K. S., Wang K. H., Arnott D., Henzel W., Goodman C. S., Tessier-Lavigne M. and Kidd T. (1999). Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell 96, 795-806. 10.1016/S0092-8674(00)80590-5 [DOI] [PubMed] [Google Scholar]
- Burr H. S. (1941). Field properties of the developing frog's egg. Proc. Natl Acad. Sci. USA 27, 276-281. 10.1073/pnas.27.6.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L., Wei D., Reid B., Zhao S., Pu J., Pan T., Yamoah E. N. and Zhao M. (2013). Endogenous electric currents might guide rostral migration of neuroblasts. EMBO Rep. 14, 184-190. 10.1038/embor.2012.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang K. S., Ionides E., Oster G., Nuccitelli R. and Isseroff R. R. (1999). Epidermal growth factor receptor relocalization and kinase activity are necessary for directional migration of keratinocytes in DC electric fields. J. Cell Sci. 112, 1967-1978. [DOI] [PubMed] [Google Scholar]
- Finkelstein E. I., Chao P.-G., Hung C. T. and Bulinski J. C. (2007). Electric field-induced polarization of charged cell surface proteins does not determine the direction of galvanotaxis. Cell Motil. Cytoskelet. 64, 833-846. 10.1002/cm.20227 [DOI] [PubMed] [Google Scholar]
- Fregni F., Nitsche M., Loo C., Brunoni A., Marangolo P., Leite J., Carvalho S., Bolognini N., Caumo W. and Paik N. (2015). Regulatory considerations for the clinical and research use of transcranial direct current stimulation (tDCS): review and recommendations from an expert panel. Clin. Res. Regul. Aff. 32, 22-35. 10.3109/10601333.2015.980944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Cázares H., Chaichana K. L. Quiñones-Hinojosa A. (2009). Neurosphere culture and human organotypic model to evaluate brain tumor stem cells. In Cancer Stem Cells, (Ed. Yu J. S.), pp. 73-83. New York, NY: Springer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Cázares H., Tzeng S. Y., Young N. P., Abutaleb A. O., Quiñones-Hinojosa A. and Green J. J. (2014). Biodegradable polymeric nanoparticles show high efficacy and specificity at DNA delivery to human glioblastoma in vitro and in vivo. ACS Nano 8, 5141-5153. 10.1021/nn501197v [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Cazares H., Lavell E., Drummond G., Ranamukhaarachchi S., Capilla-Gonzalez V., Schiapparelli P. and Quinones-Hinojosa A. (2015). Slit2 stimulation induces a chemorepellent effect on the migration of human GBM brain tumor initiating cells. Cancer Res. 75, 444-444 10.1158/1538-7445.AM2015-444 [DOI] [Google Scholar]
- Hu H. (2001). Cell-surface heparan sulfate is involved in the repulsive guidance activities of Slit2 protein. Nat. Neurosci. 4, 695-701. 10.1038/89482 [DOI] [PubMed] [Google Scholar]
- Huang Y.-J., Samorajski J., Kreimer R. and Searson P. C. (2013). The influence of electric field and confinement on cell motility. PLoS ONE 8, e59447 10.1371/journal.pone.0059447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.-J., Hoffmann G., Wheeler B., Schiapparelli P., Quinones-Hinojosa A. and Searson P. (2016). Cellular microenvironment modulates the galvanotaxis of brain tumor initiating cells. Sci. Rep. 6, 21583 10.1038/srep21583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L. F. (1977). Electrophoresis along cell membranes. Nature 265, 600-602. 10.1038/265600a0 [DOI] [PubMed] [Google Scholar]
- Kaneko N., Marín O., Koike M., Hirota Y., Uchiyama Y., Wu J. Y., Lu Q., Tessier-Lavigne M., Alvarez-Buylla A., Okano H. et al. (2010). New neurons clear the path of astrocytic processes for their rapid migration in the adult brain. Neuron 67, 213-223. 10.1016/j.neuron.2010.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondapalli K. C., Llongueras J. P., Capilla-González V., Prasad H., Hack A., Smith C., Guerrero-Cázares H., Quiñones-Hinojosa A. and Rao R. (2015). A leak pathway for luminal protons in endosomes drives oncogenic signalling in glioblastoma. Nat. Commun. 