Abstract
Background
Combined pH-impedance monitoring has been suggested as the investigation of choice for diagnosing gastro-oesophageal reflux in children. Although it is superior to oesophageal pH monitoring in detecting all types of reflux episodes (acid, weakly acidic and alkaline) with the ability to evaluate symptom association with reflux events, it is still limited by the lack of true paediatric normal value and the high cost involved (equipment and personnel).
Objective
To produce a position statement on behalf of the Motility Working Group of the British Society of Paediatric Gastroenterology, Hepatology and Nutrition on the indications and practical application of combined oesophageal pH-impedance monitoring in children.
Methods
Up-to-date review of available evidence.
Results
This document provides a practical guide to clinician on indications, methods and results interpretation of paediatric multichannel intraluminal impedance pH (MII-pH).
Conclusions
MII-pH is increasingly used by paediatricians as the diagnostic tool for assessing gastro-oesophageal reflux disease and symptom association. There is wide variation in paediatric practice and a need for standardised practice.
Keywords: PAEDIATRIC GASTROENTEROLOGY, GASTROESOPHAGEAL REFLUX DISEASE, OESOPHAGEAL DISORDERS, OESOPHAGEAL PH MONITORING
Introduction
Combined oesophageal multichannel intraluminal impedance and pH (MII-pH) monitoring is increasingly being used as the diagnostic tool for gastro-oesophageal reflux disease (GORD) in children.1 2 The main advantage over standard oesophageal pH measurement is the ability to detect the frequency and height of reflux episodes independent of pH.3 Moreover, with the increasing use of combined MII-pH monitoring, greater diagnostic information is now available, particularly in the group of patients with continuing symptoms due to suspected GORD despite adequate therapy. MII-pH is now widely available for paediatricians; however, with no agreed guidelines there is a considerable diversity in practice.4
The British Society of Paediatric Gastroenterology, Hepatology and Nutrition (BSPGHAN)—Motility Working Group therefore undertook to provide an up-to-date guidance on the indications, methodology and interpretation of MII-pH studies in children. The European Paediatric Impedance Group (EURO-PIG) under the auspices of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) published a protocol for MII-pH in 2012.1 There have since been many developments and experience that need to be assessed and incorporated in order to establish contemporary guidance for this procedure in children.
The literature was reviewed by searching all available publications, databases and conference proceedings by a core group from the BSPGHAN Motility Working Group. The group then set out specific tasks to the members before circulating and coordinating the final document.
Indications
The indications of MII-pH monitoring in children are to measure GOR and ascertain symptoms association, to quantify reflux in children not responding to therapy and in research.1 MII-pH is useful in assessing extra-oesophageal symptoms such as cough5 and respiratory symptoms6; it can be performed on or off therapy and on children fed via continuous or bolus feed. It is also useful in diagnosing children with aerophagia.7 8 The British National Institute for Health and Care Excellence in their GORD in children: diagnosis and management9 suggested performing MII-pH in infant and children with
recurrent aspiration pneumonia
unexplained apnoeas
unexplained non-epileptic seizure-like events
unexplained upper airway inflammation
dental erosion associated with neurodisability
frequent otitis media
a possible need for fundoplication
a suspected diagnosis of Sandifer's syndrome
Principle of oesophageal MII testing
Oesophageal MII detects bolus flow by measuring luminal content resistance to alternating current (AC). The impedance circuit includes an AC generator (in the recording device) and multiple metal rings placed across a non-conducting catheter. To close the circuit, the current must travel through the oesophageal mucosa or luminal content. As electrical conductivity is improved by high ironic load and impedance is inversely proportional to electrical conductivity, refluxate and liquid swallows will have good conductivity and low impedance while air has poor conductivity thus high impedance.10
The presence of a number of impedance rings in the catheter allows the sequential measurement of impedance to determine the direction of bolus flow and identification of retrograde movement (reflux) or antegrade flow (swallow).
Recording devices and software
Ambulatory and stationary systems are available and MII-pH measurements are feasible in all age groups from pre-mature infants through to adolescents. There are currently three brands of MII-pH measurement devices commercially available, each with their own software; Sandhill Scientific (BioView analysis), MMS (Ohmega software) and Vizion (Vizion software). Software from the different manufacturers offer similar capabilities regarding the automated analysis and graphic display.
