Abstract
Recently, ephedra was removed from the U.S. marketplace due to a heightened concern that dietary supplements containing ephedra may present “an unreasonable risk of illness or injury.” This is the 1st time the U.S. Food and Drug Administration has banned an herbal supplement, and the ban sheds light on the potential harm of nutritional supplements that are used for weight loss or as a boost to athletic performance. We report the case of a body builder who used Xenadrine® RFA, an ephedra-containing supplement, at recommended doses for nearly a year; he then experienced an acute myocardial infarction, which was documented to be secondary to thrombosis in situ. We ruled out other possible causes of myocardial infarction, as well a hypercoagulable state. There was no evidence of illicit drug use. Our report serves as a poignant reminder of the potential dangers of herbal supplementation, especially when used to heighten athletic performance.
Key words: Cardiovascular diseases/chemically induced/epidemiology; dietary supplements/adverse effects; drugs, Chinese herbal/adverse effects; ephedrine/adverse effects; human; male; myocardial infarction/chemically induced/epidemiology; thrombosis/chemically induced
Ephedra alkaloids are naturally occurring stimulants that are widely used to enhance athletic performance and to promote weight loss. These substances have been associated with adverse cardiovascular events, including acute myocardial infarction, severe hypertension, myocarditis, and lethal cardiac arrhythmias.1–6 They have also been reported to predispose patients to hemorrhagic and ischemic strokes.7–9 In 1999, an estimated 12 million people in the United States consumed performance-enhancing products containing ma huang, which is an ephedra alkaloid.1 Xenadrine® RFA (Cytodyne Technologies; Lakewood, NJ*) was one such preparation that contained guarana, a caffeine-like substance, and ma huang. We report the case of a young man who presented with acute myocardial infarction due to in situ coronary thrombosis after using Xenadrine RFA for 1 year.
Case Report
In September 2002, a 24-year-old man presented at the emergency room of our institution with acute onset of left precordial chest pain that radiated to the left arm, accompanied by numbness of the left arm and hand. The pain occurred during exercise. There was no family history of premature coronary artery disease. The patient had no history of chest trauma, diabetes, hypertension, hypercholesterolemia, smoking, or alcohol consumption. He was a body builder and had been using Xenadrine RFA throughout the previous year.
On physical examination, the patient was a well-built, robust young man who was 72 inches tall and weighed 204 lb. He was afebrile. The blood pressure was 124/69 mmHg, and the pulse was 92 beats/minute. The jugular venous pressure was normal, and the lung fields were clear. There was no precordial tenderness. The apex beat was not displaced. The 1st heart sound was normal, and the 2nd heart sound was widely but physiologically split. An S4 gallop was present, but no S3, rub, murmurs, or clicks were heard.
The electrocardiogram revealed sinus rhythm, right bundle branch block, and 2- to 3-mm ST-segment elevation in leads I, aVL and V2-6 (Fig. 1). A chest radiograph showed an enlarged cardiac silhouette with clear lung fields. The serum troponin T level was elevated on admission and rose further on subsequent testing.
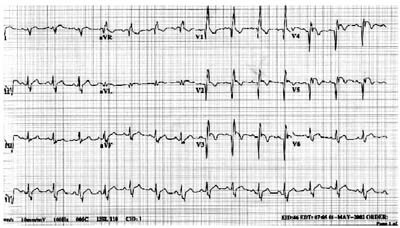
Fig. 1 Electrocardiogram at admission.
The patient was treated with aspirin, intravenous nitroglycerin, and intravenous metoprolol. The chest pain and ST-segment elevation persisted. Emergent coronary angiography revealed total occlusion of the proximal left anterior descending artery (Fig. 2). Suction of the thrombus was performed with the AngioJet® System (Possis Medical, Inc.; Minneapolis, Minn). No underlying atherosclerotic lesions were found (Fig. 3), and the left and right coronary systems appeared to be free of atherosclerosis. Persistent occlusion of the apical portion of the anterior descending artery was attributed to embolization of the proximal thrombus despite AngioJet thrombectomy, distal angioplasty, and intracoronary infusion of both nitroglycerin and adenosine. The patient had 2 episodes of ventricular tachycardia that required cardioversion.