6, 6289 10.1038/ncomms7289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozielski K. L., Tzeng S. Y., Hurtado De Mendoza B. A. and Green J. J. (2014). Bioreducible cationic polymer-based nanoparticles for efficient and environmentally triggered cytoplasmic siRNA delivery to primary human brain cancer cells. ACS Nano 8, 3232-3241. 10.1021/nn500704t [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Wijesekera O., Salas S. J., Wang J. Y., Zhu M., Aprhys C., Chaichana K. L., Chesler D. A., Zhang H. and Smith C. L. (2014). Mesenchymal stem cells from human fat engineered to secrete BMP4 are nononcogenic, suppress brain cancer, and prolong survival. Clin. Cancer Res. 20, 2375-2387. 10.1158/1078-0432.CCR-13-1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F., Baldessari F., Gyenge C. C., Sato T., Chambers R. D., Santiago J. G. and Butcher E. C. (2008). Lymphocyte electrotaxis in vitro and in vivo. J. Immunol. 181, 2465-2471. 10.4049/jimmunol.181.4.2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H. K., Marchant L., Carmona-Fontaine C., Kuriyama S., Larraín J., Holt M. R., Parsons M. and Mayor R. (2008). Directional migration of neural crest cells in vivo is regulated by Syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA. Development 135, 1771-1780. 10.1242/dev.017350 [DOI] [PubMed] [Google Scholar]
- McLaughlin S. and Poo M. M. (1981). The role of electro-osmosis in the electric-field-induced movement of charged macromolecules on the surfaces of cells. Biophys. J. 34, 85 10.1016/S0006-3495(81)84838-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerli M. A. and Graham D. M. (2011). Extracellular electrical fields direct wound healing and regeneration. Biol. Bull. 221, 79-92. 10.1086/BBLv221n1p79 [DOI] [PubMed] [Google Scholar]
- Ming G.-l., Song H.-j., Berninger B., Holt C. E., Tessier-Lavigne M. and Poo M.-m. (1997). cAMP-dependent growth cone guidance by netrin-1. Neuron 19, 1225-1235. 10.1016/S0896-6273(00)80414-6 [DOI] [PubMed] [Google Scholar]
- Mycielska M. E. and Djamgoz M. B. (2004). Cellular mechanisms of direct-current electric field effects: galvanotaxis and metastatic disease. J. Cell Sci. 117, 1631-1639. 10.1242/jcs.01125 [DOI] [PubMed] [Google Scholar]
- Nakajima K.-i., Zhu K., Sun Y.-H., Hegyi B., Zeng Q., Murphy C. J., Small J. V., Chen-Izu Y., Izumiya Y. and Penninger J. M. (2015). KCNJ15/Kir4. 2 couples with polyamines to sense weak extracellular electric fields in galvanotaxis. Nat. Commun. 6, 8532 10.1038/ncomms9532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placone A. L., McGuiggan P. M., Bergles D. E., Guerrero-Cazares H., Quinones-Hinojosa A. and Searson P. C. (2015). Human astrocytes develop physiological morphology and remain quiescent in a novel 3D matrix. Biomaterials 42, 134-143. 10.1016/j.biomaterials.2014.11.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M.-M. and Robinson K. R. (1977). Electrophoresis of concanavalin A receptors along embryonic muscle cell membrane. Nature 265, 602-605. 10.1038/265602a0 [DOI] [PubMed] [Google Scholar]
- Poo M.-M., Lam J. W., Orida N. and Chao A. W. (1979). Electrophoresis and diffusion in the plane of the cell-membrane. Biophys. J. 26, 1-21. 10.1016/S0006-3495(79)85231-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullar C. E. and Isseroff R. R. (2005). Cyclic AMP mediates keratinocyte directional migration in an electric field. J. Cell Sci. 118, 2023-2034. 10.1242/jcs.02330 [DOI] [PubMed] [Google Scholar]
- Rajnicek A. M., Robinson K. R. and McCaig C. D. (1998). The direction of neurite growth in a weak DC electric field depends on the substratum: contributions of adhesivity and net surface charge. Dev. Biol. 203, 412-423. 10.1006/dbio.1998.9039 [DOI] [PubMed] [Google Scholar]