Catheters
The principal components of MII-pH monitoring systems are a flexible catheter (6 Fr, 2 mm in diameter) with pH electrodes, multiple impedance rings and a data-recording device.3 While different types of pH electrodes are available for pH monitoring, such as glass, ion-sensitive field effect transistor or antimony sensors,11 for pH-impedance monitoring the most popular catheters are those with one antimony pH-measuring electrode and seven impedance rings with internal reference electrode. No external skin electrode is required with the use of these catheters as their internal reference electrode is located at the tip of the catheter 3–5 cm distal to the distal pH sensor, which may lead to theoretical potential to increase the GOR episodes as the catheter traverses the lower oesophageal sphincter (LOS).12
Catheter length should be age (height) appropriate (table 1). Depending on the manufacturer, the distance between impedance rings and the location of pH sensor on the catheters may vary. However, the distance between each impedance ring is usually 1.5 cm for the infant catheters, generally used in young children (height <75 cm), and 2 cm for the paediatric and adult MII-pH catheters, used in older children (height >75 cm) and young adult (height >150 cm) respectively, resulting in a total measuring length of 9 cm for infantile and 12 cm for paediatric and adult catheters. In infant and paediatric MII-pH catheters, the pH-measuring electrode is localised within the distal impedance-recording channel, while in adult MII-pH catheters is within the second most distal impedance channel. Single-use catheters should be the preferred option.
Table 1.
Characteristic of multichannel intraluminal impedance-pH catheter
| Catheter type | Catheter length | Spacing of impedance rings | Spacing of pH sensor |
|---|---|---|---|
| Infant | Height <75 cm | 1.5 cm apart | In centre of most distal impedance channel |
| Paediatric | Height >75 cm <150 cm |
2 cm apart | In centre of most distal impedance channel |
| Adult | Height >150 cm | 2 cm apart | In centre of second most distal impedance channel |
Preparation of the catheter
All catheters need to be calibrated prior to use according to the manufacturer instructions; it is of pivotal importance to carry out an accurate calibration because even small errors in the procedure can lead to erroneous results. There is always a risk of pH drift so any pH electrodes that show unstable calibration should not be used.
Placement of catheter
The catheter is passed transnasally into the oesophagus then positioned so that the distal oesophageal pH electrode is located at the appropriate position. Patient should ideally be fasted for 2–4 hours (depending on their age) before catheter insertion to avoid inducing vomiting. Lubricating gel can be used but should not be placed directly on the antimony probe to avoid inaccuracy of data captured. Sedation should be discouraged as it may influence the LOS pressure.13 14 This group suggests careful evaluation of studies where catheters were inserted under sedation or general anaesthesia.
Correct anatomic placement of the electrode is crucial for obtaining clinically useful oesophageal pH-impedance measurements. Variation from the correct position directly affects the amount of acid reflux detected. For instance, patients with excessive acid exposure whose probe is positioned proximally to the correct position may appear to have normal acid reflux, while those with normal acid exposure may be considered abnormal if the probe is placed distally to the correct position above the LOS. The current ESPGHAN consensus is that the pH electrode should be positioned two vertebral bodies above the diaphragm as noted at the level of the vertebral column.1 In infants, this position can be estimated by the use of the Strobel formula (0.252×body length in centimetres+5).15 This formula is not as accurate in older children as it overestimates the oesophageal length. Mutalib et al16 (table 2) described an accurate method to estimate catheter length in children based on their body length. The placement of the distal pH should be estimated at 1.5 cm (infants), 3 cm (<10 years old) or 5 cm (>10 years old) above the LOS if placed endoscopically; however, fluoroscopic or X-ray confirmation of position is essential. When applicable, oesophageal manometry can be used to measure distance to LOS.
Table 2.
Estimation of multichannel intraluminal impedance-pH catheter length according to height
| Height (cm) | Catheter depth (cm) |
|---|---|
| 56–68 | 15–17.5 |
| 68–80 | 17.5–20 |
| 80–86 | 20–22.5 |
| 86–92 | 22.5–25 |
| 92–98 | 25–26 |
| 98–116 | 26–27.5 |
| 116–128 | 28–30 |
| 128–140 | 30–32 |
| 140–152 | 32–35 |
| 152–176 | 35–40 |
Reproduced with permission from John Wiley & Sons Ltd.16
Patient instructions
The study analysis and interpretation is dependent upon the data acquired and the information received. The monitoring conditions should be as unrestricted as possible emphasising the daily habits that may replicate the symptoms of the patient.