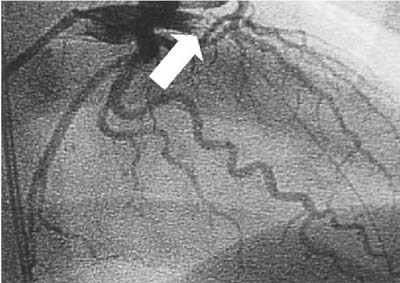
Fig. 2 Initial coronary angiogram, cranial right anterior oblique view, demonstrates total occlusion (arrow) of the proximal left anterior descending artery. A guidewire traverses the center of the occlusion, confirming the presence of thrombus.
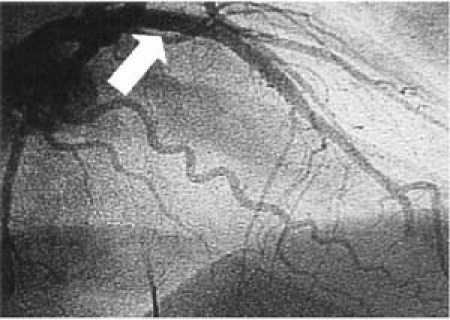
Fig. 3 Left coronary artery angiogram, cranial right anterior oblique view, after AngioJet® suctioning of the occlusive thrombus. The residual lumen (arrow) is smooth and of normal caliber, revealing neither spasm nor underlying atherosclerotic plaque.
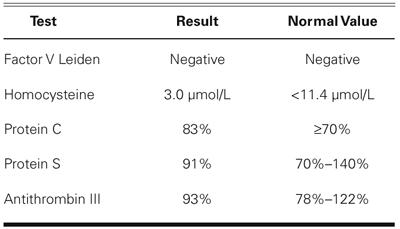
Laboratory studies showed a normal lipid profile (including high-density lipoprotein, 57 mg/dL; and low-density lipoprotein, 97 mg/dL) and a normal prothrombin time, partial thromboplastin time, and platelet count. Results of the anticardiolipin antibody test were normal, and there was no other evidence of a hypercoagulable state (Table I).
TABLE I. Coagulation Profile
The patient's hospital course was complicated by rhabdomyolysis, which gradually resolved over an 8-day period. An echocardiogram obtained on the 8th hospital day showed normal valves, a left ventricular ejection fraction of 0.35 to 0.40, and hypokinesis of the anteroseptal and anteroapical segments. The patient was discharged from the hospital in stable condition on the 9th postoperative day. The patient was lost to follow-up after several months, but was clinically stable when we last saw him.
Discussion
This young, active, previously healthy body builder had an acute myocardial infarction in the absence of structural heart disease or known risk factors for coronary artery disease. Coronary angiography revealed thrombotic occlusion of the anterior descending artery with no demonstrable underlying atherosclerotic plaque and no evidence of active coronary spasm. The myocardial infarction was likely caused by in situ coronary thrombosis, perhaps related to transient coronary spasm and transient platelet activation as a result of intense sympathetic activity. In the absence of demonstrable factors predisposing the patient to a hypercoagulable state, this infarct was likely related to the patient's use of Xenadrine RFA.
Xenadrine RFA contained 335 mg of ma huang (ephedra alkaloid) and 910 mg of guarana seed (equivalent to caffeine) per recommended dose. It was an over-the-counter dietary supplement that the patient had taken in doses that were well within the manufacturer's recommendations.
The pharmacokinetics of ma huang have been well defined. It is well absorbed after oral administration and is excreted essentially unchanged in the urine, with a serum half-life of approximately 5 hours.10 Ma huang increases the availability of catecholamines at adrenergic synapses in the brain and in the heart, directly stimulating α- and β-adrenergic receptors.11 As a result, the heart rate increases and the blood pressure rises secondary to elevated cardiac output and peripheral resistance. Coronary vasospasm is thought to be the underlying mechanism of myocardial infarction in patients taking ma huang.3,5 Ma huang has also been reported to affect the cardiac conduction system.6 The adrenergic effects of ephedra shorten cardiac refractory periods, permitting the development of reentrant cardiac arrhythmias.6
Caffeine enhances the cardiovascular and central nervous system effects of ephedra alkaloids through augmentation of catecholamine release.12,13 By competitively antagonizing adenosine receptors that mediate vasodilation, caffeine constricts blood vessels and can increase blood pressure, especially in persons prone to hypertension.