- Rueger M. A., Keuters M. H., Walberer M., Braun R., Klein R., Sparing R., Fink G. R., Graf R. and Schroeter M. (2012). Multi-session transcranial direct current stimulation (tDCS) elicits inflammatory and regenerative processes in the rat brain. PLoS ONE 7, e43776 10.1371/journal.pone.0043776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaiyan F., Kolset S. O., Prydz K., Gottfridsson E., Lindahl U. and Salmivirta M. (1999). Selective effects of sodium chlorate treatment on the sulfation of heparan sulfate. J. Biol. Chem. 274, 36267-36273. 10.1074/jbc.274.51.36267 [DOI] [PubMed] [Google Scholar]
- Sarrazin S., Lamanna W. C. and Esko J. D. (2011). Heparan sulfate proteoglycans. Cold Spring Harbor Perspect. Biol. 3, a004952 10.1101/cshperspect.a004952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M. J., Kuwayama H., van Egmond W. N., Takayama A. L. K., Takagi H., van Haastert P. J. M., Yanagida T. and Ueda M. (2009). Switching direction in electric-signal-induced cell migration by cyclic guanosine monophosphate and phosphatidylinositol signaling. Proc. Natl Acad. Sci. USA 106, 6667-6672. 10.1073/pnas.0809974106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R. and Borgens R. B. (1994). Embryonic neuroepithelial sodium transport, the resulting physiological potential, and cranial development. Dev. Biol. 165, 105-116. 10.1006/dbio.1994.1238 [DOI] [PubMed] [Google Scholar]
- Shipp E. L. and Hsieh-Wilson L. C. (2007). Profiling the sulfation specificities of glycosaminoglycan interactions with growth factors and chemotactic proteins using microarrays. Chem. Biol. 14, 195-208. 10.1016/j.chembiol.2006.12.009 [DOI] [PubMed] [Google Scholar]
- Song B., Zhao M., Forrester J. and McCaig C. (2004). Nerve regeneration and wound healing are stimulated and directed by an endogenous electrical field in vivo. J. Cell Sci. 117, 4681-4690. 10.1242/jcs.01341 [DOI] [PubMed] [Google Scholar]
- Sun Y., Do H., Gao J., Zhao R., Zhao M. and Mogilner A. (2013). Keratocyte fragments and cells utilize competing pathways to move in opposite directions in an electric field. Curr. Biol. 23, 569-574. 10.1016/j.cub.2013.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng S. Y., Guerrero-Cázares H., Martinez E. E., Sunshine J. C., Quiñones-Hinojosa A. and Green J. J. (2011). Non-viral gene delivery nanoparticles based on poly (β-amino esters) for treatment of glioblastoma. Biomaterials 32, 5402-5410. 10.1016/j.biomaterials.2011.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartanian T., Sprinkle T. J., Dawson G. and Szuchet S. (1988). Oligodendrocyte substratum adhesion modulates expression of adenylate cyclase-linked receptors. Proc. Natl Acad. Sci. USA 85, 939-943. 10.1073/pnas.85.3.939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh H. S., Johung T. B., Caretti V., Noll A., Tang Y., Nagaraja S., Gibson E. M., Mount C. W., Polepalli J. and Mitra S. S. (2015). Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell 161, 803-816. 10.1016/j.cell.2015.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight T. N., Kinsella M. G. and Qwarnström E. E. (1992). The role of proteoglycans in cell adhesion, migration and proliferation. Curr. Opin. Cell Biol. 4, 793-801. 10.1016/0955-0674(92)90102-I [DOI] [PubMed] [Google Scholar]
- Yang H.-y., Charles R.-P., Hummler E., Baines D. L. and Isseroff R. R. (2013). The epithelial sodium channel mediates the directionality of galvanotaxis in human keratinocytes. J. Cell Sci. 126, 1942-1951. 10.1242/jcs.113225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagyansky Y. A. and Jard S. (1979). Does lectin–receptor complex formation produce zones of restricted mobility within the membrane? Nature 280, 591-593. 10.1038/280591a0 [DOI] [PubMed] [Google Scholar]
- Zhao M., Song B., Pu J., Wada T., Reid B., Tai G., Wang F., Guo A., Walczysko P. and Gu Y. (2006). Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-γ and PTEN. Nature 442, 457-460. 10.1038/nature04925 [DOI] [PubMed] [Google Scholar]
- Zhu K., Sun Y., Miu A., Yen M., Liu B., Zeng Q., Mogilner A. and Zhao M. (2015). cAMP and cGMP play an essential role in galvanotaxis of cell fragments. J. Cell. Physiol. 231, 1291-1300. 10.1002/jcp.25229 [DOI] [PMC free article] [PubMed] [Google Scholar]