The study duration should be ideally for 24 hours but a minimum of 16 hours and should incorporate an overnight recording.17 Shorter studies may have reduced sensitivity and poor reproducibility.18 The study period should also include mealtimes and fasting periods.
Acid suppression therapy should be stopped before the procedure; histamine 2 (H2) blockade should be stopped 48 hours and proton pump inhibitors 7 days before the study. Prokinetics should be stopped 48 hours and antacids 6 hours before the study. This obviously is not the case if the study is performed to evaluate the efficacy of acid suppression therapy.19
The use of designated ‘event’ buttons on the data-recording device should be clearly explained and should be pressed with the occurrence of every symptom experienced. For symptom correlation, the relevant symptoms should be agreed before commencement of the study. Children should avoid acidic foods, carbonated drinks and food substances of extreme temperature. A manual symptom diary should be used to record other symptoms. Unrelated symptoms that are not being assessed during the investigation should not be included as symptom events as this will likely make interpretation more difficult.
Analysis
After completion of the recording, data are uploaded to the appropriate software. Events from the symptom diary (if a paper form is used) should be added before analysis. Symptom diaries are mostly recorded by parents or carers and the time of recoding might be delayed or approximated. When analysing symptom association, a 2 min time window before a reflux event is considered an accepted interval for reflux association.1 20 While widely accepted in paediatric clinical practice,6 21 the 2 min window is not evidence based but was extrapolated from the analysis of reflux-related non-cardiac chest pain in adults.20
Before the start of analysis, traces should be screened to ensure the study is technically valid and to remove artefacts and duplicate symptoms. This group agreed that any duplicate symptom within a given 4 min window should be deleted.
It is important to allocate sufficient resources to the process of analysing MII-pH studies as an erroneous or a hastily generated report could lead to inappropriate interventions. A gastrointestinal physiologist or nurse specialist usually performs the analysis and a physician interprets the report. It takes between 30 min and 4 hours to completely analyse a study depending on the number of reflux episodes and the experience of the person analysing the study.22 This working group suggests a minimum of 2 hours uninterrupted to analyse the study and generate the report with longer time reserved for difficult studies.
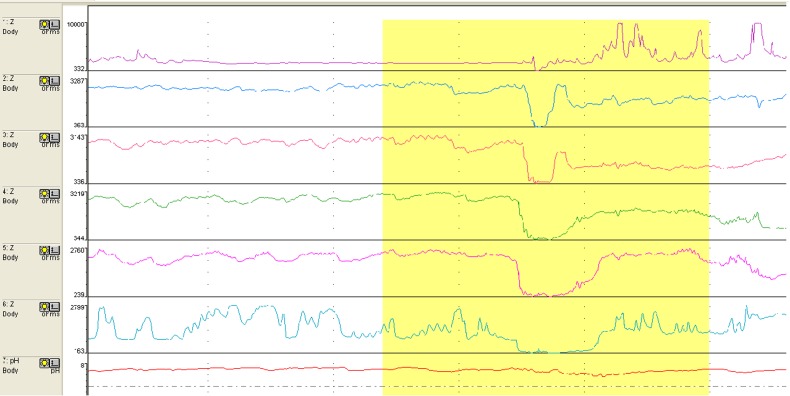
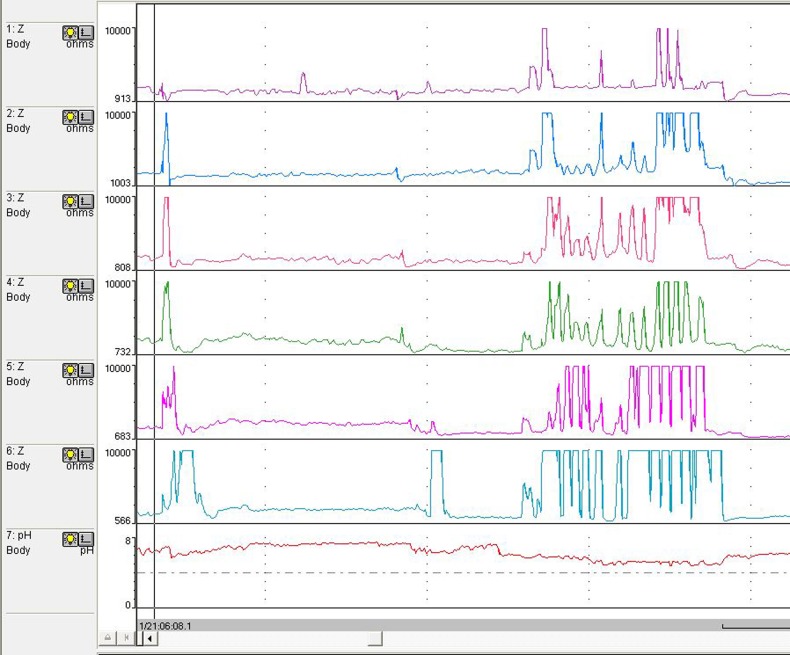
The analysis is usually begun with automated analysis but must be followed by a ‘manual’ visual inspection of the recording to add/delete reflux events throughout the study. Liquid reflux is defined as a drop in impedance to <50% of the baseline impedance (BI) value starting at the distal sensors and propagating up the oesophageal body to at least the next two proximal sensors (representing two ‘channels’)1 23 (figure 1). Gas reflux is defined as a rapid and simultaneous increase in impedance to >3000 ohms in at least two distal oesophageal sensors without an accompanying swallow23 (figure 2). Refluxate can be either pure liquid, pure gas or mixed containing features of both liquid and gas. Detection of pure gas reflux is optionally included in the automated analysis. Reflux is classified as acid when pH falls <4, weakly acidic for pH between 4 and 7 or weakly alkaline if pH is >7. Or it could simply be classified as acid (pH<4) and non-acid (pH>4).23 pH-only episodes commonly occur in infants and are not usually accompanied by MII reflux; this slow pH drift can be caused by residual reflux or oesophageal shortening is confined to the distal oesophagus and does not travel high enough in the oesophageal body to trigger GOR episode in the impedance channels.