The combined effects of caffeine and ephedra increase myocardial oxygen consumption by increas-ing heart rate, contractility, and peripheral resistance (afterload), while potentially diminishing coronary blood flow by increasing coronary resistance and promoting frank coronary spasm. The diminished blood flow may lead to the development of in situ thrombosis. Although there is a lack of prospective evidence regarding the effects of ephedra-containing alkaloids on platelet function, coagulation, and the fibrinolytic system, adverse embolic-related events associated with ephedra-containing herbal products have been reported.1 Recently, Gardner and colleagues14 found no significant change in plasminogen activator inhibitor-1 or tissue plasminogen activator levels with short-term ephedra use. However, enhanced sympathetic activity increases platelet reactivity, which likely contributes to a prothrombotic state in the presence of ephedra alkaloids. Therefore, it is not surprising that preparations such as Xenadrine RFA might precipitate myocardial infarction, even in the absence of significant atherosclerosis.
Samenuk and coworkers2 reviewed the comprehensive database of the U.S. Food and Drug Administration (FDA) for all reports of adverse experiences related to ma huang use from 1995 to 1997. In this study, 37 of the 926 cases of possible ma huang toxicity were temporally related to stroke (n = 16), myocardial infarction (n = 10), or sudden death (n = 11). In a similar analysis of FDA data from June 1997 through March 1999, 87 of 140 reported adverse events were deemed definitely, probably, or possibly related to the use of ephedra alkaloids.1 Of the reported cardiovascular adverse effects, hypertension occurred most often, followed by palpitations and tachycardia, sudden death, arrhythmia, and myocardial infarction. Stroke was reported in 17 cases and seizures in 7.1 Cockings and associates3 reported a case of myocardial infarction in a 25-year-old man after intravenous ephedra administration.3
According to its product label, Xenedrine RFA contained 325 mg of ma huang (about 20 mg of ephedra) per serving. The manufacturer recommended that it be taken 2 to 3 times a day. It has been observed, however, that herbal products such as Xenadrine RFA may contain variable amounts of ephedra alkaloids.15,16 Samenuk's group2 reported that in 36 of the 37 patients in their series who had adverse effects after using ma huang, the dose taken was within the manufacturers' guidelines. Our patient had also followed the dose recommendations written on the label but suffered a potentially fatal cardiovascular event.
As is evident from previous reports and from our case, performance-enhancing preparations that contain ma huang (especially those such as Xenadrine RFA that also contain caffeine-like substances) can lead to morbidity and mortality. To our knowledge, ours is the 1st case reported in the English-language medical literature in which in situ coronary thrombosis was demonstrated angiographically. This observation reinforces the hypothesis that coronary spasm, promoted by ephedra-caffeine combinations, may precipitate coronary thrombosis that can lead to myocardial infarction. These events can occur even in individuals with no apparent underlying coronary atherosclerosis or hypercoagulable state, as is seen with cocaine abuse. Ephedra toxicity also increases the risk of rhabdomyolysis,17 which occurred in our patient.
The present case, in the context of previous reports, supports ongoing concerns about the potential health hazards of products such as Xenadrine RFA that are promoted as performance-enhancing, weight-reducing, and thus potentially healthful substances. Such claims have not been scientifically proved. The exact incidence of serious adverse effects related to ma huang and other ephedra alkaloids may be underestimated due to a lack of suspicion at the time of diagnosis, or to inadequate reporting. Our patient had been using Xenadrine RFA for 1 year. Some might conclude that such chronic exposure decreases the likelihood that Xenadrine RFA precipitated this young man's infarction. However, it is difficult to conclude that previous event-free use indicates that the preparation is safe, since coronary thrombosis occurred in this patient without any other demonstrable cause. Prolonged exposure to the ephedra-caffeine combination leads to a state of heightened platelet reactivity and coronary vasoconstriction, which likely predisposed this patient to coronary thrombosis.
Although the FDA's adverse event reporting system has served a useful purpose in drawing attention to the potential public health concerns over Xenadrine RFA and similar over-the-counter preparations, the FDA was initially somewhat slow to react. In December 2003, however, the FDA announced that it would be removing ephedra from the market place, and the final ruling went into effect on 12 April 2004. This was the 1st time that the FDA had banned an herbal supplement. Our case serves as a reminder that the ban arrived not a moment too soon.