Figure 1.
Reflux event.
Figure 2.
Air swallow and gas reflux.
Meals are usually excluded from the automated analysis as the repeated act of swallowing will reduce the ability of automated software to detect reflux episodes, but as reflux is known to occur during meals, assessment of GOR during meals should be done manually. An isolated drop of pH to <4 that is not associated with retrograde bolus flow during meals should be interpreted with caution as many carbonated drinks and some juices have a pH value of <4.3 Nasogastric (NG) tubes transverse the LOS and increase the number of postprandial GOR episodes. This should be taken into consideration when interpreting MII-pH results of children fed via NG tubes. Studies are feasible in children on continuous bolus feed as there is no antegrade flow across the catheter; meal time analysis is relatively easy in this setting.24
GOR episode that reached the uppermost recoding sensor is considered as reaching the proximal oesophagus.25 Proximal GOR episodes are particularly useful in evaluating the correlation between extra-oesophageal symptoms and GOR.26 Some of the extra-oesophageal symptoms like apnoea and apparent life-threatening event (ALTEs) occur in low frequency during standard 24 hour monitoring, rendering statistical correlation between GOR and symptom inaccurate. We recommend careful evaluation of the trace to ascertain reflux correlation. There are a number of other parameters that are included in the analysis and are generated in the report such as, but not limited to, the total number of reflux episodes during different postures (upright and recumbent) and their pH classification and oesophageal acid exposure time.
Symptom correlation with reflux is reported as symptom index (SI), symptom sensitivity index (SSI) or symptom-associated probability (SAP). SI is defined as the percentage of reflux-associated symptoms divided by the total number of symptoms. It should be reported separately for each symptom and is regarded as positive if SI is ≥50%.27 The main limitation of SI is that it does not take into account the total number of reflux episodes leading to an increased chance of false-positive symptom association with high number of GOR episodes or small number of symptoms.1 SSI is defined as the number of symptom-associated reflux episodes divided by the total number of reflux episodes ×100%.28 An SSI value of ≥10% for each symptom is regarded as positive. However, SSI is likely to produce false-positive results when there is a high frequency of reported symptom and has very limited clinical use. SAP uses a complex statistical calculation to determine individual symptom correlation in each 2 min window of the study.29 SAP is considered the most appropriate method to characterise the association symptoms and GOR as it is least affected by the number of symptoms and/or the number of reflux episodes. SAP values >95% indicate that the observed GOR-symptom relationship is not bought by chance.1 29
There is no consensus regarding the minimum number of symptoms to produce the most reliable correlation and it is likely to differ in different symptoms. However, symptom number of <5 is more likely to produce a positive SI and a negative SAP.29 This creates a dilemma for paediatricians as it could miss the association with serious symptoms such as ALTEs, which usually occur in low numbers.
Normal values for MII-pH are results of consensus agreement, data extrapolation from adults and studying children with symptoms suggestive of GORD. ESPGHAN EURO-PIG has adopted the finding from the German Paediatric Impedance Group as the accepted normal values for children.1 30 Up to 100 reflux episodes in infants aged <1 year and oesophageal acid exposure time up to 10% and up to 70 episodes in children aged >1 year and oesophageal acid exposure time <3% are regarded as normal. Oesophageal acid exposure time of >7% is abnormal while values between 3% and 7% are indeterminate.1 A report of normal values in a cohort of normal infants and children by Mousa et al19 produced similar results (table 3).
Table 3.
Reported normal values (95th percentile) of normal infants*, children* and adults†
| Upright | Recumbent | Total | |
|---|---|---|---|
| Infants* | |||
| Acid reflux episodes | 33 | 24 | 48 |
| Non-acid episodes | 46 | 48 | 67 |
| Total GOR episodes | 68 | 61 | 93 |
| ESPGHAN (EURO PIG)‡ | 100 | ||
| Children* | |||
| Acid reflux episodes | 52 | 15 | 55 |
| Non-acid episodes | 25 | 9 | 34 |
| Total GOR episodes | 69 | 19 | 71 |
| ESPGHAN (EURO-PIG)‡ | 70 | ||
| Adults† | |||
| Acid reflux episodes | 52 | 5 | 55 |
| Non-acid episodes | 25 | 4 | 27 |
| Total GOR episodes | 67 | 7 | 73 |
Baseline impedance
BI reflects the intrinsic conductivity of oesophageal wall; its use might provide information both on oesophageal mucosal integrity and chemical clearance.31 BI measurement is usually lower in patients with GORD.32 Adults and children with erosive oesophagitis have a lower BI level compared with patients with no mucosal damage.33 In children, there appears to be an inverse relationship between distal BI and oesophageal acid exposure time, total number of reflux episodes and the number of acid reflux events, but no clear association between BI and ultrastructural changes in the oesophageal mucosa.33 Measuring distal and proximal BI of the oesophageal body can help identify a group of children with oesophageal motility disorder who may require further motility studies.
Reporting
The report for MII-pH should include a quantitative assessment of different parameters included in the analysis and a qualitative assessment of the symptom correlation. A summary including the duration of the study, symptoms under investigation and medication use should be included in the report as should a clinical interpretation of the finding with treatment recommendation.
Conclusion
MII-pH monitoring is becoming the investigation of choice for diagnosing GOR in children due to its ability both in detecting all types of reflux episodes and evaluating the association of symptoms with reflux events irrespective of their chemistry.
As the cost of the device is decreasing, combined MII-pH is rapidly replacing isolated pH monitoring as the investigation of choice for diagnosing GOR in children. However, certain limitations remain unresolved. The lack of standardisation in results interpretation and the unavailability of true paediatric normative value are areas that require further development.
Analysing MII-pH is a skill that requires specific training and can be time consuming. There remains an urgent need to standardise training and setting of a minimum standard for performing combined MII-pH in children.
Acknowledgments
The authors acknowledge the invaluable contribution of Professor Daniel Sifrim from the Wingate Institute of Neurogastroenterology at Barts and The London School of Medicine and Mrs Rachael McGhee, gastrointestinal clinical physiologist from Alder Hey Children's Hospital Liverpool, for their suggestions and review of the manuscript.