Footnotes
*Cytodyne Technologies, Inc., which has discontinued its RFA line of Xenadrine, currently markets other products bearing the name Xenadrine that do not contain ma huang.
Address for reprints: Craig A. McPherson, MD, FACC, Division of Cardiology, Bridgeport Hospital, Yale University School of Medicine, 267 Grant Street, Bridgeport, CT 06610. E-mail: pcmcph@bpthosp.org
References
- 1.Haller CA, Benowitz NL. Adverse cardiovascular and central nervous system events associated with dietary supplements containing ephedra alkaloids. N Engl J Med 2000; 343:1833–8. [DOI] [PubMed]
- 2.Samenuk D, Link MS, Homoud MK, Contreras R, Theoharides TC, Wang PJ, et al. Adverse cardiovascular events temporally associated with ma huang, an herbal source of ephedrine [published erratum appears in Mayo Clin Proc 2003;78:1055]. Mayo Clin Proc 2002;77:12–6. [DOI] [PubMed]
- 3.Cockings JG, Brown M. Ephedrine abuse causing acute myocardial infarction. Med J Aust 1997;167:199–200. [DOI] [PubMed]
- 4.Theoharides TC. Sudden death of a healthy college student related to ephedrine toxicity from a ma huang-containing drink. J Clin Psychopharmacol 1997;17:437–9. [DOI] [PubMed]
- 5.Zaacks SM, Klein L, Tan CD, Rodriguez ER, Leikin JB. Hypersensitivity myocarditis associated with ephedra use. J Toxicol Clin Toxicol 1999;37:485–9. [DOI] [PubMed]
- 6.Weesner KM, Denison M, Roberts RJ. Cardiac arrhythmias in an adolescent following ingestion of an over-the-counter stimulant. Clin Pediatr (Phila) 1982;21:700–1. [DOI] [PubMed]
- 7.Bruno A, Nolte KB, Chapin J. Stroke associated with ephedrine use. Neurology 1993;43:1313–6. [DOI] [PubMed]
- 8.Vahedi K, Domigo V, Amarenco P, Bousser MG. Ischaemic stroke in a sportsman who consumed MaHuang extract and creatine monohydrate for body building. J Neurol Neurosurg Psychiatry 2000;68:112–3. [DOI] [PMC free article] [PubMed]
- 9.Wooten MR, Khangure MS, Murphy MJ. Intracerebral hemorrhage and vasculitis related to ephedrine abuse. Ann Neurol 1983;13:337–40. [DOI] [PubMed]
- 10.Gurley BJ, Gardner SF, White LM, Wang PL. Ephedrine pharmacokinetics after the ingestion of nutritional supplements containing Ephedra sinica (ma huang). Ther Drug Monit 1998;20:439–45. [DOI] [PubMed]
- 11.Sapru HN, Theoharides TC. Autonomic nervous system. In: Theoharides TC, editor. Pharmacology (Essentials of basic science). 2nd ed. Boston: Little, Brown; 1996. p. 35–75.
- 12.Robertson D, Frolich JC, Carr RK, Watson JT, Hollifield JW, Shand DG, Oates JA. Effects of caffeine on plasma renin activity, catecholamines and blood pressure. N Engl J Med 1978;298:181–6. [DOI] [PubMed]
- 13.Benowitz NL. Clinical pharmacology of caffeine. Annu Rev Med 1990;41:277–88. [DOI] [PubMed]
- 14.Gardner SF, Franks AM, Gurley BJ, Haller CA, Singh BK, Mehta JL. Effect of a multicomponent, ephedra-containing dietary supplement (Metabolife 356) on Holter monitoring and hemostatic parameters in healthy volunteers. Am J Cardiol 2003;91:1510–3,A9. [DOI] [PubMed]
- 15.Gurley BJ, Gardner SF, Hubbard MA. Content versus label claims in ephedra-containing dietary supplements. Am J Health Syst Pharm 2000;57:963–9. [DOI] [PubMed]
- 16.Lee MK, Cheng BW, Che CT, Hsieh DP. Cytotoxicity assessment of Ma-huang (Ephedra) under different conditions of preparation. Toxicol Sci 2000;56:424–30. [DOI] [PubMed]
- 17.Mack RB. “All but death, can be adjusted”. Ma Huang (ephedrine) adversities. N C Med J 1997;58:68–70. [PubMed]