Footnotes
Contributors: The article was written jointly by the Motility Working Group.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Wenzl TG, Benninga MA, Loots CM, et al. Indications, methodology, and interpretation of combined esophageal impedance-pH monitoring in children: ESPGHAN EURO-PIG standard protocol. J Pediatr Gastroenterol Nutr 2012;55:230–4. doi:10.1097/MPG.0b013e3182592b65 [DOI] [PubMed] [Google Scholar]
- 2.Vandenplas Y, Rudolph CD Di Lorenzo C, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr 2009;49:498–547. doi:10.1097/MPG.0b013e3181b7f563 [DOI] [PubMed] [Google Scholar]
- 3.Tutuian R, Castell DO. Review article: complete gastro-oesophageal reflux monitoring—combined pH and impedance. Aliment Pharmacol Ther 2006;24(Suppl 2):27–37. doi:10.1111/j.1365-2036.2006.03039.x [DOI] [PubMed] [Google Scholar]
- 4.Loots CM, van Wijk MP, Blondeau K, et al. Interobserver and intraobserver variability in pH-impedance analysis between 10 experts and automated analysis. J Pediatr 2012;160:441–6.e1. doi:10.1016/j.jpeds.2011.08.017 [DOI] [PubMed] [Google Scholar]
- 5.Condino AA, Sondheimer J, Pan Z, et al. Evaluation of gastroesophageal reflux in pediatric patients with asthma using impedance-pH monitoring. J Pediatr 2006;149:219–19. doi:10.1016/j.jpeds.2006.03.022 [DOI] [PubMed] [Google Scholar]
- 6.Thilmany C, Beck-Ripp J, Griese M. Acid and non-acid gastro-esophageal refluxes in children with chronic pulmonary diseases. Respir Med 2007;101:969–76. doi:10.1016/j.rmed.2006.09.006 [DOI] [PubMed] [Google Scholar]
- 7.Halb C, Pomerleau M, Faure C. Multichannel intraesophageal impedance pattern of children with aerophagia. Neurogastroenterol Motil 2014;26:1010–14. doi:10.1111/nmo.12355 [DOI] [PubMed] [Google Scholar]
- 8.Rybak A, Gomez CT, Lindley K, et al. OP-15 features of aerophagia measured with multichannel ph-impedance in children. J Pediatr Gastroenterol Nutr 2015;61:515–16. doi:10.1097/01.mpg.0000472219.55927.d2 [Google Scholar]
- 9.National Institute for Health and Clinical Excellence. Gastro-oesophageal reflux disease in children and young people: diagnosis and management. NICE guideline (NG1) 2015. [PubMed] [Google Scholar]
- 10.Sifrim D, Fornari F. Esophageal impedance-pH monitoring. Dig Liver Dis 2008;40:161–6. doi:10.1016/j.dld.2007.10.023 [DOI] [PubMed] [Google Scholar]
- 11.Hemmink GJM, Weusten BLAM, Oors J, et al. Ambulatory oesophageal pH monitoring: a comparison between antimony, ISFET, and glass pH electrodes. Eur J Gastroenterol Hepatol 2010;22:572–7. doi:10.1097/MEG.0b013e328333139f [DOI] [PubMed] [Google Scholar]
- 12.Peter CS, Wiechers C, Bohnhorst B, et al. Influence of nasogastric tubes on gastroesophageal reflux in preterm infants: a multiple intraluminal impedance study. J Pediatr 2002;141:277–9. doi:10.1067/mpd.2002.126298 [DOI] [PubMed] [Google Scholar]
- 13.Marsh JK, Hoffman SM, Dmuchowski CF. Effect of intravenous midazolam on esophageal motility testing in normal human volunteers. Am J Gastroenterol 1993;88:860–3. [PubMed] [Google Scholar]
- 14.Waasdorp Hurtado C, Sheiko MA, Gralla J, et al. Effects of anesthesia on pediatric pH impedance. J Pediatr Gastroenterol Nutr 2016;63:236–41. doi:10.1097/MPG.0000000000001098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strobel CT, Byrne WJ, Ament ME, et al. Correlation of esophageal lengths in children with height: application to the Tuttle test without prior esophageal manometry. J Pediatr 1979;94:81–4. doi:10.1016/S0022-3476(79)80361-3 [DOI] [PubMed] [Google Scholar]
- 16.Mutalib M, Sintusek P, Punpanich D, et al. A new method to estimate catheter length for esophageal multichannel intraluminal impedance monitoring in children. Neurogastroenterol Motil 2015;27:728–33. doi:10.1111/nmo.12547 [DOI] [PubMed] [Google Scholar]
- 17.Dobhan R, Castell DO. Prolonged intraesophageal pH monitoring with 16-hr overnight recording. Comparison with “24-hr” analysis. Dig Dis Sci 1992;37:857–64. [DOI] [PubMed] [Google Scholar]
- 18.Pandolfino JE, Vela MF. Esophageal-reflux monitoring. Gastrointest Endosc 2009;69:917–30, 930.e1 doi:10.1016/j.gie.2008.09.022 [DOI] [PubMed] [Google Scholar]
- 19.Mousa H, Machado R, Orsi M, et al. Combined multichannel intraluminal impedance-pH (MII-pH): multicenter report of normal values from 117 children. Curr Gastroenterol Rep 2014;16:400 doi:10.1007/s11894-014-0400-6 [DOI] [PubMed] [Google Scholar]
- 20.Lam HG, Breumelhof R, Roelofs JM, et al. What is the optimal time window in symptom analysis of 24-hour esophageal pressure and pH data? Dig Dis Sci 1994;39:402–9. [DOI] [PubMed] [Google Scholar]
- 21.Rosen R, Nurko S. The importance of multichannel intraluminal impedance in the evaluation of children with persistent respiratory symptoms. Am J Gastroenterol 2004;99:2452–8. doi:10.1111/j.1572-0241.2004.40268.x [DOI] [PubMed] [Google Scholar]
- 22.van Wijk MP, Benninga MA, Omari TI. Role of the multichannel intraluminal impedance technique in infants and children. J Pediatr Gastroenterol Nutr 2009;48:2–12. doi:10.1097/MPG.0b013e31818f0902 [DOI] [PubMed] [Google Scholar]
- 23.Sifrim D, Holloway R, Silny J, et al. Acid, nonacid, and gas reflux in patients with gastroesophageal reflux disease during ambulatory 24-hour pH-impedance recordings. Gastroenterology 2001;120:1588–98. doi:10.1053/gast.2001.24841 [DOI] [PubMed] [Google Scholar]
- 24.Mousa HM, Rosen R, Woodley FW, et al. Esophageal impedance monitoring for gastroesophageal reflux. J Pediatr Gastroenterol Nutr 2011;52:129–39. doi:10.1097/MPG.0b013e3181ffde67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weusten BL, Akkermans LM, vanBerge-Henegouwen GP, et al. Spatiotemporal characteristics of physiological gastroesophageal reflux. Am J Physiol 1994;266(Pt 1):G357–62. [DOI] [PubMed] [Google Scholar]
- 26.Tolia V, Vandenplas Y. Systematic review: the extra-oesophageal symptoms of gastro-oesophageal reflux disease in children. Aliment Pharmacol Ther 2009;29:258–72. doi:10.1111/j.1365-2036.2008.03879.x [DOI] [PubMed] [Google Scholar]
- 27.Bredenoord AJ, Weusten BL, Smout AJ. Symptom association analysis in ambulatory gastro-oesophageal reflux monitoring. Gut 2005;54:1810–17. doi:10.1136/gut.2005.072629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breumelhof R, Smout AJPM. The symptom sensitivity index: additional parameter in 24-hour esophageal pH recording. Am J Gastroenterol 1991;86:161–4. [PubMed] [Google Scholar]
- 29.Weusten BL, Roelofs JM, Akkermans LM, et al. The symptom-association probability: an improved method for symptom analysis of 24-hour esophageal pH data. Gastroenterology 1994;107:1741–5. doi:10.1016/0016-5085(94)90815-X [DOI] [PubMed] [Google Scholar]
- 30.Pilic D, Fröhlich T, Nöh F, et al. Detection of gastroesophageal reflux in children using combined multichannel intraluminal impedance and pH measurement: data from the German Pediatric Impedance Group. J Pediatr 2011;158:650–4.e1. doi:10.1016/j.jpeds.2010.09.033 [DOI] [PubMed] [Google Scholar]
- 31.Heard R, Castell J, Castell DO, et al. Characterization of patients with low baseline impedance on multichannel intraluminal impedance-pH reflux testing. J Clin Gastroenterol 2012;46:e55–7. doi:10.1097/MCG.0b013e318247c319 [DOI] [PubMed] [Google Scholar]
- 32.Kessing BF, Bredenoord AJ, Weijenborg PW, et al. J. Esophageal acid exposure decreases intraluminal baseline impedance levels. Am J Gastroenterol 2011;106:2093–7. doi:10.1038/ajg.2011.276 [DOI] [PubMed] [Google Scholar]
- 33.Borrelli O, Salvatore S, Mancini V, et al. Relationship between baseline impedance levels and esophageal mucosal integrity in children with erosive and non-erosive reflux disease. Neurogastroenterol Motil 2012;24:828–e394. doi:10.1111/j.1365-2982.2012.01947.x [DOI] [PubMed] [Google Scholar]